Fabrication of Polyaniline Ni-Complex Catalytic Electrode by Plasma Deposition for Electrochemical Detection of Phosphate through Glucose Redox Reaction as Mediator
Abstract
:1. Introduction
2. Results
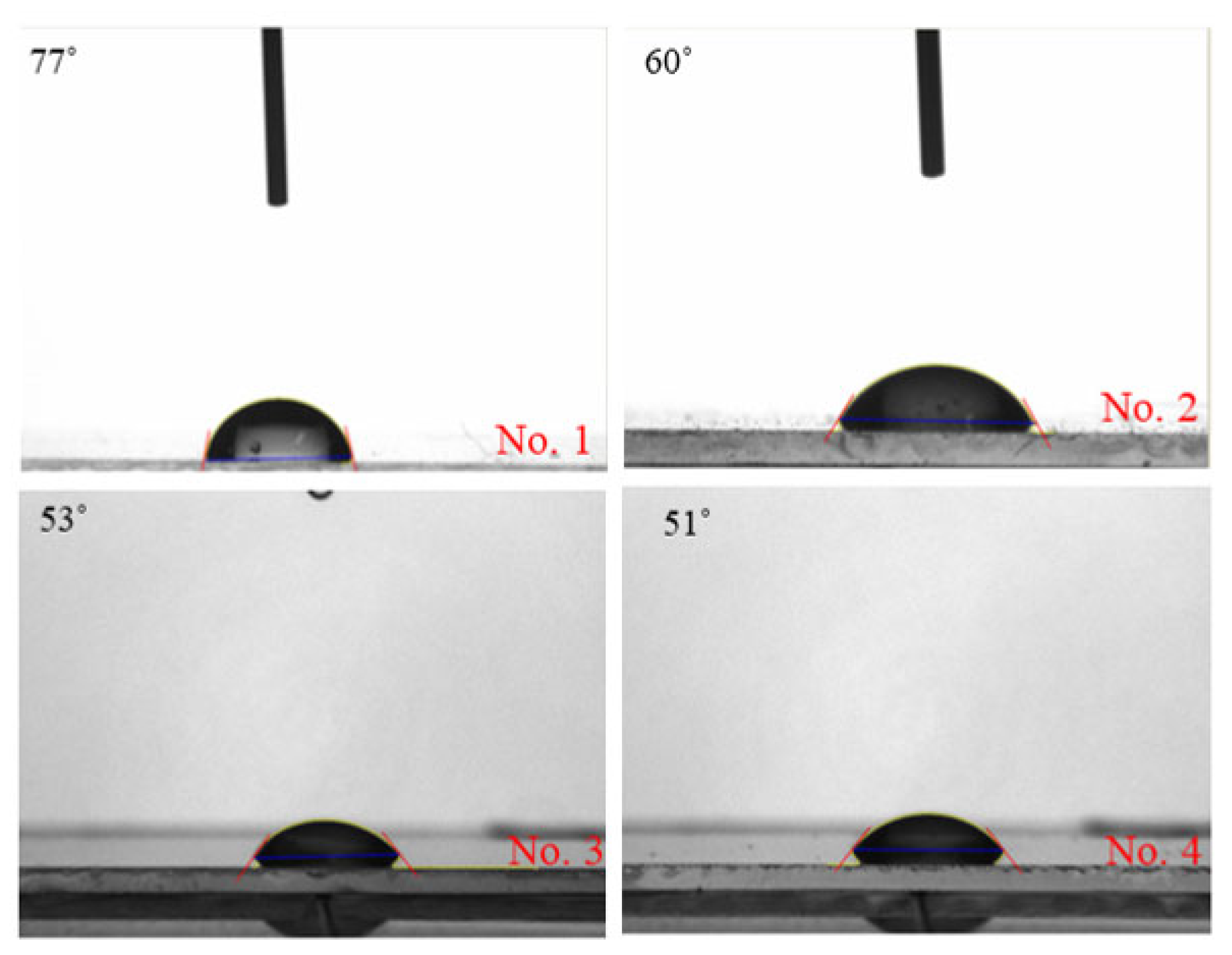
2.1. Contact Angle Analysis
2.2. SEM Data Analysis
2.3. AFM Data Analysis
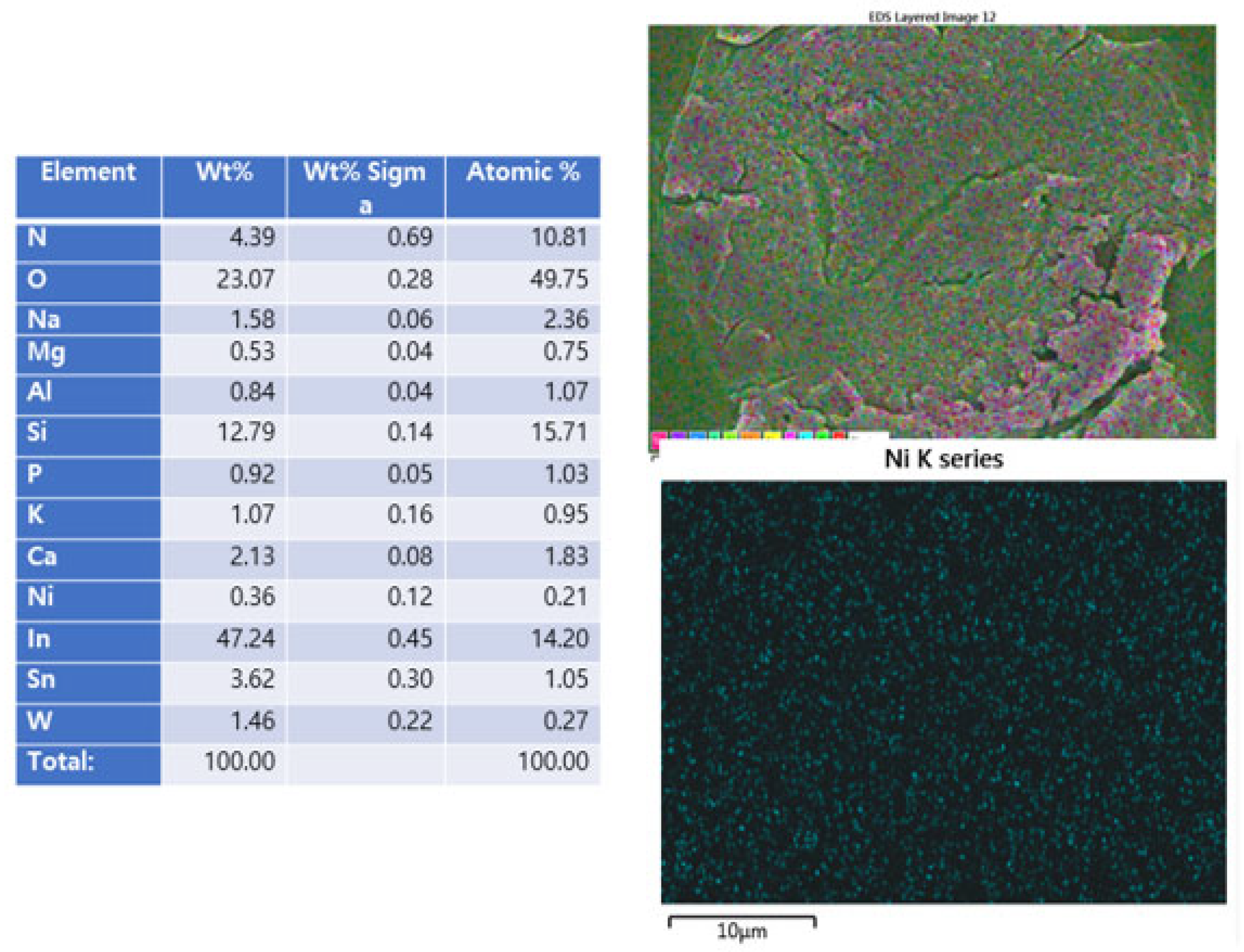
2.4. EDS Data Analysis
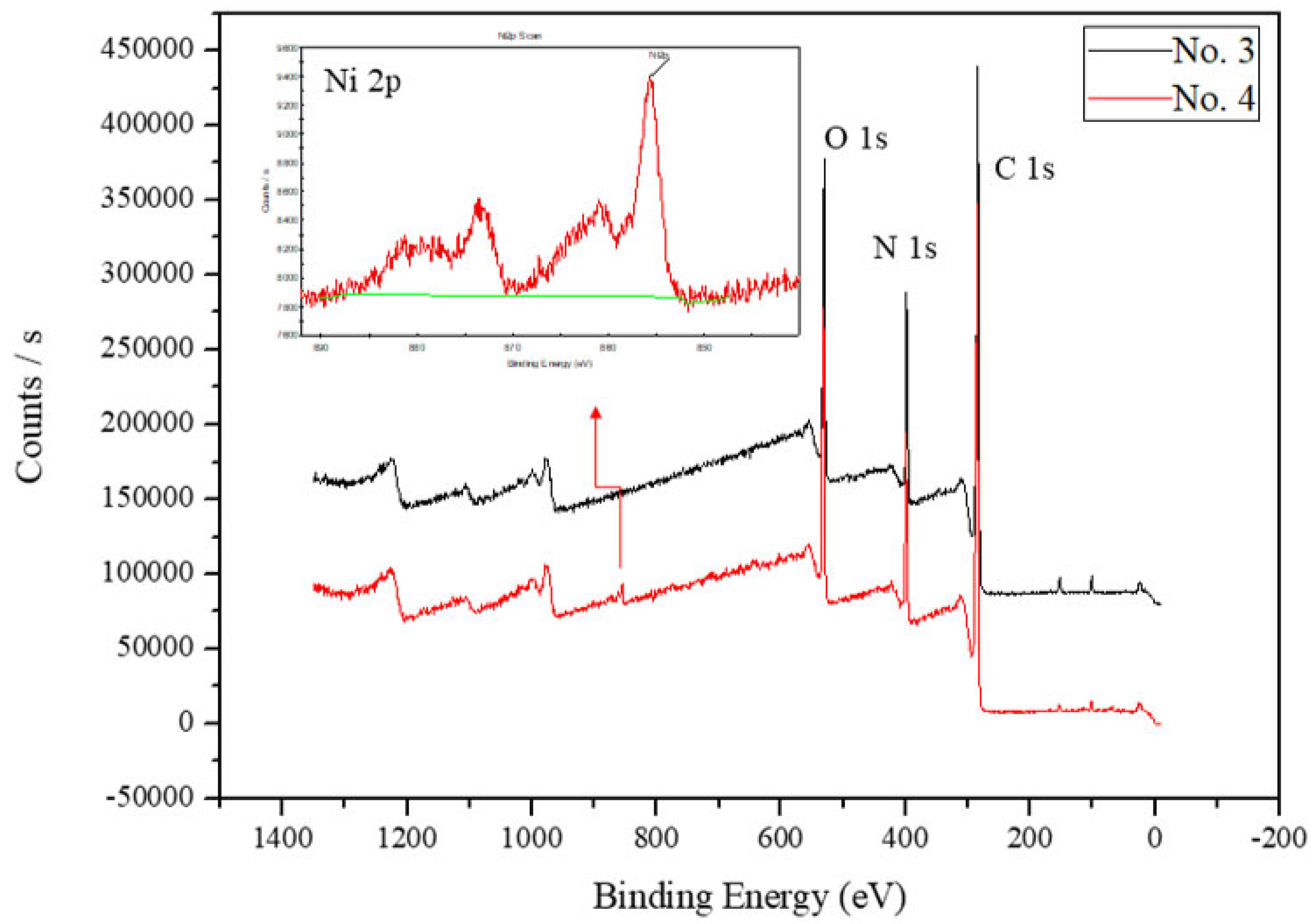
2.5. XPS Data Analysis
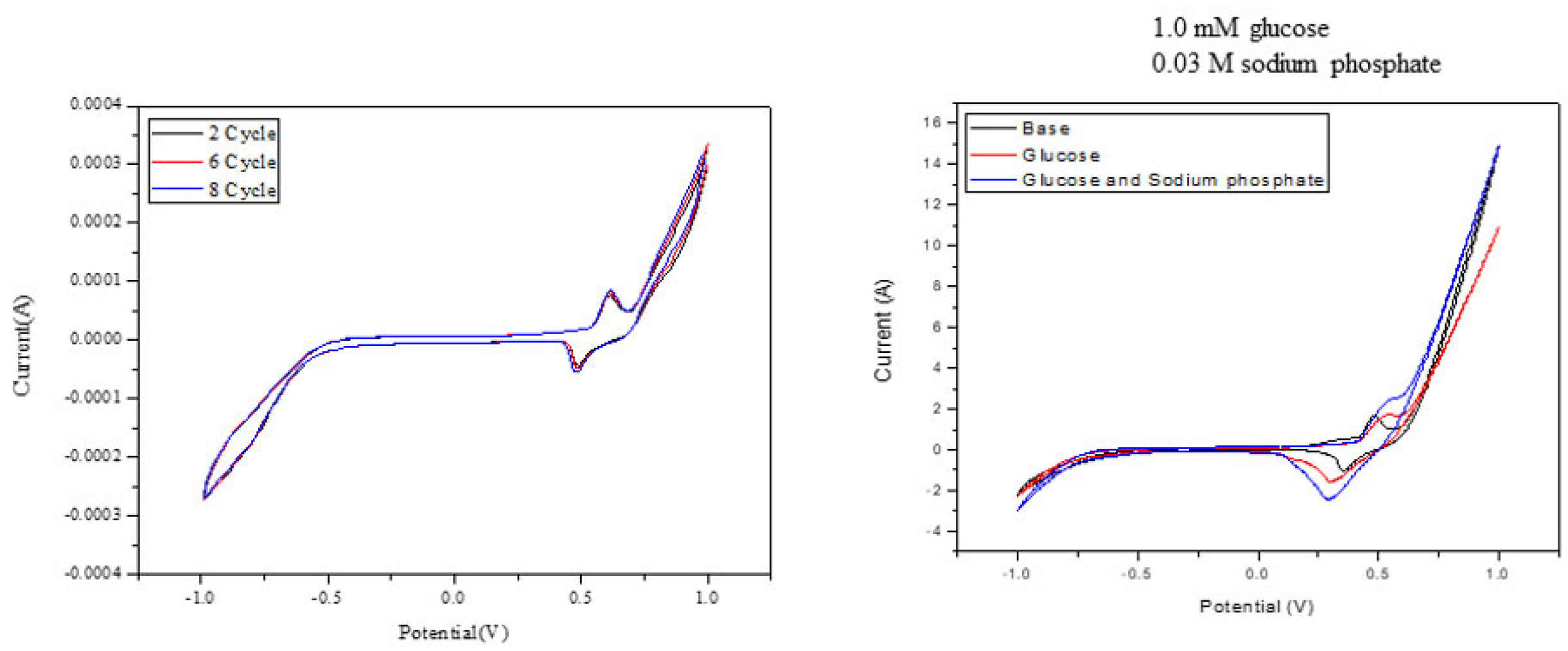
2.6. Electrochemical Data Analysis
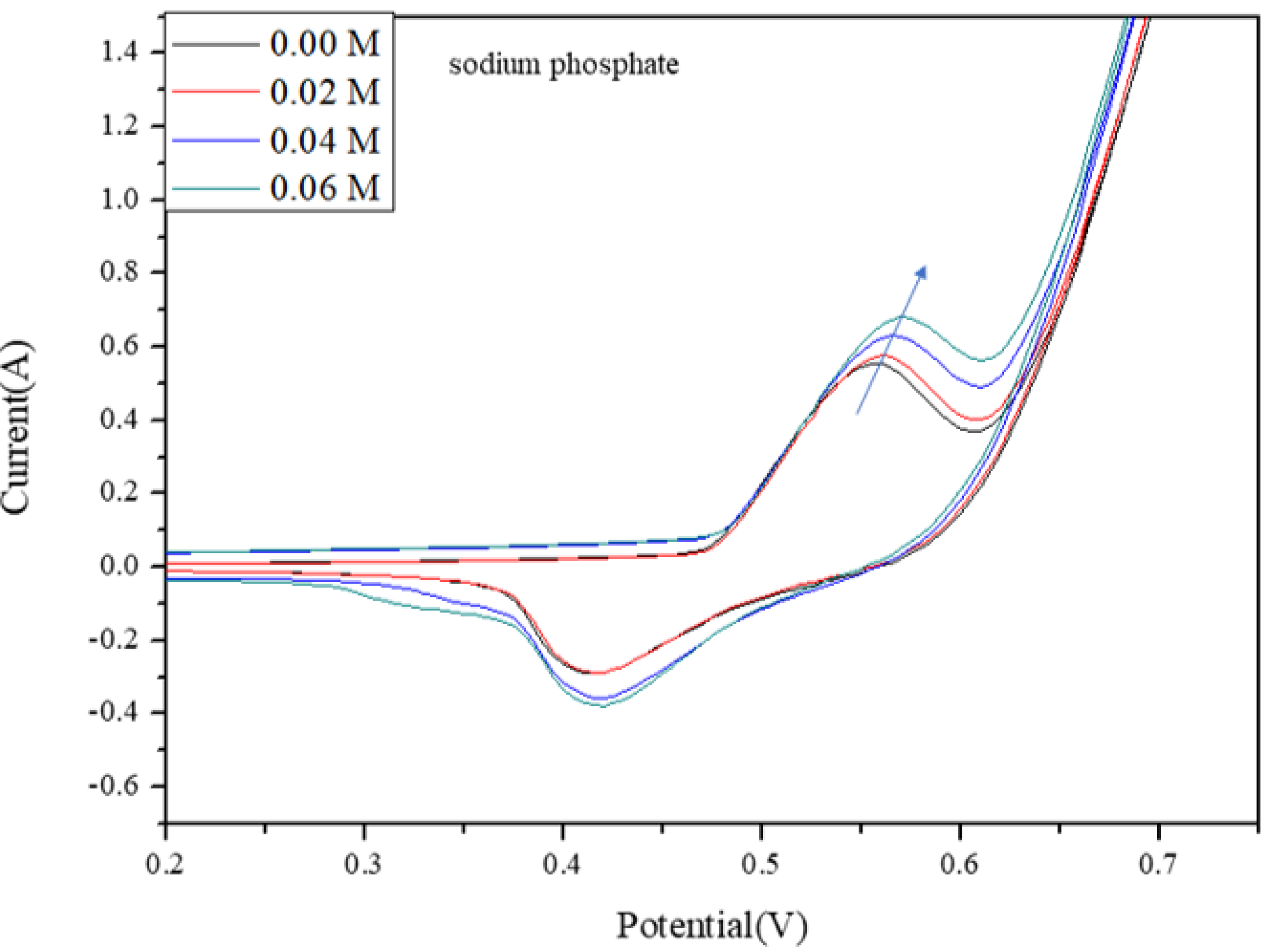
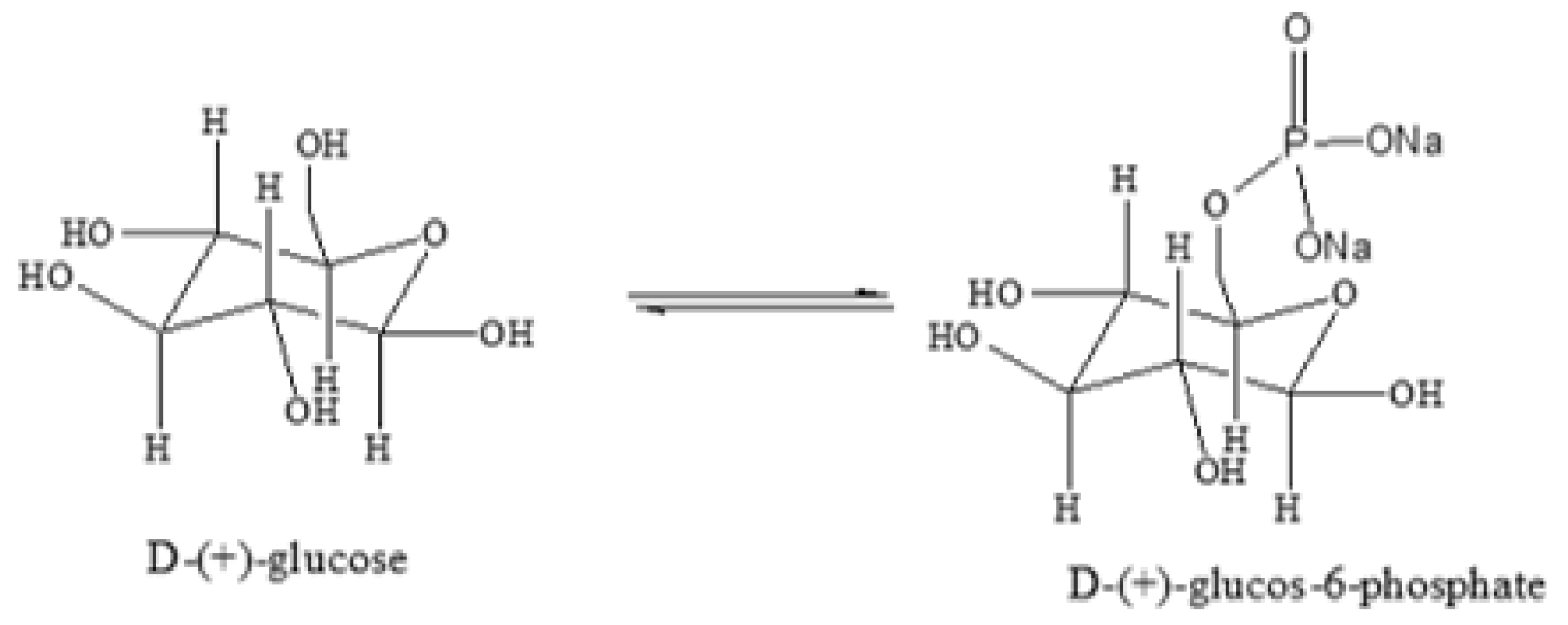
2.7. Electrochemical Detection of Phosphate
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Research Instruments
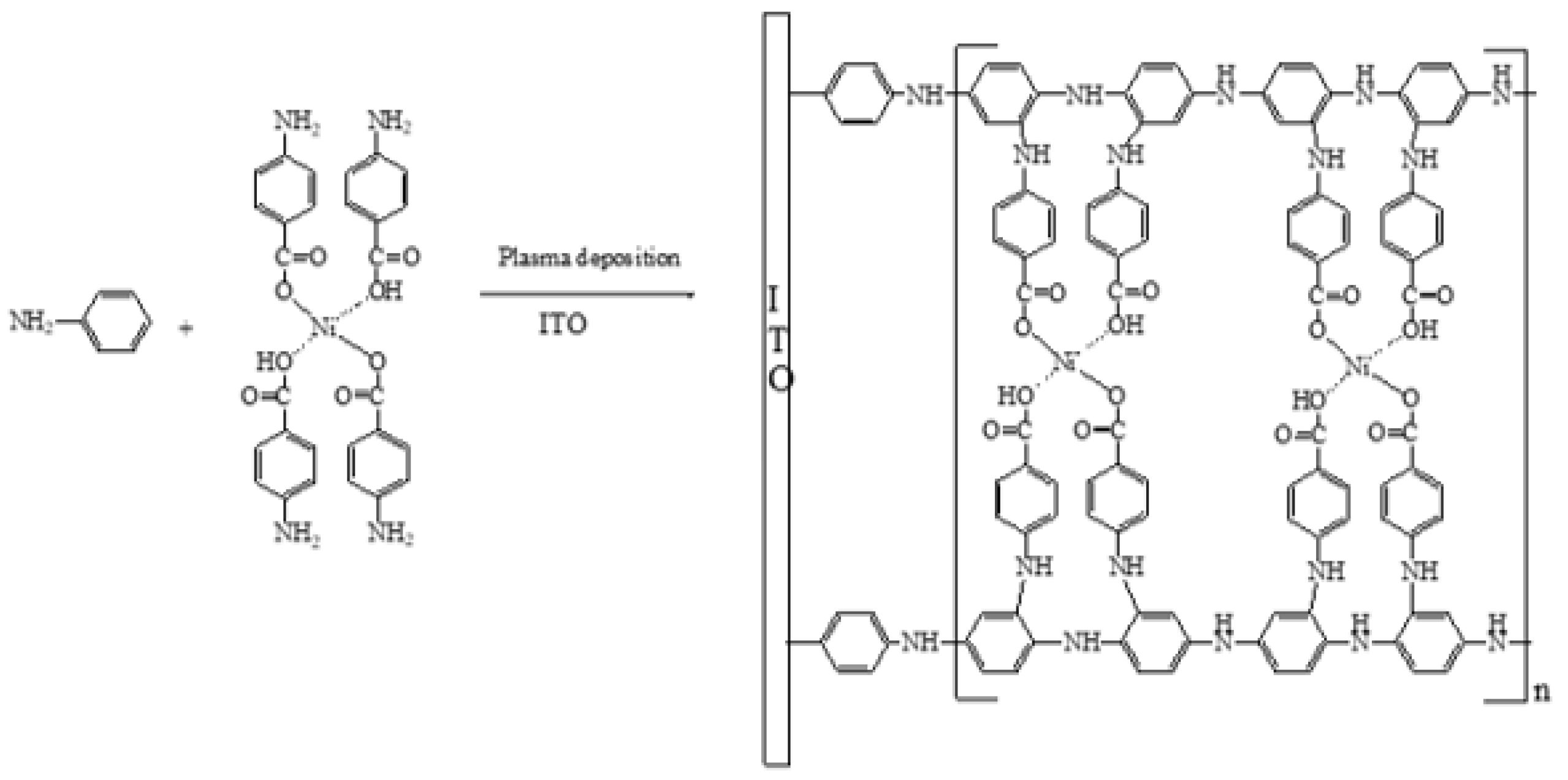
4.3. Preparation of Polyaniline Ni-Complex Catalytic Electrode
5. Conclusions
- (1)
- A polyaniline Ni-coordinated catalytic electrode can be fabricated easily and simply by one-pot plasma deposition without further treatment.
- (2)
- The catalytic activity and anodic peak potential of glucose were 11.5 mAM−1cm−2 and 0.54 V, respectively. Therefore, the polyaniline Ni-complex catalytic electrode shows higher catalytic activity for glucose oxidation.
- (3)
- By adding phosphate, the currents of anodic peak potential rose as phosphate ion concentration increased.
- (4)
- The stability of the polymeric Ni coordination electrode seems to be acceptable for practical applications.
- (5)
- The fabricated polyaniline Ni-complex catalytic electrode could be used as phosphate sensors.
Author Contributions
Funding
Conflicts of Interest
References
- Min, J.-K.; Yoo, H.-S.; Lee, E.-Y.; Lee, W.-J.; Lee, Y.-M. Simultaneous Quantitative Analysis of Sphingoid Base 1-Phosphates in Biological Samples by o-Phthalaldehyde Precolumn Derivatization after Dephosphorylation with Alkaline Phosphatase. Anal. Biochem. 2002, 303, 167–175. [Google Scholar] [CrossRef]
- Bian, X.; Li, X.; Qi, P.; Chi, Z.; Ye, R.; Lu, S.; Cai, Y. Quantitative design and analysis of marine environmental monitoring networks in coastal waters of China. Mar. Pollut. Bull. 2019, 143, 144–151. [Google Scholar] [CrossRef]
- Rouser, G.; Siakotos, A.N.; Fleischer, S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids 1966, 1, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Litke, D.W. Review of Phosphorus Control Measures in the United States and Their Effects on Water Quality. In Water-Investigations Report, USGS; US Department of the Interior: Washington, DC, USA, 1999; Volume 99. [Google Scholar]
- Poureslami, H.; Hoseinifar, R.; Khazaeli, P.; Hoseinifar, R.; Sharifi, H.; Poureslami, P. Changes in the Concentration of Ions in Saliva and Dental Plaque after Application of CPP-ACP with and without Fluoride among 6–9 Year Old Children. J. Dent. Biomater. 2017, 4, 361–366. [Google Scholar]
- Bansal, V.K. Chapter 198Serum Inorganic Phosphorus. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Khaleda, E.; Hassana, H.N.A.; Girgis, A.; Metelka, R. Construction of novel simple phosphate screen-printed and carbon paste ion-selective electrodes. Talanta 2008, 77, 737–743. [Google Scholar] [CrossRef]
- Kelani, K.M.; Badran, O.M.; Rezk, M.R.; Elghobashy, M.R.; Eid, S.M. Widening the applications of the Just-Dip-It approach: A solid contact screen-printed ion-selective electrode for the real-time assessment of pharmaceutical dissolution testing in comparison to off-line HPLC analysis. RSC Adv. 2021, 11, 31855–31864. [Google Scholar] [CrossRef]
- Altura, B.M.; Altura, B.T. Role of Magnesium and Calcium in Alcohol-Induced Hypertension and Strokes as Probed by In Vivo Television Microscopy, Digital Image Microscopy, Optical Spectroscopy, 31P-NMR, Spectroscopy and a Unique Magnesium Ion-Selective Electrode. Alcohol. Clin. Exp. Res. 1994, 18, 1057–1068. [Google Scholar] [CrossRef]
- Law, A.T.; Adeloju, S.B. Progress and recent advances in phosphate sensors: A review. Talanta 2013, 114, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Zeitoun, R.; Biswas, A. Potentiometric Determination of Phosphate Using Cobalt: A Review. J. Electrochem. Soc. 2020, 167, 127507. [Google Scholar] [CrossRef]
- Nie, H.; Yao, Z.; Zhou, X.; Yang, Z.; Huang, S. Nonenzymatic electrochemical detection of glucose using well-distributed nickel nanoparticles on straight multi-walled carbon nanotubes. Biosens. Bioelectron. 2011, 30, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Jin, J.; Yang, G.; Lu, T.; Zhang, H.; Cai, C. Nonenzymatic Electrochemical Detection of Glucose Based on Palladium−Single-Walled Carbon Nanotube Hybrid Nanostructures. Anal. Chem. 2009, 81, 7271–7280. [Google Scholar] [CrossRef]
- Benjamin, M.; Manoj, D.; Thenmozhi, K.; Bhagat, P.R.; Saravanakumar, D.; Senthilkumar, S. A bioinspired ionic liquid tagged cobalt-salophen complex for nonenzymatic detection of glucose. Biosens. Bioelectron. 2017, 91, 380–387. [Google Scholar] [CrossRef]
- Yhobu, Z.; Brinda, K.N.; Achar, G.; Małecki, J.G.; Keri, R.S.; Nagaraju, D.H.; Budagumpi, S. Glucose electrocatalysts derived from mono- or dicarbene coordinated nickel(II) complexes and their mesoporous carbon composites. Appl. Organomet. Chem. 2021, 35, e6446. [Google Scholar] [CrossRef]
- Jeon, J.S.; Yu, I.K.; Kim, W.; Choi, S.-H. Electrocatalytic Oxidation of Methanol by a Polymeric Ni Complex-modified Electrode Prepared by a One-step Cold-Plasma Process. Front. Chem. 2020, 8, 595616. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Fotouhi, L.; Ehsani, A. Recent Progress in the Development of Conducting Polymer-Based Nanocomposites for Electrochemical Biosensors Applications: A Mini-Review. Chem. Rec. 2018, 18, 599–618. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Abdelshakour, A.; Choi, J.-H.; Choi, J.-W. Application of Conducting Polymer Nanostructures to Electrochemical Biosensors. Molecules 2020, 25, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, T.K.; Prusty, S. Review on Conducting Polymers and Their Applications. Polym. Plast. Technol. Eng. 2012, 51, 1487–1500. [Google Scholar] [CrossRef]
- Stenger-Smith, J.D. Intrinsically electrically conducting polymers. Synthesis, characterization, and their applications. Prog. Polym. Sci. 1998, 23, 57–79. [Google Scholar] [CrossRef]
- Epstein, A.J. Electrically Conducting Polymers: Science and Technology. MRS Bull. 1997, 22, 16–23. [Google Scholar] [CrossRef]
- Svirskis, D.; Travas-Sejdic, J.; Rodgers, A.; Garg, S. Electrochemically controlled drug delivery based on intrinsically conducting polymers. J. Control. Release 2010, 146, 6–15. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, V.; Arias-Pardilla, J. Biomimetic electrochemistry from conducting polymers. A review Artificial muscles, smart membranes, smart drug delivery and computer/neuron interfaces. Electrochim. Acta 2012, 84, 112–128. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.; Langer, R.; Ingverg, D.E. Electrically conducting polymers can noninvasively control the shape and growth of mammalian cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3201–3204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeloju, S.B.; Wallace, G.G. Conducting Polymers and the Bioanalytical Sciences: New Tools for Biomolecular Communications-A Review. Analyst 1996, I21, 699–703. [Google Scholar] [CrossRef]
- Park, Y.; Jung, J.; Chang, M. Research Progress on Conducting Polymer-Based Biomedical Applications. Appl. Sci. 2019, 9, 1070. [Google Scholar] [CrossRef] [Green Version]
- Hackett, A.J.; Malmström, J.; Travas-Sejdic, J. Functionalization of conducting polymers for biointerface applications. Prog. Polym. Sci. 2017, 70, 18–33. [Google Scholar] [CrossRef]
- Bhattacharyaa, A.; Misra, B.N. Grafting: A versatile means to modify polymers Techniques, factors and applications. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Czerwiec, T.; Renevier, N.; Michel, H. Low-temperature plasma-assisted nitriding. Surf. Coat. Technol. 2000, 131, 267–277. [Google Scholar] [CrossRef]
- Foest, R.; Schmidt, M.; Becker, K. Microplasmas, an emerging field of low-temperature plasma science and technology. Int. J. Mass Spectrom. 2006, 248, 87–102. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, C.; Nie, C.; Li, X.; Yan, Y. A high efficiency low-temperature microwave-driven atmospheric pressure plasma jet. Appl. Phys. Lett. 2019, 114, 254106. [Google Scholar] [CrossRef]
- Zeghioud, H.; Assadi, A.A.; Khellaf, N.; Djelal, H.; Amrane, A.; Rtimi, S. Photocatalytic Performance of CuxO/TiO2 Deposited by HiPIMS on Polyester under Visible Light LEDs:Oxidants, Ions Effect, and Reactive Oxygen Species Investigation. Materials 2019, 12, 412. [Google Scholar] [CrossRef] [Green Version]
- Bui, Q.T.; Yu, I.-K.; Gopalan, A.I.; Gopalan, S.A.; Gopalan, S.; Kim, W.; Choi, S.-H. Facile Fabrication of Metal Oxide Catalytic Electrodes by AC Plasma Deposition and Electrochemical Detection of Hydrogen Peroxide. Catalysts 2019, 9, 888. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.D.; D’Arcy, J.M.; Wang, Y.; Beltramo, P.J.; Strong, V.A.; Kaner, R.B. The oxidation of aniline to produce ‘‘polyaniline’’: A process yielding many different nanoscale structures. J. Mater. Chem. 2011, 21, 3534–3550. [Google Scholar] [CrossRef]
- Lapkowski, M.; Berrada, K.; Quillard, S.; Louarn, G.; Lefrant, S.; Pron, A. Electrochemical Oxidation of Polyaniline in Nonaqueous Electrolytes: “In Situ” Raman Spectroscopic Studies. Macromolecules 1995, 28, 1233–1238. [Google Scholar] [CrossRef]
- Zeghioud, H.; Lamouri, S.; Safidine, Z.; Belbachir, M. Chemical synthesis and characterization of highly soluble conducting polyaniline in mixtures of common solvents. J. Serb. Chem. Soc. 2015, 80, 917–931. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, Z.; Yu, X.; Meng, Y. Preparation of Proton Exchange Membranes by a Plasma Polymerization Method and Application in Direct Methanol Fuel Cells (DMFCs). Plasma Processes Polym. 2010, 7, 382–389. [Google Scholar] [CrossRef]
- Choukourov, A.; Biederman, H.; Slavinska, D.; Hanley, L.; Grinevich, A.; Boldyryeva, H.; Mackova, A. Mechanistic Studies of Plasma Polymerization of Allylamine. J. Phys. Chem. B 2005, 109, 23086–23095. [Google Scholar] [CrossRef] [PubMed]
- Cech, V.; Studynka, J.; Janos, F.; Perina, V. Influence of Oxygen on the ChemicalStructure of Plasma Polymer Films Deposited from a Mixture of Tetravinylsilane and Oxygen Gas. Plasma Processes Polym. 2007, 4, S776–S780. [Google Scholar] [CrossRef]




| Aniline (mmol) | 3-Aminobenzocic Acid (mmol) | Nickel Chloride (mmol) | Water (mL) | MeOH (mL) | |
|---|---|---|---|---|---|
| No. 1 | - | - | - | - | - |
| No. 2 | 54.8 | - | - | - | - |
| No. 3 | 54.8 | 0.2 | - | 0.5 | 0.08 |
| No. 4 | 54.8 | 0.2 | 0.2 | 0.5 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-W.; Yoo, J.-N.; Yu, I.-K.; Choi, S.-H. Fabrication of Polyaniline Ni-Complex Catalytic Electrode by Plasma Deposition for Electrochemical Detection of Phosphate through Glucose Redox Reaction as Mediator. Catalysts 2022, 12, 128. https://doi.org/10.3390/catal12020128
Lee H-W, Yoo J-N, Yu I-K, Choi S-H. Fabrication of Polyaniline Ni-Complex Catalytic Electrode by Plasma Deposition for Electrochemical Detection of Phosphate through Glucose Redox Reaction as Mediator. Catalysts. 2022; 12(2):128. https://doi.org/10.3390/catal12020128
Chicago/Turabian StyleLee, Hyun-Woong, Jae-Ni Yoo, In-Keun Yu, and Seong-Ho Choi. 2022. "Fabrication of Polyaniline Ni-Complex Catalytic Electrode by Plasma Deposition for Electrochemical Detection of Phosphate through Glucose Redox Reaction as Mediator" Catalysts 12, no. 2: 128. https://doi.org/10.3390/catal12020128
APA StyleLee, H.-W., Yoo, J.-N., Yu, I.-K., & Choi, S.-H. (2022). Fabrication of Polyaniline Ni-Complex Catalytic Electrode by Plasma Deposition for Electrochemical Detection of Phosphate through Glucose Redox Reaction as Mediator. Catalysts, 12(2), 128. https://doi.org/10.3390/catal12020128






