Metal-Supported Biochar Catalysts for Sustainable Biorefinery, Electrocatalysis, and Energy Storage Applications: A Review
Abstract
:| 1. Introduction…………………………………………………………………………………………. | 2 |
| 2. Production and Activation of Biochar……………………………………………………………. | 3 |
| 2.1. Thermochemical Production…………………………………………………………………….. | 4 |
| 2.2. Activation………………………………………………………………………………………….. | 4 |
| 3. Metal Functionalization…………………………………………………………………………….. | 5 |
| 3.1. One-Step Method: In-Situ Synthesis…………………………………………………………….. | 5 |
| 3.2. Two-Step Method: Post-Activation……………………………………………………………... | 6 |
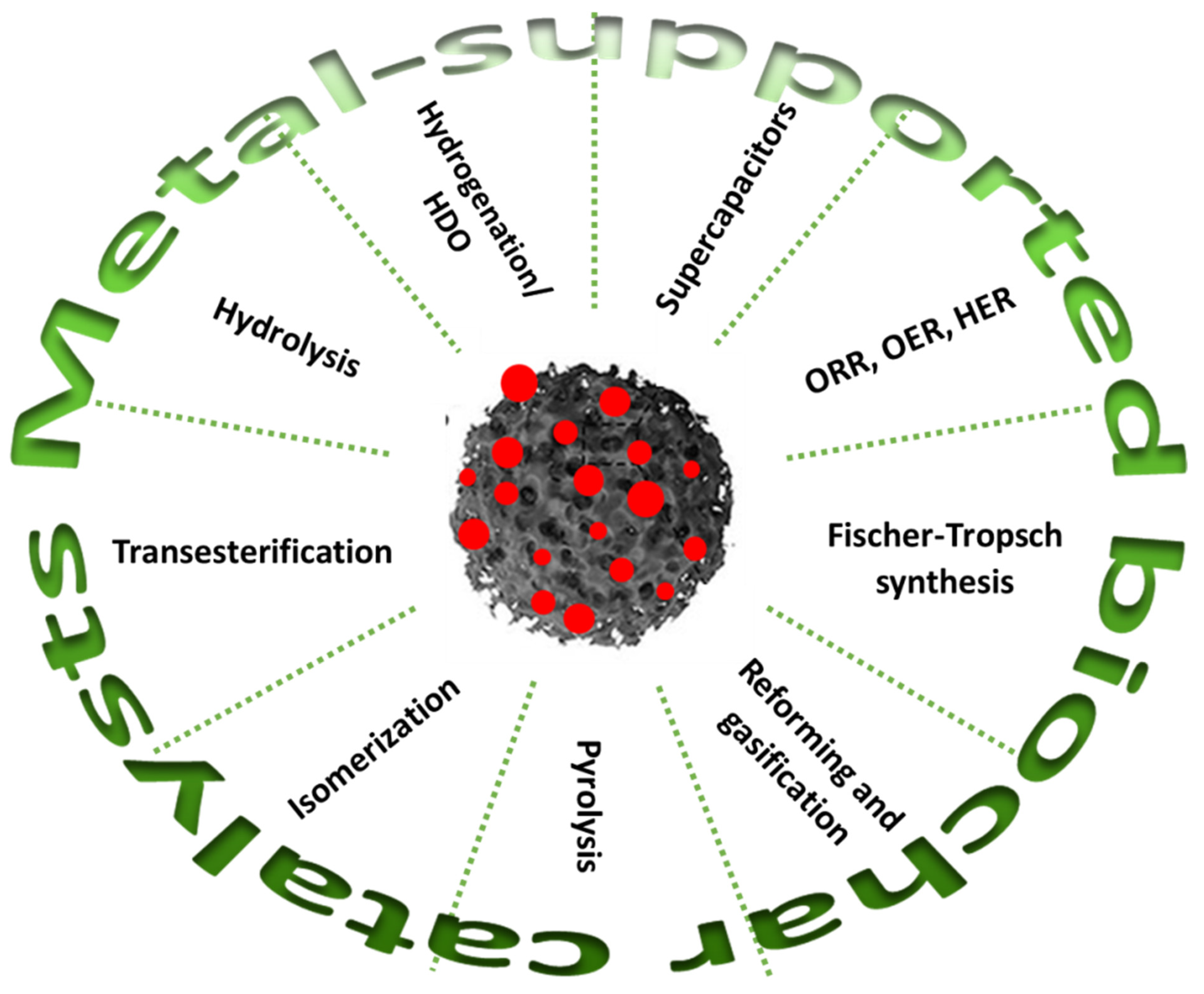
| 4. Applications for Biorefinery Processes…………………………………………………………… | 6 |
| 4.1. Transesterification Reaction……………………………………………………………………… | 7 |
| 4.2. Hydrogenation/Hydrodeoxygenation Reactions…………………………………………….... | 9 |
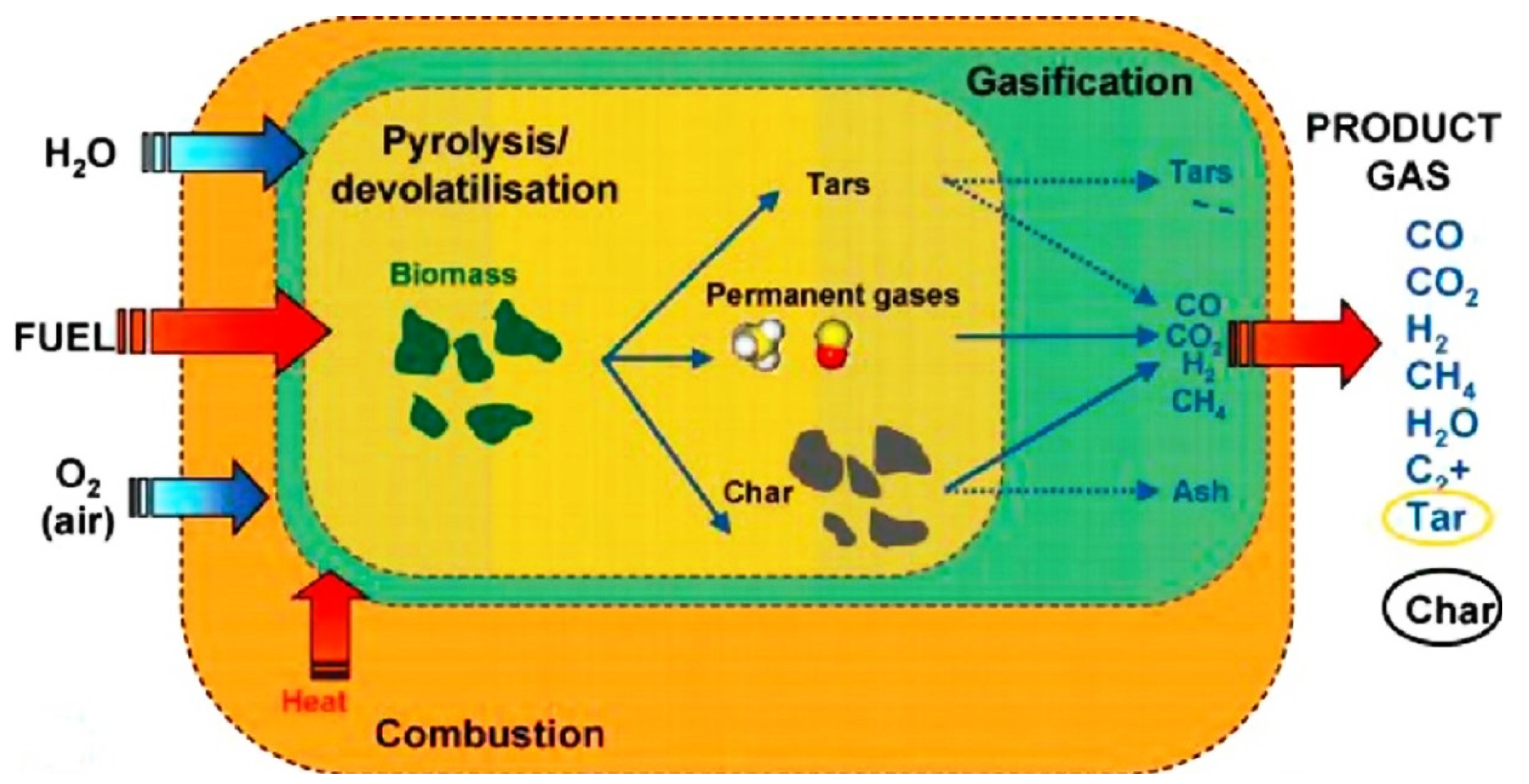
| 4.3. Reforming and Gasification Reactions………………………………………………………….. | 12 |
| 4.4. Pyrolysis Reaction………………………………………………………………………………… | 17 |
| 4.5. Hydrolysis Reactions…………………………………………………………………………….. | 19 |
| 4.6. Other Catalytic Reactions………………………………………………………………………… | 20 |
| 4.6.1. Fischer–Tropsch Synthesis……………………………………………………………………... | 20 |
| 4.6.2. Isomerization Reactions………………………………………………………………………... | 21 |
| 5. Electrochemical Applications……………………………………………………………………… | 21 |
| 5.1. Supercapacitors……………………..…………………………………………………………….. | 21 |
| 5.2. ORR, OER, HER…………………………………………………………………………………… | 37 |
| 5.2.1. Oxygen Reduction Reaction (ORR)…………………………………………………………… | 38 |
| 5.2.2. Oxygen Evolution Reaction (OER)……………………………………………………………. | 50 |
| 5.2.3. Hydrogen Evolution Reaction (HER)………………………………………………………… | 51 |
| 5.2.4. Multifunctional Electrocatalysts………………………………………………………………. | 56 |
| 6. Conclusions………………………………………………………………………………………….. | 57 |
1. Introduction
2. Production and Activation of Biochar
2.1. Thermochemical Production
2.2. Activation
3. Metal Functionalization
3.1. One-Step Method: In-Situ Synthesis
3.2. Two-Step Method: Post-Activation
4. Applications for Biorefinery Processes
4.1. Transesterification Reaction
4.2. Hydrogenation/Hydrodeoxygenation Reactions
4.3. Reforming and Gasification Reactions
4.4. Pyrolysis Reaction
4.5. Hydrolysis Reactions
4.6. Other Catalytic Reactions
4.6.1. Fischer–Tropsch Synthesis
4.6.2. Isomerization Reactions
5. Electrochemical Applications
5.1. Supercapacitors
5.2. ORR, OER, HER
5.2.1. Oxygen Reduction Reaction (ORR)
5.2.2. Oxygen Evolution Reaction (OER)
5.2.3. Hydrogen Evolution Reaction (HER)
5.2.4. Multifunctional Electrocatalysts
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Narayan, R. Biomass (renewable) resources for production of materials, chemicals, and fuels: A paradigm shift. ACS Symp. Ser. 1992, 476, 1–10. [Google Scholar]
- Patel, M.; Zhang, X.; Kumar, A. Techno-economic and life cycle assessment on lignocellulosic biomass thermochemical conversion technologies: A review. Renew. Sustain. Energy Rev. 2016, 53, 1486–1499. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Wu, M. Advances in metal/ biochar catalysts for biomass hydro-upgrading: A review. J. Clean. Prod. 2021, 303, 126825. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Li, M.; Chen, Y. Biochar facilitated bioprocessing and biorefinery for productions of biofuel and chemicals: A review. Bioresour. Technol. 2020, 295, 122252. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-C.; Jiang, H. The thermochemical conversion of non-lignocellulosic biomass to form biochar: A review on characterizations and mechanism elucidation. Bioresour. Technol. 2017, 246, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z.J.C. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N.J.E.S.; et al. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Singh, B.; Joseph, S.; Kimber, S.; Cowie, A.; Chan, K.Y. Biochar and emissions of non-CO2 greenhouse gases from soil. In Biochar for Enviromental Management Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; Volume 1, pp. 227–249. [Google Scholar]
- Liu, R.; Xiao, H.; Guan, S.; Zhang, J.; Yao, D. Technology and method for applying biochar in building materials to evidently improve the carbon capture ability. J. Clean. Prod. 2020, 273, 123154. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Recent progress in biochar-supported photocatalysts: Synthesis, role of biochar, and applications. RSC Adv. 2018, 8, 14237–14248. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Shan, R.; Wang, Y.; Lu, L.; Yuan, H. Synthesis of calcium materials in biochar matrix as a highly stable catalyst for biodiesel production. Renew. Energy 2019, 130, 41–49. [Google Scholar] [CrossRef]
- Chi, N.T.L.; Anto, S.; Ahamed, T.S.; Kumar, S.S.; Shanmugam, S.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. A review on biochar production techniques and biochar-based catalyst for biofuel production from algae. Fuel 2021, 287, 119411. [Google Scholar] [CrossRef]
- Nguyen, H.K.D.; Pham, V.V.; Do, H.T. Preparation of Ni/biochar catalyst for hydrotreating of bio-oil from microalgae biomass. Catal. Lett. 2016, 146, 2381–2391. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, W.; Ji, W. Char and char-supported nickel catalysts for secondary syngas cleanup and conditioning. Appl. Energy 2011, 88, 1656–1663. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of biochar and its composites in catalysis. Chemosphere 2020, 240, 124842. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, J.; Liu, J.; Liu, B.; Chen, G. Preparation and application of magnetic biochar in water treatment: A critical review. Sci. Total Environ. 2020, 711, 134847. [Google Scholar] [CrossRef]
- Cheng, B.-H.; Zeng, R.J.; Jiang, H. Recent developments of post-modification of biochar for electrochemical energy storage. Bioresour. Technol. 2017, 246, 224–233. [Google Scholar] [CrossRef]
- Lobos, M.L.N.; Sieben, J.M.; Comignani, V.; Duarte, M.; Volpe, M.A.; Moyano, E.L. Biochar from pyrolysis of cellulose: An alternative catalyst support for the electro-oxidation of methanol. Int. J. Hydrog. Energy 2016, 41, 10695–10706. [Google Scholar] [CrossRef]
- Yue, Y.; Lin, Q.; Irfan, M.; Chen, Q.; Zhao, X. Characteristics and potential values of bio-oil, syngas and biochar derived from Salsola collina Pall. in a fixed bed slow pyrolysis system. Bioresour. Technol. 2016, 220, 378–383. [Google Scholar] [CrossRef]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustain. Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.; Kwon, E.E.; Lee, J.; Wang, C.-H. A critical review on sustainable biochar system through gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Lee, K.; Park, K.Y. Upgrading the characteristics of biochar from cellulose, lignin, and xylan for solid biofuel production from biomass by hydrothermal carbonization. J. Ind. Eng. Chem. 2016, 42, 95–100. [Google Scholar] [CrossRef]
- Hossain, N.; Nizamuddin, S.; Griffin, G.; Selvakannan, P.; Mubarak, N.M.; Mahlia, T.M.I. Synthesis and characterization of rice husk biochar via hydrothermal carbonization for wastewater treatment and biofuel production. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Sun, R.J.R. Application of biochar-based catalysts in biomass upgrading: A review. RSC Adv. 2017, 7, 48793–48805. [Google Scholar] [CrossRef]
- Peixoto, A.F.; Ramos, R.; Moreira, M.M.; Soares, O.S.G.P.; Ribeiro, L.S.; Pereira, M.F.R.; Delerue-Matos, C.; Freire, C. Production of ethyl levulinate fuel bioadditive from 5-hydroxymethylfurfural over sulfonic acid functionalized biochar catalysts. Fuel 2021, 303, 121227. [Google Scholar] [CrossRef]
- Xiong, X.; Iris, K.; Cao, L.; Tsang, D.C.; Zhang, S.; Ok, Y.S. A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef]
- Cheng, F.; Li, X. Preparation and application of biochar-based catalysts for biofuel production. Catalysts 2018, 8, 346. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Shahbazi, A.; Wang, L.; Zhang, B.; Chung, C.-C.; Dayton, D.; Yan, Q. Nanostructured molybdenum carbide on biochar for CO2 reforming of CH4. Fuel 2018, 225, 403–410. [Google Scholar] [CrossRef]
- Thompson, E.; Danks, A.; Bourgeois, L.; Schnepp, Z. Iron-catalyzed graphitization of biomass. Green Chem. 2015, 17, 551–556. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Ling, L.-L.; Jiang, H. Selective hydrogenation of lignin to produce chemical commodities by using a biochar supported Ni–Mo2C catalyst obtained from biomass. Green Chem. 2016, 18, 4032–4041. [Google Scholar] [CrossRef]
- Shan, R.; Han, J.; Gu, J.; Yuan, H.; Luo, B.; Chen, Y. A review of recent developments in catalytic applications of biochar-based materials. Resour. Conserv. Recycl. 2020, 162, 105036. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Sun, Y.; Yu, I.K.; Tsang, D.C.; Hou, D.; Gupta, J.; Bhaskar, T.; Pandey, A. Critical review on biochar-supported catalysts for pollutant degradation and sustainable biorefinery. Adv. Sustain. Syst. 2020, 4, 1900149. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar-based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Shan, R.; Wang, Y.; Yuan, H. A novel peat biochar supported catalyst for the transesterification reaction. Energy Convers. Manag. 2017, 139, 89–96. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, P.; Yang, L.; Xing, S.; Luo, W.; Wang, Z. Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energy Convers. Manag. 2018, 160, 477–485. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Kumar, T.; Halder, G. Central composite design approach towards optimization of flamboyant pods derived steam activated carbon for its use as heterogeneous catalyst in transesterification of Hevea brasiliensis oil. Energy Convers. Manag. 2015, 100, 277–287. [Google Scholar] [CrossRef]
- di Bitonto, L.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Durán-Valle, C.J.; Pastore, C. Synthesis and characterization of nanostructured calcium oxides supported onto biochar and their application as catalysts for biodiesel production. Renew. Energy 2020, 160, 52–66. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, X.-H.; Yao, M.; Fang, Z.; Wang, Y.-T. Production of biodiesel and hydrogen from plant oil catalyzed by magnetic carbon-supported nickel and sodium silicate. Green Chem. 2016, 18, 3302–3314. [Google Scholar] [CrossRef]
- Hazmi, B.; Rashid, U.; Ibrahim, M.L.; Nehdi, I.A.; Azam, M.; Al-Resayes, S.I. Synthesis and characterization of bifunctional magnetic nano-catalyst from rice husk for production of biodiesel. Environ. Technol. Innov. 2021, 21, 101296. [Google Scholar] [CrossRef]
- Tantirungrotechai, J.; Thepwatee, S.; Yoosuk, B. Biodiesel synthesis over Sr/MgO solid base catalyst. Fuel 2013, 106, 279–284. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, L.; Xing, S.; Luo, W.; Wang, Z.; Lv, P. Biodiesel production by a highly effective renewable catalyst from pyrolytic rice husk. J. Clean. Prod. 2018, 199, 772–780. [Google Scholar] [CrossRef]
- Liu, G.-H.; Zong, Z.-M.; Liu, Z.-Q.; Liu, F.-J.; Zhang, Y.-Y.; Wei, X.-Y. Solvent-controlled selective hydrodeoxygenation of bio-derived guaiacol to arenes or phenols over a biochar supported Co-doped MoO2 catalyst. Fuel Process. Technol. 2018, 179, 114–123. [Google Scholar] [CrossRef]
- Casoni, A.I.; Hoch, P.M.; Volpe, M.A.; Gutierrez, V.S. Catalytic conversion of furfural from pyrolysis of sunflower seed hulls for producing bio-based furfuryl alcohol. J. Clean. Prod. 2018, 178, 237–246. [Google Scholar] [CrossRef]
- Ido, A.L.; de Luna, M.D.G.; Ong, D.C.; Capareda, S.C. Upgrading of Scenedesmus obliquus oil to high-quality liquid-phase biofuel by nickel-impregnated biochar catalyst. J. Clean. Prod. 2019, 209, 1052–1060. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.W.; Tsang, Y.F.; Kim, Y.T.; Lee, J. Engineered rice-straw biochar catalysts for the production of value-added chemicals from furan. Chem. Eng. J. 2020, 387, 124194. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.T.; Kwon, E.E.; Lee, J. Biochar as a catalytic material for the production of 1,4-butanediol and tetrahydrofuran from furan. Environ. Res. 2020, 184, 109325. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, H.; Li, H.; Cai, B.; Lv, W.; Cai, C.; Wang, C.; Yan, L.; Liu, Q.; Ma, L. Selective Hydrodeoxygenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran over Alloyed Cu−Ni Encapsulated in Biochar Catalysts. ACS Sustain. Chem. Eng. 2019, 7, 19556–19569. [Google Scholar] [CrossRef]
- Chen, T.; Li, D.; Jiang, H.; Xiong, C. High-performance Pd nanoalloy on functionalized activated carbon for the hydrogenation of nitroaromatic compounds. Chem. Eng. J. 2015, 259, 161–169. [Google Scholar] [CrossRef]
- Santos, J.L.; Mäki-Arvela, P.; Monzón, A.; Murzin, D.Y.; Centeno, M.Á. Metal catalysts supported on biochars: Part I synthesis and characterization. Appl. Catal. B Environ. 2020, 268, 118423. [Google Scholar] [CrossRef]
- Santos, J.L.; Mäki-Arvela, P.; Wärnå, J.; Monzón, A.; Centeno, M.A.; Murzin, D.Y. Hydrodeoxygenation of vanillin over noble metal catalyst supported on biochars: Part II: Catalytic behavior. Appl. Catal. B Environ. 2020, 268, 118425. [Google Scholar] [CrossRef]
- Kostyniuk, A.; Grilc, M.; Likozar, B. Catalytic Cracking of biomass-derived hydrocarbon tars or model compounds to form biobased benzene, toluene, and xylene isomer mixtures. Ind. Eng. Chem. Res. 2019, 58, 7690–7705. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Barea, A.; Leckner, B. Gasification of biomass and waste. In Handbook of Combustion; Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 365–397. [Google Scholar]
- Shen, Y.; Yoshikawa, K. Tar conversion and vapor upgrading via in situ catalysis using silica-based nickel nanoparticles embedded in rice husk char for biomass pyrolysis/gasification. Ind. Eng. Chem. Res. 2014, 53, 10929–10942. [Google Scholar] [CrossRef]
- Shen, Y.; Areeprasert, C.; Prabowo, B.; Takahashi, F.; Yoshikawa, K. Metal nickel nanoparticles in situ generated in rice husk char for catalytic reformation of tar and syngas from biomass pyrolytic gasification. RSC Adv. 2014, 4, 40651–40664. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, P.; Shao, Q.; Ma, D.; Takahashi, F.; Yoshikawa, K. In-situ catalytic conversion of tar using rice husk char-supported nickel-iron catalysts for biomass pyrolysis/gasification. Appl. Catal. B Environ. 2014, 152–153, 140–151. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Wang, S.; Li, X. Hydrogen production via steam reforming of acetic acid over biochar-supported nickel catalysts. Int. J. Hydrog. Energy 2018, 43, 18160–18168. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A. Reforming of lignin-derived tars over char-based catalyst using Py-GC/MS. Fuel 2015, 162, 47–54. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A. Catalytic reforming of toluene and naphthalene (model tar) by char supported nickel catalyst. Fuel 2017, 187, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Yao, D.; Hu, Q.; Wang, D.; Yang, H.; Wu, C.; Wang, X.; Chen, H. Hydrogen production from biomass gasification using biochar as a catalyst/support. Bioresour. Technol. 2016, 216, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kastner, J.R.; Mani, S.; Juneja, A. Catalytic decomposition of tar using iron supported biochar. Fuel Process. Technol. 2015, 130, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Asadullah, M.; Dong, L.; Tay, H.-L.; Li, C.-Z. An advanced biomass gasification technology with integrated catalytic hot gas cleaning. Part II: Tar reforming using char as a catalyst or as a catalyst support. Fuel 2013, 112, 646–653. [Google Scholar] [CrossRef]
- Wang, X.-B.; Yang, S.-Q.; Xu, C.; Ma, H.-D.; Zhang, Z.-H.; Du, Z.-Y.; Li, W.-Y. Effect of boron doping on the performance of Ni/Biochar catalysts for steam reforming of toluene as a tar model compound. J. Anal. Appl. Pyrolysis 2021, 155, 105033. [Google Scholar] [CrossRef]
- Kaewpanha, M.; Guan, G.; Ma, Y.; Hao, X.; Zhang, Z.; Reubroychareon, P.; Kusakabe, K.; Abudula, A. Hydrogen production by steam reforming of biomass tar over biomass char supported molybdenum carbide catalyst. Int. J. Hydrog. Energy 2015, 40, 7974–7982. [Google Scholar] [CrossRef] [Green Version]
- Postma, R.S.; Kersten, S.R.A.; van Rossum, G. Potassium-salt-catalyzed tar reduction during pyrolysis oil gasification. Ind. Eng. Chem. Res. 2016, 55, 7226–7230. [Google Scholar] [CrossRef]
- Yan, Q.; Lu, Y.; To, F.; Li, Y.; Yu, F. Synthesis of tungsten carbide nanoparticles in biochar matrix as a catalyst for dry reforming of methane to syngas. Catal. Sci. Technol. 2015, 5, 3270–3280. [Google Scholar] [CrossRef]
- Chen, Y.H.; Schmid, M.; Chang, C.C.; Chang, C.Y.; Scheffknecht, G. Lab-scale investigation of palm shell char as tar reforming catalyst. Catalysts 2020, 10, 25. [Google Scholar] [CrossRef]
- Du, Z.Y.; Zhang, Z.H.; Xu, C.; Wang, X.B.; Li, W.Y. Low-temperature steam reforming of toluene and biomass tar over biochar-supported Ni nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 3111. [Google Scholar] [CrossRef]
- Świerczyński, D.; Libs, S.; Courson, C.; Kiennemann, A. Steam reforming of tar from a biomass gasification process over Ni/olivine catalyst using toluene as a model compound. Appl. Catal. B—Environ. 2007, 74, 211–222. [Google Scholar] [CrossRef]
- Li, J.; Tao, J.; Yan, B.; Jiao, L.; Chen, G.; Hu, J. Review of microwave-based treatments of biomass gasification tar. Renew. Sustain. Energy Rev. 2021, 150, 111510. [Google Scholar] [CrossRef]
- Richardson, Y.; Motuzas, J.; Julbe, A.; Volle, G.; Blin, J. Catalytic investigation of in situ generated Ni metal nanoparticles for Tar conversion during biomass pyrolysis. J. Phys. Chem. C 2013, 117, 23812–23831. [Google Scholar] [CrossRef]
- Richardson, Y.; Blin, J.; Volle, G.; Motuzas, J.; Julbe, A. In situ generation of Ni metal nanoparticles as catalyst for H2-rich syngas production from biomass gasification. Appl. Catal. A Gen. 2010, 382, 220–230. [Google Scholar] [CrossRef]
- Poggi, L.A.; Singh, K. Thermal degradation capabilities of modified bio-chars and fluid cracking catalyst (FCC) for acetic acid. Biomass Bioenergy 2016, 90, 243–251. [Google Scholar] [CrossRef]
- Guizani, C.; Escudero Sanz, F.J.; Salvador, S. The nature of the deposited carbon at methane cracking over a nickel loaded wood-char. C. R. Chim. 2016, 19, 423–432. [Google Scholar] [CrossRef]
- Nejati, B.; Adami, P.; Bozorg, A.; Tavasoli, A.; Mirzahosseini, A.H. Catalytic pyrolysis and bio-products upgrading derived from Chlorella vulgaris over its biochar and activated biochar-supported Fe catalysts. J. Anal. Appl. Pyrolysis 2020, 152, 104799. [Google Scholar] [CrossRef]
- Liu, Q.-Y.; Yang, F.; Liu, Z.-H.; Li, G. Preparation of SnO2–Co3O4/C biochar catalyst as a Lewis acid for corncob hydrolysis into furfural in water medium. J. Ind. Eng. Chem. 2015, 26, 46–54. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, Z.; Dai, B.; Zen, S.; Liu, Y.; Xu, Q.; Kirk, S.R.; Yin, D. Biochar sulfonic acid immobilized chlorozincate ionic liquid: An efficiently biomimetic and reusable catalyst for hydrolysis of cellulose and bamboo under microwave irradiation. Cellulose 2014, 21, 1227–1237. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, Z.; Dai, B.; Zen, S.; Liu, Y.; Xu, Q.; Kirk, S.R.; Yin, D. Chlorocuprate ionic liquid functionalized biochar sulfonic acid as an efficiently biomimetic catalyst for direct hydrolysis of bamboo under microwave irradiation. Ind. Eng. Chem. Res. 2013, 52, 11537–11543. [Google Scholar] [CrossRef]
- Wei, Y.; Shen, C.; Xie, J.; Bu, Q. Study on reaction mechanism of superior bamboo biochar catalyst production by molten alkali carbonates pyrolysis and its application for cellulose hydrolysis. Sci. Total Environ. 2020, 712, 136435. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Wan, C.; Liu, J.; Gao, J.; Yu, F.; Zhang, J.; Cai, Z. Iron nanoparticles in situ encapsulated in biochar-based carbon as an effective catalyst for the conversion of biomass-derived syngas to liquid hydrocarbons. Green Chem. 2013, 15, 1631. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Yin, Q.; Zhu, L.; Yin, S. Methanation of bio-syngas over a biochar supported catalyst. New J. Chem. 2014, 38, 4471. [Google Scholar] [CrossRef]
- Moazami, N.; Mahmoudi, H.; Rahbar, K.; Panahifar, P.; Tsolakis, A.; Wyszynski, M.L. Catalytic performance of cobalt–silica catalyst for Fischer–Tropsch synthesis: Effects of reaction rates on efficiency of liquid synthesis. Chem. Eng. Sci. 2015, 134, 374–384. [Google Scholar] [CrossRef]
- Zhu, L.; Yin, S.; Yin, Q.; Wang, H.; Wang, S. Biochar: A new promising catalyst support using methanation as a probe reaction. Energy Sci. Eng. 2015, 3, 126–134. [Google Scholar] [CrossRef]
- Enslow, K.R.; Bell, A.T. SnCl4-catalyzed isomerization/dehydration of xylose and glucose to furanics in water. Catal. Sci. Technol. 2015, 5, 2839–2847. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yu, I.K.M.; Cho, D.-W.; Chen, S.S.; Tsang, D.C.W.; Shang, J.; Yip, A.C.K.; Wang, L.; Ok, Y.S. Tin-Functionalized Wood Biochar as a Sustainable Solid Catalyst for Glucose Isomerization in Biorefinery. ACS Sustain. Chem. Eng. 2019, 7, 4851–4860. [Google Scholar] [CrossRef]
- Iris, K.; Xiong, X.; Tsang, D.C.; Wang, L.; Hunt, A.J.; Song, H.; Shang, J.; Ok, Y.S.; Poon, C.S. Aluminium-biochar composites as sustainable heterogeneous catalysts for glucose isomerisation in a biorefinery. Green Chem. 2019, 21, 1267–1281. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, S.; Liu, J.; Shuang, E.; Jin, C.; Xu, Z.; Zhang, X. Hydrothermal carbonization of cellulose and xylan into hydrochars and application on glucose isomerization. J. Clean. Prod. 2019, 237, 117831. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Luo, J.D.; Qi, X.T.; Yu, J.; Cai, J.X.; Yang, Z.Y. Molten-salt-assisted synthesis of hierarchical porous MnO@Biocarbon composites as promising electrode materials for supercapacitors and lithium-ion batteries. Chemsuschem 2019, 12, 283–290. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Qi, X.T.; Yu, J.; Cai, J.X.; Yang, Z.Y. Manganese monoxide/biomass-inherited porous carbon nanostructure composite based on the high water-absorbent agaric for asymmetric supercapacitor. ACS Sustain. Chem. Eng. 2019, 7, 4284–4294. [Google Scholar] [CrossRef]
- Zhao, N.; Deng, L.B.; Luo, D.W.; Zhang, P.X. One-step fabrication of biomass-derived hierarchically porous carbon/MnO nanosheets composites for symmetric hybrid supercapacitor. Appl. Surf. Sci. 2020, 526. [Google Scholar] [CrossRef]
- Paravannoor, A. One-pot synthesis of biochar wrapped Ni/NiO nanobrick composites for supercapacitor applications. J. Electroanal. Chem. 2018, 823, 656–662. [Google Scholar] [CrossRef]
- Golmohammadi, F.; Amiri, M. Facile synthesis of nanofiber composite based on biomass-derived material conjugated with nanoparticles of Ni-Co oxides for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2020, 31, 2269–2279. [Google Scholar] [CrossRef]
- Shi, Z.J.; Xing, L.; Liu, Y.; Gao, Y.F.; Liu, J.R. A porous biomass-based sandwich-structured Co3O4@Carbon Fiber@Co3O4 composite for high-performance supercapacitors. Carbon 2018, 129, 819–825. [Google Scholar] [CrossRef]
- Thines, K.R.; Abdullah, E.C.; Ruthiraan, M.; Mubarak, N.M.; Tripathi, M. A new route of magnetic biochar-based polyaniline composites for supercapacitor electrode materials. J. Anal. Appl. Pyrolysis 2016, 121, 240–257. [Google Scholar] [CrossRef]
- Thines, K.R.; Abdullah, E.C.; Mubarak, N.M.; Ruthiraan, M. In-situ polymerization of magnetic biochar polypyrrole composite: A novel application in supercapacitor. Biomass Bioenergy 2017, 98, 95–111. [Google Scholar] [CrossRef]
- Zhao, G.; Cheng, Y.L.; Sun, P.X.; Ma, W.X.; Hao, S.H.; Wang, X.K.; Xu, X.J.; Xu, Q.Q.; Liu, M.Q. Biocarbon based template synthesis of uniform lamellar MoS2 nanoflowers with excellent energy storage performance in lithium-ion battery and supercapacitors. Electrochim. Acta 2020, 331, 135262. [Google Scholar] [CrossRef]
- Yang, Z.; Xiang, M.; Zhu, W.; Hui, J.; Qin, H.F. Biomass heteroatom carbon/cerium dioxide composite nanomaterials electrode for high-performance supercapacitors. ACS Sustain. Chem. Eng. 2020, 8, 6675–6681. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, Y.; Pei, L.; Ying, D.W.; Xu, X.Y.; Zhao, L.; Jia, J.P.; Cao, X.D. Converting Ni-loaded biochars into supercapacitors: Implication on the reuse of exhausted carbonaceous sorbents. Sci. Rep. 2017, 7, 41523. [Google Scholar] [CrossRef]
- Fu, M.; Zhu, Z.T.; Zhang, Z.H.; Zhuang, Q.R.; Chen, W.; Liu, Q.Y. Microwave deposition synthesis of Ni(OH)(2)/sorghum stalk biomass carbon electrode materials for supercapacitors. J. Alloy. Compd. 2020, 846, 156376. [Google Scholar] [CrossRef]
- Li, Q.; Lu, C.X.; Xiao, D.J.; Zhang, H.F.; Chen, C.M.; Xie, L.J.; Liu, Y.D.; Yuan, S.X.; Kong, Q.Q.; Zheng, K.; et al. β-Ni(OH)2 nanosheet arrays grown on biomass-derived hollow carbon microtubes for high-performance asymmetric supercapacitors. ChemElectroChem 2018, 5, 1279–1287. [Google Scholar] [CrossRef]
- Nagaraju, G.; Cha, S.M.; Yu, J.S. Ultrathin nickel hydroxide nanosheet arrays grafted biomass-derived honeycomblike porous carbon with improved electrochemical performance as a supercapacitive material. Sci. Rep. 2017, 7, 45201. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Le, P.A.; Phung, V.B.T. Biomass-derived carbon hooks on Ni foam with free binder for high performance supercapacitor electrode. Chem. Eng. Sci. 2021, 229, 116053. [Google Scholar] [CrossRef]
- Wang, H.L.; Wen, J. Biomass porous carbon-based composite for high performance supercapacitor. Mater. Res. Express 2020, 7, 442. [Google Scholar] [CrossRef]
- Yang, H.F.; Tang, Y.H.; Sun, X.Y.; Liu, Q.; Huang, X.G.; Wang, L.X.; Fu, Z.X.; Zhang, Q.T.; Or, S.W. Biomass-derived porous carbon materials with NiS nanoparticles for high performance supercapacitors. J. Mater. Sci. Mater. Electron. 2017, 28, 14874–14883. [Google Scholar] [CrossRef]
- Golmohammadi, F.; Amiri, M. Biomass-derived graphene-based nanocomposite: A facile template for decoration of ultrathin nickel-aluminum layered double hydroxide nanosheets as high-performance supercapacitors. Int. J. Hydrog. Energy 2020, 45, 15578–15588. [Google Scholar] [CrossRef]
- Zhang, M.M.; Song, Z.X.; Liu, H.; Ma, T.J. Biomass-derived highly porous nitrogen-doped graphene orderly supported NiMn2O4 nanocrystals as efficient electrode materials for asymmetric supercapacitors. Appl. Surf. Sci. 2020, 507, 145065. [Google Scholar] [CrossRef]
- Zhang, S.D.; Liu, J.; Huang, P.P.; Wang, H.; Cao, C.Y.; Song, W.G. Carbonaceous aerogel and CoNiAl-LDH@CA nanocomposites derived from biomass for high performance pseudo-supercapacitor. Sci. Bull. 2017, 62, 841–845. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.X.; Zhang, L.; Song, X.M.; Tan, L.C.; Ma, H.Y.; Jiao, J.; Zhu, D.; Li, F.B. NiCo2O4 nanosheets grown on interconnected honeycomb-like porous biomass carbon for high performance asymmetric supercapacitors. N. J. Chem. 2018, 42, 8478–8484. [Google Scholar] [CrossRef]
- Nan, J.X.; Shi, Y.; Xiang, Z.Q.; Wang, S.; Yang, J.W.; Zhang, B. Ultrathin NiCo2O4 nanosheets assembled on biomass-derived carbon microsheets with polydopamine for high-performance hybrid supercapacitors. Electrochim. Acta 2019, 301, 107–116. [Google Scholar] [CrossRef]
- Wang, K.; Yan, R.; Tian, X.D.; Wang, Y.; Lei, S.W.; Li, X.; Yang, T.; Wang, X.J.; Song, Y.; Liu, Y.Q.; et al. Multi-scale biomass-based carbon microtubes decorated with Ni-Co sulphides nanoparticles for supercapacitors with high rate performance. Electrochim. Acta 2019, 302, 78–91. [Google Scholar] [CrossRef]
- Ouyang, Y.H.; Xing, T.; Chen, Y.L.; Zheng, L.P.; Peng, J.; Wu, C.; Chang, B.B.; Luo, Z.G.; Wang, X.Y. Hierarchically structured spherical nickel cobalt layered double hydroxides particles grown on biomass porous carbon as an advanced electrode for high specific energy asymmetric supercapacitor. J. Energy Storage 2020, 30, 10154. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, X.M.; Tong, X.Z.; Chen, Y.F.; Duan, M.T.; Shi, J.; Jiang, C.W.; Hu, L.L.; Kong, Q.H.; Zhang, J.H. High-performance battery-type supercapacitor based on porous biocarbon and biocarbon supported Ni-Co layered double hydroxide. J. Alloy. Compd. 2020, 837, 155529. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, L.; Fang, W.; Li, W.X.; He, X.A.; Zhang, F.Q. Construction of hierarchical NiCo2S4 nanowires on 3D biomass carbon for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 9573–9581. [Google Scholar] [CrossRef]
- Wang, Q.S.; Zhang, Y.F.; Jiang, H.M.; Meng, C.G. In-situ grown manganese silicate from biomass-derived heteroatom-doped porous carbon for supercapacitors with high performance. J. Colloid Interface Sci. 2019, 534, 142–155. [Google Scholar] [CrossRef]
- Ren, Y.M.; Xu, Q.; Zhang, J.M.; Yang, H.X.; Wang, B.; Yang, D.Y.; Hu, J.H.; Liu, Z.M. Functionalization of biomass carbonaceous aerogels: Selective preparation of MnO2@CA composites for supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 9689–9697. [Google Scholar] [CrossRef]
- Li, Y.J.; Yu, N.; Yan, P.; Li, Y.G.; Zhou, X.M.; Chen, S.L.; Wang, G.L.; Wei, T.; Fan, Z.J. Fabrication of manganese dioxide nanoplates anchoring on biomass-derived cross-linked carbon nanosheets for high-performance asymmetric supercapacitors. J. Power Sources 2015, 300, 309–317. [Google Scholar] [CrossRef]
- Wan, C.C.; Jiao, Y.; Li, J. Core–shell composite of wood-derived biochar supported MnO2 nanosheets for supercapacitor applications. RSC Adv. 2016, 6, 64811–64817. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, J.H.; Wang, B.; Feng, H.X.; Yu, Y.L.; Sakai, E. Manganese dioxide/biocarbon composites with superior performance in supercapacitors. J. Electroanal. Chem. 2017, 791, 159–166. [Google Scholar] [CrossRef]
- Yang, M.H.; Kim, D.S.; Hong, S.B.; Sim, J.W.; Kim, J.; Kim, S.S.; Choi, B.G. MnO2 nanowire/biomass-derived carbon from hemp stem for high-performance supercapacitors. Langmuir 2017, 33, 5140–5147. [Google Scholar] [CrossRef]
- Yang, G.J.; Park, S.J. MnO2 and biomass-derived 3D porous carbon composites electrodes for high performance supercapacitor applications. J. Alloy. Compd. 2018, 741, 360–367. [Google Scholar] [CrossRef]
- Fang, K.L.; Chen, J.Z.; Zhou, X.Y.; Mei, C.T.; Tian, Q.H.; Xu, J.L.; Wong, C.P. Decorating biomass-derived porous carbon with Fe2O3 ultrathin film for high-performance supercapacitors. Electrochim. Acta 2018, 261, 198–205. [Google Scholar] [CrossRef]
- Yu, P.P.; Duan, W.; Jiang, Y.F. Porous Fe2O3 nanorods on hierarchical porous biomass carbon as advanced anode for high-energy-density asymmetric supercapacitors. Front. Chem. 2020, 8, 611852. [Google Scholar] [CrossRef]
- Pourhosseini, S.E.M.; Norouzi, O.; Salimi, P.; Naderi, H.R. Synthesis of a novel interconnected 3D pore network algal biochar constituting iron nanoparticles derived from a harmful marine biomass as high-performance asymmetric supercapacitor electrodes. ACS Sustain. Chem. Eng. 2018, 6, 4746–4758. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, J.H.; Wang, B.; Feng, H.X.; Ito, K.; Sakai, E. Fe3O4/biocarbon composites with superior performance in supercapacitors. J. Electroanal. Chem. 2017, 804, 232–239. [Google Scholar] [CrossRef]
- Fu, M.; Chen, W.; Ding, J.X.; Zhu, X.X.; Liu, Q.Y. Biomass waste derived multi-hierarchical porous carbon combined with CoFe2O4 as advanced electrode materials for supercapacitors. J. Alloy. Compd. 2019, 782, 952–960. [Google Scholar] [CrossRef]
- Liu, W.; Fan, H.L.; Shen, W.Z.; Qu, S.J. Facile and Sustainable Synthesis of Co3O4@ hollow-carbon-fiber for a binder-free supercapacitor electrode. Chem. Select 2016, 1, 6469–6475. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Huang, L.N.; Zhao, Y.Y.; Su, L.; Li, J.N.; Qin, H.F. Biomass carbon-modified CoZn/C as a high-performance electrode for supercapacitors. Ionics 2020, 26, 5179–5188. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Jiang, H.M.; Wang, Q.S.; Meng, C.G. In-situ hydrothermal growth of Zn4Si2O7(OH)2 H2O anchored on 3D N, S-enriched carbon derived from plant biomass for flexible solid-state asymmetrical supercapacitors. Chem. Eng. J. 2018, 352, 519–529. [Google Scholar] [CrossRef]
- Wang, Q.S.; Zhang, Y.F.; Xiao, J.Q.; Jiang, H.M.; Hu, T.; Meng, C.G. Copper oxide/cuprous oxide/hierarchical porous biomass-derived carbon hybrid composites for high-performance supercapacitor electrode. J. Alloy. Compd. 2019, 782, 1103–1113. [Google Scholar] [CrossRef]
- Genovese, M.; Lian, K. Polyoxometalate modified pine cone biochar carbon for supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 3939–3947. [Google Scholar] [CrossRef]
- Kaushal, I.; Maken, S.; Sharma, A.K. SnO2 mixed banana peel derived biochar composite for supercapacitor application. Korean Chem. Eng. Res. 2018, 56, 694–704. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Entchev, E. Ag/Biochar composite for supercapacitor electrodes. Mater. Today Energy 2017, 6, 136–145. [Google Scholar] [CrossRef]
- Yuan, H.R.; Chen, H.B.; Li, D.N.; Deng, L.F.; Chen, J.; Fan, Y.K.; He, M.Y.; Sun, F. Catalytic synthesis and simultaneous co-doping of hierarchically porous carbon with in-situ coated graphene from biomass tar as efficient catalyst for ORR. Electrochem. Commun. 2019, 100, 52–59. [Google Scholar] [CrossRef]
- Liu, Y.; Su, M.J.; Li, D.H.; Li, S.S.; Li, X.Y.; Zhao, J.W.; Liu, F.J. Soybean straw biomass-derived Fe-N co-doped porous carbon as an efficient electrocatalyst for oxygen reduction in both alkaline and acidic media. RSC Adv. 2020, 10, 6763–6771. [Google Scholar] [CrossRef]
- Hoang, V.C.; Gomes, V.G.; Dinh, K.N. Ni- and P-doped carbon from waste biomass: A sustainable multifunctional electrode for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Electrochim. Acta 2019, 314, 49–60. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, S.Q.; Zhou, Y.G.; Zhang, S.G. Template-free synthesis of biomass-derived hierarchically mesoporous carbon with ultra-small FeNi nanoparticles for oxygen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 27806–27815. [Google Scholar] [CrossRef]
- Freire, C.; Fernandes, D.M.; Nunes, M.; Abdelkader, V.K. POM & MOF-based electrocatalysts for energy-related reactions. ChemCatChem 2018, 10, 1703–1730. [Google Scholar] [CrossRef]
- Hu, J.; Shi, Z.W.; Su, C.; Lu, B.Q.; Shao, Z.P.; Huang, H. Anchoring perovskite LaMnO3 nanoparticles on biomass-derived N, P co-doped porous carbon for efficient oxygen reduction. Electrochim. Acta 2018, 274, 40–48. [Google Scholar] [CrossRef]
- Hu, S.N.; Tan, Y.; Feng, C.Q.; Wang, S.Q.; Sun, Z.G.; Wu, H.M.; Zhang, G.X. Improving biomass-derived carbon with cobalt/cobalt oxide doping for oxygen reduction reaction. J. Solid State Electrochem. 2019, 23, 2291–2299. [Google Scholar] [CrossRef]
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced biomass-derived electrocatalysts for the oxygen reduction reaction. Adv. Mater. 2018, 30, 27. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Graphene-based electrocatalysts: Hydrogen evolution reactions and overall water splitting. Int. J. Hydrog. Energy 2021, 46, 10161–10588. [Google Scholar] [CrossRef]
- Lee, D.W.; Jang, J.H.; Jang, I.; Kang, Y.S.; Jang, S.; Lee, K.Y.; Jang, J.H.; Kim, H.J.; Yoo, S.J. Bio-derived Co2P nanoparticles supported on nitrogen-doped carbon as promising oxygen reduction reaction electrocatalyst for anion exchange membrane fuel cells. Small 2019, 15, 8. [Google Scholar] [CrossRef]
- An, K.L.; Xu, X.X.; Liu, X.X. Mo2C-based electrocatalyst with biomass-derived sulfur and nitrogen Co-doped carbon as a matrix for hydrogen evolution and organic pollutant removal. ACS Sustain. Chem. Eng. 2018, 6, 1446–1455. [Google Scholar] [CrossRef]
- Pavodi, F.; Tavakkoli, M.; Lahtinen, J.; Kallio, T. Straightforward synthesis of nitrogen-doped carbon nanotubes as highly active bifunctional electrocatalysts for full water splitting. J. Catal. 2017, 353, 19–27. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, C.W.; Jia, L.C.; Gale, J.D.; Zhang, L.L.; Liu, C.; Shen, P.K.; Jiang, S.P. Pristine carbon nanotubes as non-metal electrocatalysts for oxygen evolution reaction of water splitting. Appl. Catal. B—Environ. 2015, 163, 96–104. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Q.; Cheng, N.Y.; Asiri, A.M.; Sun, X.P. Activated carbon nanotubes: A highly-active metal-free electrocatalyst for hydrogen evolution reaction. Chem. Commun. 2014, 50, 9340–9342. [Google Scholar] [CrossRef]
- Yang, H.B.; Miao, J.W.; Hung, S.F.; Chen, J.Z.; Tao, H.B.; Wang, X.Z.; Zhang, L.P.; Chen, R.; Gao, J.J.; Chen, H.M.; et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, 11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wu, Z.Y.; Jiang, H.L.; Yu, S.H. Nanowire-Directed templating synthesis of metal-organic framework nanofibers and their derived porous doped carbon nanofibers for enhanced electrocatalysis. J. Am. Chem. Soc. 2014, 136, 14385–14388. [Google Scholar] [CrossRef]
- Wu, M.G.; Wang, Y.Q.; Wei, Z.X.; Wang, L.; Zhuo, M.; Zhang, J.T.; Han, X.P.; Ma, J.M. Ternary doped porous carbon nanofibers with excellent ORR and OER performance for zinc-air batteries dagger. J. Mater. Chem. A 2018, 6, 10918–10925. [Google Scholar] [CrossRef]
- Kordek, K.; Jiang, L.X.; Fan, K.C.; Zhu, Z.J.; Xu, L.; Al-Mamun, M.; Dou, Y.H.; Chen, S.; Liu, P.R.; Yin, H.J.; et al. Two-step activated carbon cloth with oxygen-rich functional groups as a high-performance additive-free air electrode for flexible zinc-air batteries. Adv. Energy Mater. 2019, 9, 9. [Google Scholar] [CrossRef]
- Yan, X.C.; Jia, Y.; Odedairo, T.; Zhao, X.J.; Jin, Z.; Zhu, Z.H.; Yao, X.D. Activated carbon becomes active for oxygen reduction and hydrogen evolution reactions. Chem. Commun. 2016, 52, 8156–8159. [Google Scholar] [CrossRef]
- Qiao, S.L.; Zhao, J.; Zhang, B.Y.; Liu, C.H.; Li, Z.; Hu, S.Z.; Li, Q. Micrometer-Scale biomass carbon tube matrix auxiliary MoS2 heterojunction for electrocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2019, 44, 32019–32029. [Google Scholar] [CrossRef]
- Fan, Z.Y.; Li, J.; Yang, W.; Fu, Q.; Sun, K.; Song, Y.C.; Wei, Z.D.; Liao, Q.; Zhu, X. Green and facile synthesis of iron oxide nanoparticle-embedded N-doped biocarbon as an efficient oxygen reduction electrocatalyst for microbial fuel cells. Chem. Eng. J. 2020, 385, 10. [Google Scholar] [CrossRef]
- Huggins, T.M.; Pietron, J.J.; Wang, H.M.; Ren, Z.J.; Biffinger, J.C. Graphitic biochar as a cathode electrocatalyst support for microbial fuel cells. Bioresour. Technol. 2015, 195, 147–153. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Edvinsson, T.; Kwong, P. Biochar for electrochemical applications. Curr. Opin. Green Sustain. Chem. 2020, 23, 25–30. [Google Scholar] [CrossRef]
- Tran, T.; Song, M.Y.; Kang, T.H.; Samdani, J.; Park, H.Y.; Kim, H.; Jhung, S.H.; Yu, J.S. Iron phosphide incorporated into iron-treated heteroatoms-doped porous bio-carbon as efficient electrocatalyst for the oxygen reduction reaction. ChemElectroChem 2018, 5, 1944–1953. [Google Scholar] [CrossRef]
- Pi, Y.T.; Xing, X.Y.; Lu, L.M.; He, Z.B.; Ren, T.Z. Hierarchical porous activated carbon in OER with high efficiency. RSC Adv. 2016, 6, 102422–102427. [Google Scholar] [CrossRef]
- Prabu, N.; Kesavan, T.; Maduraiveeran, G.; Sasidharan, M. Bio-derived nanoporous activated carbon sheets as electrocatalyst for enhanced electrochemical water splitting. Int. J. Hydrog. Energy 2019, 44, 19995–20006. [Google Scholar] [CrossRef]
- Gong, X.B.; Peng, L.; Wang, X.H.; Wu, L.L.; Liu, Y. Duckweed derived nitrogen self-doped porous carbon materials as cost-effective electrocatalysts for oxygen reduction reaction in microbial fuel cells. Int. J. Hydrog. Energy 2020, 45, 15336–15345. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.G.; Xiao, T.F.; Wang, S.D.; Zhang, B.P.; Chen, D.Y.; Su, M.H.; Tang, J.F. Low-cost biochar derived from corncob as oxygen reduction catalyst in air cathode microbial fuel cells. Electrochim. Acta 2018, 283, 780–788. [Google Scholar] [CrossRef]
- Humagain, G.; MacDougal, K.; MacInnis, J.; Lowe, J.M.; Coridan, R.H.; MacQuarrie, S.; Dasog, M. Highly efficient, biochar-derived molybdenum carbide hydrogen evolution electrocatalyst. Adv. Energy Mater. 2018, 8, 5. [Google Scholar] [CrossRef]
- Xiong, X.L.; You, C.; Liu, Z.; Asiri, A.M.; Sun, X.P. Co-doped CuO Nanoarray: An efficient oxygen evolution reaction electrocatalyst with enhanced activity. ACS Sustain. Chem. Eng. 2018, 6, 2883–2887. [Google Scholar] [CrossRef]
- Golmohammadi, F.; Amiri, M. Fabrication of MEA from biomass-based carbon nanofibers composited with nickel-cobalt oxides as a new electrocatalyst for oxygen reduction reaction in passive direct methanol fuel cells. Electrocatalysis 2021, 11, 485–496. [Google Scholar] [CrossRef]
- Xiong, X.; Jiang, R.; Deng, B.W.; Yang, J.; Wang, D.H. Bionic structural design and electrochemical manufacture of WC/N-Doped carbon hybrids as efficient ORR catalyst. J. Electrochem. Soc. 2020, 167, 7. [Google Scholar] [CrossRef]
- Zhao, W.T.; Lu, X.Q.; Selvaraj, M.; Wei, W.; Jiang, Z.F.; Ullah, N.; Liu, J.; Xie, J.M. MXP(M = Co/Ni)@carbon core–shell nanoparticles embedded in 3D cross-linked graphene aerogel derived from seaweed biomass for hydrogen evolution reaction. Nanoscale 2018, 10, 9698–9706. [Google Scholar] [CrossRef]
- Hu, L.B.; Yu, F.; Wang, F.; Yang, S.C.; Peng, B.H.; Chen, L.; Wang, G.; Hou, J.; Dai, B.; Tian, Z.Q. Overwhelming electrochemical oxygen reduction reaction of zinc-nitrogen-carbon from biomass resource chitosan via a facile carbon bath method. Chin. Chem. Lett. 2020, 31, 1207–1212. [Google Scholar] [CrossRef]
- Panganoron, H.O.; Pascasio, J.D.A.; Esparcia, E.A.; del Rosario, J.A.D.; Ocon, J.D. Hydrothermally carbonized waste biomass as electrocatalyst support for alpha-MnO2 in oxygen reduction reaction. Catalysts 2020, 10, 18. [Google Scholar] [CrossRef] [Green Version]
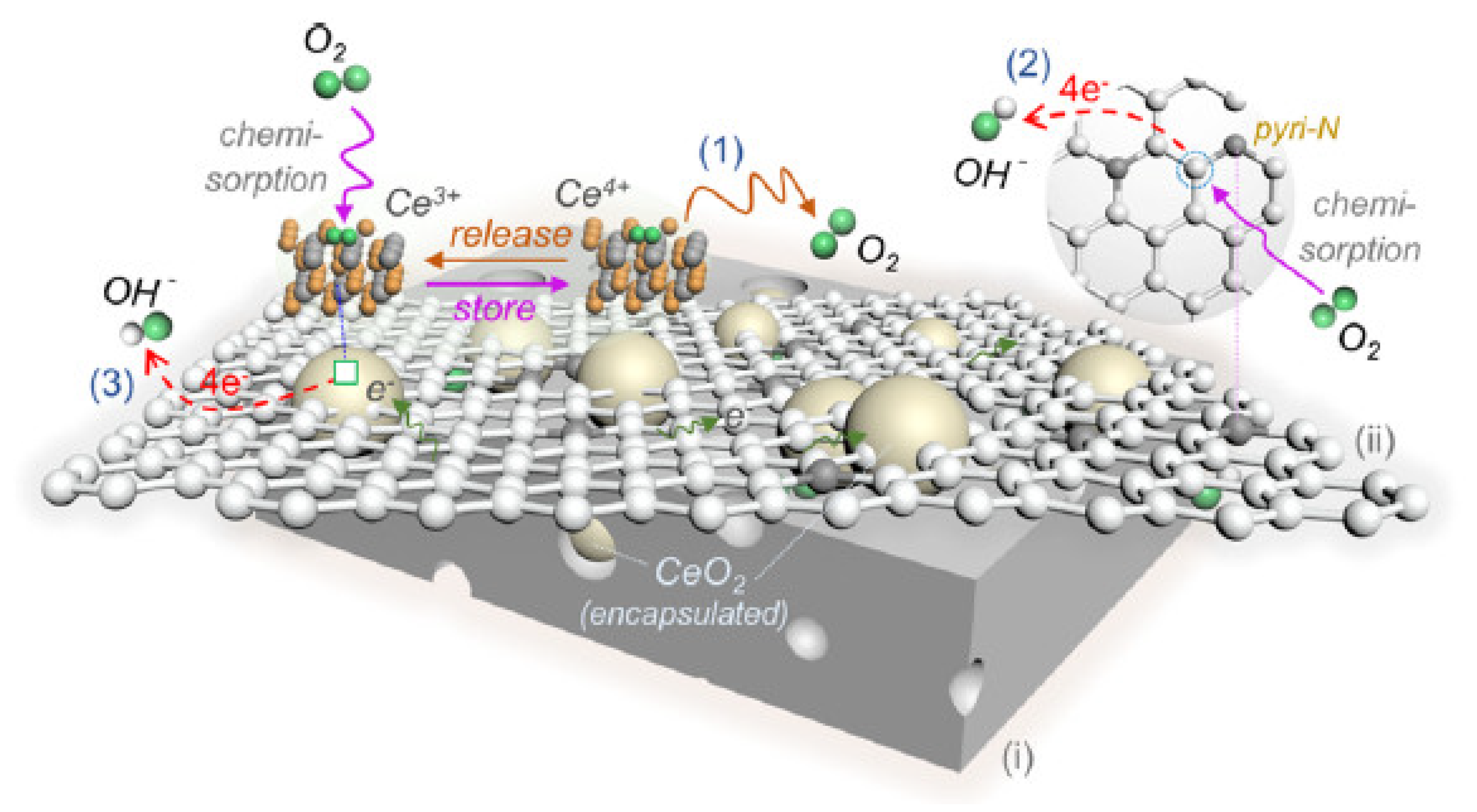
- Pi, L.; Jiang, R.; Cai, W.X.; Wang, L.; Wang, Y.Y.; Cai, J.H.; Mao, X.H. Bionic Preparation of CeO2-Encapsulated nitrogen self-doped biochars for highly efficient oxygen reduction. ACS Appl. Mater. Interfaces 2020, 12, 3642–3653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Guo, C.Z.; Ma, Z.L.; Wu, H.J.; Chen, C.G. Inexpensive ipomoea aquatica biomass-modified carbon black as an active Pt-free electrocatalyst for oxygen reduction reaction in an alkaline medium. Materials 2015, 8, 6658–6667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Jin, X.; Wang, L.N.; Sun, M.J.; Tang, Y.; Chen, Y.M.; Sun, Y.Z.; Yang, X.J.; Wan, P.Y. Improving biomass-derived carbon by activation with nitrogen and cobalt for supercapacitors and oxygen reduction reaction. Appl. Surf. Sci. 2017, 411, 251–260. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Ma, J.H.; Shi, M.H.; Xie, Y.H.; Feng, C. Biomass based iron and nitrogen co-doped 3D porous carbon as an efficient oxygen reduction catalyst. J. Colloid Interface Sci. 2018, 523, 144–150. [Google Scholar] [CrossRef]
- Wan, W.; Wang, Q.; Zhang, L.; Liang, H.W.; Chen, P.; Yu, S.H. N-, P- and Fe-tridoped nanoporous carbon derived from plant biomass: An excellent oxygen reduction electrocatalyst for zinc-air batteries. J. Mater. Chem. A 2016, 4, 8602–8609. [Google Scholar] [CrossRef]
- Wu, X.X.; Chen, K.Q.; Lin, Z.P.; Zhang, Y.M.; Meng, H. Nitrogen doped graphitic carbon from biomass as non noble metal catalyst for oxygen reduction reaction. Mater. Today Energy 2019, 13, 100–108. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Gao, X.J.; Dou, M.L.; Ji, J.; Wang, F. Biomass derived N-doped porous carbon supported single Fe atoms as superior electrocatalysts for oxygen reduction. Small 2017, 13, 8. [Google Scholar] [CrossRef]
- Jing, B.J.; You, S.J.; Ma, Y.Y.; Xing, Z.P.; Chen, H.; Dai, Y.; Zhang, C.Y.; Ren, N.Q.; Zou, J.L. Fe3Se4/FeSe heterojunctions in cornstalk-derived N-doped carbon framework enhance charge transfer and cathodic oxygen reduction reaction to boost bio-electricity generation. Appl. Catal. B—Environ. 2019, 244, 465–474. [Google Scholar] [CrossRef]
- Lu, G.L.; Li, Z.Y.; Fan, W.X.; Wang, M.; Yang, S.C.; Li, J.Y.; Chang, Z.Y.; Sun, H.; Liang, S.; Liu, Z.N. Sponge-like N-doped carbon materials with Co-based nanoparticles derived from biomass as highly efficient electrocatalysts for the oxygen reduction reaction in alkaline media. RSC Adv. 2019, 9, 4843–4848. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.; Yu, J.; Xu, X.; Yang, L.; Xing, Z.P.; Dai, Y.; Sun, Y.; Zou, J.L. Nitrogen-doped MoS2/carbon as highly oxygen-permeable and stable catalysts for oxygen reduction reaction in microbial fuel cells. J. Power Sources 2017, 339, 68–79. [Google Scholar] [CrossRef]
- Chen, B.L.; Li, R.; Ma, G.P.; Gou, X.L.; Zhu, Y.Q.; Xia, Y.D. Cobalt sulfide/N,S codoped porous carbon core–shell nanocomposites as superior bifunctional electrocatalysts for oxygen reduction and evolution reactions. Nanoscale 2015, 7, 20674–20684. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, L.Q.; Chen, X.; Lu, Y.L.; Yang, W.S. Monodisperse cobalt sulfides embedded within nitrogen-doped carbon nanoflakes: An efficient and stable electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 11342–11350. [Google Scholar] [CrossRef]
- Liu, B.; Qu, S.X.; Kou, Y.; Liu, Z.; Chen, X.; Wu, Y.T.; Han, X.P.; Deng, Y.D.; Hu, W.B.; Zhong, C. In situ electrodeposition of cobalt sulfide nanosheet arrays on carbon cloth as a highly efficient bifunctional electrocatalyst for oxygen evolution and reduction reactions. ACS Appl. Mater. Interfaces 2018, 10, 30433–30440. [Google Scholar] [CrossRef]
- Shen, M.X.; Ruan, C.P.; Chen, Y.; Jiang, C.H.; Ai, K.L.; Lu, L.H. Covalent entrapment of cobalt-iron sulfides in N-Doped mesoporous carbon: Extraordinary bifunctional electrocatalysts for oxygen reduction and evolution reactions. ACS Appl. Mater. Interfaces 2015, 7, 1207–1218. [Google Scholar] [CrossRef]
- Jiang, R.; Chen, X.; Deng, J.X.; Wang, T.Y.; Wang, K.; Chen, Y.L.; Jiang, J.Z. In-situ growth of ZnS/FeS heterojunctions on biomass -derived porous carbon for efficient oxygen reduction reaction. J. Energy Chem. 2020, 47, 79–85. [Google Scholar] [CrossRef]
- Sun, Z.H.; Wang, Y.K.; Zhang, L.B.; Wu, H.; Jin, Y.C.; Li, Y.H.; Shi, Y.C.; Zhu, T.X.; Mao, H.; Liu, J.M.; et al. Simultaneously realizing rapid electron transfer and mass transport in jellyfish-like Mott–Schottky nanoreactors for oxygen reduction reaction. Adv. Funct. Mater. 2020, 30, 12. [Google Scholar] [CrossRef]
- Lang, P.; Yuan, N.N.; Jiang, Q.Q.; Zhang, Y.C.; Tang, J.G. Recent advances and prospects of metal-based catalysts for oxygen reduction reaction. Energy Technol. 2020, 8, 18. [Google Scholar] [CrossRef]
- Liu, Y.; Kelly, T.G.; Chen, J.G.G.; Mustain, W.E. Metal carbides as alternative electrocatalyst supports. ACS Catal. 2013, 3, 1184–1194. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xiong, Y.P.; Liu, X.T.; Han, C.; Zhang, Y.F.; Bo, X.J.; Guo, L.P. Iron and nitrogen co-doped carbon nanotube@hollow carbon fibers derived from plant biomass as efficient catalysts for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 9658–9667. [Google Scholar] [CrossRef]
- Ma, M.; You, S.J.; Wang, W.; Liu, G.S.; Qi, D.P.; Chen, X.D.; Qu, J.H.; Ren, N.Q. Biomass-derived porous Fe3C/Tungsten carbide/graphitic carbon nanocomposite for efficient electrocatalysis of oxygen reduction. ACS Appl. Mater. Interfaces 2016, 8, 32307–32316. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, P.R.; Arunchander, A.; Kalpana, D.; Selvan, R.K. Bio-derived carbon as an efficient supporting electrocatalyst for the oxygen reduction reaction. J. Phys. Chem. Solids 2019, 124, 305–311. [Google Scholar] [CrossRef]
- Gong, M.; Dai, H.J. A mini review of NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.M.; Zhang, W.; Shi, R.H.; Zhu, H.W.; Qian, C.; Yang, H.X.; Zhang, J.H.; Yuan, A.H.; Zhou, Y.Z. Facile fabrication of amorphous Ni-P supported on a 3D biocarbon skeleton as an efficient electrocatalyst for the oxygen evolution reaction. ChemElectroChem 2019, 6, 3071–3076. [Google Scholar] [CrossRef]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions-A Review. Int. J. Hydrog. Energy 2015, 40, 256–274. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Rui, K.; Dou, S.X.; Sun, W.P. Heterostructures for electrochemical hydrogen evolution reaction: A review. Adv. Funct. Mater. 2018, 28, 26. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Gonullu, Y.; Pyeon, M.; Diao, Z.D.; Czympiel, L.; Singh, M.; Shen, S.H.; Mathur, S. Trace amount of platinum supported on carbonized biomorphic wood for efficient electrochemical hydrogen evolution in alkaline condition. Chem. Select 2018, 3, 2140–2143. [Google Scholar] [CrossRef]
- Min, S.X.; Duan, Y.; Li, Y.N.; Wang, F. Biomass-derived self-supported porous carbon membrane embedded with Co nanoparticles as an advanced electrocatalyst for efficient and robust hydrogen evolution reaction. Renew. Energy 2020, 155, 447–455. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, X.Y.; Liu, T.Y.; Wu, Z.Z.; Wang, D.Z. N, K Co-activated biochar-derived molybdenum carbide as efficient electrocatalysts for hydrogen evolution. Appl. Surf. Sci. 2020, 509, 8. [Google Scholar] [CrossRef]
- Kannimuthu, K.; Sangeetha, K.; Sam Sankar, S.; Karmakar, A.; Madhu, R.; Kundu, S. Investigation on nanostructured Cu-based electrocatalysts for improvising water splitting: A review. Inorg. Chem. Front. 2021, 8, 234–272. [Google Scholar] [CrossRef]
- Huang, H.; Yan, M.; Yang, C.; He, H.; Jiang, Q.; Yang, L.; Lu, Z.; Sun, Z.; Xu, X.; Bando, Y.; et al. Graphene nanoarchitectonics: Recent advances in graphene-based electrocatalysts for hydrogen evolution reaction. Adv. Mater. 2019, 31, 1903415. [Google Scholar] [CrossRef]
- Yang, J.; Voiry, D.; Ahn, S.J.; Kang, D.; Kim, A.Y.; Chhowalla, M.; Shin, H.S. Two-dimensional hybrid nanosheets of tungsten disulfide and reduced graphene oxide as catalysts for enhanced hydrogen evolution. Angew. Chem. Int. Ed. 2013, 52, 13751–13754. [Google Scholar] [CrossRef]
- Jaiswal, A.; Kumar, R.; Prakash, R. Iron/Iron carbide (Fe/Fe3C) encapsulated in S, N codoped graphitic carbon as a robust HER electrocatalyst. Energy Fuels 2021, 35, 16046–16053. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-noble metal-based carbon composites in hydrogen evolution reaction: Fundamentals to applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar] [CrossRef]
- Oh, N.K.; Seo, J.; Lee, S.; Kim, H.J.; Kim, U.; Lee, J.; Han, Y.K.; Park, H. Highly efficient and robust noble-metal free bifunctional water electrolysis catalyst achieved via complementary charge transfer. Nat. Commun. 2021, 12, 12. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, Z.-J.; Maiyalagan, T.; Manthiram, A. Cobalt oxide-coated N- and B-doped graphene hollow spheres as bifunctional electrocatalysts for oxygen reduction and oxygen evolution reactions. J. Mater. Chem. A 2016, 4, 5877–5889. [Google Scholar] [CrossRef]
- Araujo, M.P.; Nunes, M.; Rocha, I.M.; Pereira, M.F.R.; Freire, C. Co3O4 nanoparticles anchored on selectively oxidized graphene flakes as bifunctional electrocatalysts for oxygen reactions. Chem. Select 2018, 3, 10064–10076. [Google Scholar] [CrossRef]
- Wang, G.H.; Deng, Y.J.; Yu, J.N.; Zheng, L.; Du, L.; Song, H.Y.; Liao, S.J. From chlorella to nestlike framework constructed with doped carbon nanotubes: A biomass-derived, high-performance, bifunctional oxygen reduction/evolution catalyst. ACS Appl. Mater. Interfaces 2017, 9, 32168–32178. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.F.; Wang, D.; Cao, D.P. Biomass-derived FeNi alloy and nitrogen-codoped porous carbons as highly efficient oxygen reduction and evolution bifunctional electrocatalysts for rechargeable Zn-air battery. Energy Storage Mater. 2018, 12, 277–283. [Google Scholar] [CrossRef]
- Lin, C.Q.; Kang, M.Z.; Zheng, L.Z.; Fu, X.Q.; Wang, S. Ammonium nitrate-assisted low-temperature synthesis of Co, Co2P@CoP embedded in biomass-derived carbons as efficient electrocatalysts for hydrogen and oxygen evolution reaction. Chem. Select 2020, 5, 7338–7346. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, R.M.; Dong, G.X.; Xiang, M.; Hui, J.; Ou, J.F.; Qin, H.F. Biochar nanocomposite derived from watermelon peels for electrocatalytic hydrogen production. ACS Omega 2021, 6, 2066–2073. [Google Scholar] [CrossRef]
- Liu, X.; Huo, Y.Q.; Yan, L.K.; Fan, N.; Cai, K.Z.; Su, Z.M. Hollow porous MnFe2O4 sphere grown on elm-money-derived biochar towards energy-saving full water electrolysis. Chem. A Eur. J. 2020, 26, 14397–14404. [Google Scholar] [CrossRef]

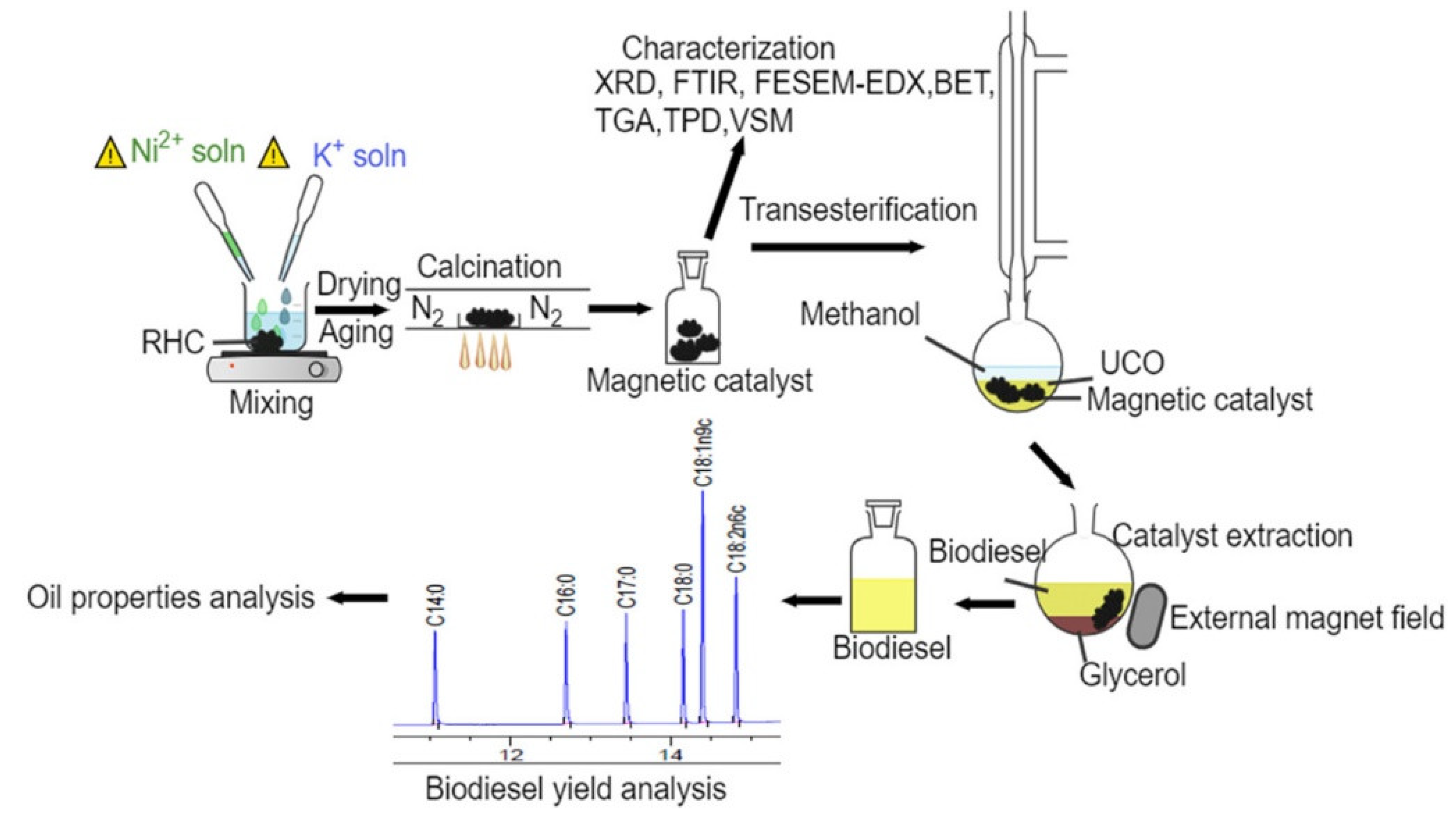








| M-BCH Catalyst Preparation | Biodiesel Production | |||||
|---|---|---|---|---|---|---|
| Biochar Feedstock | Synthesis | Catalyst M-Support | Substrate | Reaction Conditions | FAME Yield | Ref. |
| Peat | Pyrolysis: 600 °C, 2 h/WI: K2CO3 (30 wt %) | KOH | Palm oil | Methanol (8:1); 65 °C, 1.5 h | 98.6% | [39] |
| Pomelo peel | Pyrolysis: 600 °C, 2 h; treatment: 2 M KOH, 2 h; WI K2CO3 (15–35 wt %) | KOH | Palm oil | Methanol (8:1); 65 °C, 2.5 h | 98% | [40] |
| Flamboyant pods | Pyrolysis: 500 °C, 1.5 h; steam flow treatment: 1.5–2.0 kg/cm2, 300–400 °C.Impregnation: KOH, 30 °C, 24 h | KOH | Hevea brasiliensis oil | Methanol (15:1); 60 °C; 1 h | 89.3% | [41] |
| Peat | Pyrolysis at 700 °C; treatment: 2 M KOH, 1 h. WI CaO (30 wt %); dry: 105 °C; calcined for 2 h at 600–800 °C in N2 | KOH-CaO | Palm oil | Methanol (8:1); 65 °C; 2.5 h | 93.4% | [15] |
| Avocado seeds | Pyrolysis: 900 °C; 2 h (N2 flow: 100 mL/min). Precipitation: aqueous solution Ca(NO3)2·4H2O; addition of NaOH 1.5 N at 70 °C; calcination: 900 °C, 2 h, N2 | CaO | Sunflower oil | Methanol (15.6:1); 99.5 °C; 5 h | 96% | [42] |
| Bamboo powders | Pre-treatment: Aqueous solution of nickel nitrate, urea and bamboo powders, 135 °C, 10 h; dry: 105 °C. Pyrolysis: 700 °C. Aqueous sodium silicate solution at 85 °C to achieve a gel; Activation: 400 °C, 2 h, N2;milled with ZrO2 balls, 230 rpm, 12 h | Ni | Soybean oil | Methanol (9:1); 65 °C; 2 h | 98.1% | [43] |
| Rice husk | Activation: 450 °C, 3 h, N2; Treatment: 1 M KOH, 105 °C, 3 h; washing: 1 M HCl; dry: 105 °C: impregnation: K2 CO3 (20 wt %) and Ni(NO3)2·6H2O solutions; mixed for 6 h; Pyrolysis: 600 °C | Ni-KOH | Cooking oil | Methanol (12:1); 65 °C; 2 h | 98.2% | [44] |
| M-BCH Catalyst Preparation | Catalytic Biomass Upgrading | |||||
|---|---|---|---|---|---|---|
| Biomass Feedstock | Synthesis | Catalyst M-Support | Substrate | Reaction Type/Conditions | Yield (%) | Ref. |
| Microalgal | Two step synthesis: slow pyrolysis: 400 °C, 2 h/IWI: Ni(NO3)2 (0.2–M); reduction H2/N2 flow | Ni/BCH | Microalgal bio-oil | HDO batch; 300 °C; H2/N2 flow (10% volume of H2) 2 h | hydrocarbons (80%, n-heptadecane) oxygenated compounds (12%) | [17] |
| α-cellulose | One-step synthesis: Impregnation: cobalt nitrate and ammonium heptamolybdate; pyrolysis: 650 °C; passivation: RT, 2 h | Co-doped MoO2-BCH | Guaicol | HDO autoclave; 340 °C; 0.8 MPa H2; 4 h. | Arenes (70%); Phenols (91.7%) | [47] |
| Microcrystalline cellulose | Treatment: [BMim]Cl. Addition of H2SO4. Carbonization: 600 °C, 4 h, N2 flow. IWI (Bimetallic Cu−Ni) | Cu-Ni/BCH | HMF | HDO THF; 220 °C; 40 bar H2, 1 h | DMF (93.5%) | [52] |
| Fir sawdust | One-step synthesis: impregnation: nickel chloride and ammonium heptamolybdate; pyrolysis: 800 °C | Ni–Mo2C-BCH | Lignin | Hydrogenation; 250 °C, 2 h; 2 MPa H2 | 49 wt % yield to phenols, guaiacols, trimethoxybenzenes | [35] |
| Sunflower seeds hulls | Two-step synthesis: Treatment: phosphoric acid and zinc chloride; pyrolysis: 500 °C (200 mL min−1)/Precipitation: PdCl2 (5 wt %), 80 °C/Reduction: 37% formaldehyde solution | Pd/BCH | Furfural | Hydrogenation; batch; 110 °C; 0.4 MPa H2 | Furfuryl alcohol (48%) | [48] |
| Rice husk | Pré-Treatment: KOH/ Sulfonation: H2SO4; 90 °C, 9 h/Impregnation: Ni(NO3)·6H2O (20 wt %), 65 °C, 4 h/Calcination: 500 °C, 4 h | Ni/BCH | Scenedesmus obliquus microalgae oil | Hydrotreatment; 246 °C; 3.72 (w/w) dodecane/oil; 3.84 MPa H2; 6 h | 69.4% liquid-phase biofuel | [49] |
| Rice straw | Pyrolysis at 550 °C under N2 or CO2 atmosphere IWI with (HReO4 and RuCl3) dried at 110 °C; reduced at 300 °C for 2 h under an H2 flow of 300 mL/min | Ru-ReOx-BCH | Furan | HDO 160 °C under 30 bar H2 | THF (>50%) and 1,4-butanediol (10–20%) | [52,53] |
| Coconut shell | Oxidized with 20 wt % HNO3 at 85 °C for 4 h; washed and dried at 105 °C. Impregnation with PdCl2, NiCl2·6H2O and HCl aqueous solution; reduced with of 1 mol/L KBH4 | Pd–Ni–B/BCH | Nitronaphthalene and p-chloronitrobenzene | 250 mL vessel with 100 mL of tetrahydrofuran; 110 °C; 3.0 MPa H2; 4 h | 99.6% conversion | [53] |
| Vine shoots | Demineralization/Pyrolysis (800 °C): physical (CO2) and chemical activation (ZnCl2); IWI (palladium (II) acetate). | Pd/BCH | Vanillin | HDO water; 100 °C; 30 bar H2; 3 h) | 100% conversion p-creosol (92%) | [54,55] |
| M-BCH Preparation | Syngas Production and/or Tar Decomposition | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Biochar Feedstock | Synthesis | Catalyst M-Support | Substrate | Reaction Conditions | Syngas Production | Tar Removal Efficiency | |
| Rice husk | Pyrolysis at 700 °C; IWI with Ni(NO3)2·6H2O; reduction: (i) pyrolysis at 700 °C, (ii) addition of NaBH4 | Ni-BCH | Rice husk | Reforming; 1 L min−1; 800 °C | - | 96.5% | [58] |
| IWI with Ni(NO3)2·6H2O; dried at 105 °C; reduction via: (i) pyrolysis at 700 °C, (ii) addition of NaBH4 | Reforming; 1 L min−1; 750 °C | LHV = 11.32 MJ/m3 | 96.9–98.6% | [59] | |||
| IWI with Ni(NO3)2·6H2O and Fe(NO3)3·9H2O; dried at 105 °C; reduced: (i) pyrolysis at 600 °C, (ii) addition of NaBH4 | Ni-Fe-BCH | Reforming; 1 L/min; 800 °C | Higher CO/CO2 ratio | 92.3% | [60] | ||
| Pyrolysis at 500 °C; carbonization at 700 °C; treated with KOH (5 mol L−1); filtered and dried at 110 °C; calcined at 700 °C. Impregnation with nickel nitrate (10 wt %); filtrated and dried at 110 °C; pyrolyzed at 500 °C | Ni-BCH | Acetic acid | Reforming; LHSV 10 h−1; 700 °C | 71.2% H2 yield | - | [61] | |
| Red cedar | Gasification; treated with KOH; calcined at 800 °C for 1.5 h. Impregnated with Ni nitrate; reduced in H2 flow at 350 °C | Ni-BCH | Lignin | Reforming at 0.1–2.2 MPa; 700–900 °C; 1–5 μL water | - | 70% | [62] |
| Gasification; treated with KOH and NaOH. Treated with HNO3 (30 vol%); impregnated with Ni salts (10 wt %) | Ni-BCH | Toluene | Reforming with water at 800 °C | - | 99% | [63] | |
| Wheat straw, rice husk and cotton stalk | Fast pyrolysis at 500 °C. Impregnation with Ni(NO3)2·6H2O (5–20 wt %); dried at 105 °C; pyrolyzed at 800 °C | Ni-BCH | Wheat straw | Steam gasification in a two-stage fixed bed reactor;600–900 °C | 92 mg H2/g biomass | - | [64] |
| Oak wood | Mechanically mixing with NiO (5–20 wt %); reduce in Ar/H2 at 800 °C | NiO-BCH | Syngas | Reforming at lab-scale gasifier; 650–850 °C; 0.1–1.2 s | H2 and CO increase up to 34% and 32%, respectively | 97% | [18] |
| Pine bark | Pyrolysis at 950 °C for 2 h. Impregnated with iron nitrate (9–13 wt %); dried at 105 °C; calcined at 300 °C in air | Fe-BCH | Toluene | Reforming at 400–900 °C; 1 atm N2 (700 mL min−1) | - | Conversion 91%. Benzene selectivity = 0.02% | [65] |
| Mallee wood | Soaked with FeCl3 solution (0.2 mol L−1) for 24 h; pyrolyzed to 630 °C for 30 min | Fe-BCH | Mallee wood | Steam gasification; 4.4 kg h−1; 880 °C | Enhance formation of H2 and CO | 95% | [66] |
| Japanese cedar | Impregnated with ammonium heptamolybdate (5–30 wt %); calcined in Ar flow at 800 °C | Mo2C-BCH | Japanese cedar | Reforming at 650 °C; 0.09 g min−1; RT; 1 atm | H2 production x5 vs. non catalytic test | 99% | [68] |
| Pine sawdust | Fast pyrolysis; pre-treated with 0.1 mol L−1 HNO3 at 100 °C for 12 h; filtered and washed with H2O (pH ≈ 7); dried at 105 °C. IWI with ammonium molybdate tetrahydrate (15 wt %); dried at 105 °C; calcined with Ar at 800 °C for 2 h | Mo2C-BCH | CH4 | Reforming with CO2 in a fixed-bed tubular reactor at 0.5 MPa; GHSV = 4000–12,000 h−1 | CH4 conversion of 94% | [33] | |
| Pine wood | Soaking wood chips in KOH solution, (10 wt %); dried at 105 °C; calcined at 700 °C in N2 | K-BCH | Bio-oil | Gasification at 700 °C; GHSV of 237 h−1 | H2 yield ≈ 0.5 m3 kg−1 CO yield = 0.3 m3 kg−1 | ×10 vs. parent biochar | [69] |
| Fast pyrolysis. Boiled in 0.1 mol L−1 HNO3; washed and dried at 105 °C. Impregnation with ammonium tungstate; heated to 80 °C for 30 min and dried at 110 °C. Carbothermal reduction in N2 (50 mL min−1) at 1000 °C for 1–3 h | WC-BCH | Bio-gas | Reduction at 800 °C in H2/N2 flow Reforming with CO2; 0.5 MPa, 6000 h−1; 600–900 °C | 90% CO | [70] | ||
| Corncobs | 1 mol L−1 HCl solution pyrolysis at 600 °C, 5% Ni loading | Ni-BCH | Toluene | Reforming W/F = 0.5 h, S/C = 3, 1 vol.% of toluene in gas, 1 atm 400–650 °C | H2, CO, CO2, CH4 | [72] | |
| IWI with Ni(NO3)2⋅6H2O and H3BO3; dried 105 °C; calcined 600 °C in N2 for 30 min | Ni/B-BCH | Toluene | Steam reforming W/F = 0.25 h, S/C = 3; 600 °C; 1–10 h | - | >60% conversion | [67] | |
| Palm shells | Carbonized at 850 °C in N2 for 30 min Impregnation with K2CO3 and Fe(NO3)3·9H2O for 12 (5 wt %), dried at 105 °C for 24 h | Fe-BCH | Toluene and naphthalene | Steam reforming 0.17–0.51 s, 90 min, 730–900 °C | - | 80% conversion | [71] |
| M-BCH Preparation | Pyrolysis of Biomass | |||||
|---|---|---|---|---|---|---|
| Biochar Feedstock | Synthesis | Catalyst M-Support | Substrate | Reaction Type and Conditions | Yield (%) | Ref. |
| Beech wood | Impregnation with nickel nitrate; filtrated, washed and dried at 60 °C; pyrolyzed at 400–500 °C | Ni-BCH | Oak wood | Pyrolysis at 700 °C | H2 concentration increased by 260% | [76] |
| Beech wood | Vacuum impregnation with nickel nitrate solutions (0.1–1 mol L−1) at 25 °C; filtrated and dried at 60 °C; carbothermal reduction at 500 °C | Ni-BCH | Beech wood | Pyrolysis in stainless-steel horizontal tube; 40 L h−1; 700 °C | 91% H2 vs. 60–70% for pristine wood | [75] |
| Switchgrass | Pyrolysis at 500 °C; 30 min. Impregnated with iron oxide and nickel nitrate at 50 °C; kept for 24 h; dried at 103 °C | Fe-BCH | Acetic acid | Pyrolysis at 450 °C under N2 flow 42 cm3 s−1 | 40.6% conversion | [77] |
| Beech wood | Impregnated with nickel nitrate (1 mol L−1); 3 days; 293 K; filtered, washed and dried at 323 K; calcined at 850 °C | Ni-BCH | Methane | Pyrolysis; 5% of CH4 in Ar; 900 °C; 10 °C/min; 1 h; 100 mL min−1 | 527 mmol g−1 H2 production | [78] |
| Chlorella vulgaris microalgae | Washed with HCl; activated with KOH at 80 °C; calcined at 700 °C in N2. IWI with Fe3(NO3)·9H2O (2.5–10 wt %); calcined at 400 °C; dried at 105 °C | Fe-BCH | Chlorella vulgaris microalgae | Pyrolysis in dual-bed reactor 450–850 °C in Ar (30 mL min−1) | Bio-oil with HHV of 31.26 MJ kg−1 | [79] |
| M-BCH Preparation | Hydrolysis of Biomass | |||||
|---|---|---|---|---|---|---|
| Biochar Feedstock | Synthesis | Catalyst M-Support | Substrate | Reaction Type and Conditions | Yield (%) | Ref. |
| Corncob | Mixed with tin (IV) hydroxide and cobalt (II) hydroxide; dried by rotary evaporation at 45 °C under 0.09 MPa; carbonized in N2 at 100–200 °C for 38 h | SnO2–Co3O4-BCH | Corncob | Hydrolysis in batch autoclave at 180 °C, for 200 min | Furfural 30% | [80] |
| Bamboo | Carbonization at 700 °C and sulfonation with H2SO4 for 4 h at 80 °C. Treated with aqueous solution of NaCl; dried at 120 °C; treated with (IL)-ZnCl2; acidified by HCl; washed and dried at 120 °C | IL-Zn-BCH-SO3H | Cellulose and bamboo | Microwave-assisted hydrolysis; 2.45 GHz; 350–750 W; 90–110 °C for 2 h | Sugars (60%) HMF (30%) | [81] |
| Carbonization at 700 °C and sulfonation with H2SO4 for 3 h at 80 °C. Treated with NaCl; dried at 120 °C; treated with (IL)-CuCl2; acidified by HCl; washed and dried at 120 °C | IL-Cu-BCH-SO3H | Sugars (35%) HMF (10%) | [82] | |||
| Mixing with 2 mol L−1 NaOH or 1 mol L−1 Na2CO3 solutions; dried at 105 °C; pyrolyzed at 400–600 °C; washed by 1 mol L−1 H2SO4 at 100 °C | Cellulose | Hydrolysis; 150 °C; 12 h | TRS (58.6%) Glucose (43.5%) | [83] | ||
| Nanomaterial Properties | Surface Area c (m2 g−1) | Electrochemical Supercapacitor Metrics | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Biochar Feedstock a | Synthesis b | Metal | Biochar Nanocomposite | Test Cell Type/Electrolyte | Capacitance d (F g−1)/Current Density (A g−1) | Energy Density e (Wh kg−1)/Power Density (W kg−1) | ||
| Agaric | Immersion in NaCl + Mn(AcO)2·4H2O solution. Heating at 60 °C for 6 h. Carbonization in Ar atmosphere at 800 °C for 12 h | Mn | Hierarchical porous MnO/biocarbon | 139.6 | 3-electrode/3 M KOH(aq) solution | 402 (1)/2 | - | [92] |
| Immersion in Mn(AcO)2·4H2O solution. Heating at 60 °C for 6 h. Carbonization in N2 atmosphere at 800 °C for 2 h with heating rate of 5 °C min−1 | Mn | Porous MnO/biocarbon | ≈133 | Asymmetric 2-electrode/3 M KOH(aq) solution | 101/1 | 35.9/800 | [93] | |
| Bambo leaves | Carbonization at 600 °C in N2 atmosphere for 2 h with heating rate of 1 °C min−1. Hydrothermal treatment of carbon and Cu(OAc)2 at 180 °C for 24 h | Cu | CuO/Cu2O/hierarchical porous biocarbon | 301.85 | 3-electrode/3 M KOH(aq) solution | 147/1 | - | [133] |
| Carbonization at 600 °C in N2 atmosphere for 2 h with heating rate of 1 °C min−1. Hydrothermal treatment of carbon and Mn(OAc)2·4H2O at 180 °C for 24 h | Mn | MnSi/heteroatom-doped porous biocarbon | 300.3 | Asymmetric 2-electrode/Polyvinyl alcohol (PVA)/KOH gel | 54.6 (2)/12 (3) | 24.6/604.8 | [118] | |
| Carbonization at 600 °C in N2 atmosphere for 2 h with heating rate of 1 °C min−1. Hydrothermal treatment of carbon and Zn(OAc)2·2H2O at 180 °C for 24 h | Zn | Zn4Si2O7(OH)2/3D N, S-enriched biocarbon | 475 | 124 (2)/10 (3) | 0.44 (4)/40 (5) | [132] | ||
| Banana peel | Hydrothermal treatment at 120 °C for 24 h. Carbonization at 500 °C in Ar flow for 2 h. Immersion in SnCl2 2H2O water/EtOH solution. Addition of ammonia solution (keeping pH = 8) and stirring for 4 h. Annealing at 200 °C for 1 h | Sn | SnO2/biochar | 376.2 | 3-electrode/1 M H2SO4(aq) solution | 476/0.15 | - | [135] |
| Immersion in 2 mol L−1 KOH(aq) solution for 24 h. Carbonization at 900 °C in Ar atmosphere for 2 h. Hydrothermal treatment of carbon, urea and KMnO4 at 120 °C for 12 h | Mn | MnO2/3D porous biocarbon | - | 3-electrode/1 M Na2SO4(aq) solution | 70/10 | - | [124] | |
| Cellulose | Immersion in Ni(NO₃)₂(aq) solution. Carbonization at 500 °C for 1 h. Electrophoretic deposition on Ni foils using Pt wire anode, with isopropanol electrolyte, at 40 V for 1 h | Ni | Biochar wrapped Ni/NiO nanobricks | 198 | 3-electrode/ 1 M KOH(aq) solution | 640/5 | - | [95] |
| Asymmetric 2-electrode | - | 81/6000 | ||||||
| Cladophora Glomerata | Immersion in 6 mol L−1 KOH(aq) solution at 80 °C (reflux) for 6 h. Carbonization at 700 °C for 2 h. Reflux with H2SO4 and HNO3 at 80 °C for 6 h. Hydrothermal treatment with NH4OH and FeSO4·7H2O at 120 °C for 15 h | Fe | Fe NPs/interconnected 3D pore network biochar | 957 | Asymmetric 2-electrode/3 M KCl(aq) solution | 368.9/1 | 16.4/25,600 | [127] |
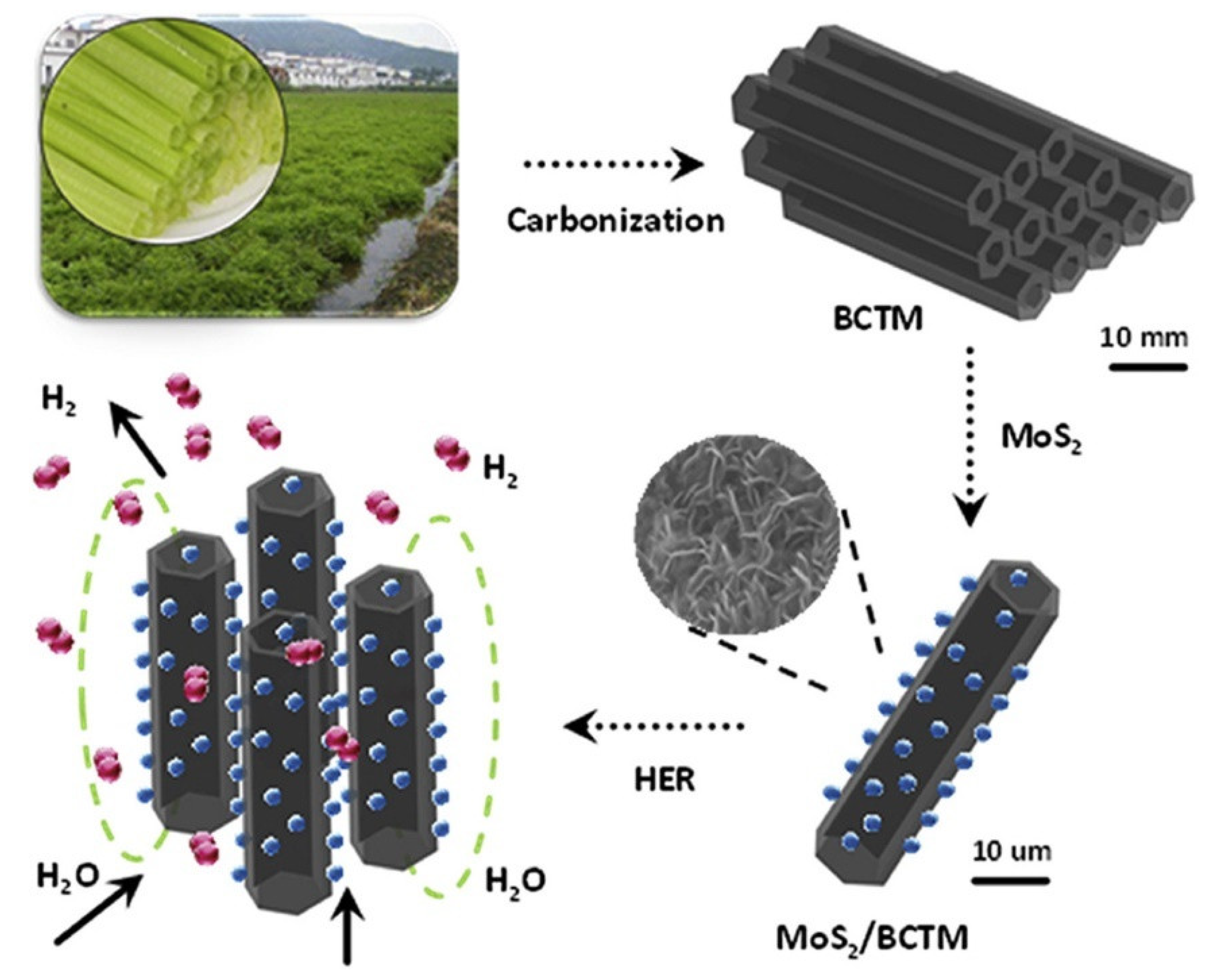
| Cornstalks | Mixing with KOH and KMnO4. Carbonization at 800 °C for 2 h under N2 atmosphere | Mo | MoS2 nanoflowers/biocarbon | 930 | Symmetric 2-electrode/6 M KOH(aq) solution | 38.1/50.0 | 12.1/37,775 | [94] |
| Cotton | Carbonization at 900 °C for 2 h under N2 atmosphere. Immersion in Co(NO3)2(aq) solution for 12 h. Calcination at 300 °C for 3 h in air | Co | Co3O4/hollow-biocarbon-fiber | 177 | Asymmetric 2-electrode/6 M KOH(aq) solution | 77.8/1 | 15.41/7500 | [130] |
| Mixing of CoCl2·6H2O with urea in water at 90 °C (reflux) for 3 h. Ultrasonic dispersion in this solution. Carbonization at 350 °C for 4 h with heating rate of 3 °C min−1 | Co | Sandwich Co3O4/biocarbon fiber/Co3O4 | - | 102/2 | 33.6/1500 | [97] | ||
| Crab waste | Carbonization at 500 °C for 1 h in N2 atmosphere. Immersion in KOH(aq) solution for 24 h. Activation at 700 °C for 2 h in N2 atmosphere. Ultrasonic dispersion in CoCl2·6H2O and FeCl3·6H2O aqueous solution. Addition of NaOH to fix pH into 11. Heat treatment at 120 °C for 12 h | Co | CoFe2O4/biocarbon | 1602 (6) | 3-electrode/6 M KOH(aq) solution | 437.4/10 | ~600/1 | [129] |
| Dairy manure/sewage sludge | Carbonization at 600 °C for 4 h under <0.5% O2 condition. Immersion in Ni(NO3)2(aq) solution for 24 h. Microwave oxidative heating under 1000 W power for 30 min | Ni | NiO-NiOOH/biochar | ≈30 | 3-electrode/0.5 M KOH(aq) solution | 123 (7) 100 (8) | - | [102] |
| Durian rind | Ultrasonic dispersion in FeCl3·6H2O, HNO3 and KMnO4 aqueous solution for 5 h at 40 °C. Drying at vacuum for 5 days at 70 °C. Carbonization at 800 °C for 25 min under vacuum condition. Ultrasonic dispersion in 1 mol L−1 HCl solution along with aniline monomer (cooled by ice batch). Slow addition of 0.125 M (NH4)2S2O8(aq) solution and stirring (2 h) for aniline polymerization | Fe | Fe3O4/biochar/polyaniline | 510.5 | 3-electrode/0.1 M KNO3(aq) solution | 615 (9) | 76.88 (9) | [98] |
| Idem to the immediately above-described protocol, except for the monomer: instead of aniline, pyrrole | Fe | Fe3O4/biochar/polypyrrole | 474.8 | 572 (9) | 71.50 (9) | [99] | ||
| Fresh bamboo strip | Carbonization at 650 °C for 2 h in N2 atmosphere with heating rate of 3 °C min−1. “In situ” growth of CoZn-ZIF-8: heating at 120 °C for 12 h of M(AcO)2·× H2O (M = Zn and Co), 2-MeIm with carbon mixture in aqueous solution. Carbonization at 900 °C for 3 h in N2 atmosphere | Co and Zn | CoZn/biocarbon | - | 3-electrode/6 M KOH(aq) solution | 757.8 (10) | - | [131] |
| Hemp stem | Carbonization at 600 °C for 2 h in N2 flow with heating rate of 10 °C min−1. Heating in steam atmosphere at 800 °C for 2 h. Immersion in KMnO4 and MnSO4 for 30 min. Hydrothermal treatmentat 140 °C for 24 h | Mn | MnO2 nanowire/biocarbon | 438 | 3-electrode/1 M Na2SO4(aq) solution | ≈299/30 | - | [123] |
| Asymmetric 2-electrode/1 M Na2SO4(aq) solution | - | 31.0/14,800 | ||||||
| Infested ash tree residue | Carbonization at 700 °C in inert gas flow with heating rate of 0.5 °C min−1. Immersion in Ag2SO4/HNO3 aqueous solution for 24 h | Ag | Ag/Biochar | 335 | Symmetric 2-electrode/2 M LiN(SO2CF3)2(aq) solution | 489 (11) | 5.8/619 | [136] |
| Luffa plant | Heat pre-treatment at 200 ℃ for 6–8 h in vacuum. Mixing/milling with K2FeO4 and dicyandiamide. Carbonization at 1000 ℃ for 2 h in N2 with heating rate of 10 ℃ min−1. Ultrasonic dispersion (for 30 min) in EtOH/ethylene glycol solution with Ni(AcO)2·4H2O and Mn(AcO)2·4H2O. Solvothermal treatment at 185 °C for 20 h | Ni and Mn | NiMn2O4 nanocrystals/3D porous graphitized biocarbon | 832 | Asymmetric 2-electrode/6 M KOH(aq) solution | 100.6/8 | 45.25/11,380 | [110] |
| Natural metasequoia wood | Immersion in ZnCl2(aq) solution under vacuum for 24 h. Carbonization at 750 °C for 3 h. Immersion in Co(NO3)2·6H2O Ni(NO3)2·6H2O aqueous solution. Hydrothermal treatment at 140 °C for 12 h. Immersion in Na2S·9H2O(aq) solution. Hydrothermal treatment at 160 °C for 12 h | Ni and Co | NiCo2S4 nanowires/3D biocarbon | - | 3-electrode/6 M KOH(aq) solution | 281.8/10 | - | [117] |
| Natural wood slice | Multi-step carbonization: (1) T = 500 °C for 1 h with heating rate of 5 °C·min−1, (2) T = 1000 °C for 2 h with heating rate of 5 °C·min−1, and (3) T = 500 °C with cooling rate of 5 °C·min−1. Immersion in KMnO4(aq) solution. Heating at 60 °C for 12 h | Mn | Core–shell MnO2 nanosheets/biochar | 683 (6) | 3-electrode/1 M Na2SO4(aq) solution | 43/10 | - | [121] |
| Peanut shells | Mixing with KOH. Carbonization at 800 °C for 1 h in vacuum. Dispersion in EtOH/ethylene glycol solution for 4 h. Hydrothermal treatment at 80 °C for 12 h in presence of Ni foam. | Ni | Biocarbon hooks on Ni foam | 1279 (6) | 3-electrode/1 M Li2SO4(aq) solution | ≈90/10 | ~12.2/4000 | [106] |
| Pinecone flowers | Carbonization at 600 °C for 3 h in N2 atmosphere with heating rate of 10 °C min−1. Immersion in KOH(aq) solution. Activation at 800 °C for 1.5 h in N2. Impregnation with H3PMo12O40 in aqueous solution for 1 h | Mo | Polyoxometalate/biochar | 510 | 3-electrode/1 M H2SO4(aq) solution | ≈850 (12) | - | [134] |
| Activation in KOH(aq) solution (reflux conditions) for 10 h. Carbonization at 900 °C for 2 h in Ar flow with heating rate of 5 °C min−1. Ultrasonic dispersion in ethanol for 30 min. Mixing with Ni(NO3)2·6H2O(aq) solution, hexamethylenetetramine and ammonia via stirring for 1 h. Solvothermal treatment at 90 °C for 10 h | Ni | Ni(OH)2 nanosheets/honeycomb-like porous biocarbon | 80.3 | 3-electrode/1 M KOH(aq) solution | 565.71/10 | - | [105] | |
| Pomelo peel | Activation in 1 M KOH(aq) solution for 12 h. Carbonization at 700 °C for 2 h in N2 atmosphere with heating rate of 5 °C min−1.Hydrothermal treatment with Co(NO3)2·6H2O, Ni(NO3)2·6H2O and urea at 120 °C for 16 h | Ni and Co | NiCo2O4 nanosheets/honeycomb-like porous biocarbon | - | Asymmetric 2-electrode/2 M KOH(aq) solution | ≈35/10 | 14.9/5346 | [112] |
| Hydrothermal treatment at 180 °C for 6.5 h. Mixing with Co(NO3)·6H2O, Ni(NO3)·6H2O, Al(NO3)·9H2O and urea in MeOH solution. Solvothermal treatment at 150 °C for 12 h | Ni, Co and Al | CoNiAl-layered double hydroxide/biocarbon aerogel | - | 3-electrode/2 M KOH(aq) solution | 902/10 | - | [111] | |
| Poplar catkins | Carbonization at 800 °C for 2 h in Ar atmosphere with heating rate of 2 °C min−1. Ultrasonic dispersion in Tris-HCl (pH = 8.5, 10 mM) buffer solution for 30 min. Addition of dopamine while stirring for 24 h to self-polymerization. Mixing with Ni(NO3)2, Co(NO3)2, hexamethylenetetramine, and citric acid trisodium salt in aqueous solution. Hydrothermal treatment at 120 °C for6 h. Calcination at 300 °C for 2 h in air with heating rate of 1 °C min−1 | Ni and Co | NiCo2O4 nanosheets/biocarbon microsheets | 143.21 | Asymmetric 2-electrode/6 M KOH(aq) solution | 96.0 (13)/10 | 21.7/8218.9 | [113] |
| Rice husk charcoal | Activation in KOH(aq) solution at 110 °C for 1 h. Annealing at 850 °C for 1 h in CO2 atmosphere. Mixing with Ni(NO3)2·6H2O and thiourea in aqueous solution. Immersion of Ni foam. Hydrothermal treatment at 120 °C for 16 h | Ni | Ni3S2/porous biocarbon | - | 89.3/2 | 27.9/1500 | [107] | |
| Seeds of Toona sinensis | Activation in KOH(aq) solution for 30 min. Carbonization at 800 °C for 2 h in Ar atmosphere. Mixing with Co(NO3)2·6H2O, Ni(NO3)2·6H2O and hexamethylenetetramine in aqueous solution. Hydrothermal treatment at 100 °C for 9 h | Ni and Co | Ni-Co layered double hydroxide/biocarbon | 111.21 | 29.6/10 | 23.5/959.7 | [116] | |
| Sorghum stalk | Carbonization at 550 °C for 0.5 h in N2 atmosphere. Activation in KOH(aq) solution at 700 °C for 1 h. Ultrasonic dispersion in Ni(NO3)2·6H2O(aq) solution. Addition of urea solution. Microwave deposition at 700 W for 3 min | Ni | Ni(OH)2/biocarbon | - | 3-electrode/3 M KOH(aq) solution | 490.1/20 | 12.3/6314.4 | [103] |
| Sugarcane bagasse | Activation with KOH in abs. EtOH at 60 °C until EtOH evaporation. Carbonization at 800 °C for 2 h in N2 atmosphere with heating rate of 10 °C min−1. Treatment with KMnO4(aq) solution at 70 °C until color change | Mn | MnO2/biocarbon | 471.33 | Asymmetric 2-electrode/1 M KOH(aq) solution | 51.3/10 | 25.9 (14)/750 | [122] |
| Activation with KOH in abs. EtOH at 60 °C until EtOH evaporation. Carbonization at 800 °C for 2 h in N2 atmosphere with heating rate of 10 °C min−1. Mixing with FeCl3·6H2O(aq) and FeCl2·4H2O(aq) solution. Addition of NaOH(aq) solution until pH = 11. Heating at 90 °C for 1 h | Fe | Fe3O4/activated biocarbon | 490.93 | 35.6/10 | 29.1 (14)/800 | [128] | ||
| Typha domingensis | Addition to a Co(NO3)2·6H2O and Ni(NO3)2·6H2O aqueous solution. Hydrothermal treatment at 200 °C for 12 h. Carbonization at 700 °C for 3 h in Ar atmosphere | Ni and Co | Ni-Co oxides NPs/biocarbon fiber | ≈110 | Asymmetric 2-electrode/6 M KOH(aq) solution | ≈92/25 | ≈30/25,600 | [96] |
| Activation in KOH(aq) solution at 80 °C for 2 h. Mixing with water-dispersed graphene oxide. Carbonization at 700 °C for 3 h. Ultrasonic dispersion in water. Mixing with Ni(NO3)2·6H2O, Al(NO3)3·9H2O and urea. Hydrothermal treatment at 95 °C for 24 h | Ni and Al | Ni-Al layered double hydroxide nanosheets/biocarbon-graphene oxide | ≈390 | 144/30 | ≈100/28,800 | [109] | ||
| Walnut shells | Activation in KOH(aq) solution. Carbonization at 600 °C for 2 h in N2 atmosphere with heating rate of 5 °C min−1. Mixing with Ni(NO3)2·6H2O, hexamethylenetetramine and thiourea in aqueous solution. Hydrothermal treatment at 180 °C for 8 h | Ni | NiS NPs/porous biocarbon | - | 3-electrode/2 M KOH(aq) solution | 350/10 | - | [108] |
| Wasted litchi shell | Mixing with thiourea and (NH4)6Mo7O24 aqueous solution. Hydrothermal treatment at 200 °C for 1 h. Carbonization at 1000 °C for 3 h in N2 atmosphere | Mn | MnO nanosheets/porous biocarbon | 326 | Symmetric 2-electrode/1 M LiPF6 in ethylene carbonate/diethyl carbonate/dimethyl carbonate solution | 250/20 | - | [100] |
| Watermelon | Hydrothermal treatment at 180 °C for 12 h. Mixing with KMnO4 and Na2S2O3·5H2O in aqueous solution. Hydrothermal treatment at 120 °C for 12 h. Carbonization at 350 °C for 2 h in N2 atmosphere | Mn | MnO2/biocarbon aerogel | Not specified | 3-electrode/6 M KOH(aq) solution | 49.3/0.5 | - | [119] |
| Water caltrop | Carbonization at 800 °C for 5 h in N2 atmosphere. Mixing with KOH. Carbonization at 850 °C for 2 h in N2 atmosphere. Ultrasonic dispersion in Co(NO3)2·6H2O, Ni(NO3)2·6H2O and polyvinylpyrrolidone MeOH solution. Solvothermal treatment at 180 °C for 12 h | Ni and Co | Ni-Co layered double hydroxide NPs/porous biocarbon | 136.6 | Asymmetric 2-electrode/6 M KOH(aq) solution | 78/10 | 29/14,736 | [115] |
| Wheat flour | Dispersion in KOH and urea aqueous solution. Carbonization at 800 °C for 1 h in N2 flux. Mixing with Fe(NO3)3·9H2O, Na2SO4 in water. Hydrothermal treatment at 120 °C for 9 h | Fe | Fe2O3 nanorods/porous biocarbon | 700 | Asymmetric 2-electrode/1 M Li2SO4(aq) solution | ≈180/10 | 102/9300 | [126] |
| Wheat straw | Immersion in KOH(aq) solution for 8 h. Carbonization at 800 °C for 2 h in Ar atmosphere. Mixing with Fe(NO3)3·9H2O in EtOH at 50 °C until EtOH evaporation. Calcination at 200 °C for 5 h in air | Fe | Ultrathin Fe2O3 film/porous biocarbon | 775.8 | Asymmetric 2-electrode/1 M KOH(aq) solution | 108/30 | 20.8/20,650 | [125] |
| Willow catkin | Immersion in KOH(aq) solution at 100 °C until water evaporation. Multi-step carbonization: (1) T = 400 °C for 3 h with heating rate of 5 °C·min−1, and (2) T = 850 °C for 1 h with heating rate of 10 °C·min−1. Mixing with KMnO4(aq) solution. Hydrothermal treatment at 120 °C for 1 h with heating rate of 2 °C min−1 | Mn | MnO2 nanoplates/cross-linked biocarbon nanosheets | 234 | Asymmetric 2-electrode/1 M Na2SO4(aq) solution | 32.1/5 | 12.1/3570.5 | [120] |
| Carbonization at 500 °C for 4 h in Ar atmosphere. Refluxing in HNO3(aq) solution at 50 °C for 5 h. Dispersion in water and mixing with Ni(NO3)2·6H2O, NH4F and urea. Hydrothermal at 150 °C for 6 h | Ni | Ni(OH)2 nanosheet arrays/hollow biocarbon microtube | 155.7 | Asymmetric 2-electrode/6 M KOH(aq) solution | 53/20 | 16.6/15,000 | [104] | |
| Carbonization at 1000 °C in Ar atmosphere. Mixing with Ni(AcO)2·4H2O, Co(AcO)2·4H2O and thiourea in ethylene glycol. Solvothermal at 180 °C for 8 h | Ni and Co | Ni-Co sulfides NPs/biocarbon microtubes | 17.3 | 57/20 | 17.7/17,200 | [114] | ||
| Yellow soybean sprouts | Carbonization at 400 °C in N2 flow. Immersion in Ce(NO3)3(aq) solution. Addition of KOH. Hydrothermal treatment at 100 °C for 12 h. Carbonization at 800 °C for 2 h in N2 flow | Ce | CeO2/biocarbon | 859 | Symmetric 2-electrode/poly(vinyl alcohol)−KOH gel electrolyte | ≈85/3 | ≈7.5/1200 | [101] |
| Nanomaterial Properties | ORR Metrics | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biochar Feedstock (a) | Synthesis | Metal Specie(s) | Biochar Nanocomposite | Electrolyte | jL (mA cm−2) | Eonset (V) | E1/2 (V) | TS (mV dec−1) | nO2 | |
| Alkaline Medium | ||||||||||
| Wetland plant (Iris sibirica L.) | The plant extracted Ce cations under a hydroponic environment; pyrolysis at 900 °C (2 h) under N2 flow | CeO2 NPs | CeO2-encapsulated N-doped biochar | 1 M KOH | 2.02 | 0.90 (vs. RHE) | 0.84 (vs. RHE) | 71 | 3.55 (a) | [172] |
| Vegetable sponge | Pre-carbonization at 500 °C (4 h, N2 atmosphere); activation with KOH at 800 °C (1 h); immersion on Co(NO3)x∙6H2O aqueous solution; ultrasonication (30 min) | Co and CoO | Biomass-derived carbon with cobalt/cobalt oxide doping | 0.1 M KOH | ≈4.7 | 0.86 (vs. RHE) | 0.77 (vs. RHE) | 64 | 3.91 (a) | [143] |
| Pomelo peel | Mixing of pomelo peels with melamine, cobalt nitrate and KOH; pyrolysis/activation at 800 °C (1 h, N2 atmosphere); acid leaching (HCl) | Co | Biomass-derived carbon with N/Co doping | 0.1 M KOH | 5 | 0.87 (vs. RHE) | 0.78 (vs. RHE) | --- | 3.90 (a) or 3.85 (b) | [174] |
| Biomass tar | Mixing of biomass tar, urea and FeCl3 in aqueous solution followed by freeze-drying; carbonization at 700 °C (3 h, N2 atmosphere); immersion in HCl solution | Fe | Hierarchically porous Fe, N-co-doped carbon with in situ coated graphene from biomass tar | 0.1 M KOH | ≈5 | 0.04 (vs. Ag/AgCl) | --- | --- | 3.98 (a) | [137] |
| Red dates | Mixing of red dated with g-C3N4 and Fe(AcO)2; pyrolysis at 800 °C (1 h, N2 atmosphere); washing with HCl | Fe | Biomass based iron and nitrogen co-doped 3D porous carbon | 0.1 M KOH | 4.5 | 0.08 (vs. Hg/HgO) | −0.05 (vs. Hg/HgO) | --- | 3.71 (a) | [175] |
| Fresh leaves of Ipomoea aquatica | Acid etching of leaves (H2SO4); mixing of the treated leaves with FeCl3∙6H2O and carbon black via ball milling; pyrolysis at 900 °C (2 h, N2 atmosphere) | Fe | Biomass-modified carbon black | 0.1 M KOH | --- | 0.05 (vs. Hg/HgO) | --- | --- | ~3.90 (a) | [173] |
| Corn silks | Hydrothermal carbonization (180 °C, 10 h); annealing in NH3 atmosphere (850 °C, 1 h); mixing with FeCl3; annealing in NH3 atmosphere (850 °C, 1 h) | Fe | N-, P- and Fe- tridoped nanoporous carbon derived from plant biomass | 0.1 M KOH | --- | 0.957 (vs. RHE) | 0.852 (vs. RHE) | --- | 3.87–3.99 (b) | [176] |
| Soybean straw | Pyrolysis at 800 °C using soybean straw, melamine, magnesium oxide and ferric nitrate (2 h, N2 atmosphere); treatment with HCl | Fe, Fe3Cand Fe3O4 | Biomass-derived Fe-N co-doped porous carbon with honeycomb structure | 0.1 M KOH | --- | 0.989 (vs. RHE) | 0.854 (vs. RHE) | 69 | 3.97 (a) | [138] |
| Catkins | Mixing of catkins with melamine and FeCl3 in aqueous medium (100 °C until complete water evaporation); pyrolysis at 800 °C (2 h); immersion in H2SO4 (80 °C) | Fe | Fe/N co-doped CNT@hollow carbon fibers derived from plant biomass | 0.1 M KOH | 4.08 | −0.098 (vs. Ag/AgCl) | −0.194 (vs. Ag/AgCl) | 65.8 | 3.915 (a) | [190] |
| Chitosan | Mixing of chitosan and Zn(NO3)2∙6H2O in aqueous solution; drying at 80 °C; thermal treatment at 900 °C (2 h, air) + treatment with HCl | Zn | Zn-N-carbon from biomass resource chitosan | 0.1 M KOH | ≈6.7 | 0.96 (vs. RHE) | 0.86 (vs. RHE) | --- | 3.88–4.01 (a) | [170] |
| Porphyra | Pre-carbonization of porphyra (300 °C, 1 h, air); carbonization/activation process with KOH (900 °C, 2 h, Ar atmosphere); washing with acid; mixing with hemin; carbonization (800 °C, 2 h, Ar atmosphere) | Single Fe atoms | Biomass derived N-doped porous carbon supported single Fe atoms | 0.1 M KOH | 5.0 | --- | 0.87 (vs. RHE) | 52 | 4.0 (a) | [178] |
| Jackfruit seed | Hydrothermal treatment of jackfruit seed (180 °C, 24 h); carbonization (700 °C, N2 atmosphere); formation of Pt NPs from adsorbed Pt ions (chemical reduction of H2PtCl6 using NaBH4) | Pt NPs | Bio-derived carbon enriched with N and decorated with Pt NPs | 0.1 M KOH | 4.9 | 0.89 (vs. RHE) | 0.75 (vs. RHE) | 74 | 3.8 (a) | [192] |
| Corncobs | Hydrothermal carbonization at 250 °C (12 h); activation with KOH (90 °C, 30 min); thermal treatment at 600 °C (1 h, N2 atmosphere); incorporation of MnO2 (hydrothermal method, 120 °C, 3 h) | α-MnO2 | Hydrochars supporting α-MnO2 | 0.1 M KOH | 2.85 | −0.23 (vs. Ag/AgCl) | −0.32 (vs. Ag/AgCl) | --- | 3.27–3.81 (a) | [171] |
| Typha domingensis | Mixing of Co(II) and Ni(II) precursors with Typha domingensis in aqueous solution; heating of the resulting solution at 200 °C (12 h); centrifugation; pyrolysis at 700 °C (3 h) under argon atmosphere | Ni-Co oxides nanocapsules | Biomass-based carbon nanofibers composited with nickel-cobalt oxides | 1 M KOH | 0.23 | −0.15 (vs. Ag/AgCl) | −0.32 (vs. Ag/AgCl) | --- | --- | [167] |
| Okara | Pyrolysis at 300 °C (2 h, N2 atmosphere); activation with KOH; pyrolysis at 700 °C (2 h, N2 atmosphere); treatment with HNO3; deposition of LaMnO3 NPs (sol-gel method); calcination at 350 °C (5 h, air) and 600 °C (4 h, vacuum) | LaMnO3 NPs | Perovskite LaMnO3 NPs anchored on biomass−derived N, P co−doped porous carbon | 0.1 M KOH | ≈4.2 | --- | --- | 76 | 3.82 (a) | [142] |
| Soybean straws | KOH activation/pyrolysis (800 °C, 1 h, N2 atmosphere); mixing with CoCl2 in aqueous medium; drying (80 °C); carbonization at 800 °C (1 h, NH3 atmosphere); washing with HCl | CoO NPs | Sponge-like N-doped carbon materials with Co-based NPs derived from biomass | 0.1 M KOH | 5.8 | 0.87 (vs. RHE) | 0.79 (vs. RHE) | --- | 4.03 (a) or 3.75 (b) | [180] |
| Sichuan pepper | Mixing of Sichuan pepper, NaSCN, FeCl3, ZnCl2 and 2,2-bipyridine in aqueous medium (25 °C); drying at 60 °C; pyrolysis at 900 °C (2 h, N2 atmosphere) | ZnS and FeS | ZnS/FeS heterojunctions on biomass-derived porous carbon | 0.1 M KOH | 5.60 | 1.00 (vs. RHE) | 0.880 (vs. RHE) | 78 | 4 (a) or ≈3.9 (b) | [186] |
| Bean sprouts | Mixing with CoCl2 in aqueous medium (18 °C); drying (80 °C); heat-treatment at 900 °C (Ar atmosphere); annealing at 750 °C (NH3 atmosphere) | Co2P NPs | Bio-derived Co2P NPs supported on N-doped carbon | 0.1 M KOH | --- | --- | 0.836 (vs. RHE) | 81.71 | 3.56 (a) | [146] |
| Microalgae | Mixing of microalgae powder with FeCl2∙5H2O; carbonization in NH3 atmosphere at 1000 °C (1 h) | Fe3C | N-doped graphitic carbon from biomass | 0.1 M KOH | --- | 1.0 (vs. RHE) | 0.864 (vs. RHE) | 65.3 | 3.91 (a) or 3.98 (b) | [177] |
| Corn crumbs | Mixing of corn crumbs with WS2 in molten NaCl-KCl; pyrolysis/electrolysis process (750 °C, 3.0 V, 24 h) | WC | WC/N-doped carbon nanocomposite | 1 M KOH | --- | 0.865 (vs. RHE) | --- | 61.52 | 3.80 (a) | [168] |
| Neutral medium | ||||||||||
| Microalgae Chlorella pyrenoidosa | Hydrothermal method at 180 °C (microalgae mixed with FeCl2 aq. solution, 12 h); pyrolysis at 900 °C (2 h, N2 atmosphere); ball milling | FeOOH, Fe3O4 and α-Fe2O3 NPs | Iron oxide NPs-embedded N-doped biocarbon | 50 mM phosphate buffer solution | 5.43 | 0.811 (vs. RHE | 0.589 (vs. RHE) | 65.1 | 3.98 (b) | [157] |
| Pine wood lumber | High temperature gasification (up to 1000 °C); alkaline pos-treatment | MnO2 nanocrystals | Graphitic biochar with supported MnO2 | 0.1 M Na2SO4 (pH adjusted) | --- | --- | --- | --- | --- | [158] |
| Cornstalk cores | Mixing of cornstalk cores, melamine, hydrazine hydrate, FeCl3 and Se powder (80 °C); drying; carbonization at 850 °C (2 h, N2 atmosphere) | Fe3Se4 and FeSe | Fe3Se4/FeSe heterojunctions in cornstalk-derived N-doped carbon | 50 mM phosphate buffer solution | --- | --- | --- | --- | 3.764–4.06 (a) | [179] |
| Pomelo peels | Mixing of pomelo peels, K4Fe(CN)6 and Na2WO4; carbonization at 600–1100 °C (1.5 h, N2 atmosphere) | Fe3C and WC NPs | Biomass-derived porous Fe3C/WC/GC nanocomposite | 50 mM phosphate buffer solution | --- | --- | --- | --- | 3.95 (a) | [191] |
| Acidic medium | ||||||||||
| Soybean straw | Pyrolysis at 800 °C using soybean straw, melamine, magnesium oxide and ferric nitrate (2 h, N2 atmosphere); treatment with HCl | Fe3C and Fe3O4 | Biomass-derived Fe-N co-doped porous carbon | 0.1 M KClO4 | --- | 0.886 (vs. RHE) | 0.754 (vs. RHE) | 76 | 3.89 (a) or 3.65 (b) | [138] |
| Catkins | Mixing of catkins with melamine and FeCl3 in aqueous medium (100 °C until complete water evaporation); pyrolysis at 800 °C (2 h, N2 atmosphere); immersion in H2SO4 (80 °C) | Fe | Fe/N co-doped CNT@hollow carbon fibers derived from plant biomass | 0.5 M H2SO4 | --- | 0.586 (vs. Ag/AgCl) | --- | --- | 3.9 (b) | [190] |
| Nanomaterial Properties | HER Metrics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Biochar Feedstock | Synthesis | Metal Specie(s) | Biochar Nanocomposite | Electrolyte | η10 (mV) | j0 (mA cm−2) | TS (mV dec−1) | Faraday Efficiency (%) | Ref. |
| Pomelo peels | Pre-carbonization with urea (400 °C); activation with KOH; mixing with (NH4)6Mo7O24; pyrolysis (800 °C, Ar) | Mo2C | N, K Co-Activated biochar-derived molybdenum carbide | 0.5 M H2SO4 | 161 | --- | 57 | --- | [199] |
| 1 M KOH | 144 | --- | 53 | --- | |||||
| Sunflower seeds | Treatment with 2 M HCl (10 h) and H3PMo12O40 (24 h); carbonization at 800 °C (4 h, Ar atmosphere) | Mo2C | Mo2C@S,N-carbon | 0.5 M H2SO4 | 146 | 0.136 | 83 | --- | [147] |
| 1 M KOH | 60 | 1.07 | 71 | ≈100 | |||||
| Pomelo peels | Mixing of pomelo peels with Co(NO3)2; annealing in air (260 °C, 6 h); carbonization (N2 atmosphere, 800 °C, 6 h) | Co NPs | Biomass-derived porous C membrane embedded with Co NPs | 1 M KOH | 154 | 1.009 | 106.4 | ≈100 | [198] |
| Wild celery | Washing with HCl; carbonization at 750 °C (3 h, N2 atmosphere); incorporation of MoS2 (heating of biochar and an aqueous solution containing thiourea and hexaammonium heptamolybdate tetrahydrate at 220 °C, 18 h) | MoS2 | Biomass carbon tube matrix auxiliary MoS2 heterojunction | 0.5 M H2SO4 | 176 | 0.01 | 51 | --- | [156] |
| Wood of Australian jarrah | Carbonization at 1000 °C, (Ar atmosphere); Pt incorporation via magnetron sputtering method (10 s, 10 mA) | Pt NPs | Platinum supported on carbonized biomorphic wood | 1 M KOH | 50 | --- | 64 | --- | [197] |
| Seaweed | Graphene oxide (GO) was prepared using a modified Hummer’s method and mixed with sodium alginate and then with Co(NO3)2∙6H2O; the mixture was dehydrated and carbonized with red phosphorus at 800 °C (240 min, N2 atmosphere) | CoP | CoXP@C Core–shell NPs Embedded in 3D Cross-linked Graphene Aerogel | 0.5 M H2SO4 | 120 | --- | 57 | --- | [169] |
| 1 M KOH | 225 | --- | 66 | --- | |||||
| Seaweed | Graphene oxide (GO) was prepared using a modified Hummer’s method and mixed with sodium alginate and then with Ni(NO3)2∙6H2O; the mixture was dehydrated and carbonized with red phosphorus at 800 °C (240 min, N2 atmosphere) | Ni2P | NiXP@C core–shell NPs embedded in 3D cross-linked graphene aerogel | 0.5 M H2SO4 | 305 | --- | 74 | --- | [169] |
| 1 M KOH | 340 | --- | 97 | --- | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, R.; Abdelkader-Fernández, V.K.; Matos, R.; Peixoto, A.F.; Fernandes, D.M. Metal-Supported Biochar Catalysts for Sustainable Biorefinery, Electrocatalysis, and Energy Storage Applications: A Review. Catalysts 2022, 12, 207. https://doi.org/10.3390/catal12020207
Ramos R, Abdelkader-Fernández VK, Matos R, Peixoto AF, Fernandes DM. Metal-Supported Biochar Catalysts for Sustainable Biorefinery, Electrocatalysis, and Energy Storage Applications: A Review. Catalysts. 2022; 12(2):207. https://doi.org/10.3390/catal12020207
Chicago/Turabian StyleRamos, Rubén, Víctor K. Abdelkader-Fernández, Renata Matos, Andreia F. Peixoto, and Diana M. Fernandes. 2022. "Metal-Supported Biochar Catalysts for Sustainable Biorefinery, Electrocatalysis, and Energy Storage Applications: A Review" Catalysts 12, no. 2: 207. https://doi.org/10.3390/catal12020207
APA StyleRamos, R., Abdelkader-Fernández, V. K., Matos, R., Peixoto, A. F., & Fernandes, D. M. (2022). Metal-Supported Biochar Catalysts for Sustainable Biorefinery, Electrocatalysis, and Energy Storage Applications: A Review. Catalysts, 12(2), 207. https://doi.org/10.3390/catal12020207









