Pd/Cu-Catalyzed Cross-Coupling of Bis(2-bromovinyl) Selenides with Terminal Acetylenes: Unusual Involvement of Selanyl Function in the Sonogashira Reaction
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Starting Selenide 1
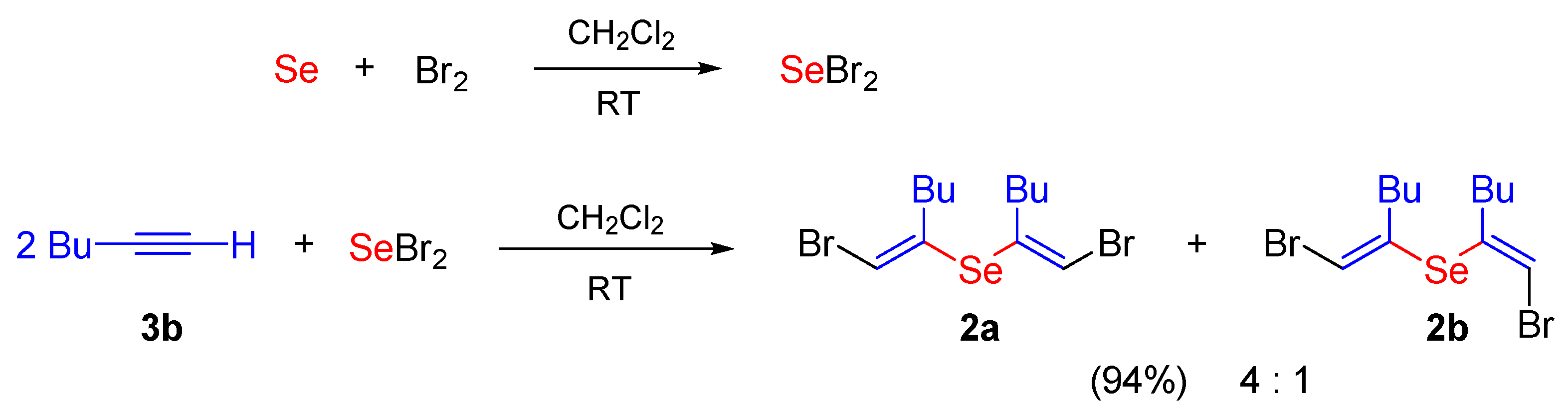
3.3. Synthesis of Selenides 2a,b
3.4. Cross-Coupling of (E,E)-bis(2-bromovinyl) selenide (1) with Terminal Acetylenes 3
3.5. Cross-Coupling of (E,E)-Bis(1-bromo-1-hexen-2-yl) selenide (2a) with Terminal Acetylenes 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Alami, M.; Crousse, B.; Ferri, F. Weakly ligated palladium complexes PdCl2(RCN)2 in piperidine: Versatile catalysts for Sonogashira reaction of vinyl chlorides at room temperature. J. Organometal. Chem. 2001, 624, 114–123. [Google Scholar] [CrossRef]
- Dai, W.-M.; Wu, J. Stereoselective synthesis of (Z)-ketoeneynes via Pd(0)-Cu(I)-catalyzed cross-coupling of (Z)-ketoenol triflate with 1-alkynes. Tetrahedron 1997, 53, 9107–9114. [Google Scholar] [CrossRef]
- Sonogashira, K. Cross-coupling reactions to sp carbon atoms. In Metal-Catalyzed Cross-coupling Reactions; Diederich, F., Stang, P., Eds.; Wiley-VCH: Weinheim, Germany, 1998; pp. 203–229. [Google Scholar] [CrossRef]
- Negishi, E.-I.; Anastasia, L. Palladium-catalyzed alkynylation. Chem. Rev. 2003, 103, 1979–2017. [Google Scholar] [CrossRef]
- Wei, Z.; Xie, Z.; Gao, L.; Wang, Y.; Sun, H.; Jian, Y.; Zhang, G.; Xu, L.; Yang, J.; Zhang, W.; et al. Highly crystallized Pd/Cu nanoparticles on activated carbon: An efficient heterogeneous catalyst for Sonogashira cross-coupling reaction. Catalysts 2020, 10, 192. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, J.; Xie, Z.; Zhang, K.; Wu, Y.; Zuo, P.; Zhang, W.; Li, J.; Gao, Z. Zeolite-enhanced sustainable Pd-catalyzed C–C cross-coupling reaction: Controlled release and capture of palladium. ACS Appl. Mater. Inter. 2020, 12, 11419–11427. [Google Scholar] [CrossRef]
- Fujiwara, S.-I.; Toyofuku, M.; Kuniyasu, H.; Kambe, N. Transition-metal-catalyzed cleavage of carbon–selenium bond and addition to alkynes and allenes. Pure Appl. Chem. 2010, 82, 565–575. [Google Scholar] [CrossRef]
- Zeni, G.; Braga, A.L.; Stefani, H.A. Palladium-catalyzed coupling of sp2-hybridized tellurides. Acc. Chem. Res. 2003, 36, 731–738. [Google Scholar] [CrossRef]
- Zeni, G.; Comasseto, J.V. Coupling of Z-vinylic tellurides with alkynes catalysed by PdCl2CuI: Synthesis of Z-enynes and Z-enediynes. Tetrahedron Lett. 1999, 40, 4619–4622. [Google Scholar] [CrossRef]
- Zeni, G.; Menezes, P.H.; Moro, A.V.; Braga, A.L.; Silveira, C.C.; Stefani, H.A. Stereoselective synthesis of (Z)-enynes via Pd(II)/CuI(I)-catalyzed cross-coupling reaction of bis-vinylic tellurides with 1-alkynes. Synlett. 2001, 2001, 1473–1475. [Google Scholar] [CrossRef]
- Braga, A.L.; Zeni, G.; Andrade, L.H.; Silveira, C.C.; Stefani, H.A. Stereospecific formation of chalcogenoenynes via palladium catalysed cross-coupling reaction of α-bromovinylic chalcogenides. Synthesis 1998, 1998, 39–41. [Google Scholar] [CrossRef]
- Fang, X.; Jiang, M.; Hu, R.; Cai, M. Facile Stereoselective Synthesis of (E)-1-arylseleno-substituted 1,3-enynes and their applications in synthesis of (E)-enediynes. Synth. Commun. 2008, 38, 4170–4181. [Google Scholar] [CrossRef]
- Silveira, C.C.; Braga, A.L.; Vieira, A.S.; Zeni, G. Stereoselective synthesis of enynes by nickel-catalyzed cross-coupling of divinylic chalcogenides with alkynes. J. Org. Chem. 2003, 68, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.L.; Bilheri, F.N.; Zeni, G. Application of organoselenides in the Suzuki, Negishi, Sonogashira and Kumada cross-coupling reactions. Chem. Commun. 2015, 51, 15522–15525. [Google Scholar] [CrossRef] [PubMed]
- Musalov, M.V.; Potapov, V.A.; Musalova, M.V.; Amosova, S.V. Stereoselective synthesis of (E,E)-bis(2-halovinyl) selenides and its derivatives based on selenium halides and acetylene. Tetrahedron 2012, 68, 10567–10572. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Kurkutov, E.O.; Yakimov, V.A.; Khabibulina, A.G.; Musalova, M.V.; Amosova, S.V.; Borodina, T.N.; Albanov, A.I. Remarkable alkene-to-alkene and alkene-to-alkyne transfer reactions of selenium dibromide and PhSeBr. Stereoselective addition of selenium dihalides to cycloalkenes. Molecules 2020, 25, 194. [Google Scholar] [CrossRef]
- Potapov, V.A.; Kurkutov, E.O.; Musalov, M.V.; Amosova, S.V. Synthesis of bis(2-haloethyl) selenides by reaction of selenium dihalides with ethylene. Russ. J. Org. Chem. 2014, 50, 291–292. [Google Scholar] [CrossRef]
- Martynov, A.V.; Larina, L.I.; Amosova, S.V. Novel bis[(E)-1-(halomethyl)-2-chlorovinyl] chalcogenides as starting materials for the efficient synthesis of bis(chloromethylidene)-1,4-dichalcogenanes. Tetrahedron Lett. 2012, 53, 1218–1221. [Google Scholar] [CrossRef]
- Johanssen, I.; Eggert, H. Selenium-77 NMR. Observation of 3JSe-Se couplings allowing cis/trans isomer assignments in substituted tetraselenafulvalenes. J. Am. Chem. Soc. 1984, 106, 1240–1243. [Google Scholar] [CrossRef]
- Potapov, V.A.; Khuriganova, O.I.; Musalov, M.V.; Larina, L.I.; Amosova, S.V. Stereospecific synthesis of E,E-bis(2-chlorovinyl)selenide. Russ. J. Gen. Chem. 2010, 80, 541–542. [Google Scholar] [CrossRef]
- Maloň, M.; Imakubo, T.; Koshino, H. 1H, 13C and 77Se NMR study of tetraselenafulvalene derivatives supported by novel H(Se)C and H(C)Se triple-resonance experiments at natural abundance. Magn. Reson. Chem. 2008, 46, 150–155. [Google Scholar] [CrossRef]
- Cao, X.; Yang, Y.; Wang, X. A direct route to conjugated enediynes from dipropargylic sulfones by a modified one-flask Ramberg–Bäcklund reaction. J. Chem. Soc. Perkin Trans. 2002, 1, 2485–2489. [Google Scholar] [CrossRef]
- Lindsell, W.E.; Preston, P.N.; Tomb, P.J. Synthesis and characterization of cobalt and molybdenum complexes derived from linear conjugated diynenes, triynedienes and tetraynetrienes. J. Organometal. Chem. 1992, 439, 201–212. [Google Scholar] [CrossRef]
- Kosinski, C.; Hirsch, A.; Heinemann, F.W.; Hampel, F. An iterative approach to cis-oligodiacetylenes. Eur. J. Org. Chem. 2001, 2001, 3879–3890. [Google Scholar] [CrossRef]
- Chemin, D.; Linstrumelle, G. Palladium-catalyzed reaction of (E) and (Z)-dichloroethenes with 1-alkynes. An efficient stereospecific synthesis of (E) and (Z)-enediynes. Tetrahedron 1994, 50, 5335–5344. [Google Scholar] [CrossRef]
- Vollhardt, K.P.C.; Winn, L.S. Stereospecific syntheses of cis- and trans-1,6-bistrimethylsilyl-hex-3-ene-1,5-diyne. Tetrahedron Lett. 1985, 26, 709–712. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martynov, A.V.; Makhaeva, N.A.; Musalov, M.V.; Albanov, A.I.; Amosova, S.V. Pd/Cu-Catalyzed Cross-Coupling of Bis(2-bromovinyl) Selenides with Terminal Acetylenes: Unusual Involvement of Selanyl Function in the Sonogashira Reaction. Catalysts 2022, 12, 1589. https://doi.org/10.3390/catal12121589
Martynov AV, Makhaeva NA, Musalov MV, Albanov AI, Amosova SV. Pd/Cu-Catalyzed Cross-Coupling of Bis(2-bromovinyl) Selenides with Terminal Acetylenes: Unusual Involvement of Selanyl Function in the Sonogashira Reaction. Catalysts. 2022; 12(12):1589. https://doi.org/10.3390/catal12121589
Chicago/Turabian StyleMartynov, Alexander V., Nataliya A. Makhaeva, Maxim V. Musalov, Alexander I. Albanov, and Svetlana V. Amosova. 2022. "Pd/Cu-Catalyzed Cross-Coupling of Bis(2-bromovinyl) Selenides with Terminal Acetylenes: Unusual Involvement of Selanyl Function in the Sonogashira Reaction" Catalysts 12, no. 12: 1589. https://doi.org/10.3390/catal12121589
APA StyleMartynov, A. V., Makhaeva, N. A., Musalov, M. V., Albanov, A. I., & Amosova, S. V. (2022). Pd/Cu-Catalyzed Cross-Coupling of Bis(2-bromovinyl) Selenides with Terminal Acetylenes: Unusual Involvement of Selanyl Function in the Sonogashira Reaction. Catalysts, 12(12), 1589. https://doi.org/10.3390/catal12121589






