Application of a Zero-Valent Iron/Cork as Permeable Reactive Barrier for In Situ Remediation of Phenanthrene in Soil
Abstract
1. Introduction
2. Results
2.1. Soil Characterization
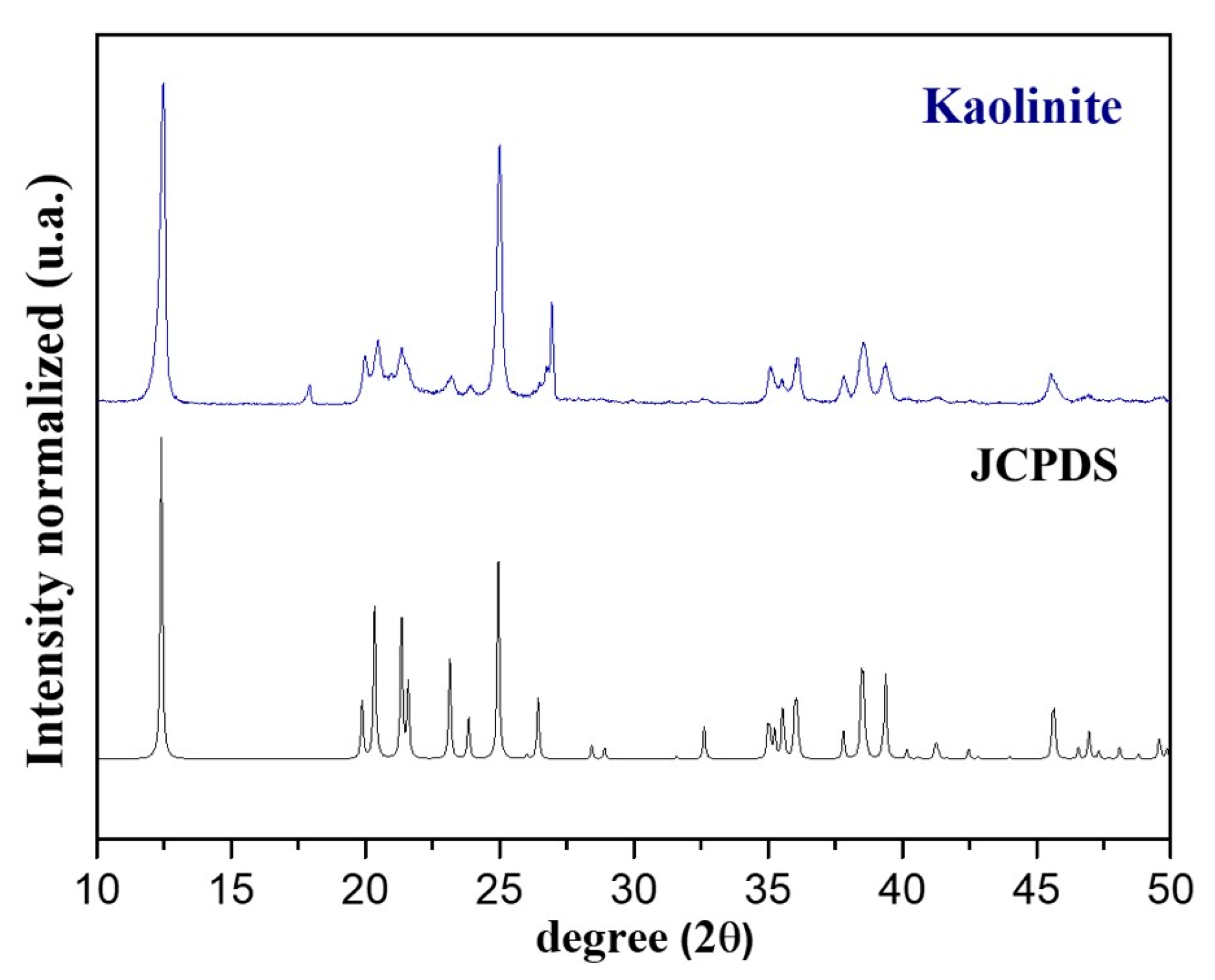
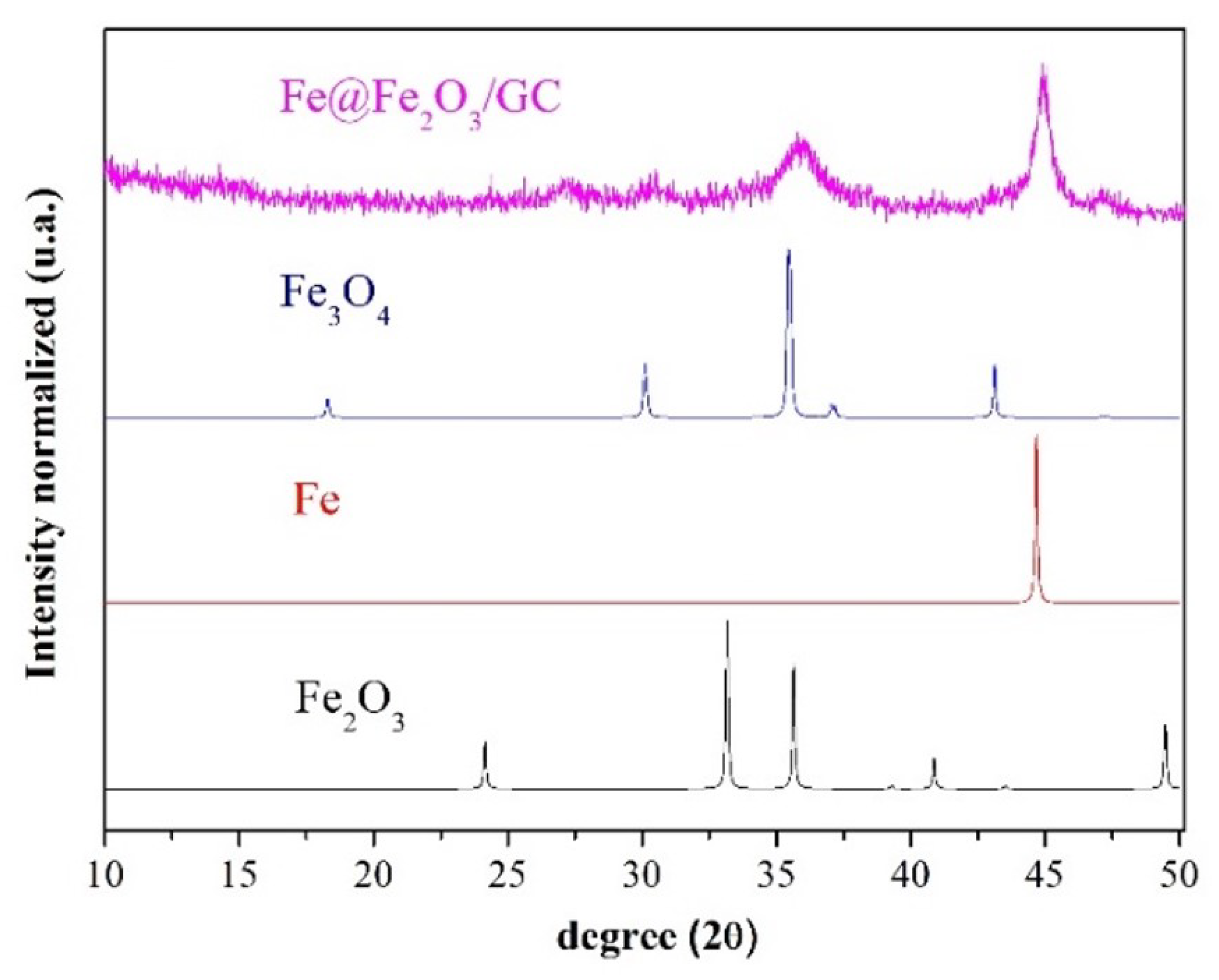
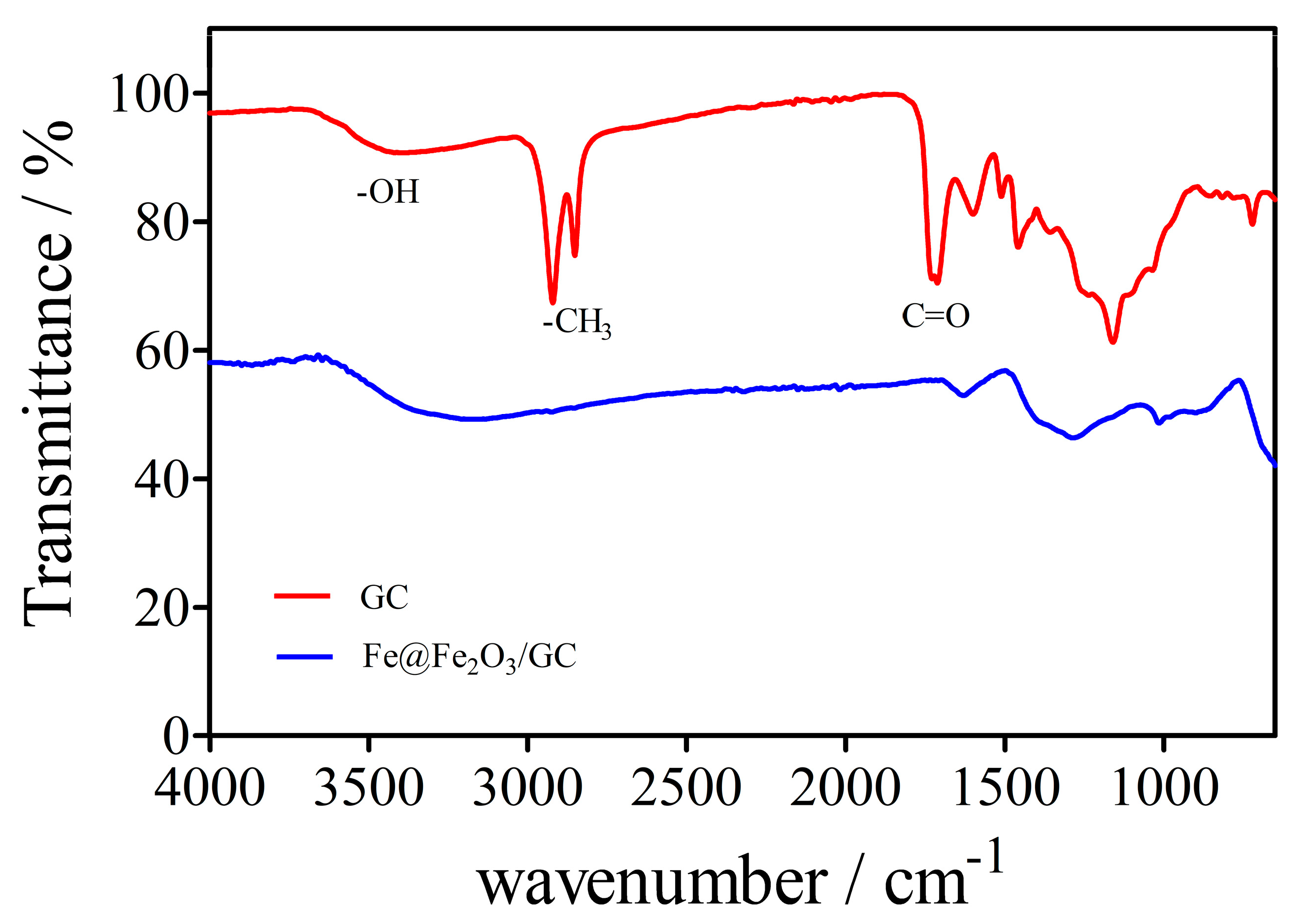
2.2. Regranulated Cork and Fe@Fe2O3/GC Characterization
2.3. pH and Conductivity
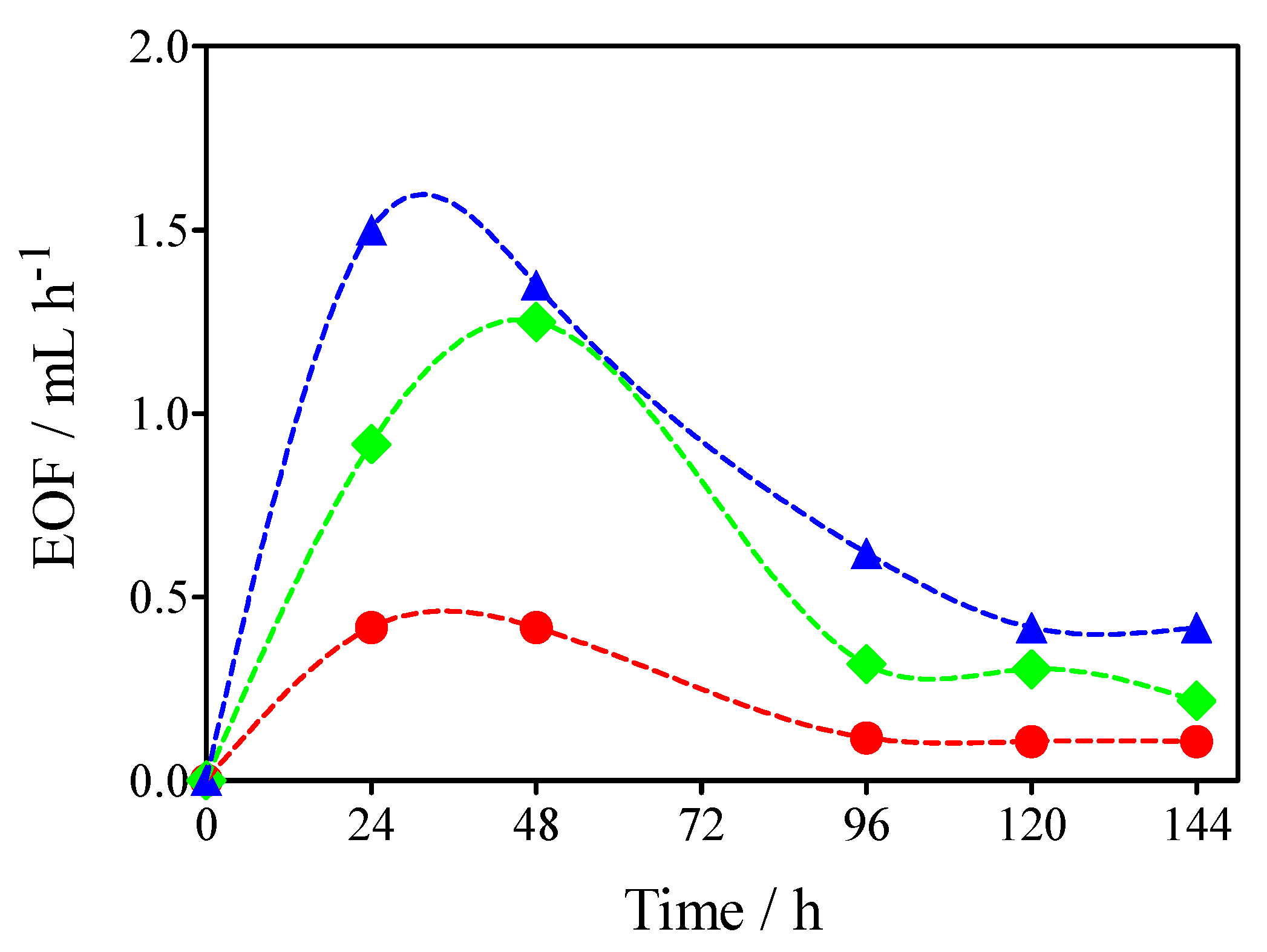
2.4. Cumulative EOF
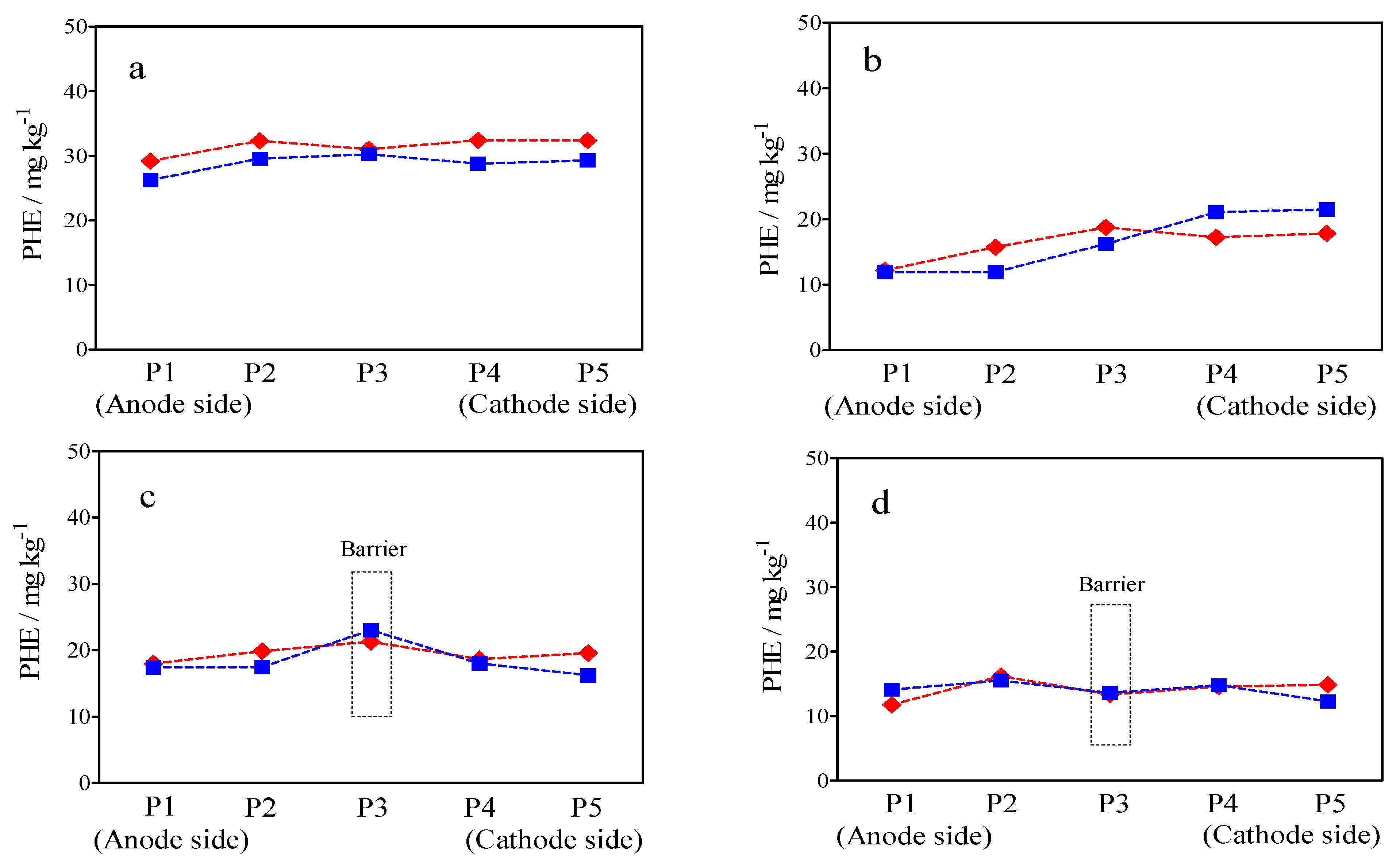
2.5. Distribution of PHE in the Soil after Treatment
3. Materials
3.1. Preparation of the Fe@Fe2O3/BC Catalyst
3.2. Characterization of the Fe@Fe2O3/GC Catalyst
3.3. Preparation of Spiked Soil
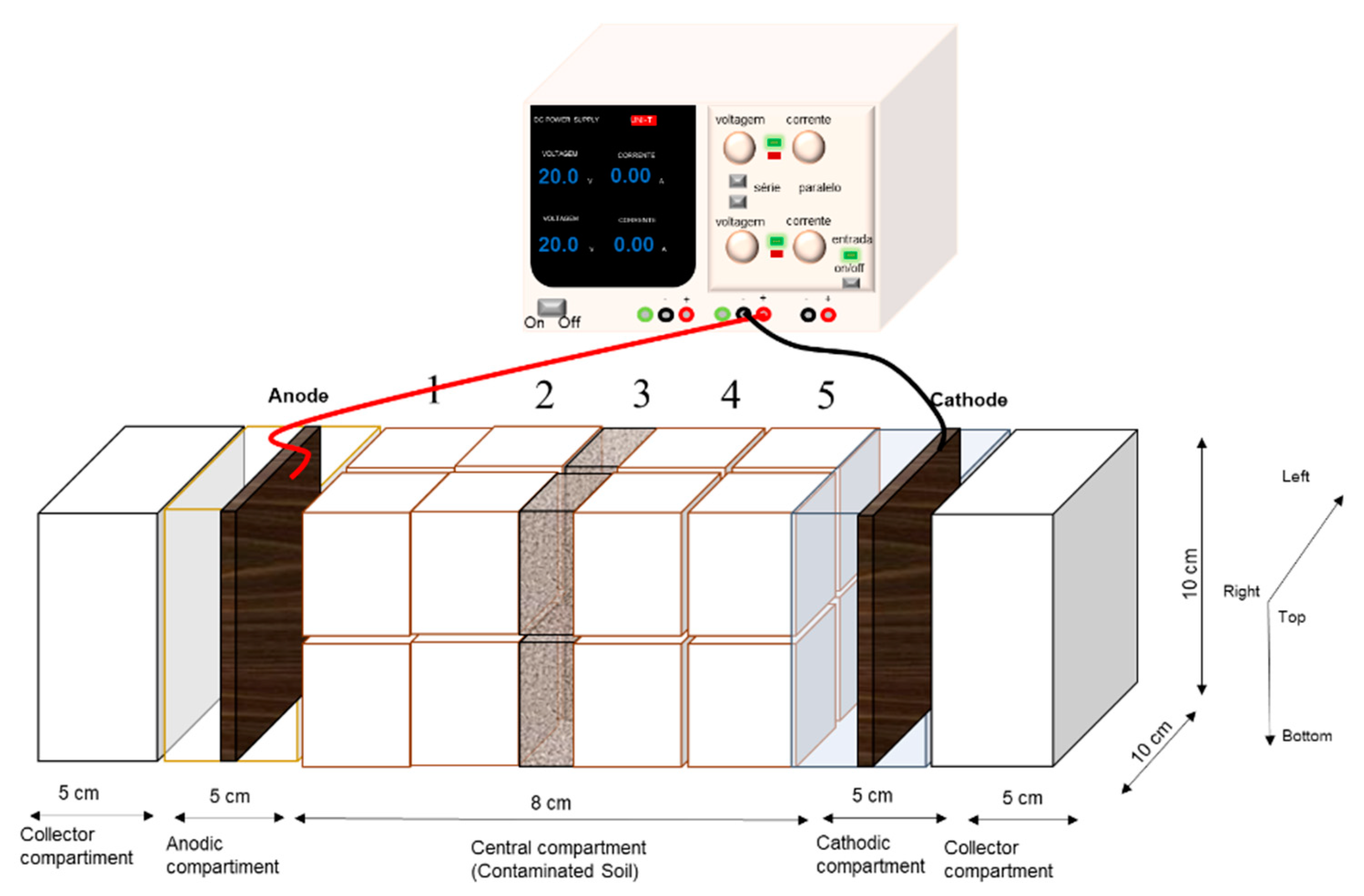
3.4. Cell Configuration
Analytical Methods
4. Conclusions
- -
- The combination of EK-PRB using GC as a reactive barrier over a period of seven days seems to be an innovative alternative for the treatment of soils polluted with PHE because the contaminants were significantly removed from the samples.
- -
- The inclusion of PRB in the polluted soil increased the EOF rate, promoting PHE migration, and electroosmosis was the main transport mechanism in the EK process, favoring the elimination of PHE.
- -
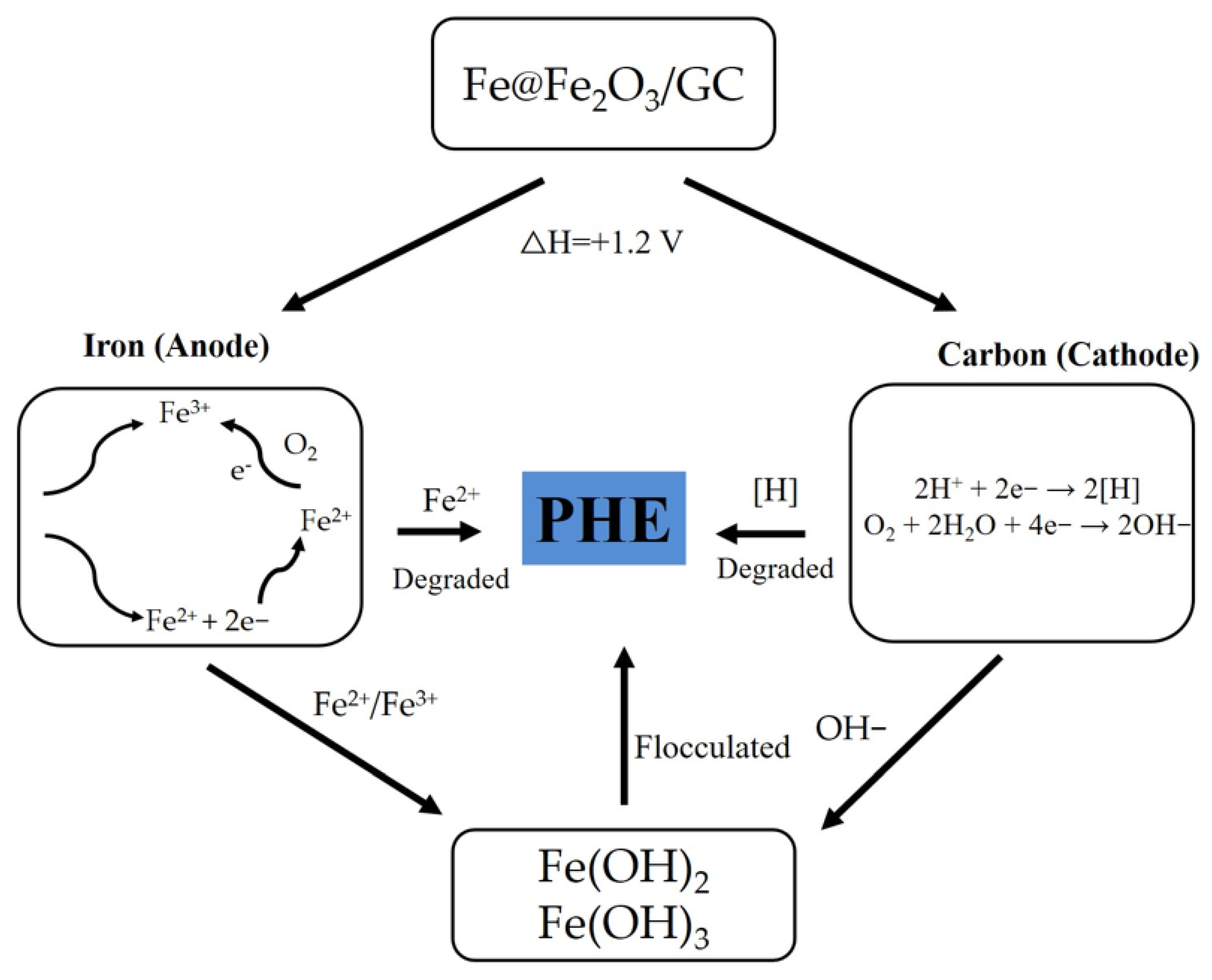
- The use of a reactive barrier formed from GC increased the efficiency of PHE removal by the EK process by 5%, whereas the EK/Fe@Fe2O3/GC process increased the efficiency of this process by 14%, indicating the degradation of PHE by the micro-electrolysis mechanism is feasible and a mechanism was proposed.
- -
- The modification of GC with Fe@Fe2O3 is not an expensive process that requires a reduced number of steps, avoiding the use of several reagents, which fits the principles of green chemistry well. The alternative use of biomass similarly connects with the goals of circular economy.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mouronte, N.; Álvarez, M.S.; Deive, F.J.; Rodríguez, A. Combining Biodegradable Surfactants and Potassium Inorganic Salts for Efficiently Removing Polycyclic Aromatic Hydrocarbons from Aqueous Effluents. J. Water Process Eng. 2022, 47, 102796. [Google Scholar] [CrossRef]
- Chu, L.; Cang, L.; Sun, Z.; Wang, X.; Fang, G.; Gao, J. Reagent-Free Electrokinetic Remediation Coupled with Anode Oxidation for the Treatment of Phenanthrene Polluted Soil. J. Hazard Mater. 2022, 433, 128724. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, H.H.; Kim, S.J.; Lee, Y.J.; Yang, J.W. Surfactant-Enhanced Electrokinetic Removal of Phenanthrene from Kaolinite. J. Hazard Mater. 2007, 140, 230–236. [Google Scholar] [CrossRef]
- de Oliveira Silva, K.N.; Rodrigo, M.A.; dos Santos, E.V. Electrochemical Treatment of Soil-Washing Effluent with Boron-Doped Diamond Electrodes: A Review. Curr. Opin. Solid State Mater. Sci. 2021, 25, 100962. [Google Scholar] [CrossRef]
- Araujo, K.C.; Silva, K.N.; da Silva, D.R.; Quiroz, M.A.; Martinez-Huitle, C.A.; Santos, E.V. The Green Use of Persulfate Electrochemically Generated with Diamond Electrodes for Depolluting Soils. In Proceedings of the 240th ECS Meeting: Symposium: L05: Electrochemical Water Remediation, Orlando, FL, USA, 10–14 October 2021; p. #L05-1530. [Google Scholar]
- Befkadu, A.A.; Chen, Q. Surfactant-Enhanced Soil Washing for Removal of Petroleum Hydrocarbons from Contaminated Soils: A Review. Pedosphere 2018, 28, 383–410. [Google Scholar] [CrossRef]
- Mena, E.; Villaseñor, J.; Rodrigo, M.A.; Cañizares, P. Electrokinetic Remediation of Soil Polluted with Insoluble Organics Using Biological Permeable Reactive Barriers: Effect of Periodic Polarity Reversal and Voltage Gradient. Chem. Eng. J. 2016, 299, 30–36. [Google Scholar] [CrossRef]
- Mena, E.; Ruiz, C.; Villaseñor, J.; Rodrigo, M.A.; Cañizares, P. Biological Permeable Reactive Barriers Coupled with Electrokinetic Soil Flushing for the Treatment of Diesel-Polluted Clay Soil. J. Hazard Mater. 2015, 283, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Huguenot, D.; Mousset, E.; van Hullebusch, E.D.; Oturan, M.A. Combination of Surfactant Enhanced Soil Washing and Electro-Fenton Process for the Treatment of Soils Contaminated by Petroleum Hydrocarbons. J. Environ. Manag. 2015, 153, 40–47. [Google Scholar] [CrossRef]
- Ng, Y.S.; sen Gupta, B.; Hashim, M.A. Stability and Performance Enhancements of Electrokinetic-Fenton Soil Remediation. Rev. Environ. Sci. Biotechnol. 2014, 13, 251–263. [Google Scholar] [CrossRef]
- Andrade, D.C.; dos Santos, E.V. Combination of Electrokinetic Remediation with Permeable Reactive Barriers to Remove Organic Compounds from Soils. Curr. Opin. Electrochem. 2020, 22, 136–144. [Google Scholar] [CrossRef]
- Rodrigo, M.A.; dos Santos, E.V. Electrochemically Assisted Remediation of Contaminated Soils; Rodrigo, M.A., Vieira dos Santos, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9783030681395. [Google Scholar]
- Rodrigo, M.A.; Oturan, M.A.; Oturan, N. Electrochemically Assisted Remediation of Pesticides in Soils and Water: A Review. Chem. Rev. 2014, 114, 8720–8745. [Google Scholar] [CrossRef] [PubMed]
- Paixão, I.C.; López-Vizcaíno, R.; Solano, A.M.S.; Martínez-Huitle, C.A.; Navarro, V.; Rodrigo, M.A.; dos Santos, E.V. Electrokinetic-Fenton for the Remediation Low Hydraulic Conductivity Soil Contaminated with Petroleum. Chemosphere 2020, 248, 126029. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Rosales, E.; Alcántara, T.; Gómez, J.; Sanromán, M.A. Decontamination of Soils Containing PAHs by Electroremediation: A Review. J. Hazard Mater. 2010, 177, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fdez-Sanromán, A.; Pazos, M.; Rosales, E.; Sanromán, M.Á. Prospects on Integrated Electrokinetic Systems for Decontamination of Soil Polluted with Organic Contaminants. Curr. Opin. Electrochem. 2021, 27, 100692. [Google Scholar] [CrossRef]
- Andrade, D.C.; Đolić, M.B.; Martínez-Huitle, C.A.; dos Santos, E.V.; Silva, T.F.; Vilar, V.J. Coupling Electrokinetic with a Cork-Based Permeable Reactive Barrier to Prevent Groundwater Pollution: A Case Study on Hexavalent Chromium- Contaminated Soil. Electrochim. Acta 2022, 429, 140936. [Google Scholar] [CrossRef]
- Vieira dos Santos, E.; Sáez, C.; Cañizares, P.; Martínez-Huitle, C.A.; Rodrigo, M.A. Reversible Electrokinetic Adsorption Barriers for the Removal of Atrazine and Oxyfluorfen from Spiked Soils. J. Hazard Mater. 2017, 322, 413–420. [Google Scholar] [CrossRef]
- Mena, E.; Villaseñor, J.; Cañizares, P.; Rodrigo, M.A. Influence of Electric Field on the Remediation of Polluted Soil Using a Biobarrier Assisted Electro-Bioremediation Process. Electrochim. Acta 2016, 190, 294–304. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Vieira, B.R.C.; Boaventura, R.A.R.; Botelho, C.M.S. Removal of Antimony from Water by Iron-Coated Cork Granulates. Sep. Purif. Technol. 2020, 233, 116020. [Google Scholar] [CrossRef]
- Amalero, E.G.; Ingua, G.L.; Erta, G.B.; Emanceau, P.L. Review Article Methods for Studying Root Colonization by Introduced. Agronomie 2003, 23, 407–418. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, Z.; Mu, J.; Chu, D.; Zang, X. Converting Industrial Waste Cork to Biochar as Cu (II) Adsorbent via Slow Pyrolysis. Waste Manag. 2020, 105, 102–109. [Google Scholar] [CrossRef]
- Abenojar, J.; López de Armentia, S.; Barbosa, A.Q.; Martínez, M.A.; Velasco, F.; da Silva, L.F.M.; del Real Romero, J.C. Coating Cork Particles with Iron Oxide: Effect on Magnetic Properties. Wood Sci. Technol. 2020, 54, 869–889. [Google Scholar] [CrossRef]
- Miranda, I.; Gominho, J.; Pereira, H. Cellular Structure and Chemical Composition of Cork from the Chinese Cork Oak (Quercus Variabilis). J. Wood Sci. 2013, 59, 1–9. [Google Scholar] [CrossRef]
- Şen, A.; Marques, A.V.; Gominho, J.; Pereira, H. Study of Thermochemical Treatments of Cork in the 150–400 °C Range Using Colour Analysis and FTIR Spectroscopy. Ind. Crops Prod. 2012, 38, 132–138. [Google Scholar] [CrossRef]
- De Paiva, S.D.S.M.; da Silva, I.B.; de M. Santos, E.C.M.; Rocha, I.M.V.; Martínez-Huitle, C.A.; dos Santos, E.V. Coupled Electrochemical Processes for Removing Dye from Soil and Water. J. Electrochem. Soc. 2018, 165, E318–E324. [Google Scholar] [CrossRef]
- Silva, K.N.O.; Paiva, S.S.M.; Souza, F.L.; Silva, D.R.; Martínez-Huitle, C.A.; Santos, E.V. Applicability of Electrochemical Technologies for Removing and Monitoring Pb2+ from Soil and Water. J. Electroanal. Chem. 2018, 816, 171–178. [Google Scholar] [CrossRef]
- de Melo Henrique, J.M.; Isidro, J.; Saez, C.; Dos Santos, E.V.; Rodrigo, M.A. Removal of Lindane Using Electrokinetic Soil Flushing Coupled with Air Stripping. J. Appl. Electrochem. 2022, 52, 1317–1326. [Google Scholar] [CrossRef]
- López-Vizcaíno, R.; Sáez, C.; Cañizares, P.; Navarro, V.; Rodrigo, M.A. Influence of the Type of Surfactant on the Mobility of Flushing Fluids for Electro-Remediation Processes. Sep. Sci. Technol. 2011, 46, 2148–2156. [Google Scholar] [CrossRef]
- Dos Santos, E.V.; Medeiros, M.O.; Dos Anjos, A.D.; Martínez-Huitle, C.A.; Da Silva, D.R. Application of Electrochemical Technologies to Treat Polluted Soil by Diesel. Chem. Eng. Trans. 2014, 41, 157–162. [Google Scholar] [CrossRef]
- Reddy, K.R.; Urbanek, A.; Khodadoust, A.P. Electroosmotic Dewatering of Dredged Sediments: Bench-Scale Investigation. J. Environ. Manag. 2006, 78, 200–208. [Google Scholar] [CrossRef]
- López Vizcaíno, R.; Yustres, A.; Asensio, L.; Saez, C.; Cañizares, P.; Rodrigo, M.A.; Navarro, V. Enhanced Electrokinetic Remediation of Polluted Soils by Anolyte PH Conditioning. Chemosphere 2018, 199, 477–485. [Google Scholar] [CrossRef]
- López-Vizcaíno, R.; dos Santos, E.V.; Yustres, A.; Rodrigo, M.A.; Navarro, V.; Martínez-Huitle, C.A. Calcite Buffer Effects in Electrokinetic Remediation of Clopyralid-Polluted Soils. Sep. Purif. Technol. 2019, 212, 376–387. [Google Scholar] [CrossRef]
- Ren, D.; Li, S.; Wu, J.; Fu, L.; Zhang, X.; Zhang, S. Remediation of Phenanthrene-Contaminated Soil by Electrokinetics Coupled with Iron/Carbon Permeable Reactive Barrier. Environ. Eng. Sci. 2019, 36, 1224–1235. [Google Scholar] [CrossRef]
- López-Vizcaíno, R.; Alonso, J.; Cañizares, P.; León, M.J.; Navarro, V.; Rodrigo, M.A.; Sáez, C. Removal of Phenanthrene from Synthetic Kaolin Soils by Eletrokinetic Soil Flushing. Sep. Purif. Technol. 2014, 132, 33–40. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, K.; Zhang, Y.; Zou, H. Remediation of Persistent Organic Pollutant-Contaminated Soil Using Biosurfactant-Enhanced Electrokinetics Coupled with a Zero-Valent Iron/Activated Carbon Permeable Reactive Barrier. Environ. Sci. Pollut. Res. 2017, 24, 28142–28151. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ai, Z.; Zhang, L. Design of a Neutral Electro-Fenton System with Fe@Fe2O3/ACF Composite Cathode for Wastewater Treatment. J. Hazard Mater. 2009, 164, 18–25. [Google Scholar] [CrossRef] [PubMed]
- López-Vizcaíno, R.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. The Use of a Combined Process of Surfactant-Aided Soil Washing and Coagulation for PAH-Contaminated Soils Treatment. Sep. Purif. Technol. 2012, 88, 46–51. [Google Scholar] [CrossRef]
- Choe, J.-H. Polynuclear Aromatic Hydrocarbons. Clim. Chang. 1986, 9, 123–128. [Google Scholar]
- Agency Environmental Protection, SW-846 Test Method 3546: Microwave Extraction, Hazard. Waste Test Methods/SW-846. 2007, 1–13. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-3546-microwave-extraction (accessed on 17 September 2022).




| Chemical Composition | |
|---|---|
| SiO2 | 63.06 |
| Al2O3 | 35.60 |
| Fe2O3 | 0.76 |
| K2O | 0.56 |
| MgO | 0.02 |
| Mineralogical composition | |
| Kaolinite (%) | 95 |
| Quartz | 5 |
| pH | 4.8 |
| Conductivity (µS cm−1) | 470 |
| PHE/mg kg−1 | 50 |
| Trial | Supporting Electrolyte/Reservoir | Electric Field (V cm−1) | Time (Days) | ||
|---|---|---|---|---|---|
| # | Anodic | Cathodic | |||
| 1 | Without EK and PRB | - | - | - | 7 |
| 2 | EK without PRB | Tap water | Tap water | 1 | 7 |
| 3 | EK with GC-PRB | Tap water | Tap water | 1 | 7 |
| 4 | EK/Fe@Fe2O3/GC | Tap water | Tap water | 1 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvão, Á.G.P.; Costa, L.G.A.; Costa, E.C.T.d.A.; da Silva, D.R.; Martínez-Huitle, C.A.; Vieira dos Santos, E. Application of a Zero-Valent Iron/Cork as Permeable Reactive Barrier for In Situ Remediation of Phenanthrene in Soil. Catalysts 2022, 12, 1591. https://doi.org/10.3390/catal12121591
Galvão ÁGP, Costa LGA, Costa ECTdA, da Silva DR, Martínez-Huitle CA, Vieira dos Santos E. Application of a Zero-Valent Iron/Cork as Permeable Reactive Barrier for In Situ Remediation of Phenanthrene in Soil. Catalysts. 2022; 12(12):1591. https://doi.org/10.3390/catal12121591
Chicago/Turabian StyleGalvão, Álvaro G. P., Letícia G. A. Costa, Emily C. T. de A. Costa, Djalma R. da Silva, Carlos A. Martínez-Huitle, and Elisama Vieira dos Santos. 2022. "Application of a Zero-Valent Iron/Cork as Permeable Reactive Barrier for In Situ Remediation of Phenanthrene in Soil" Catalysts 12, no. 12: 1591. https://doi.org/10.3390/catal12121591
APA StyleGalvão, Á. G. P., Costa, L. G. A., Costa, E. C. T. d. A., da Silva, D. R., Martínez-Huitle, C. A., & Vieira dos Santos, E. (2022). Application of a Zero-Valent Iron/Cork as Permeable Reactive Barrier for In Situ Remediation of Phenanthrene in Soil. Catalysts, 12(12), 1591. https://doi.org/10.3390/catal12121591











