Excessive Na-Doped La0.75Sr0.25Cr0.5Fe0.4Cu0.1O3-δ Perovskite as an Additional Internal Reforming Catalyst for Direct Carbon Dioxide-Ethanol Solid Oxide Fuel Cells
Abstract
:1. Introduction
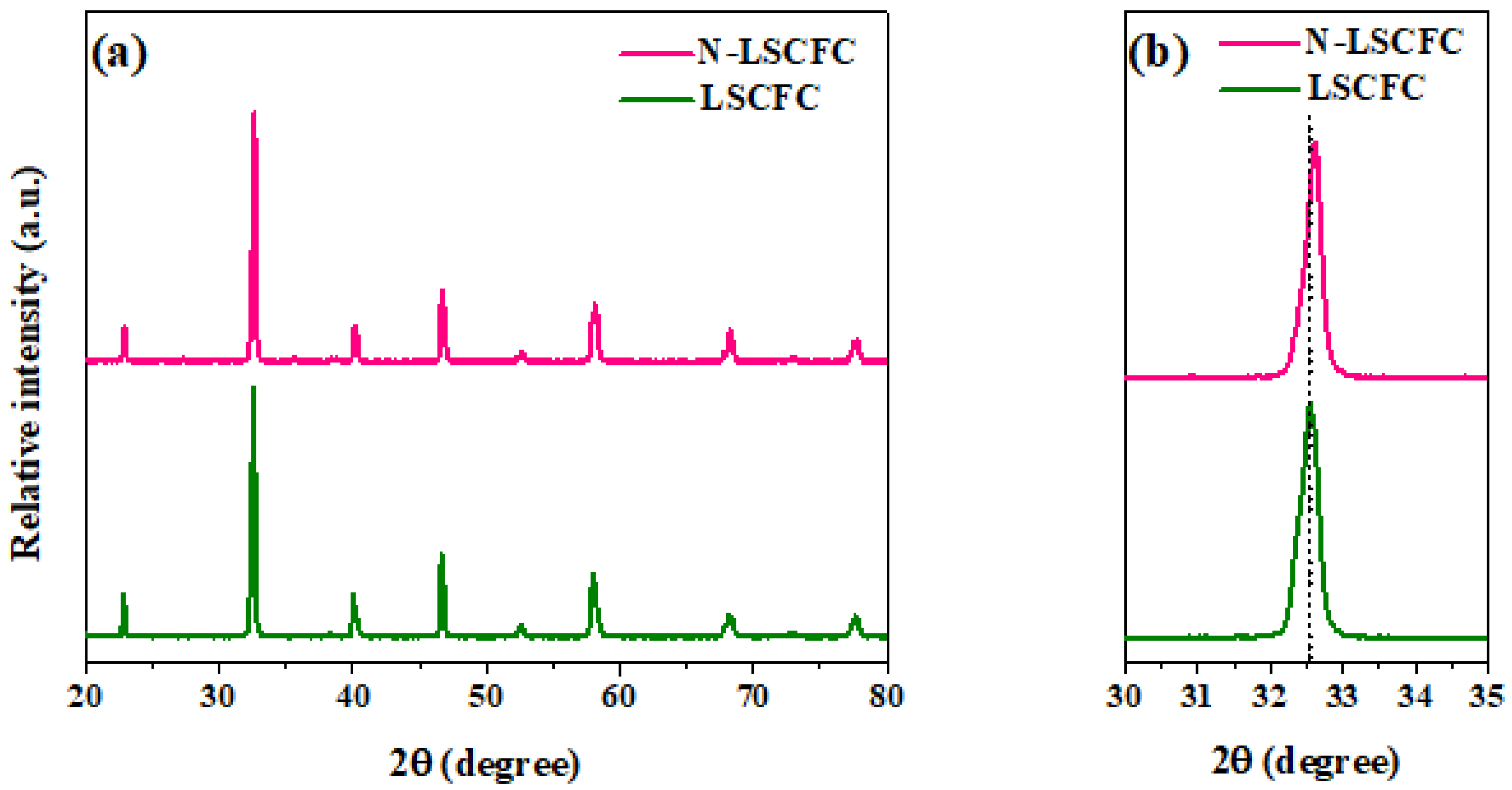
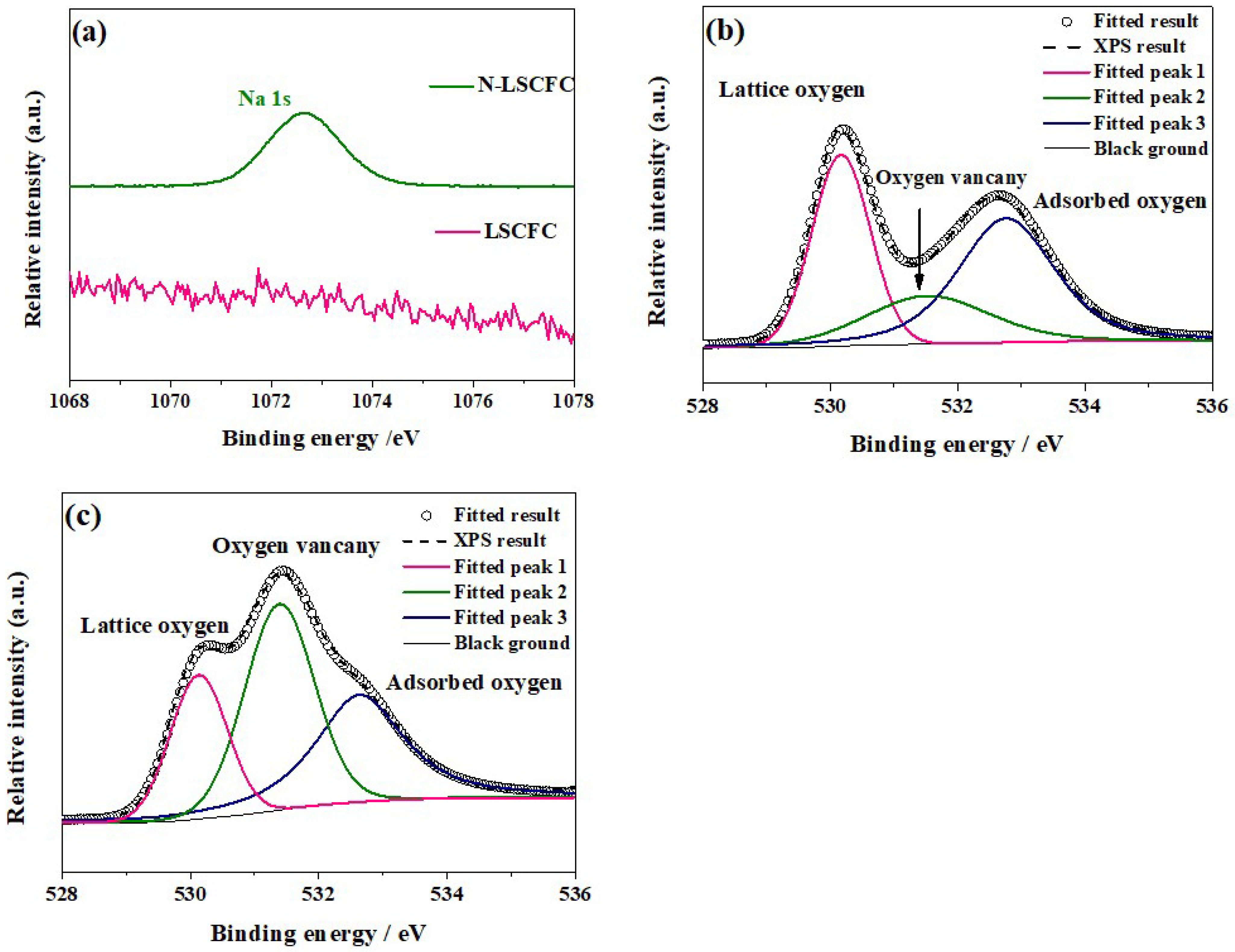
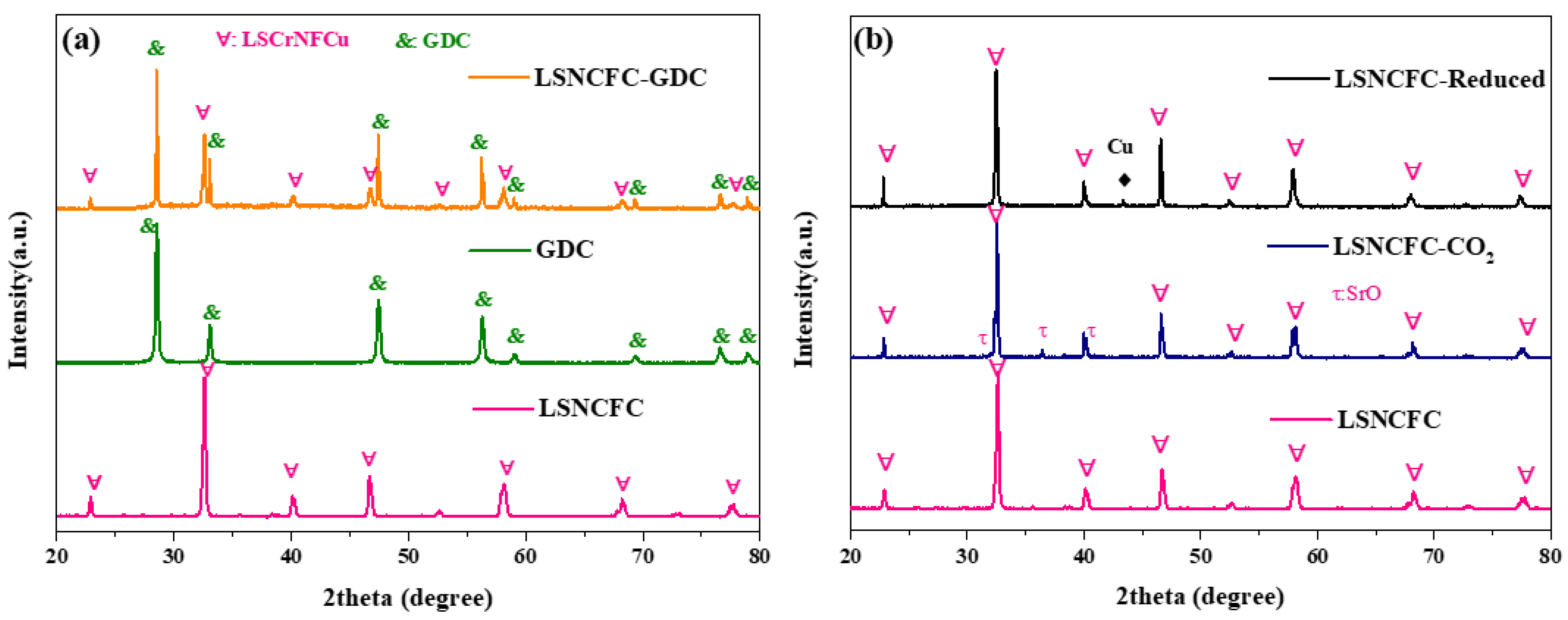
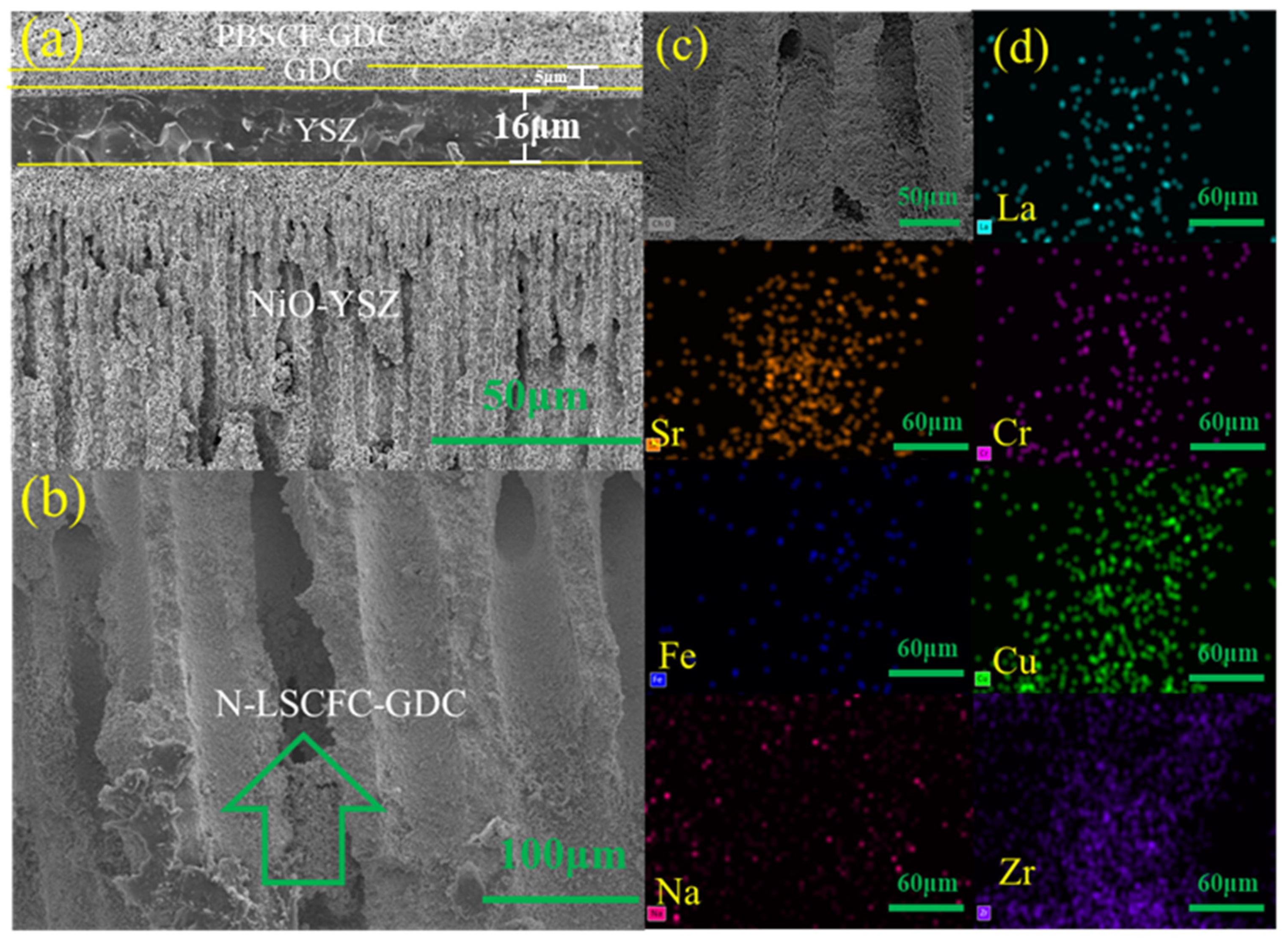
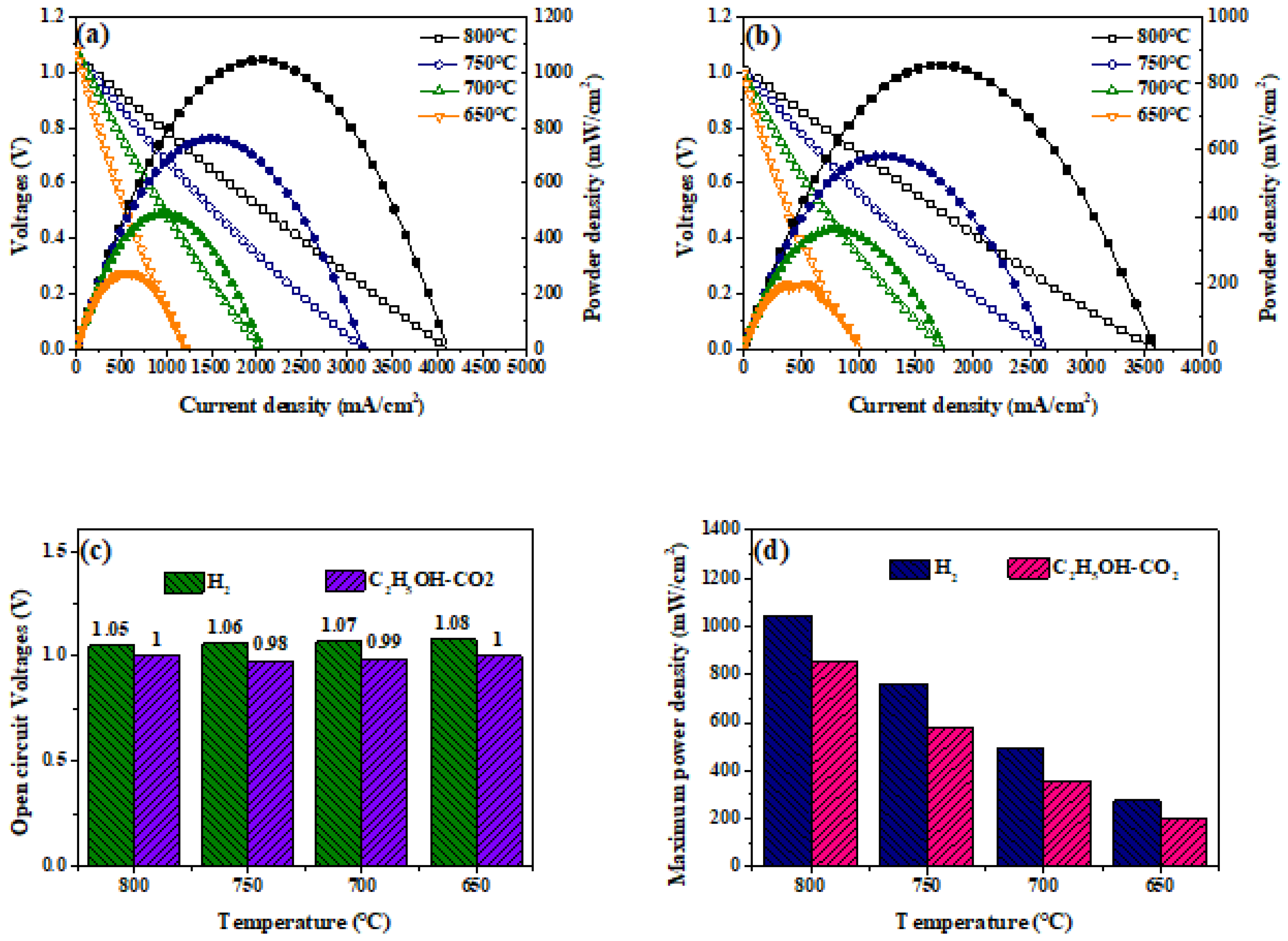
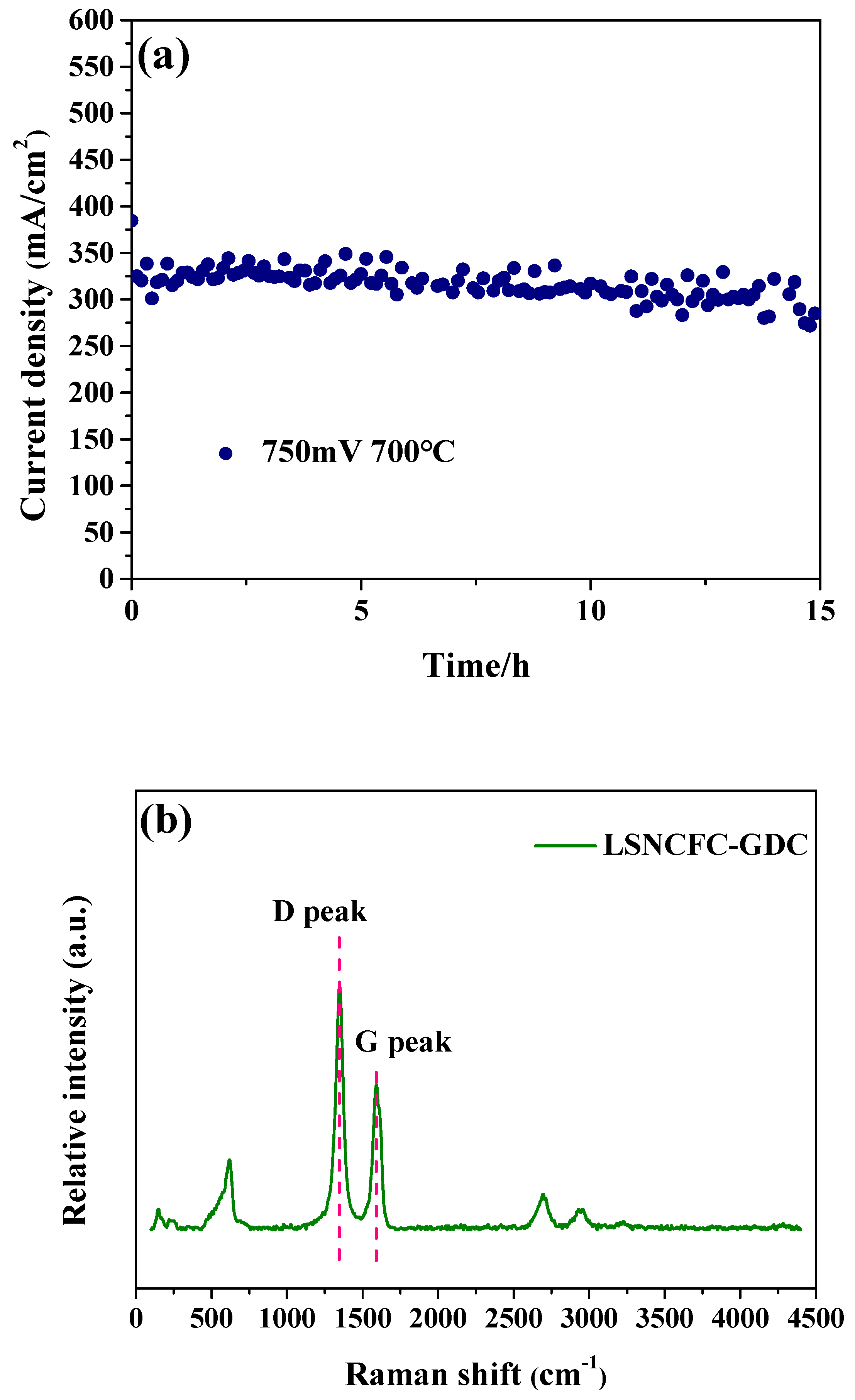
2. Result and Discussion
3. Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.L.; Tu, H.Y.; Li, S.L.; Yu, Q.C. Fabrication and characterization of functionally graded cathodes based on in-situ formed La0.6Sr0.4CoO3-δ for intermediate temperature SOFCs. J. Inorg. Mater. 2014, 29, 621–626. [Google Scholar] [CrossRef]
- Ling, Y.; Guo, T.; Guo, Y.; Yang, Y.; Tian, Y.; Wang, X.; Ou, X.; Feng, P. New two-layer Ruddlesden-Popper cathode materials for protonic ceramics fuel cells. J. Adv. Ceram. 2021, 10, 1052–1060. [Google Scholar] [CrossRef]
- Prakash, B.S.; Kumar, S.S.; Aruna, S.T. Properties and development of Ni/YSZ as an anode material in solid oxide fuel cell: A review. Renew. Sustain. Energy Rev. 2014, 36, 149–179. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Liu, Z.J.; Yan, X.M.; Tan, K.; Tian, F.Y.; Liu, J. Simultaneous electrochemical reduction of carbon dioxide and partial oxidation of methane in a solid oxide cell with silver-based cathode and nickel-based anode. J. Electrochem. Soc. 2022, 169, 034502. [Google Scholar] [CrossRef]
- Yin, W.B.; Chuang, S.S.C. CH4 internal dry reforming over a Ni/YSZ/ScSZ anode catalyst in a SOFC: A transient kinetic study. Catal. Commun. 2017, 102, 62–66. [Google Scholar] [CrossRef]
- Lyu, Z.W.; Shi, W.Y.; Han, M.F. Electrochemical characteristics and carbon tolerance of solid oxide fuel cells with direct internal dry reforming of methane. Appl. Energy. 2018, 228, 556–567. [Google Scholar] [CrossRef]
- Rismanchian, A.; Chuang, S.S. Electroless deposited Cu on the Ni/YSZ anode for the direct CH4-SOFC. In Proceedings of the 13th International Symposium on Solid Oxide Fuel Cells (SOFC-XIII), Okinawa, Japan, 3–11 October 2013; pp. 1429–1436. [Google Scholar] [CrossRef]
- Wongsawatgul, N.; Chaianansutcharit, S.; Yamamoto, K.; Nanko, M.; Sato, K. Cobalt alloying effect on improvement of Ni/YSZ anode-supported solid oxide fuel cell operating with dry methane. Mater. Trans. 2021, 62, 1541–1548. [Google Scholar] [CrossRef]
- Sun, C.W.; Xie, Z.; Xia, C.R.; Li, H.; Chen, L.Q. Investigations of mesoporous CeO2-Ru as a reforming catalyst layer for solid oxide fuel cells. Electrochem. Commun. 2006, 8, 833–838. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Wang, X.X.; Zheng, K.Q.; Shen, S.L.; Ou, X.M.; Lin, B.; Feng, P.Z.; Wang, S.R.; Ling, Y.H. Enhance coking tolerance of high-performance direct carbon dioxide-methane solid oxide fuel cells with an additional internal reforming catalyst. J. Power Sources 2021, 512, 230533. [Google Scholar] [CrossRef]
- Wei, T.; Qiu, P.; Yang, J.; Jia, L.C.; Chi, B.; Pu, J.; Li, J. High-performance direct carbon dioxide-methane solid oxide fuel cell with a structure-engineered double-layer anode. J. Power Sources 2021, 484, 229199. [Google Scholar] [CrossRef]
- Jiang, C.R.; Ma, J.J.; Bonaccorso, A.D.; Irvine, J.T.S. Demonstration of high power, direct conversion of waste-derived carbon in a hybrid direct carbon fuel cell. Energy Environ. Sci. 2012, 5, 6973–6980. [Google Scholar] [CrossRef]
- Enneffatia, M.; Rasheed, M.; Louatia, B.; Guidaraa, K.; Shihab, S.; Barillé, R. Investigation of structural, morphology, optical properties and electrical transport conduction of Li0.25Na0.75CdVO4 compound. J. Phys. Conf. Ser. 2021, 1795, 012050. [Google Scholar] [CrossRef]
- Aukstuolis, A.; Girtan, M.; Mousdis, G.A.; Mallet, R.; Socol, M.; Rasheed, M.; Stanculescu, A. Measurement of Charge Carrier Mobility in Perovskite Nanowire Films by Photo-Celiv Method. Proc. Rom. Acad. Ser. a-Math. Phys. Tech. Sci. Inf. Sci. 2017, 18, 34–41. Available online: https://hal.archives-ouvertes.fr/hal-02443179 (accessed on 18 November 2022).
- Zhai, S.; Xie, H.P.; Chen, B.; Ni, M. A rational design of FeNi alloy nanoparticles and carbonate-decorated perovskite as a highly active and coke-resistant anode for solid oxide fuel cells. Chem. Eng. J. 2022, 430, 132615. [Google Scholar] [CrossRef]
- Wang, W.; Wang, F.; Chen, Y.B.; Qu, J.F.; Tade, M.O.; Shao, Z.P. Ceramic lithium ion conductor to solve the anode coking problem of practical solid oxide fuel cells. ChemSusChem 2015, 8, 2978–2986. [Google Scholar] [CrossRef] [PubMed]
- Ebiad, M.A.; El-Hafiz, D.R.A.; Elsalamony, R.A.; Mohamed, L.S. Ni supported high surface area CeO2-ZrO2 catalysts for hydrogen production from ethanol steam reforming. RSC Adv. 2012, 2, 8145–8156. [Google Scholar] [CrossRef]
- Zanchet, D.; Santos, J.B.O.; Damyanova, S.; Gallo, J.M.R.; Bueno, J.M.C. Toward understanding metal-catalyzed ethanol reforming. ACS Catal. 2015, 5, 3841–3863. [Google Scholar] [CrossRef]
- Ogo, S.; Sekine, Y. Recent progress in ethanol steam reforming using non-noble transition metal catalysts: A review. Fuel Process. Technol. 2020, 199, 106238. [Google Scholar] [CrossRef]
- Kale, G.R.; Gaikwad, T.M. Thermodynamic Analysis of Ethanol Dry Reforming: Effect of Combined Parameters. Int. Sch. Res. Not. 2014, 2014, 929676. [Google Scholar] [CrossRef]
- Jankhah, S.; Abatzoglou, N.; Gitzhofer, F. Thermal and catalytic dry reforming and cracking of ethanol for hydrogen and carbon nanofilaments production. Int. J. Hydrogen Energy 2008, 33, 4769–4779. [Google Scholar] [CrossRef]
- Papazisi, K.M.; Balomenou, S.; Tsiplakides, D. Synthesis and characterization of La0.75Sr0.25Cr0.9M0.1O3 perovskites as anodes for CO-fuelled solid oxide fuel cells. J. Appl. Electrochem. 2010, 40, 1875–1881. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Xu, Y.H.; Gan, L.Z. Exsolved metallic iron nanoparticles in perovskite cathode to enhance CO2 electrolysis. J. Solid State Chem. 2022, 26, 409–417. [Google Scholar] [CrossRef]
- Park, G.S.; Mo, S.I.; Kim, J.H.; Yun, J.W. Characteristics of Li2CO3 as sintering aid for Ce0.8Sm0.2O2-δ electrolyte in solid oxide fuel cells. Korean J. Chem. Eng. 2022, 39, 1796–1804. [Google Scholar] [CrossRef]
- Alami, A.H.; Hawili, A.A. Synthesis, characterization and applications of FeCu alloys. Appl. Surf. Sci. 2020, 1, 100027. [Google Scholar] [CrossRef]
- Xu, Y.S.; Xu, X.; Bi, L. A high-entropy spinel ceramic oxide as the cathode for proton-conducting solid oxide fuel cells. J. Adv. Ceram. 2022, 11, 794–804. [Google Scholar] [CrossRef]
- Artrith, N.; Lin, Z.X.; Chen, J.G. Predicting the activity and selectivity of bimetallic metal catalysts for ethanol reforming using machine learning. ACS Catal. 2020, 10, 9438–9444. [Google Scholar] [CrossRef]
- Shafiqah, M.N.N.; Siang, T.J.; Kumar, P.S.; Ahmad, Z.; Jalil, A.A.; Bahari, M.B.; Le, Q.V.; Xiao, L.L.; Mofijur, M.; Xia, C.L.; et al. Advanced catalysts and effect of operating parameters in ethanol dry reforming for hydrogen generation. A review. Environ. Chem. Lett. 2022, 20, 1695–1718. [Google Scholar] [CrossRef]
- Song, W.C.; Ma, Z.K.; Yang, Y.; Zhang, S.H.; Ou, X.M.; Ling, Y.H. Characterization and polarization DRT analysis of direct ethanol solid oxide fuel cells using low fuel partial pressures. Int. J. Hydrogen Energy 2020, 45, 14480–14490. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, J.; Zeng, X.Y.; Li, X.; Yang, J.; Zou, Y.L.; Liu, S.D.F.; Dong, P.; Zhang, Y.J.; Liu, J. A high performance direct carbon solid oxide fuel cell-a green pathway for brown coal utilization. Appl. Energy 2019, 248, 679–687. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, L.Y.; Zhang, L.Q.; An, W.T.; Wang, W.; Zhou, W.; Tade, M.O.; Shao, Z.P.; Bai, J.P.; Li, S.D. Direct operation of solid oxide fuel cells on low-concentration oxygen-bearing coal-bed methane with high stability. Energy Fuels 2018, 32, 4547–4558. [Google Scholar] [CrossRef]
- Bao, H.; Chen, R.J.; Wang, X.R.; Yang, Y.; Lin, T.Q.; Wang, X.X.; Ou, X.M.; Tian, Y.F.; Ling, Y.H. Characterization of one-step co-fired BaZr0.8Y0.2O3-delta-La2Ce2O7 composite electrolyte for low-temperature solid oxide fuel cells. J. Eur. Ceram. Soc. 2021, 41, 5531–5540. [Google Scholar] [CrossRef]
- Shao, X.; Rickard, W.D.A.; Dong, D.H.; Dang, H.; Saunders, M.; Dodd, A.; Parkinson, G.; Li, C.Z. High performance anode with dendritic porous structure for low temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2018, 43, 17849–17856. [Google Scholar] [CrossRef]
- Li, T.; Ling, Y.H.; Lin, B.; Ou, X.M.; Wang, S.R. A simple, feasible, and non-hazardous laboratory evaluation of direct ammonia solid oxide fuel cells fueled by aqueous ammonia. Sep. Purif. Technol. 2022, 297, 121511. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Dong, J.; Chen, Z.; Huang, K.; Xiong, K.; Li, R.; Rao, M.; Chen, C.; Ling, Y.; Lin, B. Excessive Na-Doped La0.75Sr0.25Cr0.5Fe0.4Cu0.1O3-δ Perovskite as an Additional Internal Reforming Catalyst for Direct Carbon Dioxide-Ethanol Solid Oxide Fuel Cells. Catalysts 2022, 12, 1600. https://doi.org/10.3390/catal12121600
Li M, Dong J, Chen Z, Huang K, Xiong K, Li R, Rao M, Chen C, Ling Y, Lin B. Excessive Na-Doped La0.75Sr0.25Cr0.5Fe0.4Cu0.1O3-δ Perovskite as an Additional Internal Reforming Catalyst for Direct Carbon Dioxide-Ethanol Solid Oxide Fuel Cells. Catalysts. 2022; 12(12):1600. https://doi.org/10.3390/catal12121600
Chicago/Turabian StyleLi, Mingfei, Jiangbo Dong, Zhengpeng Chen, Kairu Huang, Kai Xiong, Ruoyu Li, Mumin Rao, Chuangting Chen, Yihan Ling, and Bin Lin. 2022. "Excessive Na-Doped La0.75Sr0.25Cr0.5Fe0.4Cu0.1O3-δ Perovskite as an Additional Internal Reforming Catalyst for Direct Carbon Dioxide-Ethanol Solid Oxide Fuel Cells" Catalysts 12, no. 12: 1600. https://doi.org/10.3390/catal12121600
APA StyleLi, M., Dong, J., Chen, Z., Huang, K., Xiong, K., Li, R., Rao, M., Chen, C., Ling, Y., & Lin, B. (2022). Excessive Na-Doped La0.75Sr0.25Cr0.5Fe0.4Cu0.1O3-δ Perovskite as an Additional Internal Reforming Catalyst for Direct Carbon Dioxide-Ethanol Solid Oxide Fuel Cells. Catalysts, 12(12), 1600. https://doi.org/10.3390/catal12121600







