Synthesis of Catalytic Precursors Based on Mixed Ni-Al Oxides by Supercritical Antisolvent Co-Precipitation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
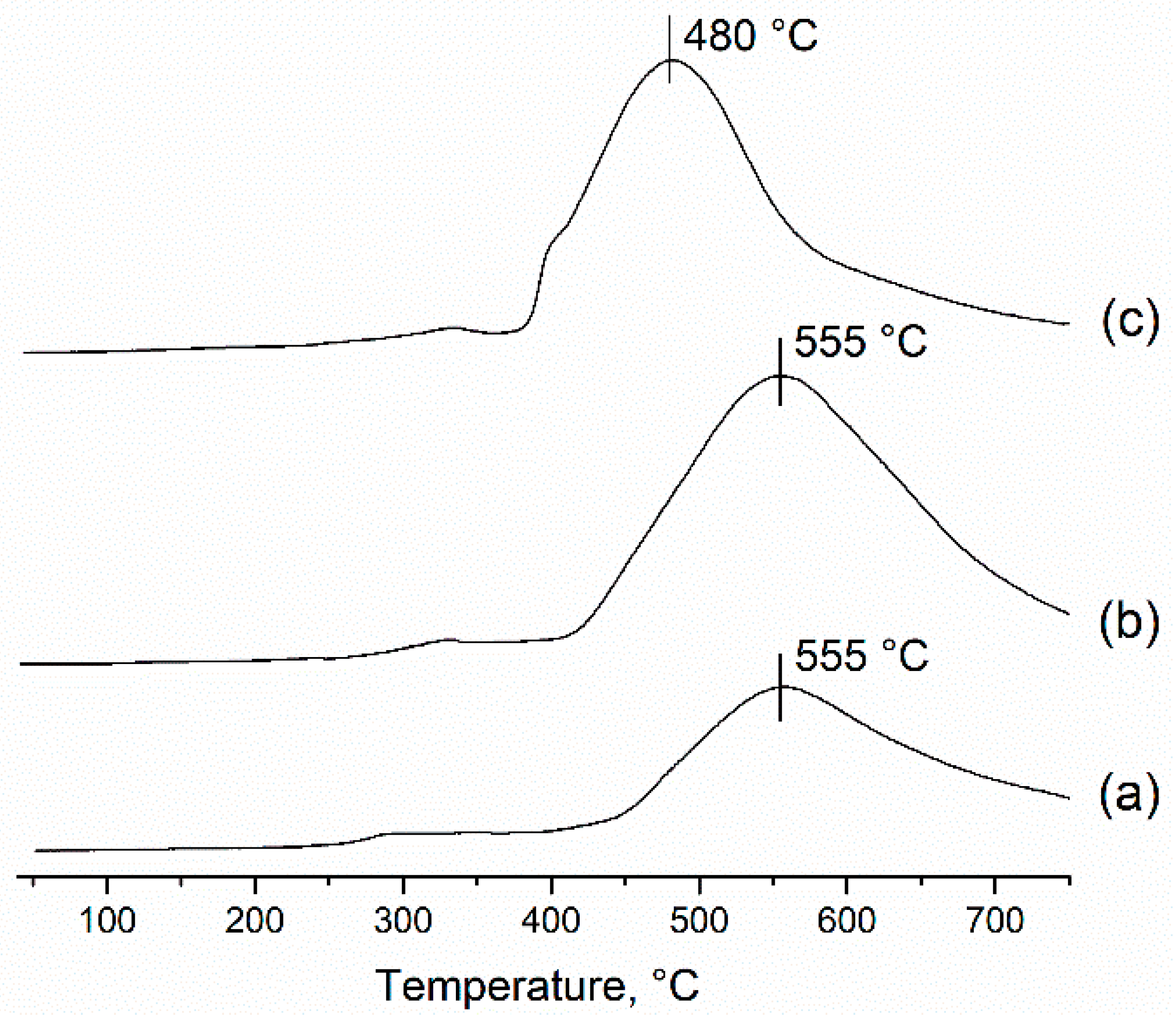
2.1.1. Ni-Al Oxide Precursors
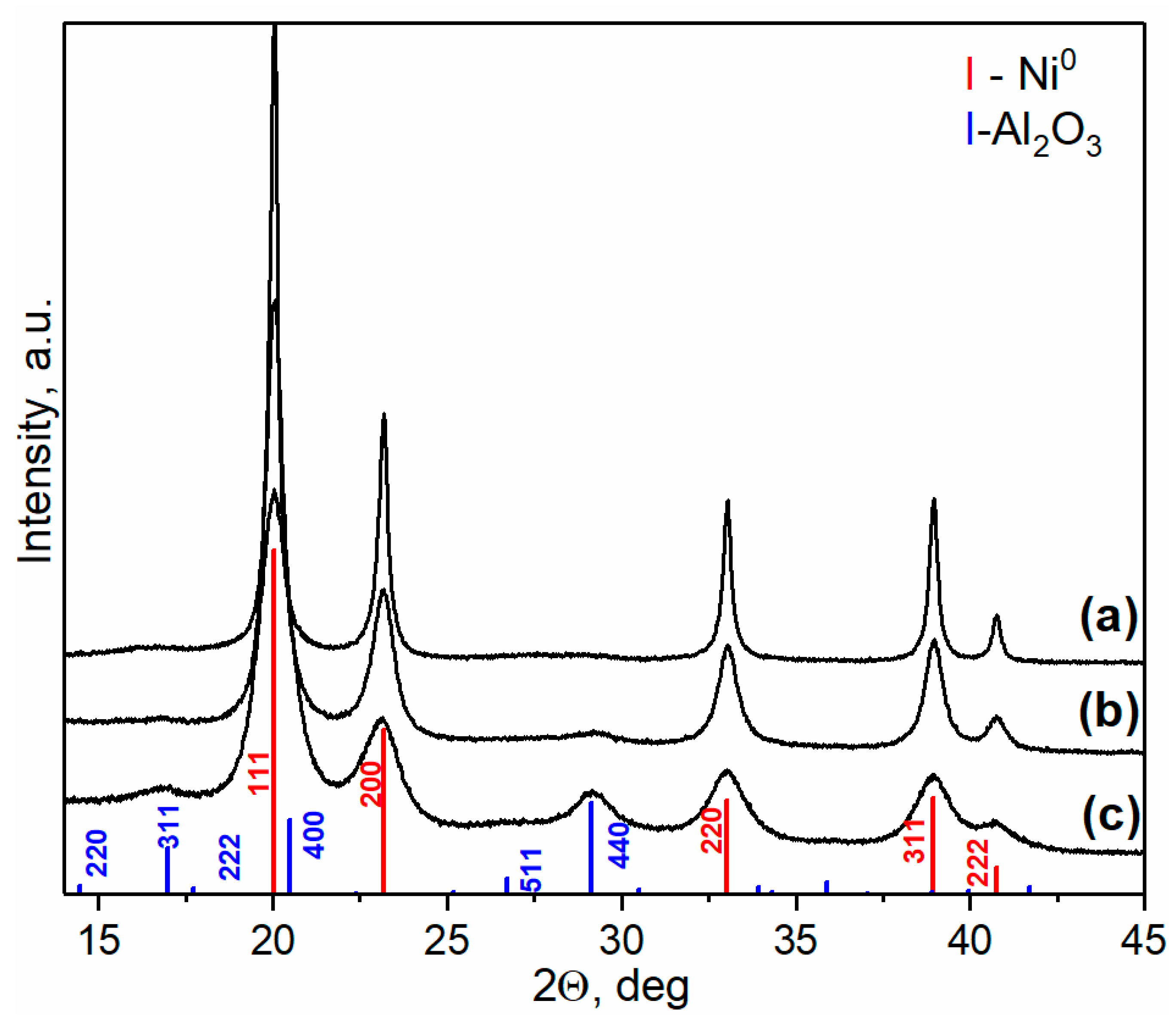
2.1.2. Reduced Ni-Al Catalysts
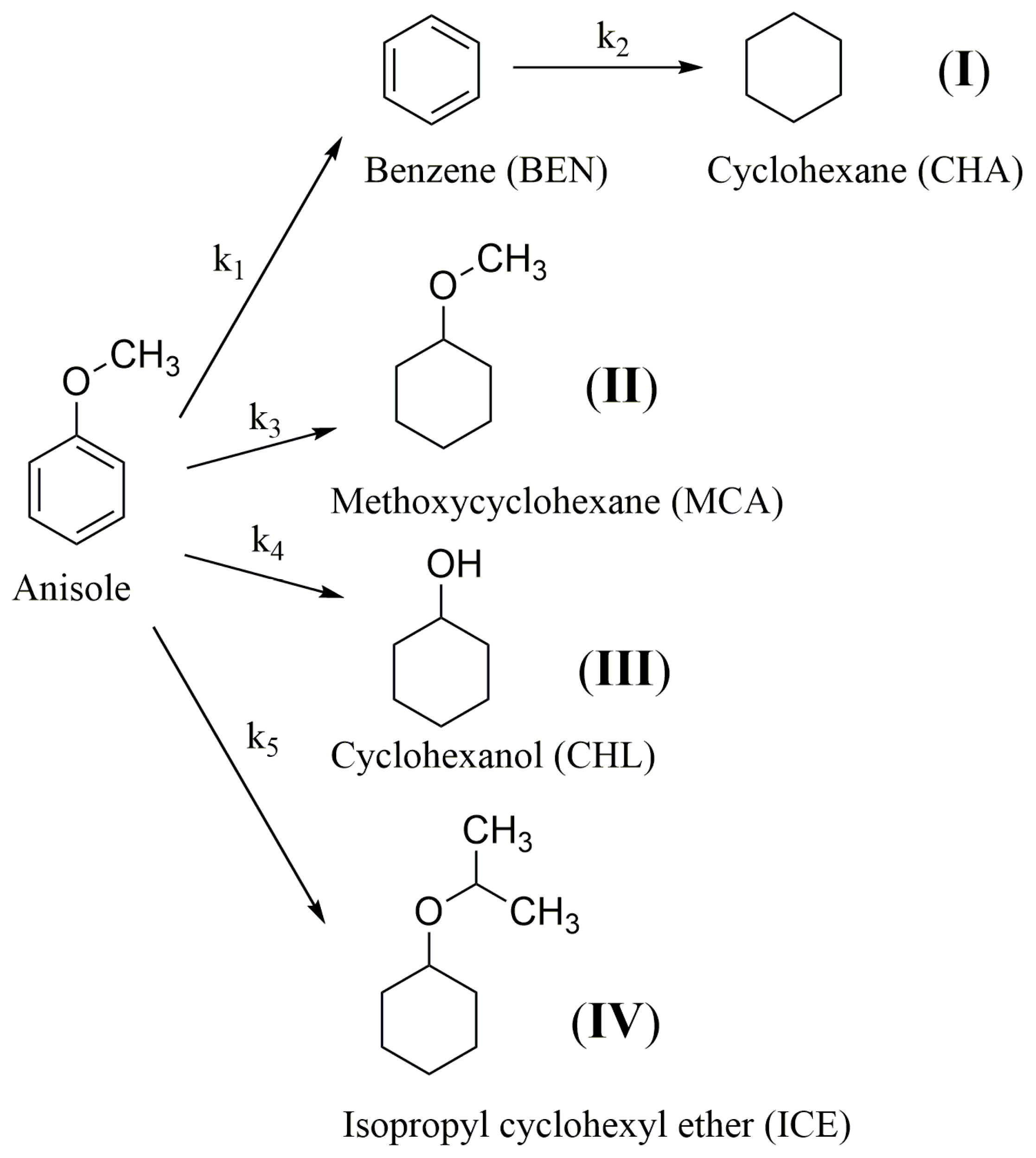
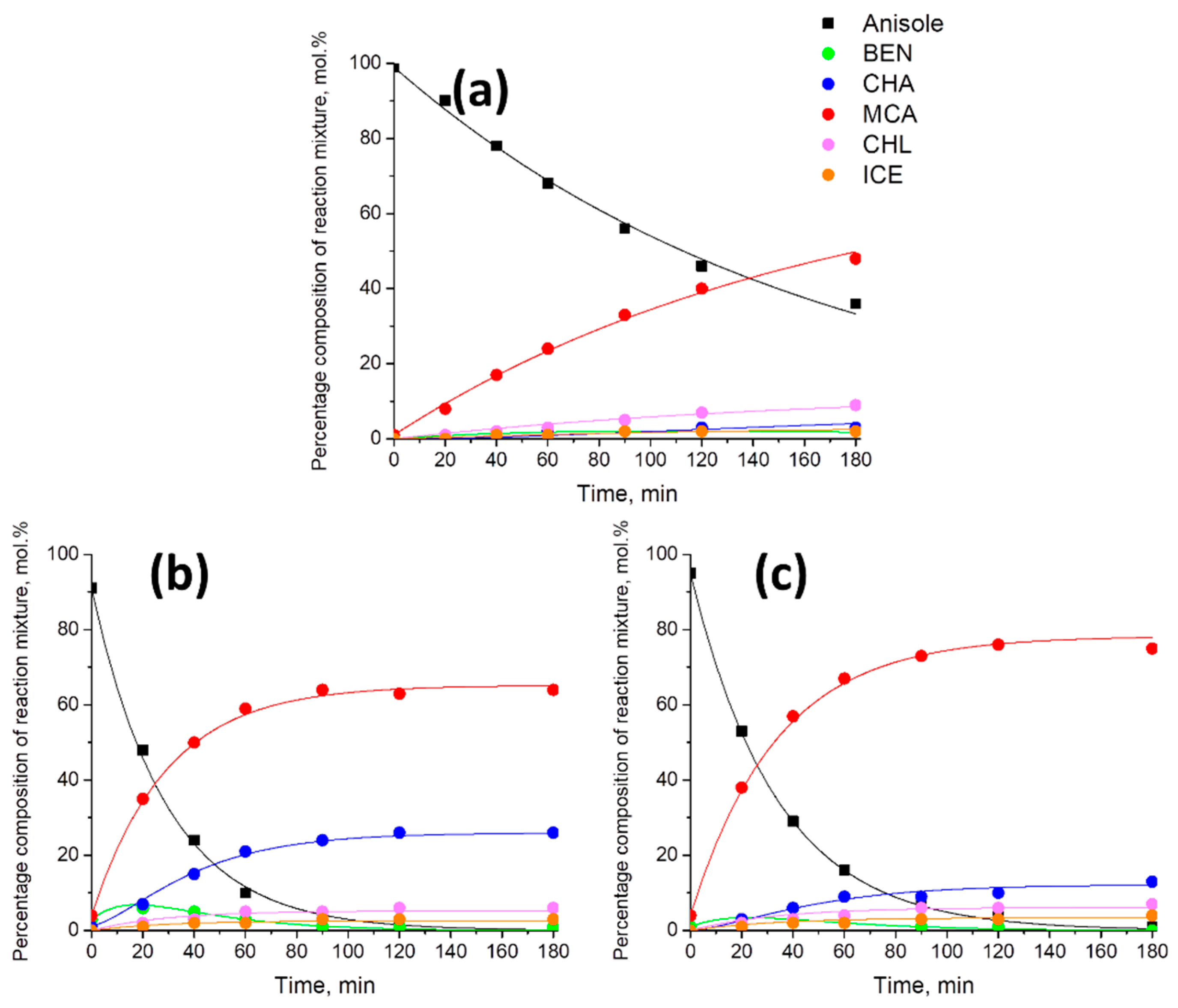
2.2. Transfer Hydrogenation of Anisole
3. Materials and Methods
3.1. Reagents
3.2. Catalysts Synthesis
3.3. CO Pulse Chemisorption and H2-Temperature Programming Reduction Measurements
3.4. ICP-AES
3.5. XRD Characterization
3.6. HRTEM Characterization
3.7. Batch Experiments
3.8. Product Analysis
3.9. Calculations of Kinetic Data
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kwon, Y.; Eichler, J.E.; Mullins, C.B. NiAl2O4 as a Beneficial Precursor for Ni/Al2O3 Catalysts for the Dry Reforming of Methane. J. CO2 Util. 2022, 63, 102112. [Google Scholar] [CrossRef]
- Alreshaidan, S.B.; Ibrahim, A.A.; Fakeeha, A.H.; Almutlaq, A.M.; Ali, F.A.A.; Al-Fatesh, A.S. Effect of Modified Alumina Support on the Performance of Ni-Based Catalysts for CO2 Reforming of Methane. Catalysts 2022, 12, 1066. [Google Scholar] [CrossRef]
- Weber, S.; Abel, K.L.; Zimmermann, R.T.; Huang, X.; Bremer, J.; Rihko-Struckmann, L.K.; Batey, D.; Cipiccia, S.; Titus, J.; Poppitz, D.; et al. Porosity and Structure of Hierarchically Porous Ni/Al2O3 Catalysts for CO2 Methanation. Catalysts 2020, 10, 1471. [Google Scholar] [CrossRef]
- Zhou, L.; Li, L.; Wei, N.; Li, J.; Basset, J.-M. Effect of NiAl2O4 Formation on Ni/Al2O3 Stability during Dry Reforming of Methane. ChemCatChem 2015, 7, 2508–2516. [Google Scholar] [CrossRef] [Green Version]
- Gericke, S.M.; Rissler, J.; Bermeo, M.; Wallander, H.; Karlsson, H.; Kollberg, L.; Scardamaglia, M.; Temperton, R.; Zhu, S.; Sigfridsson Clauss, K.G.V.; et al. In Situ H2 Reduction of Al2O3-Supported Ni- and Mo-Based Catalysts. Catalysts 2022, 12, 755. [Google Scholar] [CrossRef]
- Philippov, A.A.; Nesterov, N.N.; Pakharukova, V.P.; Martyanov, O.N. High-Loaded Ni-Based Catalysts Obtained via Supercritical Antisolvent Coprecipitation in Transfer Hydrogenation of Anisole: Influence of the Support. Appl. Catal. A Gen. 2022, 643, 118792. [Google Scholar] [CrossRef]
- Yoon, J.S.; Park, M.B.; Kim, Y.; Hwang, D.W.; Chae, H.-J. Effect of Metal Oxide-Support Interactions on Ethylene Oligomerization over Nickel Oxide/Silica–Alumina Catalysts. Catalysts 2019, 9, 933. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ren, J. Conversion of Methane and Carbon Dioxide into Synthesis Gas over Alumina-Supported Nickel Catalysts. Effect of Ni-Al2O3 Interactions. Catal. Lett. 1994, 29, 39–48. [Google Scholar] [CrossRef]
- Margossian, T.; Larmier, K.; Kim, S.M.; Krumeich, F.; Fedorov, A.; Chen, P.; Müller, C.R.; Copéret, C. Molecularly Tailored Nickel Precursor and Support Yield a Stable Methane Dry Reforming Catalyst with Superior Metal Utilization. J. Am. Chem. Soc. 2017, 139, 6919–6927. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, S.; Li, Y.; Yang, F.; Yu, H.; Chu, Y.; Li, T.; Yin, H. Improving Anti-Coking Properties of Ni/Al2O3 Catalysts via Synergistic Effect of Metallic Nickel and Nickel Phosphides in Dry Methane Reforming. Materials 2022, 15, 3044. [Google Scholar] [CrossRef]
- Bian, Z.; Zhong, W.; Yu, Y.; Wang, Z.; Jiang, B.; Kawi, S. Dry Reforming of Methane on Ni/Mesoporous-Al2O3 Catalysts: Effect of Calcination Temperature. Int. J. Hydrogen Energy 2021, 46, 31041–31053. [Google Scholar] [CrossRef]
- Zhang, T.; Ai, H.; Liu, Q. La2O3-Promoted Ni/Al2O3 Catalyst for CO Methanation: Enhanced Catalytic Activity and Stability. Energy Technol. 2019, 7, 1900531. [Google Scholar] [CrossRef]
- Sahli, N.; Petit, C.; Roger, A.C.; Kiennemann, A.; Libs, S.; Bettahar, M.M. Ni Catalysts from NiAl2O4 Spinel for CO2 Reforming of Methane. Catal. Today 2006, 113, 187–193. [Google Scholar] [CrossRef]
- Alekseev, E.S.; Alentiev, A.Y.; Belova, A.S.; Bogdan, V.I.; Bogdan, T.V.; Bystrova, A.V.; Gafarova, E.R.; Golubeva, E.N.; Grebenik, E.A.; Gromov, O.I.; et al. Supercritical Fluids in Chemistry. Russ. Chem. Rev. 2020, 89, 1337–1427. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Simentsova, I.I.; Yudanov, V.F.; Martyanov, O.N. A Comparative FMR Study of the Reduction of Co-Containing Catalysts for the Fischer-Tropsch Process in Hydrogen and Supercritical Isopropanol. J. Struct. Chem. 2016, 57, 90–96. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000; ISBN 978-0-19-850698-0. [Google Scholar]
- Siril, P.F.; Türk, M. Synthesis of Metal Nanostructures Using Supercritical Carbon Dioxide: A Green and Upscalable Process. Small 2020, 16, 2001972. [Google Scholar] [CrossRef]
- Hutchings, G.J. Catalyst Synthesis Using Supercritical Carbon Dioxide: A Green Route to High Activity Materials. Top. Catal. 2009, 52, 982–987. [Google Scholar] [CrossRef]
- Martín, A.; Cocero, M.J. Numerical Modeling of Jet Hydrodynamics, Mass Transfer, and Crystallization Kinetics in the Supercritical Antisolvent (SAS) Process. J. Supercrit. Fluids 2004, 32, 203–219. [Google Scholar] [CrossRef]
- Kondrat, S.A.; Smith, P.J.; Wells, P.P.; Chater, P.A.; Carter, J.H.; Morgan, D.J.; Fiordaliso, E.M.; Wagner, J.B.; Davies, T.E.; Lu, L.; et al. Stable Amorphous Georgeite as a Precursor to a High-Activity Catalyst. Nature 2016, 531, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.J.; Kondrat, S.A.; Carter, J.H.; Chater, P.A.; Bartley, J.K.; Taylor, S.H.; Spencer, M.S.; Hutchings, G.J. Supercritical Antisolvent Precipitation of Amorphous Copper-Zinc Georgeite and Acetate Precursors for the Preparation of Ambient-Pressure Water-Gas-Shift Copper/Zinc Oxide Catalysts. ChemCatChem 2017, 9, 1621–1631. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; De Marco, I.; Vaiano, V. Zinc Oxide Nanoparticles Obtained by Supercritical Antisolvent Precipitation for the Photocatalytic Degradation of Crystal Violet Dye. Catalysts 2019, 9, 346. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, E.P.; Winkler, M.E.G.; Giufrida, W.M.; Cardozo-Filho, L.; Alonso, C.G.; Lopes, J.B.O.; Rubira, A.F.; Silva, R. Effect of Phase Composition on the Photocatalytic Activity of Titanium Dioxide Obtained from Supercritical Antisolvent. J. Colloid Interface Sci. 2019, 535, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Marin, R.P.; Ishikawa, S.; Bahruji, H.; Shaw, G.; Kondrat, S.A.; Miedziak, P.J.; Morgan, D.J.; Taylor, S.H.; Bartley, J.K.; Edwards, J.K.; et al. Supercritical Antisolvent Precipitation of TiO2 with Tailored Anatase/Rutile Composition for Applications in Redox Catalysis and Photocatalysis. Appl. Catal. A Gen. 2015, 504, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Nesterov, N.S.; Shalygin, A.S.; Pakharukova, V.P.; Glazneva, T.S.; Martyanov, O.N. Mesoporous Aerogel-like Al-Si Oxides Obtained via Supercritical Antisolvent Precipitation of Alumina and Silica Sols. J. Supercrit. Fluids 2019, 149, 110–119. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Pakharukova, V.P.; Martyanov, O.N. Water as a Cosolvent—Effective Tool to Avoid Phase Separation in Bimetallic Ni-Cu Catalysts Obtained via Supercritical Antisolvent Approach. J. Supercrit. Fluids 2017, 130, 133–139. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Paharukova, V.P.; Yakovlev, V.A.; Martyanov, O.N. The Facile Synthesis of Ni–Cu Catalysts Stabilized in SiO2 Framework via a Supercritical Antisolvent Approach. J. Supercrit. Fluids 2016, 112, 119–127. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Shalygin, A.S.; Pakharukova, V.P.; Martyanov, O.N. Coprecipitation of Au Clusters and Alumina Sol in Supercritical CO2—The Facile Way to Stabilize Gold Nanoparticles within Oxide Matrix. J. Sol. Gel. Sci. Technol. 2019, 92, 523–528. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Shalygin, A.S.; Glazneva, T.S.; Pakharukova, V.P.; Martyanov, O.N. The Facile Synthesis of Aerogel-like Alumina Highly-Loaded with Gold Nanoparticles. Gold Bull. 2021, 54, 69–74. [Google Scholar] [CrossRef]
- Nesterov, N.S.; Smirnov, A.A.; Pakharukova, V.P.; Yakovlev, V.A.; Martyanov, O.N. Advanced Green Approaches for the Synthesis of NiCu-Containing Catalysts for the Hydrodeoxygenation of Anisole. Catal. Today 2021, 379, 262–271. [Google Scholar] [CrossRef]
- Philippov, A.; Nesterov, N.; Pakharukova, V.; Kozhevnikov, I.; Martyanov, O. Advanced High-Loaded Ni–Cu Catalysts in Transfer Hydrogenation of Anisole: Unexpected Effect of Cu Addition. Catalysts 2022, 12, 1307. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Xu, Y.; Gao, X.; Dai, Y.; Tang, Y. Palladium-Incorporated α-MoC Mesoporous Composites for Enhanced Direct Hydrodeoxygenation of Anisole. Catalysts 2021, 11, 370. [Google Scholar] [CrossRef]
- Mudhulu, S.; Gong, Z.-J.; Ku, H.-C.; Lu, Y.-H.; Yu, W.-Y. Recent Advances in Heterogeneous Catalytic Hydrodeoxygenation of Biomass-Derived Oxygenated Furanics Mediated by Formic Acid. Mater. Today Sustain. 2022, 19, 100199. [Google Scholar] [CrossRef]
- Song, L.; Ouyang, Y.; Huang, S.; Li, Z.; Sun, M. Insight into Liquefaction Process of Sawdust with Hydrogen Donor Solvents. Biomass Bioenergy 2022, 160, 106444. [Google Scholar] [CrossRef]
- Xu, H.; Li, H. Alcohol-Assisted Hydrodeoxygenation as a Sustainable and Cost-Effective Pathway for Biomass Derivatives Upgrading. J. Energy Chem. 2022, 73, 133–159. [Google Scholar] [CrossRef]
- Philippov, A.A.; Chibiryaev, A.M.; Martyanov, O.N. Raney® Nickel-Catalyzed Hydrodeoxygenation and Dearomatization under Transfer Hydrogenation Conditions—Reaction Pathways of Non-Phenolic Compounds. Catal. Today 2020, 355, 35–42. [Google Scholar] [CrossRef]
- Philippov, A.A.; Chibiryaev, A.M.; Martyanov, O.N. Catalyzed Transfer Hydrogenation by 2-Propanol for Highly Selective PAHs Reduction. Catal. Today 2021, 379, 15–22. [Google Scholar] [CrossRef]
- Shafaghat, H.; Tsang, Y.F.; Jeon, J.K.; Kim, J.M.; Kim, Y.; Kim, S.; Park, Y.K. In-Situ Hydrogenation of Bio-Oil/Bio-Oil Phenolic Compounds with Secondary Alcohols over a Synthesized Mesoporous Ni/CeO2 Catalyst. Chem. Eng. J. 2020, 382, 122912. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Q.; Zhang, D.; Liu, W.; Liu, X.; Yin, D. Highly Efficient Synthesis of γ-Valerolactone by Catalytic Conversion of Biomass-Derived Levulinate Esters over Support-Free Mesoporous Ni. Renew. Energy 2021, 163, 1023–1032. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway in Biomass Upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Philippov, A.A.A.; Chibiryaev, A.M.; Martyanov, O.N. Base-Free Transfer Hydrogenation of Menthone by Sub- and Supercritical Alcohols. J. Supercrit. Fluids 2019, 145, 162–168. [Google Scholar] [CrossRef]
- Jin, W.; Pastor-Pérez, L.; Villora-Picó, J.J.; Sepúlveda-Escribano, A.; Gu, S.; Reina, T.R. Investigating New Routes for Biomass Upgrading: “H2-Free” Hydrodeoxygenation Using Ni-Based Catalysts. ACS Sustain. Chem. Eng. 2019, 7, 16041–16049. [Google Scholar] [CrossRef]
- Zangouei, M.; Moghaddam, A.Z.; Arasteh, M. The Influence of Nickel Loading on Reducibility of NiO/Al2O3 Catalysts Synthesized by Sol-Gel Method. Chem. Eng. Res. Bull. 2010, 14, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Gamliel, D.P.; Karakalos, S.; Valla, J.A. Liquid Phase Hydrodeoxygenation of Anisole, 4-Ethylphenol and Benzofuran Using Ni, Ru and Pd Supported on USY Zeolite. Appl. Catal. A Gen. 2018, 559, 20–29. [Google Scholar] [CrossRef]
- Khan, T.S.; Singh, D.; Samal, P.P.; Krishnamurty, S.; Dhepe, P.L. Mechanistic Investigations on the Catalytic Transfer Hydrogenation of Lignin-Derived Monomers over Ru Catalysts: Theoretical and Kinetic Studies. ACS Sustain. Chem. Eng. 2021, 9, 14040–14050. [Google Scholar] [CrossRef]
- Rios-Escobedo, R.; Ortiz-Santos, E.; Colín-Luna, J.A.; Díaz de León, J.N.; del Angel, P.; Escobar, J.; de los Reyes, J.A. Anisole Hydrodeoxygenation: A Comparative Study of Ni/TiO2-ZrO2 and Commercial TiO2 Supported Ni and NiRu Catalysts. Top. Catal. 2022, 65, 1448–1461. [Google Scholar] [CrossRef]
- Li, S.; Guo, L.; He, X.; Qiao, C.; Tian, Y. Synthesis of Uniform Ni Nanoparticles Encapsulated in ZSM–5 for Selective Hydrodeoxygenation of Phenolics. Renew. Energy 2022, 194, 89–99. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, G.; Lin, Y.; Yang, L.; Li, F. Synergies of Surface-Interface Multiple Active Sites over Al-Zr Oxide Solid Solution Supported Nickel Catalysts for Enhancing the Hydrodeoxygenation of Anisole. Appl. Catal. A Gen. 2022, 631, 118481. [Google Scholar] [CrossRef]
- Vargas-Villagrán, H.; Flores-Villeda, M.A.; Puente-Lee, I.; Solís-Casados, D.A.; Gómez-Cortés, A.; Díaz-Guerrero, G.; Klimova, T.E. Supported Nickel Catalysts for Anisole Hydrodeoxygenation: Increase in the Selectivity to Cyclohexane. Catal. Today 2018, 349, 26–41. [Google Scholar] [CrossRef]
- Taghvaei, H.; Moaddeli, A.; Khalafi-Nezhad, A.; Iulianelli, A. Catalytic Hydrodeoxygenation of Lignin Pyrolytic-Oil over Ni Catalysts Supported on Spherical Al-MCM-41 Nanoparticles: Effect of Si/Al Ratio and Ni Loading. Fuel 2021, 293, 120493. [Google Scholar] [CrossRef]
- Egami, T.; Billinge, S.J.L. Underneath the Bragg Peaks Structural Analysis of Complex Materials; Elsevier: Pergamon, Turkey, 2012. [Google Scholar]
- Qiu, X.; Thompson, J.W.; Billinge, S.J.L. PDFgetX2: A GUI-Driven Program to Obtain the Pair Distribution Function from X-Ray Powder Diffraction Data. J. Appl. Crystallogr. 2004, 37, 678. [Google Scholar] [CrossRef]
- Farrow, C.L.; Juhas, P.; Liu, J.W.; Bryndin, D.; Božin, E.S.; Bloch, J.; Proffen, T.; Billinge, S.J.L. PDFfit2 and PDFgui: Computer Programs for Studying Nanostructure in Crystals. J. Phys. Condens. Matter 2007, 19, 335219. [Google Scholar] [CrossRef] [PubMed]
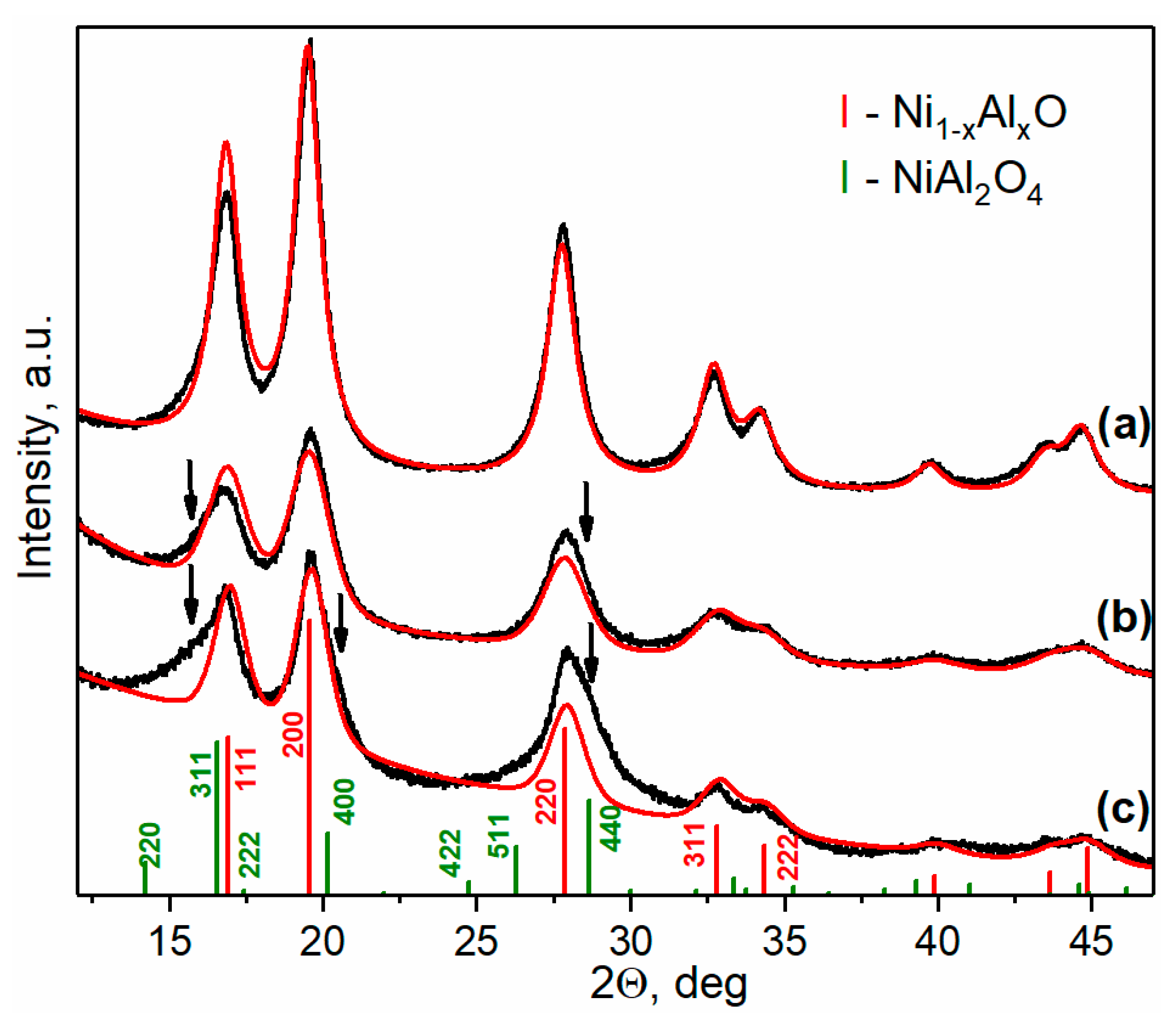
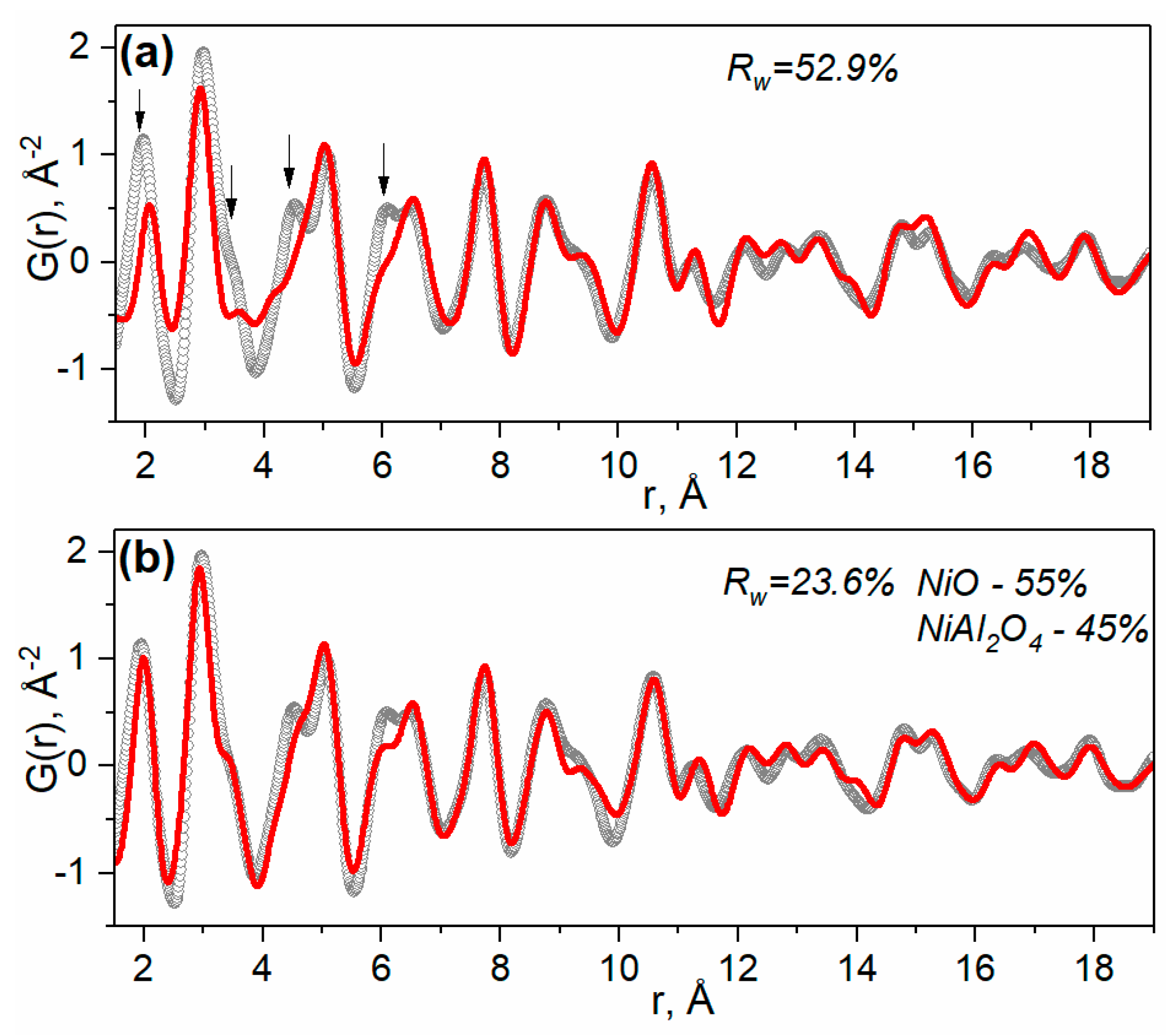
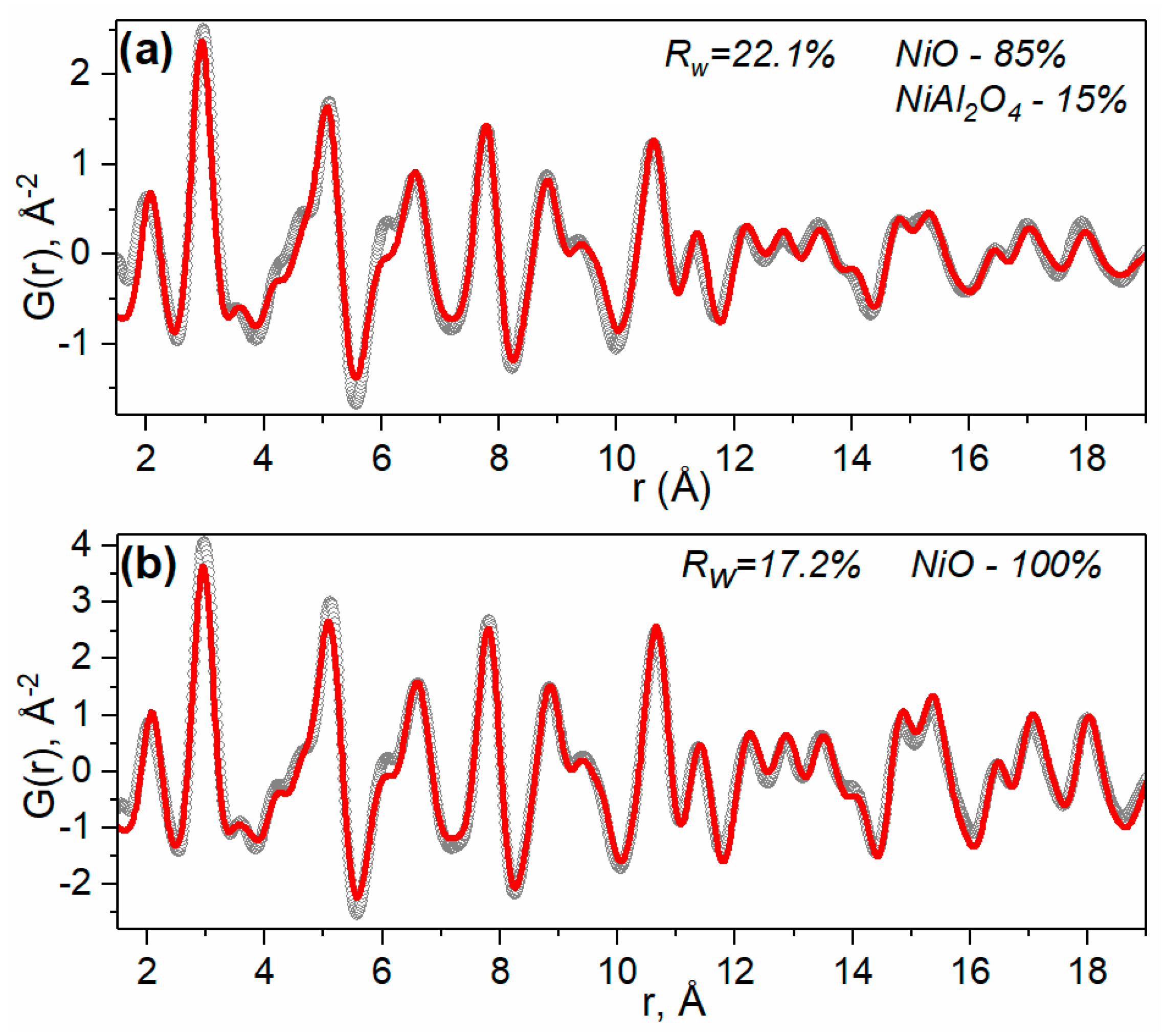
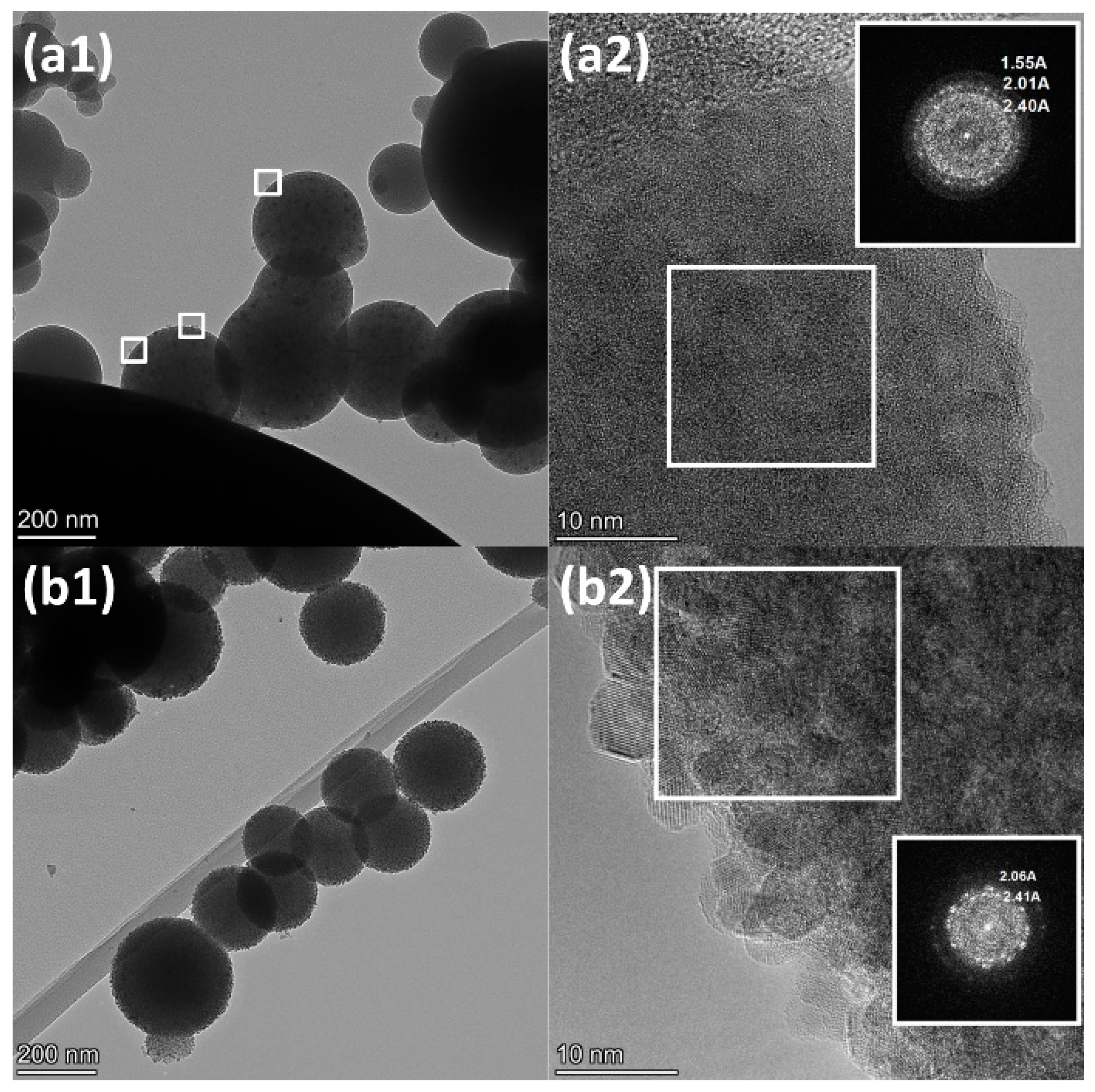
| Sample | Lattice Parameter (nm) | dXRD (nm) |
|---|---|---|
| Ni80_Alum | 0.4164(1) | 4.0 |
| Ni50_Alum | 0.4157(1) | 2.5 |
| Ni20_Alum | 0.4147(2) | ~2.5–3 |
| Sample | Ni0 | Al2O3 | |||
|---|---|---|---|---|---|
| Lattice Parameter (nm) | dXRD (nm) | Ni Content, wt.% | Lattice Parameter (nm) | dXRD (nm) | |
| Ni80_Alum | 0.3524(1) | 15.5 | 85.6 | - | - |
| Ni50_Alum | 0.3523(1) | 6.5 | 57.8 | - | <2.5 |
| Ni20_Alum | 0.3526(1) | 3.5 | 26.8 | 0.7951(2) | ~2.5 |
| Rate Constants | |||||
|---|---|---|---|---|---|
| Sample | k1 × 104, s−1 | k2 × 104, s−1 | k3 × 104, s−1 | k4 × 104, s−1 | k5 × 104, s−1 |
| Ni20_Alum | 0.09 ± 0.01 | 2.23 ± 0.58 | 0.75 ± 0.01 | 0.13 ± 0.01 | 0.04 ± 0.01 |
| Ni50_Alum | 1.38 ± 0.03 | 10.15 ± 0.75 | 3.85 ± 0.05 | 0.33 ± 0.02 | 0.16 ± 0.02 |
| Ni80_Alum | 0.58 ± 0.02 | 8.18 ± 1.19 | 3.87 ± 0.05 | 0.32 ± 0.02 | 0.18 ± 0.02 |
| Specific rate constants | |||||
| Sample | k1S × 104, s−1 × m−2 | k2S ×1 04, s−1 × m−2 | k3S × 104, s−1 × m−2 | k4S × 104, s−1 × m−2 | k5S × 104, s−1 × m−2 |
| Ni20_Alum | 0.16 ± 0.02 | 4.05 ± 1.05 | 1.36 ± 0.02 | 0.24 ± 0.02 | 0.07 ± 0.02 |
| Ni50_Alum | 0.62 ± 0.01 | 4.58 ± 0.34 | 1.74 ± 0.02 | 0.15 ± 0.01 | 0.07 ± 0.01 |
| Ni80_Alum | 0.33 ± 0.01 | 4.71 ± 0.69 | 2.23 ± 0.03 | 0.18 ± 0.01 | 0.10 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesterov, N.S.; Pakharukova, V.P.; Philippov, A.A.; Gerasimov, E.Y.; Tsybulya, S.V.; Martyanov, O.N. Synthesis of Catalytic Precursors Based on Mixed Ni-Al Oxides by Supercritical Antisolvent Co-Precipitation. Catalysts 2022, 12, 1597. https://doi.org/10.3390/catal12121597
Nesterov NS, Pakharukova VP, Philippov AA, Gerasimov EY, Tsybulya SV, Martyanov ON. Synthesis of Catalytic Precursors Based on Mixed Ni-Al Oxides by Supercritical Antisolvent Co-Precipitation. Catalysts. 2022; 12(12):1597. https://doi.org/10.3390/catal12121597
Chicago/Turabian StyleNesterov, Nikolay S., Vera P. Pakharukova, Alexey A. Philippov, Evgeny Y. Gerasimov, Sergey V. Tsybulya, and Oleg N. Martyanov. 2022. "Synthesis of Catalytic Precursors Based on Mixed Ni-Al Oxides by Supercritical Antisolvent Co-Precipitation" Catalysts 12, no. 12: 1597. https://doi.org/10.3390/catal12121597
APA StyleNesterov, N. S., Pakharukova, V. P., Philippov, A. A., Gerasimov, E. Y., Tsybulya, S. V., & Martyanov, O. N. (2022). Synthesis of Catalytic Precursors Based on Mixed Ni-Al Oxides by Supercritical Antisolvent Co-Precipitation. Catalysts, 12(12), 1597. https://doi.org/10.3390/catal12121597









