Fusion-Assisted Hydrothermal Synthesis and Post-Synthesis Modification of Mesoporous Hydroxy Sodalite Zeolite Prepared from Waste Coal Fly Ash for Biodiesel Production
Abstract
1. Introduction
2. Results and Discussion
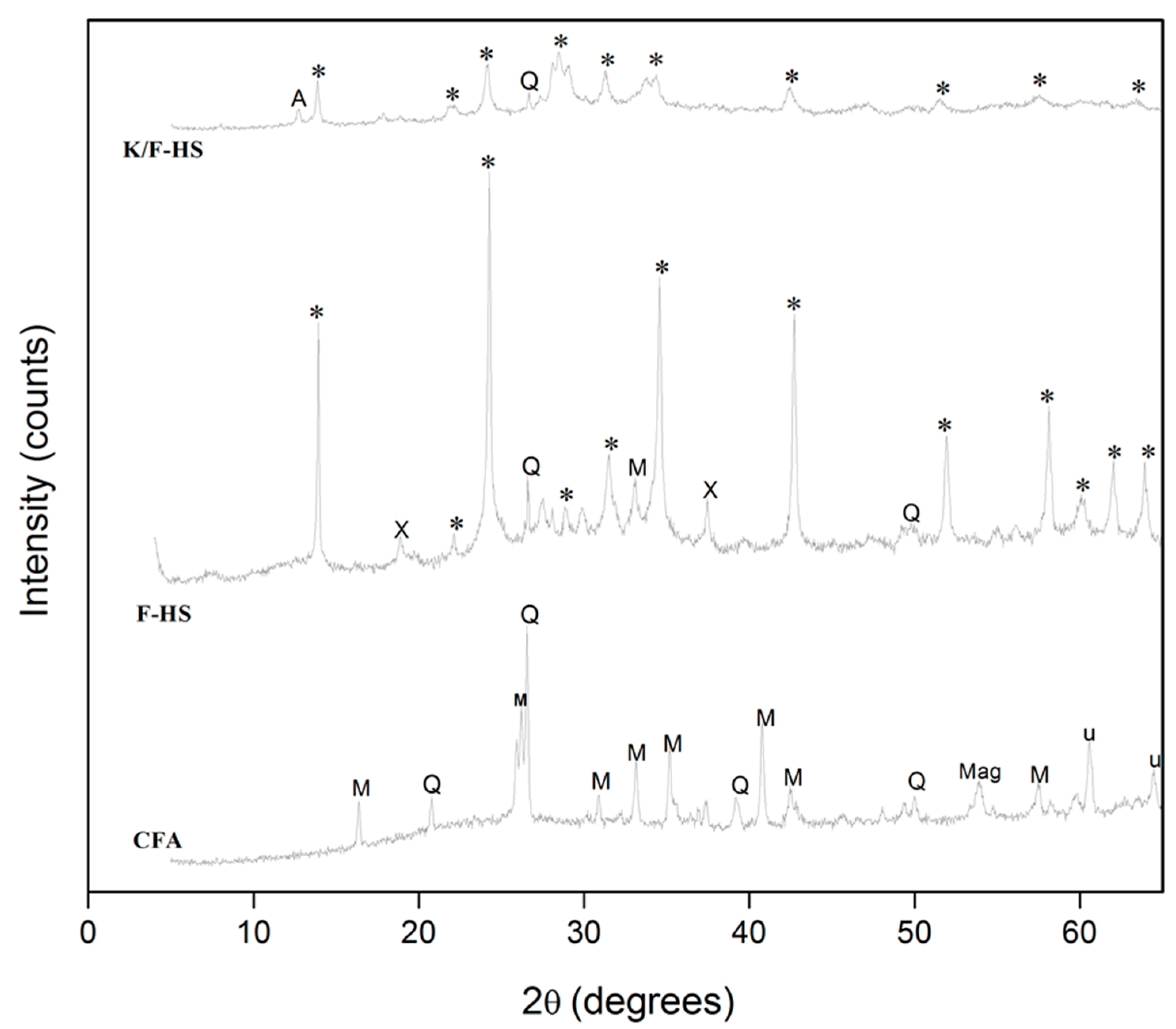
2.1. XRD Analysis
2.2. Crystal Morphology
2.3. Elemental Composition
2.4. Fourier-Transform Infrared
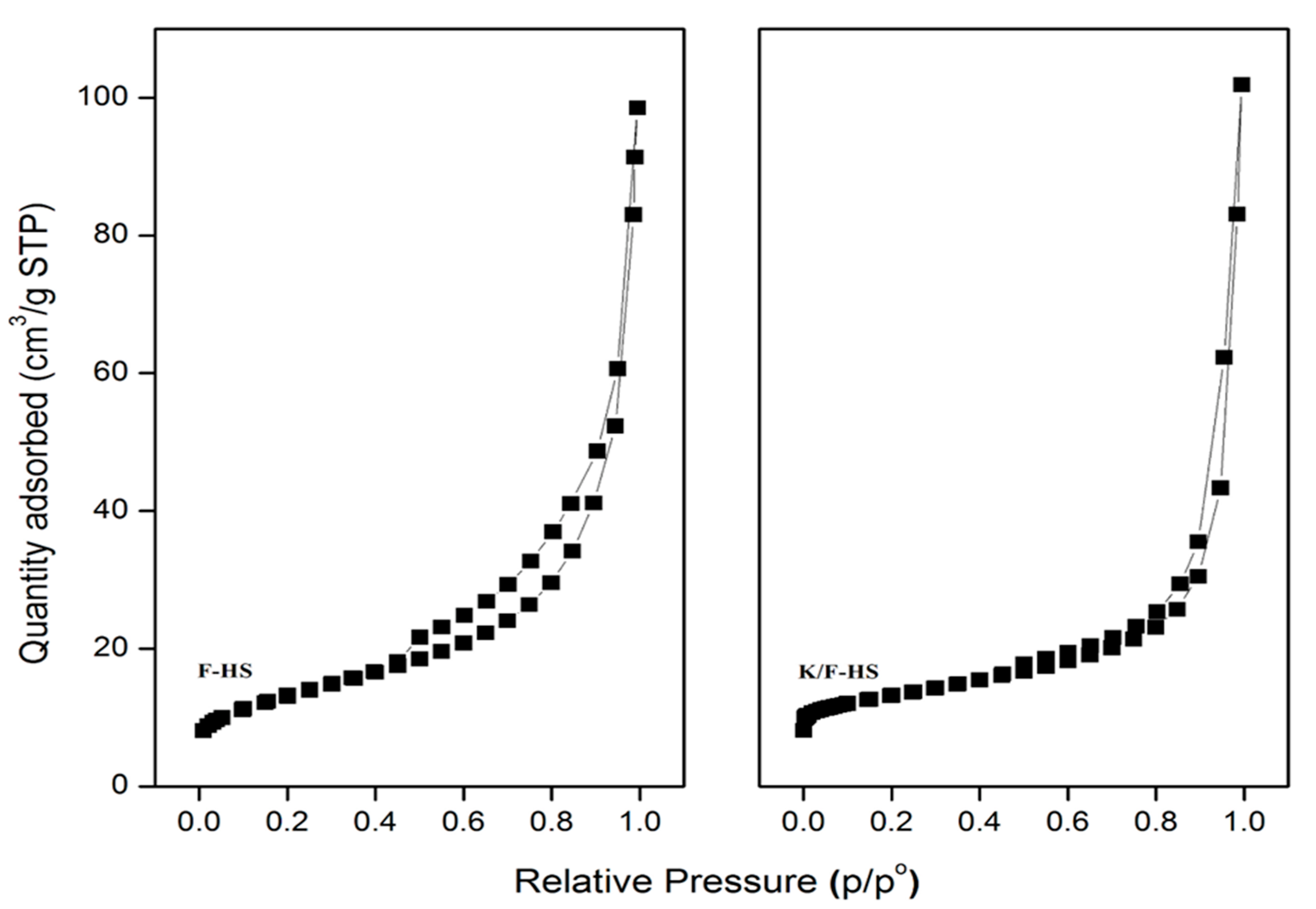
2.5. Textural Properties
Pore Distribution and Isotherm Curves of Produced Catalysts
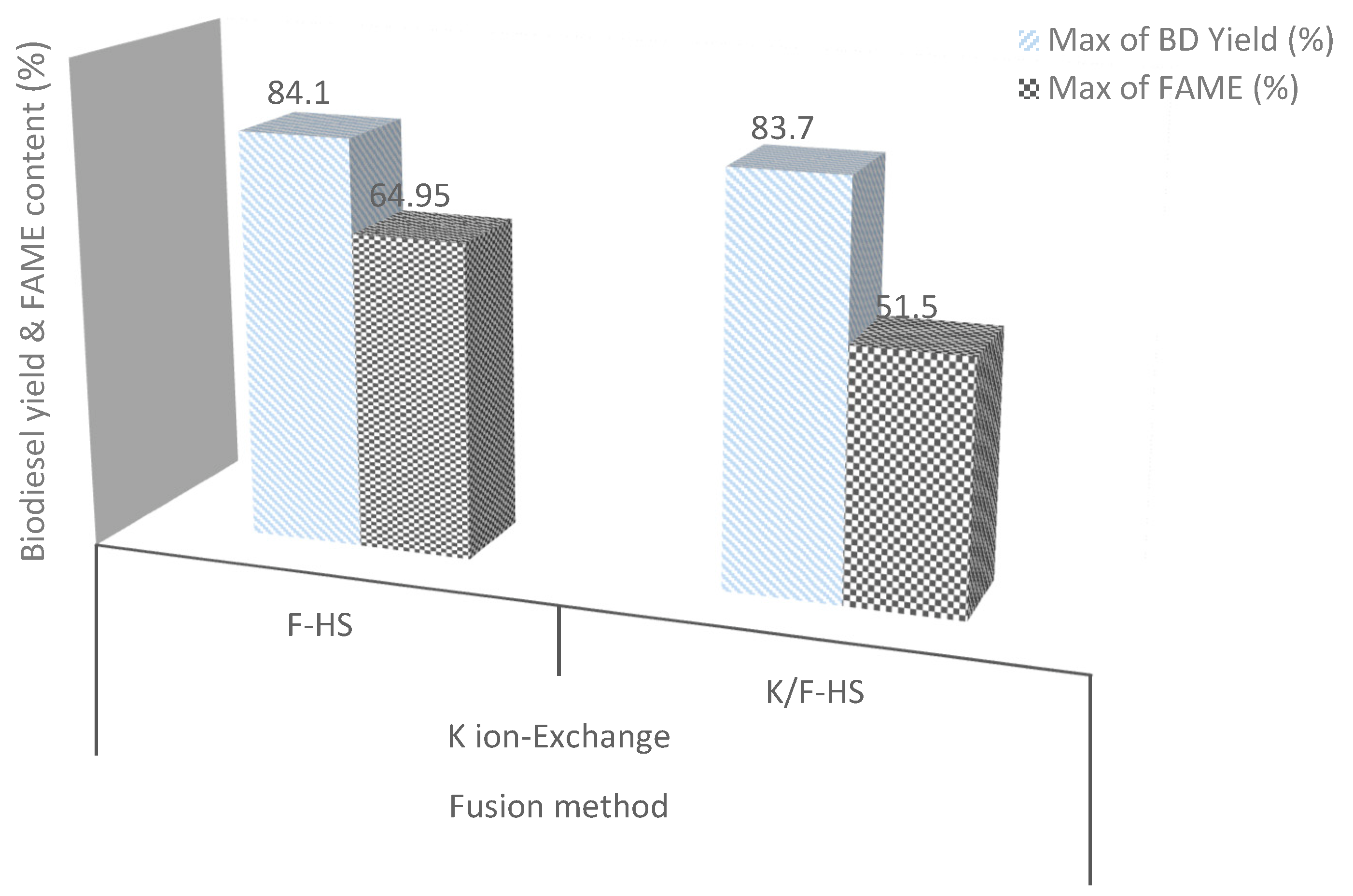
2.6. Biodiesel Production over CFA-Synthesised and Modified HS Zeolite
2.7. Techno-Economic and Environmental Impact of Hydroxyl Sodalite (F-HS) as a Heterogeneous Catalyst Compared to a Conventional Homogenous Catalyst in Biodiesel Production
3. Materials and Methods
3.1. Materials
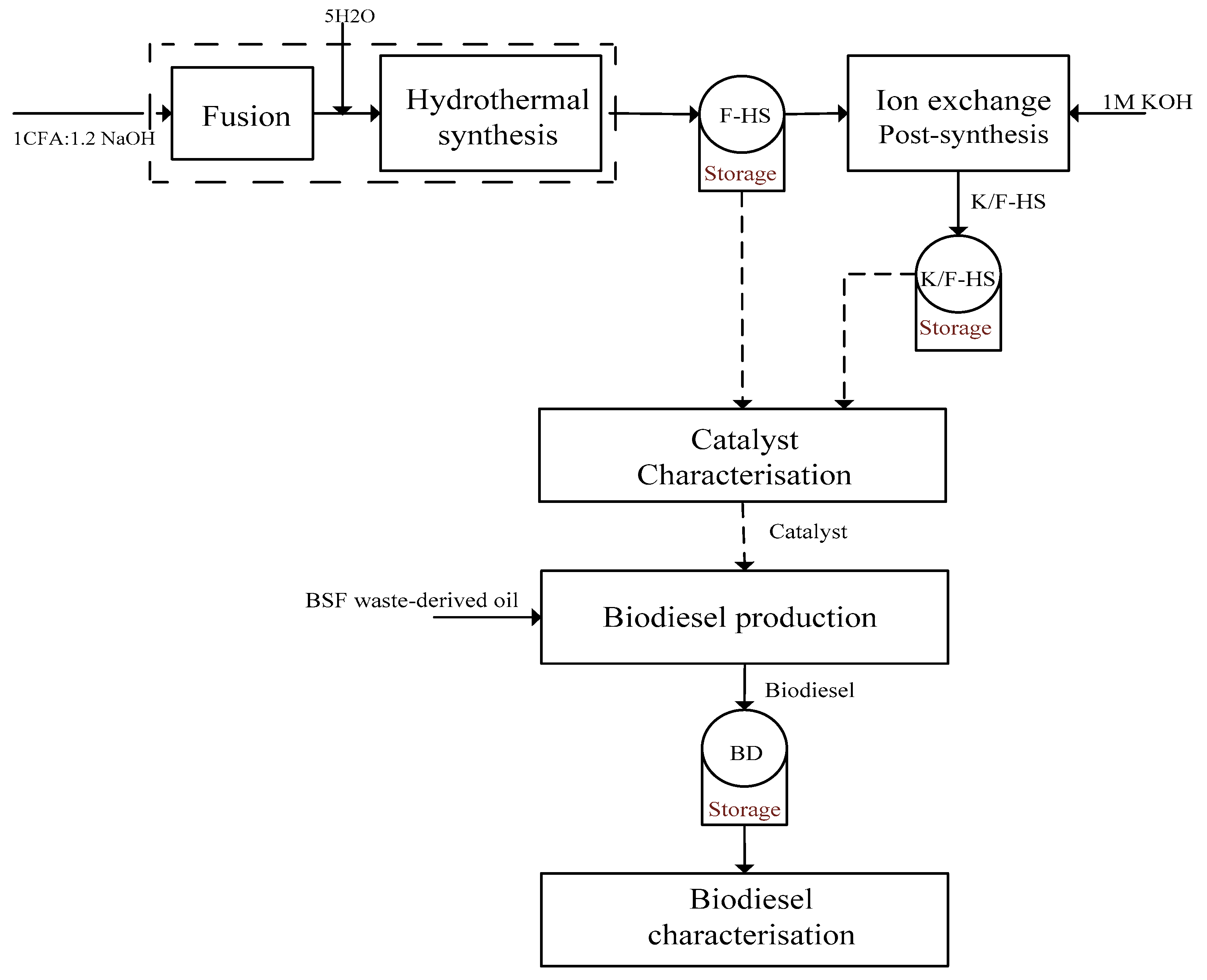
3.2. Catalyst Preparation and Ion Exchange Modification
3.2.1. Fusion-Assisted Hydrothermal Synthesis
3.2.2. Post-Synthesis Ion-Exchange Modification
3.2.3. Activity Tests of Synthesized Catalysts in Biodiesel Production from BSF Oil
3.3. Catalyst and Biodiesel Sample Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naylor, R.L.; Higgins, M.M. The rise in global biodiesel production: Implications for food security. Glob. Food Secur. 2017, 16, 75–84. [Google Scholar] [CrossRef]
- Gonfa Keneni, Y.; Mario Marchetti, J. Oil extraction from plant seeds for biodiesel production. AIMS Energy 2017, 5, 316–340. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- You, Y.-D.; Shie, J.-L.; Chang, C.-Y.; Huang, S.-H.; Pai, C.-Y.; Yu, Y.-H.; Chang, C.H. Economic cost analysis of biodiesel production: Case in soybean oil. Energy Fuels 2008, 22, 182–189. [Google Scholar] [CrossRef]
- Okechukwu, O.D.; Joseph, E.; Nonso, U.C.; Kenechi, N.-O. Improving heterogeneous catalysis for biodiesel production process. Clean. Chem. Eng. 2022, 3, 100038. [Google Scholar] [CrossRef]
- Shabani, J.; Babajide, O.; Oyekola, O. An Investigation into the Potential of Maggot Oil as a Feedstock for Biodiesel Production. In Valorization of Biomass to Value-Added Commodities; Springer: Berlin/Heidelberg, Germany, 2020; pp. 285–301. [Google Scholar]
- Wang, C.; Qian, L.; Wang, W.; Wang, T.; Deng, Z.; Yang, F.; Xiong, J.; Feng, W. Exploring the potential of lipids from black soldier fly: New paradigm for biodiesel production (I). Renew. Energy 2017, 111, 749–756. [Google Scholar] [CrossRef]
- Leong, S.Y.; Kutty, S.R.M.; Malakahmad, A.; Tan, C.K. Feasibility study of biodiesel production using lipids of Hermetia illucens larva fed with organic waste. Waste Manag. 2016, 47, 84–90. [Google Scholar] [CrossRef]
- Oonincx, D.; Van Huis, A.; Van Loon, J.J.A. Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. J. Insects Food Feed. 2015, 1, 131–139. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Inseco. Our Products. 2022. Available online: https://store.inseco.co.za/product/entooil/ (accessed on 20 September 2022).
- Ecosystem. Our product. 2010. Available online: http://www.eco-system.com/products.php (accessed on 15 June 2022).
- Dwivedi, A.; Jain, M.K. Fly ash—Waste management and overview: A Review. Recent Res. Sci. Technol. 2014, 6, 30–35. [Google Scholar]
- Heidrich, C.; Feuerborn, H.-J.; Weir, A. Coal combustion products: A global perspective. In Proceedings of the World of Coal Ash Conference, Covington, KY, USA, 16–19 May 2022; 2022; p. 25. [Google Scholar]
- Brassell, J.P. Investigation of some Scale-up Conditions on the Synthesis of Faujasite Zeolites from South African Coal Fly Ash. Ph.D. Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2017. [Google Scholar]
- Murayama, N.; Yamamoto, H.; Shibata, J. Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. Int. J. Miner. Process. 2002, 64, 1–17. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.t.; Alastuey, A.; Hernández, E.; Lopez-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Vilakazi, A.Q.; Ndlovu, S.; Chipise, L.; Shemi, A. The recycling of coal fly ash: A review on sustainable developments and economic considerations. Sustainability 2022, 14, 1958. [Google Scholar] [CrossRef]
- Zielke-Olivier, J.; Vermeulen, D. Fine Ash Leaching in Tailings Dams–An Impact on the Underlying Aquifers? Proc. IMWA 2016, 353–360. [Google Scholar]
- Chouhan, A.P.S.; Sarma, A.K. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Sun, K.; Lu, J.; Ma, L.; Han, Y.; Fu, Z.; Ding, J. A comparative study on the catalytic performance of different types of zeolites for biodiesel production. Fuel 2015, 158, 848–854. [Google Scholar] [CrossRef]
- Suppes, G.J.; Dasari, M.A.; Doskocil, E.J.; Mankidy, P.J.; Goff, M.J. Transesterification of soybean oil with zeolite and metal catalysts. Appl. Catal. A: Gen. 2004, 257, 213–223. [Google Scholar] [CrossRef]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Catalytic Applications in the Production of Biodiesel from Vegetable Oils. ChemSusChem 2009, 2, 278–300. [Google Scholar] [CrossRef]
- Fawaz, E.G.; Salam, D.A.; Rigolet, S.S.; Daou, T.J. Hierarchical zeolites as catalysts for biodiesel production from waste frying oils to overcome mass transfer limitations. Molecules 2021, 26, 4879. [Google Scholar] [CrossRef]
- Volli, V.; Purkait, M.K. Selective preparation of zeolite X and A from flyash and its use as catalyst for biodiesel production. J. Hazard. Mater. 2015, 297, 101–111. [Google Scholar] [CrossRef]
- Nanganoa, L.T.; Mbadcam, K.J.; Kang, S. Synthesis of Hydroxy-sodalite from Fine Fractions of Sandy Clay Loam Soil (Natural Aluminosilicate). Int. J. Chem. Tech. Res. 2016, 9, 725–732. [Google Scholar]
- Musyoka, N.M.; Petrik, L.F.; Balfour, G.; Gitari, W.M.; Hums, E. Synthesis of hydroxy sodalite from coal fly ash using waste industrial brine solution. J. Environ. Sci. Health Part A 2011, 46, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Madeira, F.; Ben Tayeb, K.; Pinard, L.; Vezin, H.; Maury, S.; Cadran, N. Ethanol transformation into hydrocarbons on ZSM-5 zeolites: Influence of Si/Al ratio on catalytic performances and deactivation rate. Study of the radical species role. Appl. Catal. A Gen. 2012, 443–444, 171–180. [Google Scholar] [CrossRef]
- Xie, W.; Huang, X.; Li, H. Soybean oil methyl esters preparation using NaX zeolites loaded with KOH as a heterogeneous catalyst. Bioresour. Technol. 2007, 98, 936–939. [Google Scholar] [CrossRef]
- Babajide, O.; Musyoka, N.; Petrik, L.; Ameer, F. Novel zeolite Na-X synthesized from fly ash as a heterogeneous catalyst in biodiesel production. Catal. Today 2012, 190, 54–60. [Google Scholar] [CrossRef]
- Shirani Lapari, S.; Ramli, Z.; Triwahyono, S. Effect of Different Templates on the Synthesis of Mesoporous Sodalite. J. Chem. 2015, 2015, 272613. [Google Scholar] [CrossRef]
- Bukhari, S.S.; Behin, J.; Kazemian, H.; Rohani, S. A comparative study using direct hydrothermal and indirect fusion methods to produce zeolites from coal fly ash utilizing single-mode microwave energy. J. Mater. Sci. 2014, 49, 8261–8271. [Google Scholar] [CrossRef]
- Viswanadham, N.; Saxena, S.K.; Kumar, J.; Sreenivasulu, P.; Nandan, D. Catalytic performance of nano crystalline H-ZSM-5 in ethanol to gasoline (ETG) reaction. Fuel 2012, 95, 298–304. [Google Scholar] [CrossRef]
- Hollman, G.; Steenbruggen, G.; Janssen-Jurkovičová, M. A two-step process for the synthesis of zeolites from coal fly ash. Fuel 1999, 78, 1225–1230. [Google Scholar] [CrossRef]
- Mezni, M.; Hamzaoui, A.; Hamdi, N.; Srasra, E. Synthesis of zeolites from the low-grade Tunisian natural illite by two different methods. Appl. Clay Sci. 2011, 52, 209–218. [Google Scholar] [CrossRef]
- Makgaba, C.P.; Daramola, M.O. Transesterification of Waste Cooking Oil to Biodiesel over Calcined Hydroxy Sodalite (HS) Catalyst: A preliminary investigation. In Proceedings of the 2015 International Conference on Sustainable Energy and Environmental Engineering (SEEE 2015), Bangkok, Thailand, 25–26 October 2015. [Google Scholar]
- Aniokete, T.; Ozonoh, M.; Daramola, M.O. Synthesis of pure and high surface area sodalite catalyst from waste industrial brine and coal fly ash for conversion of waste cooking oil (WCO) to biodiesel. Int. J. Renew. Energy Res. (IJRER) 2019, 9, 1924–1937. [Google Scholar]
- Xu, M.; Mukarakate, C.; Robichaud, D.J.; Nimlos, M.R.; Richards, R.M.; Trewyn, B.G. Elucidating zeolite deactivation mechanisms during biomass catalytic fast pyrolysis from model reactions and zeolite syntheses. Top. Catal. 2016, 59, 73–85. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, H.; Yang, J. Hydrothermal Synthesis of Sodalite on Alkali-Activated Coal Fly Ash for Removal of Lead Ions. Procedia Environ. Sci. 2016, 31, 605–614. [Google Scholar] [CrossRef]
- Treacy, M.M.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites Fifth Revised Edition, 5th ed.; Elsevier: Amsterdam, Netherlands, 2007. [Google Scholar]
- Rayalu, S.; Meshram, S.; Hasan, M. Highly crystalline faujasitic zeolites from flyash. J. Hazard. Mater. 2000, 77, 123–131. [Google Scholar] [CrossRef]
- Suppes, G.; Bockwinkel, K.; Lucas, S.; Botts, J.; Mason, M.; Heppert, J. Calcium carbonate catalyzed alcoholysis of fats and oils. J. Am. Oil Chem. Soc. 2001, 78, 139–146. [Google Scholar] [CrossRef]
- Rios, C.A.; Williams, C.D.; Fullen, M.A. Nucleation and growth history of zeolite LTA synthesized from kaolinite by two different methods. Appl. Clay Sci. 2009, 42, 446–454. [Google Scholar] [CrossRef]
- Golbad, S.; Khoshnoud, P.; Abu-Zahra, N. Hydrothermal synthesis of hydroxy sodalite from fly ash for the removal of lead ions from water. Int. J. Environ. Sci. Technol. 2017, 14, 135–142. [Google Scholar] [CrossRef]
- Endalew, A.K.; Kiros, Y.; Zanzi, R. Inorganic heterogeneous catalysts for biodiesel production from vegetable oils. Biomass Bioenergy 2011, 35, 3787–3809. [Google Scholar] [CrossRef]
- Buhl, J.-C.; Schuster, K.; Robben, L. Nanocrystalline sodalite grown from superalkaline NaCl bearing gels at low temperature (333 K) and the influence of TEA on crystallization process. Microporous Mesoporous Mater. 2011, 142, 666–671. [Google Scholar] [CrossRef]
- Sotomayor, F.J.; Cychosz, K.A.; Thommes, M. Characterization of micro/mesoporous materials by physisorption: Concepts and case studies. Acc. Mater. Surf. Res. 2018, 3, 36–37. [Google Scholar]
- Shabani, J.M. Synthesis of Fly Ash-Based Zeolites for Use as Catalysts in the Transesterification of Waste-Derived Maggot Oil for Biodiesel Production. Ph.D. Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2021. [Google Scholar]
- Nathan. Atomic Radius of Elements. 2022. Available online: https://www.breakingatom.com/learn-the-periodic-table/atomic-radius-of-elements (accessed on 10 July 2022).
- Volli, V. Preparation and Characterization of Flyash Based Catalyst for Transesterification of Mustard Oil. Ph.D. Thesis, Indian Institute of Technology Guwahati, Guwahati, India, 2015. [Google Scholar]
- Weitkamp, J. Zeolites and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Dehkhoda, A.M. Developing Biochar-Based Catalyst for Biodiesel Production. Master’s thesis, University of British Columbia, Vancouver, BC, Canada, 2010. [Google Scholar]
- Endalew, A.K.; Kiros, Y.; Zanzi, R. Heterogeneous catalysis for biodiesel production from Jatropha curcas oil (JCO). Energy 2011, 36, 2693–2700. [Google Scholar] [CrossRef]
- Jayasinghe, P.; Hawboldt, K. A review of bio-oils from waste biomass: Focus on fish processing waste. Renew. Sustain. Energy Rev. 2012, 16, 798–821. [Google Scholar] [CrossRef]
- Sakthivel, R.; Ramesh, K.; Purnachandran, R.; Shameer, P.M. A review on the properties, performance and emission aspects of the third generation biodiesels. Renew. Sustain. Energy Rev. 2018, 82, 2970–2992. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.H.; Mijan, N.-A.; Taufiq-Yap, Y.H. Efficient reaction for biodiesel manufacturing using bi-functional oxide catalyst. Catal. Commun. 2021, 149, 106201. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D.; Shoumkova, A. Recycling of lignite coal fly ash by its conversion into zeolites. Coal Combust. Gasif. Prod. 2015, 7, 1–8. [Google Scholar]
- Faria, D.N.; Cipriano, D.F.; Schettino, M.A., Jr.; Neto, A.C.; Cunha, A.G.; Freitas, J.C. Na, Ca-based catalysts supported on activated carbon for synthesis of biodiesel from soybean oil. Mater. Chem. Phys. 2020, 249, 123173. [Google Scholar] [CrossRef]
- Saifuddin, N.; Samiuddin, A.; Kumaran, P. A review on processing technology for biodiesel production. Trends Appl. Sci. Res. 2015, 10, 1. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of vegetable oils with ethanol and characterization of the key fuel properties of ethyl esters. Energies 2009, 2, 362–376. [Google Scholar] [CrossRef]
- AMSEC. Rheometer Concise Operating Procedures. Available online: https://amsec.wwu.edu/files/2020-08/Rheometer_SOPs.pdf (accessed on 15 June 2022).
- Lucas, J. How do I Determine the Free Fatty Acid (FFA) Percentage in Non-Edible Oils? Available online: https://www.researchgate.net/post/how_do_i_determine_the_Free_Fatty_Acid_FFA_Percentage_in_non-edible_oils (accessed on 15 June 2022).
- Babajide, O.O. Optimisation of Biodiesel Production via Different Catalytic and Process Systems. Ph.D. Thesis, University of the Werstern Cape, Cape Town, South Africa, 2011. [Google Scholar]
- Japir, A.A.-W.; Salimon, J.; Derawi, D.; Bahadi, M.; Al-Shuja’a, S.; Yusop, M.R. Physicochemical characteristics of high free fatty acid crude palm oil. OCL 2017, 24, D506. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Rodríguez-Rodríguez, J.E.; del Rayo Jaramillo-Jacob, A. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Malonda Shabani, J.; Babajide, O.; Oyekola, O.; Petrik, L. Synthesis of Hydroxy Sodalite from Coal Fly Ash for Biodiesel Production from Waste-Derived Maggot Oil. Catalysts 2019, 9, 1052. [Google Scholar] [CrossRef]
- World of Science. Product. Available online: https://worldofscience.co.za/product/sodium-hydroxide-ar-500g/ (accessed on 15 September 2022).
- CCT. Water and sanitation services and costs in formal housing. Available online: https://www.capetown.gov.za/Family%20and%20home/residential-utility-services/residential-water-and-sanitation-services/water-and-sanitation-services-and-costs-for-formal-housing (accessed on 6 June 2022).
- Hong, J.L.X.; Maneerung, T.; Koh, S.N.; Kawi, S.; Wang, C.-H. Conversion of coal fly ash into zeolite materials: Synthesis and characterizations, process design, and its cost-benefit analysis. Industrial & Engineering Chemistry Research 2017, 56, 11565–11574. [Google Scholar]



| Sample | Element (Atomic, w/w%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Al | Si | Na | Mg | K | Ca | Ti | Fe | P | Total | Si/Al | Na/Al | |
| CFA | 65.15 | 11.23 | 14.86 | - | 1.54 | 0.23 | 4.32 | 0.30 | 1.13 | 1.25 | 100.0 | 1.32 | - |
| F-HS | 65.35 | 12.42 | 14.65 | 11.55 | 0.39 | - | 1.18 | 0.29 | 0.57 | - | 100.0 | 1.18 | 0.93 |
| K/F-HS | 59.75 | 10.1 | 13.67 | 8.17 | 0.8 | 3.77 | 1.97 | 0.44 | 1.13 | - | 100.0 | 1.35 | 0.81 |
| Synthesis Method | Sample | Source | Surface Area (m2/g) | Reference | ||
|---|---|---|---|---|---|---|
| Total (a) | Meso. (b) | Micro. (b) | ||||
| Fusion | F-HS | CFA | 45 | 42.2 | 2.7 | This study |
| Direct | HS | CFA | 13.2 | 12.6 | 0.6 | Shabani [48] |
| Conventional direct | HS | Pure chemicals | 43.6 | - | 9.6 | Golbad, Khoshnoud and Abu-Zahra [44] |
| Conventional direct | HS | Pure chemicals | 11 | - | - | Shirani Lapari, Ramli and Triwahyono [31] |
| Ion exchange | K/F-HS | 25.8 | 25 | 0.8 | This study | |
| Biodiesel Properties | Catalyst | B-Standard (a) | |
|---|---|---|---|
| F-HS | K/F-HS | ENS (b)/ASTM (c) | |
| Acid value (mg KOH/g) | 0.53 | 0.74 | 0.5/0.8 Max |
| Saponification value (mg KOH/g) | 147.87 | 145.43 | - |
| Ester content (d) (% m/m) | 64.95 | 51.5 | 96.5 |
| Iodine value (g of /100 g) | 65.01 | 61.4 | /120–130 |
| Density at 40 °C (g/mL) | 0.877 | 0.893 | 0.86–0.90 |
| Kinematics viscosity at 40 °C (mm2/s) | 5.16 | 4.68 | 3.5–5.0/1.9–6.0 |
| Refractive index | 1.4455 | 1.4435 | /1.479 |
| Cetane number | 36.63 | 36.66 | 51/47 |
| Biodiesel Feedstock | Zeolite Catalyst | Ion Exchange Post-Synthesis | Biodiesel | Reference | ||
|---|---|---|---|---|---|---|
| Type (Source) | Hydrothermal Synthesis Method | Yield (%) | Properties | |||
| WCO | ZSM-5 (* pure chem. reagents) | Direct | × √(NH4Cl) | - | - | Fawaz, Salam, S. Rigolet and Daou [24] |
| Oleic acid | ZSM-5, ZRP-5, Beta (* pure chem. reagents) | - | × √(NH4Cl) | - | - | Sun, Lu, Ma, Han, Fu and Ding [21] |
| Mustard oil | Na-X (CFA) | Fusion | × √(CH3COOK) | 41.90 53.7 | - - | Volli and Purkait [25] |
| Sunflower oil | Na-X (CFA) | Fusion | × √(CH3COOK) | 56 83.52 | - - | Babajide, Musyoka, Petrik and Ameer [30] |
| WCO | HS (* pure chem. reagents) | Direct | × | - | - | Makgaba and Daramola [36] |
| * BSF oil | HS (CFA) | Fusion | × √(KOH) | 84.10 83.70 | √√ √√ | This study |
| * Catalysed-Transesterification | Techno-Economic Impact | Enviromental Impact | Reference |
|---|---|---|---|
| NaOH homogeneous- |
|
| |
| HS-catalysed |
|
| |
| - |
| |
|
| ||
| - |
| |
|
|
| Characterisation | Instrument Model | Detector | Operating Conditions |
|---|---|---|---|
| Catalyst | |||
| XRD | Bruker AXS (Bruker, Billerica, MA, USA) | LynxEye detector | Cu-Kα radiation (ń;Kα1 = 1.5406 Å), 40 kV, 40 mA. |
| EDS-SEM | ZeiSS Gemini Auriga (high mag, Carl Zeiss AG, Jena, Germany) | CDU-lad detector | 25 kV |
| FT-IR | Perkin Elmer 100 FT-IR (Perkin Elmer, Waltham, MA, USA | - | Sample configuration data set-range (450–2000 cm−1) |
| BET-BJH | 3Flex version 5.00, serial # 990 (Micromeritics, Norcross, GA, USA) | - | Adsorption-desorption isotherms (77.6 K, 5 h) |
| ** Biodiesel | Instrument/analytical method | - | Reference |
| Density | Density meter (DMA, Anton Paar) | - | Anastopoulos, et al. [62] |
| Viscosity at 40 °C | Rheometer (* DHR) (ISO 3104) | - | AMSEC [63] |
| Acid value | Titrimetric methods (ISO 1242) Titrimetric method (AOCS CD3) | - | Lucas [64] Babajide [65] |
| Saponification | - | ||
| Iodine value | Wij’s method (EN 14111) | - | Japir, et al. [66] |
| FAME/ester | * GC-FID (HP88GC) (EN 14103) | Polar capillary column | Anastopoulos, Zannikou, Stournas and Kalligeros [62] |
| Refractive index, n | Refractometer (#PA203 Misco) | - | - |
| Cetane value, ∅𝑖 | Empirical correlation | - | Ramírez-Verduzco, et al. [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabani, J.M.; Ameh, A.E.; Oyekola, O.; Babajide, O.O.; Petrik, L. Fusion-Assisted Hydrothermal Synthesis and Post-Synthesis Modification of Mesoporous Hydroxy Sodalite Zeolite Prepared from Waste Coal Fly Ash for Biodiesel Production. Catalysts 2022, 12, 1652. https://doi.org/10.3390/catal12121652
Shabani JM, Ameh AE, Oyekola O, Babajide OO, Petrik L. Fusion-Assisted Hydrothermal Synthesis and Post-Synthesis Modification of Mesoporous Hydroxy Sodalite Zeolite Prepared from Waste Coal Fly Ash for Biodiesel Production. Catalysts. 2022; 12(12):1652. https://doi.org/10.3390/catal12121652
Chicago/Turabian StyleShabani, Juvet Malonda, Alechine E. Ameh, Oluwaseun Oyekola, Omotola O. Babajide, and Leslie Petrik. 2022. "Fusion-Assisted Hydrothermal Synthesis and Post-Synthesis Modification of Mesoporous Hydroxy Sodalite Zeolite Prepared from Waste Coal Fly Ash for Biodiesel Production" Catalysts 12, no. 12: 1652. https://doi.org/10.3390/catal12121652
APA StyleShabani, J. M., Ameh, A. E., Oyekola, O., Babajide, O. O., & Petrik, L. (2022). Fusion-Assisted Hydrothermal Synthesis and Post-Synthesis Modification of Mesoporous Hydroxy Sodalite Zeolite Prepared from Waste Coal Fly Ash for Biodiesel Production. Catalysts, 12(12), 1652. https://doi.org/10.3390/catal12121652







