Enhancing the Catalytic Activity of Eggshell-Derived CaO Catalyst and Its Application in Biodiesel Production from Waste Chicken Fat
Abstract
:1. Introduction
2. Result and Discussions
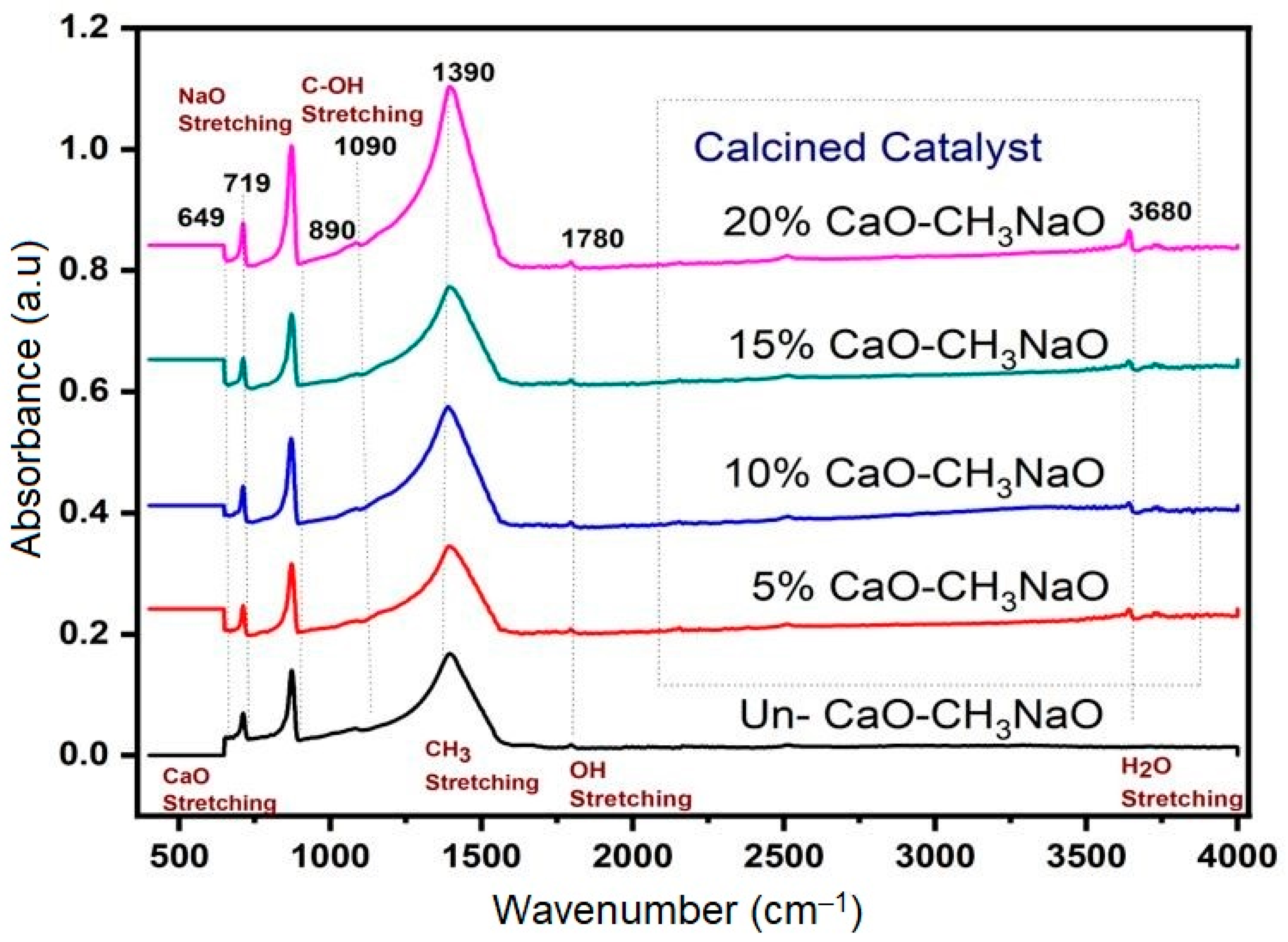
2.1. FTIR Analysis
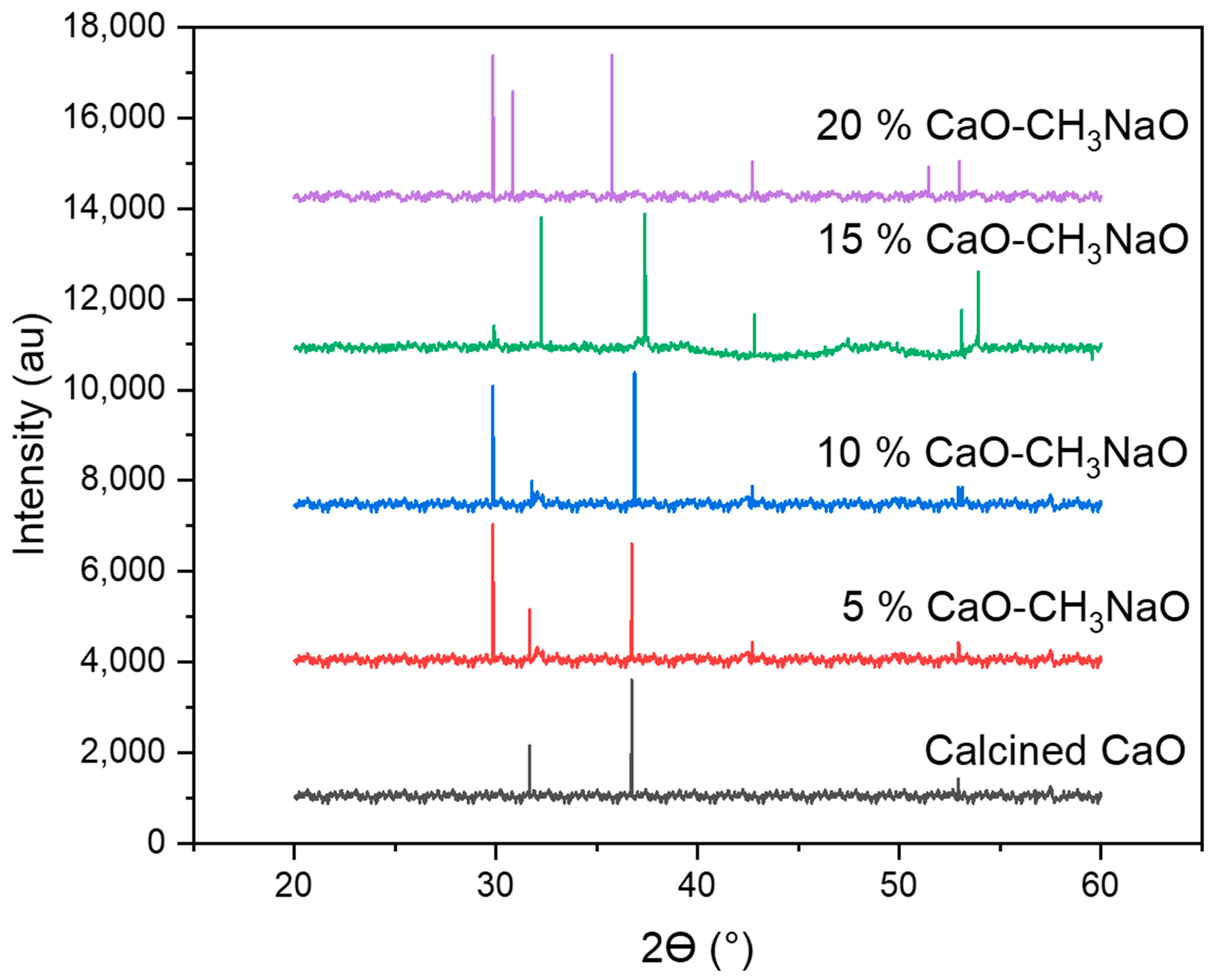
2.2. XRD Characterization
2.3. GC-MS Analysis
2.4. Effect of Reaction Parameters
2.4.1. Effect of Oil to Methanol Ratio
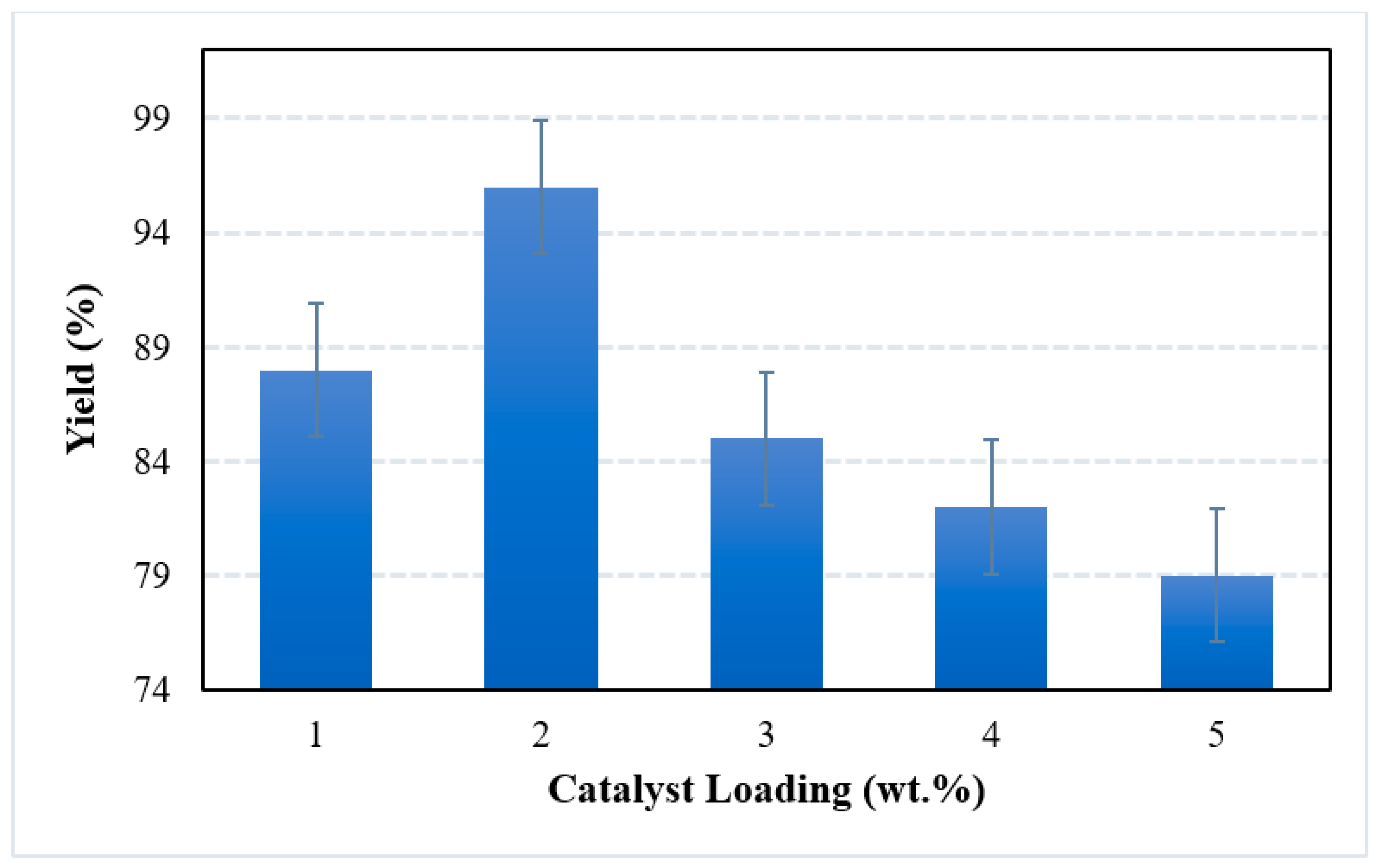
2.4.2. Effect of Catalyst Loading
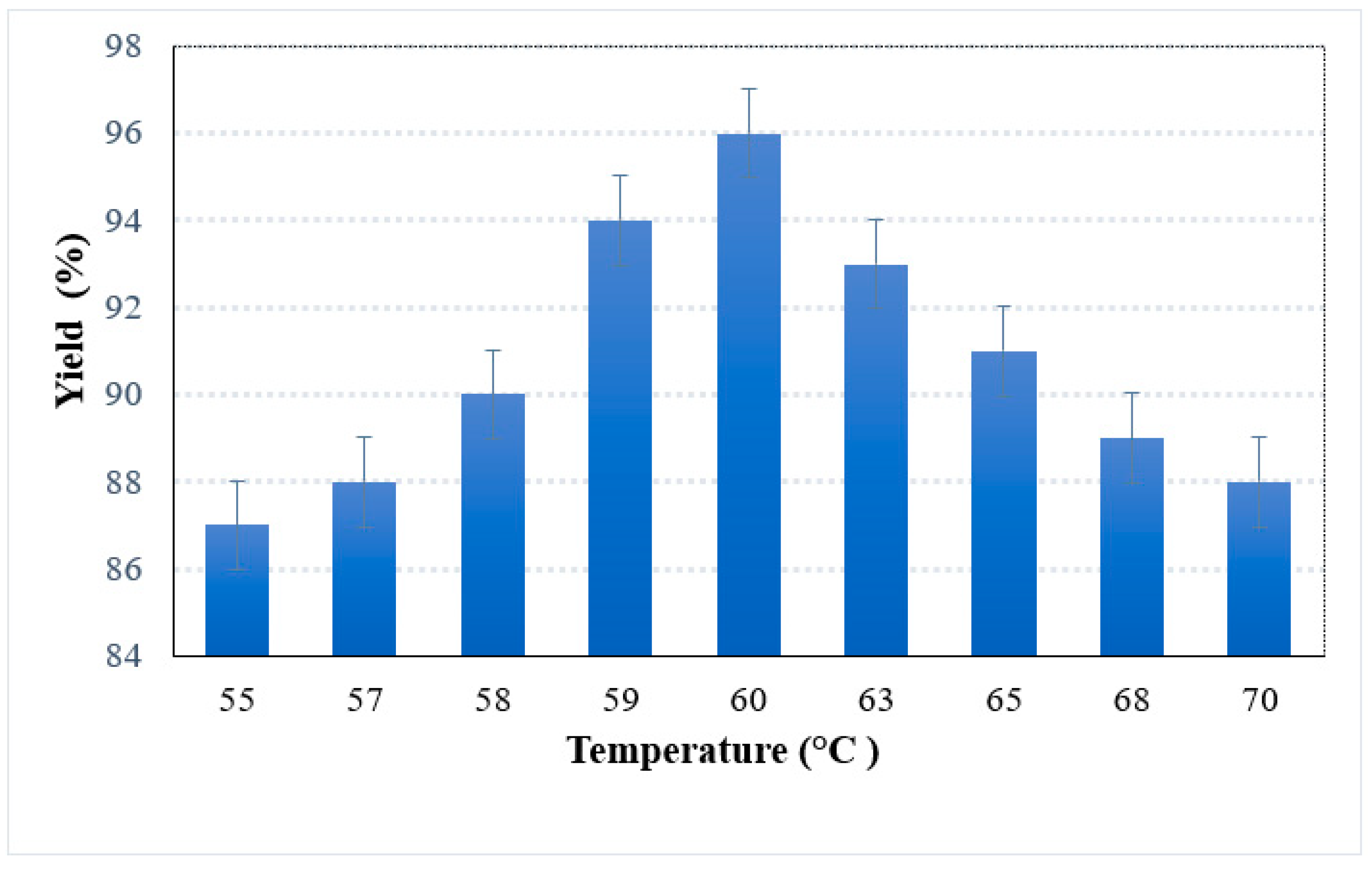
2.4.3. Effect of Temperature
2.4.4. Effect of Reaction Time
2.4.5. Comparative Study of Biodiesel Yield from Waste Chicken Fat
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Oil-Extraction Method
3.2.2. Determination of Acid Value
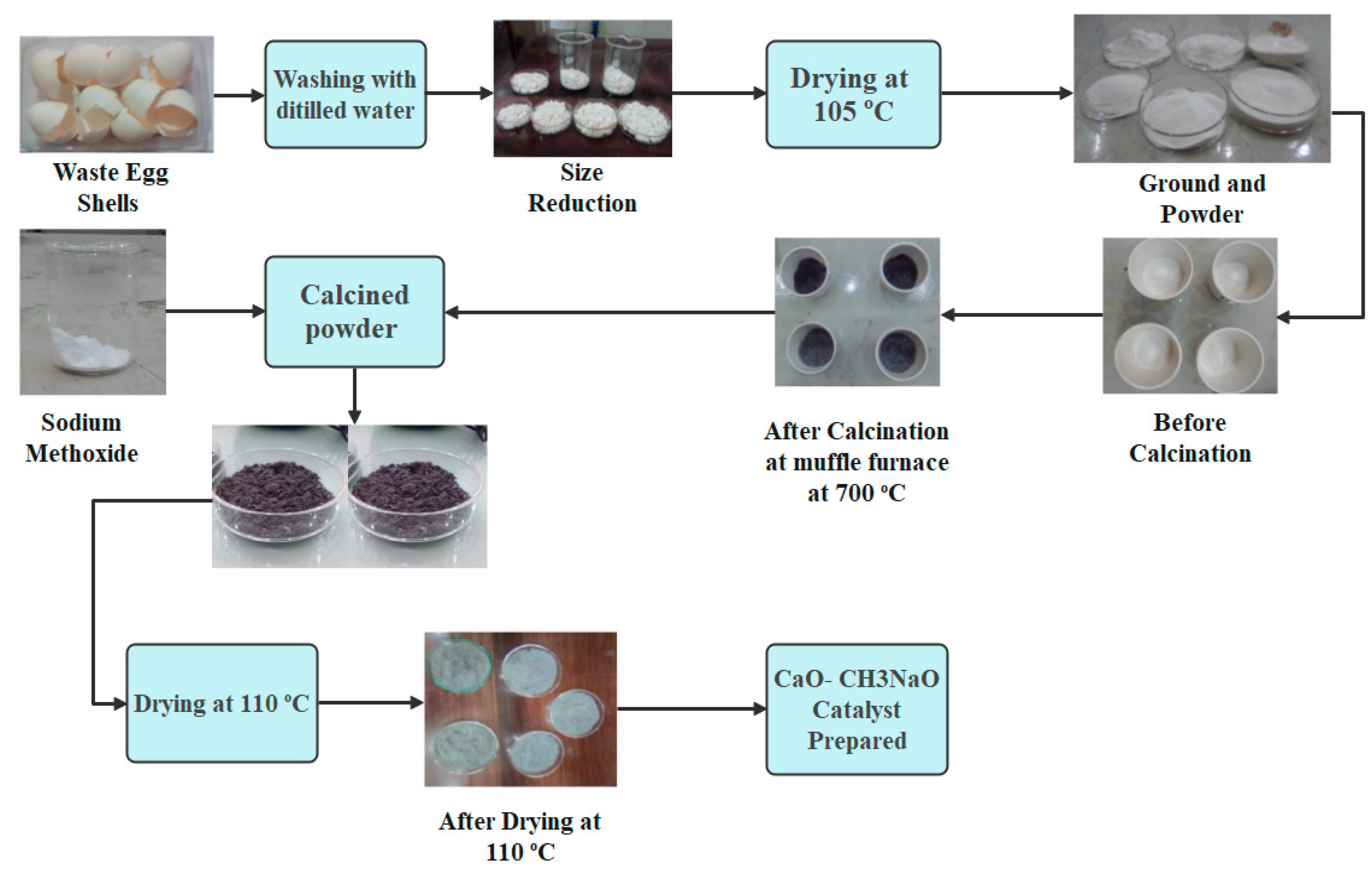
3.2.3. Catalyst Synthesis and Characterization
3.2.4. Transesterification of Chicken Fat Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sajid, M.; Raheem, A.; Ullah, N.; Asim, M.; Ur Rehman, M.S.; Ali, N. Gasification of municipal solid waste: Progress, challenges, and prospects. Renew. Sustain. Energy Rev. 2022, 168, 112815. [Google Scholar] [CrossRef]
- Osman, A.I.; Qasim, U.; Jamil, F.; Al-Muhtaseb, A.H.; Jrai, A.A.; Al-Riyami, M.; Al-Maawali, S.; Al-Haj, L.; Al-Hinai, A.; Al-Abri, M.; et al. Bioethanol and biodiesel: Bibliometric mapping, policies and future needs. Renew. Sustain. Energy Rev. 2021, 152, 111677. [Google Scholar] [CrossRef]
- Inayat, A.; Jamil, F.; Ghenai, C.; Kamil, M.; Bokhari, A.; Waris, A.; Rafiq, S.; Khurram, S. Biodiesel synthesis from neem oil using neem seeds residue as sustainable catalyst support. Biomass Convers. Biorefinery 2021, 1–6. [Google Scholar] [CrossRef]
- Bashir, M.A.; Wu, S.; Zhu, J.; Krosuri, A.; Khan, M.U.; Ndeddy Aka, R.J. Recent development of advanced processing technologies for biodiesel production: A critical review. Fuel Processing Technol. 2022, 227, 107120. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, L.; Esmaeili, H. A review on biodiesel production using various heterogeneous nanocatalysts: Operation mechanisms and performances. Biomass Bioenergy 2022, 158, 106356. [Google Scholar] [CrossRef]
- Ali Qamar, O.; Jamil, F.; Hussain, M.; Al-Muhtaseb, A.H.; Inayat, A.; Waris, A.; Akhter, P.; Park, Y.-K. Feasibility-to-applications of value-added products from biomass: Current trends, challenges, and prospects. Chem. Eng. J. 2022, 454, 140240. [Google Scholar] [CrossRef]
- Basumatary, S.F.; Patir, K.; Das, B.; Saikia, P.; Brahma, S.; Basumatary, B.; Nath, B.; Basumatary, B.; Basumatary, S. Production of renewable biodiesel using metal organic frameworks based materials as efficient heterogeneous catalysts. J. Clean. Prod. 2022, 358, 131955. [Google Scholar] [CrossRef]
- Zulkepli, S.; Lee, H.V.; Rahman, N.A.; Chuan, L.T.; Show, P.L.; Chen, W.-H.; Juan, J.C. Highly active iron-promoted hexagonal mesoporous silica (HMS) for deoxygenation of triglycerides to green hydrocarbon-like biofuel. Fuel 2022, 308, 121860. [Google Scholar] [CrossRef]
- Shaah, M.A.H.; Hossain, M.S.; Salem Allafi, F.A.; Alsaedi, A.; Ismail, N.; Ab Kadir, M.O.; Ahmad, M.I. A review on non-edible oil as a potential feedstock for biodiesel: Physicochemical properties and production technologies. RSC Adv. 2021, 11, 25018–25037. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of biodiesel production from waste cooking oil and its use as fuel in compression ignition engines: 3rd generation cleaner feedstock. J. Clean. Prod. 2021, 307, 127299. [Google Scholar] [CrossRef]
- Abu-Jrai, A.M.; Jamil, F.; Al-Muhtaseb, A.H.; Baawain, M.; Al-Haj, L.; Al-Hinai, M.; Al-Abri, M.; Rafiq, S. Valorization of waste Date pits biomass for biodiesel production in presence of green carbon catalyst. Energy Convers. Manag. 2017, 135, 236–243. [Google Scholar] [CrossRef]
- Sitepu, E.K.; Heimann, K.; Raston, C.L.; Zhang, W. Critical evaluation of process parameters for direct biodiesel production from diverse feedstock. Renew. Sustain. Energy Rev. 2020, 123, 109762. [Google Scholar] [CrossRef]
- Ningaraju, C.; Yatish, K.V.; Sakar, M. Simultaneous refining of biodiesel-derived crude glycerol and synthesis of value-added powdered catalysts for biodiesel production: A green chemistry approach for sustainable biodiesel industries. J. Clean. Prod. 2022, 363, 132448. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Tan, X.; Khoo, K.S.; Ng, H.S.; Jonglertjunya, W.; Yew, G.Y.; Show, P.L. Microalgae-based bioplastics: Future solution towards mitigation of plastic wastes. Environ. Res. 2022, 206, 112620. [Google Scholar] [CrossRef] [PubMed]
- Jamil, F.; Saleem, M.; Qamar, O.A.; Khurram, M.S.; Al-Muhtaseb, A.H.; Inayat, A.; Akhter, P.; Hussain, M.; Rafiq, S.; Yim, H.; et al. State-of-the-art catalysts for clean fuel (methyl esters) production—A comprehensive review. J. Phys. Energy 2022, 5, 014005. [Google Scholar] [CrossRef]
- Chanthon, N.; Ngaosuwan, K.; Kiatkittipong, W.; Wongsawaeng, D.; Appamana, W.; Quitain, A.T.; Assabumrungrat, S. High-efficiency biodiesel production using rotating tube reactor: New insight of operating parameters on hydrodynamic regime and biodiesel yield. Renew. Sustain. Energy Rev. 2021, 151, 111430. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, J. Valorisation of food waste using salt to alleviate inhibition by animal fats and vegetable oils during anaerobic digestion. Biomass Bioenergy 2020, 143, 105826. [Google Scholar] [CrossRef]
- Ndiaye, M.; Arhaliass, A.; Legrand, J.; Roelens, G.; Kerihuel, A. Reuse of waste animal fat in biodiesel: Biorefining heavily-degraded contaminant-rich waste animal fat and formulation as diesel fuel additive. Renew. Energy 2020, 145, 1073–1079. [Google Scholar] [CrossRef]
- Sai Akhil, U.; Alagumalai, A. A Short Review on Valorization of Slaughterhouse Wastes for Biodiesel Production. ChemistrySelect 2019, 4, 13356–13362. [Google Scholar] [CrossRef]
- Kirubakaran, M.; Selvan, V.A.M. A comprehensive review of low cost biodiesel production from waste chicken fat. Renew. Sustain. Energy Rev. 2018, 82, 390–401. [Google Scholar] [CrossRef]
- Riaz, I.; Shafiq, I.; Jamil, F.; Al-Muhtaseb, A.H.; Akhter, P.; Shafique, S.; Park, Y.-K.; Hussain, M. A review on catalysts of biodiesel (methyl esters) production. Catal. Rev. 2022, 1–53. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Teo, S.H.; Rashid, U.; Islam, A.; Hussien, M.Z.; Lee, K.T. Transesterification of Jatropha curcas crude oil to biodiesel on calcium lanthanum mixed oxide catalyst: Effect of stoichiometric composition. Energy Convers. Manag. 2014, 88, 1290–1296. [Google Scholar] [CrossRef]
- Munyentwali, A.; Li, H.; Yang, Q. Review of advances in bifunctional solid acid/base catalysts for sustainable biodiesel production. Appl. Catal. A Gen. 2022, 633, 118525. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, H.; Hu, Y.; Rao, K.T.V.; Xu, C.; Yang, S. Advances in production of bio-based ester fuels with heterogeneous bifunctional catalysts. Renew. Sustain. Energy Rev. 2019, 114, 109296. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.-L.; Tan, X.; Li, H.; Yang, S. Catalytic high-yield biodiesel production from fatty acids and non-food oils over a magnetically separable acid nanosphere. Ind. Crops Prod. 2021, 173, 114126. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, H.; Li, H.; Yang, S. Electrovalent bifunctional acid enables heterogeneously catalytic production of biodiesel by (trans)esterification of non-edible oils. Fuel 2022, 310, 122273. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.H.; Rashid, U.; Saiman, M.I.; Tan, Y.P.; Alsultan, G.A.; Taufiq-Yap, Y.H. Modified waste egg shell derived bifunctional catalyst for biodiesel production from high FFA waste cooking oil. A review. Renew. Sustain. Energy Rev. 2018, 82, 3645–3655. [Google Scholar] [CrossRef]
- Sronsri, C.; Sittipol, W.; U-yen, K. Performance of CaO catalyst prepared from magnetic-derived CaCO3 for biodiesel production. Fuel 2021, 304, 121419. [Google Scholar] [CrossRef]
- Kesserwan, F.; Ahmad, M.N.; Khalil, M.; El-Rassy, H. Hybrid CaO/Al2O3 aerogel as heterogeneous catalyst for biodiesel production. Chem. Eng. J. 2020, 385, 123834. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M.H. Biodiesel production from microalgal biomass using CaO catalyst synthesized from natural waste material. Renew. Energy 2019, 136, 837–845. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N.; Inuwa, I.M. New route for the synthesis of silica-supported calcium oxide catalyst in biodiesel production. Renew. Energy 2020, 156, 1266–1277. [Google Scholar] [CrossRef]
- Badnore, A.U.; Jadhav, N.L.; Pinjari, D.V.; Pandit, A.B. Efficacy of newly developed nano-crystalline calcium oxide catalyst for biodiesel production. Chem. Eng. Process.-Process Intensif. 2018, 133, 312–319. [Google Scholar] [CrossRef]
- Das, V.; Tripathi, A.M.; Borah, M.J.; Dunford, N.T.; Deka, D. Cobalt-doped CaO catalyst synthesized and applied for algal biodiesel production. Renew. Energy 2020, 161, 1110–1119. [Google Scholar] [CrossRef]
- Zul, N.A.; Ganesan, S.; Hamidon, T.S.; Oh, W.-D.; Hussin, M.H. A review on the utilization of calcium oxide as a base catalyst in biodiesel production. J. Environ. Chem. Eng. 2021, 9, 105741. [Google Scholar] [CrossRef]
- Poosumas, J.; Ngaosuwan, K.; Quitain, A.T.; Assabumrungrat, S. Role of ultrasonic irradiation on transesterification of palm oil using calcium oxide as a solid base catalyst. Energy Convers. Manag. 2016, 120, 62–70. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Ryu, J.-H.; Bae, S.-Y.; Crofcheck, C.; Crocker, M. Lipid extraction from Scenedesmus sp. microalgae for biodiesel production using hot compressed hexane. Fuel 2014, 130, 66–69. [Google Scholar] [CrossRef] [Green Version]
- Meher, L.C.; Kulkarni, M.G.; Dalai, A.K.; Naik, S.N. Transesterification of karanja (Pongamia pinnata) oil by solid basic catalysts. Eur. J. Lipid Sci. Technol. 2006, 108, 389–397. [Google Scholar] [CrossRef]
- Cho, Y.B.; Seo, G. High activity of acid-treated quail eggshell catalysts in the transesterification of palm oil with methanol. Bioresour. Technol. 2010, 101, 8515–8519. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Chervonnyi, A.D. Infrared Spectroscopy of Minerals and Related Compounds, 1st ed.; Springer: Cham, Switzerland, 2016; p. 1109. [Google Scholar]
- Fiquet, G.; Richet, P.; Montagnac, G. High-temperature thermal expansion of lime, periclase, corundum and spinel. Phys. Chem. Miner. 1999, 27, 103–111. [Google Scholar] [CrossRef]
- Barrett, C.S. X-ray study of the alkali metals at low temperatures. Acta Crystallogr. 1956, 9, 671–677. [Google Scholar] [CrossRef]
- Verbraeken, M.C.; Suard, E.; Irvine, J.T.S. Structural and electrical properties of calcium and strontium hydrides. J. Mater. Chem. 2009, 19, 2766–2770. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Crystal Structures 1, 2nd ed.; Interscience Publishers: New York, NY, USA, 1963; pp. 7–83. [Google Scholar]
- Eboibi, B.E.; Eboibi, O.; Okputu, J.; Okpohwo, K.A. Production and analysis of biodiesel from Jatropha curcas seed. J. Appl. Sci. Environ. Manag. 2018, 22, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Mata, T.M.; Cardoso, N.; Ornelas, M.; Neves, S.; Caetano, N.S. Evaluation of Two Purification Methods of Biodiesel from Beef Tallow, Pork Lard, and Chicken Fat. Energy Fuels 2011, 25, 4756–4762. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M.; Sanli, H. Methyl ester production from chicken fat with high FFA. In Proceedings of the World Renewable Energy Congress-Sweden, Linköping, Sweden, 8–13 May 2011; pp. 319–326. [Google Scholar]
- Odetoye, T.E.; Agu, J.O.; Ajala, E.O. Biodiesel production from poultry wastes: Waste chicken fat and eggshell. J. Environ. Chem. Eng. 2021, 9, 105654. [Google Scholar] [CrossRef]
- Seffati, K.; Honarvar, B.; Esmaeili, H.; Esfandiari, N. Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 2019, 235, 1238–1244. [Google Scholar] [CrossRef]
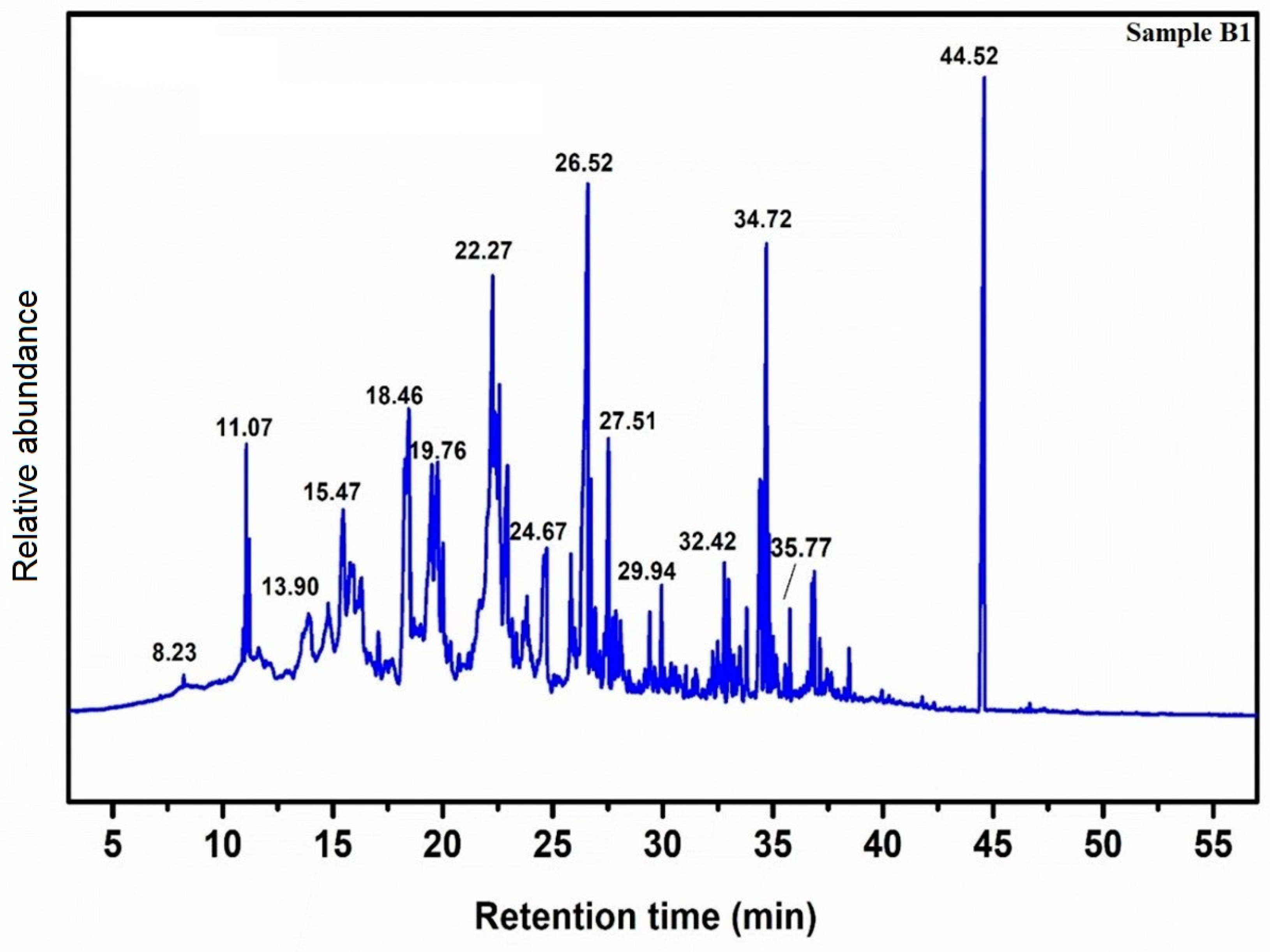

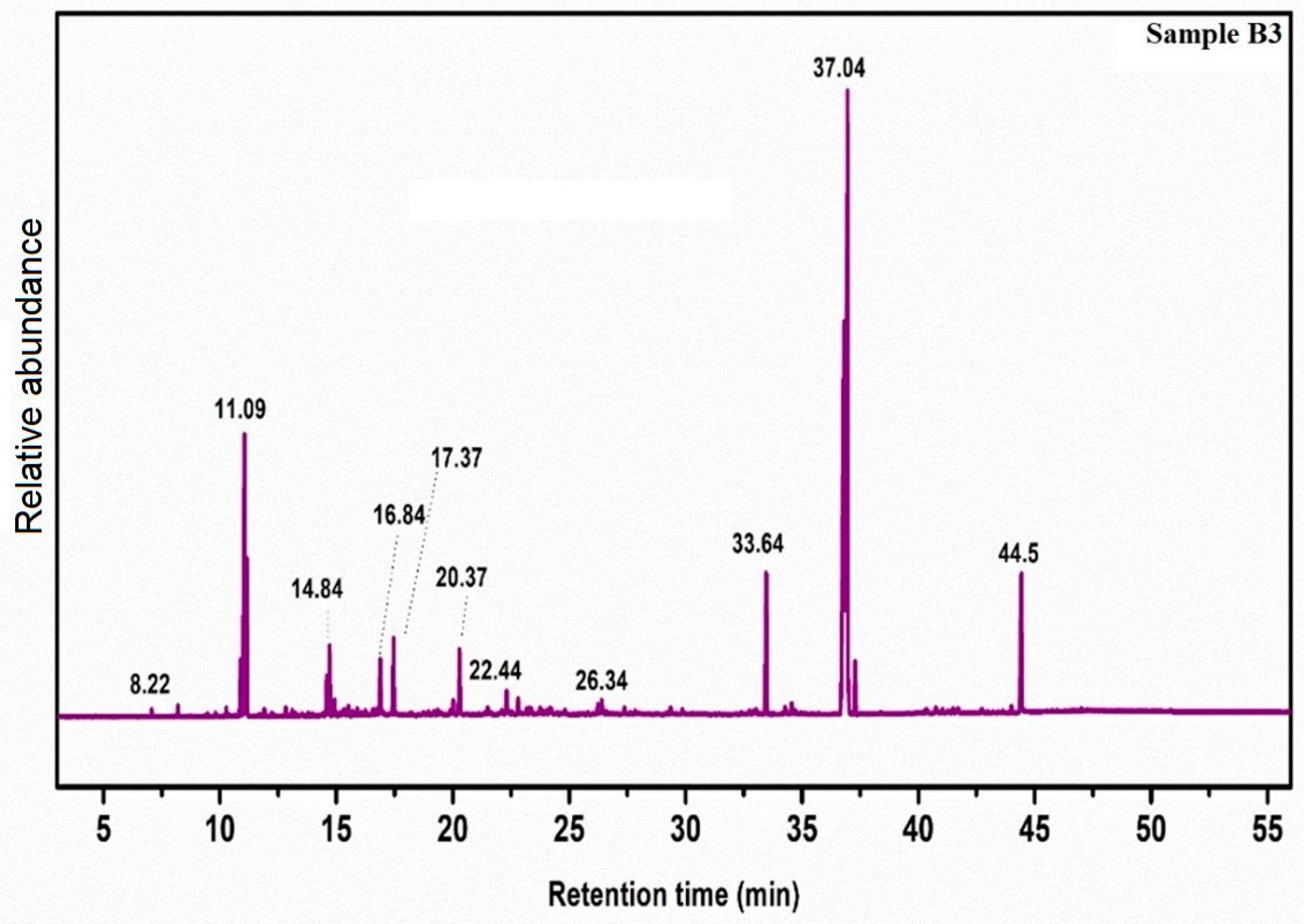


| 5% CaO-CH3NaO | 10% CaO-CH3NaO | 15% CaO-CH3NaO | ||||||
|---|---|---|---|---|---|---|---|---|
| Ret. Time | Composition | Mw | Ret. Time | Composition | Mw | Ret. Time | Composition | Mw |
| 8.23 | Cyclohexene,1-methyl-5 | 270 | 8.23 | Cyclohexene,1-methyl-5 | 128 | 8.22 | Methyl ethyl ester | 186 |
| 13.90 | 5-dimethyl-benzene methanol | 398 | 8.24 | Isobutyl ester | 128 | 11.09 | Encalyptol | 284 |
| 18.46 | 6-heptanoic acid, methyl ester | 438 | 11.41 | 3-methyl butyl ester | 284 | 14.84 | Cyclo hexene | 296 |
| 19.76 | 2,4-hexadenoic acid, methyl ester | 264 | 14.71 | 2-propenoic acid, methyl ester | 296 | 16.84 | Citronellol | 208 |
| 22.27 | 3-hexadiene-1-ol | 100 | 17.01 | 7-methylene-2,4,4-timethyl, methyl ester | 320 | 22.24 | 2-cyclo hexe-1one | 296 |
| 32.42 | 6- Octenoic acid | 214 | 17.61 | Mono-(2,2-dimethyl cyclo hexyl acid) ester | 250 | 26.34 | Napthalene | 156 |
| 33.49 | Hexadecanoic acid, methyl ester | 438 | 20.12 | Hexa decanoic acid, methyl ester | 138 | 29.86 | Docosaptenoic acid, methyl ester | 214 |
| 35.577 | Pentadecanoic acid, methyl ester | 466 | 26.62 | 6-octenoic acid, methyl ester | 284 | 33.64 | Hexa decenoic acid, methyl ester | 310 |
| 44.52 | 7-dimethyl-6-octenyl ester | 389 | 34.65 | Tetra methyl thioctic acid | 160 | 37.04 | Hepta deconic acid, methyl ester | 348 |
| 36.85 | 9-octenoic acid, methyl ester | 203 | 40.35 | 11-eicosenoic acid, methyl ester | 408 | |||
| 44.49 | Mono ethyl hexyl ester | 307 | 44.5 | Docasenoic acid, methyl ester | 450 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, M.; Jamil, F.; Qamar, O.A.; Akhter, P.; Hussain, M.; Khurram, M.S.; Al-Muhtaseb, A.H.; Inayat, A.; Shah, N.S. Enhancing the Catalytic Activity of Eggshell-Derived CaO Catalyst and Its Application in Biodiesel Production from Waste Chicken Fat. Catalysts 2022, 12, 1627. https://doi.org/10.3390/catal12121627
Saleem M, Jamil F, Qamar OA, Akhter P, Hussain M, Khurram MS, Al-Muhtaseb AH, Inayat A, Shah NS. Enhancing the Catalytic Activity of Eggshell-Derived CaO Catalyst and Its Application in Biodiesel Production from Waste Chicken Fat. Catalysts. 2022; 12(12):1627. https://doi.org/10.3390/catal12121627
Chicago/Turabian StyleSaleem, Muhammad, Farrukh Jamil, Obaid Ali Qamar, Parveen Akhter, Murid Hussain, Muhammad Shahzad Khurram, Ala’a H. Al-Muhtaseb, Abrar Inayat, and Noor Samad Shah. 2022. "Enhancing the Catalytic Activity of Eggshell-Derived CaO Catalyst and Its Application in Biodiesel Production from Waste Chicken Fat" Catalysts 12, no. 12: 1627. https://doi.org/10.3390/catal12121627
APA StyleSaleem, M., Jamil, F., Qamar, O. A., Akhter, P., Hussain, M., Khurram, M. S., Al-Muhtaseb, A. H., Inayat, A., & Shah, N. S. (2022). Enhancing the Catalytic Activity of Eggshell-Derived CaO Catalyst and Its Application in Biodiesel Production from Waste Chicken Fat. Catalysts, 12(12), 1627. https://doi.org/10.3390/catal12121627











