Hydrothermal Liquefaction of Lignocellulosic and Protein-Containing Biomass: A Comprehensive Review
Abstract
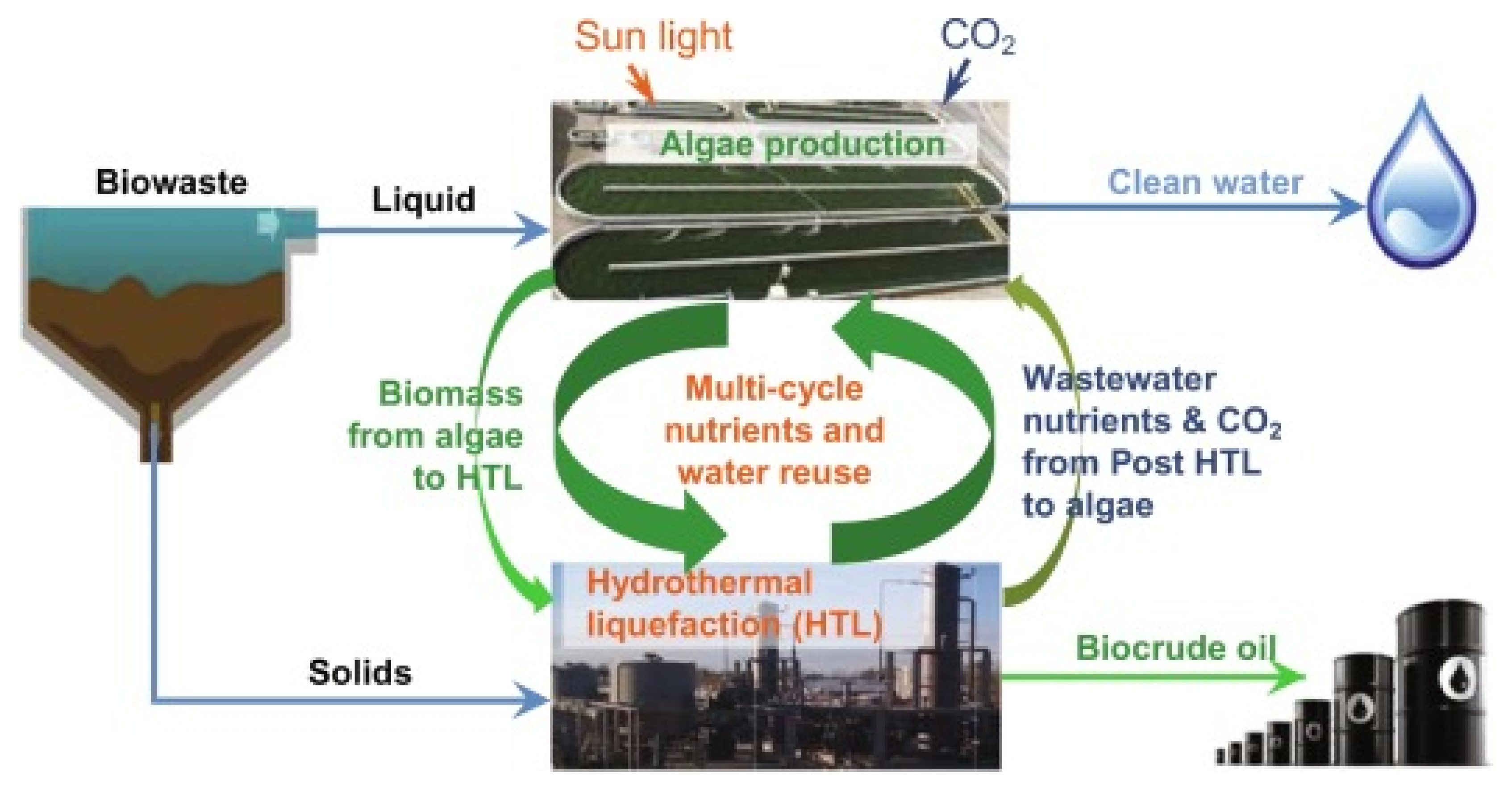
1. Introduction
2. Liquefaction Mechanism of Lignocellulose
2.1. Hydrothermal Liquefaction of Cellulose
2.2. Hydrothermal Liquefaction of Hemicellulose
2.3. Hydrothermal Liquefaction of Lignin
3. Protein-Containing Feed for Hydrothermal Liquefaction
4. Factors Affecting the Hydrothermal Liquefaction
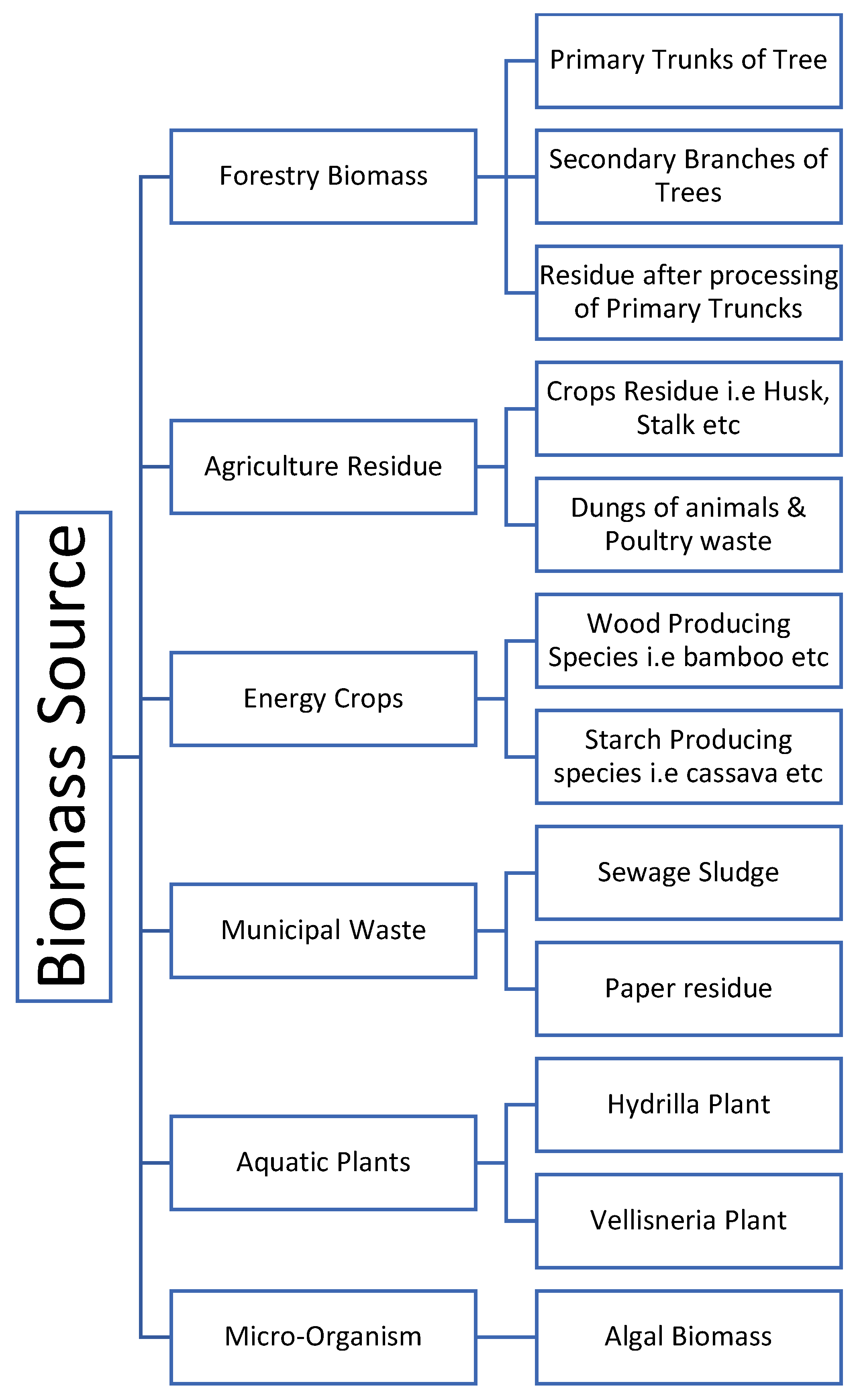
4.1. Types of Lignocellulose
4.2. Process Mode
4.3. Process Conditions
4.4. Catalysis
| Feedstocks | Temp (°C) | Catalyst | Non-Cat-Yield (%) | Cat-Yield (%) | Change in Yield (%), by Value | Change in C (%) by Value | Change in N (%) by Value | Change in HHV (MJ/kg) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Lignocellulosic biomass | |||||||||
| Wood (birchwood sawdust) | 300 | K2CO3 | 19.11 | 38 | 19 | −4.00 | −0.03 | −3.00 | [139] |
| Wood (birchwood sawdust) | 300 | KOH | 19.11 | 39 | 20 | −5.00 | 0.00 | −3.00 | [139] |
| Wood (birchwood sawdust) | 300 | FeSO4 | 19.11 | 32 | 13 | −4.00 | 0.03 | 2.00 | [139] |
| Wood (birchwood sawdust) | 300 | MgO | 19.11 | 30 | 11 | −5.00 | 0.03 | −2.00 | [139] |
| Oak Wood | 330 | Nickel Powder | 33.12 | 35 | 2 | −1.00 | 0.00 | 0.31 | [140] |
| Eucalyptus | 350 | K2CO3 | 33.12 | 37 | 4 | 3.53 | −0.09 | 1.54 | [141] |
| Eucalyptus | 400 | K2CO3 | 27 | 29 | 2 | −6.50 | −0.15 | 2.93 | [141] |
| Wheat straw | 350 | K2CO3 | 26 | 32 | 6 | 2.61 | −0.19 | 0.54 | [142] |
| Wheat straw | 400 | K2CO3 | 24 | 23 | −1 | −1.18 | 0.27 | −0.47 | [142] |
| Barley straw | 300 | K2CO3 | 18 | 34 | 16 | 5.26 | 0.06 | 2.42 | [99] |
| Animal Manures | |||||||||
| Cow manure | 350 | K2CO3 | 41 | 35 | −6 | 9.00 | 0.90 | 3.00 | [142] |
| Cow manure | 400 | K2CO3 | 32.37 | 32.29 | −0.01 | 4.41 | 0.00 | 0.00 | [142] |
| Swine manure | 350 | K2CO3 | 41 | 37 | −4 | 5.00 | 0.60 | 3.30 | [142] |
| Swine manure | 400 | K2CO3 | 36.97 | 34.76 | −2 | −0.33 | −0.09 | 3.09 | [142] |
| Protein-containing biomass | |||||||||
| Fish sludge | 350 | K2CO3 | 59 | 51 | −8 | 0.92 | 0.17 | 0.50 | [142] |
| Fish sludge | 400 | K2CO3 | 51.27 | 47.17 | −4 | 0.85 | 0.35 | 0.12 | [142] |
| Sewage sludge | 350 | K2CO3 | 40.65 | 45 | 4.45 | 2.51 | −1.02 | 1.30 | [12] |
| Sewage sludge | 400 | K2CO3 | 40.13 | 43 | 2.87 | 1.15 | −1.43 | 0.26 | [12] |
| Sewage sludge | 300 | NiMo/Al2O3 | 27 | 24 | −3 | 4 | 0.03 | 2.42 | [143] |
| Sewage sludge | 300 | CoMo/Al2O3 | 27 | 21 | −6 | 1 | 2.29 | 0.42 | [143] |
| Sewage sludge | 300 | Activated Carbon | 27 | 23 | −4 | 1 | 1.30 | 3.42 | [143] |
| Biopulp (Food waste) | 350 | K2CO3 | 28.9 | 36.6 | 7.5 | 1.24 | −0.09 | 0.80 | [144] |
| Spent compost mushroom | 400 | K2CO3 | 22.86 | 20.42 | −1.8 | −1.47 | −0.65 | −0.67 | [145] |
| Macroalgae (Ulva prolifera) | 280 | MgO | 17 | 16 | −1 | 13 | −0.4 | −2.80 | [146] |
| Macroalgae (Ulva prolifera) | 280 | Al2O3 | 17 | 26 | 9 | 10 | −0.7 | −3.40 | [146] |
| Macroalgae (Ulva prolifera) | 280 | MgCl2 | 17 | 27 | 10 | 11 | −0.2 | 0.60 | [146] |
| Microalgae (Chlorella vulgaris) | 350 | Na2CO3 | 38 | 23 | −15 | 2.90 | −1.60 | 2.00 | [142] |
| Microalgae (Nannochloropsis) | 350 | Na2CO3 | 37 | 21 | −16 | 1.50 | −0.30 | 1.00 | [142] |
| Microalgae (Porphyridium) | 350 | Na2CO3 | 21 | 21 | 0.00 | −26.70 | −2.20 | −12.90 | [142] |
| Microalgae (Nannochloropsis) | 350 | Pd/C | 35 | 57 | 22 | −2 | −0.38 | 0.00 | [147] |
| Microalgae (Chlorella vulgaris) | 300 | NiMo/ Al2O3 | 32 | 29 | −3 | 10.77 | 3.52 | 4.10 | [143] |
| Microalgae (Chlorella vulgaris) | 300 | CoMo/ Al2O3 | 32 | 35 | 3 | 12.19 | 3.49 | 4.97 | [143] |
| Soyabean oil (Triyglycerides) | 320 | KH2PO4 | 85.9 | 93.5 | 8.85 | 1.40 | 0.22 | −1.00 | [148] |
| Soy protein | 320 | K2HPO4 | 28.85 | 31.25 | 2.95 | 0.54 | 0.39 | −0.14 | [149] |
| Potato starch | 320 | K2HPO4 | 11.07 | 22.1 | 11 | 2.60 | −0.01 | 3.04 | [148] |
| Potato starch | 320 | Na2CO3 | 11.07 | 18.77 | 7.6 | −1.32 | 0.00 | 2.14 | [148] |
| Human feces | 330 | Ni-Tm/TiO2 | 40 | 44 | 4 | −4.8 | 1.18 | −4.7 | [149] |
| Human feces | 330 | Tm/ TiO2 | 40 | 40 | 0.0 | −2.6 | 0.47 | −2.7 | [149] |
| Human feces | 330 | Ni/ TiO2 | 40 | 41 | 1 | −3.8 | 0.64 | −2.7 | [149] |
| Human feces | 330 | TiO2 | 40 | 40 | 0.0 | 0.79 | 0.79 | −0.78 | [149] |
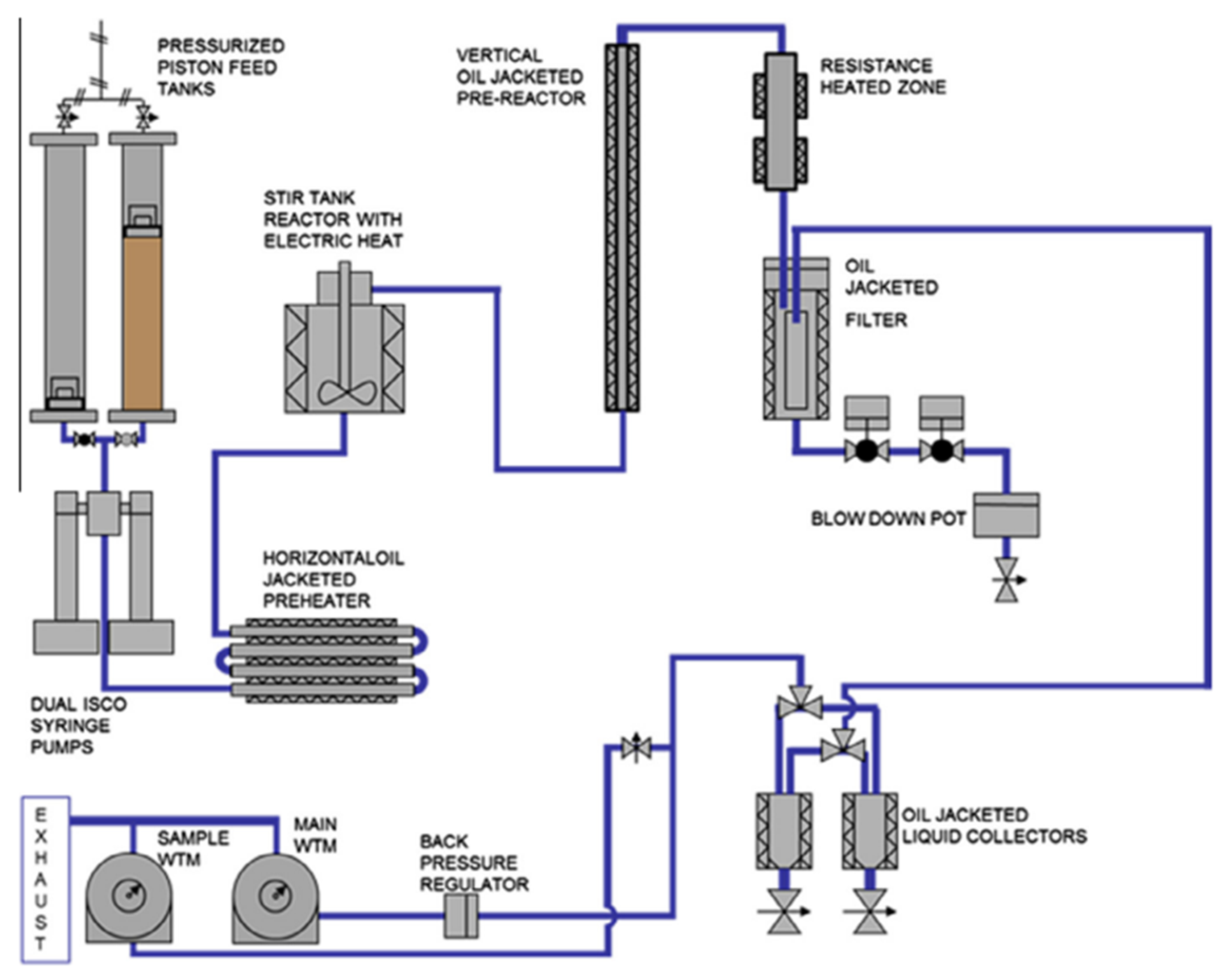
4.5. HTL Products Separation
5. Challenges in HTL and Recommendations
5.1. Feedstocks Perspective
5.2. Setting a Common Paradigm for Product Processing
5.3. Process Mode
5.4. Undertaking the Whole Chain (HTL with Catalytic Hydrotreatment)
Author Contributions
Funding
Conflicts of Interest
References
- Moodley, P.; Sewsynker-Sukai, Y.; Kana, E.G. Progress in the development of alkali and metal salt catalysed lignocellulosic pretreatment regimes: Potential for bioethanol production. Bioresour. Technol. 2020, 310, 123372. [Google Scholar] [CrossRef] [PubMed]
- Sewsynker-Sukai, Y.; David, A.N.; Kana, E.G. Recent developments in the application of kraft pulping alkaline chemicals for lignocellulosic pretreatment: Potential beneficiation of green liquor dregs waste. Bioresour. Technol. 2020, 306, 123225. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-A.; Zhou, Y.; Qin, Y.; Liu, D.; Zhao, X. Evaluation of the action of Tween 20 non-ionic surfactant during enzymatic hydrolysis of lignocellulose: Pretreatment, hydrolysis conditions and lignin structure. Bioresour. Technol. 2018, 269, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Toor, S.S.; Seehar, T.H.; Sadetmahaleh, K.K.; Pedersen, T.H.; Nielsen, A.H.; Rosendahl, L.A. Biocrude production through co-hydrothermal processing of swine manure with sewage sludge to enhance pumpability. Fuel 2021, 288, 119407. [Google Scholar] [CrossRef]
- Beig, B.; Riaz, M.; Naqvi, S.R.; Hassan, M.; Zheng, Z.; Karimi, K.; Pugazhendhi, A.; Atabani, A.; Chi, N.T.L. Current challenges and innovative developments in pretreatment of lignocellulosic residues for biofuel production: A review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Li, M.-F. Hydrothermal liquefaction of lignocellulose for value-added products: Mechanism, parameter and production application. Bioresour. Technol. 2021, 342, 126035. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.S.; Shah, A.A.; Sharma, K.; Seehar, T.H.; Pedersen, T.H.; Rosendahl, L.A. Biocrude Production from Protein-Extracted Grass Residue through Hydrothermal Liquefaction. Energies 2022, 15, 364. [Google Scholar] [CrossRef]
- Ding, Y.; Shan, B.; Cao, X.; Liu, Y.; Huang, M.; Tang, B. Development of bio oil and bio asphalt by hydrothermal liquefaction using lignocellulose. J. Clean. Prod. 2021, 288, 125586. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef]
- Vo, T.K.; Lee, O.K.; Lee, E.Y.; Kim, C.H.; Seo, J.-W.; Kim, J.; Kim, S.-S. Kinetics study of the hydrothermal liquefaction of the microalga Aurantiochytrium sp. KRS101. Chem. Eng. J. 2016, 306, 763–771. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, Q.; Zhang, Q.; Wu, K.; Pu, W.; Yang, M.; Wu, Y. Catalytic hydrothermal liquefaction of Euglena sp. microalgae over zeolite catalysts for the production of biocrude. RSC Adv. 2017, 7, 8944–8951. [Google Scholar] [CrossRef]
- Shah, A.A.; Toor, S.S.; Conti, F.; Nielsen, A.H.; Rosendahl, L.A. Hydrothermal liquefaction of high ash containing sewage sludge at sub and supercritical conditions. Biomass Bioenergy 2020, 135, 105504. [Google Scholar] [CrossRef]
- Maag, A.R.; Paulsen, A.D.; Amundsen, T.J.; Yelvington, P.E.; Tompsett, G.A.; Timko, M.T. Catalytic hydrothermal liquefaction of food waste using CeZrOx. Energies 2018, 11, 564. [Google Scholar] [CrossRef]
- Alper, K.; Wang, Y.-Y.; Meng, X.; Tekin, K.; Karagoz, S.; Ragauskas, A.J. Use of a Lewis acid, a Brønsted acid, and their binary mixtures for the hydrothermal liquefaction of lignocellulose. Fuel 2021, 304, 121398. [Google Scholar] [CrossRef]
- Shah, A.A.; Sharma, K.; Haider, M.S.; Toor, S.S.; Rosendahl, L.A.; Pedersen, T.H.; Castello, D. The Role of Catalysts in Biomass Hydrothermal Liquefaction and Biocrude Upgrading. Processes 2022, 10, 207. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- dos Passos, J.S.; Chiaberge, S.; Biller, P. Combined Hydrothermal Liquefaction of Polyurethane and Lignocellulosic Biomass for improved carbon recovery. Energy Fuels 2021, 35, 10630–10640. [Google Scholar] [CrossRef]
- Mahssin, Z.Y.; Zainol, M.M.; Hassan, N.A.; Yaacob, H.; Puteh, M.H.; Amin, N.A.S. Hydrothermal liquefaction bioproduct of food waste conversion as an alternative composite of asphalt binder. J. Clean. Prod. 2021, 282, 125422. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Yang, T.; Feng, B.; Kai, X.; Wang, S.; Li, R. Aqueous phase reforming of biocrude derived from lignocellulose hydrothermal liquefaction: Conditions optimization and mechanism study. Renew. Energy 2021, 175, 98–107. [Google Scholar] [CrossRef]
- Basar, I.A.; Liu, H.; Carrere, H.; Trably, E.; Eskicioglu, C. A review on key design and operational parameters to optimize and develop hydrothermal liquefaction of biomass for biorefinery applications. Green Chem. 2021, 23, 1404–1446. [Google Scholar] [CrossRef]
- Miyata, Y.; Sagata, K.; Yamazaki, Y.; Teramura, H.; Hirano, Y.; Ogino, C.; Kita, Y. Mechanism of the Fe-assisted hydrothermal liquefaction of lignocellulosic biomass. Ind. Eng. Chem. Res. 2018, 57, 14870–14877. [Google Scholar] [CrossRef]
- Stablein, M.J.; Aierzhati, A.; Watson, J.; Si, B.; Zhang, Y. Characterization and bioremediation potential of byproducts from hydrothermal liquefaction of food wastes. Bioresour. Technol. Rep. 2020, 12, 100555. [Google Scholar] [CrossRef]
- Garba, N.A.; Usman, J.; Anka, M.M. Hydrothermal Liquefaction of Lignocellulosic Biomass to Liquid Biofuels: A Review. Int. J. Sci. Glob. Sustain. 2020, 6, 8. [Google Scholar]
- Shah, A.A.; Seehar, T.H.; Sharma, K.; Toor, S.S. Biomass pretreatment technologies. In Hydrocarbon Biorefinery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–228. [Google Scholar]
- Ellersdorfer, M. Hydrothermal co-liquefaction of Chlorella vulgaris with food processing residues, green waste and sewage sludge. Biomass Bioenergy 2020, 142, 105796. [Google Scholar] [CrossRef]
- Howard, R.; Abotsi, E.; Van Rensburg, E.J.; Howard, S. Lignocellulose biotechnology: Issues of bioconversion and enzyme production. Afr. J. Biotechnol. 2003, 2, 602–619. [Google Scholar] [CrossRef]
- Ludueña, L.; Fasce, D.; Alvarez, V.A.; Stefani, P.M. Nanocellulose from rice husk following alkaline treatment to remove silica. BioResources 2011, 6, 1440–1453. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef]
- Gouvea, B.M.; Torres, C.; Franca, A.S.; Oliveira, L.S.; Oliveira, E.S. Feasibility of ethanol production from coffee husks. Biotechnol. Lett. 2009, 31, 1315–1319. [Google Scholar] [CrossRef]
- Kim, M.; Day, D.F. Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microbiol. Biotechnol. 2011, 38, 803–807. [Google Scholar] [CrossRef]
- Schmitt, E.; Bura, R.; Gustafson, R.; Cooper, J.; Vajzovic, A. Converting lignocellulosic solid waste into ethanol for the State of Washington: An investigation of treatment technologies and environmental impacts. Bioresour. Technol. 2012, 104, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Singh, A.; Joshi, H.C. Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour. Conserv. Recycl. 2007, 50, 1–39. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Yang, Y. Biofibers from agricultural byproducts for industrial applications. Trends Biotechnol. 2005, 23, 22–27. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, Y.Y.; Sunwoo, C.; Kim, J.S. Pretreatment of corn stover by low-liquid ammonia recycle percolation process. Appl. Biochem. Biotechnol. 2006, 133, 41–57. [Google Scholar] [CrossRef]
- Sun, N.; Rodríguez, H.; Rahman, M.; Rogers, R.D. Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass? Chem. Commun. 2011, 47, 1405–1421. [Google Scholar] [CrossRef]
- Sawan, S.P.; Sawan, S.P. Supercritical Fluid Cleaning: Fundamentals, Technology and Applications; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Xu, Z.-X.; Cheng, J.-H.; He, Z.-X.; Wang, Q.; Shao, Y.-W.; Hu, X. Hydrothermal liquefaction of cellulose in ammonia/water. Bioresour. Technol. 2019, 278, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, F.-S. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef]
- de Caprariis, B.; Scarsella, M.; Bavasso, I.; Bracciale, M.P.; Tai, L.; De Filippis, P. Effect of Ni, Zn and Fe on hydrothermal liquefaction of cellulose: Impact on biocrude yield and composition. J. Anal. Appl. Pyrolysis 2021, 157, 105225. [Google Scholar] [CrossRef]
- do Couto Fraga, A.; de Almeida, M.B.B.; Sousa-Aguiar, E.F. Hydrothermal liquefaction of cellulose and lignin: A new approach on the investigation of chemical reaction networks. Cellulose 2021, 28, 2003–2020. [Google Scholar] [CrossRef]
- Beims, R.F.; Hu, Y.; Shui, H.; Xu, C.C. Hydrothermal liquefaction of biomass to fuels and value-added chemicals: Products applications and challenges to develop large-scale operations. Biomass Bioenergy 2020, 135, 105510. [Google Scholar] [CrossRef]
- Chen, H.; He, Z.; Zhang, B.; Feng, H.; Kandasamy, S.; Wang, B. Effects of the aqueous phase recycling on biocrude yield in hydrothermal liquefaction of Spirulina platensis, α-cellulose, and lignin. Energy 2019, 179, 1103–1113. [Google Scholar] [CrossRef]
- Ding, X.; Subramanya, S.M.; Waltz, K.E.; Wang, Y.; Savage, P.E. Hydrothermal liquefaction of polysaccharide feedstocks with heterogeneous catalysts. Bioresour. Technol. 2022, 352, 127100. [Google Scholar] [CrossRef]
- Kryeziu, A.; Slovák, V.; Parchaňská, A. Liquefaction of Cellulose for Production of Advanced Porous Carbon Materials. Polymers 2022, 14, 1621. [Google Scholar] [CrossRef]
- Deniz, İ. Hydrothermal Liquefaction of Marine Biomass: An Integrated Process. Acad. Platf. J. Eng. Sci. 2018, 6, 36–39. [Google Scholar]
- Yang, C.; Wang, S.; Yang, J.; Xu, D.; Li, Y.; Li, J.; Zhang, Y. Hydrothermal liquefaction and gasification of biomass and model compounds: A review. Green Chem. 2020, 22, 8210–8232. [Google Scholar] [CrossRef]
- Li, B.; Yang, T.; Li, R.; Kai, X. Co-generation of liquid biofuels from lignocellulose by integrated biochemical and hydrothermal liquefaction process. Energy 2020, 200, 117524. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, F.; Djandja, J.O.; Zhang, S.-L.; Xu, Y.-P.; Duan, P.-G. Hydrothermal liquefaction of crop straws: Effect of feedstock composition. Fuel 2020, 265, 116946. [Google Scholar] [CrossRef]
- Gu, X.; Fu, X.; Chen, S. Hydrothermal liquefaction conversion of lignocelluloses with enhanced fungal pretreatment. Ind. Crops Prod. 2021, 162, 113268. [Google Scholar] [CrossRef]
- Wu, H.; Shakeel, U.; Zhang, Q.; Zhang, K.; Xu, X.; Yuan, Y.; Xu, J. Catalytic degradation of poplar by Na2CO3 and Na2CO3/Fe under various hydrothermal liquefaction processes. Energy 2022, 259, 125055. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Wang, Z.; Liu, B. Catalytic hydrothermal liquefaction of lignin for production of aromatic hydrocarbon over metal supported mesoporous catalyst. Bioresour. Technol. 2021, 323, 124569. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, C.; Tsang, D.C.W.; Fan, J.; Clark, J.H.; Zhang, S. Hydrothermal Liquefaction of Lignin to Aromatic Chemicals: Impact of Lignin Structure. Ind. Eng. Chem. Res. 2020, 59, 16957–16969. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, A.; Kaur, R.; Krishna, B.B.; Bhaskar, T. Catalytic hydrothermal liquefaction of alkali lignin over activated bio-char supported bimetallic catalyst. Bioresour. Technol. 2021, 337, 125439. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, Z.; Zhang, Y.; Savage, P.E. Synergistic and Antagonistic Interactions during Hydrothermal Liquefaction of Soybean Oil, Soy Protein, Cellulose, Xylose, and Lignin. ACS Sustain. Chem. Eng. 2018, 6, 14501–14509. [Google Scholar] [CrossRef]
- Collett, J.R.; Billing, J.M.; Meyer, P.A.; Schmidt, A.J.; Remington, A.B.; Hawley, E.R.; Hofstad, B.A.; Panisko, E.A.; Dai, Z.; Hart, T.R.; et al. Renewable diesel via hydrothermal liquefaction of oleaginous yeast and residual lignin from bioconversion of corn stover. Appl. Energy 2019, 233–234, 840–853. [Google Scholar] [CrossRef]
- Nagel, E.; Zhang, C. Hydrothermal Decomposition of a Lignin Dimer under Neutral and Basic Conditions: A Mechanism Study. Ind. Eng. Chem. Res. 2019, 58, 18866–18880. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzym. Res. 2011, 2011, 787532. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, I.; Oliva, J.; Negro, M.; Manzanares, P.; Ballesteros, M. Enzymic hydrolysis of steam exploded herbaceous agricultural waste (Brassica carinata) at different particule sizes. Process Biochem. 2002, 38, 187–192. [Google Scholar] [CrossRef]
- Pérez, J.; Ballesteros, I.; Ballesteros, M.; Sáez, F.; Negro, M.; Manzanares, P. Optimizing liquid hot water pretreatment conditions to enhance sugar recovery from wheat straw for fuel-ethanol production. Fuel 2008, 87, 3640–3647. [Google Scholar] [CrossRef]
- Kim, Y.; Mosier, N.S.; Ladisch, M.R. Enzymatic digestion of liquid hot water pretreated hybrid poplar. Biotechnol. Prog. 2009, 25, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Hornung, U.; Raffelt, K.; Dahmen, N. The influence of lipids on the fate of nitrogen during hydrothermal liquefaction of protein-containing biomass. J. Anal. Appl. Pyrolysis 2020, 147, 104798. [Google Scholar] [CrossRef]
- Fan, Y.; Hornung, U.; Dahmen, N.; Kruse, A. Hydrothermal liquefaction of protein-containing biomass: Study of model compounds for Maillard reactions. Biomass Convers. Biorefinery 2018, 8, 909–923. [Google Scholar] [CrossRef]
- Déniel, M.; Haarlemmer, G.; Roubaud, A.; Weiss-Hortala, E.; Fages, J. Modelling and predictive study of hydrothermal liquefaction: Application to food processing residues. Waste Biomass Valorization 2017, 8, 2087–2107. [Google Scholar] [CrossRef]
- Toor, S.S.; Reddy, H.; Deng, S.; Hoffmann, J.; Spangsmark, D.; Madsen, L.B.; Holm-Nielsen, J.B.; Rosendahl, L.A. Hydrothermal liquefaction of Spirulina and Nannochloropsis salina under subcritical and supercritical water conditions. Bioresour. Technol. 2013, 131, 413–419. [Google Scholar] [CrossRef]
- Rout, P.R.; Goel, M.; Mohanty, A.; Pandey, D.S.; Halder, N.; Mukherjee, S.; Bhatia, S.K.; Sahoo, N.K.; Varjani, S. Recent advancements in microalgal mediated valorisation of wastewater from hydrothermal liquefaction of biomass. BioEnergy Res. 2022, 1–16. [Google Scholar] [CrossRef]
- Bayat, H.; Dehghanizadeh, M.; Jarvis, J.M.; Brewer, C.E.; Jena, U. Hydrothermal Liquefaction of Food Waste: Effect of Process Parameters on Product Yields and Chemistry. Front. Sustain. Food Syst. 2021, 5, 658592. [Google Scholar] [CrossRef]
- Anastasakis, K.; Biller, P.; Madsen, R.B.; Glasius, M.; Johannsen, I. Continuous hydrothermal liquefaction of biomass in a novel pilot plant with heat recovery and hydraulic oscillation. Energies 2018, 11, 2695. [Google Scholar] [CrossRef]
- Yang, J.; Niu, H.; Dalai, A.; Corscadden, K.; Zhou, N. Microwave-assisted hydrothermal liquefaction of biomass model components and comparison with conventional heating. Fuel 2020, 277, 118202. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Liu, Q.; Zhou, N.; Wu, Y. Is it feasible to replace freshwater by seawater in hydrothermal liquefaction of biomass for biocrude production? Fuel 2020, 282, 118870. [Google Scholar] [CrossRef]
- Nallasivam, J.; Prashanth, P.F.; Vinu, R. Hydrothermal liquefaction of biomass for the generation of value-added products. Biomass Biofuels Biochem. 2022, 65–107. [Google Scholar]
- Yang, J.; Yang, L. A review on hydrothermal co-liquefaction of biomass. Appl. Energy 2019, 250, 926–945. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.; Niu, H.; Corscadden, K.; Astatkie, T. Hydrothermal liquefaction of biomass model components for product yield prediction and reaction pathways exploration. Appl. Energy 2018, 228, 1618–1628. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.-T. Hydrothermal liquefaction of protein-containing feedstocks. In Direct Thermochemical Liquefaction for Energy Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 127–168. [Google Scholar]
- Gong, Z.; Shen, H.; Zhou, W.; Wang, Y.; Yang, X.; Zhao, Z.K. Efficient conversion of acetate into lipids by the oleaginous yeast Cryptococcus curvatus. Biotechnol. Biofuels 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Schwenzfeier, A.; Wierenga, P.A.; Gruppen, H. Isolation and characterization of soluble protein from the green microalgae Tetraselmis sp. Bioresour. Technol. 2011, 102, 9121–9127. [Google Scholar] [CrossRef] [PubMed]
- Reddy, H.K.; Muppaneni, T.; Ponnusamy, S.; Sudasinghe, N.; Pegallapati, A.; Selvaratnam, T.; Seger, M.; Dungan, B.; Nirmalakhandan, N.; Schaub, T. Temperature effect on hydrothermal liquefaction of Nannochloropsis gaditana and Chlorella sp. Appl. Energy 2016, 165, 943–951. [Google Scholar] [CrossRef]
- Chen, W.-T.; Zhang, Y.; Zhang, J.; Yu, G.; Schideman, L.C.; Zhang, P.; Minarick, M. Hydrothermal liquefaction of mixed-culture algal biomass from wastewater treatment system into biocrude. Bioresour. Technol. 2014, 152, 130–139. [Google Scholar] [CrossRef]
- Chen, W.-T.; Zhang, Y.; Zhang, J.; Schideman, L.; Yu, G.; Zhang, P.; Minarick, M. Co-liquefaction of swine manure and mixed-culture algal biomass from a wastewater treatment system to produce biocrude. Appl. Energy 2014, 128, 209–216. [Google Scholar] [CrossRef]
- Biller, P.; Friedman, C.; Ross, A.B. Hydrothermal microwave processing of microalgae as a pre-treatment and extraction technique for bio-fuels and bio-products. Bioresour. Technol. 2013, 136, 188–195. [Google Scholar] [CrossRef]
- He, Z.; Xu, D.; Liu, L.; Wang, Y.; Wang, S.; Guo, Y.; Jing, Z. Product characterization of multi-temperature steps of hydrothermal liquefaction of Chlorella microalgae. Algal Res. 2018, 33, 8–15. [Google Scholar] [CrossRef]
- Lu, J.; Watson, J.; Zeng, J.; Li, H.; Zhu, Z.; Wang, M.; Zhang, Y.; Liu, Z. Biocrude production and heavy metal migration during hydrothermal liquefaction of swine manure. Process Saf. Environ. Prot. 2018, 115, 108–115. [Google Scholar] [CrossRef]
- Phukan, M.M.; Chutia, R.S.; Konwar, B.; Kataki, R. Microalgae Chlorella as a potential bio-energy feedstock. Appl. Energy 2011, 88, 3307–3312. [Google Scholar] [CrossRef]
- Valdez, P.J.; Tocco, V.J.; Savage, P.E. A general kinetic model for the hydrothermal liquefaction of microalgae. Bioresour. Technol. 2014, 163, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-T.; Haque, M.A.; Lu, T.; Aierzhati, A.; Reimonn, G. A perspective on hydrothermal processing of sewage sludge. Curr. Opin. Environ. Sci. Health 2020, 14, 63–73. [Google Scholar] [CrossRef]
- Muzammil, N.; Shah, S.A.A.; Shahzad, A.; Khan, M.A.; Ghoniem, R.M. Multifilters-Based Unsupervised Method for Retinal Blood Vessel Segmentation. Appl. Sci. 2022, 12, 6393. [Google Scholar] [CrossRef]
- Toor, S.S.; Conti, F.; Shah, A.A.; Seehar, T.H.; Rosendahl, L.A. Hydrothermal Liquefaction: A Sustainable Solution to the Sewage Sludge Disposal Problem. In Advances in Waste-to-Energy Technologies; CRC Press: Boca Raton, FL, USA, 2019; pp. 143–163. [Google Scholar]
- Feng, S.; Yuan, Z.; Leitch, M.; Xu, C.C. Hydrothermal liquefaction of barks into biocrude—Effects of species and ash content/composition. Fuel 2014, 116, 214–220. [Google Scholar] [CrossRef]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y. Comparative studies of oil compositions produced from sawdust, rice husk, lignin and cellulose by hydrothermal treatment. Fuel 2005, 84, 875–884. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.B. Biocrude production from switchgrass using subcritical water. Energy Fuels 2009, 23, 5151–5159. [Google Scholar] [CrossRef]
- Cheng, L.; Ye, X.P.; He, R.; Liu, S. Investigation of rapid conversion of switchgrass in subcritical water. Fuel Process. Technol. 2009, 90, 301–311. [Google Scholar] [CrossRef]
- Bhaskar, T.; Sera, A.; Muto, A.; Sakata, Y. Hydrothermal upgrading of wood biomass: Influence of the addition of K2CO3 and cellulose/lignin ratio. Fuel 2008, 87, 2236–2242. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal conversion of lignin: A review. Renew. Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Belkheiri, T.; Andersson, S.-I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal liquefaction of kraft lignin in sub-critical water: The influence of the sodium and potassium fraction. Biomass Convers. Biorefinery 2018, 8, 585–595. [Google Scholar] [CrossRef]
- Jasiūnas, L.; Pedersen, T.H.; Toor, S.S.; Rosendahl, L.A. Biocrude production via supercritical hydrothermal co-liquefaction of spent mushroom compost and aspen wood sawdust. Renew. Energy 2017, 111, 392–398. [Google Scholar] [CrossRef]
- Zhu, Z.; Toor, S.S.; Rosendahl, L.; Yu, D.; Chen, G. Influence of alkali catalyst on product yield and properties via hydrothermal liquefaction of barley straw. Energy 2015, 80, 284–292. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Yu, D.; Chen, G. Hydrothermal liquefaction of barley straw to biocrude: Effects of reaction temperature and aqueous phase recirculation. Appl. Energy 2015, 137, 183–192. [Google Scholar] [CrossRef]
- Xu, D.; Savage, P.E. Characterization of biocrudes recovered with and without solvent after hydrothermal liquefaction of algae. Algal Res. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Schmidt, A.J.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Albrecht, K.O.; Hallen, R.T.; Holladay, J.E. Process development for hydrothermal liquefaction of algae feedstocks in a continuous-flow reactor. Algal Res. 2013, 2, 445–454. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Neuenschwander, G.G.; Rotness, L.J.; Roesijadi, G.; Zacher, A.H.; Magnuson, J.K. Hydrothermal processing of macroalgal feedstocks in continuous-flow reactors. ACS Sustain. Chem. Eng. 2014, 2, 207–215. [Google Scholar] [CrossRef]
- Albrecht, K.O.; Zhu, Y.; Schmidt, A.J.; Billing, J.M.; Hart, T.R.; Jones, S.B.; Maupin, G.; Hallen, R.; Ahrens, T.; Anderson, D. Impact of heterotrophically stressed algae for biofuel production via hydrothermal liquefaction and catalytic hydrotreating in continuous-flow reactors. Algal Res. 2016, 14, 17–27. [Google Scholar] [CrossRef]
- Elliott, D.C.; Schmidt, A.J.; Hart, T.R.; Billing, J.M. Conversion of a wet waste feedstock to biocrude by hydrothermal processing in a continuous-flow reactor: Grape pomace. Biomass Convers. Biorefinery 2017, 7, 455–465. [Google Scholar] [CrossRef]
- Marrone, P.A.; Elliott, D.C.; Billing, J.M.; Hallen, R.T.; Hart, T.R.; Kadota, P.; Moeller, J.C.; Randel, M.A.; Schmidt, A.J. Bench-Scale Evaluation of Hydrothermal Processing Technology for Conversion of Wastewater Solids to Fuels. Water Environ. Res. 2018, 90, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Jazrawi, C.; Biller, P.; Ross, A.B.; Montoya, A.; Maschmeyer, T.; Haynes, B.S. Pilot plant testing of continuous hydrothermal liquefaction of microalgae. Algal Res. 2013, 2, 268–277. [Google Scholar] [CrossRef]
- Ocfemia, K.; Zhang, Y.; Funk, T. Hydrothermal processing of swine manure into oil using a continuous reactor system: Development and testing. Trans. ASABE 2006, 49, 533–541. [Google Scholar] [CrossRef]
- Ocfemia, K.S.; Zhang, Y.; Funk, T. Hydrothermal processing of swine manure to oil using a continuous reactor system: Effects of operating parameters on oil yield and quality. Trans. ASABE 2006, 49, 1897–1904. [Google Scholar] [CrossRef]
- Suesse, A.R.; Norton, G.A.; van Leeuwen, J. Pilot-scale continuous-flow hydrothermal liquefaction of filamentous fungi. Energy Fuels 2016, 30, 7379–7386. [Google Scholar] [CrossRef]
- Nguyen, T.D.H.; Maschietti, M.; Belkheiri, T.; Åmand, L.-E.; Theliander, H.; Vamling, L.; Olausson, L.; Andersson, S.-I. Catalytic depolymerisation and conversion of Kraft lignin into liquid products using near-critical water. J. Supercrit. Fluids 2014, 86, 67–75. [Google Scholar] [CrossRef]
- Nguyen, T.D.H.; Maschietti, M.; Åmand, L.-E.; Vamling, L.; Olausson, L.; Andersson, S.-I.; Theliander, H. The effect of temperature on the catalytic conversion of Kraft lignin using near-critical water. Bioresour. Technol. 2014, 170, 196–203. [Google Scholar] [CrossRef]
- Belkheiri, T.; Andersson, S.-I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal liquefaction of Kraft lignin in subcritical water: Influence of phenol as capping agent. Energy Fuels 2018, 32, 5923–5932. [Google Scholar] [CrossRef]
- Leng, L.; Zhang, W.; Peng, H.; Li, H.; Jiang, S.; Huang, H. Nitrogen in biocrude produced from hydrothermal liquefaction of biomass: A review. Chem. Eng. J. 2020, 401, 126030. [Google Scholar] [CrossRef]
- Chen, P.H.; Quinn, J.C. Microalgae to biofuels through hydrothermal liquefaction: Open-source techno-economic analysis and life cycle assessment. Appl. Energy 2021, 289, 116613. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Snowden-Swan, L.J.; Askander, J.A.; Schmidt, A.J.; Billing, J.M. Techno-economic uncertainty analysis of wet waste-to-biocrude via hydrothermal liquefaction. Appl. Energy 2021, 283, 116340. [Google Scholar] [CrossRef]
- Aierzhati, A.; Stablein, M.J.; Wu, N.E.; Kuo, C.-T.; Si, B.; Kang, X.; Zhang, Y. Experimental and model enhancement of food waste hydrothermal liquefaction with combined effects of biochemical composition and reaction conditions. Bioresour. Technol. 2019, 284, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, A.; Boukis, N.; Hauer, E.; Galla, U.; Dinjus, E.; Hitzmann, B.; Larsen, T.; Nygaard, S.D. Catalytic conversion of waste biomass by hydrothermal treatment. Fuel 2011, 90, 555–562. [Google Scholar] [CrossRef]
- Hammerschmidt, A.; Boukis, N.; Galla, U.; Dinjus, E.; Hitzmann, B. Conversion of yeast by hydrothermal treatment under reducing conditions. Fuel 2011, 90, 3424–3432. [Google Scholar] [CrossRef]
- Hammerschmidt, A.; Boukis, N.; Galla, U.; Zevaco, T.; Dinjus, E.; Hitzmann, B. Influence of the heating rate and the potassium concentration of the feed solution on the hydrothermal liquefaction of used yeast and apple pomace under reducing conditions. Biomass Convers. Biorefinery 2015, 5, 125–139. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Grigoras, I.; Hoffmann, J.; Toor, S.S.; Daraban, I.M.; Jensen, C.U.; Iversen, S.; Madsen, R.; Glasius, M.; Arturi, K.R. Continuous hydrothermal co-liquefaction of aspen wood and glycerol with water phase recirculation. Appl. Energy 2016, 162, 1034–1041. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Jensen, C.U.; Sandström, L.; Rosendahl, L.A. Full characterization of compounds obtained from fractional distillation and upgrading of a HTL biocrude. Appl. Energy 2017, 202, 408–419. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Abbasi, S.A.; Hashmi, Z.; Shah, A.K.; Alam, M.S.; Bhatti, Z.A.; Maitlo, G.; Hussain, S.; Khandro, G.A.; Usto, M.A. Recent trends and future perspectives of lignocellulose biomass for biofuel production: A comprehensive review. Biomass Convers. Biorefinery 2021, 1–13. [Google Scholar] [CrossRef]
- Ni, J.; Qian, L.; Wang, Y.; Zhang, B.; Gu, H.; Hu, Y.; Wang, Q. A review on fast hydrothermal liquefaction of biomass. Fuel 2022, 327, 125135. [Google Scholar] [CrossRef]
- He, J.; Lu, L.; Zhao, C.; Mei, D.; Lercher, J.A. Mechanisms of catalytic cleavage of benzyl phenyl ether in aqueous and apolar phases. J. Catal. 2014, 311, 41–51. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, S.; Yang, X.; Xi, W.; Zheng, K.; Chu, C.; Ju, M.; Liu, L. Effect of operating parameters on hydrothermal liquefaction of corn straw and its life cycle assessment. Environ. Sci. Pollut. Res. 2020, 27, 6362–6374. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Zhang, W.; Chen, Q.; Zhou, J.; Peng, H.; Zhan, H.; Li, H. Machine learning prediction of nitrogen heterocycles in biocrude produced from hydrothermal liquefaction of biomass. Bioresour. Technol. 2022, 362, 127791. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhang, Y.; Schideman, L.; Funk, T.; Wang, Z. Hydrothermal liquefaction of low lipid content microalgae into biocrude. Trans. ASABE 2011, 54, 239–246. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, P.; Mohanty, K. Hydrothermal liquefaction of biomass for biocrude production: A review on feedstocks, chemical compositions, operating parameters, reaction kinetics, techno-economic study, and life cycle assessment. Fuel 2022, 316, 123377. [Google Scholar] [CrossRef]
- Yin, S.; Dolan, R.; Harris, M.; Tan, Z. Subcritical hydrothermal liquefaction of cattle manure to biocrude: Effects of conversion parameters on biocrude yield and characterization of biocrude. Bioresour. Technol. 2010, 101, 3657–3664. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.; Chen, M.; Zhao, G.; Wu, S. Influence of catalyst and solvent on the hydrothermal liquefaction of woody biomass. Bioresour. Technol. 2022, 346, 126354. [Google Scholar] [CrossRef]
- Ravichandran, S.R.; Venkatachalam, C.D.; Sengottian, M.; Sekar, S.; Kandasamy, S.; Subramanian, K.P.R.; Purushothaman, K.; Chandrasekaran, A.L.; Narayanan, M. A review on hydrothermal liquefaction of algal biomass on process parameters, purification and applications. Fuel 2022, 313, 122679. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.C.; Kastner, J.R. Comparison of the effects of Na2CO3, Ca3(PO4)2, and NiO catalysts on the thermochemical liquefaction of microalga Spirulina platensis. Appl. Energy 2012, 98, 368–375. [Google Scholar] [CrossRef]
- Ratha, S.K.; Renuka, N.; Abunama, T.; Rawat, I.; Bux, F. Hydrothermal liquefaction of algal feedstocks: The effect of biomass characteristics and extraction solvents. Renew. Sustain. Energy Rev. 2022, 156, 111973. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Li, H.; Han, X.; Zhang, M.; Sun, Y.; Fan, X.; Tu, R.; Zeng, Y.; Xu, C.C. Applications of catalysts in thermochemical conversion of biomass (pyrolysis, hydrothermal liquefaction and gasification): A critical review. Renew. Energy 2022, 196, 462–481. [Google Scholar] [CrossRef]
- Anastasakis, K.; Ross, A.B. Hydrothermal liquefaction of the brown macro-alga Laminaria saccharina: Effect of reaction conditions on product distribution and composition. Bioresour. Technol. 2011, 102, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Theegala, C.S.; Midgett, J.S. Hydrothermal liquefaction of separated dairy manure for production of biocrudes with simultaneous waste treatment. Bioresour. Technol. 2012, 107, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Ong, M. Evaluation of Anaerobic Membrane Bioreactors and Hydrothermal Catalytic Gasification for Enhanced Conversion of Organic Wastes to Renewable Fuels. Master’s Thesis, University of Illinois Urbana-Champaign, Champaign, IL, USA, 2014. [Google Scholar]
- Nazari, L.; Yuan, Z.; Souzanchi, S.; Ray, M.B.; Xu, C.C. Hydrothermal liquefaction of woody biomass in hot-compressed water: Catalyst screening and comprehensive characterization of biocrudes. Fuel 2015, 162, 74–83. [Google Scholar] [CrossRef]
- de Caprariis, B.; Bracciale, M.P.; Bavasso, I.; Chen, G.; Damizia, M.; Genova, V.; Marra, F.; Paglia, L.; Pulci, G.; Scarsella, M.; et al. Unsupported Ni metal catalyst in hydrothermal liquefaction of oak wood: Effect of catalyst surface modification. Sci. Total Environ. 2020, 709, 136215. [Google Scholar] [CrossRef] [PubMed]
- Seehar, T.H.; Toor, S.S.; Sharma, K.; Nielsen, A.H.; Pedersen, T.H.; Rosendahl, L.A. Influence of process conditions on hydrothermal liquefaction of eucalyptus biomass for biocrude production and investigation of the inorganics distribution. Sustain. Energy Fuels 2021, 5, 1477–1487. [Google Scholar] [CrossRef]
- Seehar, T.H.; Toor, S.S.; Shah, A.A.; Pedersen, T.H.; Rosendahl, L.A. Biocrude Production from Wheat Straw at Sub and Supercritical Hydrothermal Liquefaction. Energies 2020, 13, 3114. [Google Scholar] [CrossRef]
- Prestigiacomo, C.; Costa, P.; Pinto, F.; Schiavo, B.; Siragusa, A.; Scialdone, O.; Galia, A. Sewage sludge as cheap alternative to microalgae as feedstock of catalytic hydrothermal liquefaction processes. J. Supercrit. Fluids 2019, 143, 251–258. [Google Scholar] [CrossRef]
- Kohansal, K.; Toor, S.; Sharma, K.; Chand, R.; Rosendahl, L.; Pedersen, T.H. Hydrothermal liquefaction of pre-treated municipal solid waste (biopulp) with recirculation of concentrated aqueous phase. Biomass Bioenergy 2021, 148, 106032. [Google Scholar] [CrossRef]
- Toor, S.S.; Jasiunas, L.; Xu, C.; Sintamarean, I.M.; Yu, D.; Nielsen, A.H.; Rosendahl, L.A. Reduction of inorganics from macroalgae Laminaria digitata and spent mushroom compost (SMC) by acid leaching and selective hydrothermal liquefaction. Biomass Convers. Biorefinery 2018, 8, 369–377. [Google Scholar] [CrossRef]
- Xu, J.; Dong, X.; Wang, Y. Hydrothermal liquefaction of macroalgae over various solids, basic or acidic oxides and metal salt catalyst: Products distribution and characterization. Ind. Crops Prod. 2020, 151, 112458. [Google Scholar] [CrossRef]
- Duan, P.; Savage, P.E. Hydrothermal Liquefaction of a Microalga with Heterogeneous Catalysts. Ind. Eng. Chem. Res. 2011, 50, 52–61. [Google Scholar] [CrossRef]
- Ding, X.; Mahadevan Subramanya, S.; Fang, T.; Guo, Y.; Savage, P.E. Effects of Potassium Phosphates on Hydrothermal Liquefaction of Triglyceride, Protein, and Polysaccharide. Energy Fuels 2020, 34, 15313–15321. [Google Scholar] [CrossRef]
- Wang, W.; Yang, L.; Yin, Z.; Kong, S.; Han, W.; Zhang, J. Catalytic liquefaction of human feces over Ni-Tm/TiO2 catalyst and the influence of operating conditions on products. Energy Convers. Manag. 2018, 157, 239–245. [Google Scholar] [CrossRef] [PubMed]

| Biomass | Lignin (%) | Hemicellulose (%) | Cellulose (%) | Ref. |
|---|---|---|---|---|
| Nut shells | 30–40 | 25–30 | 25–30 | [27] |
| Rice husk | 26–31 | 18–21 | 25–35 | [28] |
| Corn stover | 7–19 | 24–26 | 38–40 | [29] |
| Coffee husk | 9 | 7 | 43 | [30] |
| Sugar cane (bagasse) | 20–42 | 19–25 | 42–48 | [31] |
| Leaves and grass | 43.8 | 10.5 | 15.3 | [32] |
| Wheat straw | 17–19 | 26–32 | 33–38 | [29] |
| Coffee husk | 9 | 7 | 43 | [30] |
| Bamboo | 21–31 | 15–26 | 26–43 | [33] |
| Coir | 41–45 | 0.15–0.25 | 36–43 | [29] |
| Corn cob | 14–15 | 35–39 | 42–45 | [34] |
| Banana waste | 14 | 14.8 | 13.2 | [33] |
| Solid cattle manure | 2.7–5.7 | 1.6–1.33 | 16–4.7 | [33] |
| Corn fiber | 8.4 | 16.8 | 14.28 | [35] |
| Coir | 41–45 | 0.15–0.25 | 36–43 | [29] |
| Rice straw | 12–14 | 23–28 | 28–36 | [29] |
| Barley straw | 14–19 | 27–38 | 31–45 | [29] |
| Wheat straw | 17–19 | 26–32 | 33–38 | [29] |
| Pineapple leaf fiber | 5–1 | 18 | 70–82 | [36] |
| Sweet sorghum bagasse | 14–21 | 18–27 | 34–45 | [29] |
| Oat straw | 16–19 | 27–38 | 31–37 | [33] |
| Aspen | 19.5 | 21.7 | 52.7 | [37] |
| Eucalyptus | 26.9–28.2 | 12.7–14.4 | 46.6–50.3 | [38] |
| Japanese beech | 24 | 28.4 | 43.9 | [39] |
| Pine | 20 | 24–27 | 42–50 | [37] |
| Hardwood stem | 18–25 | 24–40 | 40–55 | [38] |
| Softwood stem | 25–35 | 25–35 | 45–50 | [39] |
| Paper | 0–15 | 0 | 85–99 | [28] |
| Cotton or seed hairs | 0 | 5–20 | 80–85 | [33] |
| Newspaper | 18–30 | 25–40 | 40–55 | [32] |
| Solid waste water | — | — | 8–15 | [30] |
| Waste paper from chemical pulp | 5–10 | 10–20 | 60–70 | [33] |
| Solid cattle manure | 2.7–5.7 | 1.4–3.3 | 1.6–4.7 | [34] |
| Bermuda grass | 6.4 | 35.7 | 25 | [34] |
| Swine waste | — | 28 | 6.0 | [30] |
| Pretreatment Methods | Advantages | Disadvantages | Comments | References |
|---|---|---|---|---|
| Alkali |
|
| If this pretreatment method linked with the mechanical process addressed the mentioned issues | [35,60] |
| Acid |
|
| Recycling of acids inhibitors (e.g., acetic acids) can be converted to valuable products and combined with a steam explosion. Addressed the mentioned issues or adoption of this process with catalyst leads to enhance the activity and selectivity of the process. That leads to an optimum quantity of acid, making the process more economical | [38,61] |
| Green solvent |
|
| The deep eutectic solvent can be easily prepared. DES could be prepared easily by different hydrogen bond acceptors (HBA) and hydrogen bond donors (HBD) based on different molar ratios | [38] |
| Steam |
|
| The process linked with acidic and alkali leads to addressing the issues mentioned slightly | [62] |
| LHW a |
|
| Combined liquid hot water with sodium carbonate-oxygen pretreatment | [63,64] |
| AFEX b |
|
| LAT process overcome this issue | [60,61] |
| ARP c |
|
| Recycling of liquids/solvents combined with co-solvent to lower energy demand | [37] |
| Ozonolysis |
|
| Combined with other pretreatments to reduce ozone consumption, e.g., aqueous ammonia | [38] |
| Super critical fluid |
|
| Combined with microwave to reduce utility usage | [39] |
| Organosolv | Removal of lignin and hemicellulose, leaving a high-purity solid glucan-rich fraction after solid-liquid separation |
| Acid-catalyzed organosolv pretreatment address this issue with a solvent recovery route | [39] |
| Wet oxidation | Efficient removal of lignin with the low formation of inhibitors | High cost of oxygen and alkaline catalyst | Recovery of alkaline catalyst can make the process more feasible | [39] |
| Concentrated acid | High glucose yield, ambient temperature | High cost of acid, corrosion, and inhibitor formation | A recovery route must be adopted to make the process feasible; corrosion can be addressed by adopting alloying material for construction | [63,64] |
| Diluted acid | Less corrosion problem with less inhibitor formation | Generation of degradation products, low sugar concentration | Combined with a steam explosion. Addressed the mentioned issues or adoption of this process with catalyst leads to enhance the activity as well as selectivity of the process | [39] |
| Pretreatment | Advantage | Disadvantage | Comments |
|---|---|---|---|
| Micro-organisms, i.e., fungi and bacteria | Low energy, cost with non-chemical degradation efficient for lignin | High reaction time with loss in carbohydrates | In-shortage pretreatment of wet biomass providing year-long delignification |
| Ligninolytic enzymes | Selective degradation of minimal lignin toxins | High cost of extraction and purification | Recycle the enzyme to economize the process |
| Cellulosic enzymes, i.e., cellulases | Hydrolysis of β-1, 4 linkages in cellulose | High cost of extraction and purification | Recycle the enzyme to economize the process |
| Xylanases | Efficient hydrolysis of hemicellulose fraction becomes crucial, and supplementation of accessory enzymes increases hydrolysis yields and thereby reduces enzyme costs and dosages | High cost of extraction and purification | Recycle the enzyme to economize the process |
| Feedstock | Value-Added Products during Pretreatment | Pretreatment Mechanism | Benefit | Limitation | References |
|---|---|---|---|---|---|
| Yeast (C. curvatus) | Polysaccharides and proteins derivatives | Low-temperature liquefaction (160–300 °C) | Produce value-added chemical and biocrude oil with lower N | Multistep processes, potential loss of organic matter | [78] |
| Microalgae (Tetraselmis sp.) | Protein derivatives | Low-temperature liquefaction (130–200 °C) | Produce biocrude with improved yields and lower N | Multistep processes, organic carbon lost to the pretreatment process water, high energy output | [79] |
| Microalgae (Spirulina, Nannochloropsis, Chlorella, and Scenedesmus) | Nitrogen-rich nutrient streams | Low-temperature liquefaction (125–225 °C) | Produce nutrient streams and biocrude oil with lower N | Multistep processes, organic matter lost to the pretreatment process | [80] |
| Mixed-culture algal biomass | N/A | Centrifugation and ultrasonication | Produce biocrude with improved yields and heating value | Multistep processes, require additional energy for pretreatment | [81] |
| Microalgae (Nannochloropsis) | Biodiesel | Microwave irradiation | Produce biodiesel and biocrude simultaneously | Multistep processes, biocrude with lower HHV | [82] |
| Microalgae (Nannochloropsis, Chlorogloeopsis | Lipids and phytochemicals | Microwave irradiation | Produce biocrude with lower N | Multistep processes, organic matter lost to the pretreatment process water | [83] |
| Microalgae (Chlorella) | Polysaccharides | Low-temperature liquefaction (140–200 °C) | Lower solid residue yield, produce biocrude oil with lower N | Multistep processes, loss of some organic matters | [84] |
| Swine manure | N/A | Filtration and centrifugation | Achieve a higher solid content of the feedstock, improve the energy recovery of HTL | Require additional energy for pretreatment | [85] |
| Microalgae (Chlorella) | Protein derivatives | Extracted with sulphuric or formic | Produce biocrude with lower N | Unselective removal of nitrogen-containing compounds | [86] |
| Microalgae (Nannochloropsis and Scenedesmus) | Crude lipids | Soxhlet extraction with hexane | Produce lipids and biocrude simultaneously | Produce biocrude with higher N, lower the yield of biocrude | [87] |
| Microalgae (Scenedesmus) | Crude lipids | Soxhlet extraction with hexane | Produce lipids and biocrude simultaneously | Produce biocrude with higher N, lower the yield of biocrude | [87] |
| Sewage sludge | Solid residue or biocharcontaining metals | Extracted with acetic acid, hydroxylammonium chloride, and hydrogen peroxide | Produce biocrude and biochar with lower metal concentrations | Required hazardous chemicals for demetalization, lower biocrude yield | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jatoi, A.S.; Shah, A.A.; Ahmed, J.; Rehman, S.; Sultan, S.H.; Shah, A.K.; Raza, A.; Mubarak, N.M.; Hashmi, Z.; Usto, M.A.; et al. Hydrothermal Liquefaction of Lignocellulosic and Protein-Containing Biomass: A Comprehensive Review. Catalysts 2022, 12, 1621. https://doi.org/10.3390/catal12121621
Jatoi AS, Shah AA, Ahmed J, Rehman S, Sultan SH, Shah AK, Raza A, Mubarak NM, Hashmi Z, Usto MA, et al. Hydrothermal Liquefaction of Lignocellulosic and Protein-Containing Biomass: A Comprehensive Review. Catalysts. 2022; 12(12):1621. https://doi.org/10.3390/catal12121621
Chicago/Turabian StyleJatoi, Abdul Sattar, Ayaz Ali Shah, Jawad Ahmed, Shamimur Rehman, Syed Hasseb Sultan, Abdul Karim Shah, Aamir Raza, Nabisab Mujawar Mubarak, Zubair Hashmi, Muhammad Azam Usto, and et al. 2022. "Hydrothermal Liquefaction of Lignocellulosic and Protein-Containing Biomass: A Comprehensive Review" Catalysts 12, no. 12: 1621. https://doi.org/10.3390/catal12121621
APA StyleJatoi, A. S., Shah, A. A., Ahmed, J., Rehman, S., Sultan, S. H., Shah, A. K., Raza, A., Mubarak, N. M., Hashmi, Z., Usto, M. A., & Murtaza, M. (2022). Hydrothermal Liquefaction of Lignocellulosic and Protein-Containing Biomass: A Comprehensive Review. Catalysts, 12(12), 1621. https://doi.org/10.3390/catal12121621







