Assessment of Pretreatments for Highly Concentrated Leachate Waters to Enhance the Performance of Catalytic Wet Peroxide Oxidation with Sustainable Low-Cost Catalysts
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
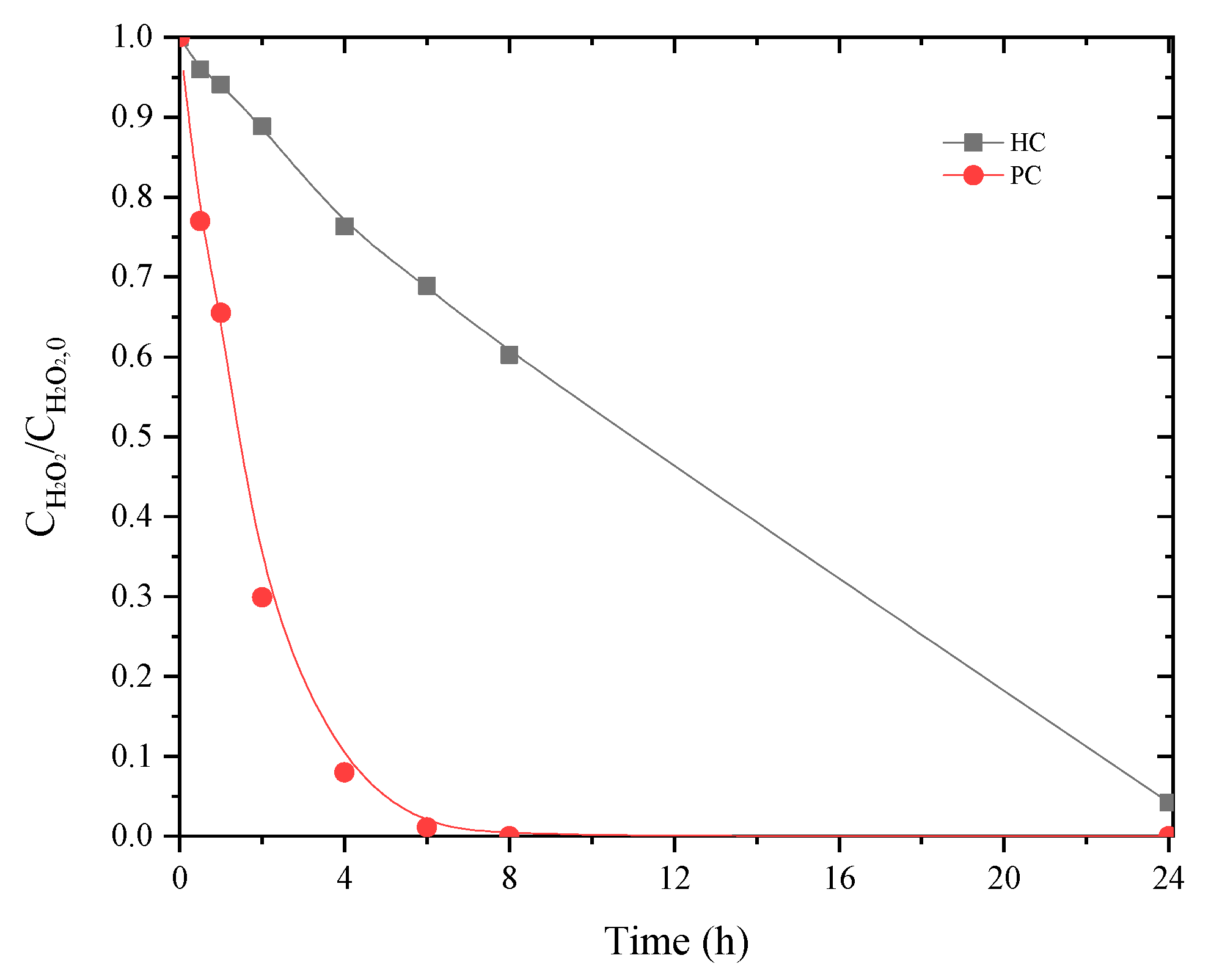
2.2. CWPO of Leachate Waters as a Single Step
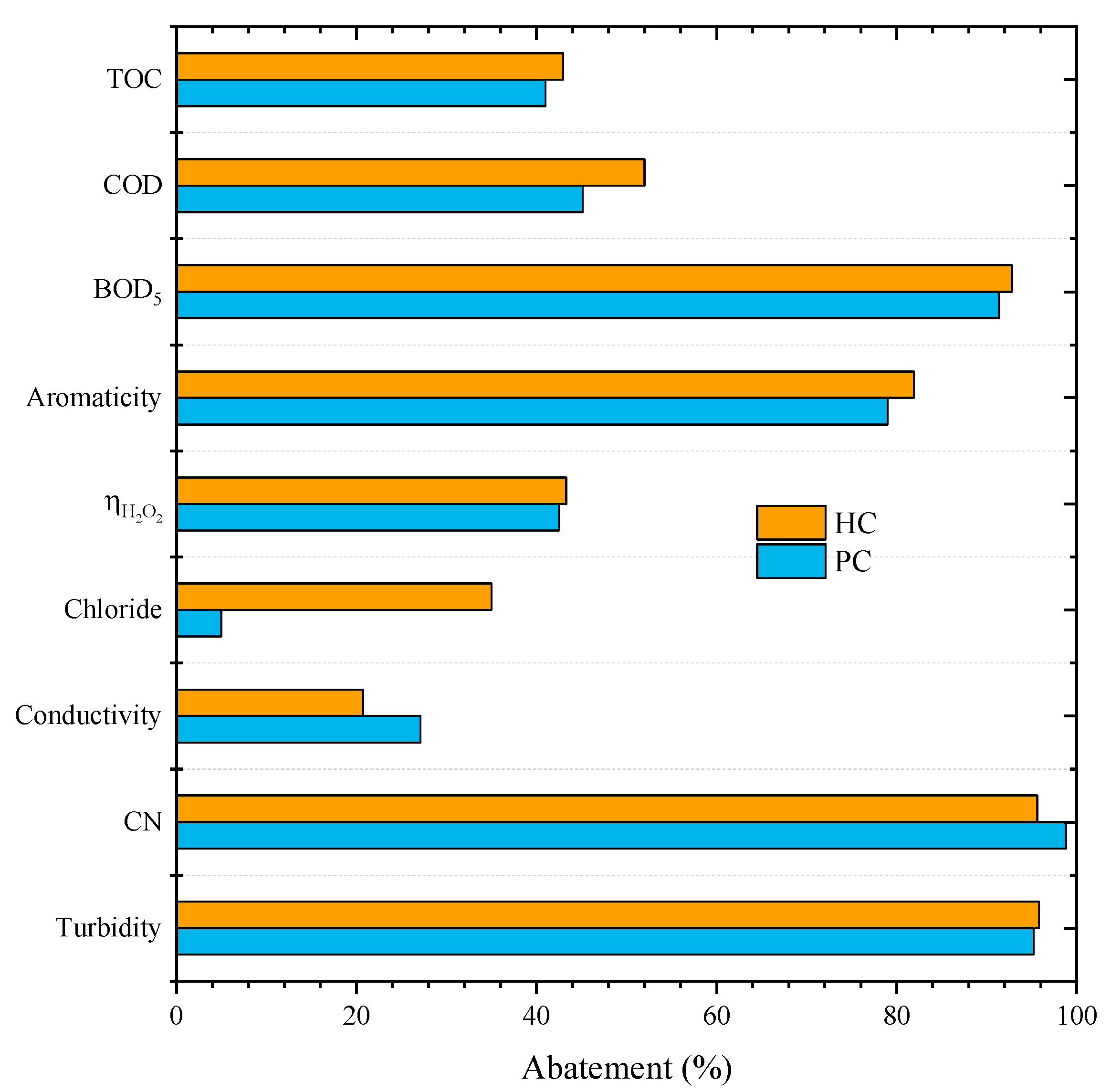
2.3. Ion Exchange Resin and CWPO
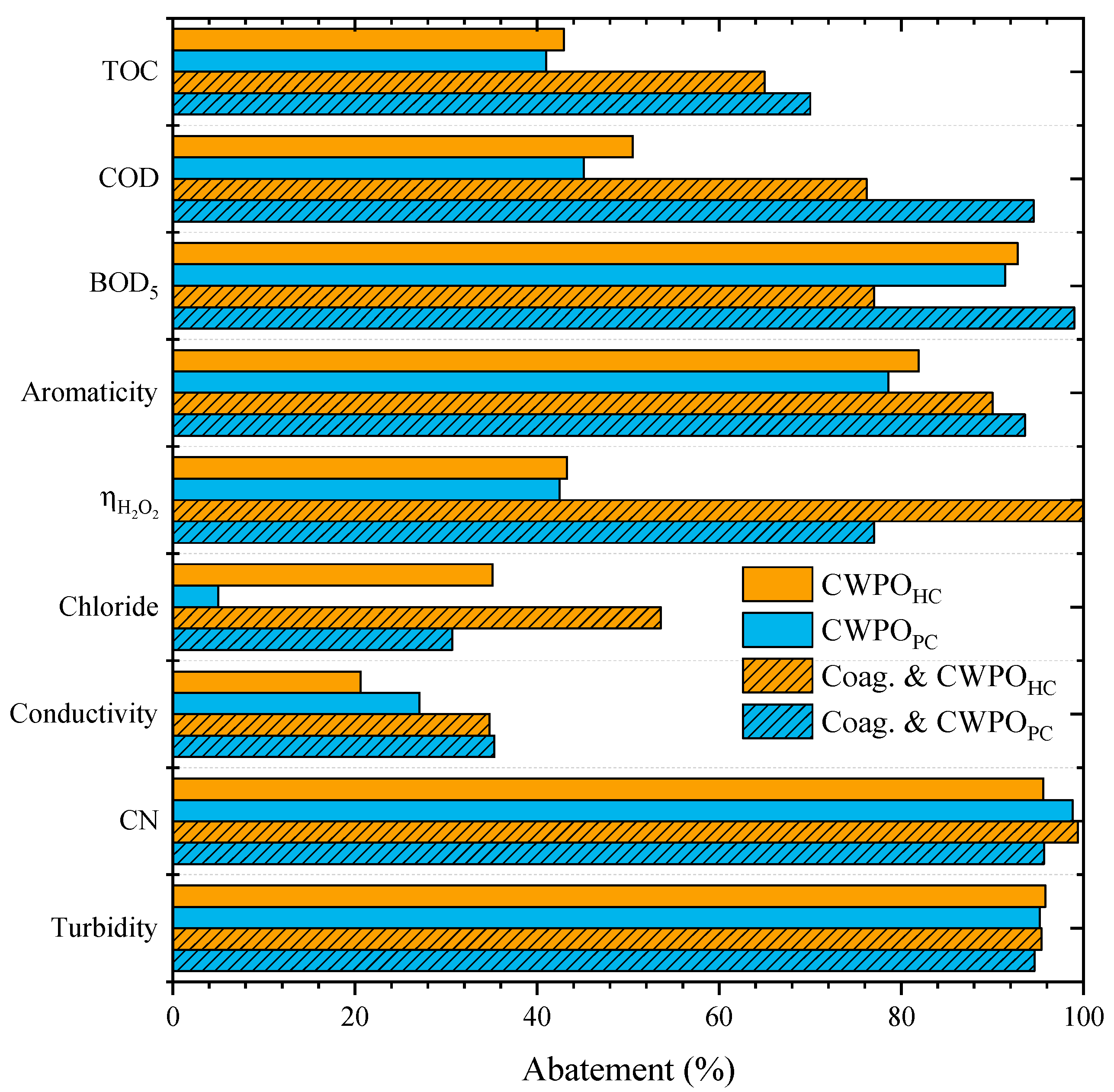
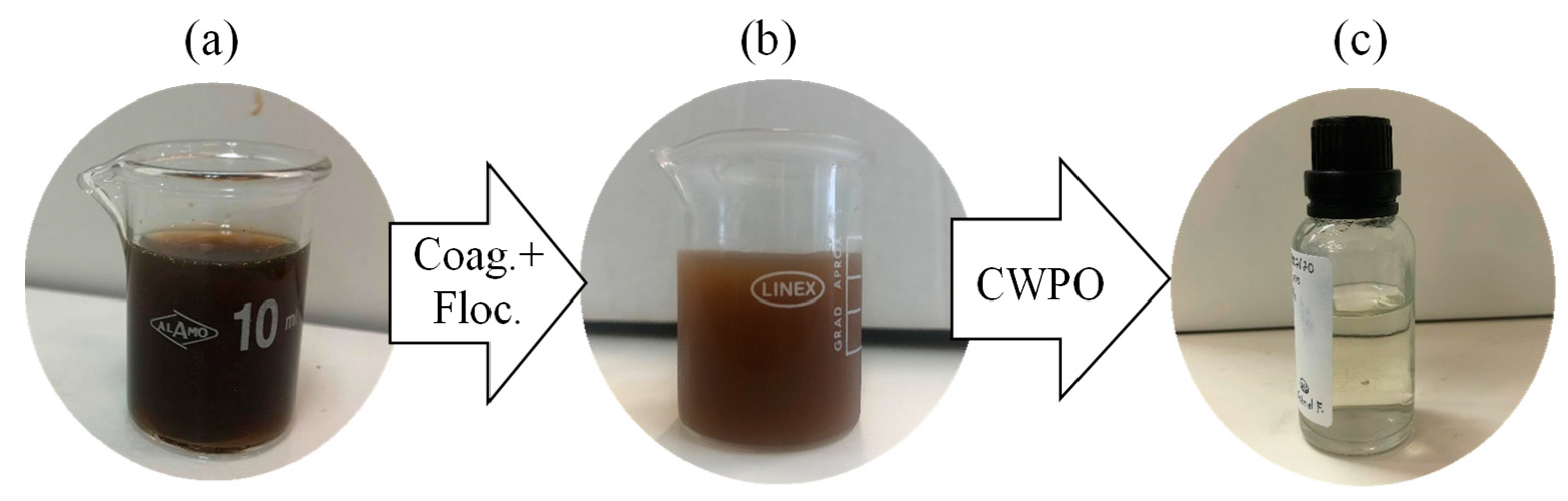
2.4. Coagulation–Flocculation and CWPO
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation and Characterization of Catalysts
3.3. Treatment of the Leachate Waters
3.3.1. Ion-Exchange Resin
3.3.2. Coagulation–Flocculation
3.3.3. CWPO Experiments
3.4. Analytical Techniques
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roman, F.F.; de Tuesta, J.L.D.; Praça, P.; Silva, A.M.; Faria, J.L.; Gomes, H.T. Hydrochars from compost derived from municipal solid waste: Production process optimization and catalytic applications. J. Environ. Chem. Eng. 2021, 9, 104888. [Google Scholar] [CrossRef]
- Eurostat—Statistics Explained. Municipal Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Municipal_waste_statistics (accessed on 3 November 2019).
- Wei, Y.; Li, J.; Shi, D.; Liu, G.; Zhao, Y.; Shimaoka, T. Environmental challenges impeding the composting of biodegradable municipal solid waste: A critical review. Resour. Conserv. Recycl. 2017, 122, 51–65. [Google Scholar] [CrossRef]
- De Tuesta, J.L.D.; de Almeida, F.V.; Oliveira, J.R.; Praça, P.; Guerreiro, M.C.; Gomes, H.T. Kinetic insights on wet peroxide oxidation of caffeine using EDTA-functionalized low-cost catalysts prepared from compost generated in municipal solid waste treatment facilities. Environ. Technol. Innov. 2021, 24, 101984. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Rodrigues, R.O.; Silva, A.M.; Tavares, P.B.; Carvalho, A.M.; Figueiredo, J.L.; Faria, J.; Gomes, H.T. Hybrid magnetic graphitic nanocomposites towards catalytic wet peroxide oxidation of the liquid effluent from a mechanical biological treatment plant for municipal solid waste. Appl. Catal. B Environ. 2017, 219, 645–657. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; Cheng, Y.; He, D.; Pan, X. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Sci. Total Environ. 2020, 703, 135468. [Google Scholar] [CrossRef]
- Gautam, P.; Kumar, S.; Lokhandwala, S. Advanced oxidation processes for treatment of leachate from hazardous waste landfill: A critical review. J. Clean. Prod. 2019, 237, 117639. [Google Scholar] [CrossRef]
- Ishak, A.R.; Hamid, F.S.; Mohamad, S.; Tay, K.S. Removal of organic matter from stabilized landfill leachate using Coagulation-Flocculation-Fenton coupled with activated charcoal adsorption. Waste Manag. Res. 2017, 35, 739–746. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Rueda Márquez, J.J.; Levchuk, I.; Sillanpää, M. Application of Catalytic Wet Peroxide Oxidation for Industrial and Urban Wastewater Treatment: A Review. Catalysts 2018, 8, 673. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.; Figueiredo, J.; Faria, J.; Gomes, H.T. Catalytic wet peroxide oxidation: A route towards the application of hybrid magnetic carbon nanocomposites for the degradation of organic pollutants. A review. Appl. Catal. B Environ. 2016, 187, 428–460. [Google Scholar] [CrossRef]
- De Tuesta, J.D.; Quintanilla, A.; Casas, J.; Rodriguez, J. Kinetic modeling of wet peroxide oxidation with a carbon black catalyst. Appl. Catal. B Environ. 2017, 209, 701–710. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; Casas, J.A.; Zazo, J.A.; Rodriguez, J.J. Highly stable Fe/γ-Al2O3 catalyst for catalytic wet peroxide oxidation. J. Chem. Technol. Biotechnol. 2011, 86, 497–504. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Q.; Hua, T. Removal of Organic Matter from Landfill Leachate by Advanced Oxidation Processes: A Review. Int. J. Chem. Eng. 2010, 2010, 270532. [Google Scholar] [CrossRef]
- Torretta, V.; Ferronato, N.; Katsoyiannis, I.A.; Tolkou, A.K.; Airoldi, M. Novel and Conventional Technologies for Landfill Leachates Treatment: A Review. Sustainability 2016, 9, 9. [Google Scholar] [CrossRef]
- Galeano, L.A.; Vicente, M.Á.; Gil, A. Treatment of municipal leachate of landfill by Fenton-like heterogeneous catalytic wet peroxide oxidation using an Al/Fe-pillared montmorillonite as active catalyst. Chem. Eng. J. 2011, 178, 146–153. [Google Scholar] [CrossRef]
- Sruthi, T.; Gandhimathi, R.; Ramesh, S.; Nidheesh, P. Stabilized landfill leachate treatment using heterogeneous Fenton and electro-Fenton processes. Chemosphere 2018, 210, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Mena, I.F.; Diaz, E.; Rodriguez, J.J.; Mohedano, A.F. CWPO of bisphenol A with iron catalysts supported on microporous carbons from grape seeds activation. Chem. Eng. J. 2017, 318, 153–160. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Pang, Y.; Li, X.; Chen, F.; Liao, X.; Lei, M.; Song, Y. Landfill leachate treatment by coagulation/flocculation combined with microelectrolysis-Fenton processes. Environ. Technol. 2019, 40, 1862–1870. [Google Scholar] [CrossRef]
- Bakraouy, H.; Souabi, S.; Digua, K.; Dkhissi, O.; Sabar, M.; Fadil, M. Optimization of the treatment of an anaerobic pretreated landfill leachate by a coagulation–flocculation process using experimental design methodology. Process Saf. Environ. Prot. 2017, 109, 621–630. [Google Scholar] [CrossRef]
- Oloibiri, V.; De Coninck, S.; Chys, M.; Demeestere, K.; Van Hulle, S.W. Characterisation of landfill leachate by EEM-PARAFAC-SOM during physical-chemical treatment by coagulation-flocculation, activated carbon adsorption and ion exchange. Chemosphere 2017, 186, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Zamri, M.F.M.A.; Kamaruddin, M.A.; Yusoff, M.S.; Aziz, H.A.; Foo, K.Y. Semi-aerobic stabilized landfill leachate treatment by ion exchange resin: Isotherm and kinetic study. Appl. Water Sci. 2017, 7, 581–590. [Google Scholar] [CrossRef]
- Niveditha, S.; Gandhimathi, R. Flyash augmented Fe3O4 as a heterogeneous catalyst for degradation of stabilized landfill leachate in Fenton process. Chemosphere 2020, 242, 125189. [Google Scholar] [CrossRef] [PubMed]
- Orkun, M.O.; Kuleyin, A. Treatment performance evaluation of chemical oxygen demand from landfill leachate by electro-coagulation and electro-fenton technique. Environ. Prog. Sustain. Energy 2012, 31, 59–67. [Google Scholar] [CrossRef]
- Moravia, W.G.; Amaral, M.C.; Lange, L.C. Evaluation of landfill leachate treatment by advanced oxidative process by Fenton’s reagent combined with membrane separation system. Waste Manag. 2013, 33, 89–101. [Google Scholar] [CrossRef]
- Zhang, H.; Choi, H.J.; Huang, C.-P. Optimization of Fenton process for the treatment of landfill leachate. J. Hazard. Mater. 2005, 125, 166–174. [Google Scholar] [CrossRef]
- Tizaoui, C.; Mansouri, L.; Bousselmi, L. Ozone catalysed with solids as an advanced oxidation process for landfill leachate treatment. Water Sci. Technol. 2007, 55, 237–243. [Google Scholar] [CrossRef]
- De Tuesta, J.L.D.; Pantuzza, G.F.; Silva, A.M.T.; Praça, P.; Faria, J.L.; Gomes, H.T. Catalysts Prepared with Matured Compost Derived from Mechanical-Biological Treatment Plants for the Wet Peroxide Oxidation of Pollutants with Different Lipophilicity. Catalysts 2020, 10, 1243. [Google Scholar] [CrossRef]
- Gomes, H.; Miranda, S.; Sampaio, M.J.; Silva, A.; Faria, J. Activated carbons treated with sulphuric acid: Catalysts for catalytic wet peroxide oxidation. Catal. Today 2010, 151, 153–158. [Google Scholar] [CrossRef]
- de Tuesta, J.L.D.; Saviotti, M.C.; Roman, F.F.; Pantuzza, G.F.; Sartori, H.J.; Shinibekova, A.; Kalmakhanova, M.S.; Massalimova, B.K.; Pietrobelli, J.M.; Lenzi, G.G.; et al. Assisted hydrothermal carbonization of agroindustrial byproducts as effective step in the production of activated carbon catalysts for wet peroxide oxidation of micro-pollutants. J. Environ. Chem. Eng. 2021, 9, 105004. [Google Scholar] [CrossRef]
- Wang, C.; Jin, X.; Wang, Y.; Yan, Y.; Cui, J.; Liu, Y.; Che, D. Release and Transformation of Sodium during Pyrolysis of Zhundong Coals. Energy Fuels 2014, 29, 78–85. [Google Scholar] [CrossRef]
- Smith, A.; Singh, S.; Ross, A.B. Fate of inorganic material during hydrothermal carbonisation of biomass: Influence of feedstock on combustion behaviour of hydrochar. Fuel 2016, 169, 135–145. [Google Scholar] [CrossRef]
- Huaccallo-Aguilar, Y.; de Tuesta, J.D.; Álvarez-Torrellas, S.; Gomes, H.; Larriba, M.; Ovejero, G.; García, J. New insights on the removal of diclofenac and ibuprofen by CWPO using a magnetite-based catalyst in an up-flow fixed-bed reactor. J. Environ. Manag. 2021, 281, 111913. [Google Scholar] [CrossRef] [PubMed]
- De Tuesta, J.L.D.; Quintanilla, A.; Casas, J.A.; Morales-Torres, S.; Faria, J.L.; Silva, A.M.; Gomes, H.T. The pH effect on the kinetics of 4-nitrophenol removal by CWPO with doped carbon black catalysts. Catal. Today 2020, 356, 216–225. [Google Scholar] [CrossRef]
- Domínguez, C.; Quintanilla, A.; Casas, J.A.; Rodriguez, J. Kinetics of wet peroxide oxidation of phenol with a gold/activated carbon catalyst. Chem. Eng. J. 2014, 253, 486–492. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Rey, A.; Faraldos, M.; Bahamonde, A.; Casas, J.A.; Zazo, J.A.; Rodríguez, J.J. Role of the Activated Carbon Surface on Catalytic Wet Peroxide Oxidation. Ind. Eng. Chem. Res. 2008, 47, 8166–8174. [Google Scholar] [CrossRef]
- Pinho, M.T.; Ribeiro, R.S.; Gomes, H.T.; Faria, J.L.; Silva, A.M.T. Screening of Activated Carbons for the Treatment of Highly Concentrated Phenol Solutions Using Catalytic Wet Peroxide Oxidation: The Effect of Iron Impurities on the Catalytic Activity. Catalysts 2020, 10, 1318. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, H.; Liu, P.; Yu, Y.; Zhao, Y.; Li, X.; Jiang, W.; Wang, J.; Yang, X.; Sun, C. Effect of structural defects on activated carbon catalysts in catalytic wet peroxide oxidation of m-cresol. Catal. Today 2015, 258, 120–131. [Google Scholar] [CrossRef]
- Martin-Martinez, M.; Ribeiro, R.S.; Machado, B.F.; Serp, P.; Morales-Torres, S.; Silva, A.M.T.; Figueiredo, J.; Faria, J.L.; Gomes, H.T. Role of Nitrogen Doping on the Performance of Carbon Nanotube Catalysts: A Catalytic Wet Peroxide Oxidation Application. ChemCatChem 2016, 8, 2068–2078. [Google Scholar] [CrossRef]
- De Tuesta, J.D.; Quintanilla, A.; Casas, J.; Rodriguez, J. P-, B- and N-doped carbon black for the catalytic wet peroxide oxidation of phenol: Activity, stability and kinetic studies. Catal. Commun. 2017, 102, 131–135. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.; Pinho, M.T.; Figueiredo, J.L.; Faria, J.; Gomes, H.T. Development of glycerol-based metal-free carbon materials for environmental catalytic applications. Catal. Today 2015, 240, 61–66. [Google Scholar] [CrossRef]
- De Tuesta, J.L.D.; Quintanilla, A.; Moreno, D.; Ferro, V.R.; Casas, J.A. Simulation and Optimization of the CWPO Process by Combination of Aspen Plus and 6-Factor Doehlert Matrix: Towards Autothermal Operation. Catalysts 2020, 10, 548. [Google Scholar] [CrossRef]
- Vedrenne, M.; Vasquez-Medrano, R.; Prato, D.; Frontana-Uribe, B.A.; Ibanez, J.G. Characterization and detoxification of a mature landfill leachate using a combined coagulation–flocculation/photo Fenton treatment. J. Hazard. Mater. 2012, 205-206, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gu, Z.; Wen, P.; Li, Q. Degradation of refractory organic contaminants in membrane concentrates from landfill leachate by a combined coagulation-ozonation process. Chemosphere 2019, 217, 411–422. [Google Scholar] [CrossRef]
- Amor, C.; De Torres-Socías, E.; Peres, J.; Maldonado, M.I.; Oller, I.; Malato, S.; Lucas, M.S. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J. Hazard. Mater. 2015, 286, 261–268. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, W.; Shi, P.; Guo, J.; Cheng, J. Characterization of dissolved organic matter in landfill leachate during the combined treatment process of air stripping, Fenton, SBR and coagulation. Waste Manag. 2015, 41, 111–118. [Google Scholar] [CrossRef]
- Segundo, I.D.B.; Gomes, A.I.; Souza-Chaves, B.M.; Park, M.; dos Santos, A.B.; Boaventura, R.A.; Moreira, F.C.; Silva, T.F.; Vilar, V.J. Incorporation of ozone-driven processes in a treatment line for a leachate from a hazardous industrial waste landfill: Impact on the bio-refractory character and dissolved organic matter distribution. J. Environ. Chem. Eng. 2021, 9, 105554. [Google Scholar] [CrossRef]
- Tejera, J.; Miranda, R.; Hermosilla, D.; Urra, I.; Negro, C.; Blanco, Á. Treatment of a Mature Landfill Leachate: Comparison between Homogeneous and Heterogeneous Photo-Fenton with Different Pretreatments. Water 2019, 11, 1849. [Google Scholar] [CrossRef]
- Muthulakshmi, S.; Uma, S.; Hemalatha, G. Feasibility study on compost as partial replacement for fine aggregate in concrete. Mater. Today Proc. 2021, 46, 3775–3778. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Corpas-Iglesias, F.A.; Pérez-Villarejo, L.; Iglesias-Godino, F.J. Recycling of sawdust, spent earth from oil filtration, compost and marble residues for brick manufacturing. Constr. Build. Mater. 2012, 34, 275–284. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Patel, S.; Kundu, S.; Halder, P.; Ratnnayake, N.; Marzbali, M.H.; Aktar, S.; Selezneva, E.; Paz-Ferreiro, J.; Surapaneni, A.; de Figueiredo, C.C.; et al. A critical literature review on biosolids to biochar: An alternative biosolids management option. Rev. Environ. Sci. Bio/Technol. 2020, 19, 807–841. [Google Scholar] [CrossRef]
- Munir, M.T.; Mansouri, S.S.; Udugama, I.A.; Baroutian, S.; Gernaey, K.V.; Young, B.R. Resource recovery from organic solid waste using hydrothermal processing: Opportunities and challenges. Renew. Sustain. Energy Rev. 2018, 96, 64–75. [Google Scholar] [CrossRef]
- Basso, D.; Weiss-Hortala, E.; Patuzzi, F.; Castello, D.; Baratieri, M.; Fiori, L. Hydrothermal carbonization of off-specification compost: A byproduct of the organic municipal solid waste treatment. Bioresour. Technol. 2015, 182, 217–224. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.; Pastrana-Martínez, L.M.; Figueiredo, J.; Faria, J.; Gomes, H. Graphene-based materials for the catalytic wet peroxide oxidation of highly concentrated 4-nitrophenol solutions. Catal. Today 2015, 249, 204–212. [Google Scholar] [CrossRef]
- De Tuesta, J.L.D.; García-Figueruelo, C.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Application of high-temperature Fenton oxidation for the treatment of sulfonation plant wastewater. J. Chem. Technol. Biotechnol. 2015, 90, 1839–1846. [Google Scholar] [CrossRef]
- Tizaoui, C.; Bousselmi, L.; Mansouri, L.; Ghrabi, A. Landfill leachate treatment with ozone and ozone/hydrogen peroxide systems. J. Hazard. Mater. 2007, 140, 316–324. [Google Scholar] [CrossRef]
- Krull, R.; Döpkens, E. Recycling of dyehouse effluents by biological and chemical treatment. Water Sci. Technol. 2004, 49, 311–317. [Google Scholar] [CrossRef][Green Version]
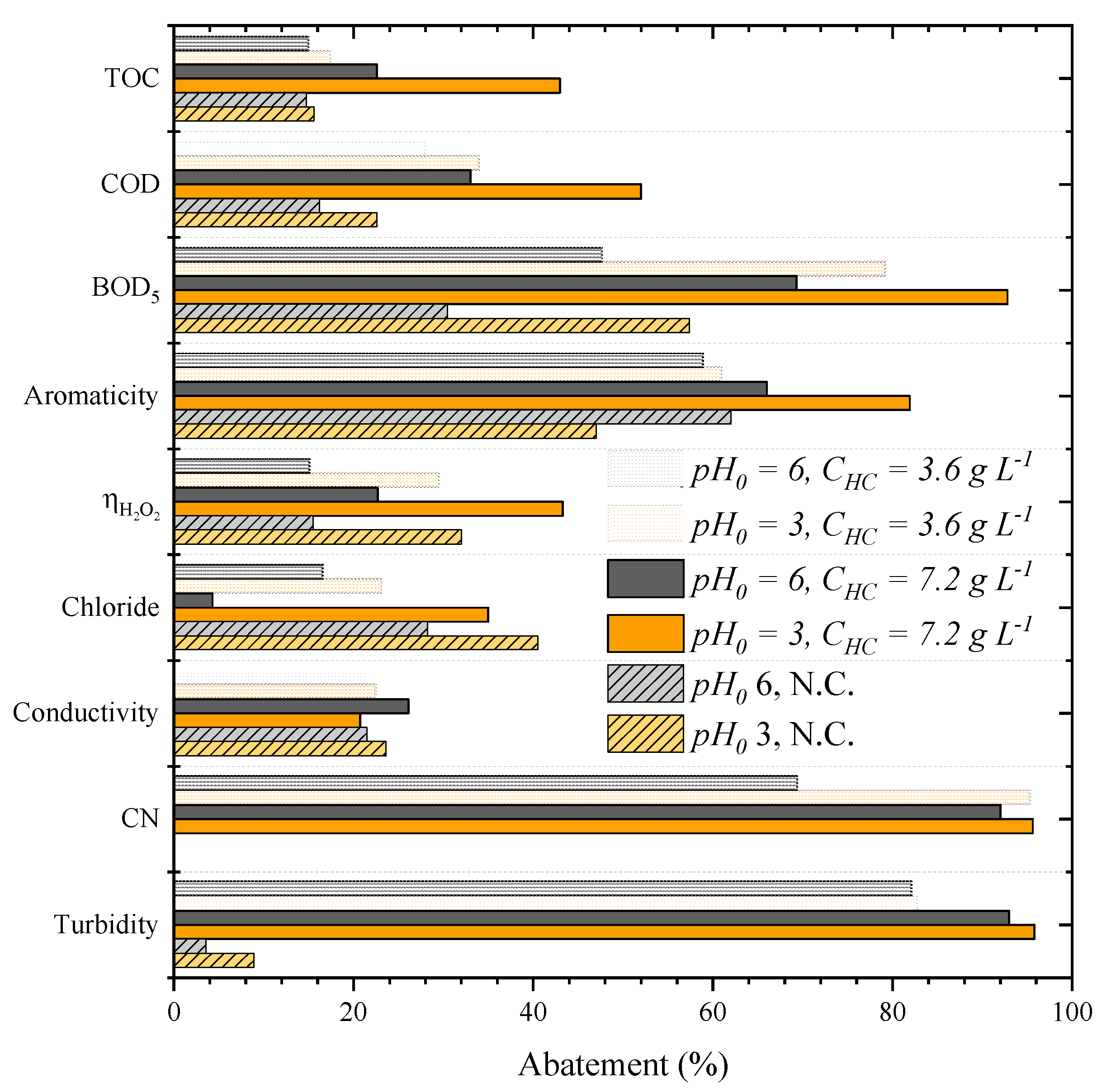


| Catalyst | C/H Ratio | C (%) | H (%) | S (%) | N (%) | Ashes (%) | Mg (%) | K (%) | Si (%) | Al (%) | Na (%) | Ca (%) | Fe (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RP | 9.2 | 21.3 | 2.3 | 0.6 | 1.7 | 55.5 | 1.7 | 1.8 | 0.40 | 3.4 | 0.74 | 11 | 2.3 |
| PC | 44 | 17.6 | 0.4 | 0.5 | 0.0 | 81.5 | 2.0 | 1.9 | 0.41 | 3.6 | 0.63 | 17 | 2.7 |
| HC | 11.3 | 19.3 | 1.7 | 1.2 | 0.1 | 32.6 | 1.6 | 1.0 | 0.47 | 3.5 | 0.47 | 12 | 2.5 |
| Catalyst | Acidity (mmol g−1) | Basicity (mmol g−1) | pHPZC | SBET (m2 g−1) |
|---|---|---|---|---|
| PC | 0.9 | 2.5 | 11.0 | 77 |
| HC | 1.1 | 1.4 | 7.5 | 12 |
| Parameter | Unity | Value |
|---|---|---|
| TOC | g L−1 | 26.8 ± 0.4 |
| COD | g L−1 | 59.9 ± 3.9 |
| BOD5 | g L−1 | 23.3 ± 1.1 |
| Aromaticity | g L−1 | 10.2 ± 0.6 |
| Chlorides | g L−1 | 5.01 ± 0.01 |
| Conductivity | mS cm−1 | 38.8 ± 0.1 |
| pH at 25 °C | - | 7.2 ± 0.1 |
| Color number (CN) | m−1 | 40.0 |
| Turbidity | NTU | 410 ± 6 |
| Iron | mg L−1 | 38.9 ± 2.8 |
| Parameter | Original Leachate | [Al2(SO4)3] = 5 g L−1 | [Al2(SO4)3] = 10 g L−1 | ||
|---|---|---|---|---|---|
| pH = 7 | pH = 8 | pH = 7 | pH = 8 | ||
| COD (g L−1) | 59.9 | 59.9 | 50.2 | 59.9 | 46.2 |
| TOC (g L−1) | 27.0 | 27.0 | 23.8 | 27.0 | 22.4 |
| Sequential Treatment | Leachate Characteristics | Catalyst | Abatement | Ref. |
|---|---|---|---|---|
| Coagulation–flocculation + Photo-Fenton | [COD] = 15 g L−1 [TC] = 8 g L−1 | Fe2+ | 63% and 96% for COD and TC, respectively | [45] |
| Coagulation–flocculation + Ozonation | [COD] = 4 g L−1 [BOD5/COD] = 0.01 [CN] = 3.5 cm−1 | N.A. * | 88% and 98% for COD and CN, respectively BOD5/COD increased to 0.34 | [46] |
| Coagulation–flocculation + Photo-Fenton | [COD] = 6 g L−1 Turbidity = 140 NTU | Fe2+ | 63% and 80% for COD and turbidity, respectively | [47] |
| Air stripping + Fenton + sequencing batch reactor (SBR) + coagulation–flocculation | [COD] = 4.5 g L−1 [BOD5] = 0.8 g L−1 | Fe2+ | 93% and 88% for COD and BOD5, respectively | [48] |
| Coagulation–flocculation + microeletrolysis-Fenton | [COD] = 6 g L−1 | Fe-C | 90% of COD | [20] |
| Catalytic oxidation + chemical precipitation + perozonation (O3/H2O2) + biological oxidation | [COD] = 2.8 g L−1 [BOD5] = 0.36 g L−1 Turbidity = 29 NTU | N.A. * | 75% and 95% for COD and turbidity, respectively | [49] |
| Coagulation + heterogeneous photo-Fenton | [COD] = 4.9 g L−1 [BOD5] = 0.15 g L−1 | Iron microspheres (Fe0) | 85–90% for COD | [50] |
| Coagulation–flocculation + CWPO | [COD] = 60 g L−1 [TOC] = 27 g L−1 [BOD5] = 23 g L−1 Turbidity = 410 NTU | Compost-based | 94%, 70% and 99% for COD, TOC and BOD5, respectively | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Freitas Batista, G.; Roman, F.F.; de Tuesta, J.L.D.; Mambrini, R.V.; Praça, P.; Gomes, H.T. Assessment of Pretreatments for Highly Concentrated Leachate Waters to Enhance the Performance of Catalytic Wet Peroxide Oxidation with Sustainable Low-Cost Catalysts. Catalysts 2022, 12, 238. https://doi.org/10.3390/catal12020238
de Freitas Batista G, Roman FF, de Tuesta JLD, Mambrini RV, Praça P, Gomes HT. Assessment of Pretreatments for Highly Concentrated Leachate Waters to Enhance the Performance of Catalytic Wet Peroxide Oxidation with Sustainable Low-Cost Catalysts. Catalysts. 2022; 12(2):238. https://doi.org/10.3390/catal12020238
Chicago/Turabian Stylede Freitas Batista, Gabriel, Fernanda F. Roman, Jose L. Diaz de Tuesta, Raquel Vieira Mambrini, Paulo Praça, and Helder T. Gomes. 2022. "Assessment of Pretreatments for Highly Concentrated Leachate Waters to Enhance the Performance of Catalytic Wet Peroxide Oxidation with Sustainable Low-Cost Catalysts" Catalysts 12, no. 2: 238. https://doi.org/10.3390/catal12020238
APA Stylede Freitas Batista, G., Roman, F. F., de Tuesta, J. L. D., Mambrini, R. V., Praça, P., & Gomes, H. T. (2022). Assessment of Pretreatments for Highly Concentrated Leachate Waters to Enhance the Performance of Catalytic Wet Peroxide Oxidation with Sustainable Low-Cost Catalysts. Catalysts, 12(2), 238. https://doi.org/10.3390/catal12020238









