In Situ Partial Sulfidation for Preparing Cu/Cu2−xS Core/Shell Nanorods with Enhanced Photocatalytic Degradation
Abstract
:1. Introduction
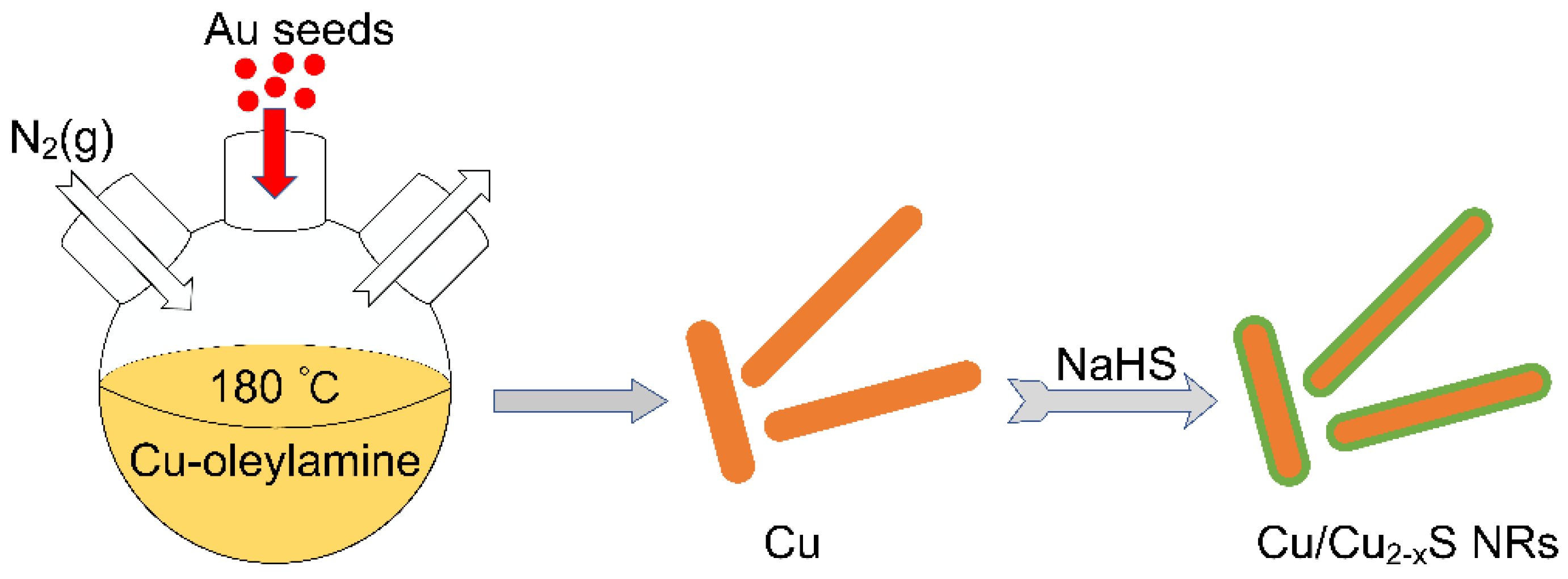
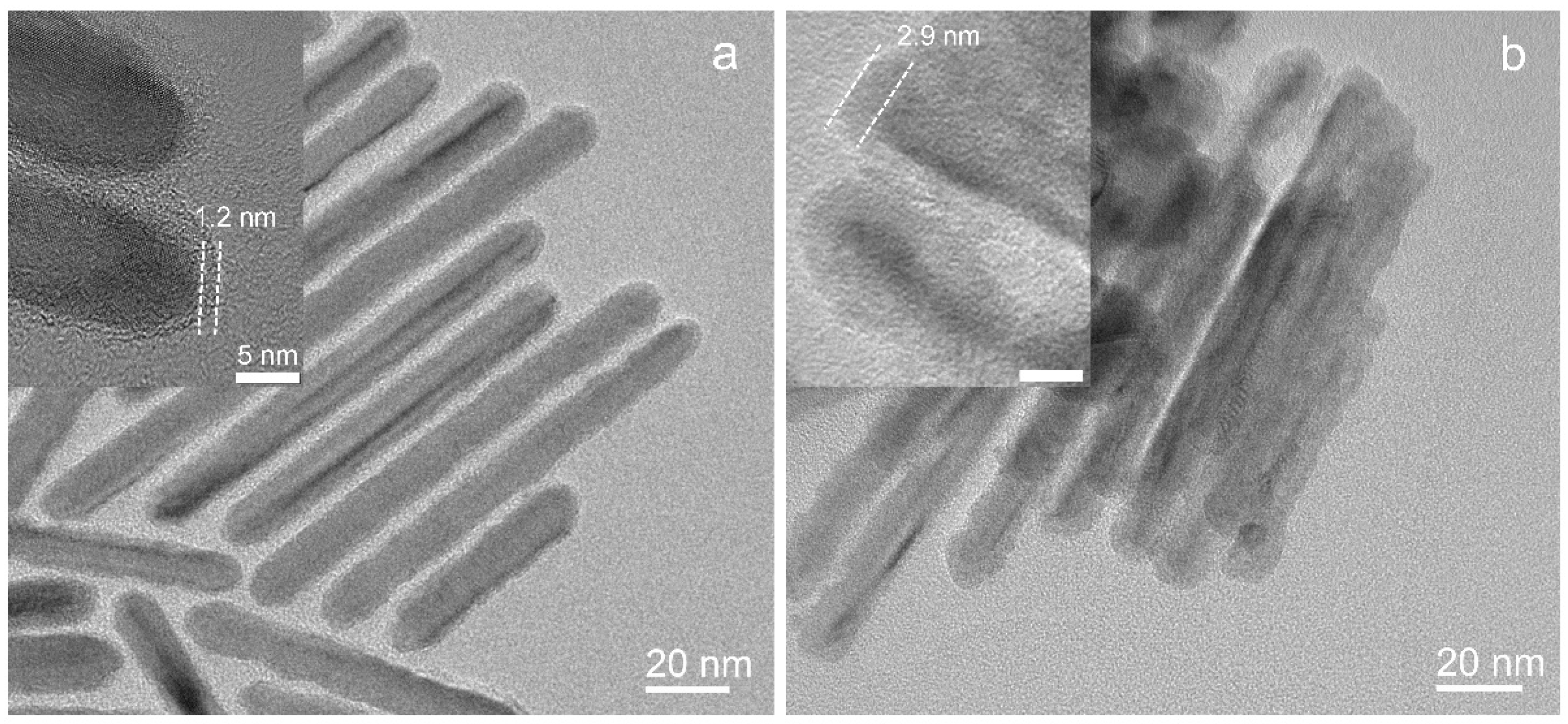
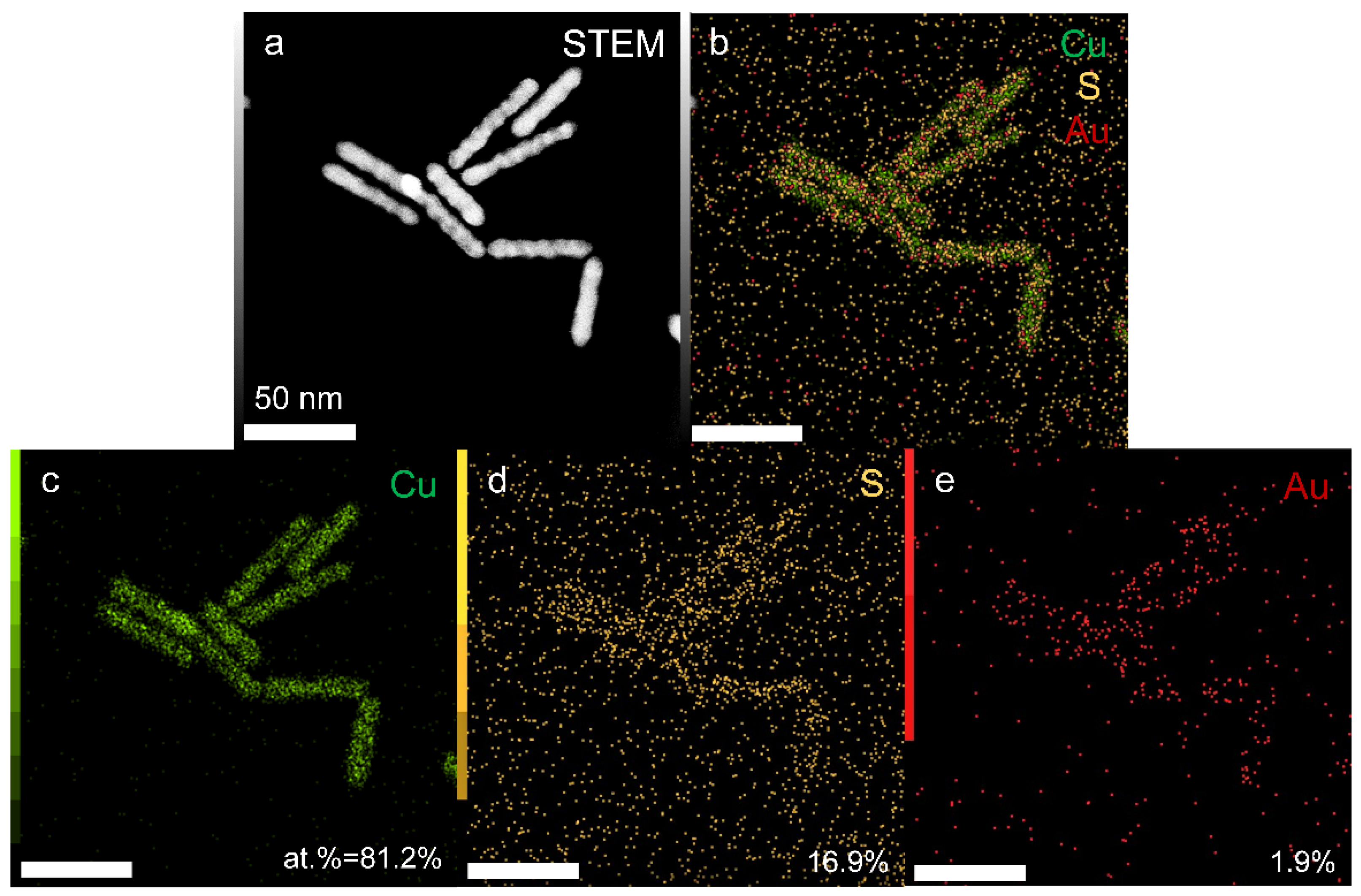
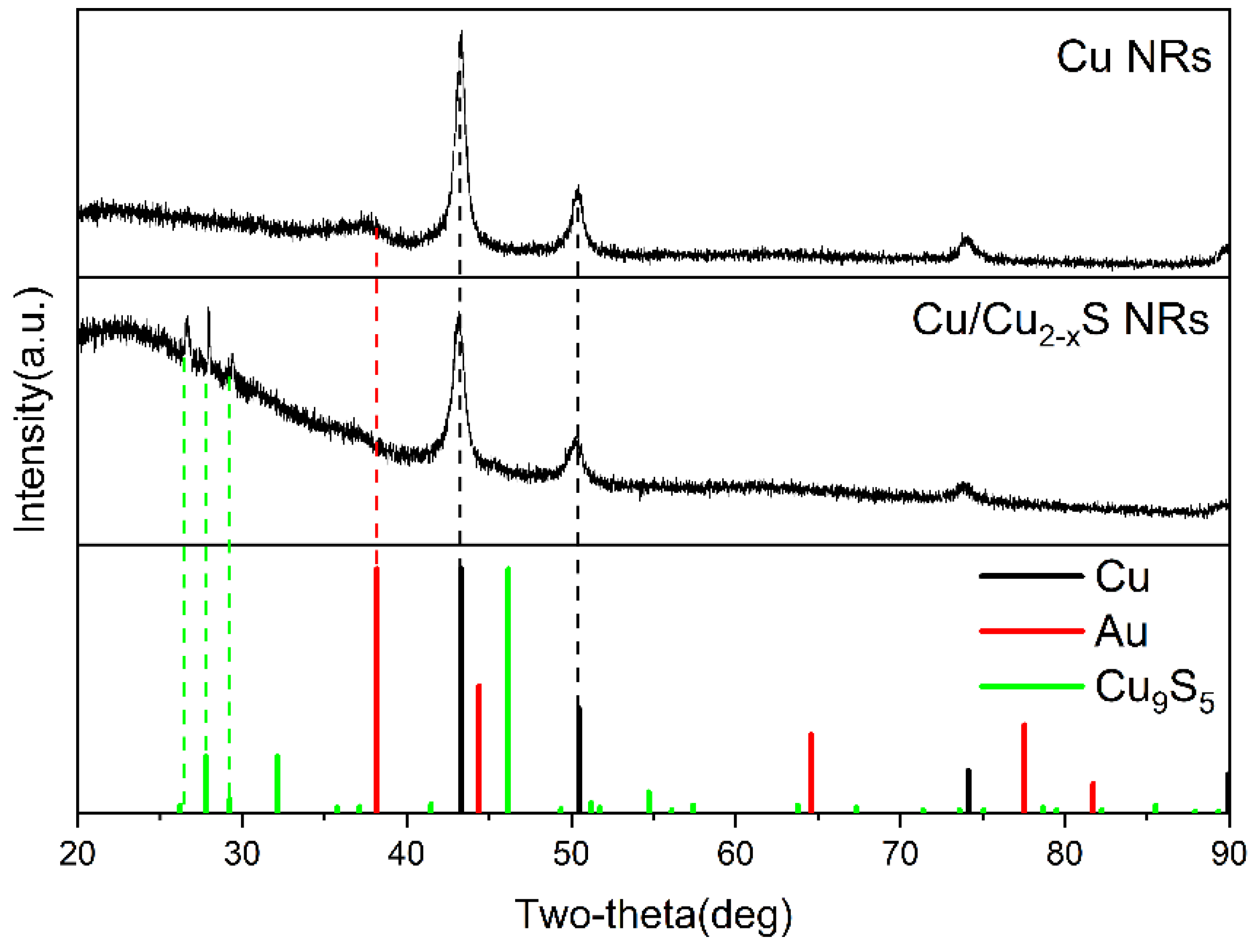
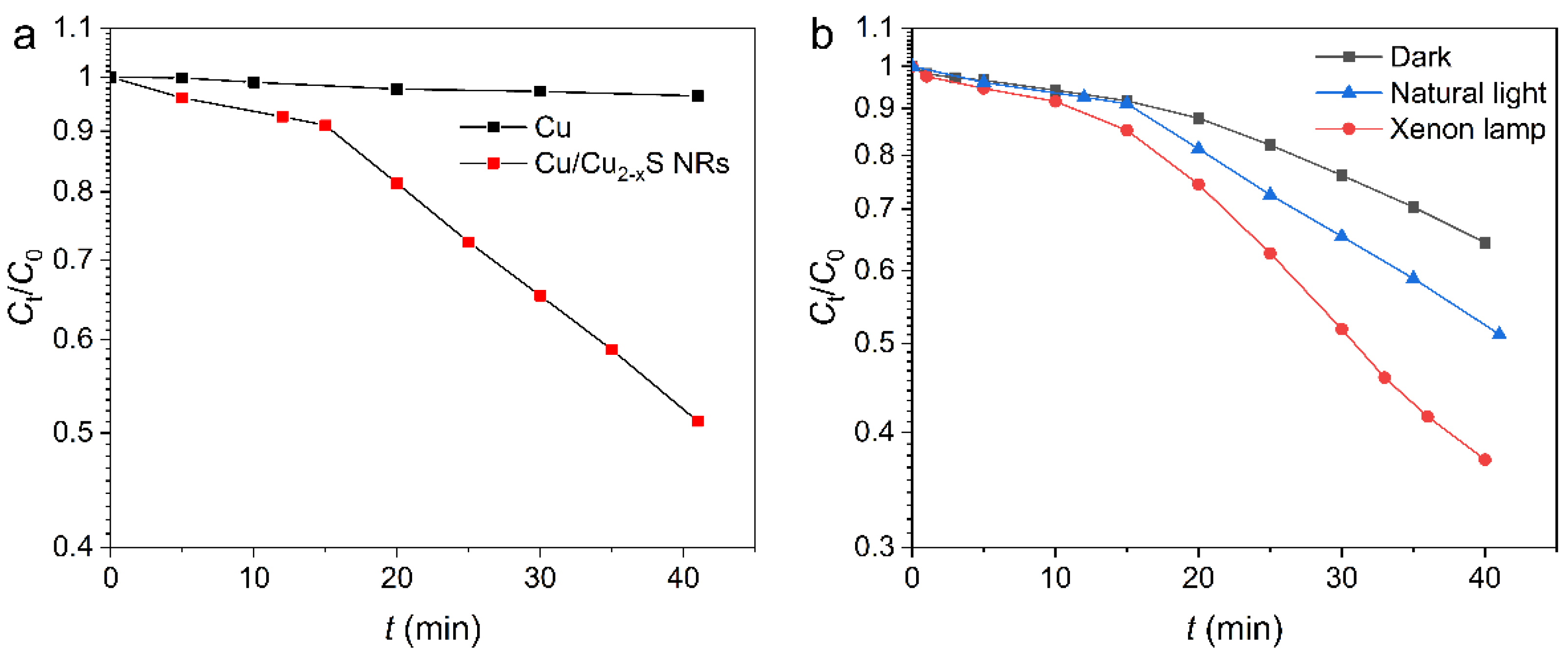
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Au Seeds
3.3. Synthesis of Cu NRs with Tunable Length
3.4. In Situ Sulfidation of Cu NRs
3.5. Characterization
3.6. Photocatalytic Degradation of Dye
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeong, S.; Liu, Y.; Zhong, Y.; Zhan, X.; Li, Y.; Wang, Y.; Cha, P.M.; Chen, J.; Ye, X. Heterometallic seed-mediated growth of monodisperse colloidal copper nanorods with widely tunable plasmonic resonances. Nano Lett. 2020, 20, 7263–7271. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jenkins, S.V.; Tao, J.; Zhu, Y.; Chen, J. Anisotropic seeded growth of Cu–M (M = Au, Pt, or Pd) Bimetallic nanorods with tunable optical and catalytic properties. J. Phys. Chem. C 2013, 117, 8924–8932. [Google Scholar] [CrossRef]
- Cui, F.; Yu, Y.; Dou, L.; Sun, J.; Yang, Q.; Schildknecht, C.; Schierle-Arndt, K.; Yang, P. Synthesis of ultrathin copper nanowires using Tris(trimethylsilyl)silane for high-performance and low-haze transparent conductors. Nano Lett. 2015, 15, 7610–7615. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Kim, M.J.; Lyu, Z.; Shi, Y.; Wiley, B.J.; Xia, Y. One-dimensional metal nanostructures: From colloidal syntheses to applications. Chem. Rev. 2019, 119, 8972–9073. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Zhang, H.; Zhang, Z.; Wu, H.; Jin, M.; Wu, C.; Yang, D.; Yin, Y. Lattice-mismatch-induced twinning for seeded growth of anisotropic nanostructures. ACS Nano 2015, 9, 3307–3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Liu, X.L.; Yang, Y.Z.; Wang, Y.L.; Wang, J.H.; Yang, Z.J.; Wang, L.B.; Jia, S.F.; Yu, X.F.; Zhou, L.; et al. Symmetric and asymmetric Au-AgCdSe hybrid nanorods. Nano Lett. 2012, 12, 5281–5286. [Google Scholar] [CrossRef]
- Ma, L.; Liang, S.; Liu, X.L.; Yang, D.J.; Zhou, L.; Wang, Q.Q. Synthesis of dumbbell-like gold-metal sulfide core-shell nanorods with largely enhanced transverse plasmon resonance in visible region and efficiently improved photocatalytic activity. Adv. Funct. Mater. 2015, 25, 898–904. [Google Scholar] [CrossRef]
- Wang, P.F.; Chen, K.; Ma, S.; Wang, W.; Qiu, Y.H.; Ding, S.J.; Liang, S.; Wang, Q.Q. Asymmetric synthesis of Au-CdSe core-semishell nanorods for plasmon-enhanced visible-light-driven hydrogen evolution. Nanoscale 2020, 12, 687–694. [Google Scholar] [CrossRef]
- Wu, B.; Wang, P.F.; Qiu, Y.H.; Liang, S.; Wu, Z.Y.; Zhou, L.; Wang, Q.Q. Enhanced second-harmonic generation of asymmetric Au@CdSe heterorods. Sci. China Mater. 2020, 63, 1472–1479. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Ma, S.; Chen, K.; Wang, P.F.; Qiu, Y.H.; Liang, S.; Zhou, L.; Chen, Y.; Wang, Q.Q. Controlled growth of plasmonic heterostructures and their applications. Sci. China Mater. 2020, 63, 1398–1417. [Google Scholar] [CrossRef] [Green Version]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Sulfur-doped mesoporous carbon nitride decorated with Cu particles for efficient photocatalytic degradation under visible-light irradiation. J. Phys. Chem. C 2017, 121, 19239–19253. [Google Scholar] [CrossRef]
- Chen, L.; Hu, H.; Chen, Y.; Li, Y.; Gao, J.; Li, G. Sulfur precursor reactivity affecting the crystal phase and morphology of Cu2−xS nanoparticles. Chem. Eur. J. 2021, 27, 1057–1065. [Google Scholar] [CrossRef]
- Luo, M.; Ruditskiy, A.; Peng, H.C.; Tao, J.; Figueroa-Cosme, L.; He, Z.K.; Xia, Y.N. Penta-twinned copper nanorods: Facile synthesis via seed-mediated growth and their tunable plasmonic properties. Adv. Funct. Mater. 2016, 26, 1209–1216. [Google Scholar] [CrossRef]
- Fu, W.; Liu, L.; Yang, G.; Deng, L.; Zou, B.; Ruan, W.; Zhong, H. Oleylamine-assisted phase-selective synthesis of Cu2−xS nanocrystals and the mechanism of phase control. Part. Part. Syst. Char. 2015, 32, 907–914. [Google Scholar] [CrossRef]
- Hsu, S.W.; Ngo, C.; Bryks, W.; Tao, A.R. Shape focusing during the anisotropic growth of CuS triangular nanoprisms. Chem. Mater. 2015, 27, 4957–4963. [Google Scholar] [CrossRef]
- Sun, C.; Liu, M.; Zou, Y.; Wei, J.; Jiang, J. Synthesis of plasmonic Au–CuS hybrid nanocrystals for photothermal transduction and chemical transformations. RSC Adv. 2016, 6, 26374–26379. [Google Scholar] [CrossRef]
- Lyu, Z.; Zhu, S.; Xie, M.; Zhang, Y.; Chen, Z.; Chen, R.; Tian, M.; Chi, M.; Shao, M.; Xia, Y. Controlling the surface oxidation of cu nanowires improves their catalytic selectivity and stability toward C2+ Products in CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 1909–1915. [Google Scholar] [CrossRef]
- Sun, B.; Li, H.; Li, X.; Liu, X.; Zhang, C.; Xu, H.; Zhao, X.S. Degradation of organic dyes over fenton-like Cu2O–Cu/C catalysts. Ind. Eng. Chem. Res. 2018, 57, 14011–14021. [Google Scholar] [CrossRef]
- Chakraborty, A.; Islam, D.A.; Acharya, H. One pot synthesis of ZnO-CuO nanocomposites for catalytic peroxidase like activity and dye degradation. Mater. Res. Bull. 2019, 120, 110592. [Google Scholar] [CrossRef]
- Jyothi, N.S.; Ravichandran, K. Optimum pH for effective dye degradation: Mo, Mn, Co and Cu doped ZnO photocatalysts in thin film form. Ceram. Int. 2020, 46, 23289–23292. [Google Scholar] [CrossRef]
- Kale, G.; Arbuj, S.; Kawade, U.; Kadam, S.; Nikam, L.; Kale, B. Paper templated synthesis of nanostructured Cu–ZnO and its enhanced photocatalytic activity under sunlight. J. Mater. Sci. Mater. Electron. 2019, 30, 7031–7042. [Google Scholar] [CrossRef]
- Vasanthi, D.S.; Ravichandran, K.; Kavitha, P.; Sriram, S.; Praseetha, P.K. Combined effect of Cu and N on bandgap modification of ZnO film towards effective visible light responsive photocatalytic dye degradation. Superlattices Microstruct. 2020, 145, 106637. [Google Scholar] [CrossRef]
- Hoseini, S.J.; Fath, R.H. Formation of nanoneedle Cu(0)/CuS nanohybrid thin film by the disproportionation of a copper(i) complex at an oil–water interface and its application for dye degradation. RSC Adv. 2016, 6, 76964–76971. [Google Scholar] [CrossRef]
- Wan, M.; Cui, S.; Wei, W.; Cui, S.; Chen, K.; Chen, W.; Mi, L. Bi-component synergic effect in lily-like CdS/Cu7S4 QDs for dye degradation. RSC Adv. 2019, 9, 2441–2450. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.S.; Lakshmi Prabavathi, S.; Indurani, P.; Karuthapandian, S.; Muthuraj, V. Light assisted synthesis of hierarchically structured Cu/CdS nanorods with superior photocatalytic activity, stability and photocatalytic mechanism. Sep. Purif. Technol. 2017, 172, 192–201. [Google Scholar] [CrossRef]
- Alhebshi, N.; Huang, H.; Ghandour, R.; Alghamdi, N.K.; Alharbi, O.; Aljurban, S.; He, J.H.; Al-Jawhari, H. Green synthesized CuxO@Cu nanocomposites on a Cu mesh with dual catalytic functions for dye degradation and hydrogen evaluation. J. Alloys Compd. 2020, 848, 156284. [Google Scholar] [CrossRef]
- Basu, M.; Sinha, A.K.; Pradhan, M.; Sarkar, S.; Negishi, Y.; Govind; Pal, T. Evolution of hierarchical hexagonal stacked plates of CuS from liquid−liquid interface and its photocatalytic application for oxidative degradation of different dyes under indoor lighting. Environ. Sci. Technol. 2010, 44, 6313–6318. [Google Scholar] [CrossRef]
- Jiang, Q.; Jiang, J.; Deng, R.; Xie, X.; Meng, J. Controllable preparation of CuO/Cu2O composite particles with enhanced photocatalytic performance. New J. Chem. 2020, 44, 6369–6374. [Google Scholar] [CrossRef]
- Periyayya, U.; Madhu, D.; Subramaniyam, K.; Son, H.; Lee, I.H. Enhanced cyclic performance initiated via an in situ transformation of Cu/CuO nanodisk to Cu/CuO/Cu2O nanosponge. Environ. Sci. Pollut. Res. 2021, 28, 6459–6469. [Google Scholar]
- Solomane, N.; Ajibade, P.A. Synthesis and crystal structure of bis(thiomorpholinyldithiocarbamato)Cu(II) complex and its use as precursor for CuS nanoparticles photocatalyst for the degradation of organic dyes. J. Sulphur. Chem. 2020, 42, 167–179. [Google Scholar] [CrossRef]
- Bhowmick, S.; Moi, C.T.; Kalita, N.; Sahu, A.; Suman, S.; Qureshi, M. Spontaneous Fenton-like dye degradation in clustered-petal di-manganese copper oxide by virtue of self-cyclic redox couple. J. Environ. Chem. Eng. 2021, 9, 106094. [Google Scholar] [CrossRef]
- Hou, Y.; Li, X.; Zou, X.; Quan, X.; Chen, G. Photoeletrocatalytic activity of a Cu2O-loaded self-organized highly oriented TiO2 nanotube array electrode for 4-chlorophenol degradation. Environ. Sci. Technol. 2009, 43, 858–863. [Google Scholar] [CrossRef]
- Rahmatolahzadeh, R.; Ebadi, M.; Motevalli, K. Preparation and characterization of Cu clusters and Cu–Ag alloy via galvanic replacement method for azo dyes degradation. J. Mater. Sci. Mater. Electron. 2017, 28, 6056–6063. [Google Scholar] [CrossRef]
- Li, Y.Y.; Pan, G.M.; Liu, Q.Y.; Ma, L.; Xie, Y.; Zhou, L.; Hao, Z.H.; Wang, Q.Q. Coupling resonances of surface plasmon in gold nanorod/copper chalcogenide core-shell nanostructures and their enhanced photothermal effect. ChemPhysChem 2018, 19, 1852–1858. [Google Scholar] [CrossRef]
- Ma, S.; Chen, K.; Qiu, Y.H.; Gong, L.L.; Pan, G.M.; Lin, Y.J.; Hao, Z.H.; Zhou, L.; Wang, Q.Q. Controlled growth of CdS-Cu2−xS lateral heteroshells on Au nanoparticles with improved photocatalytic activity and photothermal efficiency. J. Mater. Chem. A 2019, 7, 3408–3414. [Google Scholar] [CrossRef]
- Wang, W.; Ma, S.; Chen, K.; Wang, P.F.; Liu, Q.Y.; Zhou, L.; Wang, Q.Q. Controlled growth of Cu2−xS sheet-like nanoshells and Cu2−xS-CdS p-n junctions on Au nanorods with coupled plasmon resonances and enhanced photocatalytic activities. J. Mater. Chem. C 2020, 8, 3058–3068. [Google Scholar] [CrossRef]
- Kuo, K.Y.; Chen, S.H.; Hsiao, P.H.; Lee, J.T.; Chen, C.Y. Day-night active photocatalysts obtained through effective incorporation of Au@CuxS nanoparticles onto ZnO nanowalls. J. Hazard. Mater. 2022, 421, 126674. [Google Scholar] [CrossRef]
- Deng, S.Q.; Miao, Y.L.; Tan, Y.L.; Fang, H.N.; Li, Y.T.; Mo, X.J.; Cai, S.L.; Fan, J.; Zhang, W.G.; Zheng, S.R. An anionic nanotubular metal-organic framework for high-capacity dye adsorption and dye degradation in darkness. Inorg. Chem. 2019, 58, 13979–13987. [Google Scholar] [CrossRef]
- Nandi, P.; Das, D. ZnO-CuO heterostructure photocatalyst for efficient dye degradation. J. Phys. Chem. Solids 2020, 143, 109463. [Google Scholar] [CrossRef]
- Song, C.; Wang, X.; Zhang, J.; Chen, X.; Li, C. Enhanced performance of direct Z-scheme CuS-WO3 system towards photocatalytic decomposition of organic pollutants under visible light. Appl. Surf. Sci. 2017, 425, 788–795. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.; Xie, C.; Wang, Y.; Wei, L.; Yang, J. Synthesis and photocatalytic mechanism of visible-light-driven Ag/AgBr/(I/S) composites for organic dyes degradation. Opt. Mater. 2022, 123, 111947. [Google Scholar] [CrossRef]
- Tavakoli Joorabi, F.; Kamali, M.; Sheibani, S. Effect of aqueous inorganic anions on the photocatalytic activity of CuO–Cu2O nanocomposite on MB and MO dyes degradation. Mater. Sci. Semicon. Proc. 2022, 139, 106335. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Zhong, Y.-T.; Wang, Q.-Q.; Zhou, L. In Situ Partial Sulfidation for Preparing Cu/Cu2−xS Core/Shell Nanorods with Enhanced Photocatalytic Degradation. Catalysts 2022, 12, 147. https://doi.org/10.3390/catal12020147
Cheng L, Zhong Y-T, Wang Q-Q, Zhou L. In Situ Partial Sulfidation for Preparing Cu/Cu2−xS Core/Shell Nanorods with Enhanced Photocatalytic Degradation. Catalysts. 2022; 12(2):147. https://doi.org/10.3390/catal12020147
Chicago/Turabian StyleCheng, Li, Yu-Ting Zhong, Qu-Quan Wang, and Li Zhou. 2022. "In Situ Partial Sulfidation for Preparing Cu/Cu2−xS Core/Shell Nanorods with Enhanced Photocatalytic Degradation" Catalysts 12, no. 2: 147. https://doi.org/10.3390/catal12020147
APA StyleCheng, L., Zhong, Y.-T., Wang, Q.-Q., & Zhou, L. (2022). In Situ Partial Sulfidation for Preparing Cu/Cu2−xS Core/Shell Nanorods with Enhanced Photocatalytic Degradation. Catalysts, 12(2), 147. https://doi.org/10.3390/catal12020147






