Natural Clay Modified with ZnO/TiO2 to Enhance Pollutant Removal from Water
Abstract
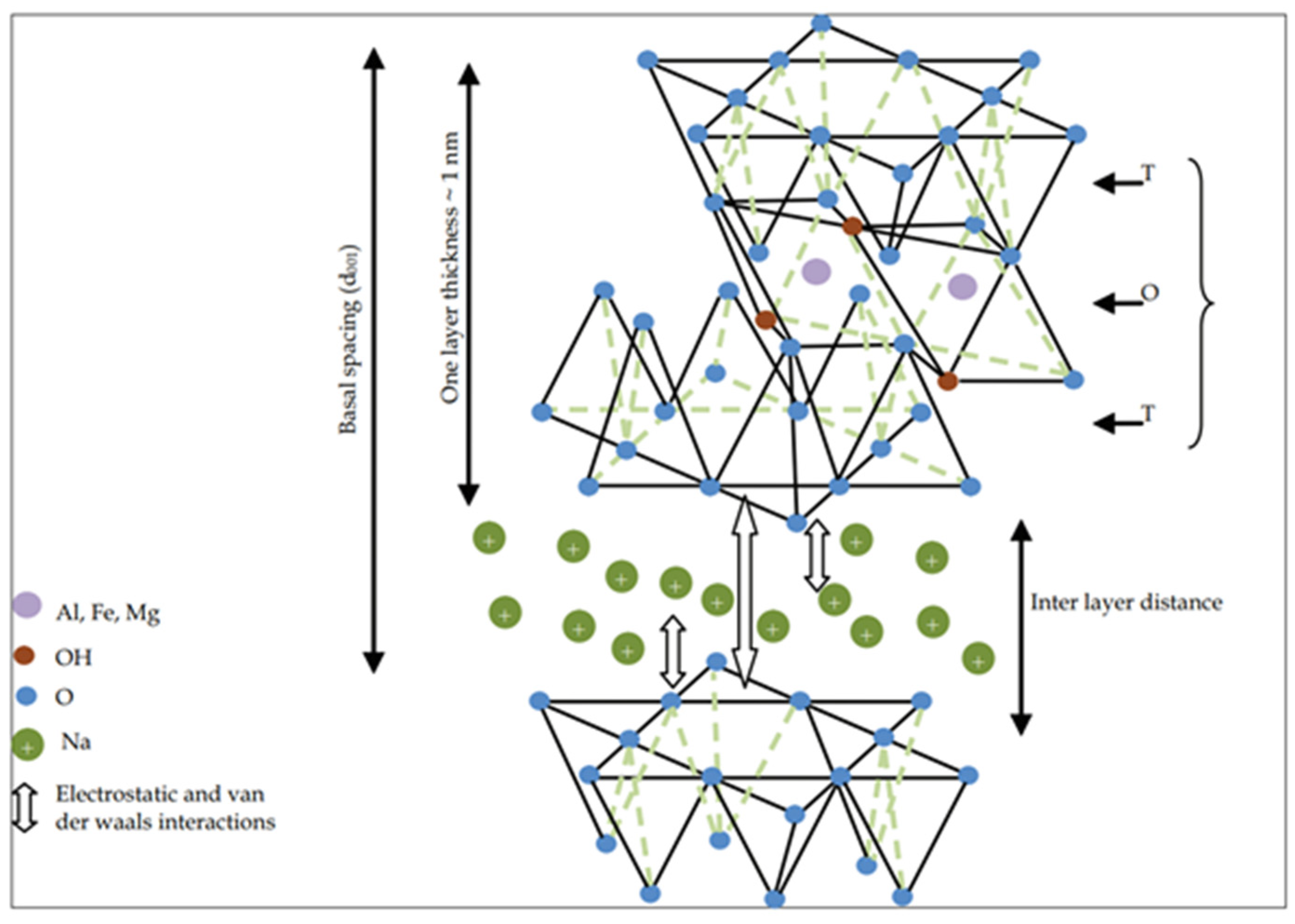
1. Introduction
2. Results and Discussion
2.1. Composition
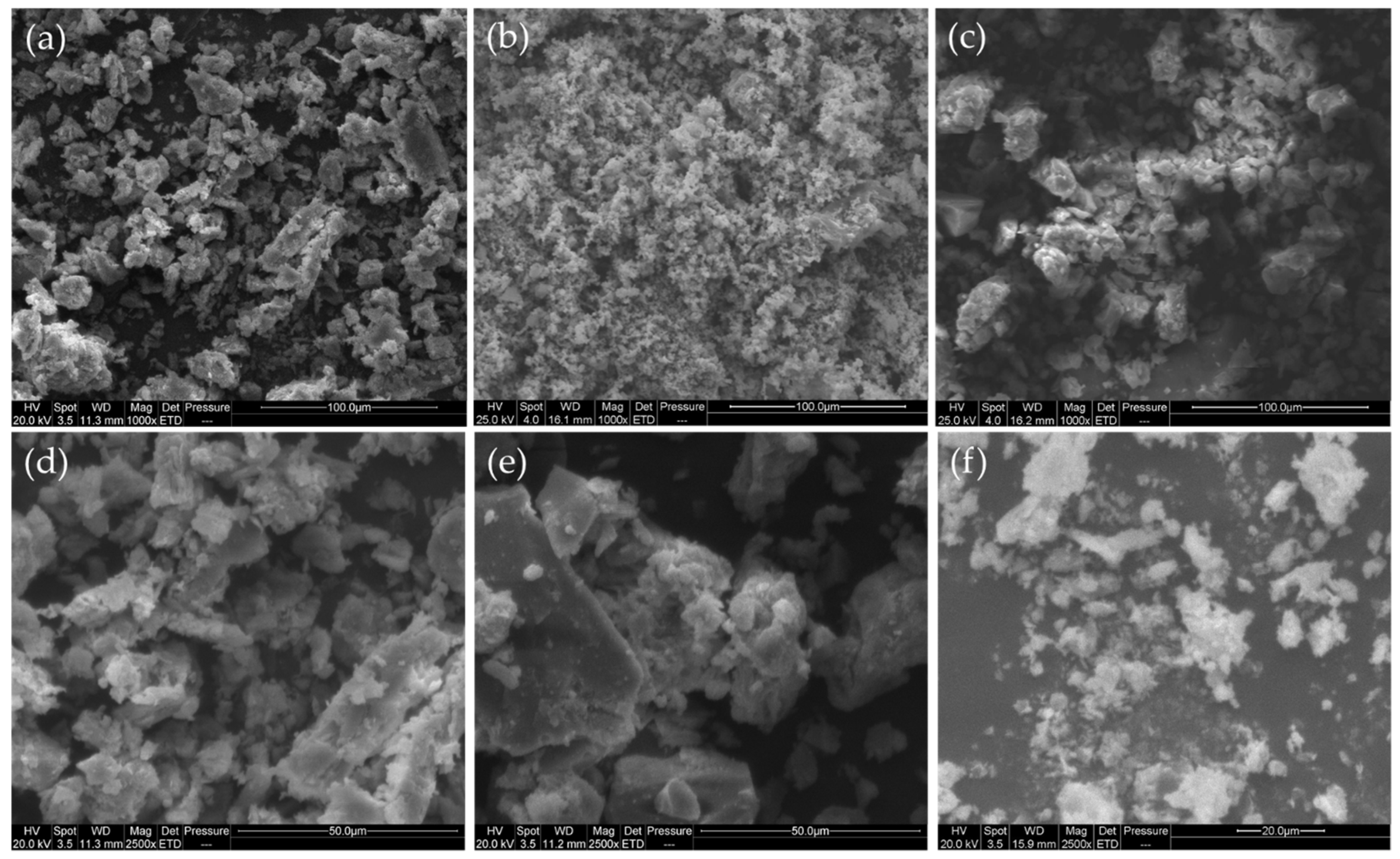
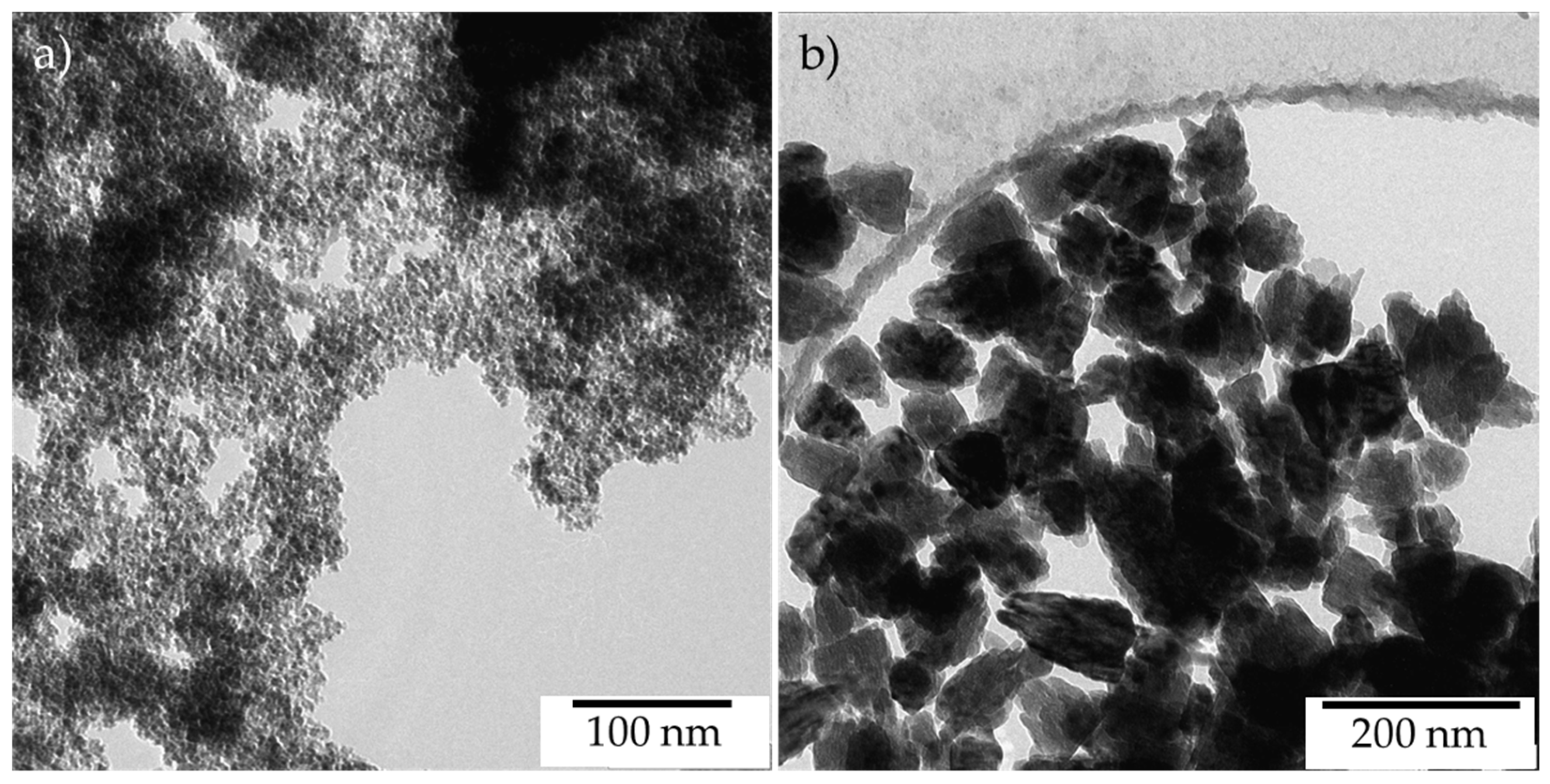
2.2. Texture and Morphology
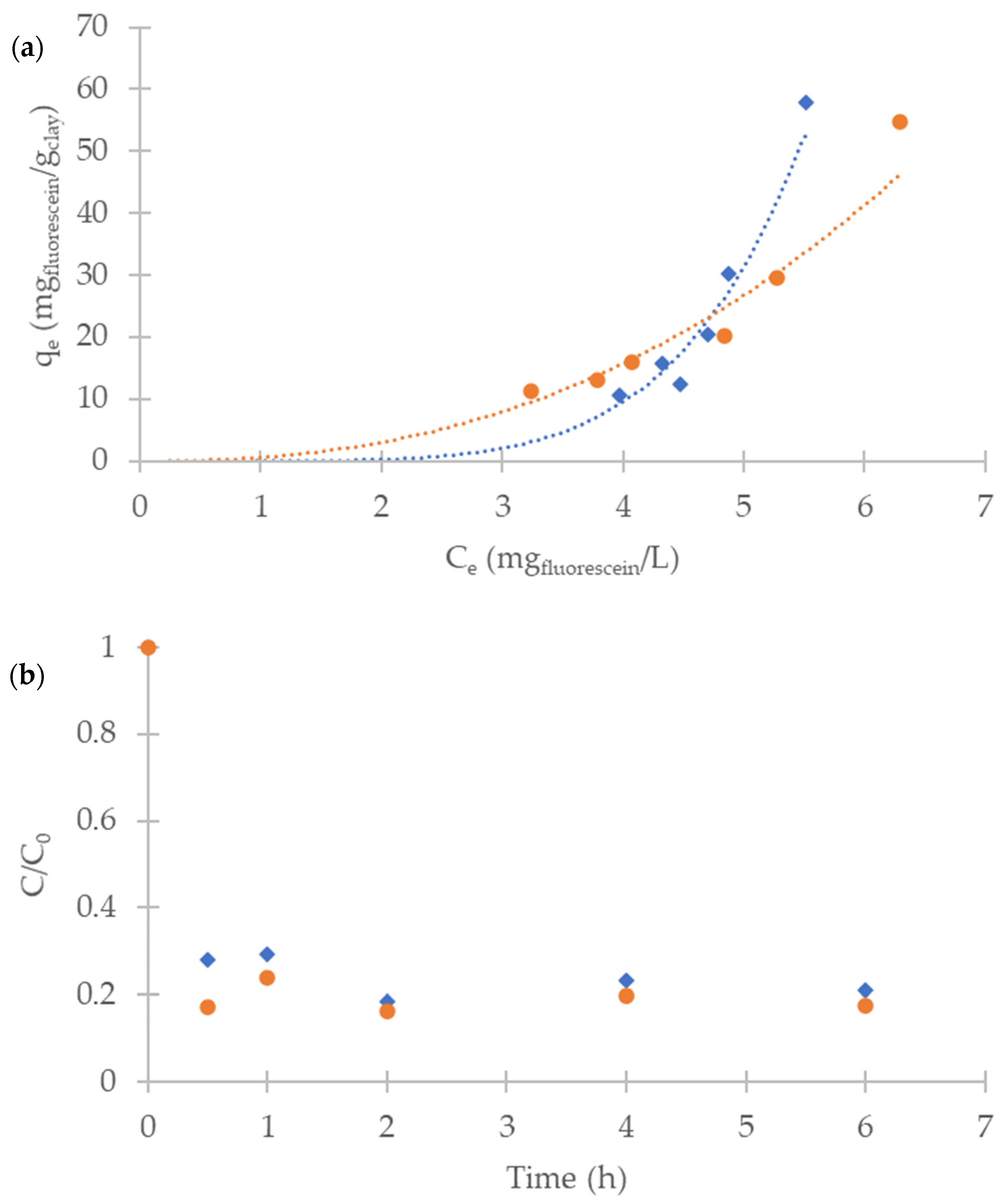
2.3. Adsorption Study
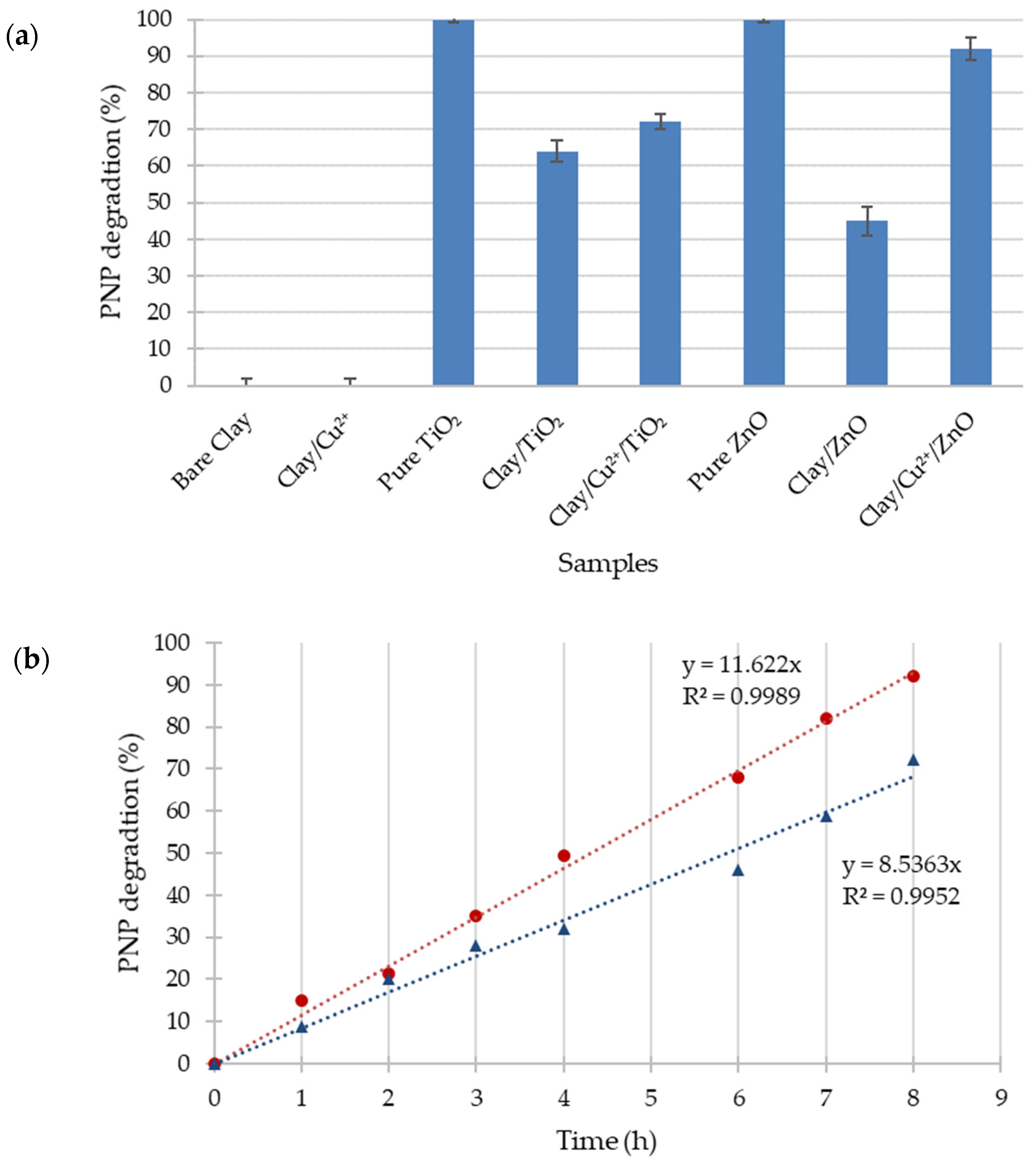
2.4. Photocatalytic Activity
3. Materials and Methods
3.1. Description of the Clay and Modification with Cu2+ Ions
3.1.1. Presentation of the Clay
3.1.2. Modification of Clay with Ions Cu2+ or Interfoliar Cation Exchange
3.2. Synthesis of Pure TiO2 and ZnO Photocatalysts
3.2.1. ZnO Sample
3.2.2. TiO2 Sample
3.3. Synthesis of Hybrid Clay/Photocatalyst Materials
3.3.1. Clay/ZnO Materials
3.3.2. Clay/TiO2 Materials
3.4. Characterization of Samples
3.5. Adsorption Experiments
3.6. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kemgang Lekomo, Y.; Mwebi Ekengoue, C.; Douola, A.; Fotie Lele, R.; Christian Suh, G.; Obiri, S.; Kagou Dongmo, A. Assessing Impacts of Sand Mining on Water Quality in Toutsang Locality and Design of Waste Water Purification System. Clean. Eng. Technol. 2021, 2, 100045. [Google Scholar] [CrossRef]
- Auriol, M.; Filali-Meknassi, Y.; Dayal Tyagi, R. Présence et Devenir Des Hormones Stéroïdiennes Dans Les Stations de Traitement Des Eaux Usées. Occurrence and Fate of Steroid Hormones in Wastewater Treatment Plants. Rev. Des Sci. L’eau 2007, 20, 89–108. [Google Scholar]
- Ekengoue, C.M.; Lele, R.F.; Dongmo, A.K. Influence De L’exploitation Artisanale Du Sable Sur La Santé Et La Sécurité Des Artisans Et L’environnement: Cas De La Carrière De Nkol’Ossananga, Région Du Centre Cameroun. Eur. Sci. J. ESJ 2018, 14, 246. [Google Scholar] [CrossRef]
- Available online: Https://Minepded.Gov.Cm/Fr/ (accessed on 18 November 2021).
- Nkoumbou, C.; Njopwouo, D.; Villiéras, F.; Njoya, A.; Yonta Ngouné, C.; Ngo Ndjock, L.; Tchoua, F.M.; Yvon, J. Talc Indices from Boumnyebel (Central Cameroon), Physico-Chemical Characteristics and Geochemistry. J. Afr. Earth Sci. 2006, 45, 61–73. [Google Scholar] [CrossRef]
- Filice, S.; Bongiorno, C.; Libertino, S.; Compagnini, G.; Gradon, L.; Iannazzo, D.; la Magna, A.; Scalese, S. Structural Characterization and Adsorption Properties of Dunino Raw Halloysite Mineral for Dye Removal from Water. Materials 2021, 14, 3676. [Google Scholar] [CrossRef]
- Jacques Richard, M. Mineralogie et Proprietes Physico-Chimiques des Smectites de Bana et Sabga (Cameroun). Utilisation Dans La Décoloration d’ Une Huile Végétale Alimentaire; Université de Liège: Liège, Belgique, 2013. [Google Scholar]
- Djoufac Woumfo, E.; Elimbi, A.; Panczer, G.; Nyada Nyada, R.; Njopwouo, D. Physico-Chemical and Mineralogical Characterization of Garoua Vertisols (North Cameroon). Ann. Chim. 2006, 31, 75–90. [Google Scholar]
- Léonard, G.L.-M.; Malengreaux, C.M.; Mélotte, Q.; Lambert, S.D.; Bruneel, E.; van Driessche, I.; Heinrichs, B. Doped Sol–Gel Films vs. Powders TiO2: On the Positive Effect Induced by the Presence of a Substrate. J. Environ. Chem. Eng. 2016, 4, 449–459. [Google Scholar] [CrossRef]
- Parsons, S. Advanced Oxidation Processes for Water and Wastewater Treatment; IWA Publishing: London, UK, 2004; ISBN 1843390175. [Google Scholar]
- Bhowmick, M.; Semmens, M.J. Ultraviolet Photooxidation for the Destruction f VOCs in Air. Wat. Res. 1994, 28, 2407–2415. [Google Scholar] [CrossRef]
- Ikehata, K.; El-Din, M.G. Aqueous Pesticide Degradation by Hydrogen Peroxide/Ultraviolet Irradiation and Fenton-Type Advanced Oxidation Processes: A Review. J. Environ. Eng. Sci. 2006, 5, 81–135. [Google Scholar] [CrossRef]
- Filice, S.; Fiorenza, R.; Reitano, R.; Scalese, S.; Sciré, S.; Fisicaro, G.; Deretzis, I.; la Magna, A.; Bongiorno, C.; Compagnini, G. TiO2 Colloids Laser-Treated in Ethanol for Photocatalytic H2 Production. ACS Appl. Nano Mater. 2020, 3, 9127–9140. [Google Scholar] [CrossRef]
- Goncharuk, V.V.; Potapchenko, N.G.; Savluk, O.S.; Kosinova, V.N.; Sova, A.N. Study of Various Conditions for O3/UV Disinfection of Water. Khimiya Tecknol. Vody 2003, 25, 487–496. [Google Scholar]
- Drogui, P.; Blais, J.-F.; Mercier, G. Review of Electrochemical Technologies for Environmental Applications. Recent Pat. Eng. 2007, 1, 257–272. [Google Scholar] [CrossRef]
- Douven, S.; Mahy, J.G.; Wolfs, C.; Reyserhove, C.; Poelman, D.; Devred, F.; Gaigneaux, E.M.; Lambert, S.D. Efficient N, Fe Co-Doped TiO2 Active under Cost-Effective Visible LED Light: From Powders to Films. Catalysts 2020, 10, 547. [Google Scholar] [CrossRef]
- Mahy, J.G.; Wolfs, C.; Vreuls, C.; Drot, S.; Dircks, S.; Boergers, A.; Tuerk, J.; Hermans, S.; Lambert, S.D. Advanced Oxidation Processes for Waste Water Treatment: From Lab-Scale Model Water to on-Site Real Waste Water. Environ. Technol. 2021, 42, 3974–3986. [Google Scholar] [CrossRef] [PubMed]
- Mahy, J.G.; Lejeune, L.; Haynes, T.; Body, N.; de Kreijger, S.; Elias, B.; Marcilli, R.H.M.; Fustin, C.A.; Hermans, S. Crystalline ZnO Photocatalysts Prepared at Ambient Temperature: Influence of Morphology on p-Nitrophenol Degradation in Water. Catalysts 2021, 11, 1182. [Google Scholar] [CrossRef]
- Bodson, C.J.; Heinrichs, B.; Tasseroul, L.; Bied, C.; Mahy, J.G.; Man, M.W.C.; Lambert, S.D. Efficient P- and Ag-Doped Titania for the Photocatalytic Degradation of Waste Water Organic Pollutants. J. Alloys Compd. 2016, 682, 144–153. [Google Scholar] [CrossRef]
- Cheng, T.; Gao, H.; Liu, G.; Pu, Z.; Wang, S.; Yi, Z.; Wu, X.; Yang, H. Preparation of Core-Shell Heterojunction Photocatalysts by Coating CdS Nanoparticles onto Bi4Ti3O12 Hierarchical Microspheres and Their Photocatalytic Removal of Organic Pollutants and Cr(VI) Ions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127918. [Google Scholar] [CrossRef]
- Xiong, S.; Yin, Z.; Zhou, Y.; Peng, X.; Yan, W.; Liu, Z.; Zhang, X. The Dual-Frequency (20/40 KHz) Ultrasound Assisted Photocatalysis with the Active Carbon Fiber-Loaded Fe3+-TiO2 as Photocatalyst for Degradation of Organic Dye. Bull. Korean Chem. Soc. 2013, 34, 3039–3045. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Li, M.; Xu, P.; Tang, S.; Liu, C. Efficient Photocatalytic Degradation of Acid Orange 7 over N-Doped Ordered Mesoporous Titania on Carbon Fibers under Visible-Light Irradiation Based on Three Synergistic Effects. Appl. Catal. A Gen. 2016, 524, 163–172. [Google Scholar] [CrossRef]
- Tang, N.; Li, Y.; Chen, F.; Han, Z. In Situ Fabrication of a Direct Z-Scheme Photocatalyst by Immobilizing CdS Quantum Dots in the Channels of Graphene-Hybridized and Supported Mesoporous Titanium Nanocrystals for High Photocatalytic Performance under Visible Light. RSC Adv. 2018, 8, 42233–42245. [Google Scholar] [CrossRef]
- Lin, X.; Li, M.; Li, Y.; Chen, W. Enhancement of the Catalytic Activity of Ordered Mesoporous TiO2 by Using Carbon Fiber Support and Appropriate Evaluation of Synergy between Surface Adsorption and Photocatalysis by Langmuir-Hinshelwood (L-H) Integration Equation. RSC Adv. 2015, 5, 105227–105238. [Google Scholar] [CrossRef]
- Ndé, H.S.; Tamfuh, P.A.; Clet, G.; Vieillard, J.; Mbognou, M.T.; Woumfo, E.D. Comparison of HCl and H2SO4 for the Acid Activation of a Cameroonian Smectite Soil Clay: Palm Oil Discolouration and Landfill Leachate Treatment. Heliyon 2019, 5, e02926. [Google Scholar] [CrossRef] [PubMed]
- Olad, A. 7 Polymer/Clay Nanocomposites. In Advances in Diverse Industrial Applications of Nanocomposites; Reddy, B., Ed.; InTechOpen: London, UK, 2011. [Google Scholar]
- Yeop Lee, S.; Jin Kim, S. Expansion of smectite by hexadecyltrimethylammonium. Clays Clay Miner. 2002, 50, 435–445. [Google Scholar]
- Theo Kloprogge, J.; Komarnenl, S.; Amonetie, J.E. Synthesis of smectite clay minerals: A critical review. Clays Clay Miner. 1999, 47, 529–554. [Google Scholar] [CrossRef]
- Mahy, J.G.; Léonard, G.L.-M.; Pirard, S.; Wicky, D.; Daniel, A.; Archambeau, C.; Liquet, D.; Heinrichs, B. Aqueous Sol-Gel Synthesis and Film Deposition Methods for the Large-Scale Manufacture of Coated Steel with Self-Cleaning Properties. J. Sol-Gel Sci. Technol. 2017, 81, 27–35. [Google Scholar] [CrossRef]
- Benhebal, H.; Chaib, M.; Leonard, A.; Lambert, S.D.; Crine, M. Photodegradation of Phenol and Benzoic Acid by Sol-Gel-Synthesized Alkali Metal-Doped ZnO. Mater. Sci. Semicond. Process. 2012, 15, 264–269. [Google Scholar] [CrossRef]
- Léonard, G.L.-M.; Pàez, C.A.; Ramírez, A.E.; Mahy, J.G.; Heinrichs, B. Interactions between Zn2+ or ZnO with TiO2 to Produce an Efficient Photocatalytic, Superhydrophilic and Aesthetic Glass. J. Photochem. Photobiol. A Chem. 2018, 350, 32–43. [Google Scholar] [CrossRef]
- Mahy, J.G.; Lambert, S.D.; Léonard, G.L.M.; Zubiaur, A.; Olu, P.Y.; Mahmoud, A.; Boschini, F.; Heinrichs, B. Towards a Large Scale Aqueous Sol-Gel Synthesis of Doped TiO2: Study of Various Metallic Dopings for the Photocatalytic Degradation of p-Nitrophenol. J. Photochem. Photobiol. A Chem. 2016, 329, 189–202. [Google Scholar] [CrossRef]
- Geology, K.G. Geology, Petrology and Geochemistry of the Tertiary Bana Volcano-Plutonic Complex, West Cameroon, Central Africa. Ph.D. Thesis, Kobe University, Kobe, Japan, 2004. [Google Scholar]
- Aboubakar, Y. Etude Pédologique Du Terroir de Bana; ORSTOM: Yaounde, Cameroon, 1974. [Google Scholar]
- Bi Tra, T. Etude Pédologique et Cartographique à L’échelle 1/50000 d’un Secteur de L’ouest-Cameroun (Région de Bafang); ORSTOM: Yaounde, Cameroon, 1980. [Google Scholar]
- Benhebal, H.; Chaib, M.; Crine, M.; Leonard, A.; Lambert, S.D. Photocatalytic Decolorization of Gentian Violet with Na-Doped (SnO2 and ZnO). Chiang Mai J. Sci. 2016, 43, 584–589. [Google Scholar]


| Al | Si | Fe | Cu | TiO2 | ZnO | |
|---|---|---|---|---|---|---|
| wt % | wt % | wt % | wt % | wt % | wt % | |
| Bare Clay | 10.1 | 20.9 | 3.7 | <0.1 | <0.1 | <0.1 |
| Clay/Cu2+ | 11.5 | 21.2 | 4.2 | 0.8 | <0.1 | <0.1 |
| Clay/ZnO | 5.2 | 9.2 | 1.6 | <0.1 | <0.1 | 28.1 |
| Clay/ZnO/ Cu2+ | 6.4 | 11.6 | 1.8 | 0.4 | <0.1 | 30.3 |
| Clay/TiO2 | 5.7 | 10.2 | 1.6 | <0.01 | 28.8 | <0.1 |
| Clay/TiO2/Cu2+ | 5.9 | 11.6 | 1.2 | 0.3 | 27.6 | <0.1 |
| Sample | Specific Surface Area (m2/g) ± 5 |
|---|---|
| Bare Clay | 45 |
| Clay/Cu2+ | 55 |
| Pure TiO2 | 180 |
| Clay/TiO2 | 325 |
| Clay/Cu2+/TiO2 | 240 |
| Pure ZnO | 30 |
| Clay/ZnO calcine à 300 °C | 125 |
| Clay/Cu2+/ZnO | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahy, J.G.; Tsaffo Mbognou, M.H.; Léonard, C.; Fagel, N.; Woumfo, E.D.; Lambert, S.D. Natural Clay Modified with ZnO/TiO2 to Enhance Pollutant Removal from Water. Catalysts 2022, 12, 148. https://doi.org/10.3390/catal12020148
Mahy JG, Tsaffo Mbognou MH, Léonard C, Fagel N, Woumfo ED, Lambert SD. Natural Clay Modified with ZnO/TiO2 to Enhance Pollutant Removal from Water. Catalysts. 2022; 12(2):148. https://doi.org/10.3390/catal12020148
Chicago/Turabian StyleMahy, Julien G., Marlène Huguette Tsaffo Mbognou, Clara Léonard, Nathalie Fagel, Emmanuel Djoufac Woumfo, and Stéphanie D. Lambert. 2022. "Natural Clay Modified with ZnO/TiO2 to Enhance Pollutant Removal from Water" Catalysts 12, no. 2: 148. https://doi.org/10.3390/catal12020148
APA StyleMahy, J. G., Tsaffo Mbognou, M. H., Léonard, C., Fagel, N., Woumfo, E. D., & Lambert, S. D. (2022). Natural Clay Modified with ZnO/TiO2 to Enhance Pollutant Removal from Water. Catalysts, 12(2), 148. https://doi.org/10.3390/catal12020148








