Abstract
Visible-responsive photocatalysts for environmental purification and fuel generation are, currently, highly sought after. Among the possible candidates, Bi2WO6 (BWO) has been considered due to its efficient light harvesting, stability, and promising activities. Here, hierarchical BWO microballs have been prepared using a hydrothermal method, and additionally modified with deposits of noble metals (gold, silver, copper, palladium and platinum) by the photodeposition method. The structure, morphology, photoabsorption properties, and surface composition of bare and metal-modified BWO samples were investigated by XRD, SEM, DRS and XPS analyses. The photocatalytic activity was evaluated by the oxidative degradation of model dye (methyl orange (MO)) under UV/vis, and hydrogen generation under vis and/or UV irradiation. It was found that hierarchical morphology is detrimental for high photocatalytic activity in both tested systems, resulting in the improved degradation of MO (ca. 65% during 90 min of UV/vis irradiation), and hydrogen evolution (0.1 and 0.4 μmol h−1 under vis and UV/vis irradiation, respectively). Moreover, the type of noble metal and its properties influence the overall photocatalytic performance. It was found that, under UV/vis irradiation, only platinum accelerates hydrogen evolution, whereas under vis irradiation the activity follows the order: BWO < BWO/Cu < BWO/Ag < BWO/Pt < BWO/Pd < BWO/Au. It was concluded that zero-valent metal is recommended for high vis response, probably due to plasmonic photocatalysis, efficient light harvesting ability, and co-catalytic role.
1. Introduction
Global energy and environmental crises are considered the most urgent problems to be solved. Among various solutions, hydrogen has been proposed as an alternative fuel due to its high specific energy (three times the heating value of petroleum) and environmentally friendly nature, i.e., its combustion results in the formation of only a clean product—water (“maker of water” in Greek) [1]. However, the most significant is the clean production of hydrogen itself, and thus, usage of fossil fuels should be avoided. Accordingly, green processes, such as photocatalysis, have been considered for hydrogen generation [2,3,4,5]. For example, semiconductor photocatalysis might solve both energy and environmental problems (including ones related to drinking water), particularly when performed under natural solar radiation [6,7,8]. The photocatalysts could split water into hydrogen and oxygen, as well as decompose all pollutants (microorganisms and organic and inorganic compounds), and thus heterogeneous photocatalysis has attracted a lot of attention in recent decades [9,10,11,12]. Among the various semiconductors, titanium(IV) oxide (TiO2, titania, titanium dioxide) has been the most explored due to its low cost, high activity, low toxicity, abundance, and pollution-free nature [13,14,15]. However, titania also has some shortcomings. First, titania is only responsive to the UV part of the solar spectrum due to its large bandgap, and thus, only a minor part of solar energy can be efficiently used (3–4%). Second, the recombination of charge carriers results in a lower than 100% quantum efficiency of photocatalytic reactions. Third, the most active titania photocatalysts are usually in the form of fine particles, which raises some problems with photocatalyst reuse and circulation (high costs of ultrafiltration). In contrast, the application of immobilized photocatalysts is connected with a low specific surface area, and thus a decrease in photocatalytic efficiency [16,17,18]. Therefore, the synthesis of highly efficient materials for the broad range of solar radiation, and easy recyclability, is a hot topic of present studies. For example, wide-bandgap semiconductors were either surface modified [19,20,21] or doped [22,23,24] with various elements, as well as coupled with other semiconductors of narrower bandgaps [25,26,27,28]. Moreover, new chemical compounds have been synthesized and proposed as efficient photocatalysts, such as oxynitrides [29], graphitic carbon nitride [30], germanium nitride [31], and graphene derivatives [32]. However, a narrower bandgap allows for fast charge carriers’ recombination. Therefore, these new materials have also been further modified/coupled (e.g., MoS2-C-g-C3N4 [33]) to obtain an efficient transfer of charge carriers (via type II heterojunction or/and Z-scheme [5,34]), and thus, high yields of photocatalytic reactions.
For example, as a material with a narrower bandgap than that of titania, bismuth-based compounds have attracted much attention [35]. It has been shown that bismuth tungstate (Bi2WO6, BWO) with a layered structure of WO6 and [Bi2O2]2+ (perovskite-like plates) has exhibited good photocatalytic efficiency under vis irradiation [36,37,38,39]. However, the photogenerated charge carriers recombine easily, and thus result in a low photocatalytic efficiency. Accordingly, many methods have been used for BWO modification, such as morphology optimization [37,38,40,41], elemental doping [42,43], surface modification (e.g., with noble metals [44,45,46,47]), and the formation of heterojunctions, e.g., Bi2WO6/Zn-Al [48], Bi2Fe4O9/Bi2WO6 [49], MoS2/Bi2WO6 [50].
It is known that noble metals can effectively improve the photocatalytic performance of photocatalysts, being an electron pool, and thus hinder the recombination of charge carriers. Various semiconductors with deposited noble metals have already been prepared, such as titania [51,52,53,54] and other photocatalysts, e.g., Bi2MoO6 nanoplates (Ag) [55], BiOCl nanospheres (Ag) [56], carbon nitride nanorods (Au) [57], g-C3N4 (Cu) [58], and BiOBr (Pd) [59]. It should be mentioned that though modification with noble metals (to improve UV-photocatalytic activity) has been known for more than 40 years, novel research has been started recently on plasmonic photocatalysis, in which noble metals play another function, i.e., the activation of wide-bandgap semiconductors towards a vis response due to plasmonic properties [60,61,62].
Although both pristine and modified BWO samples have been investigated for various photocatalysts reactions, there are no reports showing a direct comparison between BWO photocatalysts modified with different noble metals. In this study, the influence of five metals (Au, Ag, Cu, Pd and Pt) on the properties, and thus overall activity, of BWO (in the form of hierarchical microballs for cheap recycling) is investigated for the first time. The photocatalytic activity has been tested for hydrogen generation and the decomposition of methyl orange (MO; common and toxic dye [63]) since both “green” fuel production and environmental purification (especially the degradation of organic compounds) are the most urgent problems facing humanity.
2. Results and Discussion
2.1. Characterization of Samples
The successful preparation of BWO by a hydrothermal method has been confirmed by XRD analysis, as shown in Figure 1. All pristine samples, prepared at different temperatures, show almost identical diffraction patterns with clear peaks at ca. 28.3, 32.8, 47.2, 55.8, 58.5, 68.7, 76.1 and 78.5 degrees, indicating (131), (002), (202), (133), (262), (004), (333) and (460) crystal planes of russellite Bi2WO6, respectively. An increase in hydrothermal temperature results in peak sharpening, suggesting that larger crystallites have been formed. Indeed, Scherrer analysis confirms that the crystallites prepared at 140 °C reach 13.6 nm, whereas higher temperatures (180 °C and 160 °C) cause the formation of larger crystallites (14.8 nm and 16.2 nm, respectively).

Figure 1.
XRD patterns of BWO samples prepared at different temperatures.
The microscopic observations revealed that a hydrothermal reaction results in the formation of microballs composed of nanosheet subunits, as shown in Figure 2. The best morphology was obtained at the lowest temperature of reaction (140 °C), as the S1 sample exhibits a regular spherical flower-like structure with a relatively uniform size distribution, and the microballs are composed of flat sheets (Figure 2a,b). A worsening of the morphology was noticed after increasing the temperature of the hydrothermal reaction. Accordingly, though microballs in the S2 sample are still mainly spherical (Figure 2c,d), the substructured sheets are irregular and uneven in size. Finally, the worst morphology was achieved for the S3 sample (Figure 2e,f), for which the spherical morphology is no longer obvious, and the structure of built-in flakes is likewise incomplete. Based on the presented data, it has been found that the spherical structure of BWO was destroyed gradually, and substructure sheets were fragmented by increasing the hydrothermal reaction temperature, indicating that temperature is crucial for the morphology of BWO samples. Accordingly, the S1 sample with the best morphology (and highest photocatalytic efficiency, as discussed further) was selected for the modification with noble metals.

Figure 2.
BWO samples obtained at different temperatures: (a,b) S1 (140 °C), (c,d) S2 (160 °C) and (e,f) S3 (180 °C).
The XPS was used for the surface characterization of both pristine and modified samples. All samples are composed of Bi2WO6 (Figure 3). Although slight shifts in the binding energy were observed with an increase in the temperature of the hydrothermal reaction, it seems to be irrelevant for the chemical composition, as previously reported [64]. Taking the S1 sample as an example, the XPS spectra of Bi 4f and W 4f show the binding energies of 159.0 eV (Bi 4f7/2), 164.31 eV (Bi 4f5/2), 37.33 eV (W 4f5/2) and 35.2 eV (W 4f7/2), indicating that bismuth and tungsten exist in tri- [65] and six-valent [66] oxidation states, respectively. In the case of the oxygen (O 1s) spectrum, two peaks at 530.05 eV and 531.53 eV could be seen, relating to W-O and Bi-O, respectively. Thus, the chemical formula of Bi2WO6 was confirmed [67].


Figure 3.
XPS results for: (a) Bi 4f, (b) O 1s and (c) W 4f.
In the case of modified S1 samples, XPS data have confirmed that noble metals were efficiently loaded onto the BWO surface, as shown in Figure 4 and Table 1. It has been found that silver exists in two oxidation forms, i.e., Ag+ and Ag(0), with binding energies of 367.51 eV and 368.3 eV, respectively, with the oxidized form being predominant (ca. 90%). In contrast, three forms of gold, i.e., Au(δ+), Au(0) and Au(δ−), were observed with binding energies of 82.89 eV, 83.6 eV and 83.94 eV, respectively. Here, the zero-valent form of gold is the most predominant, reaching 92%. In the case of copper, only its oxidized forms could be detected, i.e., Cu+ (932.16 eV [68]; 58%) and Cu2+ (932.28 eV [68]; 42%), confirming the instability of zero-valent copper under ambient conditions [69,70,71]. As expected, palladium and platinum exist mainly in the zero-valent form, reaching 80% and 79%, respectively, making up the minority of the oxidized forms of metals (probably PdO [72] and Pt(δ+) [68]). Similar data have been commonly found for semiconductors modified with noble metals, where less noble metals (silver and copper) exist, predominantly in the oxidized forms, whereas precious metals (gold, platinum and palladium) are present as zero-valent deposits [69,70,71,73,74,75]. It must be underlined that, during photodeposition (anaerobic environment), though metal cations are reduced efficiently, forming zero-valent NPs (as confirmed by the color change, e.g., violet for Cu/S1 due to the plasmonic properties), the direct contact of the sample with air (after photodeposition) results in the partial oxidation (surface) of less noble metals.

Figure 4.
XPS results for S1 sample modified with noble metals: (a) Ag 3d5/2, (b) Au 4f7/2, (c) Cu 2p3/2, (d) Pd 3d5 and (e) Pt 4f7/2.

Table 1.
Surface composition of pristine and modified S1 samples.
The photoabsorption properties of bare and modified BWO materials are presented in Figure 5. It has been confirmed that BWO can absorb visible light up to ca. 450 nm, which corresponds to bandgap of ca. 2.75 eV (evidently narrower than that in titania). Moreover, as expected (considering the same crystalline form and similar crystallite sizes), all bare samples (S1–S3) show almost identical photoabsorption properties (Figure 5a). Furthermore, a significant increase in vis absorption was observed after BWO modification with noble metals. The characteristic plasmonic peaks (localized surface plasmon resonance; LSPR) were observed for Au/S1 and Ag/S1 at ca. 545 nm and ca. 490 nm, respectively, suggesting the formation of fine gold NPs (10–20 nm) and larger silver deposits (ca. 80 nm [76]). It should be mentioned that the LSPR wavelength is influenced by both the properties (size/shape/surroundings) and the type of the metal, and thus smaller and spherical NPs possess LSPR at shorter wavelengths [77]. For example, spherical NPs of 10–20 nm show LSPR at ca. 200 nm [78], 395–405 nm [79], 420–430 nm [80], 530–540 nm [81] and 560–570 nm [82] when composed of palladium, platinum, silver, gold, and copper, respectively. The formation of a Ag2O/Bi2WO6 heterojunction should also be considered as a similar photoabsorption feature has been observed (the peak at ca. 500 nm) for other semiconductors (e.g., titania) modified with silver(I) oxide (even though a very different photoabsorption feature of sole Ag2O is obtained due to its dark brown color) [26]. In the case of samples containing palladium and platinum, it is difficult to observe a clear LSPR peak (expected at UV range), and thus the efficient light absorption in the whole vis region could be caused by either the presence of large particles or scattering of the light on the metal deposits. In contrast, though a clear peak at near IR was observed for Cu/S1 sample, its origin is due to CuO rather than zero-valent copper (the lowest light absorption at main LSPR wavelength of 560 nm for spherical Cu NPs), thus it has been confirmed (similar to XPS data) that copper exists mainly in the oxidized form [83,84,85,86].
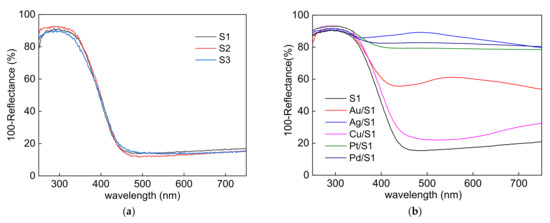
Figure 5.
DRS spectra of: (a) bare BWO samples and (b) bare and modified S1 samples.
2.2. Photocatalytic Activity
The photocatalytic activities of obtained photocatalysts were evaluated in two reaction systems: (1) the decomposition of MO (aerobic environment) and (2) generation of hydrogen (anaerobic environment) under vis and/or UV irradiation.
First, the photocatalytic activity of bare BWO samples was examined in comparison to two reference samples, pure commercial WO3 (purchased by Aladdin (Vienna, VA, USA)) and Bi2WO6 (homemade), and the obtained data for MO degradation are presented in Figure 6. It is obvious that both reference samples are barely active, which is reasonable considering that oxides with narrower bandgap than that in titania suffer from charge carriers’ recombination due to negligible activity for the one-electron reduction of oxygen. Conduction band (CB) position must be more negative than −0.284/−0.046 V vs. NHE (O2 + e− = O2− (aq.)/O2 + H+ + e− = HO2 (aq.)) for the consumption of photogenerated electrons and degradation of organic matter by photogenerated holes [87,88]. In contrast, hierarchical microballs exhibit superior photocatalytic activity, which correlates with their morphology; i.e., the most active sample (S1) has the best morphology. It should be emphasized that all samples possess nearly identical properties, such as the crystallite size, crystallinity, composition and photoabsorption properties, except for morphology, and thus the difference in the activity should be caused by the morphology. Accordingly, it could be concluded that “flake balls” are responsible for the oxidative decomposition of organic compounds with the possibility of a multi-electron transfer mode, as suggested by Hori et al. [89].
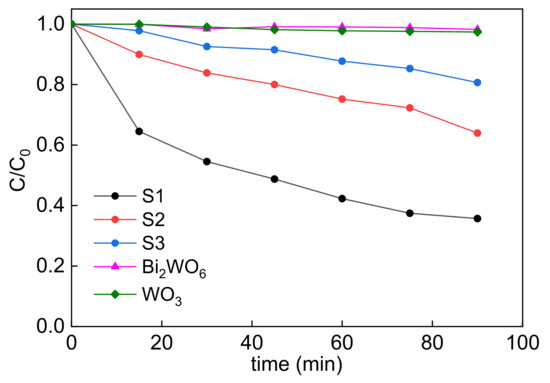
Figure 6.
Photocatalytic activity of MO discoloration under UV/vis irradiation for synthesized (S1, S2, S3) and reference (commercial WO3 and homemade BWO) samples.
Similarly, S1 sample shows to be the most active for hydrogen evolution, as shown in Figure 7a. However, the reaction rates for S1 and S2 photocatalysts are very similar, reaching 0.38 μmol/h and 0.33 μmol/h, respectively. In contrast, a twice-as-slow rate has been obtained for S3 sample with partly destroyed sheets’ structure. Therefore, it is obvious that the morphology is detrimental to achieving a high photocatalytic activity. However, it seems that a perfect sheet structure is more important for hydrogen generation, whereas microball morphology is detrimental for the decomposition of organic compounds.

Figure 7.
Photocatalytic hydrogen generation on: (a) BWO microballs (S1, S2 and S3) under UV/vis irradiation, and (b) bare and modified S1 samples under UV/vis (black) and vis (red) irradiation.
Since the photocatalytic activity data for pristine samples have indicated that S1 is the most active, this sample was selected for the modification with noble metals, and the obtained results are presented in Figure 7b. Interestingly, only modification with platinum resulted in a significant generation of hydrogen under UV/vis irradiation. It must be emphasized that this differs greatly from the titania case, for which modification with any noble metal increases the photocatalytic activity for hydrogen evolution [70,81,90,91,92,93,94]. It has been proposed that molecular hydrogen is directly formed on the deposits of noble metals [95,96]. It should be mentioned that the low activity of BWO for hydrogen evolution has already been suggested (even after modification with platinum) [97], likely resulting from insufficient redox properties (too positive a conduction band). However, there are also some reports showing the photocatalytic activity of BWO for hydrogen generation [98]. Here, hierarchical microballs modified with platinum have exhibited a noticeable generation of hydrogen. Accordingly, it is proposed that platinum might work as an electron pool and a catalyst (dark) for the formation of molecular hydrogen.
Interestingly, the activity under sole vis irradiation is quite different, where gold-modified sample shows the highest activity. Additionally, samples with platinum and palladium are much more active than pristine BWO sample. Therefore, it might be concluded that zero-valent metals are responsible for efficient hydrogen evolution. Two reasons could be considered, i.e., (i) plasmonic photocatalysis or (ii) catalytic “dark” reaction on the metallic deposits. Considering the catalytic “dark” properties of metals (volcano plot), the activity should be in the following order: platinum > palladium > gold ≥ copper > silver [99]. Here, Au/S1 is the most active, with a broad plasmonic peak (500–650 nm), and thus it is expected that the efficient light absorption by plasmonic metal might be the main reason for enhanced activity under vis irradiation. There are two mechanisms of plasmonic photocatalysis, i.e., an energy transfer, and an electron transfer [60,100,101,102]. It seems that both could be involved as even a slight overlapping of photoabsorption bands (LSPR and semiconductor) could result in the energy transfer. Moreover, the double function of noble metals must also be considered: (i) plasmonic sensitizer, and (ii) catalytic center for hydrogen generation. Obviously, zero-valent metals are crucial for both activities. The slight increase in activity by silver modification might also result from the presence of zero-valent silver (ca. 10%). Of course, the formation of heterojunctions between two oxides, e.g., Ag2O/BWO, Cu2O/BWO and CuO/BWO, could also be inspected. However, the inactivity of the copper-modified sample suggests that these types of heterojunctions are not working for hydrogen evolution (again, in contrast to the case of titania [26,27,103,104]). Therefore, it could be concluded that zero-valent noble metals are necessary for the efficient enhancement of photocatalytic efficiency of BWO under vis light.
3. Materials and Methods
3.1. Preparation of BWO
Na2WO4·2H2O, Bi(NO3)3·5H2O, and cetyltrimethylammonium bromide (CTAB) were purchased from Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China) (AR, 99%), and used without further treatment. BWO was synthesized by hydrothermal reaction. In brief, 2 mmol of Na2WO4·2H2O and 4 mmol of Bi(NO3)3·5H2O were placed in a 100 mL beaker, to which 60 mL deionized water was added, and then the content was stirred magnetically for half an hour. Afterwards, 0.05 g of CTAB was added, the content was stirred for 15 min, and the white suspension (the pH value of 1.44) was poured to a 100 mL Teflon tube. The tube was placed inside the oven for a 24 h hydrothermal reaction, performed at three different temperatures. The final product was collected, washed with deionized water, centrifuged, and freeze dried. The obtained yellowish powders were marked as S1, S2 and S3 for BWO samples prepared at 140 °C, 160 °C and 180 °C, respectively.
3.2. Modification of BWO with Noble Metals
H2PtCl6·6H2O, HAuCl4·3H2O, AgNO3, CuSO4·5H2O, and K2PdCl6 were purchased from Aladdin company (Vienna, VA, USA) (AR, 99%). For the modification, S1 sample (prepared at 140 °C) and aqueous solution (40 g/L) of respective salts, i.e., H2PtCl6·6H2O (Aladdin ((Vienna, VA, USA)), HAuCl4·3H2O (Aladdin (Vienna, VA, USA)), AgNO3 (Aladdin (Vienna, VA, USA)), CuSO4·5H2O (Aladdin (Vienna, VA, USA)) and K2PdCl6 (Aladdin (Vienna, VA, USA)), were used, and the obtained samples were named accordingly, i.e., Pt/S1, Au/S1, Ag/S1, Cu/S1 and Pd/S1. In brief, 500 mg of S1 sample was added to a glass tube containing 25 mL of water/methanol (1:1) solution, and then the aqueous solution of noble metal (2 wt% of noble metal in respect to BWO) was added gradually by a micropipette under continuous stirring. Afterwards, the glass tube was covered with a latex stopper, and argon gas was purged through a thin plastic tube placed at the bottom of the tube for 15 min to remove oxygen (working as an electron scavenger). Then, the tube was sealed and wrapped with tape. The tube was irradiated with a xenon lamp (300 W, 100 mW cm−2, 10 cm distance from the lamp) and the content was continuously stirred during 1 h irradiation. Finally, the obtained powder was collected after finishing operations, i.e., centrifugation, washing (2 times with ethanol and 2 times with deionized water), freeze drying and grinding.
3.3. Sample Characterization
The crystalline properties of samples were examined by X-ray diffraction (XRD; PANalytical Empyrean X-ray diffractometer). The morphology was observed with scanning electron microscopy (SEM, SU8010, Hitachi Limited Company, Tokyo, Japan) The surface properties (chemical composition and state of elements) were evaluated by X-ray photoelectron spectroscopy (XPS, PHI5000, ULVAC-PHI, Inc, Kanagawa, Japan) with C 1s (284.8 eV) as a reference for binding energy. The photoabsorption features were estimated by UV/vis diffuse reflectance spectroscopy (DRS, UV3600, Shimadzu Corporation, Kyoto, Japan).
3.4. Photocatalytic Activity
The photocatalytic performance was evaluated for two model reactions: oxidation (photodegradation of methyl orange) and reduction (generation of hydrogen). For the oxidative decomposition of methyl orange, a 300 W xenon lamp (100 mW cm−2) was settled in 15 cm distance from the glass reactor. First, 150 mg of photocatalyst and 150 mL of MO (20 mg/L) were placed into the glass reactor, stirred for half an hour in a dark box to reach the equilibrium of adsorption/desorption, and then irradiated. Every 15 min the portion of suspension (ca. 6 mL) was withdrawn from the reactor, centrifuged for 5 min (5000 rpm), and then the absorbance of the supernatant was analyzed by a UV/vis spectrophotometer (UV-1800PC).
For hydrogen generation, a closed glass reaction system—an automatic on-line trace gas analysis system (Labsolar-6A, Beijing Perfectlight Technology Co., Ltd., Beijing, China) was used. First, the photocatalyst (50 mg) was added to 100 mL aqueous solution with triethanolamine (TEOA, 10 vol%, a sacrificial electron donor). The reactor was evacuated with a vacuum pump and irradiated with a xenon lamp (300 W, 100 mW cm−2). For vis activity measurements, a cut-off filter (λ > 420 nm) was installed on the front of the lamp. The amount of generated hydrogen was determined by on-line gas chromatography system (GC7900II).
4. Conclusions
BWO hierarchical microballs can be easily prepared by a simple hydrothermal method and then modified with nanoparticles of different noble metals. It has been found that a uniform morphology of microballs, composed of similar sheets, is necessary for the efficient degradation of methyl orange under UV/vis, which results in 65% decomposition over 90 min of illumination. In contrast, the flat sheet surface seems detrimental to hydrogen generation, as two different samples (S1 and S2) have caused similar effects, generating ca. 0.4 μmol h−1 of hydrogen under UV/vis irradiation. The type of noble metal and its properties are crucial for the photocatalytic performance. Under UV/vis irradiation, only platinum enhances the evolution of hydrogen (2.77 μmol h−1), probably due to the best catalytic (dark) property. In contrast, under vis irradiation, gold enhances the activity of BWO the most, reaching ca. 0.25 μmol h−1 of hydrogen generation. Based on the XPS analysis, it has been found that zero-valent metals are the most highly recommended for activity enhancement under vis light, probably due to plasmonic photocatalysis, efficient light harvesting, and the catalytic effect.
Although the obtained data indicated that BWO hierarchical balls are prospective for both hydrogen generation and decomposition of organic compounds, further experiments are necessary to confirm the complete degradation (mineralization) of pollutants, e.g., with the use of a total organic carbon (TOC) analyzer. Moreover, the application of another test compound (colorless) for activity testing to avoid the possibility of photocatalyst sensitization [54,105,106,107] is under study for the detailed discussion on the reaction mechanisms.
Author Contributions
Conceptualization, Z.W.; methodology, Z.W.; investigation, Z.L. and J.Z.; resources, Z.W.; writing—original draft preparation, Z.W.; writing—review and editing, E.K.; visualization, K.W.; supervision, Y.C., Z.W., and E.K.; funding acquisition, Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (NSFC) (51802087), the Natural Science Foundation of the Hubei province of China (2019CFB524).
Data Availability Statement
The data presented in this study are available on request from corresponding author (Z.W.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Esswein, A.J.; Nocera, D.G. Hydrogen production by molecular photocatalysis. Chem. Rev. 2007, 107, 4022–4047. [Google Scholar] [CrossRef]
- Domen, K.; Naito, S.; Soma, M.; Onishi, T.; Tamaru, K. Photocatalytic decomposition of water vapour on an NiO-SrTiO3 catalyst. Chem. Commun. 1980, 12, 543–544. [Google Scholar] [CrossRef]
- Kudo, A. Recent progress in the development of visible light-driven powdered photocatalysts for water splitting. Int. J. Hydrog. Energ. 2007, 32, 2673–2678. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Wei, Z.; Mogan, T.R.; Wang, K.; Janczarek, M.; Kowalska, E. Morphology-governed performance of multi-dimensional photocatalysts for hydrogen generation. Energies 2021, 14, 7223. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Bahnemann, D.W.; Kholuiskaya, S.N.; Dillert, R.; Kulak, A.I.; Kokorin, A.I. Photodestruction of dichloroacetic acid catalyzed by nano-sized TiO2 particles. Appl. Catal. B Environ. 2002, 36, 161–169. [Google Scholar] [CrossRef]
- Bahnemann, D.; Cunningham, J.; Fox, M.A.; Pelizzetti, E.; Pichat, P.; Serpone, N. Photocatalytic treatment of waters. In Aquatic and Surface Photochemistry; CRC Press: Boca Raton, FL, USA, 1994; pp. 261–316. [Google Scholar]
- Markowska-Szczupak, A.; Ulfig, K.; Morawski, W.A. The application of titanium dioxide for deactivation of bioparticulates: An overview. Catal. Today 2011, 161, 249–257. [Google Scholar] [CrossRef]
- Mitoraj, D.; Kisch, H. The nature of nitrogen-modified titanium dioxide photocatalysts active in visible light. Angew. Chem. Int. Ed. 2008, 47, 9975–9978. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ming, H.; Zhang, H.; Li, H.; Pan, K.; Liu, Y.; Wang, F.; Gong, J.; Kang, Z. Au/ZnO nanocomposite: Facile fabrication and enhanced photocatalytic activity for degradation of benzene. Mat. Chem. Phys. 2012, 137, 113–117. [Google Scholar] [CrossRef]
- Abe, R.; Shinmei, K.; Koumura, N.; Hara, K.; Ohtani, B. Visible-light-induced water splitting based on two-step photoexcitation between dye-sensitized layered niobate and tungsten oxide photocatalysts in the presence of a triiodide/iodide shuttle redox mediator. J. Am. Chem. Soc. 2013, 135, 16872–16884. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Caceres, J.; Fernandez-Alba, A.R.; Aguera, A.; Rodriguez, A. Photocatalytic treatment of water-soluble pesticides by photo-Fenton and TiO2 using solar energy. Catal. Today 2002, 76, 209–220. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photoch. Photobio. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photoch. Photobio. C 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Kowalska, E.; Rau, S. Photoreactors for wastewater treatment: A review. Recent Pat. Engin. 2010, 4, 242–266. [Google Scholar] [CrossRef]
- Bielan, Z.; Dudziak, S.; Kubiak, A.; Kowalska, E. Application of spinel and hexagonal ferrites in heterogeneous photocatalysis. Appl. Sci. 2021, 11, 10160. [Google Scholar] [CrossRef]
- Grzechulska, J.; Morawski, A.W. Photocatalytic labyrinth flow reactor with immobilized P25 TiO2 bed for removal of phenol from water. Appl. Catal. B Environ. 2003, 46, 415–419. [Google Scholar] [CrossRef]
- Zabek, P.; Eberl, J.; Kisch, H. On the origin of visible light activity in carbon-modified titania. Photochem. Photobiol. Sci. 2009, 8, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Dozzi, M.V.; Prati, L.; Canton, P.; Selli, E. Effects of gold nanoparticles deposition on the photocatalytic activity of titanium dioxide under visible light. Phys. Chem. Chem. Phys. 2009, 11, 7171–7180. [Google Scholar] [CrossRef]
- Janus, M.; Tryba, B.; Inagaki, M.; Morawski, A.W. New preparation of a carbon-TiO2 photocatalyst by carbonization of n-hexane deposited on TiO2. Appl. Catal. B Environ. 2004, 52, 61–67. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A review. Rec. Patent. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Ohno, T.; Mitsui, T.; Matsumura, M. Photocatalytic activity of S-doped TiO2 photocatalyst under visible light. Chem. Lett. 2003, 32, 364–365. [Google Scholar] [CrossRef]
- Hsu, M.-H.; Chang, C.-J.; Weng, H.-T. Efficient H2 production using Ag2S-coupled ZnO@ZnS core–shell nanorods decorated metal wire mesh as an immobilized hierarchical photocatalyst. ACS Sustain. Chem. Eng. 2016, 4, 1381–1391. [Google Scholar] [CrossRef]
- Endo-Kimura, M.; Janczarek, M.; Bielan, Z.; Zhang, D.; Wang, K.; Markowska-Szczupak, A.; Kowalska, E. Photocatalytic and antimicrobial properties of Ag2O/TiO2 heterojunction. ChemEngineering 2019, 3, 3. [Google Scholar] [CrossRef]
- Janczarek, M.; Endo, M.; Zhang, D.; Wang, K.; Kowalska, E. Enhanced photocatalytic and antimicrobial performance of cuprous oxide/titania: The effect of titania matrix. Materials 2018, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, L.; Dong, E.; Zhang, X.; Zhang, W.; Wang, Q.; Xu, S.; Li, H. Synthesis of CaIn2S4/TiO2 heterostructures for enhanced UV–visible light photocatalytic activity. J. Alloys Compd. 2021, 885, 161027. [Google Scholar] [CrossRef]
- Yashima, M.; Maeda, K.; Teramura, K.; Takata, T.; Domen, K. Crystal structure and optical properties of (Ga1-xZnx)(N1-xOx) oxynitride photocatalyst (x = 0.13). Chem. Phys. Lett. 2005, 416, 225–228. [Google Scholar] [CrossRef]
- Maeda, K.; Wang, X.; Nishihara, Y.; Lu, D.; Antonietti, M.; Domen, K. Photocatalytic activities of graphitic carbon nitride powder for water reduction and oxidation under visible light. J. Phys. Chem. C 2009, 113, 4940–4947. [Google Scholar] [CrossRef]
- Sato, J.; Saito, N.; Yamada, Y.; Maeda, K.; Takata, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K.; Inoue, Y. RuO2-loaded beta-Ge3N4 as a non-oxide photocatalyst for overall water splitting. J. Am. Chem. Soc. 2005, 127, 4150–4151. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, H.; Farzadkia, M.; Boukherroub, R.; Srivastava, V.; Sillanpaa, M. Design and preparation of core-shell structured magnetic graphene oxide@MIL-101(Fe): Photocatalysis under shell to remove diazinon and atrazine pesticides. Sol. Energy 2020, 208, 990–1000. [Google Scholar] [CrossRef]
- Liang, H.; Bai, J.; Xu, T.; Li, C. In-situ synthesized and photocatalytic performance evaluation of MoS2-C-g-C3N4 heterostructure photocatalyts. Adv. Powder. Technol. 2021, 32, 4805–4813. [Google Scholar] [CrossRef]
- Enesca, A.; Isac, L.; Duta, A. Charge carriers injection in tandem semiconductors for dyes mineralization. Appl. Catal. B Environ. 2015, 162, 352–363. [Google Scholar] [CrossRef]
- Jin, X.; Ye, L.; Xie, H.; Chen, G. Bismuth-rich bismuth oxyhalides for environmental and energy photocatalysis. Coord. Chem. Rev. 2017, 349, 84–101. [Google Scholar] [CrossRef]
- Zhang, L.W.; Baumanis, C.; Robben, L.; Kandiel, T.; Bahnemann, D. Bi2WO6 inverse opals: Facile fabrication and efficient visible-light-driven photocatalytic and photoelectrochemical water-splitting activity. Small 2011, 7, 2714–2720. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Zhou, L.; Xu, H. Bi2WO6 nano—and microstructures: Shape control and associated visible-light-driven photocatalytic activities. Small 2007, 3, 1618–1625. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Y. Synthesis of square Bi2WO6 nanoplates as high-activity visible-light-driven photocatalysts. Chem. Mater. 2005, 17, 3537–3545. [Google Scholar] [CrossRef]
- Fu, H.; Pan, C.; Yao, W.; Zhu, Y. Visible-light-induced degradation of rhodamine B by nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, L.; Xie, Y.; Lin, Z.; Fan, Y.; Liu, D.; Chen, L.; Zhang, Z.; Wang, X. Controllable synthesis of Bi2WO6 nanoplate self-assembled hierarchical erythrocyte microspheres via a one-pot hydrothermal reaction with enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2017, 403, 326–334. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Huang, X. Synthesis and visible-light photocatalytic property of Bi2WO6 hierarchical octahedron-like structures. Nanoscale Res. Lett. 2008, 3, 365–371. [Google Scholar] [CrossRef]
- Deng, X.-X.; Tian, S.; Chai, Z.-M.; Bai, Z.-J.; Tan, Y.-X.; Chen, L.; Guo, J.-K.; Shen, S.; Cai, M.-Q.; Au, C.-T.; et al. Boosted activity for toluene selective photooxidation over Fe-doped Bi2WO6. Ind. Eng. Chem. Res. 2020, 59, 13528–13538. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Novel Bi2WO6 modified by N-doped graphitic carbon nitride photocatalyst for efficient photocatalytic degradation of phenol under visible light. Appl. Cata. B Environ. 2020, 268, 118426. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, H.; Xu, Q.; Song, X.; Zhai, C.; Zhu, M. Pt decorated 2D/3D heterostructure of Bi2WO6 nanosheet/Cu2S snowflake for improving electrocatalytic methanol oxidation with visible-light assistance. Appl. Surf. Sci. 2020, 521, 146431. [Google Scholar] [CrossRef]
- Wang, R.; Li, B.; Xiao, Y.; Tao, X.; Su, X.; Dong, X. Optimizing Pd and Au-Pd decorated Bi2WO6 ultrathin nanosheets for photocatalytic selective oxidation of aromatic alcohols. J. Catal. 2018, 364, 154–165. [Google Scholar] [CrossRef]
- Yu, Y.-N.; Lu, S.-Y.; Bao, S.-J. Photocatalytic activity of Pt-modified Bi2WO6 nanoporous wall under sunlight. J. Nanopart. Res. 2015, 17, 323. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Cui, Y.; Yang, L.; Zhang, G.-Y.; Gao, D.Z. Facile in-situ photocatalysis of Ag/Bi2WO6 heterostructure with obviously enhanced performance. Sep. Purif. Technol. 2015, 142, 168–175. [Google Scholar] [CrossRef]
- Ma, T.; Liu, C.; Li, Z.; Zheng, R.; Chen, M.; Dai, S.; Zhao, T. Mechanochemically constructed Bi2WO6/Zn-Al layered double hydroxide heterojunction with prominent visible light-driven photocatalytic efficiency. Appl. Clay Sci. 2021, 215, 106328. [Google Scholar] [CrossRef]
- Li, B.; Lai, C.; Zeng, G.; Qin, L.; Yi, H.; Huang, D.; Zhou, C.; Liu, X.; Cheng, M.; Xu, P.; et al. Facile hydrothermal synthesis of Z-Scheme Bi2Fe4O9/Bi2WO6 heterojunction photocatalyst with enhanced visible light photocatalytic activity. ACS Appl. Mater. Interf. 2018, 10, 18824–18836. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Gu, S.; Li, H.; Wu, X.; Ren, C.; Liu, X. Facile fabrication of direct Z-scheme MoS2/Bi2WO6 heterojunction photocatalyst with superior photocatalytic performance under visible light irradiation. J. Photochem. Photobiol. A Chem. 2017, 335, 140–148. [Google Scholar] [CrossRef]
- Kraeutler, B.; Bard, A.J. Heterogeneous photocatalytic preparation of supported catalysts. Photodeposition of platinum on TiO2 powder and other substrates. J. Am. Chem. Soc. 1978, 100, 4317–4318. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Disdier, J.; Pichat, P.; Fernandez, A.; Gonzalez-Elipe, A.; Munuera, G.; Leclercq, C. Titania-supported bimetallic catalyst synthesis by photocatalytic codeposition at ambient temperature: Preparation and characterization of platinum-rhodium, silver-rhodium, and platinum-palladium couples. J. Catal. 1991, 132, 490–497. [Google Scholar] [CrossRef]
- Ohtani, B.; Kakimoto, M.; Nishimoto, S.; Kagiya, T. Photocatalytic reaction of neat alcohols by metal-loaded titanium(IV) oxide particles. J. Phys. Chem. A Chem. 1993, 70, 265–272. [Google Scholar] [CrossRef]
- Kowalska, E.; Remita, H.; Colbeau-Justin, C.; Hupka, J.; Belloni, J. Modification of titanium dioxide with platinum ions and clusters: Application in photocatalysis. J. Phys. Chem. C 2008, 112, 1124–1131. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Putdum, S.; Dumrongrojthanath, P.; Ekthammathat, N.; Thongtem, S.; Thongtem, T. Enhanced properties for visible-light-driven photocatalysis of Ag nanoparticle modified Bi2MoO6 nanoplates. Mater. Sci. Semicond. Process. 2015, 34, 175–181. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Li, Z.; Li, C.; Cao, X.; Zhou, A.; Hu, Q. Microwave-assisted synthesis of flower-like Ag–BiOCl nanocomposite with enhanced visible-light photocatalytic activity. Mater. Lett. 2014, 136, 295–297. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, Z.; Shen, X.; Zhu, B.; Macharia, D.K.; Chen, Z.; Zhang, L. Synthesis of Au nanoparticle-decorated carbon nitride nanorods with plasmon-enhanced photoabsorption and photocatalytic activity for removing various pollutants from water. J. Hazard. Mater. 2018, 344, 1188–1197. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, Y.; Wang, L.; Ai, S.; Ding, H. Cu-modified alkalinized g-C3N4 as photocatalytically assisted heterogeneous Fenton-like catalyst. Appl. Surf. Sci. 2017, 426, 1133–1140. [Google Scholar] [CrossRef]
- Meng, X.; Li, Z.; Chen, J.; Xie, H.; Zhang, Z. Enhanced visible light-induced photocatalytic activity of surface-modified BiOBr with Pd nanoparticles. Appl. Surf. Sci. 2018, 433, 76–87. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, S.W. TiO2 photocatalysis for the degradation of pollutants in gas phase: From morphological design to plasmonic enhancement. J. Photoch. Photobio. C 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Kowalska, E.; Abe, R.; Ohtani, B. Visible light-induced photocatalytic reaction of gold-modified titanium(IV) oxide particles: Action spectrum analysis. Chem. Commun. 2009, 2, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.; Dhabbe, R.; Gophane, A.; Sathe, T.; Garadkar, K. Template free synthesis of ZnO/Ag2O nanocomposites as a highly efficient visible active photocatalyst for detoxification of methyl orange. J. Photochem. Photobiol. B 2016, 154, 24–33. [Google Scholar] [CrossRef]
- Morgan, W.E.; Stec, W.J.; Van Wazer, J.R. Inner-orbital binding-energy shifts of antimony and bismuth compounds. Inorg. Chem. 1973, 12, 953–955. [Google Scholar] [CrossRef]
- Fu, Y.; Chang, C.; Chen, P.; Chu, X.; Zhu, L. Enhanced photocatalytic performance of boron doped Bi2WO6 nanosheets under simulated solar light irradiation. J. Hazard. Mater. 2013, 254–255, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-J.; Zhu, S.-F.; Xie, F.-Z.; Zhang, J.; Meng, Z.-D. Plate-on-plate structured Bi2MoO6/Bi2WO6 heterojunction with high-efficiently gradient charge transfer for decolorization of MB. Sep. Purif. Technol. 2013, 113, 1–8. [Google Scholar] [CrossRef]
- Xu, X.; Meng, L.; Li, Y.; Sun, C.; Yang, S.; He, H. Bi2S3 nanoribbons-hybridized {001} facets exposed Bi2WO6 ultrathin nanosheets with enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2019, 479, 410–422. [Google Scholar] [CrossRef]
- Fleisch, T.H.; Zajac, G.W.; Schreiner, J.O.; Mains, G.J. An XPS study of the UV photoreduction of transition and noble metal oxides. Appl. Surf. Sci. 1986, 26, 488–497. [Google Scholar] [CrossRef]
- Endo-Kimura, M.; Karabiyik, B.; Wang, K.; Wei, Z.; Ohtani, B.; Markowska-Szczupak, A.; Kowalska, E. Vis-responsive copper-modified titania for decomposition of organic compounds and microorganisms. Catalysts 2020, 10, 1194. [Google Scholar] [CrossRef]
- Wei, Z.; Endo, M.; Wang, K.; Charbit, E.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Noble metal-modified octahedral anatase titania particles with enhanced activity for decomposition of chemical and microbiological pollutants. Chem. Eng. J. 2017, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Wei, Z.; Endo, M.; Ohtani, B.; Kowalska, E. Silver- and copper-modified decahedral anatase tiania particles as visible light-responsive plasmonic photocatalyst. J. Photon. Energy 2017, 7, 1–16. [Google Scholar]
- Zhang, J.; Chen, T.; Lu, H.; Yang, Z.; Yin, F.; Gao, J.; Liu, Q.; Tu, Y. Hierarchical Bi2WO6 architectures decorated with Pd nanoparticles for enhanced visible-light-driven photocatalytic activities. Appl. Surf. Sci. 2017, 404, 282–290. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Endo, M.; Colbeau-Justin, C.; Ohtani, B.; Kowalska, E. Silver-modified octahedral anatase particles as plasmonic photocatalyst. Catal. Today 2018, 310, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Janczarek, M.; Endo, M.; Wang, K.L.; Balcytis, A.; Nitta, A.; Mendez-Medrano, M.G.; Colbeau-Justin, C.; Juodkazis, S.; Ohtani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl. Catal. B Environ. 2018, 237, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wei, Z.; Yoshiiri, K.; Braumuller, M.; Ohtani, B.; Rau, S.; Kowalska, E. Titania modification with ruthenium(II) complex and gold nanoparticles for photocatalytic degradation of organic compounds. Photochem. Photobiol. Sci. 2016, 15, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, S.; Bharti, S.; Shukla, G.; Mukherji, S. Synthesis and characterization of size- and shape-controlled silver nanoparticles. Phys. Sci. Rev. 2018, 4, 20170082. [Google Scholar]
- Xia, Y.N.; Halas, N.J. Shape-controlled synthesis and surface plasmonic properties of metallic nanostructures. Mrs Bull. 2005, 30, 338–344. [Google Scholar] [CrossRef]
- Sugawa, K.; Tahara, H.; Yamashita, A.; Otsuki, J.; Sagara, T.; Harumoto, T.; Yanagida, S. Refractive index susceptibility of the plasmonic palladium nanoparticle: Potential as the third plasmonic sensing material. ACS Nano 2015, 9, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Zielinska-Jurek, A.; Wei, Z.; Wysocka, I.; Szweda, P.; Kowalska, E. The effect of nanoparticles size on photocatalytic and antimicrobial properties of Ag-Pt/TiO2 photocatalysts. Appl. Surf. Sci. 2015, 353, 317–325. [Google Scholar] [CrossRef]
- Kowalska, E.; Wei, Z.; Karabiyik, B.; Herissan, A.; Janczarek, M.; Endo, M.; Markowska-Szczupak, A.; Remita, H.; Ohtani, B. Silver-modified titania with enhanced photocatalytic and antimicrobial properties under UV and visible light irradiation. Catal. Today 2015, 252, 136–142. [Google Scholar] [CrossRef]
- Kowalska, E.; Mahaney, O.O.P.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Gellini, C.; Simonelli, A.; Tiberi, M.; Giammanco, F.; Giorgetti, E. Characterization of copper nanoparticles obtained by laser ablation in liquids. Appl. Phys. A Mater. 2013, 110, 829–833. [Google Scholar] [CrossRef]
- Mendez-Medrano, M.G.; Kowalska, E.; Ohtani, B.; Uribe, D.B.; Colbeau-Justin, C.; Rau, S.; Rodriguez-Lopez, J.L.; Remita, H. Heterojunction of CuO nanoclusters with TiO2 for photo-oxidation of organic compounds and for hydrogen production. J. Chem. Phys. 2020, 153, 034705. [Google Scholar] [CrossRef]
- Mendez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Bahena, D.; Briois, V.; Colbeau-Justin, C.; Rodriguez-Lopez, J.; Remita, H. Surface modification of TiO2 with Ag nanoparticles and CuO nanoclusters for applications in photocatalysis. J. Phys. Chem. C 2016, 120, 5143–5154. [Google Scholar] [CrossRef]
- Luna, A.L.; Valenzuela, M.A.; Colbeau-Justin, C.; Vazquez, P.; Rodriguez, J.L.; Avendano, J.R.; Alfaro, S.; Tirado, S.; Garduno, A.; De la Rosa, J.M. Photocatalytic degradation of gallic acid over CuO–TiO2 composites under UV/Vis LEDs irradiation. Appl. Catal. A Gen. 2016, 521, 140–148. [Google Scholar] [CrossRef]
- Ito, T.; Yamaguchi, H.; Masumi, T.; Adachi, S. Optical properties of CuO studied by spectroscopic ellipsometry. J. Phys. Soc. Jpn. 1998, 67, 3304–3309. [Google Scholar] [CrossRef]
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photoch. Photobio. C 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Abe, R.; Takami, H.; Murakami, N.; Ohtani, B. Pristine simple oxides as visible light driven photocatalysts: Highly efficient decomposition of organic compounds over platinum-loaded tungsten oxide. J. Am. Chem. Soc. 2008, 130, 7780–7781. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Takashima, M.; Takase, M.; Ohtani, B. Kinetic analysis supporting multielectron reduction of oxygen in bismuth tungstate-photocatalyzed oxidation of organic compounds. Catal. Today 2018, 313, 218–223. [Google Scholar] [CrossRef]
- Wang, K.L.; Wei, Z.S.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Wei, Z.; Kowalska, E.; Wang, K.; Colbeau-Justin, C.; Ohtani, B. Enhanced photocatalytic activity of octahedral anatase particles prepared by hydrothermal reaction. Catal. Today 2017, 280, 29–36. [Google Scholar] [CrossRef][Green Version]
- Wei, Z.; Rosa, L.; Wang, K.; Endo, M.; Juodkazi, S.; Ohtani, B.; Kowalska, E. Size-controlled gold nanoparticles on octahedral anatase particles as efficient plasmonic photocatalyst. Appl. Catal. B Environ. 2017, 206, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Yoshiiri, K.; Wei, Z.; Zheng, S.; Kastl, E.; Remita, H.; Ohtani, B.; Rau, S. Hybrid photocatalysts composed of titania modified with plasmonic nanoparticles and ruthenium complexes for decomposition of organic compounds. Appl. Catal. B Environ. 2015, 178, 133–143. [Google Scholar] [CrossRef]
- Kowalska, E.; Janczarek, M.; Rosa, L.; Juodkazi, S.; Ohtani, B. Mono- and bi-metallic plasmonic photocatalysts for degradation of organic compounds under UV and visible light irradiation. Catal. Today 2014, 230, 131–137. [Google Scholar] [CrossRef]
- Luna, A.L.; Dragoe, D.; Wang, K.L.; Beaunier, P.; Kowalska, E.; Ohtani, B.; Uribe, D.B.; Valenzuela, M.A.; Remita, H.; Colbeau-Justin, C. Photocatalytic hydrogen evolution using Ni-Pd/TiO2: Correlation of light absorption, charge-carrier dynamics, and quantum efficiency. J. Phys. Chem. C 2017, 121, 14302–14311. [Google Scholar] [CrossRef]
- Luna, A.L.; Novoseltceva, E.; Louran, E.; Beaunier, P.; Kowalska, E.; Ohtani, B.; Valenzuela, M.A.; Remita, H.; Colbeau-Justin, C. Synergetic effect of Ni and Au nanoparticles synthesized on titania particles for efficient photocatalytic hydrogen production. Appl. Catal. B Environ. 2016, 191, 18–28. [Google Scholar] [CrossRef]
- Hori, H.; Takashima, M.; Takase, M.; Ohtani, B. Pristine bismuth-tungstate photocatalyst particles driving organics decomposition through multielectron reduction of oxygen. Chem. Lett. 2017, 46, 1376–1378. [Google Scholar] [CrossRef]
- Wu, S.; Sun, J.; Li, Q.; Hood, Z.D.; Yang, S.; Su, T.; Peng, R.; Wu, Z.; Sun, W.; Kent, P.R.C.; et al. Effects of surface terminations of 2D Bi2WO6 on photocatalytic hydrogen evolution from water splitting. ACS Appl. Mater. Interfaces 2020, 12, 20067–20074. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Li, Y. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Wang, K.; Zheng, S.; Kowalska, E. Morphology-governed performance of plasmonic photocatalysts. Catalysts 2020, 10, 1070. [Google Scholar] [CrossRef]
- Furube, A.; Du, L.; Hara, K.; Katoh, R.; Tachiya, M. Ulltrafast plasmon-induced electron transfer from gold nanodots into TiO2 nanoparticles. J. Am. Chem. Soc. 2007, 129, 14852–14853. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.K.; Wu, N.Q. Progress and perspectives of plasmon-enhanced solar energy conversion. J. Phys. Chem. Lett. 2016, 7, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Bielan, Z.; Endo-Kimura, M.; Janczarek, M.; Zhang, D.; Kowalski, D.; Zielińska-Jurek, A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. On the mechanism of photocatalytic reactions on CuxO@TiO2 core–shell photocatalysts. J. Mat. Chem. A 2021, 9, 10135–10145. [Google Scholar] [CrossRef]
- Janczarek, M.; Wang, K.L.; Kowalska, E. Synergistic effect of Cu2O and urea as modifiers of TiO2 for enhanced visible light activity. Catalysts 2018, 8, 240. [Google Scholar] [CrossRef]
- Yan, X.; Ohno, T.; Nishijima, K.; Abe, R.; Ohtani, B. Is Methylene blue an appropriate substrate for a photocatalytic activity test? A study with visible-light responsive titania. Chem. Phys. Lett. 2006, 429, 606–610. [Google Scholar] [CrossRef]
- Rochkind, M.; Pasternak, S.; Paz, Y. Using dyes for evaluating photocatalytic properties: A critical review. Molecules 2015, 20, 88–110. [Google Scholar] [CrossRef]
- Barbero, N.; Vione, D. Why dyes should not be used to test the photocatalytic activity of semiconductor oxides. Environ. Sci. Technol. 2016, 50, 2130–2131. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).