Molecular Insights into Adsorption and Diffusion Mechanism of N-Hexane in MFI Zeolites with Different Si-to-Al Ratios and Counterions
Abstract
:1. Introduction
2. Results
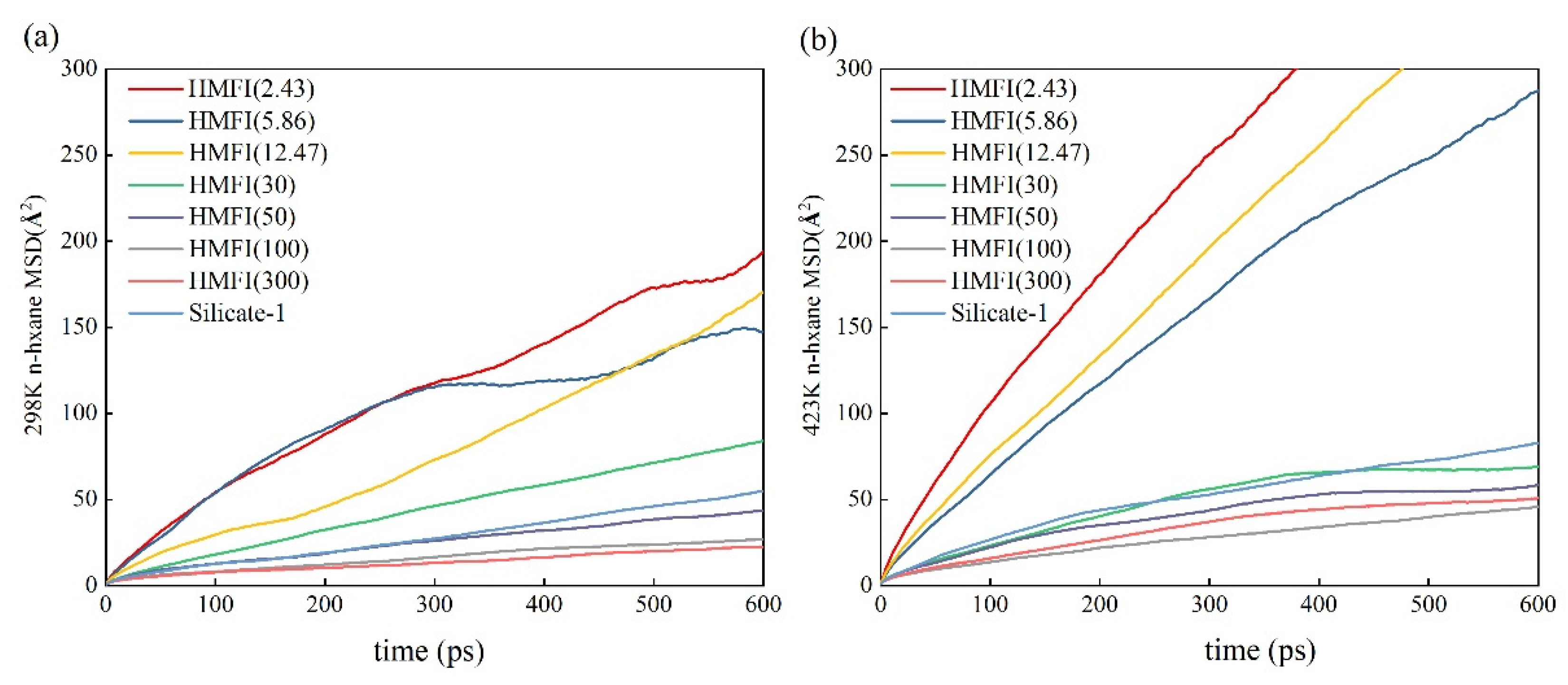
2.1. Adsorption and Diffusion Properties of N-Hexane Molecules on/in the HMFI Models
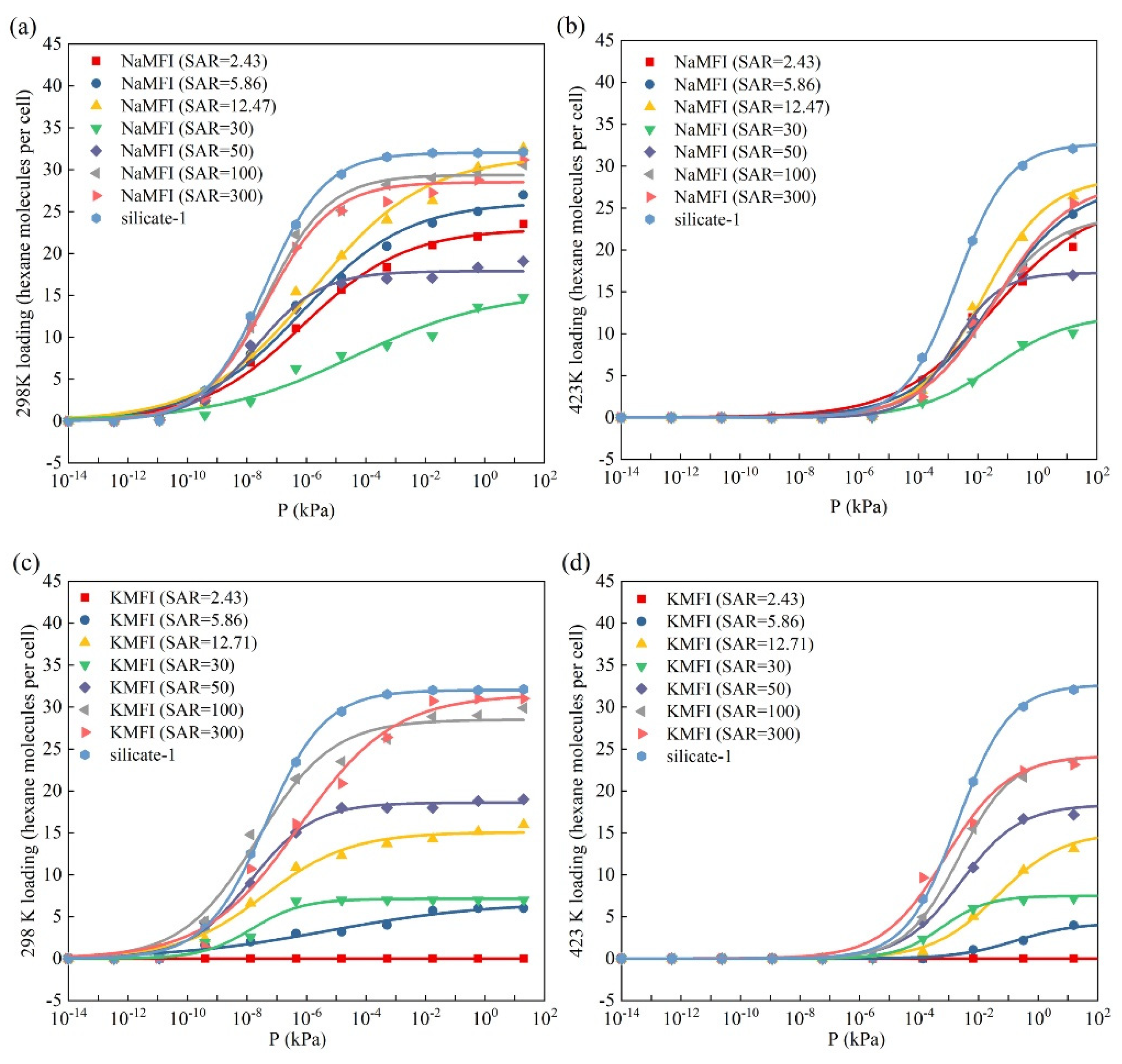
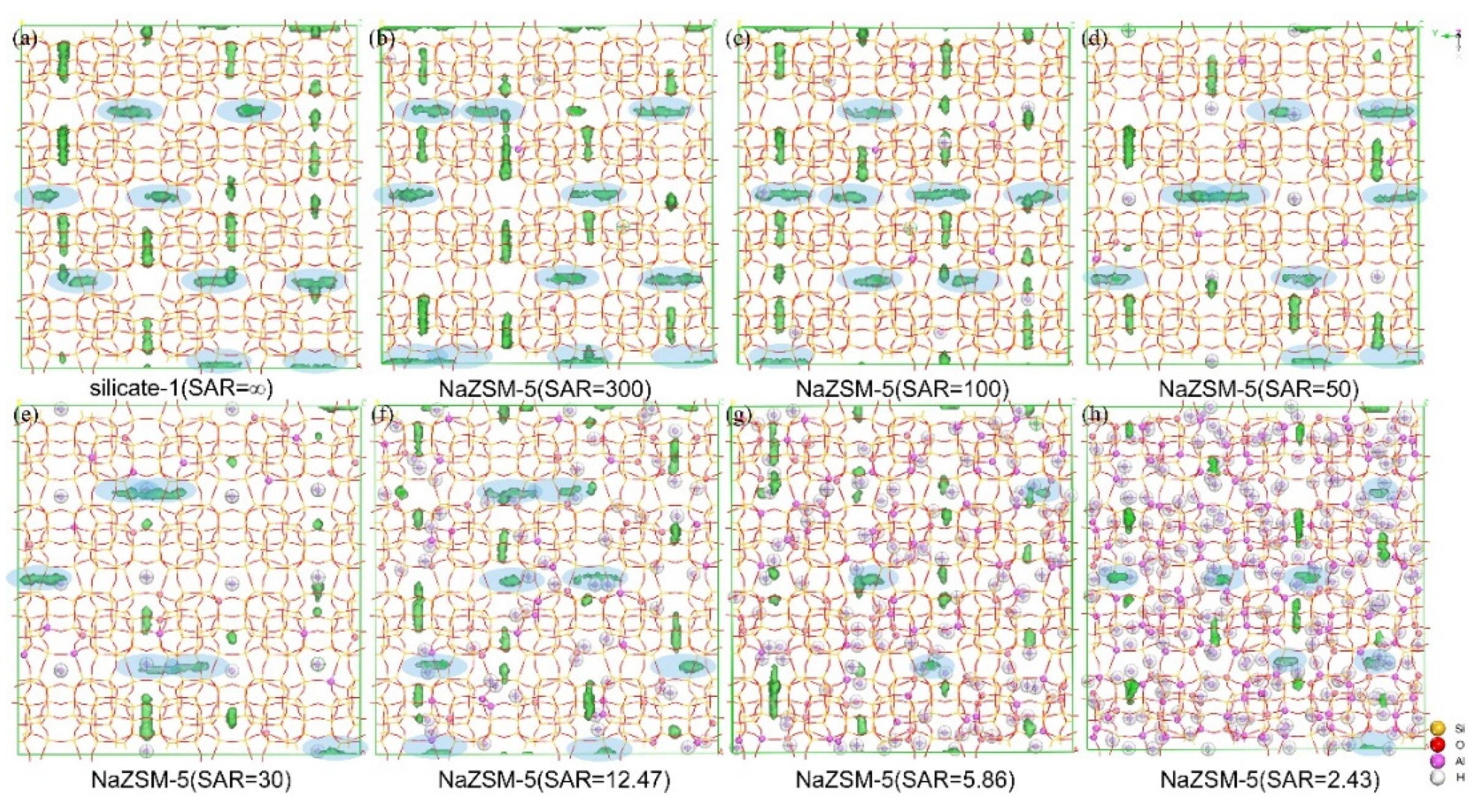
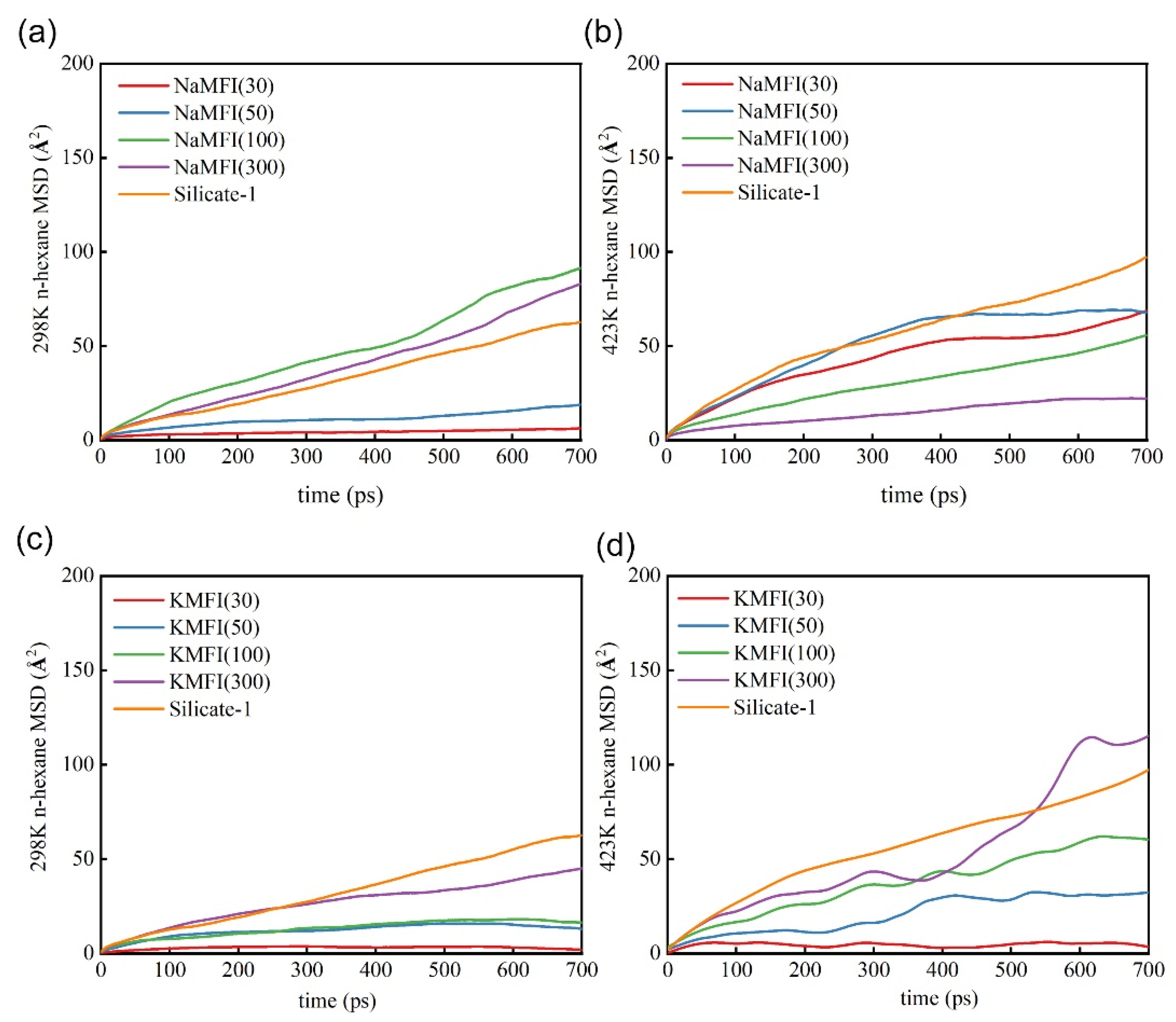
2.2. Adsorption and Diffusion Properties of N-Hexane Molecules on the NaMFI and KMFI Models
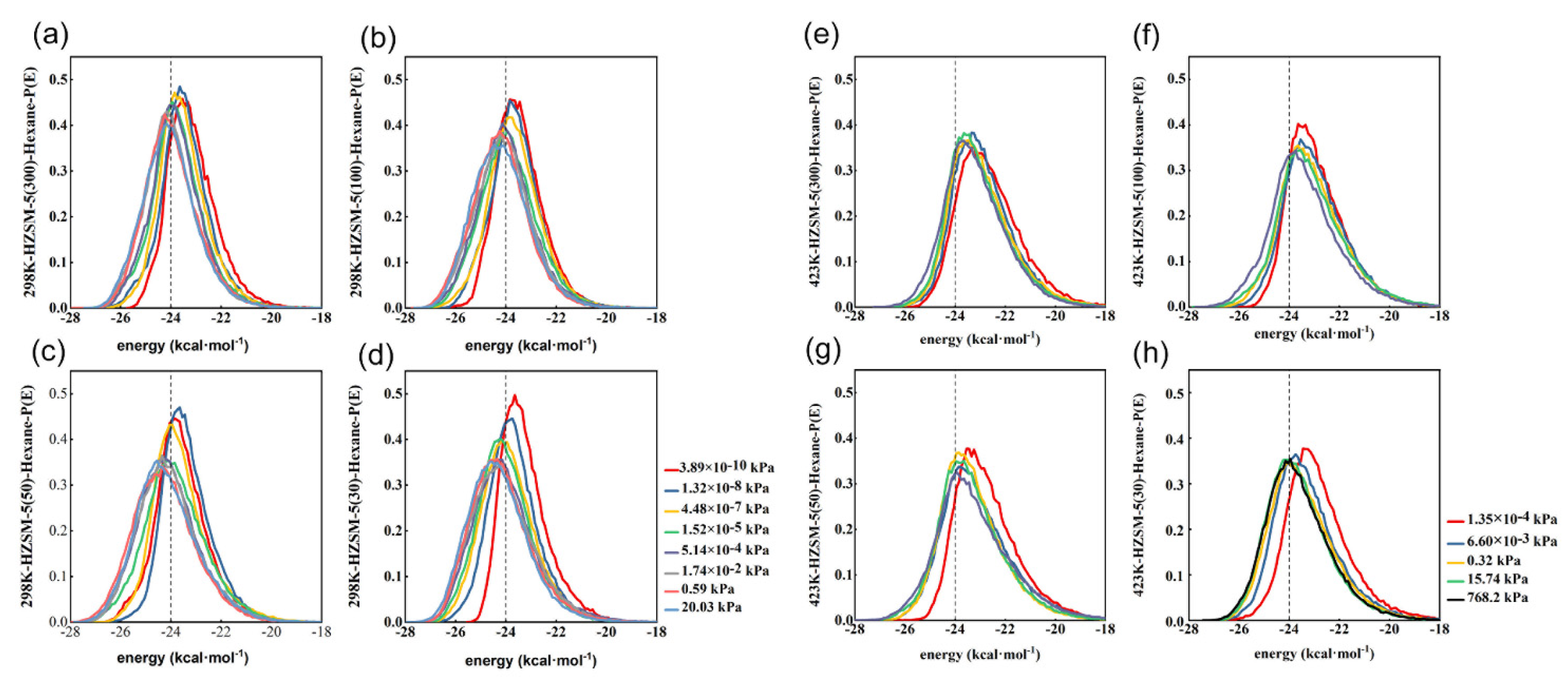
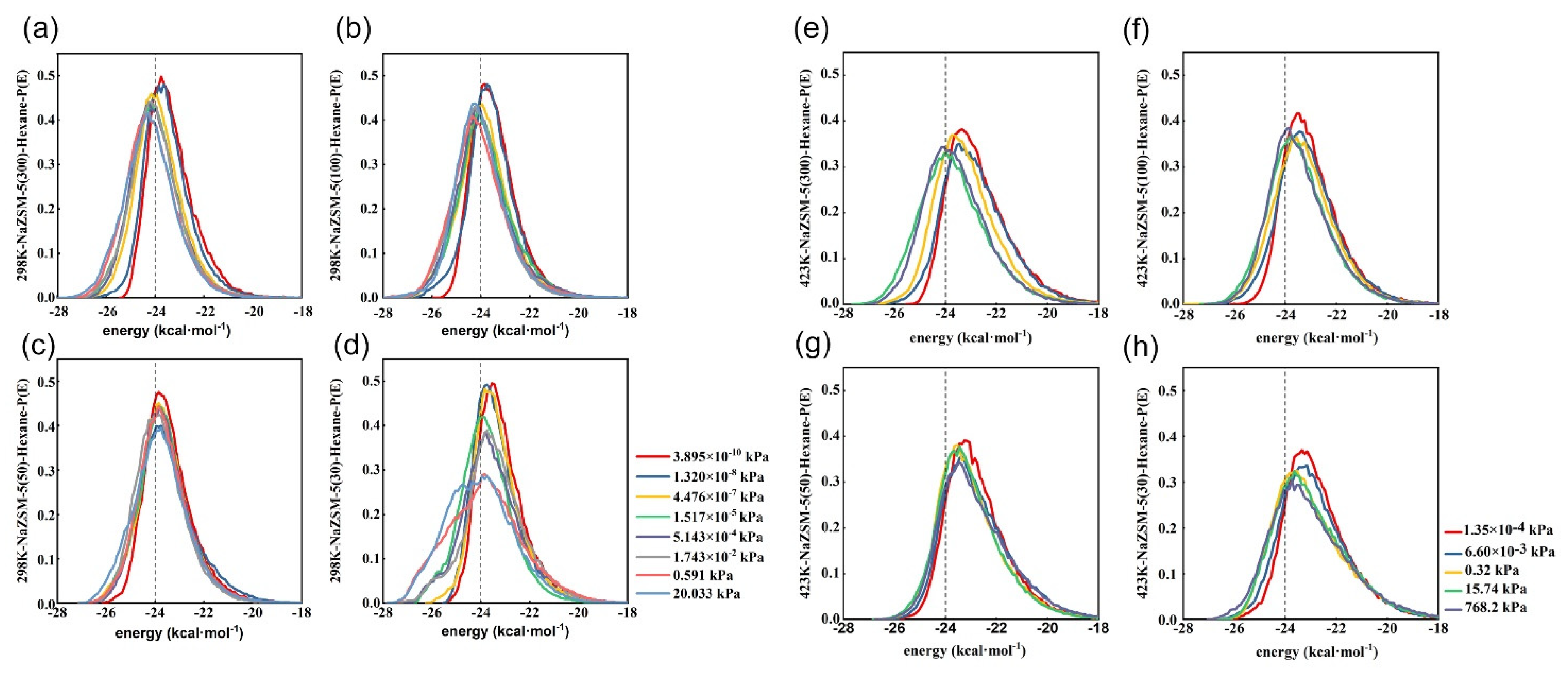
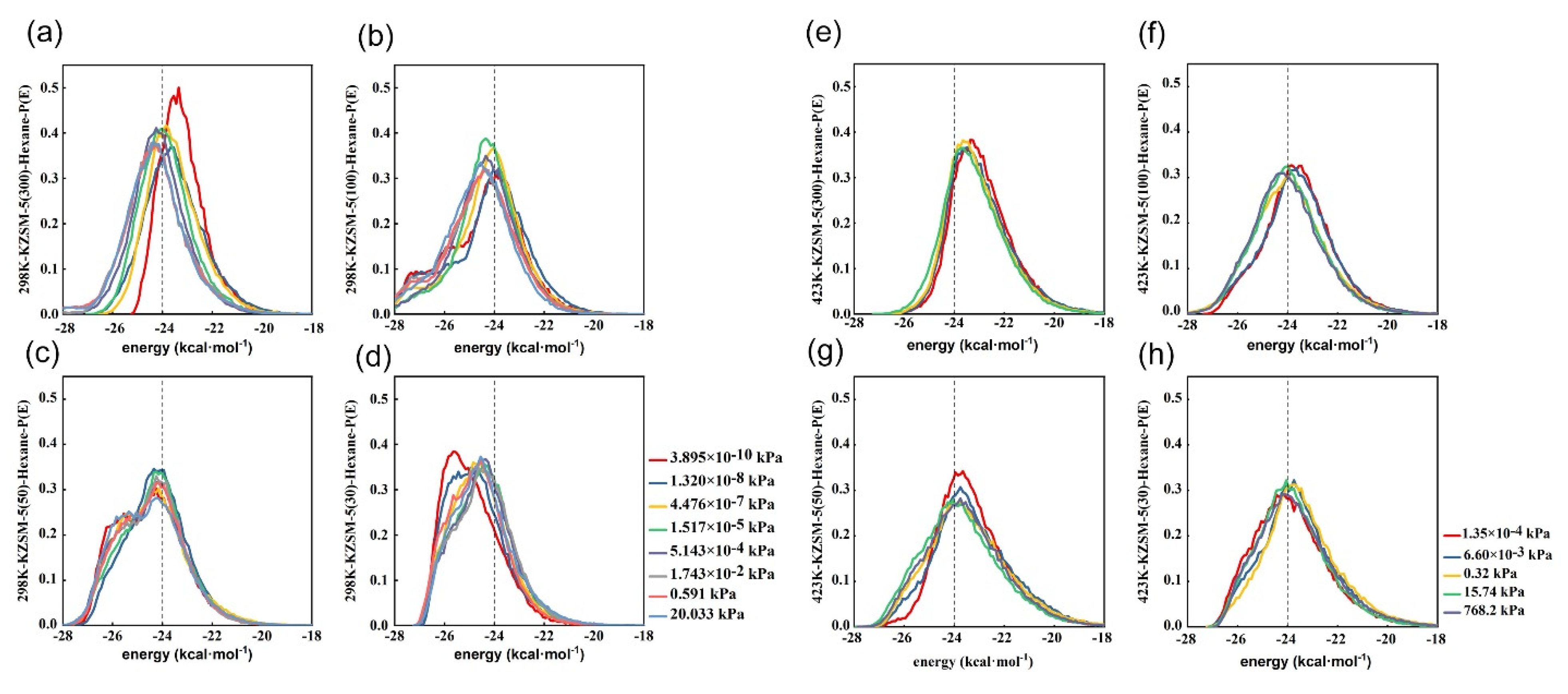
2.3. Comparative Analysis of Energy Distribution of N-Hexane Molecules on the HMFI, NaMFI and KMFI Zeolites with Typical SAR
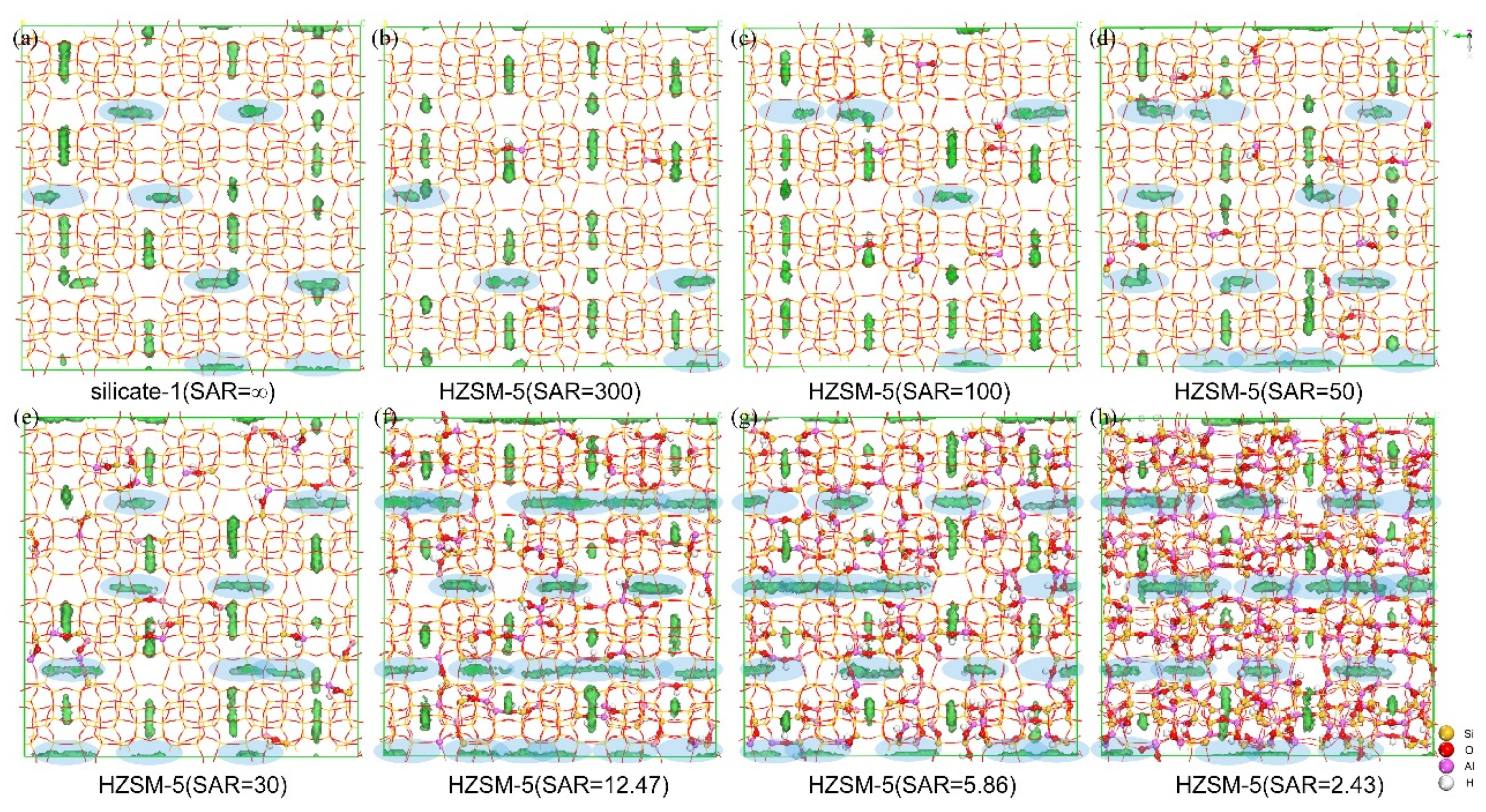
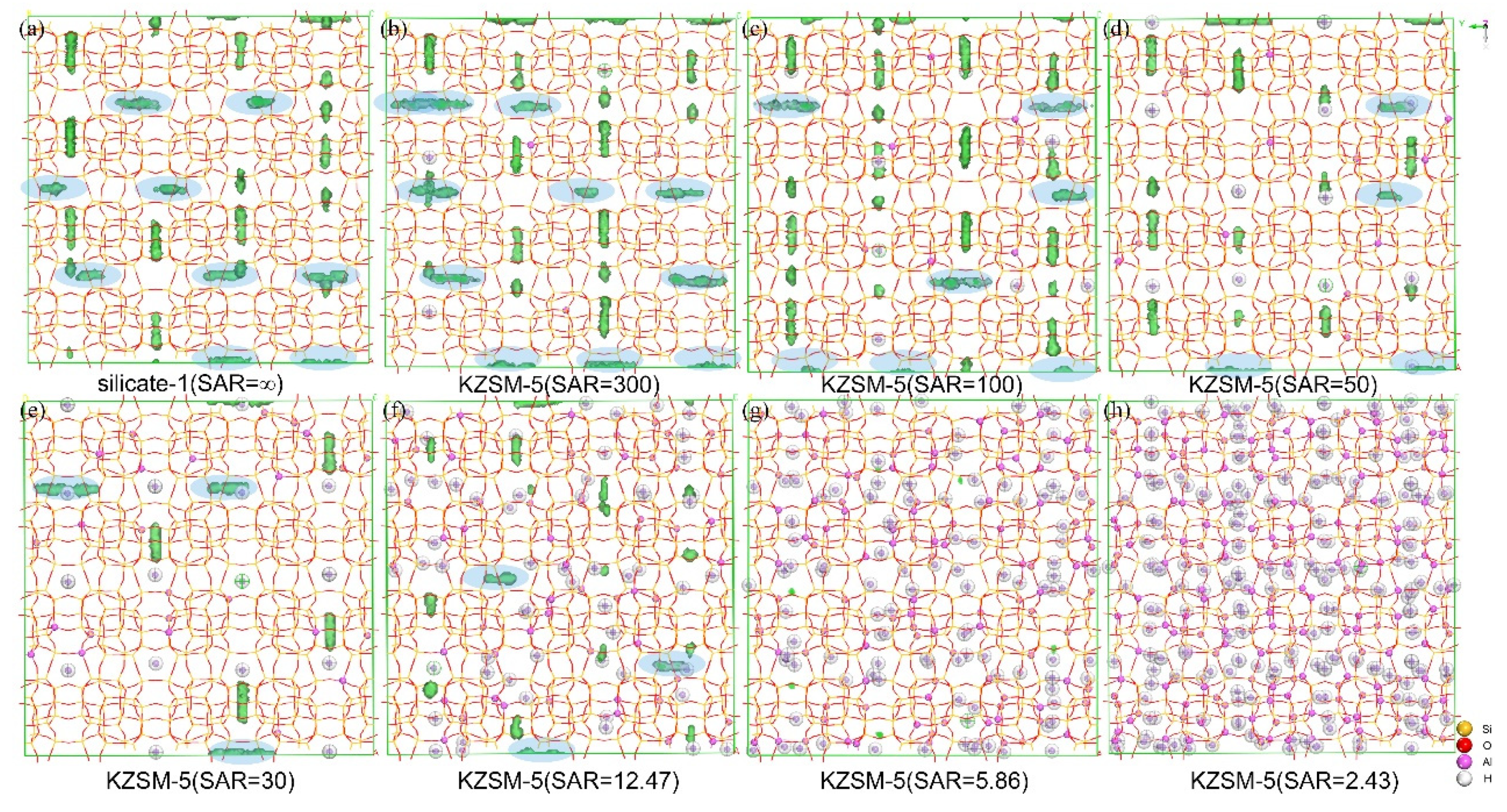
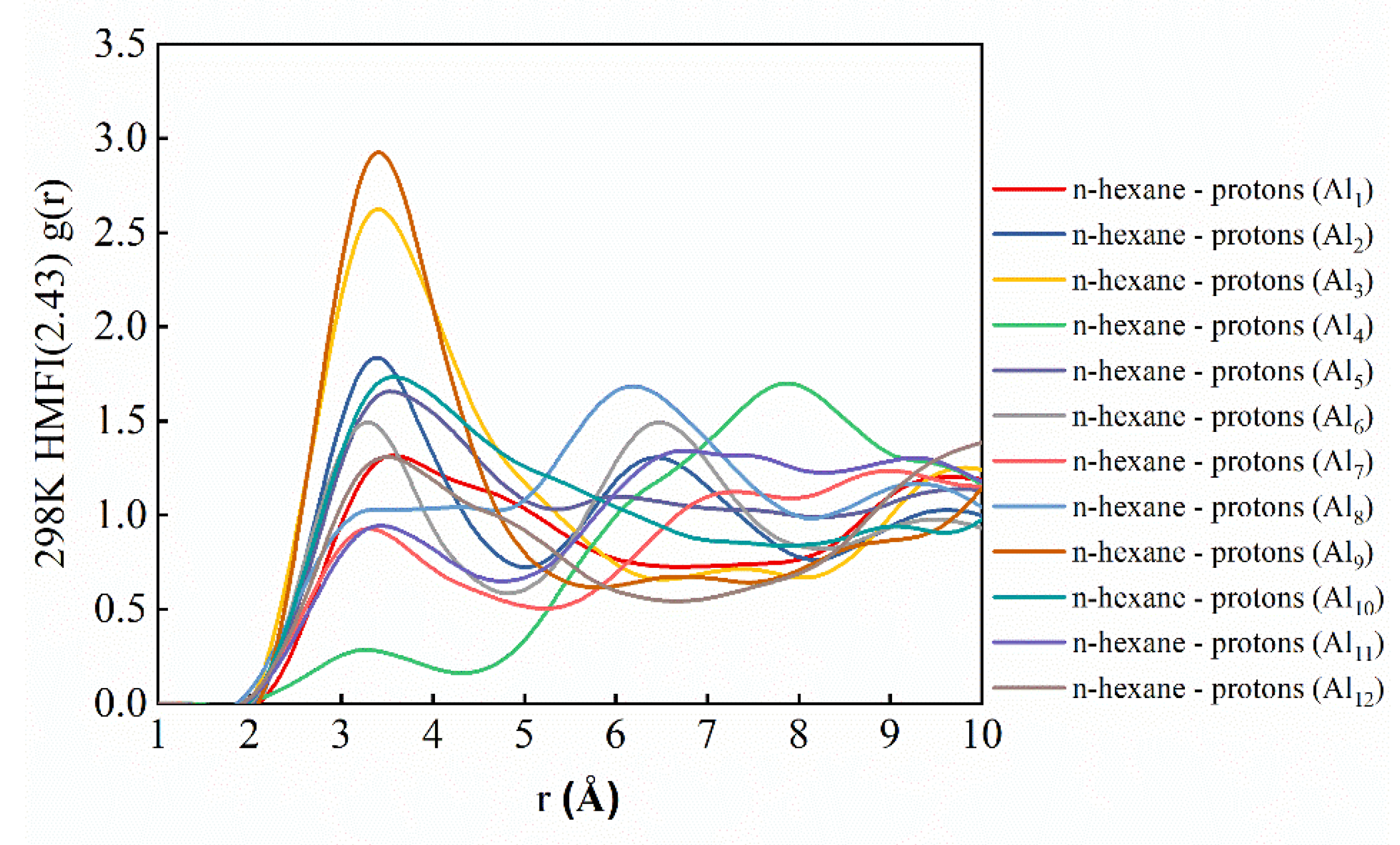
2.4. Identification of Adsorption Sites of N-Hexane Molecules on HZSM-5
3. Discussion
4. Computational Model and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Li, G.; Hensen, E.J.M.; Pidko, E.A. Nature and catalytic role of extraframework aluminum in faujasite zeolite: A theoretical perspective. ACS Catal. 2015, 5, 7024–7033. [Google Scholar] [CrossRef]
- Liu, C.; Li, G.; Hensen, E.J.M.; Pidko, E.A. Relationship between acidity and catalytic reactivity of faujasite zeolite: A periodic DFT study. J. Catal. 2016, 344, 570–577. [Google Scholar] [CrossRef]
- Zheng, J.; Qin, Y.; Li, Q.; Zhang, L.; Gao, X.; Song, L. A periodic DFT study of the synergistic mechanisms between extraframework aluminum species and Brønsted acid sites in HY zeolites. Ind. Eng. Chem. Res. 2020, 59, 2736–2744. [Google Scholar] [CrossRef]
- Huang, C.; Han, D.; Guan, L.; Zhu, L.; Mei, Y.; He, D.; Zu, Y. Bimetallic Ni-Zn site anchored in siliceous zeolite framework for synergistically boosting propane dehydrogenation. Fuel 2022, 307, 121790. [Google Scholar] [CrossRef]
- Park, S.; Biligetu, T.; Wang, Y.; Nishitoba, T.; Kondo, J.N.; Yokoi, T. Acidic and catalytic properties of ZSM-5 zeolites with different Al distributions. Catal. Today 2018, 303, 64–70. [Google Scholar] [CrossRef]
- Vjunov, A.; Fulton, J.L.; Huthwelker, T.; Pin, S.; Mei, D.; Schenter, G.K.; Govind, N.; Camaioni, D.M.; Hu, J.Z.; Lercher, J.A. Quantitatively probing the Al distribution in zeolites. J. Am. Chem. Soc. 2015, 136, 8296. [Google Scholar] [CrossRef] [PubMed]
- Alswat, A.A.; Ahmad, M.B.; Hussein, M.Z.; Ibrahim, N.A.; Saleh, T.A. Copper oxide nanoparticles-loaded zeolite and its characteristics and antibacterial activities. J. Mater. Sci. Technol. 2017, 33, 889–896. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, C.; Ma, J.; Li, R.; Jiao, H. Determining the structures, acidity and adsorption properties of Al substituted HZSM-5. Phys. Chem. Chem. Phys. 2019, 21, 18758–18768. [Google Scholar] [CrossRef]
- Jones, A.J.; Iglesia, E. The strength of Brønsted acid sites in microporous aluminosilicates. ACS Catal. 2015, 5, 5741–5755. [Google Scholar] [CrossRef]
- Védrine, J.C.; Auroux, A.; Dejaifve, P.; Ducarme, V.; Hoser, H.; Zhou, S. Catalytic and physical properties of phosphorus-modified ZSM-5 zeolite. J. Catal. 1982, 73, 147–160. [Google Scholar] [CrossRef]
- Castella-Ventura, M.; Moissette, A.; Kassab, E. A theoretical study of the confinement effects on the energetics and vibrational properties of 4,4-bipyridine adsorption on H-ZSM-5 zeolite. Phys. Chem. Chem. Phys. 2018, 20, 6354–6364. [Google Scholar] [CrossRef] [PubMed]
- Thivasasith, A.; Maihom, T.; Pengpanich, S.; Limtrakul, J.; Wattanakit, C. Insights into the reaction mechanism of n-hexane dehydroaromatization to benzene over gallium embedded HZSM-5: Effect of H2 incorporated on active sites. Phys. Chem. Chem. Phys. 2019, 21, 5359–5367. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, C.; Mccusker, L.B. Database of zeolite structures. Microporous Mesoporous Mater. 2020, 297, 110000. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Qin, Z.; Chen, Y.Y.; Fan, W. Relation of catalytic performance to the aluminum siting of acidic zeolites in the conversion of methanol to olefins, viewed via a comparison between ZSM-5 and ZSM-11. ACS Catal. 2018, 8, 5485–5505. [Google Scholar] [CrossRef]
- Sastre, G.; Fornes, V.; Corma, A. On the preferential location of Al and proton siting in zeolites: A computational and infrared study. J. Phys. Chem. B 2002, 106, 701–708. [Google Scholar] [CrossRef]
- Sklenak, S.; Dedecek, J.; Li, C.; Wichterlová, B.; Sauer, J. Aluminium siting in the ZSM-5 framework by combination of high resolution 27Al NMR and DFT/MM calculations. Phys. Chem. Chem. Phys. 2009, 11, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Iorio, J.; Gounder, R. Controlling the isolation and pairing of aluminum in chabazite zeolites using mixtures of organic and inorganic structure-directing agents. Chem. Mater. 2016, 28, 2236–2247. [Google Scholar] [CrossRef]
- SchrDer, K.P.; Sauer, J.; Leslie, M.; Richard, C.; Catlow, A. Siting of Al and bridging hydroxyl groups in ZSM-5: A computer simulation study. Zeolites 1992, 12, 20–23. [Google Scholar] [CrossRef]
- Ricchiardi, G.; Newsam, J.M. Predicted effects of site-specific aluminum substitution on the framework geometry and unit cell dimensions of zeolite ZSM-5 materials. J. Phys. Chem. B 2001, 101, 9943–9950. [Google Scholar] [CrossRef]
- Olson, D.H.; Khosrovani, N.; Peters, A.W.; Toby, B.H. Crystal structure of dehydrated CsZSM-5 (5.8Al): evidence for nonrandom aluminum distribution. J. Phys. Chem. B 2000, 104, 4844–4848. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Rimer, J.D.; Grabow, L.C. Periodic, VDW-corrected density functional theory investigation of the effect of Al siting in H-ZSM-5 on chemisorption properties and site-specific acidity. Catal. Commun. 2014, 52, 98–102. [Google Scholar] [CrossRef]
- Xing, B.; Ma, J.; Li, R.; Jiao, H. Location, distribution and acidity of Al substitution in ZSM-5 with different Si/Al ratios—A periodic DFT computation. Catal. Sci. Technol. 2017, 7, 5694–5708. [Google Scholar] [CrossRef]
- Dědeček, J.; Kaucký, D.; Wichterlová, B.; Gonsiorová, O. Co2+ ions as probes of Al distribution in the framework of zeolites. ZSM-5 study. Phys. Chem. Chem. Phys. 2002, 4, 5406–5413. [Google Scholar] [CrossRef]
- Gábová, V.; Dědeček, J.; Čejka, J. Control of Al distribution in ZSM-5 by conditions of zeolite synthesis. Chem. Commun. 2003, 10, 1196–1197. [Google Scholar] [CrossRef]
- Mann, S.; Perry, C.C.; Williams, R.; Fyfe, C.A.; Gobbi, G.C.; Kennedy, G.J. The characterisation of the nature of silica in biological systems. J. Chem. Soc. Chem. Commun. 1983, 4, 168–170. [Google Scholar] [CrossRef]
- Gonzales, N.O.; Chakraborty, A.K.; Bell, A.T. A density functional theory study of the effects of metal cations on the Brøsted acidity of H-ZSM-5. Catal. Lett. 1998, 50, 135–139. [Google Scholar] [CrossRef]
- Li, C.; Vidal-Moya, A.; Miguel, P.J.; Dedecek, J.; Boronat, M.; Corma, A. Selective introduction of acid sites in different confined positions in ZSM-5 and its catalytic implications. ACS Catal. 2018, 8, 7688–7697. [Google Scholar] [CrossRef]
- Rice, M.J.; Chakraborty, A.K.; Bell, A.T. Al Next Nearest Neighbor, Ring occupation, and proximity statistics in ZSM-5. J. Catal. 1999, 186, 222–227. [Google Scholar] [CrossRef] [Green Version]
- Yokoi, T.; Mochizuki, H.; Namba, S.; Kondo, J.N.; Tatsumi, T. Control of the Al distribution in the framework of ZSM-5 zeolite and its evaluation by solid-state NMR technique and catalytic properties. J. Phys. Chem. C 2015, 119, 15303–15315. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Moliner, M.; Davis, M.E. Impact of controlling the site distribution of al atoms on catalytic properties in Ferrierite-type zeolites. J. Phys. Chem. C 2010, 115, 1096–1102. [Google Scholar] [CrossRef]
- Ajj, A.; Rtc, A.; Siza, B.; Ei, A. Acid strength and solvation in catalysis by MFI zeolites and effects of the identity, concentration and location of framework heteroatoms. J. Catal. 2014, 312, 58–68. [Google Scholar] [CrossRef]
- von Ballmoos, R.; Meier, W.M. Zoned aluminium distribution in synthetic zeolite ZSM-5. Nature 1981, 289, 782–783. [Google Scholar] [CrossRef]
- Groen, J.C.; Bach, T.; Ziese, U.; Paulaime-van Donk, A.M.; de Jong, K.P.; Moulijn, J.A.; Pérez-Ramírez, J. Creation of hollow zeolite architectures by controlled desilication of Al-zoned ZSM-5 crystals. J. Am. Chem. Soc. 2005, 127, 10792–10793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deguchi, M.; Iyoki, K.; Anand, C.; Yanaba, Y.; Yoshikawa, T.; Okubo, T.; Wakihara, T. Temperature-controlled, two-stage synthesis of ZSM-5 zeolite nanoparticles with Al atoms tetrahedrally coordinated in the framework. Microporous Mesoporous Mater. 2018, 270, 200–203. [Google Scholar] [CrossRef]
- Li, J.; Ma, H.; Chen, Y.; Xu, Z.; Li, C.; Ying, W. Conversion of methanol to propylene over hierarchical HZSM-5: The effect of Al spatial distribution. Chem. Commun. 2018, 54, 6032–6035. [Google Scholar] [CrossRef]
- Voogd, P.; Bekkum, H.V. Limitation of n-hexane and 3-methylpentane conversion over zeolite ZSM-5 by intracrystalline diffusion. Appl. Catal. 1990, 59, 311–331. [Google Scholar] [CrossRef]
- Afroukhteh-Langaroudi, N.; Tarighi, S.; Khonakdara, H.A. Catalytic cracking of n -hexane and n -heptane over ZSM-5 zeolite: Influence of SiO2/Al2O3 ratio. Pet. Chem. 2018, 58, 457–463. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Huang, C.; Sun, X. Density functional theory study of Cu-ZSM-5-catalyzed C–H bond activation: The importance of active centers. J. Phys. Chem. C 2018, 122, 28645–28651. [Google Scholar] [CrossRef]
- Jobic, H.; Laloué, N.; Laroche, C.; van Baten, J.M. Influence of isotherm inflection on the loading dependence of the diffusivities of n-hexane and n-heptane in MFI zeolite. J. Phys. Chem. B 2006, 110, 2195–2201. [Google Scholar] [CrossRef]
- Ruthven, D.M.; Kaul, B.K. Adsorption of n-hexane and intermediate molecular weight aromatic hydrocarbons on LaY zeolite. Ind. Eng. Chem. Res. 1996, 35, 2060–2064. [Google Scholar] [CrossRef]
- Bozbiyik, B.; Duerinck, T.; Lannoeye, J.; De Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Adsorption and separation of n-hexane and cyclohexane on the UiO-66 metal-organic framework. Microporous Mesoporous Mater. 2014, 183, 143–149. [Google Scholar] [CrossRef]
- Vlugt, T.; Krishna, R.; Smit, B. Molecular simulations of adsorption isotherms of linear and branched alkanes and their mixtures in silicalite. J. Phys. Chem. B 1999, 103, 1102–1118. [Google Scholar] [CrossRef] [Green Version]
- Ju, S.G.; Zeng, Y.P.; Xing, W.H.; Chen, C.L. Computer simulation of the adsorption of thiophene in all-silica Y and Na-Y. ACS J. Surf. Colloids 2006, 22, 8353. [Google Scholar] [CrossRef] [PubMed]
- Pfriem, N.; Hintermeier, P.H.; Eckstein, S.; Kim, S.; Liu, Q.; Shi, H.; Milakovic, L.; Liu, Y.; Haller, G.L.; Baráth, E. Role of the ionic environment in enhancing the activity of reacting molecules in zeolite pores. Science 2021, 372, 952–957. [Google Scholar] [CrossRef]
- Loewenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Miner. 1954, 39, 92–97. [Google Scholar]
- Zheng, H.; Liang, Z.; Yang, Q.; Gao, J.; Xu, C. Influence of framework protons on the adsorption sites of the benzene/HY system. Ind. Eng. Chem. Res. 2014, 53, 13610–13617. [Google Scholar] [CrossRef]
- Liu, Y.B.; Li, Y.Z.; Ding, X. Adsorption simulation of basic nitrogen compounds in ZSM-5 and USY zeolites by grand canonical monte carlo method. In Advanced Materials Research; Trans Tech Publications Ltd.: Baech, Switzerland, 2015; Volume 1096, Available online: https://doi.org/10.4028/www.scientific.net/AMR.1096.189 (accessed on 17 December 2021).
- Dang, S.; Zhao, L.; Gao, J.; Xu, C. Loading dependence of the adsorption mechanism of thiophene in FAU zeolite. Ind. Eng. Chem. Res. 2016, 55, 11801–11808. [Google Scholar] [CrossRef]
- Bai, P.; Siepmann, J.I.; Deem, M.W. Adsorption of glucose into zeolite beta from aqueous solution. AIChE J. 2013, 59, 3523–3529. [Google Scholar] [CrossRef]
- Brémard, C.; Ginestet, G.; Le Maire, M. Sorption sites, energetics, and reactions of molybdenum hexacarbonyl and benzene cosorbed in faujasitic zeolites. J. Am. Chem. Soc. 1996, 118, 12724–12734. [Google Scholar] [CrossRef]
- Yang, J.Z.; Liu, Q.L.; Wang, H.T. Analyzing adsorption and diffusion behaviors of ethanol/water through silicalite membranes by molecular simulation. J. Membr. Sci. 2007, 291, 1–9. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Huang, L.; Wei, P.; Li, Q.; Fan, X.; Qin, Y.; Song, L. Molecular Insights into Adsorption and Diffusion Mechanism of N-Hexane in MFI Zeolites with Different Si-to-Al Ratios and Counterions. Catalysts 2022, 12, 144. https://doi.org/10.3390/catal12020144
Zheng J, Huang L, Wei P, Li Q, Fan X, Qin Y, Song L. Molecular Insights into Adsorption and Diffusion Mechanism of N-Hexane in MFI Zeolites with Different Si-to-Al Ratios and Counterions. Catalysts. 2022; 12(2):144. https://doi.org/10.3390/catal12020144
Chicago/Turabian StyleZheng, Jian, Le Huang, Panpeng Wei, Qiang Li, Xiaolei Fan, Yucai Qin, and Lijuan Song. 2022. "Molecular Insights into Adsorption and Diffusion Mechanism of N-Hexane in MFI Zeolites with Different Si-to-Al Ratios and Counterions" Catalysts 12, no. 2: 144. https://doi.org/10.3390/catal12020144
APA StyleZheng, J., Huang, L., Wei, P., Li, Q., Fan, X., Qin, Y., & Song, L. (2022). Molecular Insights into Adsorption and Diffusion Mechanism of N-Hexane in MFI Zeolites with Different Si-to-Al Ratios and Counterions. Catalysts, 12(2), 144. https://doi.org/10.3390/catal12020144






