Controlled Synthesis and Photoelectrochemical Performance Enhancement of Cu2−xSe Decorated Porous Au/Bi2Se3 Z-Scheme Plasmonic Photoelectrocatalyst
Abstract
:1. Introduction
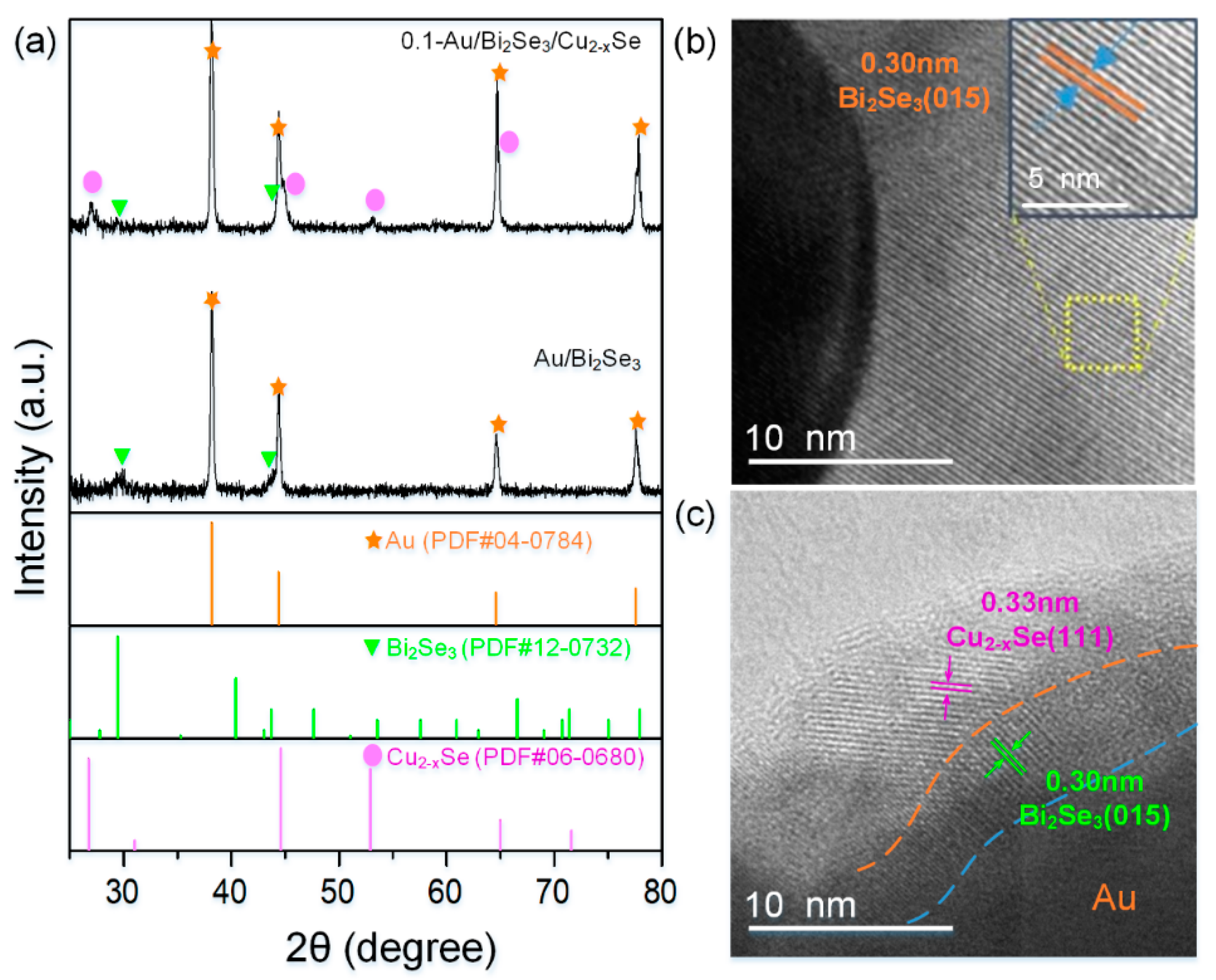
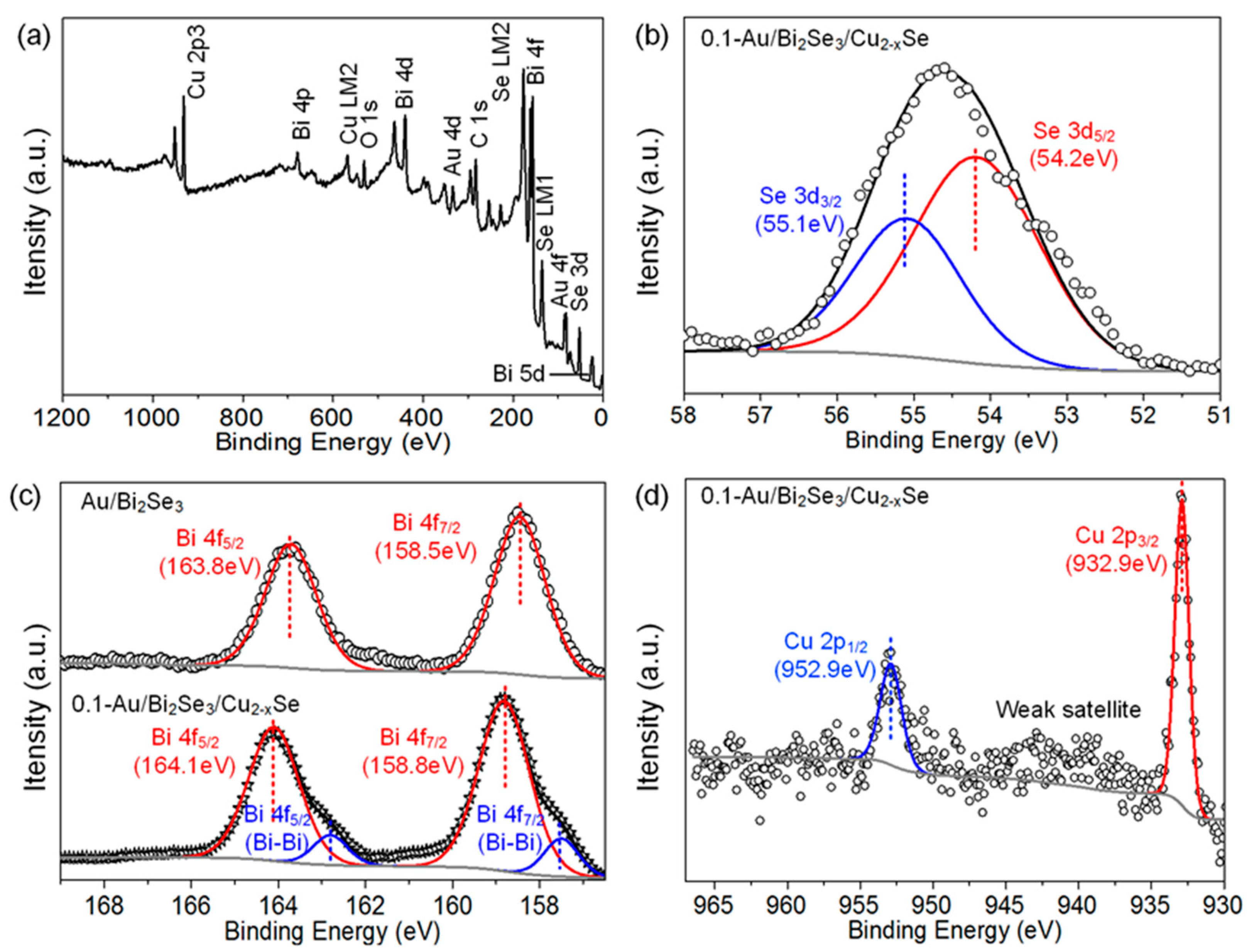
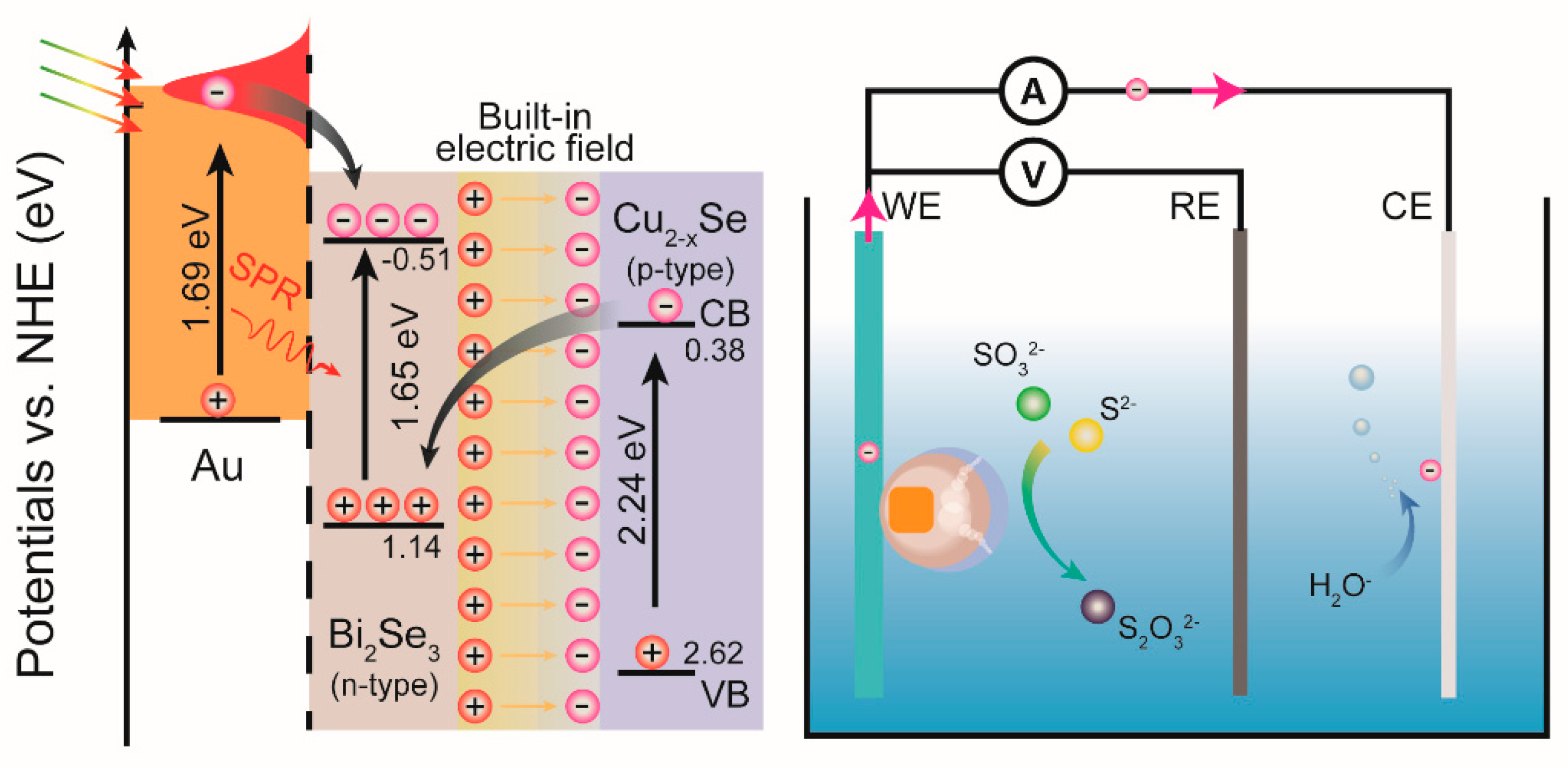
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of the Au Nanocubes
3.3. Synthesis of Porous Au/Bi2Se3 Nanoparticles
3.4. Synthesis of the Porous Au/Bi2Se3/Cu2−xSe Nanoparticles
3.5. Photoelectrochemical (PEC) Tests
3.6. Morphology and Structure Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Kudo, A. Visible light responsive photocatalysts developed by substitution with metal cations aiming at artificial photosynthesis. Front. Energy 2021, 15, 568–576. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M. Recent progress in structural development and band engineering of perovskites materials for photocatalytic solar hydrogen production: A review. Int. J. Hydrogen Energy 2020, 45, 19078–19111. [Google Scholar] [CrossRef]
- Hong, J.; Xu, C.; Deng, B.; Gao, Y.; Zhu, X.; Zhang, X.; Zhang, Y. Photothermal chemistry based on solar energy: From Synergistic Effects to Practical Applications. Adv. Sci. 2021, 9, e2103926. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Xu, P.; Xue, W.; Lei, L.; Cheng, M.; Wang, R.; Liu, X.; Deng, R. Semiconductor-based photocatalysts for photocatalytic and photoelectrochemical water splitting: Will we stop with photocorrosion. J. Mater. Chem. A 2020, 8, 2286–2322. [Google Scholar] [CrossRef]
- Yao, T.; An, X.; Han, H.; Chen, J.Q.; Li, C. Photoelectrocatalytic materials for solar water splitting. Adv. Energy Mater. 2018, 8, 1800210. [Google Scholar] [CrossRef]
- Tournet, J.; Lee, Y.; Karuturi, S.K.; Tan, H.H.; Jagadish, C. III–V semiconductor materials for solar hydrogen production: Status and prospects. ACS Energy Lett. 2020, 5, 611–622. [Google Scholar] [CrossRef]
- Niu, F.; Wang, D.; Li, F.; Liu, Y.; Shen, S.; Meyer, T.J. Hybrid photoelectrochemical water splitting systems: From interface design to system assembly. Adv. Energy Mater. 2019, 10, 1900399. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Dai, L.; Jin, Y.; Liu, J.; Zhang, Q.; Wang, H. Design strategies toward transition metal selenide-based catalysts for electrochemical water splitting. Sustain. Energy Fuels 2021, 5, 1347–1365. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Zhang, Q.; Pan, S.; Zhao, Y.; Zhang, X. Controlling the crystallographic orientation of Sb2Se3 film for efficient photoelectrochemical water splitting. Sol. RRL 2021, 2100798. [Google Scholar] [CrossRef]
- Wang, H.; Xia, Y.; Wen, N.; Shu, Z.; Jiao, X.; Chen, D. Surface states regulation of sulfide-based photoanode for photoelectrochemical water splitting. Appl. Catal. B-Environ. 2022, 300, 120717. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Yao, L.; Deng, L.; Bowen, C.; Zhang, Y.; Chen, S.; Lin, Z.; Peng, F.; Zhang, P. Recent advances in metal sulfides: From controlled fabrication to electrocatalytic, photocatalytic and photoelectrochemical water splitting and beyond. Chem. Soc. Rev. 2019, 48, 4178–4280. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, Q.; Zhang, M.; Liu, L. Photoelectric properties of Bi2Se3 films grown by thermal evaporation method. Mater. Res. Express 2020, 7, 016429. [Google Scholar] [CrossRef]
- Liao, G.; Zhou, Y.; Huang, Z.; Ma, Q.; Luo, S.; Liu, Y.; Qi, X. Bi2Se3 nanosheets hybridized with reduced graphene oxide for enhanced photoelectrochemical activity. Appl. Phys. A 2021, 127, 705. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Zhang, T.; Zuo, Y.; Xiao, K.; Arbiol, J.; Llorca, J.; Liu, Y.; Cabot, A. Enhanced thermoelectric performance of n-type Bi2Se3 nanosheets through Sn doping. Nanomaterials 2021, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Fang, M.; Jiang, C.; Lin, H.; Luo, C.; Qi, R.; Huang, R.; Peng, H. Size-controlled synthesis of hierarchical bismuth selenide nanoflowers and their photocatalytic performance in the presence of H2O2. J. Nanopart. Res. 2018, 20, 228. [Google Scholar] [CrossRef]
- Wang, C.C.; Shieu, F.S.; Shih, H.C. Photosensing and characterizing of the pristine and In-, Sn-Doped Bi2Se3 nanoplatelets fabricated by thermal V-S Process. Nanomaterials 2021, 11, 1352. [Google Scholar] [CrossRef]
- Tang, L.; Liang, S.; Li, J.B.; Zhang, D.; Chen, W.B.; Yang, Z.J.; Xiao, S.; Wang, Q.Q. Controlled synthesis of Au nanocrystals-metal selenide hybrid nanostructures toward plasmon-enhanced photoelectrochemical energy conversion. Nanomaterials 2020, 10, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.-D.; Chen, B.; Wang, G.-G.; Ma, S.; Cheng, L.; Liu, W.; Zhou, L.; Wang, Q.-Q. Controlled growth of hierarchical Bi2Se3/CdSe-Au nanorods with optimized photothermal conversion and demonstrations in photothermal therapy. Adv. Funct. Mater. 2021, 31, 2104424. [Google Scholar] [CrossRef]
- Liu, D.; Chen, S.; Li, R.; Peng, T. Review of Z-scheme heterojunctions for photocatalytic energy conversion. Acta Phys.-Chim. Sin. 2020, 37, 2010017. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Wang, Y.; Shuai, X.; Zhang, J.; Zhao, R.; Guo, T.; Du, J.; Zheng, J.; Li, J. Engineering of band structure of bismuth selenide ultrathin nanosheets as multifunctional material for photocatalytic application. Adv. Mater. Interfaces 2022, 2102417. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, S.; Zhang, J.; Zhong, W.; Huang, X. Cu2−xSe/CdS composite photocatalyst with enhanced visible light photocatalysis activity. Appl. Surf. Sci. 2019, 478, 762–769. [Google Scholar] [CrossRef]
- Marbella, L.E.; Gan, X.Y.; Kaseman, D.C.; Millstone, J.E. Correlating carrier density and emergent plasmonic features in Cu2−xSe nanoparticles. Nano Lett. 2017, 17, 2414–2419. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Wang, W.; Ma, S.; Chen, K.; Wang, P.-F.; Liu, Q.-Y.; Zhou, L.; Wang, Q.-Q. Controlled growth of Cu2−xS sheet-like nanoshells and Cu2−xS-CdS p-n junctions on Au nanorods with coupled plasmon resonances and enhanced photocatalytic activities. J. Mater. Chem. C 2020, 8, 3058–3068. [Google Scholar] [CrossRef]
- Thongam, D.D.; Chaturvedi, H. Advances in nanomaterials for heterogeneous photocatalysis. Nano Express 2021, 2, 012005. [Google Scholar] [CrossRef]
- Ng, B.J.; Putri, L.K.; Kong, X.Y.; Teh, Y.W.; Pasbakhsh, P.; Chai, S.P. Z-scheme photocatalytic systems for solar water splitting. Adv. Sci. 2020, 7, 1903171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, L.; Yu, X.; Zhang, J.; Yang, R.; Zhang, X.; Ji, Y.; Wu, M.; Deng, L.; Li, L.; et al. Piezoelectric-effect-enhanced full-spectrum photoelectrocatalysis in p-n heterojunction. Adv. Funct. Mater. 2019, 29, 1807279. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, D.; Lin, S.; Guo, W.; Zhan, D.; Sun, L.; Lin, C. Enhanced photoelectrocatalytic activity of Bi2S3–TiO2 nanotube arrays hetero-structure under visible light irradiation. Int. J. Hydrogen Energy 2020, 45, 32012–32021. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Baviskar, V.S.; Salunkhe, D.B.; Patil, G.P.; Jadhav, C.D.; Nerkar, D.M.; Baviskar, P.K.; Patil, R.S. Effect of deposition time on photoelectrochemical performance of chemically grown Bi2Se3-sensitized TiO2 nanostructure solar cells. Acta Phys.-Chim. Sin. 2020, 31, 17440–17450. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Sang, D.; Yu, K.; Lin, H.; Qu, F. Cu2−xSe/Bi2Se3@PEG Z-scheme heterostructure: A multimode bioimaging guided theranostic agent with enhanced photo/chemodynamic and photothermal therapy. Biomater. Sci. 2021, 9, 4473–4483. [Google Scholar] [CrossRef]
- Chen, S.; Ma, G.; Wang, Q.; Sun, S.; Hisatomi, T.; Higashi, T.; Wang, Z.; Nakabayashi, M.; Shibata, N.; Pan, Z.; et al. Metal selenide photocatalysts for visible-light-driven Z-scheme pure water splitting. J. Mater. Chem. A 2019, 7, 7415–7422. [Google Scholar] [CrossRef] [Green Version]
- Subramanyam, P.; Meena, B.; Suryakala, D.; Subrahmanyam, C. TiO2 photoanodes sensitized with Bi2Se3 nanoflowers for visible–near-infrared photoelectrochemical water splitting. ACS Appl. Nano Mater. 2021, 4, 739–745. [Google Scholar] [CrossRef]
- Zhuang, T.T.; Liu, Y.; Sun, M.; Jiang, S.L.; Zhang, M.W.; Wang, X.C.; Zhang, Q.; Jiang, J.; Yu, S.H. A unique ternary semiconductor-(semiconductor/metal) nano-architecture for efficient photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2015, 54, 11495–11500. [Google Scholar] [CrossRef]
- Cai, X.; Chen, Q.; Wang, R.; Wang, A.; Wang, J.; Zhong, S.; Liu, Y.; Chen, J.; Bai, S. Integration of plasmonic metal and cocatalyst: An efficient strategy for boosting the visible and broad spectrum photocatalytic H2 evolution. Adv. Mater. Interfaces 2019, 6, 1900775. [Google Scholar] [CrossRef]
- Chen, K.; Gong, L.-L.; Ding, S.-J.; Liu, J.; Ma, S.; Wang, J.-H.; Yang, D.-J.; Pan, G.-M.; Hao, Z.-H.; Zhou, L.; et al. Tunable charge transfer and dual plasmon resonances of Au@WO3−x hybrids and applications in photocatalytic hydrogen generation. Plasmonics 2019, 15, 21–29. [Google Scholar] [CrossRef]
- Chen, W.-B.; Hu, L.-Y.; Meng, F.; Tang, L.; Liang, S.; Li, J.-B. Dual-plasmon-induced photocatalytic performance enhancement in Au-PbS-CdS nanodumbbells with double Au caps on the ends. Opt. Mater. 2021, 117, 111210. [Google Scholar] [CrossRef]
- Li, D.; Lao, J.; Jiang, C.; Luo, C.; Qi, R.; Lin, H.; Huang, R.; Waterhouse, G.I.N.; Peng, H. Plasmonic Au nanoparticle-decorated Bi2Se3 nanoflowers with outstanding electrocatalytic performance for hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 30876–30884. [Google Scholar] [CrossRef]
- Ma, S.; Chen, K.; Qiu, Y.-H.; Gong, L.-L.; Pan, G.-M.; Lin, Y.-J.; Hao, Z.-H.; Zhou, L.; Wang, Q.-Q. Controlled growth of CdS–Cu2-xS lateral heteroshells on Au nanoparticles with improved photocatalytic activity and photothermal efficiency. J. Mater. Chem. A 2019, 7, 3408–3414. [Google Scholar] [CrossRef]
- Li, D.; Lao, J.; Jiang, C.; Shen, Y.; Luo, C.; Qi, R.; Lin, H.; Huang, R.; Waterhouse, G.I.N.; Peng, H. Heterostructured MoS2@Bi2Se3 nanoflowers: A highly efficient electrocatalyst for hydrogen evolution. J. Catal. 2020, 381, 590–598. [Google Scholar] [CrossRef]
- Mohammadniaei, M.; Lee, T.; Bharate, B.G.; Yoon, J.; Choi, H.K.; Park, S.J.; Kim, J.; Kim, J.; Choi, J.W. Bifunctional Au@Bi2Se3 core−shell nanoparticle for synergetic therapy by SERS-Traceable AntagomiR delivery and photothermal treatment. Small 2018, 14, 1802934. [Google Scholar] [CrossRef]
- Pang, Y.; Zang, W.; Kou, Z.; Zhang, L.; Xu, G.; Lv, J.; Gao, X.; Pan, Z.; Wang, J.; Wu, Y. Assembling of Bi atoms on TiO2 nanorods boosts photoelectrochemical water splitting of semiconductors. Nanoscale 2020, 12, 4302–4308. [Google Scholar] [CrossRef]
- Liu, X.-L.; Liang, S.; Li, M.; Yu, X.-F.; Zhou, L.; Wang, Q.-Q. Facile synthesis of Au nanocube-CdS core-shell nanocomposites with enhanced photocatalytic activity. Chin. Phys. Lett. 2014, 31, 064203. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893–2939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ran, J.; Qiao, S.Z.; Jaroniec, M. Characterization of semiconductor photocatalysts. Chem. Soc. Rev. 2019, 48, 5184–5206. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Li, Y.; Chen, W.; Liu, X.; Liang, S.; Cheng, Z.; Li, J.; Zhou, L. Controlled Synthesis and Photoelectrochemical Performance Enhancement of Cu2−xSe Decorated Porous Au/Bi2Se3 Z-Scheme Plasmonic Photoelectrocatalyst. Catalysts 2022, 12, 359. https://doi.org/10.3390/catal12040359
Hu L, Li Y, Chen W, Liu X, Liang S, Cheng Z, Li J, Zhou L. Controlled Synthesis and Photoelectrochemical Performance Enhancement of Cu2−xSe Decorated Porous Au/Bi2Se3 Z-Scheme Plasmonic Photoelectrocatalyst. Catalysts. 2022; 12(4):359. https://doi.org/10.3390/catal12040359
Chicago/Turabian StyleHu, Linyu, Yuqi Li, Wenbo Chen, Xiaogang Liu, Shan Liang, Ziqiang Cheng, Jianbo Li, and Li Zhou. 2022. "Controlled Synthesis and Photoelectrochemical Performance Enhancement of Cu2−xSe Decorated Porous Au/Bi2Se3 Z-Scheme Plasmonic Photoelectrocatalyst" Catalysts 12, no. 4: 359. https://doi.org/10.3390/catal12040359
APA StyleHu, L., Li, Y., Chen, W., Liu, X., Liang, S., Cheng, Z., Li, J., & Zhou, L. (2022). Controlled Synthesis and Photoelectrochemical Performance Enhancement of Cu2−xSe Decorated Porous Au/Bi2Se3 Z-Scheme Plasmonic Photoelectrocatalyst. Catalysts, 12(4), 359. https://doi.org/10.3390/catal12040359






