Photocatalytic Degradation and Mineralization of Estriol (E3) Hormone Using Boron-Doped TiO2 Catalyst
Abstract
1. Introduction
2. Results and Discussion
2.1. Photocatalysts Characterization
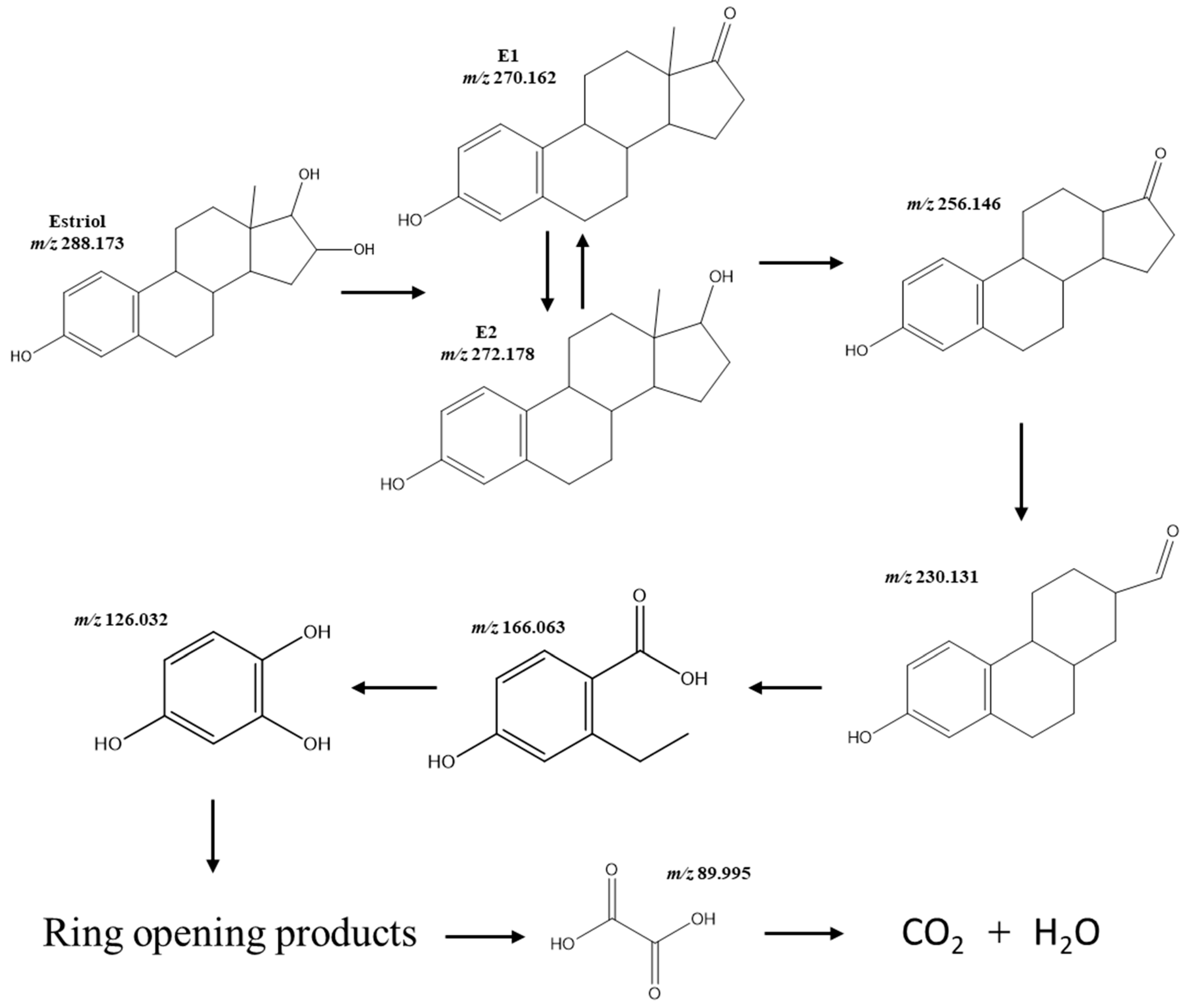
2.2. Photocatalytic Activity of the Prepared Catalysts
3. Material and Methods
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Characterization
3.4. Photocatalysts Performance
3.5. Analytical Measurements
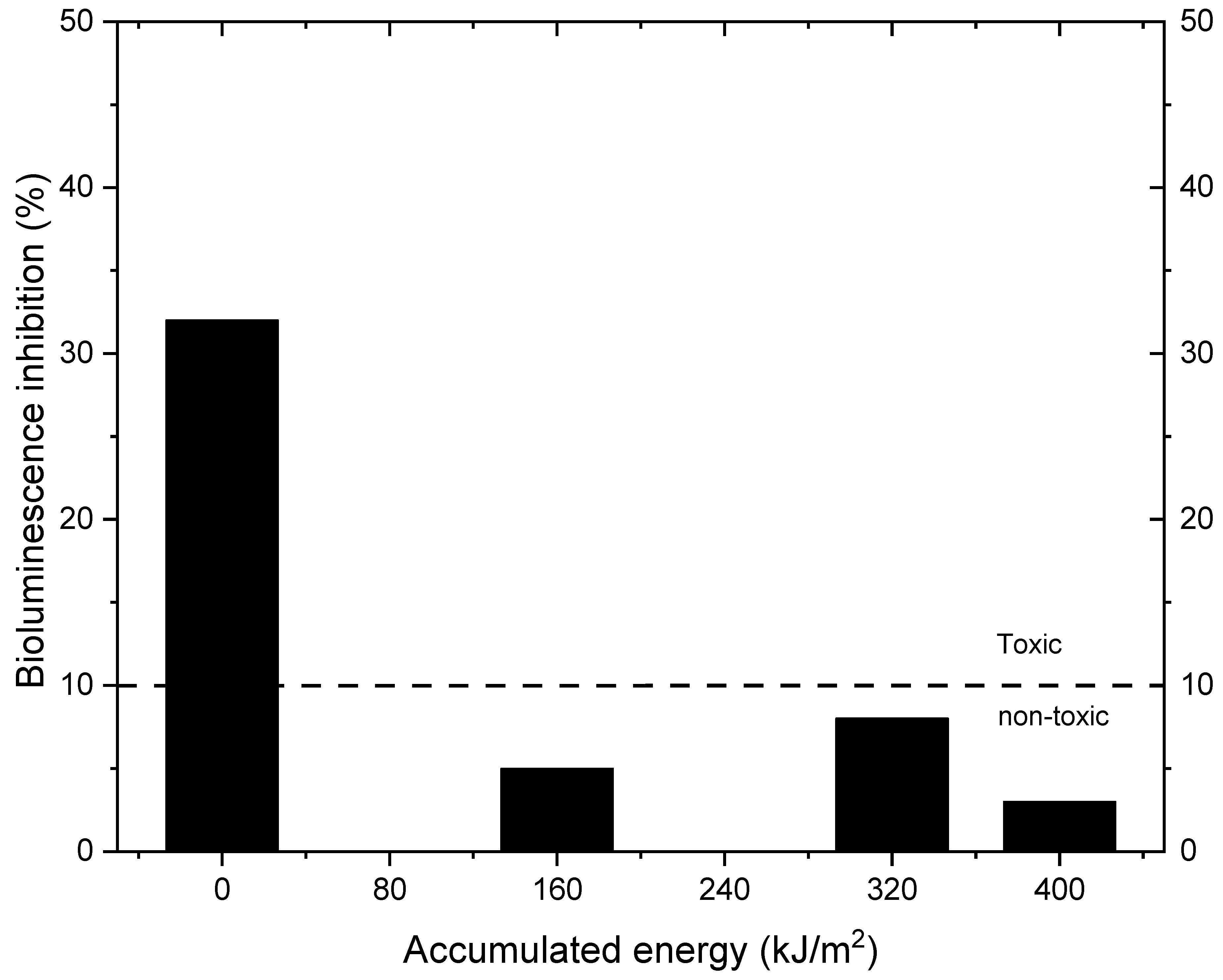
3.6. Toxicity Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salgueiro-González, N.; Turnes-Carou, I.; Besada, V.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Occurrence, distribution and bioaccumulation of endocrine disrupting compounds in water, sediment and biota samples from a European river basin. Sci. Total Environ. 2015, 529, 121–130. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, C.; Niu, L.; Cai, W. Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: A review. Crit. Rev. Environ. Sci. Technol. 2015, 46, 1–59. [Google Scholar] [CrossRef]
- López-Velázquez, K.; Guzmán-Mar, J.L.; Saldarriaga-Noreña, H.A.; Murillo-Tovar, M.A.; Hinojosa-Reyes, L.; Villanueva-Rodríguez, M. Occurrence and seasonal distribution of five selected endocrine-disrupting compounds in wastewater treatment plants of the Metropolitan Area of Monterrey, Mexico: The role of water quality parameters. Environ. Pollut. 2020, 269, 116223. [Google Scholar] [CrossRef]
- Bilal, M.; Barceló, D.; Iqbal, H.M. Occurrence, environmental fate, ecological issues, and redefining of endocrine disruptive estrogens in water resources. Sci. Total. Environ. 2021, 800, 149635. [Google Scholar] [CrossRef]
- Bistan, M.; Tišler, T.; Pintar, A. Ru/TiO2 catalyst for efficient removal of estrogens from aqueous samples by means of wet-air oxidation. Catal. Commun. 2012, 22, 74–78. [Google Scholar] [CrossRef]
- Baynes, A.; Green, C.; Nicol, E.; Beresford, N.; Kanda, R.; Henshaw, A.; Churchley, J.; Jobling, S. Additional Treatment of Wastewater Reduces Endocrine Disruption in Wild Fish—A Comparative Study of Tertiary and Advanced Treatments. Environ. Sci. Technol. 2012, 46, 5565–5573. [Google Scholar] [CrossRef][Green Version]
- Zhang, A.; Li, Y. Removal of phenolic endocrine disrupting compounds from waste activated sludge using UV, H2O2, and UV/H2O2 oxidation processes: Effects of reaction conditions and sludge matrix. Sci. Total. Environ. 2014, 493, 307–323. [Google Scholar] [CrossRef]
- Bertanza, G.; Papa, M.; Pedrazzani, R.; Repice, C.; Mazzoleni, G.; Steimberg, N.; Feretti, D.; Ceretti, E.; Zerbini, I. EDCs, estrogenicity and genotoxicity reduction in a mixed (domestic+textile) secondary effluent by means of ozonation: A full-scale experience. Sci. Total Environ. 2013, 458–460, 160–168. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Ueda, M.; Shintani, K.; Nakanishi-Anjyuin, A.; Nakazawa, M.; Kusuda, M.; Nakatani, F.; Kawaguchi, T.; Tsujiyama, S.-I.; Kawanishi, M.; Yagi, T.; et al. A protein from Pleurotus eryngii var. tuoliensis C.J. Mou with strong removal activity against the natural steroid hormone, estriol: Purification, characterization, and identification as a laccase. Enzym. Microb. Technol. 2012, 51, 402–407. [Google Scholar] [CrossRef]
- Shen, B.; Wen, X.-H.; Huang, X. Enhanced removal performance of estriol by a three-dimensional electrode reactor. Chem. Eng. J. 2017, 327, 597–607. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.; Liu, H.; Liu, Y.; Yang, Y.; Zhang, Y. Performance and Mechanism on Degradation of Estriol Using O3/PS Process. Ozone Sci. Eng. 2016, 38, 358–366. [Google Scholar] [CrossRef]
- Padovan, R.N.; de Carvalho, L.S.; Bergo, P.L.d.S.; Xavier, C.; Leitão, A.; Neto, J.D.S.; Lanças, F.M.; Azevedo, E.B. Degradation of hormones in tap water by heterogeneous solar TiO2-photocatalysis: Optimization, degradation products identification, and estrogenic activity removal. J. Environ. Chem. Eng. 2021, 9, 106442. [Google Scholar] [CrossRef]
- Gao, X.; Chen, P.; Liu, J. Enhanced visible-light absorption of nitrogen-doped titania induced by shock wave. Mater. Lett. 2011, 65, 685–687. [Google Scholar] [CrossRef]
- Japa, M.; Tantraviwat, D.; Phasayavan, W.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Simple preparation of nitrogen-doped TiO2 and its performance in selective oxidation of benzyl alcohol and benzylamine under visible light. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 610, 125743. [Google Scholar] [CrossRef]
- Ibrahim, F.A.; Al-Ghobashy, M.A.; Abo-Elmagd, I.F. Energy-efficient carbon-doped titanium dioxide nanoparticles: Synthesis, characterization, and catalytic properties under visible LED irradiation for degradation of gemifloxacin. SN Appl. Sci. 2019, 1, 631. [Google Scholar] [CrossRef]
- Helmy, E.T.; Abouellef, E.M.; Soliman, U.A.; Pan, J.H. Novel green synthesis of S-doped TiO2 nanoparticles using Malva parviflora plant extract and their photocatalytic, antimicrobial and antioxidant activities under sunlight illumination. Chemosphere 2020, 271, 129524. [Google Scholar] [CrossRef]
- Mohamed, R.; Aazam, E. Synthesis and characterization of P-doped TiO2 thin-films for photocatalytic degradation of butyl benzyl phthalate under visible-light irradiation. Chin. J. Catal. 2013, 34, 1267–1273. [Google Scholar] [CrossRef]
- Feng, N.; Zheng, A.; Wang, Q.; Ren, P.; Gao, X.; Liu, S.-B.; Shen, Z.; Chen, T.; Deng, F. Boron Environments in B-Doped and (B, N)-Codoped TiO2 Photocatalysts: A Combined Solid-State NMR and Theoretical Calculation Study. J. Phys. Chem. C 2011, 115, 2709–2719. [Google Scholar] [CrossRef]
- Barkul, R.P.; Sutar, R.S.; Patil, M.K.; Delekar, S.D. Photocatalytic Degradation of Organic Pollutants by Using Nanocrystalline Boron-doped TiO2 Catalysts. ChemistrySelect 2021, 6, 3360–3369. [Google Scholar] [CrossRef]
- Niu, P.; Wu, G.; Chen, P.; Zheng, H.; Cao, Q.; Jiang, H. Optimization of Boron Doped TiO2 as an Efficient Visible Light-Driven Photocatalyst for Organic Dye Degradation With High Reusability. Front. Chem. 2020, 8, 172. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Doping TiO2 with p-block elements: Effects on photocatalytic activity. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 13–28. [Google Scholar] [CrossRef]
- Grabowska-Musiał, E.; Zaleska-Medynska, A.; Sobczak, J.; Gazda, M.; Hupka, J. Boron-doped TiO2: Characteristics and photoactivity under visible light. Procedia Chem. 2009, 1, 1553–1559. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Liu, X.; Fan, R.; Shi, Y.; Li, S.; Zhang, L.; Fan, X.; Tang, P.; Xu, R.; et al. A direct synthesis of B-doped TiO2 and its photocatalytic performance on degradation of RhB. Appl. Surf. Sci. 2013, 265, 36–40. [Google Scholar] [CrossRef]
- Quiñones, D.; Rey, A.; Álvarez, P.; Beltrán, F.; Puma, G.L. Boron doped TiO2 catalysts for photocatalytic ozonation of aqueous mixtures of common pesticides: Diuron, o-phenylphenol, MCPA and terbuthylazine. Appl. Catal. B Environ. 2015, 178, 74–81. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Yang, H.; Xue, X.; Liu, Z. Doping TiO2 with boron or/and cerium elements: Effects on photocatalytic antimicrobial activity. Vacuum 2016, 131, 58–64. [Google Scholar] [CrossRef]
- Wang, W.-K.; Chen, J.-J.; Gao, M.; Huang, Y.-X.; Zhang, X.; Yu, H.-Q. Photocatalytic degradation of atrazine by boron-doped TiO2 with a tunable rutile/anatase ratio. Appl. Catal. B: Environ. 2016, 195, 69–76. [Google Scholar] [CrossRef]
- Simsek, E.B. Solvothermal synthesized boron doped TiO2 catalysts: Photocatalytic degradation of endocrine disrupting compounds and pharmaceuticals under visible light irradiation. Appl. Catal. B: Environ. 2017, 200, 309–322. [Google Scholar] [CrossRef]
- Bin Arifin, N.; Tarek, M.; Khan, M.R. Efficient treatment of organic pollutants by boron doped TiO2 photocatalysts under visible light radiation. Chem. Eng. Res. Des. 2022, 180, 212–219. [Google Scholar] [CrossRef]
- Cano-Casanova, L.; Ansón-Casaos, A.; Hernández-Ferrer, J.; Benito, A.M.; Maser, W.K.; Garro, N.; Lillo-Ródenas, M.A.; Román-Martínez, M.C. Surface-Enriched Boron-Doped TiO2 Nanoparticles as Photocatalysts for Propene Oxidation. ACS Appl. Nano Mater. 2022, 5, 12527–12539. [Google Scholar] [CrossRef]
- Cavalcante, R.P.; Dantas, R.F.; Bayarri, B.; González, O.; Giménez, J.; Esplugas, S.; Machulek, A. Synthesis and characterization of B-doped TiO2 and their performance for the degradation of metoprolol. Catal. Today 2015, 252, 27–34. [Google Scholar] [CrossRef]
- Khan, R.; Kim, S.W.; Kim, T.-J.; Nam, C.-M. Comparative study of the photocatalytic performance of boron–iron Co-doped and boron-doped TiO2 nanoparticles. Mater. Chem. Phys. 2008, 112, 167–172. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-H.; Song, C.-L.; Liu, Y.; Han, G.-R. Band structures of TiO2 doped with N, C and B. J. Zhejiang Univ. Sci. B 2006, 7, 299–303. [Google Scholar] [CrossRef]
- Frank, O.; Zukalova, M.; Laskova, B.; Kürti, J.; Koltai, J.; Kavan, L. Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Phys. Chem. Chem. Phys. 2012, 14, 14567–14572. [Google Scholar] [CrossRef]
- Yan, J.; Wu, G.; Guan, N.; Li, L.; Li, Z.; Cao, X. Understanding the effect of surface/bulk defects on the photocatalytic activity of TiO2: Anatase versus rutile. Phys. Chem. Chem. Phys. 2013, 15, 10978–10988. [Google Scholar] [CrossRef]
- Chen, D.; Yang, D.; Wang, A.Q.; Jiang, Z. Effects of Boron Doping on Photocatalytic Activity and Microstructure of Titanium Dioxide Nanoparticles. Ind. Eng. Chem. Res. 2006, 45, 4110–4116. [Google Scholar] [CrossRef]
- Deng, L.; Chen, Y.; Yao, M.; Wang, S.; Zhu, B.; Huang, W.; Zhang, S. Synthesis, characterization of B-doped TiO2 nanotubes with high photocatalytic activity. J. Sol-Gel Sci. Technol. 2009, 53, 535–541. [Google Scholar] [CrossRef]
- Quesada-González, M.; Boscher, N.D.; Carmalt, C.J.; Parkin, I.P. Interstitial Boron-Doped TiO2 Thin Films: The Significant Effect of Boron on TiO2 Coatings Grown by Atmospheric Pressure Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2016, 8, 25024–25029. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, I.M.; Bandala, E.R. Photocatalytic Degradation of Estriol Using Iron-Doped TiO2 under High and Low UV Irradiation. Catalysts 2018, 8, 625. [Google Scholar] [CrossRef]
- Perondi, T.; Michelon, W.; Basso, A.; Bohrer, J.K.; Viancelli, A.; Fonseca, T.G.; Treichel, H.; Moreira, R.F.P.M.; Peralta, R.A.; Düsman, E.; et al. Degradation of estriol (E3) and transformation pathways after applying photochemical removal processes in natural surface water. Water Sci. Technol. 2020, 82, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Pino-Sandoval, D.A.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Murillo-Sierra, J.C.; Hernández-Ramírez, A. Solar Photocatalysis for Degradation of Pharmaceuticals in Hospital Wastewater: Influence of the Type of Catalyst, Aqueous Matrix, and Toxicity Evaluation. Water Air Soil Pollut. 2021, 233, 14. [Google Scholar] [CrossRef]
- Kowalska, K.; Maniakova, G.; Carotenuto, M.; Sacco, O.; Vaiano, V.; Lofrano, G.; Rizzo, L. Removal of carbamazepine, diclofenac and trimethoprim by solar driven advanced oxidation processes in a compound triangular collector based reactor: A comparison between homogeneous and heterogeneous processes. Chemosphere 2019, 238, 124665. [Google Scholar] [CrossRef]
- Hao, Z.; Huang, Y.; Wang, Y.; Meng, X.; Wang, X.; Liu, X. Enhanced degradation and mineralization of estriol over ZrO2/OMS-2 nanocomposite: Kinetics, pathway and mechanism. Chemosphere 2022, 308, 136521. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, K.; Zuo, Y. Direct and indirect photodegradation of estriol in the presence of humic acid, nitrate and iron complexes in water solutions. Sci. Total Environ. 2013, 463–464, 802–809. [Google Scholar] [CrossRef] [PubMed]
- AlSalka, Y.; Hakki, A.; Fleisch, M.; Bahnemann, D.W. Understanding the degradation pathways of oxalic acid in different photocatalytic systems: Towards simultaneous photocatalytic hydrogen evolution. J. Photochem. Photobiol. A Chem. 2018, 366, 81–90. [Google Scholar] [CrossRef]
- Spina, M.; Venâncio, W.; Rodrigues-Silva, C.; Pivetta, R.C.; Diniz, V.; Rath, S.; Guimarães, J.R. Degradation of antidepressant pharmaceuticals by photoperoxidation in diverse water matrices: A highlight in the evaluation of acute and chronic toxicity. Environ. Sci. Pollut. Res. 2021, 28, 24034–24045. [Google Scholar] [CrossRef]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.; Faria, J.L.; Hentati, O.; Silva, A.M.; Ksibi, M. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Solar reclamation of wastewater effluent polluted with bisphenols, phthalates and parabens by photocatalytic treatment with TiO2/Na2S2O8 at pilot plant scale. Chemosphere 2018, 212, 95–104. [Google Scholar] [CrossRef]
- COTEMARNAT (Government of México). Mexican Norm NMX-AA-112-SCFI-2017, Water and Sediment Analysis—Acute Toxicity Evaluation with Vibrio Fischeri—Test Method; COTEMARNAT (Government of México): Mexico City, Mexico, 2017.
- Ramos-Delgado, N.; Hinojosa-Reyes, L.; Guzman-Mar, I.; Gracia-Pinilla, M.; Hernández-Ramírez, A. Synthesis by sol–gel of WO3/TiO2 for solar photocatalytic degradation of malathion pesticide. Catal. Today 2013, 209, 35–40. [Google Scholar] [CrossRef]
- Floquet, C.F.; Sieben, V.J.; MacKay, B.A.; Mostowfi, F. Determination of boron in produced water using the carminic acid assay. Talanta 2016, 150, 240–252. [Google Scholar] [CrossRef] [PubMed]
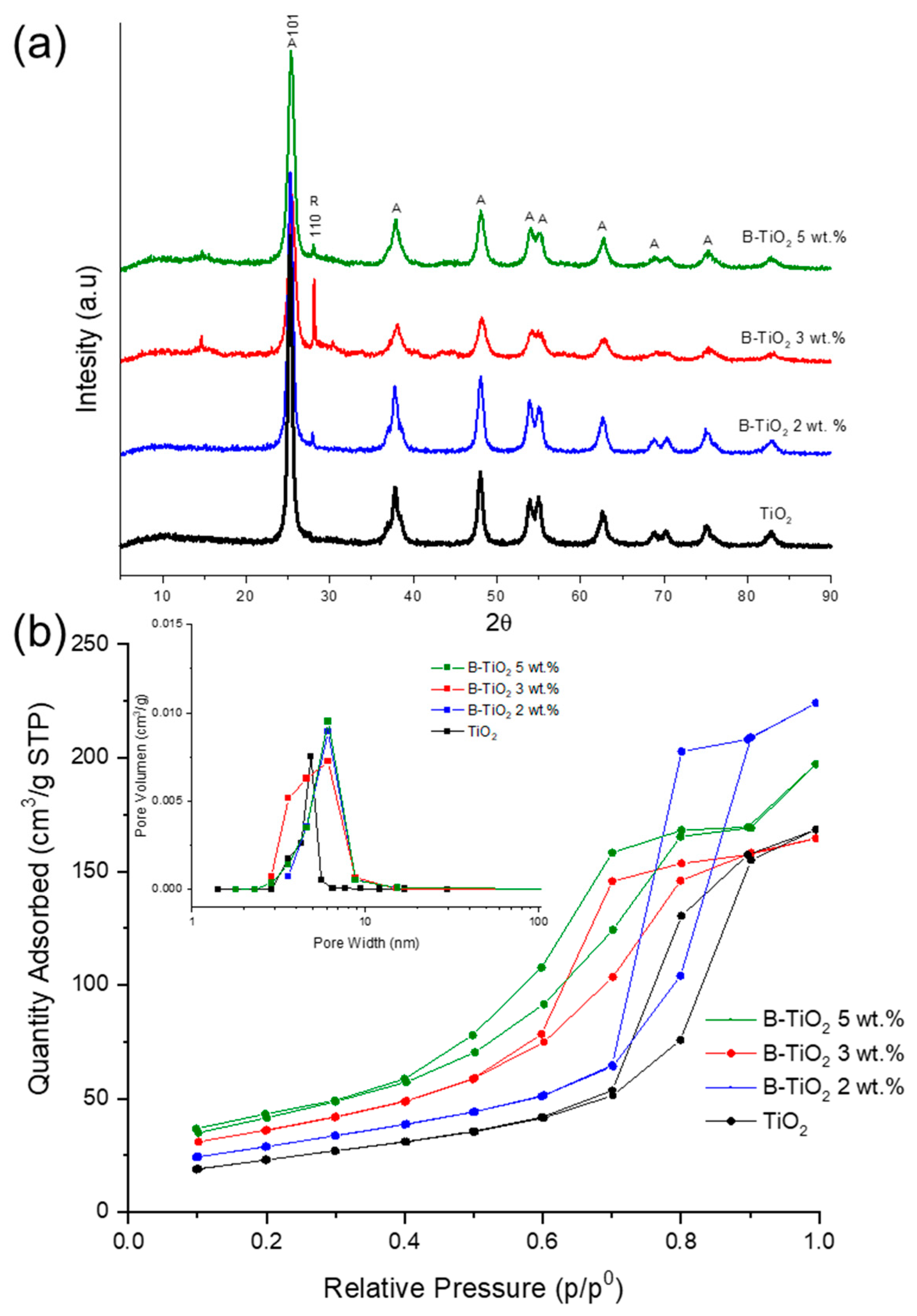
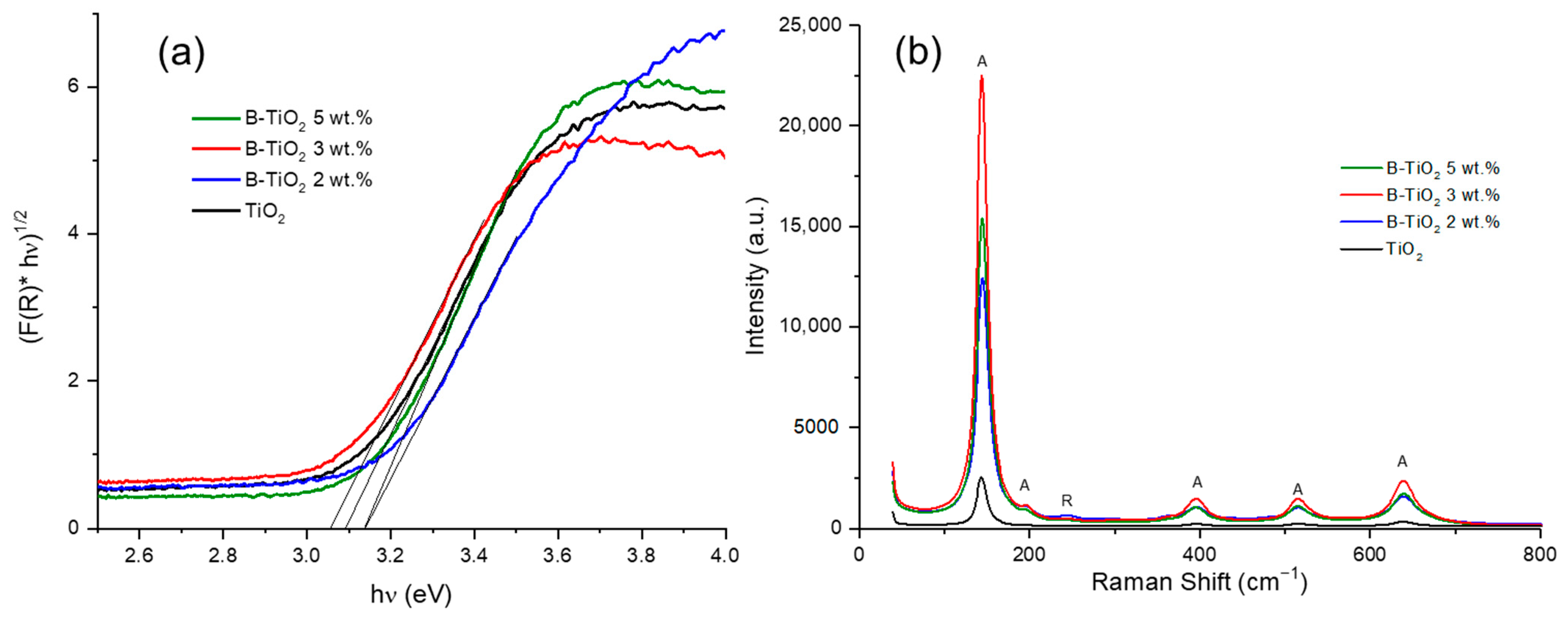

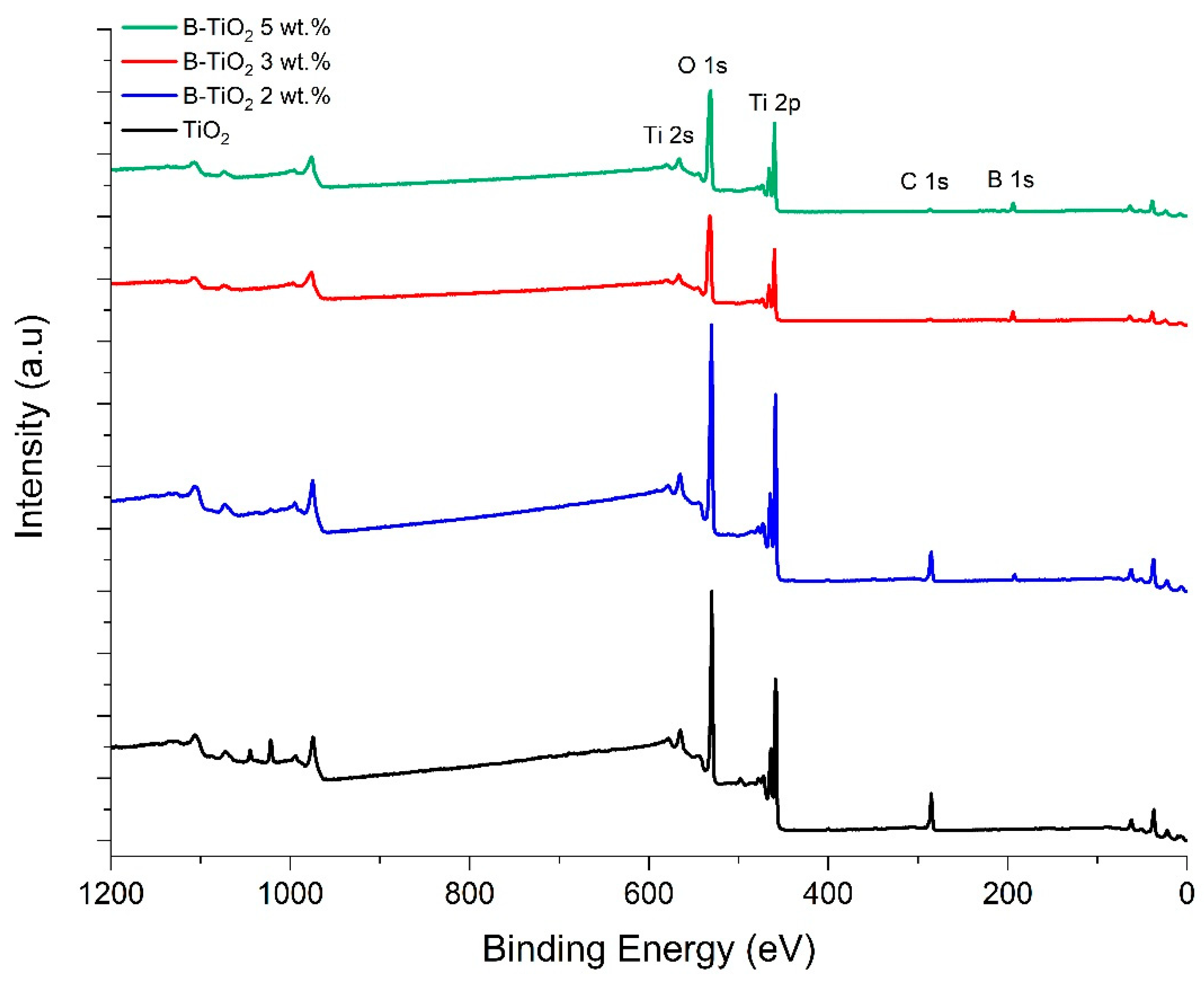
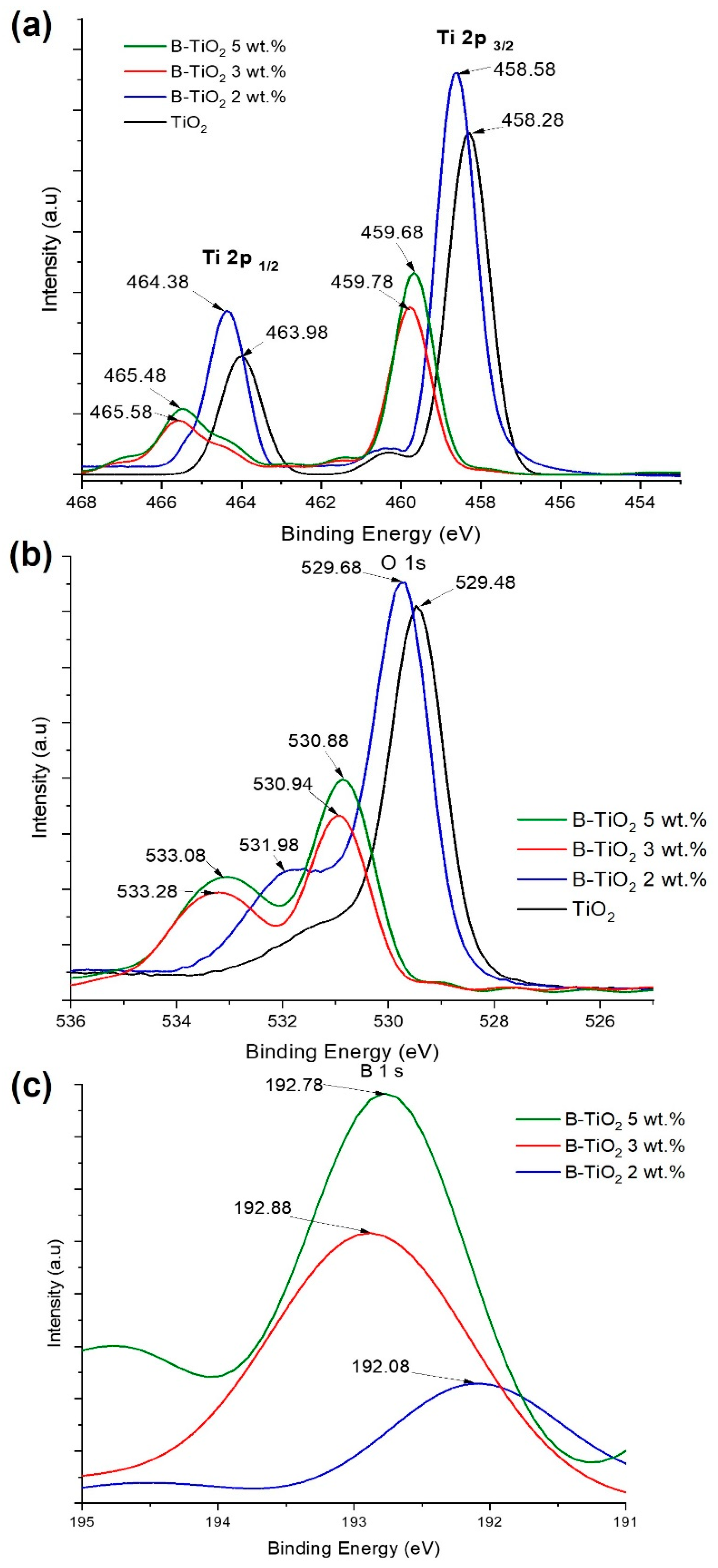

| Catalysts | a Crystallite Size (nm) | SBET (m2/g) | b Average Pore Size (nm) | Eg (eV) | c B Incorporated (wt. %) |
|---|---|---|---|---|---|
| TiO2 | 17.81 | 85 | 4.88 | 3.09 | n.d |
| B-TiO2 2 wt.% | 9.13 | 121.3 | 6.11 | 3.13 | 1.3 ± 0.05 |
| B-TiO2 3 wt.% | 7.33 | 153.2 | 6.11 | 3.06 | 2.7 ± 0.03 |
| B-TiO2 5 wt.% | 6.06 | 130.7 | 6.12 | 3.13 | 2.2 ± 0.04 |
| Material/Catalyst Loading (g/L) | Kapp × 10−3 (m2/kJ) | R2 | ||||
|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | 0.5 | 1.0 | 1.5 | |
| B-TiO2 2 wt.% | 7.17 | 6.52 | 8.17 | 0.9748 | 0.9693 | 0.9578 |
| B-TiO2 3 wt.% | 7.87 | 12.69 | 9.07 | 0.944 | 0.9132 | 0.9760 |
| B-TiO2 5 wt.% | 5.09 | 5.98 | 5.59 | 0.9397 | 0.9429 | 0.9636 |
| Material/Catalyst Loading (g/L) | % Mineralization at 400 kJ/m2 | ||
|---|---|---|---|
| 0.5 | 1.0 | 1.5 | |
| B-TiO2 2 wt.% | 58 | 68 | 69 |
| B-TiO2 3 wt.% | 71 | 61 | 61 |
| B-TiO2 5 wt.% | 47 | 67 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Quintanilla, L.Y.; Pino-Sandoval, D.; Murillo-Sierra, J.C.; Guzmán-Mar, J.L.; Ruiz-Ruiz, E.J.; Hernández-Ramírez, A. Photocatalytic Degradation and Mineralization of Estriol (E3) Hormone Using Boron-Doped TiO2 Catalyst. Catalysts 2023, 13, 43. https://doi.org/10.3390/catal13010043
Ramírez-Quintanilla LY, Pino-Sandoval D, Murillo-Sierra JC, Guzmán-Mar JL, Ruiz-Ruiz EJ, Hernández-Ramírez A. Photocatalytic Degradation and Mineralization of Estriol (E3) Hormone Using Boron-Doped TiO2 Catalyst. Catalysts. 2023; 13(1):43. https://doi.org/10.3390/catal13010043
Chicago/Turabian StyleRamírez-Quintanilla, Laura Yanneth, Diego Pino-Sandoval, Juan Camilo Murillo-Sierra, Jorge Luis Guzmán-Mar, Edgar J. Ruiz-Ruiz, and Aracely Hernández-Ramírez. 2023. "Photocatalytic Degradation and Mineralization of Estriol (E3) Hormone Using Boron-Doped TiO2 Catalyst" Catalysts 13, no. 1: 43. https://doi.org/10.3390/catal13010043
APA StyleRamírez-Quintanilla, L. Y., Pino-Sandoval, D., Murillo-Sierra, J. C., Guzmán-Mar, J. L., Ruiz-Ruiz, E. J., & Hernández-Ramírez, A. (2023). Photocatalytic Degradation and Mineralization of Estriol (E3) Hormone Using Boron-Doped TiO2 Catalyst. Catalysts, 13(1), 43. https://doi.org/10.3390/catal13010043






