Structurally Modified MXenes-Based Catalysts for Application in Hydrogen Evolution Reaction: A Review
Abstract
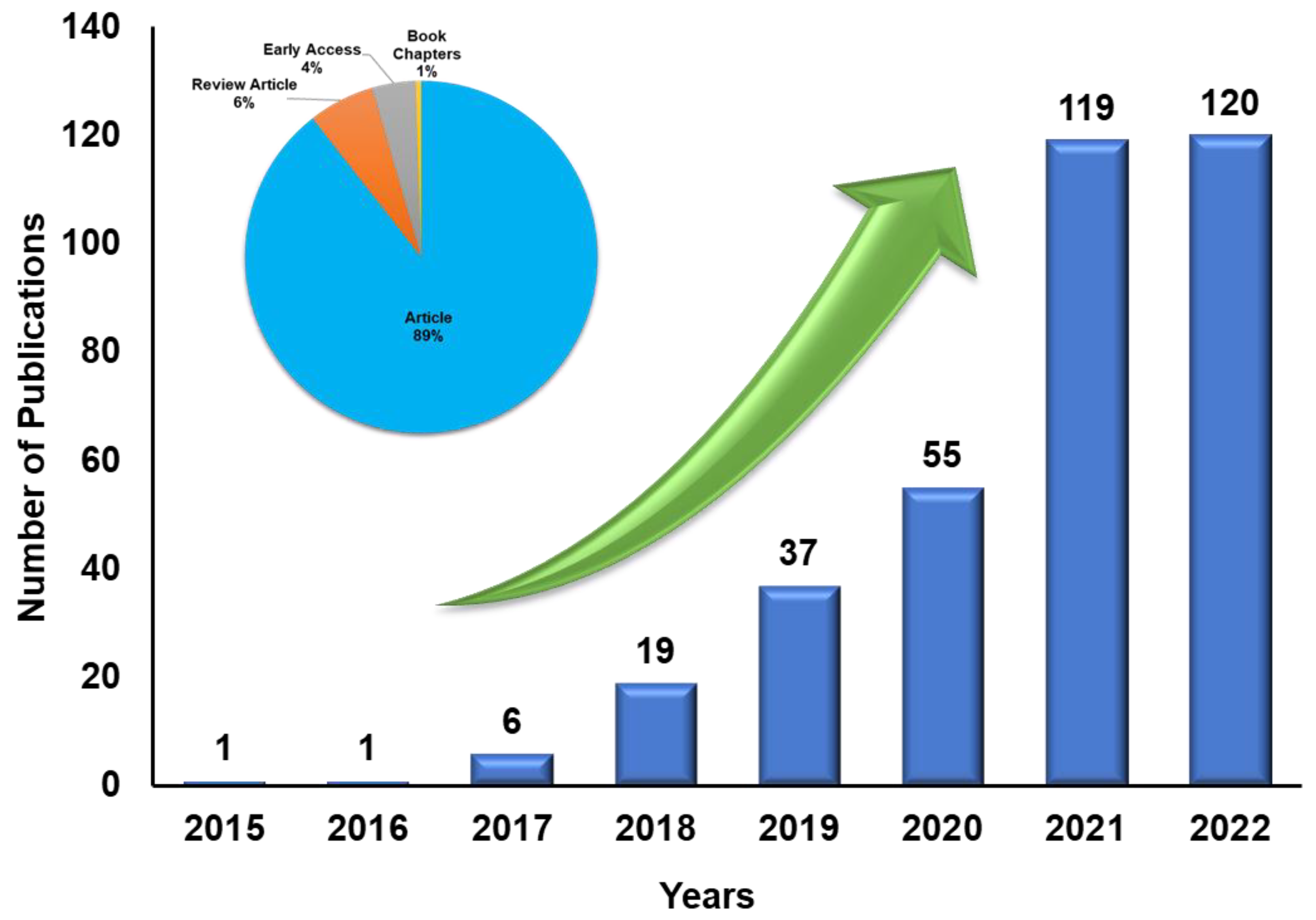
1. Introduction
2. Hydrogen Evolution Reaction
2.1. Mechanism of Electrochemical HER
2.2. Catalyst Activity toward Overpotential, Current Density and Tafel Slope
2.3. Catalyst Activity for Current–Time Curve
2.4. Efficiency toward Turnover Frequency (TOF) and Faradaic Efficiency
3. State-of-Art HER Electrocatalysts
3.1. Noble-Metal-Based Electrocatalysts
3.1.1. Pt-Based Electrocatalysts
3.1.2. Ru-Based Electrocatalysts
3.1.3. Ir-Based Electrocatalysts
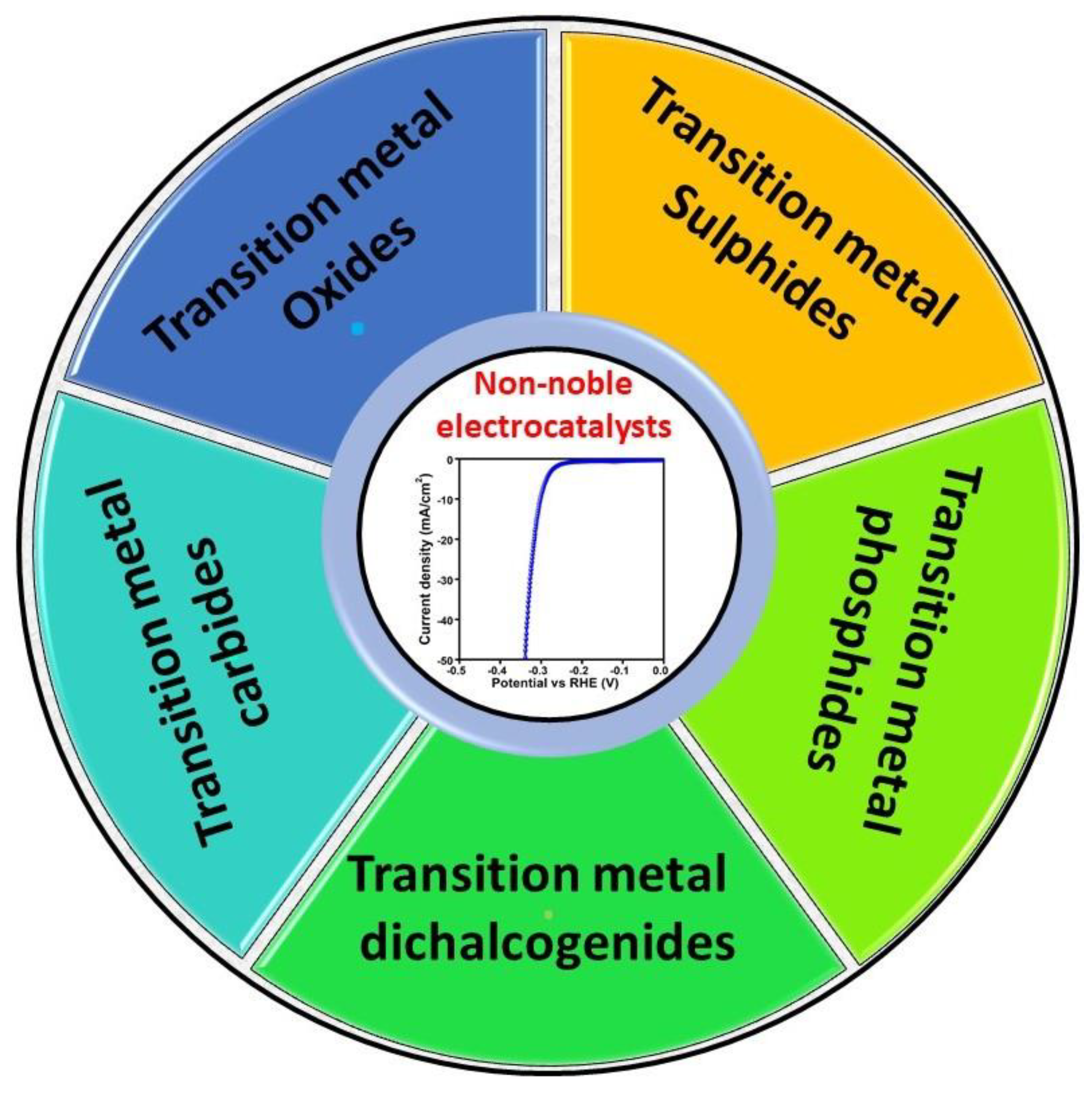
3.2. Non-Noble-Metal-Based Electrocatalysts
3.2.1. Transition-Metal Oxides
3.2.2. Transition-Metal Sulphides
3.2.3. Transition-Metal Carbides (TMCs)
3.2.4. Transition-Metal Phosphides
3.2.5. Transition-Metal Dichalcogenides
4. MXenes as Emerging Materials for HER
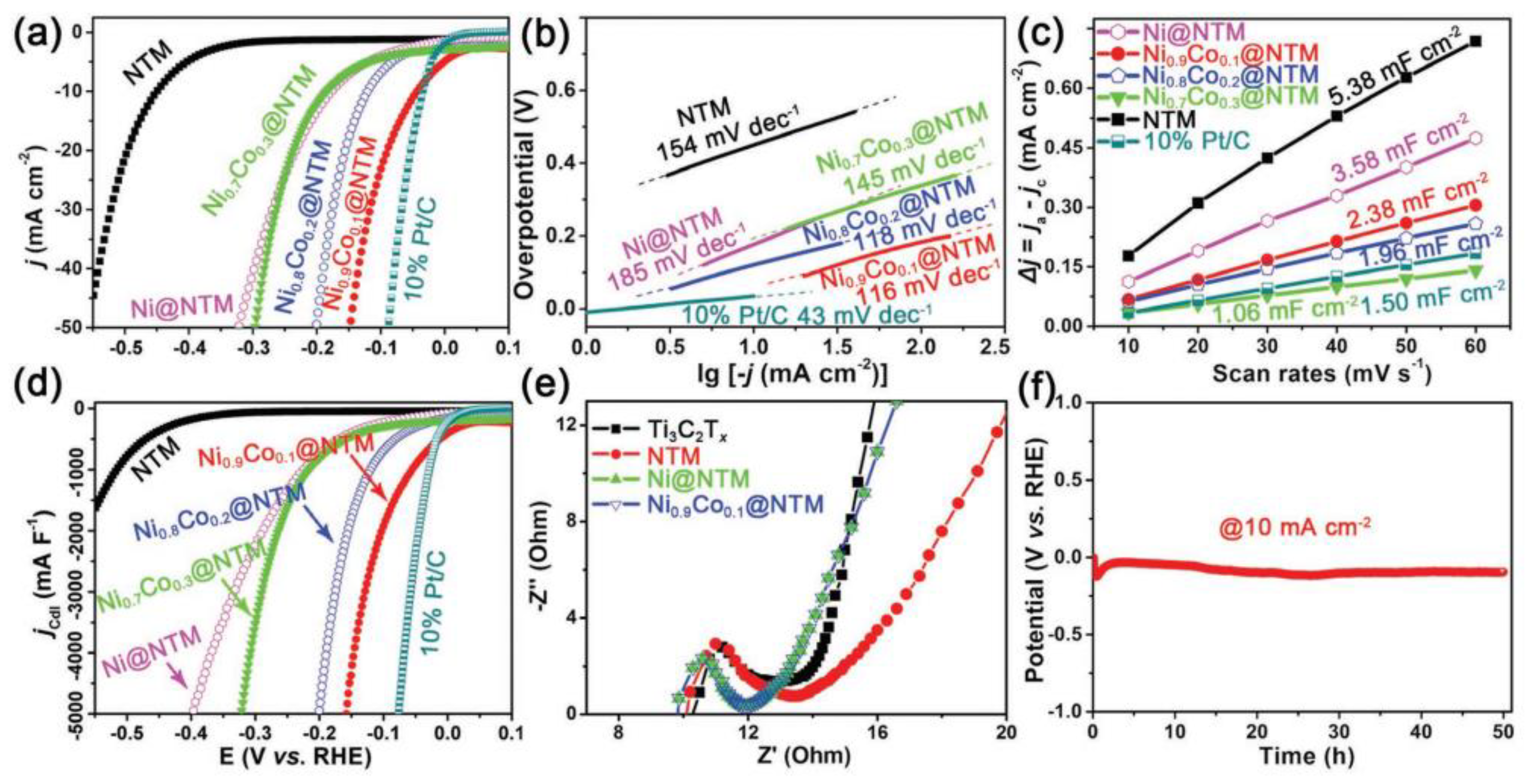
5. HER Properties of Various MXene Structures
5.1. Multilayer and Few/Single-Layer MXenes
5.2. Porous MXenes
5.3. Special Structures—Crumple and Rolled MXenes
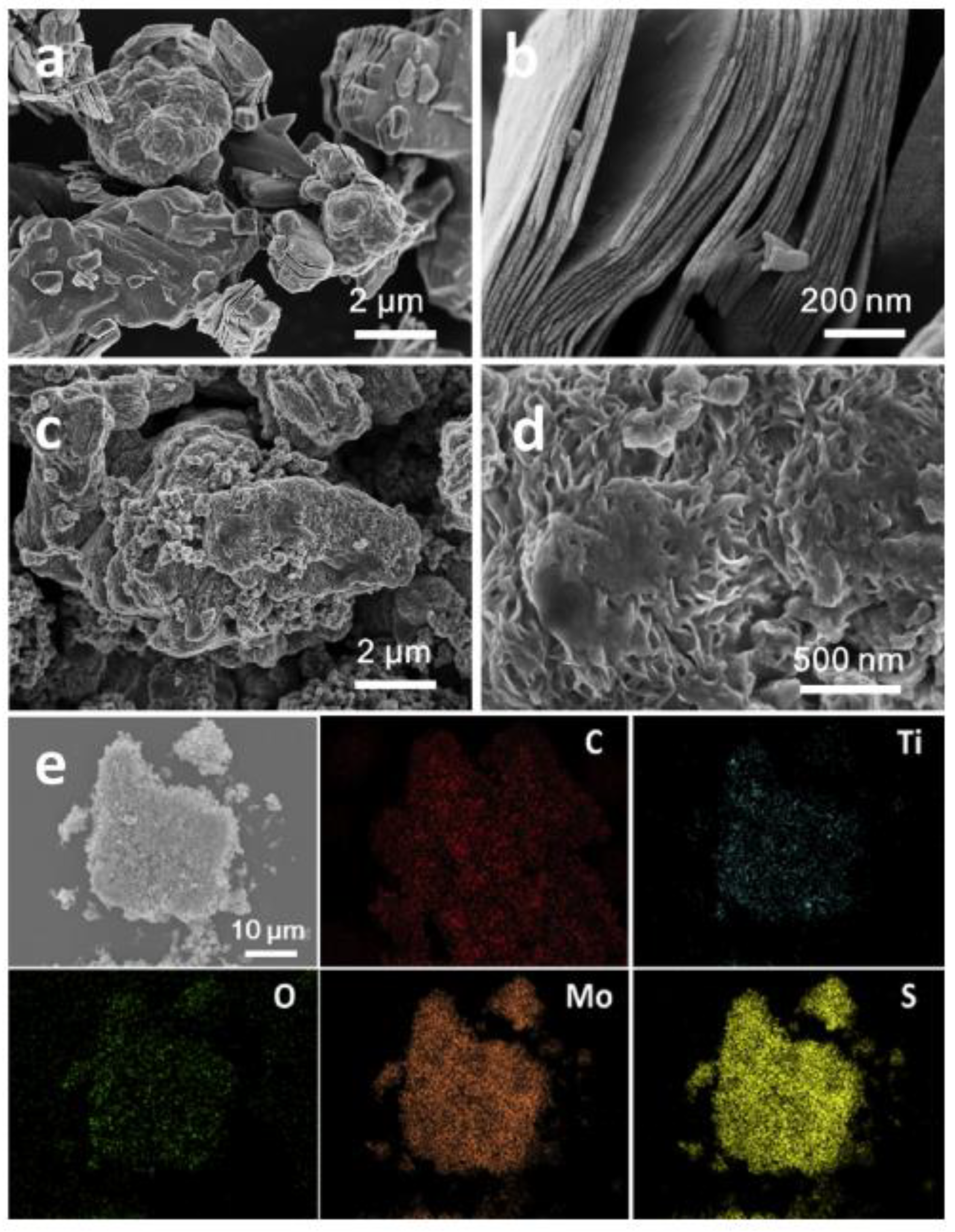
6. Synthesis of Different MXene Morphologies
6.1. Multilayer and Few-Layer MXenes
6.2. Preparation of Porous MXenes
6.2.1. Coating Porous Scaffold—Dip-Coating
6.2.2. In-Plane Porous MXenes
6.2.3. Self-Assembly of MXene Nanosheets
6.3. Crumpled and Rolled MXenes
7. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perry, M.; Fuller, T.F. A Historical Perspective of Fuel Cell Technology in the 20th Century. J. Electrochem. Soc. 2002, 149, S59. [Google Scholar] [CrossRef]
- Lubitz, W.; Tumas, W. Hydrogen: An Overview. Chem. Rev. 2007, 107, 3900–3903. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A.; Remhof, A.; Borgschulte, A.; Friedrichs, O. Hydrogen: The future energy carrier. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3329–3342. [Google Scholar] [CrossRef]
- Mosca, L.; Jimenez, J.A.M.; Wassie, S.A.; Gallucci, F.; Palo, E.; Colozzi, M.; Taraschi, S.; Galdieri, G. Process design for green hydrogen production. Int. J. Hydrog. Energy 2020, 45, 7266–7277. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weissgaerber, T.; Röntzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Martinez-Burgos, W.J.; Candeo, E.D.S.; Medeiros, A.B.P.; de Carvalho, J.C.; Tanobe, V.O.D.A.; Soccol, C.R.; Sydney, E.B. Hydrogen: Current advances and patented technologies of its renewable production. J. Clean. Prod. 2020, 286, 124970. [Google Scholar] [CrossRef]
- Xu, X.; Su, C.; Shao, Z. Fundamental Understanding and Application of Ba0.5Sr0.5Co0.8Fe0.2O3-δ Perovskite in Energy Storage and Conversion: Past, Present, and Future. Energy Fuels 2021, 35, 13585–13609. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Hughes, J.P.; Clipsham, J.; Chavushoglu, H.; Rowley-Neale, S.J.; Banks, C.E. Polymer electrolyte electrolysis: A review of the activity and stability of non-precious metal hydrogen evolution reaction and oxygen evolution reaction catalysts. Renew. Sustain. Energy Rev. 2021, 139, 110709. [Google Scholar] [CrossRef]
- Salehmin, M.N.I.; Husaini, T.; Goh, J.; Sulong, A.B. High-pressure PEM water electrolyser: A review on challenges and mitigation strategies towards green and low-cost hydrogen production. Energy Convers. Manag. 2022, 268, 115985. [Google Scholar] [CrossRef]
- Bazarah, A.; Majlan, E.H.; Husaini, T.; Zainoodin, A.; Alshami, I.; Goh, J.; Masdar, M.S. Factors influencing the performance and durability of polymer electrolyte membrane water electrolyzer: A review. Int. J. Hydrog. Energy 2022, 47, 35976–35989. [Google Scholar] [CrossRef]
- Teuku, H.; Alshami, I.; Goh, J.; Masdar, M.S.; Loh, K.S. Review on bipolar plates for low-temperature polymer electrolyte membrane water electrolyzer. Int. J. Energy Res. 2021, 45, 20583–20600. [Google Scholar] [CrossRef]
- Zhou, F.; Zhou, Y.; Liu, G.-G.; Wang, C.-T.; Wang, J. Recent advances in nanostructured electrocatalysts for hydrogen evolution reaction. Rare Met. 2021, 40, 3375–3405. [Google Scholar] [CrossRef]
- Lasia, A. Mechanism and kinetics of the hydrogen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 19484–19518. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Quaino, P.; Juarez, F.; Santos, E.; Schmickler, W. Volcano plots in hydrogen electrocatalysis—Uses and abuses. Beilstein J. Nanotechnol. 2014, 5, 846–854. [Google Scholar] [CrossRef]
- Xu, X.; Shao, Z.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 2200573. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Yang, Y.; Sirisomboonchai, S.; Wang, P.; Ma, X.; Abudula, A.; Guan, G. Earth-abundant transition-metal-based bifunctional catalysts for overall electrochemical water splitting: A review. J. Alloy. Compd. 2020, 819, 153346. [Google Scholar] [CrossRef]
- Zhang, M.; Lai, C.; Li, B.; Liu, S.; Huang, D.; Xu, F.; Liu, X.; Qin, L.; Fu, Y.; Li, L.; et al. MXenes as Superexcellent Support for Confining Single Atom: Properties, Synthesis, and Electrocatalytic Applications. Small 2021, 17, 2007113. [Google Scholar] [CrossRef]
- Zubair, M.; Hassan, M.M.U.; Mehran, M.T.; Baig, M.M.; Hussain, S.; Shahzad, F. 2D MXenes and their heterostructures for HER, OER and overall water splitting: A review. Int. J. Hydrog. Energy 2022, 47, 2794–2818. [Google Scholar] [CrossRef]
- Shi, L.-N.; Cui, L.-T.; Ji, Y.-R.; Xie, Y.; Zhu, Y.-R.; Yi, T.-F. Towards high-performance electrocatalysts: Activity optimization strategy of 2D MXenes-based nanomaterials for water-splitting. Coord. Chem. Rev. 2022, 469, 214668. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Zhou, W.; Zhu, Z.; Su, C.; Liu, M.; Shao, Z. A Perovskite Electrocatalyst for Efficient Hydrogen Evolution Reaction. Adv. Mater. 2016, 28, 6442–6448. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Nguyen, D.M.T.; Tran, D.L.; Le, H.K.; Vo, D.-V.N.; Lam, S.S.; Varma, R.S.; Shokouhimehr, M.; Nguyen, C.C.; Van Le, Q. MXenes: Applications in electrocatalytic, photocatalytic hydrogen evolution reaction and CO2 reduction. Mol. Catal. 2020, 486, 110850. [Google Scholar] [CrossRef]
- Shen, J.; Wu, Z.; Li, C.; Zhang, C.; Genest, A.; Rupprechter, G.; He, L. Emerging applications of MXene materials in CO2 photocatalysis. FlatChem 2021, 28, 100252. [Google Scholar] [CrossRef]
- Li, Z.; Attanayake, N.H.; Blackburn, J.L.; Miller, E.M. Carbon dioxide and nitrogen reduction reactions using 2D transition metal dichalcogenide (TMDC) and carbide/nitride (MXene) catalysts. Energy Environ. Sci. 2021, 14, 6242–6286. [Google Scholar] [CrossRef]
- Feng, S.; Yu, Y.; Li, J.; Luo, J.; Deng, P.; Jia, C.; Shen, Y.; Tian, X. Recent progress in seawater electrolysis for hydrogen evolution by transition metal phosphides. Catal. Commun. 2022, 162, 106382. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W.; Liu, S.; Chen, X.; Ma, T.; Wang, G.; Lu, Z.; Sun, J. Enhanced interface interaction in Cu2S@Ni core-shell nanorod arrays as hydrogen evolution reaction electrode for alkaline seawater electrolysis. J. Power Sources 2021, 506, 230235. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Boron-Doped Carbon nanoparticles supported palladium as an efficient hydrogen evolution electrode in PEM water electrolysis. Renew. Energy 2020, 146, 2281–2290. [Google Scholar] [CrossRef]
- Du, L.; Sun, Y.; You, B. Hybrid water electrolysis: Replacing oxygen evolution reaction for energy-efficient hydrogen production and beyond. Mater. Rep. Energy 2021, 1, 100004. [Google Scholar] [CrossRef]
- Peera, S.G.; Koutavarapu, R.; Chao, L.; Singh, L.; Murugadoss, G.; Rajeshkhanna, G. 2D MXene Nanomaterials as Electrocatalysts for Hydrogen Evolution Reaction (HER): A Review. Micromachines 2022, 13, 1499. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Xia, M.; Li, Z.; Chen, Z.; Ma, Z.; Qin, X.; Shao, G. Ni nanoparticles supported on graphene layers: An excellent 3D electrode for hydrogen evolution reaction in alkaline solution. J. Power Sources 2017, 347, 220–228. [Google Scholar] [CrossRef]
- Tan, Y.; Che, Q.; Li, Q. Generating highly active Ni11(HPO3)8(OH)6/Mn3O4 catalyst for electrocatalytic hydrogen evolution reaction by electrochemical activation. J. Colloid Interface Sci. 2020, 560, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Navadeepthy, D.; Rebekah, A.; Viswanthan, C.; Ponpandian, N. Boosting the kinetics of oxygen and hydrogen evolution in alkaline water splitting using nickel ferrite/N-graphene nanocomposite as a bifunctional electrocatalyst. Int. J. Hydrog. Energy 2021, 46, 21512–21524. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, M.; Long, A.; Xue, Y.; Liao, H.; Wei, C.; Xu, Z.J. Heterostructured Electrocatalysts for Hydrogen Evolution Reaction Under Alkaline Conditions. Nano-Micro Lett. 2018, 10, 75. [Google Scholar] [CrossRef]
- Jebaslinhepzybai, B.T.; Prabu, N.; Sasidharan, M. Facile galvanic replacement method for porous Pd@Pt nanoparticles as an efficient HER electrocatalyst. Int. J. Hydrog. Energy 2020, 45, 11127–11137. [Google Scholar] [CrossRef]
- Ma, Z.; Tian, H.; Meng, G.; Peng, L.; Chen, Y.; Chen, C.; Chang, Z.; Cui, X.; Wang, L.; Jiang, W.; et al. Size effects of platinum particles@CNT on HER and ORR performance. Sci. China-Mater. 2020, 63, 2517–2529. [Google Scholar] [CrossRef]
- Hanan, A.; Ahmed, M.; Lakhan, M.N.; Shar, A.H.; Cao, D.; Asif, A.; Alic, A.; Gul, M. Novel rGO@Fe3O4 nanostructures: An active electrocatalyst for hydrogen evolution reaction in alkaline media. J. Indian Chem. Soc. 2022, 99, 100442. [Google Scholar] [CrossRef]
- Tan, H.; Tang, B.; Lu, Y.; Ji, Q.; Lv, L.; Duan, H.; Na Li, N.; Wang, Y.; Feng, S.; Li, Z.; et al. Engineering a local acid-like environment in alkaline medium for efficient hydrogen evolution reaction. Nat. Commun. 2022, 13, 2024. [Google Scholar] [CrossRef]
- Wiensch, J.D.; John, J.; Velazquez, J.M.; Torelli, D.A.; Pieterick, A.P.; McDowell, M.T.; Sun, K.; Zhao, X.; Brunschwig, B.S.; Lewis, N.S. Comparative Study in Acidic and Alkaline Media of the Effects of pH and Crystallinity on the Hydrogen-Evolution Reaction on MoS2 and MoSe2. ACS Energy Lett. 2017, 2, 2234–2238. [Google Scholar] [CrossRef]
- Rajpure, M.M.; Bandal, H.A.; Jadhav, H.S.; Kim, H. Systematic development of bimetallic MOF and its phosphide derivative as an efficient multifunctional electrocatalyst for urea-assisted water splitting in alkaline medium. J. Electroanal. Chem. 2022, 923, 116825. [Google Scholar] [CrossRef]
- Mohammadi, O.; Bahari, Y.; Daryakenari, A.A.; Koldeh, F.J.; Zhang, X.; Tian, Z.Q.; Shen, P.K. NiCoP nanoarchitectures: One-step controlled electrodeposition and their application as efficient electrocatalysts for boosting hydrogen evolution reaction. Int. J. Hydrog. Energy 2022, 47, 34943–34954. [Google Scholar] [CrossRef]
- Saji, V.S. A mini-review on transition metals-based 1D nanotubular bifunctional electrocatalysts for overall water splitting. Int. J. Hydrog. Energy 2022, 47, 32372–32393. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Kemppainen, E.; Dorbandt, I.; Bors, R.; Xi, F.; Schlatmann, R.; van de Krol, R.; Calnan, S. Understanding the Hydrogen Evolution Reaction Kinetics of Electrodeposited Nickel-Molybdenum in Acidic, Near-Neutral, and Alkaline Conditions. ChemElectroChem 2021, 8, 195–208. [Google Scholar] [CrossRef]
- Hosoi, T.; Yonekura, T.; Sunada, K.; Sasaki, K. Exchange Current Density of SOFC Electrodes: Theoretical Relations and Partial Pressure Dependencies Rate-Determined by Electrochemical Reactions. J. Electrochem. Soc. 2014, 162, F136–F152. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, L.; Hensen, E.J.M.; Hofmann, J.P. Evaluating the Stability of Co2P Electrocatalysts in the Hydrogen Evolution Reaction for Both Acidic and Alkaline Electrolytes. ACS Energy Lett. 2018, 3, 1360–1365. [Google Scholar] [CrossRef]
- Inzelt, G. Chronoamperometry, Chronocoulometry, and Chronopotentiometry. In Encyclopedia of Applied Electrochemistry; Kreysa, G., Ota, K.-I., Savinell, R.F., Eds.; Springer: New York, NY, USA, 2014; pp. 207–214. [Google Scholar]
- Søndergaard-Pedersen, F.; Lakhotiya, H.; Bøjesen, E.D.; Bondesgaard, M.; Myekhlai, M.; Benedetti, T.M.; Gooding, J.J.; Tilley, R.D.; Iversen, B.B. Highly efficient and stable Ru nanoparticle electrocatalyst for the hydrogen evolution reaction in alkaline conditions. Catal. Sci. Technol. 2022, 12, 3606–3613. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J.; Sun, X. Recent progress on earth abundant electrocatalysts for hydrogen evolution reaction (HER) in alkaline medium to achieve efficient water splitting—A review. J. Energy Chem. 2019, 34, 111–160. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.-J. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg 2021, 8, 4. [Google Scholar] [CrossRef]
- Anantharaj, S.; Karthik, P.E.; Noda, S. The Significance of Properly Reporting Turnover Frequency in Electrocatalysis Research. Angew. Chem. Int. Ed. 2021, 60, 23051–23067. [Google Scholar] [CrossRef]
- Shin, S.; Jin, Z.; Kwon, D.H.; Bose, R.; Min, Y.-S. High Turnover Frequency of Hydrogen Evolution Reaction on Amorphous MoS2 Thin Film Directly Grown by Atomic Layer Deposition. Langmuir 2015, 31, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, Y.; Wang, Y.; Ge, J.; Liu, C.; Xing, W. Enhanced electrocatalytic performance for the hydrogen evolution reaction through surface enrichment of platinum nanoclusters alloying with ruthenium in situ embedded in carbon. Energy Environ. Sci. 2018, 11, 1232–1239. [Google Scholar] [CrossRef]
- Kweon, D.H.; Okyay, M.S.; Kim, S.-J.; Jeon, J.-P.; Noh, H.-J.; Park, N.; Mahmood, J.; Baek, J.-B. Ruthenium anchored on carbon nanotube electrocatalyst for hydrogen production with enhanced Faradaic efficiency. Nat. Commun. 2020, 11, 1278. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Liu, Q.; Asiri, A.M.; Sun, X. High-Efficiency Electrochemical Hydrogen Evolution Catalyzed by Tungsten Phosphide Submicroparticles. ACS Catal. 2015, 5, 145–149. [Google Scholar] [CrossRef]
- Zhou, B.; Ou, P.; Rashid, R.T.; Vanka, S.; Sun, K.; Yao, L.; Sun, H.; Song, J.; Mi, Z. Few-Atomic-Layers Iron for Hydrogen Evolution from Water by Photoelectrocatalysis. iScience 2020, 23, 101613. [Google Scholar] [CrossRef]
- Wang, M.; Liu, S.; Qian, T.; Liu, J.; Zhou, J.; Ji, H.; Xiong, J.; Zhong, J.; Yan, C. Over 56.55% Faradaic efficiency of ambient ammonia synthesis enabled by positively shifting the reaction potential. Nat. Commun. 2019, 10, 341. [Google Scholar] [CrossRef]
- Hanus, F.J.; Carter, K.R.; Evans, H.J. [67] Techniques for Measurement of Hydrogen Evolution by Nodules. In Methods in Enzymology; San Pietro, A., Ed.; Academic Press: New York, NY, USA, 1980; pp. 731–738. [Google Scholar]
- Lotfi, N.; Farahani, T.S.; Yaghoubinezhad, Y.; Darband, G.B. Evaluation of the electrocatalytic activity and stability of graphene oxide nanosheets coated by Co/Ni elements toward hydrogen evolution reaction. Mater. Res. Express 2019, 6, 085524. [Google Scholar] [CrossRef]
- Zhai, W.; Ma, Y.; Chen, D.; Ho, J.C.; Dai, Z.; Qu, Y. Recent progress on the long-term stability of hydrogen evolution reaction electrocatalysts. InfoMat 2022, 4, e12357. [Google Scholar] [CrossRef]
- Xiong, J.; Yan, C.; Liu, W.; Guo, X.; Ma, J.; Yi, W.; Han, M. Insights into the principles, design methodology and applications of electrocatalysts towards hydrogen evolution reaction. Energy Rep. 2021, 7, 8577–8596. [Google Scholar] [CrossRef]
- Martinez, U.; Babu, S.K.; Holby, E.F.; Chung, H.T.; Yin, X.; Zelenay, P. Progress in the Development of Fe-Based PGM-Free Electrocatalysts for the Oxygen Reduction Reaction. Adv. Mater. 2019, 31, 1806545. [Google Scholar] [CrossRef] [PubMed]
- Martinez, U.; Holby, E.F.; Babu, S.K.; Artyushkova, K.; Lin, L.; Choudhury, S.; Purdy, G.M.; Zelenay, P. Experimental and Theoretical Trends of PGM-Free Electrocatalysts for the Oxygen Reduction Reaction with Different Transition Metals. J. Electrochem. Soc. 2019, 166, F3136–F3142. [Google Scholar] [CrossRef]
- Abbas, S.A.; Kim, S.-H.; Iqbal, M.I.; Muhammad, S.; Yoon, W.-S.; Jung, K.-D. Synergistic effect of nano-Pt and Ni spine for HER in alkaline solution: Hydrogen spillover from nano-Pt to Ni spine. Sci. Rep. 2018, 8, 2986. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Liu, Q.; Shi, X.; Asiri, A.M.; Luo, Y.; Sun, X. Superior alkaline hydrogen evolution electrocatalysis enabled by an ultrafine PtNi nanoparticle-decorated Ni nanoarray with ultralow Pt loading. Inorg. Chem. Front. 2018, 5, 1365–1369. [Google Scholar] [CrossRef]
- Lu, Q.; Hutchings, G.S.; Yu, W.; Zhou, Y.; Forest, R.V.; Tao, R.; Rosen, J.; Yonemoto, B.T.; Cao, Z.; Zheng, H.; et al. Highly porous non-precious bimetallic electrocatalysts for efficient hydrogen evolution. Nat. Commun. 2015, 6, 6567. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Haid, R.W.; Kluge, R.M.; Ding, X.; Garlyyev, B.; Fichtner, J.; Watzele, S.; Hou, S.; Bandarenka, A.S. Enhancing the Hydrogen Evolution Reaction Activity of Platinum Electrodes in Alkaline Media Using Nickel-Iron Clusters. Angew. Chem. Int. Ed. Engl. 2020, 59, 10934–10938. [Google Scholar] [CrossRef]
- FitzGerald, S.A.; Burkholder, B.; Friedman, M.; Hopkins, J.B.; Pierce, C.J.; Schloss, J.M.; Thompson, B.; Rowsell, J.L. Metal-Specific Interactions of H2 Adsorbed within Isostructural Metal–Organic Frameworks. J. Am. Chem. Soc. 2011, 133, 20310–20318. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, H.; Gao, W.; Xue, W.; Liu, Z.; Huang, J.; Pan, X.; Huang, Y. Surface-Engineered PtNi-O Nanostructure with Record-High Performance for Electrocatalytic Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 9046–9050. [Google Scholar] [CrossRef]
- Li, W.; Zou, S. PtNi Nanoparticles Encapsulated in Few Carbon Layers as High-Performance Catalysts for Oxygen Reduction Reaction. ACS Appl. Energy Mater. 2019, 2, 2769–2778. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Wang, N.; Zhou, Y.; Zheng, J.; Chu, W. Plasma-assisted highly dispersed Pt single atoms on Ru nanoclusters electrocatalyst for pH-universal hydrogen evolution. Chem. Eng. J. 2022, 448, 137611. [Google Scholar] [CrossRef]
- Zhao, W.; Luo, C.; Lin, Y.; Wang, G.-B.; Chen, H.M.; Kuang, P.; Yu, J. Pt-Ru Dimer Electrocatalyst with Electron Redistribution for Hydrogen Evolution Reaction. ACS Catal. 2022, 12, 5540–5548. [Google Scholar] [CrossRef]
- Peng, J.; Chen, Y.; Wang, K.; Tang, Z.; Chen, S. High-performance Ru-based electrocatalyst composed of Ru nanoparticles and Ru single atoms for hydrogen evolution reaction in alkaline solution. Int. J. Hydrog. Energy 2020, 45, 18840–18849. [Google Scholar] [CrossRef]
- Jiang, P.; Yang, Y.; Shi, R.; Xia, G.; Chen, J.; Su, J.; Chen, Q. Pt-like electrocatalytic behavior of Ru–MoO2 nanocomposites for the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 5475–5485. [Google Scholar] [CrossRef]
- Pierozynski, B.; Mikolajczyk, T. Hydrogen evolution reaction at Ru-modified nickel-coated carbon fibre in 0.1 M NaOH. Pol. J. Chem. Technol. 2015, 17, 18–22. [Google Scholar] [CrossRef]
- Jiang, R.; Tran, D.T.; Li, J.; Chu, D. Ru@RuO2 Core-Shell Nanorods: A Highly Active and Stable Bifunctional Catalyst for Oxygen Evolution and Hydrogen Evolution Reactions. Energy Environ. Mater. 2019, 2, 201–208. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, X.; Hu, G.; Feng, L. Ru Nanoclusters Coupled on Co/N-Doped Carbon Nanotubes Efficiently Catalyzed the Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2020, 8, 9136–9144. [Google Scholar] [CrossRef]
- Zhou, G.; Dou, R.; Bi, H.; Xie, S.; Pei, Y.; Fan, K.; Qiao, M.; Sun, B.; Zong, B. Ru nanoparticles on rutile/anatase junction of P25 TiO2: Controlled deposition and synergy in partial hydrogenation of benzene to cyclohexene. J. Catal. 2015, 332, 119–126. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, X.; Li, X.; Long, X.; Chang, C.; Yang, Z. Stable and high-performance Ir electrocatalyst with boosted utilization efficiency in acidic overall water splitting. Chem. Eng. J. 2021, 424, 130337. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Chen, L.-W.; Liang, H.-W. Ir-based bifunctional electrocatalysts for overall water splitting. Catal. Sci. Technol. 2021, 11, 4673–4689. [Google Scholar] [CrossRef]
- Liu, J.; Wu, W.; Wang, X. Electrodeposition of Iridium-Nickel Thin Films on Copper Foam: Effects of Loading and Solution Temperature on HER Performance of Electrocatalyst in Alkaline Water. Johns. Matthey Technol. Rev. 2020, 65, 94–111. [Google Scholar] [CrossRef]
- Dai, Q.; Meng, Q.; Du, C.; Ding, F.; Huang, J.; Nie, J.; Zhang, X.; Chen, J. Spontaneous deposition of Ir nanoparticles on 2D siloxene as a high-performance HER electrocatalyst with ultra-low Ir loading. Chem. Commun. 2020, 56, 4824–4827. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Goei, R.; Ong, S.-A.; Ong, A.J.; Huang, J.; Tok, A.I.Y. Development of Core-Shell Rh@Pt and Rh@Ir Nanoparticle Thin Film Using Atomic Layer Deposition for HER Electrocatalysis Applications. Processes 2022, 10, 1008. [Google Scholar] [CrossRef]
- Yao, W.; Jiang, X.; Li, Y.; Zhao, C.; Ding, L.; Sun, D.; Tang, Y. N-doped graphene anchored ultrasmall Ir nanoparticles as bifunctional electrocatalyst for overall water splitting. Green Energy Environ. 2022, 7, 1111–1118. [Google Scholar] [CrossRef]
- Majhi, K.C.; Yadav, M. Palladium oxide decorated transition metal nitride as efficient electrocatalyst for hydrogen evolution reaction. J. Alloy. Compd. 2021, 855, 157511. [Google Scholar] [CrossRef]
- Ilanchezhiyan, P.; Kumar, G.M.; Tamilselvan, S.; Kang, T.W.; Kim, D.Y. Highly efficient overall water splitting performance of gadolinium-indium-zinc ternary oxide nanostructured electrocatalyst. Int. J. Energy Res. 2020, 44, 6819–6827. [Google Scholar] [CrossRef]
- Ilanchezhiyan, P.; Kumar, G.M.; Siva, C.; Madhankumar, A.; Jeon, H.; Kang, T.; Kim, D. Evidencing enhanced oxygen and hydrogen evolution reactions using In-Zn-Co ternary transition metal oxide nanostructures: A novel bifunctional electrocatalyst. Int. J. Hydrog. Energy 2019, 44, 23081–23090. [Google Scholar] [CrossRef]
- Sarika, S.; Abhilash, S.; Sumi, V.; Rijith, S. Synthesis and characterization of transition metal mixed oxide doped graphene embedded durable electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 2021, 46, 16387–16403. [Google Scholar] [CrossRef]
- Sarika, S.; Abhilash, S.; Sumi, V.; Rijith, S. Graphene oxide supported transition metal mixed oxide nanourchins onto bimetallic phosphide coatings as high performance hydrogen evolution electrodes in alkaline media. J. Alloy. Compd. 2021, 875, 160033. [Google Scholar] [CrossRef]
- Liu, H.; He, P.; Wang, S.; Gao, J.; Zhou, L.; Li, C.; Zhang, Y.; Yang, D.; He, M.; Jia, L.; et al. Facile one-step fabrication of bimetallic Co–Ni–P hollow nanospheres anchored on reduced graphene oxide as highly efficient electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 24140–24150. [Google Scholar] [CrossRef]
- Kumar, G.M.; Ilanchezhiyan, P.; Siva, C.; Madhankumar, A.; Kang, T.; Kim, D. Co-Ni based hybrid transition metal oxide nanostructures for cost-effective bi-functional electrocatalytic oxygen and hydrogen evolution reactions. Int. J. Hydrog. Energy 2020, 45, 391–400. [Google Scholar] [CrossRef]
- Nouseen, S.; Singh, P.; Lavate, S.; Chattopadhyay, J.; Kuchkaev, A.M.; Yakhvarov, D.G.; Srivastava, R. Transition metal based ternary hierarchical metal sulphide microspheres as electrocatalyst for splitting of water into hydrogen and oxygen fuel. Catal. Today 2022, 397, 618–630. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, C.; Wang, S.; Hou, Y. 3D arrays of molybdenum sulphide nanosheets on Mo meshes: Efficient electrocatalysts for hydrogen evolution reaction. Electrochim. Acta 2015, 174, 653–659. [Google Scholar] [CrossRef]
- Quan, B.; Tong, H.; Liu, C.; Li, D.; Meng, S.; Chen, M.; Zhu, J.; Jiang, D. Integration of ZnCo2S4 nanowires arrays with NiFe-LDH nanosheet as water dissociation promoter for enhanced electrocatalytic hydrogen evolution. Electrochim. Acta 2019, 324, 134861. [Google Scholar] [CrossRef]
- Bhat, K.S.; Nagaraja, H.S. Performance evaluation of molybdenum dichalcogenide (MoX2; X = S, Se, Te) nanostructures for hydrogen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 17878–17886. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Pillai, V.V.; Alhassan, S.M. Scalable solid-state synthesis of MoS2-NiS2/graphene nanohybrids as bifunctional electrocatalysts for enhanced overall water splitting. Mater. Adv. 2020, 1, 794–803. [Google Scholar] [CrossRef]
- Dhandapani, B.; Jagannathan, M.; AlSalhi, M.S.; Aljaafreh, M.J.; Prasad, S. N-doped carbon embedded Ni3S2 electrocatalyst material towards efficient hydrogen evolution reaction in broad pH range. Colloids Surf. A-Physicochem. Eng. Asp. 2020, 603, 125194. [Google Scholar] [CrossRef]
- Govindaraju, V.R.; Sreeramareddygari, M.; Hanumantharayudu, N.D.; Devaramani, S.; Thippeswamy, R.; Surareungchai, W. Solvothermal decoration of Cu3SnS4 on reduced graphene oxide for enhanced electrocatalytic hydrogen evolution reaction. Environ. Prog. Sustain. Energy 2021, 40, e13558. [Google Scholar] [CrossRef]
- Kim, T.; Park, J.; Jin, H.; Oh, A.; Baik, H.; Joo, S.H.; Lee, K. A facet-controlled Rh3Pb2S2 nanocage as an efficient and robust electrocatalyst toward the hydrogen evolution reaction. Nanoscale 2018, 10, 9845–9850. [Google Scholar] [CrossRef]
- Ganesan, P.; Sivanantham, A.; Shanmugam, S. Inexpensive electrochemical synthesis of nickel iron sulphides on nickel foam: Super active and ultra-durable electrocatalysts for alkaline electrolyte membrane water electrolysis. J. Mater. Chem. A 2016, 4, 16394–16402. [Google Scholar] [CrossRef]
- Du, H.; Kong, R.-M.; Guo, X.; Qu, F.; Li, J. Recent progress in transition metal phosphides with enhanced electrocatalysis for hydrogen evolution. Nanoscale 2018, 10, 21617–21624. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Rodriguez, J.A. Catalysts for hydrogen evolution from the [NiFe] hydrogenase to the Ni2P(001) surface: The importance of ensemble effect. J. Am. Chem. Soc. 2005, 127, 14871–14878. [Google Scholar] [CrossRef] [PubMed]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Krane, N.; Lotze, C.; Franke, K. Moiré structure of MoS2 on Au(111): Local structural and electronic properties. Surf. Sci. 2018, 678, 136–142. [Google Scholar] [CrossRef]
- Faber, M.S.; Dziedzic, R.; Lukowski, M.A.; Kaiser, N.S.; Ding, Q.; Jin, S. High-Performance Electrocatalysis Using Metallic Cobalt Pyrite (CoS2) Micro- and Nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061. [Google Scholar] [CrossRef]
- Sulaiman, R.R.R.; Wong, W.Y.; Loh, K.S. Recent developments on transition metal–based electrocatalysts for application in anion exchange membrane water electrolysis. Int. J. Energy Res. 2022, 46, 2241–2276. [Google Scholar] [CrossRef]
- Miles, M.H.; Thomason, M.A. Periodic Variations of Overvoltages for Water Electrolysis in Acid Solutions from Cyclic Voltammetric Studies. J. Electrochem. Soc. 1976, 123, 1459. [Google Scholar] [CrossRef]
- Hu, S.; Feng, C.; Wang, S.; Liu, J.; Wu, H.; Zhang, L.; Zhang, J. Ni3N/NF as Bifunctional Catalysts for Both Hydrogen Generation and Urea Decomposition. ACS Appl. Mater. Interfaces 2019, 11, 13168–13175. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, S.; Feng, C.; Wu, H.; Zhang, L.; Zhang, J. Novel Cobalt-Doped Ni0.85Se Chalcogenides (CoxNi0.85–xSe) as High Active and Stable Electrocatalysts for Hydrogen Evolution Reaction in Electrolysis Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 40491–40499. [Google Scholar] [CrossRef]
- Hanan, A.; Shu, D.; Aftab, U.; Cao, D.; Laghari, A.J.; Solangi, M.Y.; Abro, M.I.; Nafady, A.; Vigolo, B.; Tahira, A.; et al. Co2FeO4@rGO composite: Towards trifunctional water splitting in alkaline media. Int. J. Hydrog. Energy 2022, 47, 33919–33937. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High Catalytic Activity of Nitrogen and Sulfur Co-Doped Nanoporous Graphene in the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2015, 54, 2131–2136. [Google Scholar] [CrossRef]
- Hu, C.; Xiao, Y.; Zou, Y.; Dai, L. Carbon-Based Metal-Free Electrocatalysis for Energy Conversion, Energy Storage, and Environmental Protection. Electrochem. Energy Rev. 2018, 1, 84–112. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Y.; Miao, S.; Ni, M.; Zhou, W.; Shao, Z. Spherical Ruthenium Disulfide-Sulfur-Doped Graphene Composite as an Efficient Hydrogen Evolution Electrocatalyst. ACS Appl. Mater. Interfaces 2018, 10, 34098–34107. [Google Scholar] [CrossRef] [PubMed]
- Parvez, K.; Yang, S.; Hernandez, Y.; Winter, A.; Turchanin, A.; Feng, X.; Müllen, K. Nitrogen-Doped Graphene and Its Iron-Based Composite As Efficient Electrocatalysts for Oxygen Reduction Reaction. ACS Nano 2012, 6, 9541–9550. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Bawari, S.; Vineesh, T.V.; Shyaga, N.; Narayanan, T.N. Selenium-Coupled Reduced Graphene Oxide as Single-Atom Site Catalyst for Direct Four-Electron Oxygen Reduction Reaction. ACS Appl. Energy Mater. 2019, 2, 3624–3632. [Google Scholar] [CrossRef]
- Sun, X.; Liu, F.; Chen, X.; Li, C.; Yu, J.; Pan, M. Iridium-doped ZIFs-derived porous carbon-coated IrCo alloy as competent bifunctional catalyst for overall water splitting in acid medium. Electrochim. Acta 2019, 307, 206–213. [Google Scholar] [CrossRef]
- Babar, N.-U.; Joya, Y.F.; Khalil, H.; Hussain, F.; Joya, K.S. Thin-film iron-oxide nanobeads as bifunctional electrocatalyst for high activity overall water splitting. Int. J. Hydrog. Energy 2021, 46, 7885–7902. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, R.; Zhang, J.; Wang, Z.; Xue, H.; Mao, W.; Huang, W. Highly active and stable electrocatalytic hydrogen evolution catalyzed by nickel, iron doped cobalt disulfide@reduced graphene oxide nanohybrid electrocatalysts. Mater. Today Energy 2018, 7, 44–50. [Google Scholar] [CrossRef]
- Kumar, R.S.; Ramakrishnan, S.; Prabhakaran, S.; Kim, A.R.; Kumar, D.R.; Kim, D.H.; Yoo, D.J. Structural, electronic, and electrocatalytic evaluation of spinel transition metal sulfide supported reduced graphene oxide. J. Mater. Chem. A 2022, 10, 1999–2011. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, Q.; Qi, X.; Peng, B.; Xu, L.; Liu, C.; Liang, T. High-performance bifunctional Fe-doped molybdenum oxide-based electrocatalysts with in situ grown epitaxial heterojunctions for overall water splitting. Int. J. Hydrog. Energy 2020, 45, 24828–24839. [Google Scholar] [CrossRef]
- Saad, A.; Shen, H.; Cheng, Z.; Ju, Q.; Guo, H.; Munir, M.; Turak, A.; Wang, J.; Yang, M. Three-Dimensional Mesoporous Phosphide-Spinel Oxide Heterojunctions with Dual Function as Catalysts for Overall Water Splitting. ACS Appl. Energy Mater. 2020, 3, 1684–1693. [Google Scholar] [CrossRef]
- Liu, M.; Yang, L.; Liu, T.; Tang, Y.; Luo, S.; Liu, C.; Zeng, Y. Fe2P/reduced graphene oxide/Fe2P sandwich-structured nanowall arrays: A high-performance non-noble-metal electrocatalyst for hydrogen evolution. J. Mater. Chem. A 2017, 5, 8608–8615. [Google Scholar] [CrossRef]
- He, W.; Wang, F.; Gao, Y.; Hao, Q.; Liu, C. One-step synthesis of amorphous transition metal sulfides as bifunctional electrocatalysts for the hydrogen evolution reaction and oxygen evolution reaction. Sustain. Energy Fuels 2022, 6, 3852–3857. [Google Scholar] [CrossRef]
- Weng, B.; Wang, X.; Grice, C.R.; Xu, F.; Yan, Y. A new metal-organic open framework enabling facile synthesis of carbon encapsulated transition metal phosphide/sulfide nanoparticle electrocatalysts. J. Mater. Chem. A 2019, 7, 7168–7178. [Google Scholar] [CrossRef]
- Zhao, Y.; Mavrokefalos, C.K.; Zhang, P.; Erni, R.; Li, J.; Triana, C.A.; Patzke, G.R. Self-Templating Strategies for Transition Metal Sulfide Nanoboxes as Robust Bifunctional Electrocatalysts. Chem. Mater. 2020, 32, 1371–1383. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, L.; Xu, W.; Xiong, C.; Chen, W.; Xiang, X.; Zhang, B.; Shang, H. Carbon-supported high-entropy Co-Zn-Cd-Cu-Mn sulfide nanoarrays promise high-performance overall water splitting. Nano Res. 2022, 15, 6054–6061. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, H.; Zhang, L.; Zhang, S.; Zhang, M.; Du, M. Activating MoS2 by interface engineering for efficient hydrogen evolution catalysis. Mater. Res. Bull. 2019, 112, 46–52. [Google Scholar] [CrossRef]
- Wang, K.; Yan, L.; Mu, X.; Feng, B.; Lv, K.; Yu, X.; Li, L.; Zhang, X.; Yang, X.; Lu, Z. One-Step Hydrothermal Synthesis of Mo-Doped Ni3S2 Nanorods for Efficient Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5, 11498–11505. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Liu, Y.; Zhang, C.; Jia, K.; Su, J.; Wang, K. The High Electrocatalytic Performance of NiFeSe/CFP for Hydrogen Evolution Reaction Derived from a Prussian Blue Analogue. Catalysts 2022, 12, 739. [Google Scholar] [CrossRef]
- Li, Q.; Yan, W.; Liu, Y.; Du, H.; Wang, Z.; Hao, X.; Guan, G. One-step electrodeposition of cauliflower-like CozNiySx@polypyrrole electrocatalysts on carbon fiber paper for hydrogen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 12931–12940. [Google Scholar] [CrossRef]
- Masud, J.; Swesi, A.T.; Liyanage, W.P.R.; Nath, M. Cobalt Selenide Nanostructures: An Efficient Bifunctional Catalyst with High Current Density at Low Coverage. ACS Appl. Mater. Interfaces 2016, 8, 17292–17302. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Yang, W.; Liu, Y.; Yu, Y. Engineering sulfur vacancies in basal plane of MoS2 for enhanced hydrogen evolution reaction. J. Catal. 2020, 391, 91–97. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Z.; Liu, F.; Zhang, J.; Zheng, L.; Hu, Y.; Mao, J.; Liu, H.; Xue, Y.; Tang, C. Sulfur vacancy-tailored NiCo2S4 nanosheet arrays for the hydrogen evolution reaction at all pH values. Catal. Sci. Technol. 2020, 10, 1056–1065. [Google Scholar] [CrossRef]
- Che, Q.; Li, Q.; Tan, Y.; Chen, X.; Xu, X.; Chen, Y. One-step controllable synthesis of amorphous (Ni-Fe)S-x/NiFe(OH)(y) hollow microtube/sphere films as superior bifunctional electrocatalysts for quasi industrial water splitting at large-current-density. Appl. Catal. B-Environ. 2019, 246, 337–348. [Google Scholar] [CrossRef]
- Cao, Q.; Gu, W.; Li, J.; Wang, E. The Effect of Metal Components in the Quaternary Electrocatalysts on the Morphology and Catalytic Performance of Transition Metal Phosphides. Electroanalysis 2018, 30, 2584–2588. [Google Scholar] [CrossRef]
- Huo, D.; Sun, Z.; Liu, Y.; Yu, Z.; Wang, Y.; Wang, A. Synthesis of Co-Doped Tungsten Phosphide Nanoparticles Supported on Carbon Supports as High-Efficiency HER Catalysts. ACS Sustain. Chem. Eng. 2021, 9, 12311–12322. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Ting, N.-H.; Ting, J.-M. Multi-metal phosphide as bi-functional electrocatalyst for enhanced water splitting performance. J. Power Sources 2022, 552, 232249. [Google Scholar] [CrossRef]
- Zhai, M.; Wang, F.; Du, H. Transition-Metal Phosphide Carbon Nanosheet Composites Derived from Two-Dimensional Metal-Organic Frameworks for Highly Efficient Electrocatalytic Water-Splitting. ACS Appl. Mater. Interfaces 2017, 9, 40171–40179. [Google Scholar] [CrossRef]
- Ren, L.; Yang, D.; Yang, J.-H. Ruthenium-manganese phosphide nanohybrid supported on graphene for efficient hydrogen evolution reaction in acid and alkaline conditions. Int. J. Hydrog. Energy 2022, 47, 13876–13886. [Google Scholar] [CrossRef]
- Kawashima, K.; Shin, K.; Wygant, B.R.; Kim, J.-H.; Cao, C.L.; Lin, J.; Son, Y.J.; Liu, Y.; Henkelman, G.; Mullins, C.B. Cobalt Metal-Cobalt Carbide Composite Microspheres for Water Reduction Electrocatalysis. ACS Appl. Energy Mater. 2020, 3, 3909–3918. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.-A.; Huang, Y.; Mu, H.; Gu, X.; Li, F.; Wang, Z.; Li, J. Impacts of Metal-Support Interaction on Hydrogen Evolution Reaction of Cobalt-Nitride-Carbide Catalyst. Front. Chem. 2022, 9, 828964. [Google Scholar] [CrossRef] [PubMed]
- An, T.-Y.; Surendran, S.; Kim, H.; Choe, W.-S.; Kim, J.K.; Sim, U. A polydopamine-mediated biomimetic facile synthesis of molybdenum carbide-phosphide nanodots encapsulated in carbon shell for electrochemical hydrogen evolution reaction with long-term durability. Compos. Part B-Eng. 2019, 175, 107071. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, C.; Wang, S.; Gao, B.; Fan, X.; Xu, S.; Jia, Y.; Zhang, Y.; Gao, Q.; Cao, X.; et al. N-doped molybdenum carbides embedded in porous carbon for efficient hydrogen evolution. Mater. Today Energy 2022, 26, 100992. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, T.; Li, B.; Wei, S.; Gao, W. Enhancing the electrochemical hydrogen evolution of CoP3/CoMoP nanosheets through the support of black TiO2-x nanotube arrays. J. Alloy. Compd. 2022, 905, 164165. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Su, T.; Wang, X.; Sun, C.; Su, Z. Graphene-coated hybrid electrocatalysts derived from bimetallic metal-organic frameworks for efficient hydrogen generation. J. Mater. Chem. A 2017, 5, 5000–5006. [Google Scholar] [CrossRef]
- Ab Latif, F.E.; Numan, A.; Mubarak, N.M.; Khalid, M.; Abdullah, E.C.; Manaf, N.A.; Walvekar, R. Evolution of MXene and its 2D heterostructure in electrochemical sensor applications. Coord. Chem. Rev. 2022, 471, 214755. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Huang, Q. MXenes: Two-Dimensional Building Blocks for Future Materials and Devices. ACS Nano 2021, 15, 5775–5780. [Google Scholar] [CrossRef]
- Junaidi, N.H.A.; Wong, W.Y.; Loh, K.S.; Rahman, S.; Daud, W.R.W. A comprehensive review of MXenes as catalyst supports for the oxygen reduction reaction in fuel cells. Int. J. Energy Res. 2021, 45, 15760–15782. [Google Scholar] [CrossRef]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-Dimensional Molybdenum Carbide (MXene) as an Efficient Electrocatalyst for Hydrogen Evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Gao, G.; O’Mullane, A.P.; Du, A. 2D MXenes: A New Family of Promising Catalysts for the Hydrogen Evolution Reaction. ACS Catal. 2017, 7, 494–500. [Google Scholar] [CrossRef]
- Wei, Y.; Soomro, R.A.; Xie, X.; Xu, B. Design of efficient electrocatalysts for hydrogen evolution reaction based on 2D MXenes. J. Energy Chem. 2021, 55, 244–255. [Google Scholar] [CrossRef]
- Yoon, Y.; Tiwari, A.P.; Lee, M.; Choi, M.; Song, W.; Im, J.; Zyung, T.; Jung, H.-K.; Lee, S.S.; Jeon, S.; et al. Enhanced electrocatalytic activity by chemical nitridation of two-dimensional titanium carbide MXene for hydrogen evolution. J. Mater. Chem. A 2018, 6, 20869–20877. [Google Scholar] [CrossRef]
- Asokan, A.; Liu, C.; Peera, S.G.; Hussain, A.M. Electrocatalysts Derived from 2D Mxenes for Oxygen Reduction and Hydrogen Evolution Reactions, in Adapting 2D Nanomaterials for Advanced Applications. Am. Chem. Soc. 2020, 1353, 167–189. [Google Scholar]
- Handoko, A.D.; Fredrickson, K.D.; Anasori, B.; Convey, K.W.; Johnson, L.R.; Gogotsi, Y.; Vojvodic, A.; Seh, Z.W. Tuning the Basal Plane Functionalization of Two-Dimensional Metal Carbides (MXenes) To Control Hydrogen Evolution Activity. ACS Appl. Energy Mater. 2018, 1, 173–180. [Google Scholar] [CrossRef]
- He, H.; Chen, Y.; Yang, C.; Yang, L.; Jiang, Q.; Huang, H. Constructing 3D interweaved MXene/graphitic carbon nitride nanosheets/graphene nanoarchitectures for promoted electrocatalytic hydrogen evolution. J. Energy Chem. 2022, 67, 483–491. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, T.; Xie, X.; Jiang, W.; Li, J.; Tian, B.; Su, C. Oxygen-Functionalized Ultrathin Ti3C2Tx MXene for Enhanced Electrocatalytic Hydrogen Evolution. ChemSusChem 2019, 12, 1368–1373. [Google Scholar] [CrossRef]
- Benchakar, M.; Natu, V.; Elmelegy, T.A.; Sokol, M.; Snyder, J.; Comminges, C.; Morais, C.; Célérier, S.; Habrioux, A.; Barsoum, M.W. On a Two-Dimensional MoS2/Mo2CTx Hydrogen Evolution Catalyst Obtained by the Topotactic Sulfurization of Mo2CTx MXene. J. Electrochem. Soc. 2020, 167, 124507. [Google Scholar] [CrossRef]
- Liang, J.; Ding, C.; Liu, J.; Chen, T.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Heterostructure engineering of Co-doped MoS2 coupled with Mo2CTx MXene for enhanced hydrogen evolution in alkaline media. Nanoscale 2019, 11, 10992–11000. [Google Scholar] [CrossRef]
- Schultz, T.; Frey, N.C.; Hantanasirisakul, K.; Park, S.; May, S.J.; Shenoy, V.B.; Gogotsi, Y.; Koch, N. Surface Termination Dependent Work Function and Electronic Properties of Ti3C2Tx MXene. Chem. Mater. 2019, 31, 6590–6597. [Google Scholar] [CrossRef]
- Dillon, A.D.; Ghidiu, M.J.; Krick, A.L.; Griggs, J.; May, S.J.; Gogotsi, Y.; Barsoum, M.W.; Fafarman, A.T. Highly Conductive Optical Quality Solution-Processed Films of 2D Titanium Carbide. Adv. Funct. Mater. 2016, 26, 4162–4168. [Google Scholar] [CrossRef]
- Miranda, A.; Halim, J.; Barsoum, M.W.; Lorke, A. Electronic properties of freestanding Ti3C2Tx MXene monolayers. Appl. Phys. Lett. 2016, 108, 033102. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, L.; Liu, J. Recent advances in cobalt-based electrocatalysts for hydrogen and oxygen evolution reactions. J. Alloy. Compd. 2020, 821, 153542. [Google Scholar] [CrossRef]
- Kong, Q.; An, X.; Huang, L.; Wang, X.; Feng, W.; Qiu, S.; Wang, Q.; Sun, C. A DFT study of Ti3C2O2 MXenes quantum dots supported on single layer graphene: Electronic structure an hydrogen evolution performance. Front. Phys. 2021, 16, 53506. [Google Scholar] [CrossRef]
- Ren, J.; Zong, H.; Sun, Y.; Gong, S.; Feng, Y.; Wang, Z.; Hu, L.; Yu, K.; Zhu, Z. 2D organ-like molybdenum carbide (MXene) coupled with MoS2 nanoflowers enhances the catalytic activity in the hydrogen evolution reaction. CrystEngComm 2020, 22, 1395–1403. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Liu, Q.; Sun, P.; Wang, Y.; Chou, S.; Hu, Z.; Zhang, Z. Single-atom Ru anchored in nitrogen-doped MXene (Ti3C2Tx) as an efficient catalyst for the hydrogen evolution reaction at all pH values. J. Mater. Chem. A 2020, 8, 24710–24717. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, X.; Lu, Q.; Li, Y.; Xiao, F.; Tang, B.; Wang, S.; Yu, D.Y.W.; Rogach, A.L. Electrocatalytic enhancement mechanism of cobalt single atoms anchored on different MXene substrates in oxygen and hydrogen evolution reactions. EcoMat 2022, e12293. [Google Scholar] [CrossRef]
- Bu, F.; Zagho, M.M.; Ibrahim, Y.; Ma, B.; Elzatahry, A.; Zhao, D. Porous MXenes: Synthesis, structures, and applications. Nano Today 2020, 30, 100803. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, W.; Yu, R.; Xia, L.; Hong, X.; Zhu, J.; Li, J.; Lv, L.; Chen, W.; Zhao, Y.; et al. Anchoring Sub-Nanometer Pt Clusters on Crumpled Paper-Like MXene Enables High Hydrogen Evolution Mass Activity. Adv. Funct. Mater. 2022, 32, 2110910. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Xie, X.; Ren, C.E.; Makaryan, T.; Anasori, B.; Wang, G.; Gogotsi, Y. Hollow MXene Spheres and 3D Macroporous MXene Frameworks for Na-Ion Storage. Adv. Mater. 2017, 29, 1702410. [Google Scholar] [CrossRef]
- Pandey, M.; Thygesen, K.S. Two-Dimensional MXenes as Catalysts for Electrochemical Hydrogen Evolution: A Computational Screening Study. J. Phys. Chem. C 2017, 121, 13593–13598. [Google Scholar] [CrossRef]
- Li, S.; Tuo, P.; Xie, J.; Zhang, X.; Xu, J.; Bao, J.; Pan, B.; Xie, Y. Ultrathin MXene nanosheets with rich fluorine termination groups realizing efficient electrocatalytic hydrogen evolution. Nano Energy 2018, 47, 512–518. [Google Scholar] [CrossRef]
- Han, M.; Yang, J.; Jiang, J.; Jing, R.; Ren, S.; Yan, C. Efficient tuning the electronic structure of N-doped Ti-based MXene to enhance hydrogen evolution reaction. J. Colloid Interface Sci. 2021, 582, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ai, L.; Wang, M.; Jiang, J.; Wang, S. Hierarchical MoS2 nanosheets integrated Ti3C2 MXenes for electrocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2019, 44, 965–976. [Google Scholar] [CrossRef]
- Wu, F.; Xu, C.; Yang, X.; Yang, L.; Yin, S. S-doped multilayer niobium carbide (Nb4C3Tx) electrocatalyst for efficient hydrogen evolution in alkaline solutions. Int. J. Hydrog. Energy 2022, 47, 17233–17240. [Google Scholar] [CrossRef]
- Cui, C.; Cheng, R.; Zhang, H.; Zhang, C.; Ma, Y.; Shi, C.; Fan, B.; Wang, H.; Wang, X. Ultrastable MXene@Pt/SWCNTs’ Nanocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2020, 30, 2000693. [Google Scholar] [CrossRef]
- Yue, Q.; Sun, J.; Chen, S.; Zhou, Y.; Li, H.; Chen, Y.; Zhang, R.; Wei, G.; Kang, Y. Hierarchical Mesoporous MXene–NiCoP Electrocatalyst for Water-Splitting. ACS Appl. Mater. Interfaces 2020, 12, 18570–18577. [Google Scholar] [CrossRef]
- Du, C.; Sun, X.; Yu, H.; Liang, Q.; Dinh, K.N.; Zheng, Y.; Luo, Y.; Wang, Z.; Yan, Q. Synergy of Nb Doping and Surface Alloy Enhanced on Water–Alkali Electrocatalytic Hydrogen Generation Performance in Ti-Based MXene. Adv. Sci. 2019, 6, 1900116. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Liu, J.; Sun, F.; Yang, P.; Qiu, J. A hierarchically porous and hydrophilic 3D nickel–iron/MXene electrode for accelerating oxygen and hydrogen evolution at high current densities. Nano Energy 2019, 63, 103880. [Google Scholar] [CrossRef]
- Le, T.A.; Tran, N.Q.; Hong, Y.; Kim, M.; Lee, H. Porosity-Engineering of MXene as a Support Material for a Highly Efficient Electrocatalyst toward Overall Water Splitting. ChemSusChem 2020, 13, 945–955. [Google Scholar] [CrossRef]
- Kang, S.M.; Kim, M.; Lee, J.B.; Xu, S.; Selvam, N.C.S.; Yoo, P.J. A NiCoP nanocluster-anchored porous Ti3C2Tx monolayer as high performance hydrogen evolution reaction electrocatalysts. Nanoscale 2021, 13, 12854–12864. [Google Scholar] [CrossRef]
- Kong, W.; Li, L.; Yu, X.; Xiang, Z.; Cao, Y.; Tahir, M.; Lu, Z.; Deng, J.; Song, Y. Platinum nickel alloy-MXene catalyst with inverse opal structure for enhanced hydrogen evolution in both acidic and alkaline solutions. Nano Res. 2022. [Google Scholar] [CrossRef]
- Lin, W.; Lu, Y.-R.; Peng, W.; Luo, M.; Chan, T.-S.; Tan, Y. Atomic bridging modulation of Ir–N, S co-doped MXene for accelerating hydrogen evolution. J. Mater. Chem. A 2022, 10, 9878–9885. [Google Scholar] [CrossRef]
- Peng, W.; Han, J.; Lu, Y.-R.; Luo, M.; Chan, T.-S.; Peng, M.; Tan, Y. A General Strategy for Engineering Single-Metal Sites on 3D Porous N, P Co-Doped Ti3C2TX MXene. ACS Nano 2022, 16, 4116–4125. [Google Scholar] [CrossRef]
- Shen, B.; Huang, H.; Liu, H.; Jiang, Q.; He, H. Bottom-up construction of three-dimensional porous MXene/nitrogen-doped graphene architectures as efficient hydrogen evolution electrocatalysts. Int. J. Hydrog. Energy 2021, 46, 29984–29993. [Google Scholar] [CrossRef]
- Kuang, P.; He, M.; Zhu, B.; Yu, J.; Fan, K.; Jaroniec, M. 0D/2D NiS2/V-MXene composite for electrocatalytic H2 evolution. J. Catal. 2019, 375, 8–20. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Guo, X.; Chen, C.; Dong, C.-L.; Liu, R.-S.; Han, C.-P.; Li, Y.; Gogotsi, Y.; Wang, G. Single platinum atoms immobilized on an MXene as an efficient catalyst for the hydrogen evolution reaction. Nat. Catal. 2018, 1, 985–992. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Yang, Q.; Tan, L.; Dong, L.; Dong, M. Ultrathin Ti3C2Tx (MXene) Nanosheet-Wrapped NiSe2 Octahedral Crystal for Enhanced Supercapacitor Performance and Synergetic Electrocatalytic Water Splitting. Nano-Micro Lett. 2019, 11, 31. [Google Scholar] [CrossRef]
- Li, M.; Sun, R.; Li, Y.; Jiang, J.; Xu, W.; Cong, H.; Han, S. The 3D porous “celosia” heterogeneous interface engineering of layered double hydroxide and P-doped molybdenum oxide on MXene promotes overall water-splitting. Chem. Eng. J. 2022, 431, 133941. [Google Scholar] [CrossRef]
- Shen, B.; Huang, H.; Jiang, Y.; Xue, Y.; He, H. 3D interweaving MXene–graphene network–confined Ni–Fe layered double hydroxide nanosheets for enhanced hydrogen evolution. Electrochim. Acta 2022, 407, 139913. [Google Scholar] [CrossRef]
- Cui, C.; Cheng, R.; Zhang, C.; Wang, X. Pt immobilized spontaneously on porous MXene/MAX hybrid monolith for hydrogen evolution reaction. Chin. Chem. Lett. 2020, 31, 988–991. [Google Scholar] [CrossRef]
- Lim, K.R.G.; Shekhirev, M.; Wyatt, B.C.; Anasori, B.; Gogotsi, Y.; Seh, Z.W. Fundamentals of MXene synthesis. Nat. Synth. 2022, 1, 601–614. [Google Scholar] [CrossRef]
- Zhan, X.; Si, C.; Zhou, J.; Sun, Z. MXene and MXene-based composites: Synthesis, properties and environment-related applications. Nanoscale Horiz. 2020, 5, 235–258. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, S.J.; Seo, D.; Chae, Y.; Anayee, M.; Lee, Y.; Gogotsi, Y.; Ahn, C.W.; Jung, H.-T. Etching Mechanism of Monoatomic Aluminum Layers during MXene Synthesis. Chem. Mater. 2021, 33, 6346–6355. [Google Scholar] [CrossRef]
- Numan, A.; Rafique, S.; Khalid, M.; Zaharin, H.A.; Radwan, A.; Mokri, N.A.; Ching, O.P.; Walvekar, R. Microwave-assisted rapid MAX phase etching and delamination: A paradigm shift in MXene synthesis. Mater. Chem. Phys. 2022, 288, 126429. [Google Scholar] [CrossRef]
- Hu, X.; Tian, X.; Lin, Y.-W.; Wang, Z. Nickel foam and stainless steel mesh as electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and overall water splitting in alkaline media. RSC Adv. 2019, 9, 31563–31571. [Google Scholar] [CrossRef]
- Park, Y.S.; Jang, M.J.; Jeong, J.; Park, S.M.; Wang, X.; Seo, M.H.; Choi, S.M.; Yang, J. Hierarchical chestnut-burr like structure of copper cobalt oxide electrocatalyst directly grown on Ni foam for anion exchange membrane water electrolysis. ACS Sustain. Chem. Eng. 2020, 8, 2344–2349. [Google Scholar] [CrossRef]
- Song, D.; Li, X.; Li, X.-P.; Jia, X.; Min, P.; Yu, Z.-Z. Hollow-structured MXene-PDMS composites as flexible, wearable and highly bendable sensors with wide working range. J. Colloid Interface Sci. 2019, 555, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, Y.; Sun, Z.; Lu, X. Ga doped Ni3S2 ultrathin nanosheet arrays supported on Ti3C2-MXene/Ni foam: An efficient and stable 3D electrocatalyst for oxygen evolution reaction. Int. J. Hydrog. Energy 2022, 47, 2958–2966. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, H.-K.; Li, T.-T.; Shiu, B.-C.; Ren, H.-T.; Zhang, X.; Lou, C.-W.; Lin, J.-H. MXene-coated conductive composite film with ultrathin, flexible, self-cleaning for high-performance electromagnetic interference shielding. Chem. Eng. J. 2021, 412, 128681. [Google Scholar] [CrossRef]
- Lai, L.; Clark, M.; Su, S.; Li, R.; Ivey, D.G.; Zhu, X. Dip-coating synthesis of rGO/α-Ni(OH)2@nickel foam with layer-by-layer structure for high performance binder-free supercapacitors. Electrochim. Acta 2021, 368, 137589. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, F.; Zhang, L.; Liu, L.; Chen, J.; Yang, B.; Yan, X. Rolling up MXene sheets into scrolls to promote their anode performance in lithium-ion batteries. J. Energy Chem. 2020, 46, 256–263. [Google Scholar] [CrossRef]
- Wu, Z.; Shang, T.; Deng, Y.; Tao, Y.; Yang, Q. The Assembly of MXenes from 2D to 3D. Adv. Sci. 2020, 7, 1903077. [Google Scholar] [CrossRef] [PubMed]


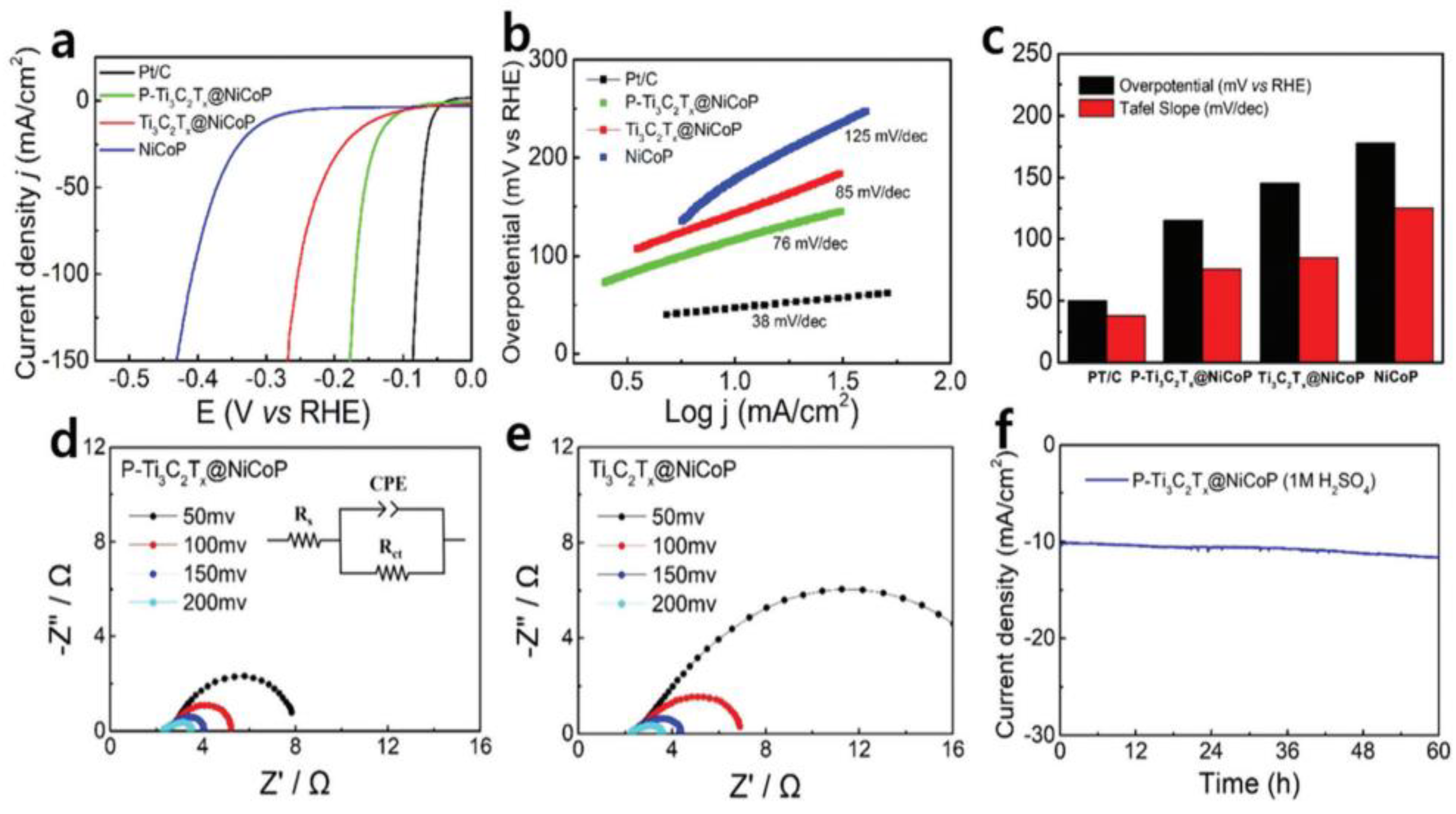

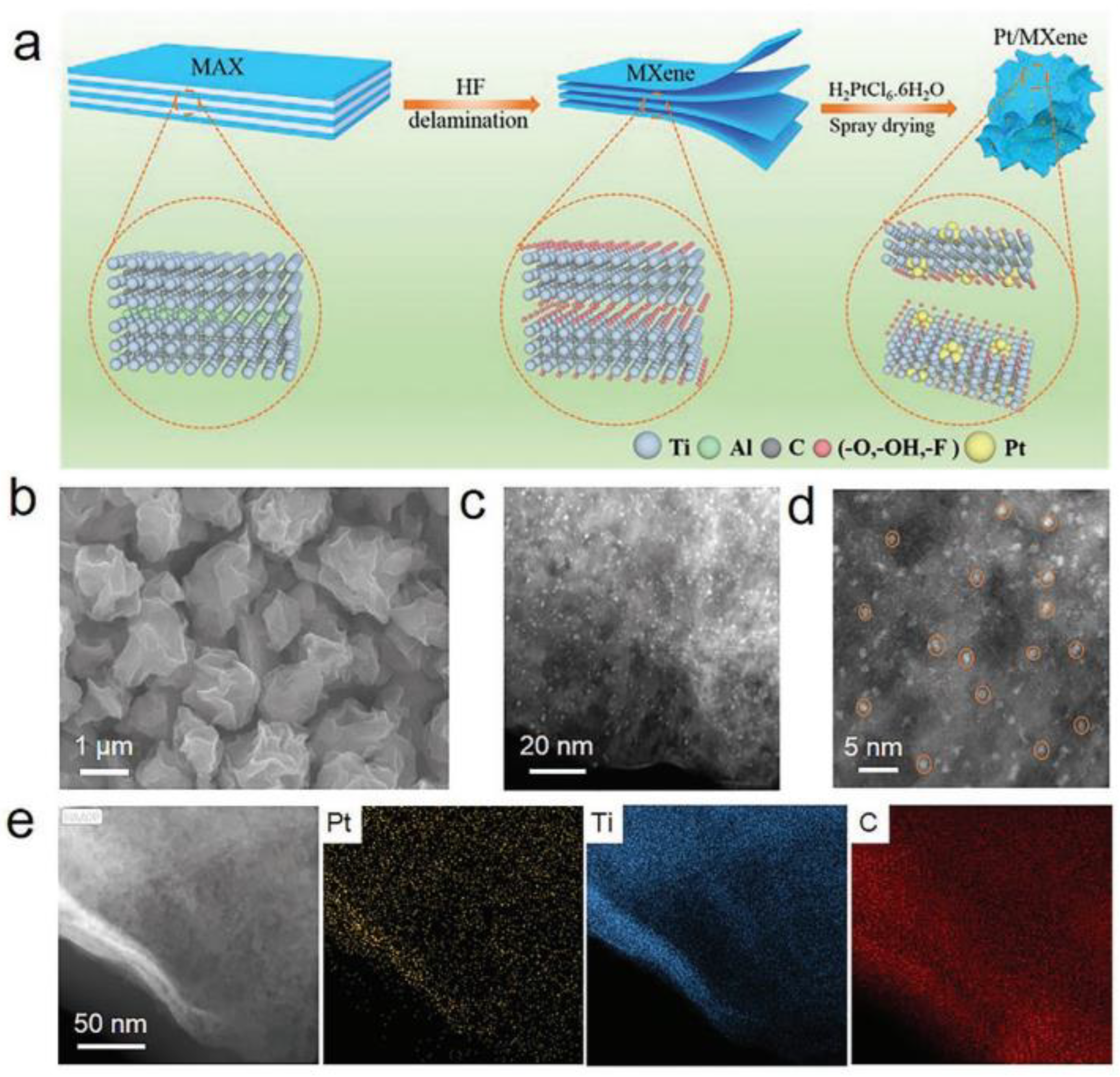
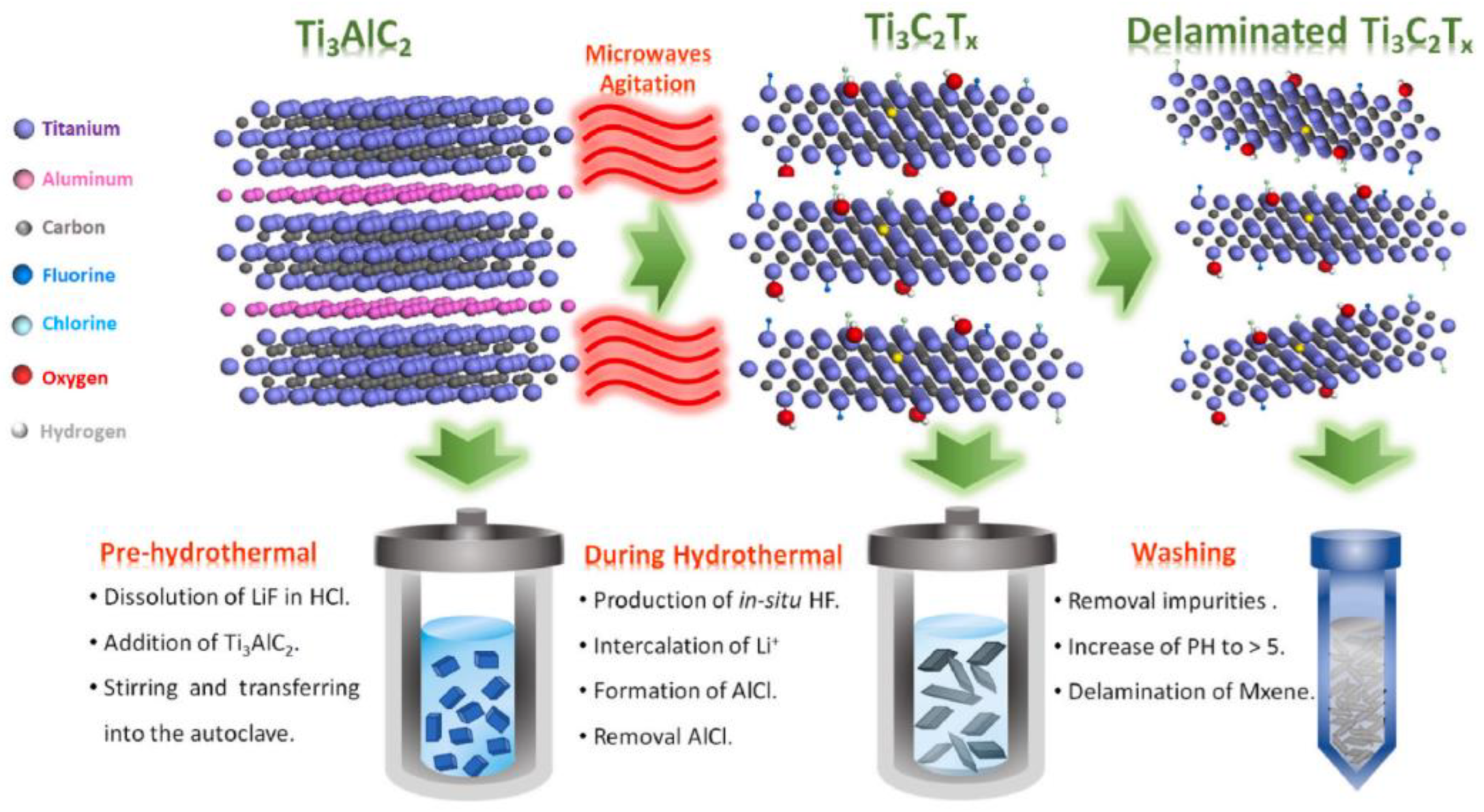
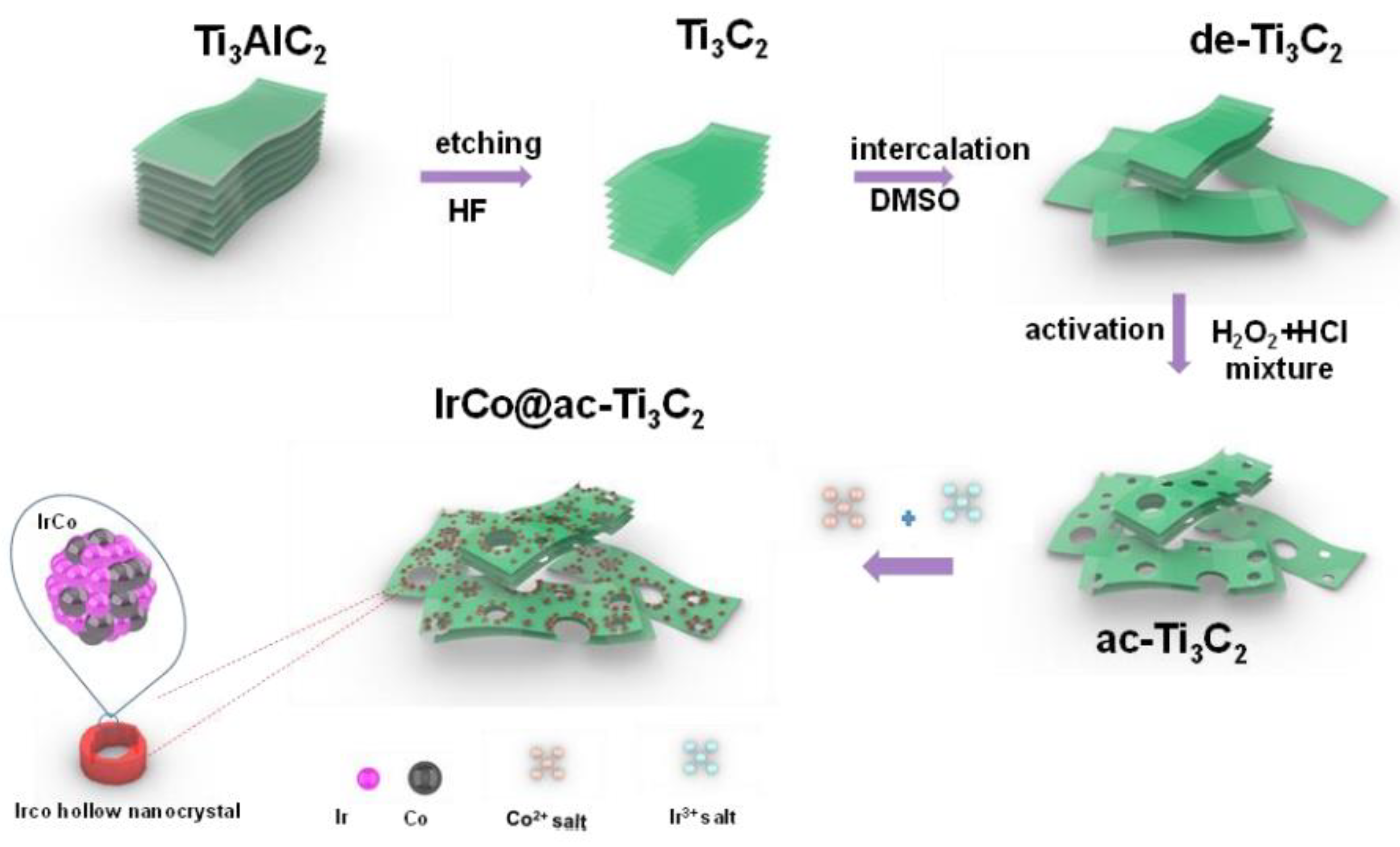
| Catalyst | Electrolyte | BET Surface Area (m2/g) | Overpotential, η (mV) @ 10 mA/cm2 | Tafel Slope, b (mV/dec) | Durability 1 |
|---|---|---|---|---|---|
| NiFe/Pt [68] | 0.1 M KOH | - | 128 | - | - |
| NiO/PtNi [70] | 1 M KOH | - | 39.8 | 78.8 | 10 h @ 10 mA/cm2 |
| Ru-MoO2 [75] | 1 M KOH and 0.5 M H2SO4 | - | 29 (1 M KOH), 55 (0.5 M H2SO4) | 31 (1 M KOH), 44 (0.5 M H2SO4) | |
| Ru-NMCNs-T [74] | 1 M KOH | 684 (Ru-NMCNs-T), 755 (NMCNs) | 28 | 57.6 | - |
| Ru-NiCCF [76] | 0.1 M NaOH | - | 100–300 | - | - |
| Ru@RuO2 [77] | 0.1 M KOH | 39.8 | 137 | 112 | - |
| Ru@Co/N-CNTs [78] | 1 M KOH and 0.5 M H2SO4 | - | 48 (1 M KOH), 92 (0.5 M H2SO4) | 33 (1 M KOH), 45 (0.5 M H2SO4) | - |
| Ir80Ni20/Cu foam [83] | 1 M KOH | - | 60 | 40 | 10 h @ 10 mA/cm2 |
| Ir NP/Siloxene [84] | - | ||||
| Rh@Pt [85] | - | - | 139 | 84.8 | - |
| Rh@Ir [85] | - | - | 169 | 112 | - |
| Ir-Co [118] | 0.5 M H2SO4 | - | 23.9 | 25.7 | - |
| rGO-Fe3O4 [38] | 1 M KOH | 310 | 80 | 24 h @ 10 mA/cm2 | |
| FeOx-NBs [119] | 0.5 M KOH | 450 | 85 | - | |
| Co0.8Ni0.1Fe0.1S2 [120] | 0.5 M H2SO4 | 138 | 49 | 33 h @10 mA/cm2 | |
| ZCS@rGO [121] | - | 135 | 47 | 36 h @10 mA/cm2 | |
| FeMoO2/MoO3/ENF [122] | - | 36 | - | 95 h @100 mA/cm2 | |
| CoCr2O4 [123] | 1 M KOH | 212 | - | 24 h @10 mA/cm2 | |
| Fe2P@rGO [124] | - | 101 | 55.2 | 12 h@10 mA/cm2 | |
| NiSx/NF [125] | 1 M KOH | 53 | - | 100 h @100 mA/cm2 | |
| NiP/NiFeP/C [126] | 1 M KOH | 1.53 V (cell) | - | 20 h @100 mA/cm2 | |
| Co-Fe-S@PB [127] | - | 286 | 37.8 | 33 h @10 mA/cm2 | |
| CoZnCdCuMnS@CF [128] | 1 M KOH | 173 | - | 70 h @10 mA/cm2 | |
| Au-MoS2/CNFs [129] | - | 92 | 126 | 50 h @10 mA/cm2 | |
| Ni3S2 [130] | 1 M KOH | 37.7 | - | 24 h @10 mA/cm2 | |
| NiFeSe/CFP [131] | 1 M KOH | 186 | 52 | - | |
| CozNiySx@PPy/CFP-6 (A-6) [132] | 1 M KOH | 185 | 78 | 100 h @10 mA/cm2 | |
| Co7Se8 [133] | 1 M KOH | 260 | 32.6 | 12 h @10 mA/cm2 | |
| MoS2 [134] | - | 190 | 54 | ||
| NiCo2S4 nanosheet [135] | 1 M KOH | 150 | 82 | - | |
| (Ni-Fe)S-x/NiFe(OH)(y) [136] | 1 M KOH | 1.46 V (cell) | - | 50 h @100 mA/cm2 | |
| NiP/NiFeP/C [126] | 1 M KOH | 1.53 V (cell) | - | 20 h @100 mA/cm2 | |
| Co0.75Ni0.125Mn0.125P [137] | - | 137 | 49 | 12.5 @10 mA/cm2 | |
| CoWP-CA/KB [138] | - | 111 | 58 | 60 h @10 mA/cm2 | |
| Multi-metal phosphide [139] | 0.5 M H2SO4 | 220 | - | 100 h @100 mA/cm2 | |
| Cobalt phosphide nanoparticles [140] | 0.5 M H2SO4 | 98 | 74 | - | |
| MnRuPOGO-500 [141] | 1 M KOH | 109 | 38.55 | 60 h @ 10 mA/cm2 | |
| Co-CoxC [142] | - | 78 | 87 | 1 h @10 mA/cm2 | |
| Co-N-C [143] | 0.5 M H2SO4 | 145 | - | - | |
| Mo2C-MoP hybrid nanodots [144] | 1 M KOH | 147 | 64 | 120 @ 10 mA/cm2 | |
| N-Mo2C/PC [145] | 0.5 M H2SO4 | 109 | - | - | |
| CoP3/CoMoP/TiO2-x@Ti [146] | - | 143 | 61 | 48 h @ 10 mA/cm2 | |
| NiMo2C@C [147] | 1 M KOH | 181 | - | 10 h @ 10 mA/cm2 |
| MXene | Structure | Electrolyte | Overpotential (mV) @ 10 mA/cm2 | Tafel Slope (mV/dec) |
|---|---|---|---|---|
| Ti3C2Tx [156,157] | Multilayer | 1 M KOH | >600 | - |
| Ti3C2Tx [158] | Few layer | 1 M KOH | >500 | >100 |
| Ti3C2Tx [152] | Few-layer | 0.5 M H2SO4 | 609 | 124 |
| Ti3C2Ox [159] | Few-layer | 0.5 M H2SO4 | 190 | 60.7 |
| Ti3C2(OH)x [159] | Few-layer | 0.5 M H2SO4 | 217 | 88.5 |
| Mo2CTx [152] | Few-layer | 0.5 M H2SO4 | 283 | 82 |
| Mo2CTx [160] | Few-layer | 1 M KOH | 300 | 110 |
| Mo2CTx [161] | Multilayer | 1 M KOH | 280 | 118 |
| MXenes | |||||||
|---|---|---|---|---|---|---|---|
| Catalyst | MXene Type | MXene Morphology | Electrolyte | BET Surface Area (m2/g) | Overpotential, η (mV) @ 10 mA/cm2 | Tafel Slope, b (mV/dec) | Durability |
| Multilayer MXene | |||||||
| Co-MoS2/Mo2CTx [161] | Mo2CTx | Multilayer | 1 M KOH | - | 112 | 82 | LSV loss (1000 cycles): negligible. CA @ 10 mA/cm2, 18 h: slight decline in current density. |
| S-ML-Nb4C3Tx [177] | Nb4C3Tx | Multilayer | 1 M KOH | 45.15 (S-ML-Nb4C3Tx) 31.33 (ML-Nb4C3Tx) | 118 | 104 | LSV loss (2000 cycles): slight increase in overpotential at 10 mA/cm2. CA @ 10 mA/cm2, 24 h: steady current density. |
| NiS2/T-MXene [188] | Ti3C2Tx | Multilayer | 1 M KOH | 28.1 (NiS2/Ti-MXene) 4.7 (pristine multilayer Ti-MXene) | - | 100 | - |
| MoS2/Ti3C2 [176] | Ti3C2 | Multilayer | 0.5 M H2SO4 | - | 280 | 68 | CP @ 10 mA/cm2, 35 h: slight increase in potential within 22 h. Potential decrease until 35 h. |
| N-MXene-35 [175] | Ti3C2Tx | Multilayer | 0.5 M H2SO4 | 23.6 (N-MXene-35) 12.3 (pristine multilayer Ti3C2Tx) | 162 | 69 | LSV loss (24 h CV cycling): slight increase in overpotential. CP @ 10 mA/cm2, 35 h: steady potential. |
| LiF + HCl-etched Ti3C2Tx [157] | Ti3C2Tx | Multilayer | 0.5 M H2SO4 | - | 538 | 128 | - |
| Mo2CTx [157] | Mo2CTx | Multilayer | 0.5 M H2SO4 | - | 189 | 75 | LSV loss (1000 cycles): negligible |
| Ti2CTx [152] | Ti2CTx | Multilayer | 0.5 M H2SO4 | - | 609 | CP @ 10 mA/cm2, 120 h: steady potential. | |
| Few-layer MXene | |||||||
| NiS2/V-MXene [188] | V2CTx | Few-layer | 1 M KOH | 44.4 (NiS2/V-MXene) 7.5 (pristine V-MXene) | 179 | 85 | CP @ 10 mA/cm2, 96 h: Slight potential increase. Post-CP LSV test also shows overpotential increase. |
| Ti3C2Tx@mNiCoP [179] | Ti3C2Tx | Few-layer | 1 M KOH | 143.5 (Ti3C2@mNiCoP) | 127 | 103 | CA @ 10 mA/cm2, 10 h: <5% current density loss. |
| NiCo@Nb-doped Ti3C2Tx [180] | Ti3C2Tx | Few-layer | 1 M KOH | - | 43.4 | 116 | CP @ 10 mA/cm2, 50 h: steady potential |
| Ti3C2Ox [159] | Ti3C2Tx | Few-layer | 0.5 M H2SO4 | - | 190 | 60.7 | LSV loss (2000 cycles): slight overpotential increase. |
| Pt SA-Mo2TiC2Tx [189] | Mo2TiC2Tx | Few-layer | 0.5 M H2SO4 | - | 30 | 30 | LSV loss (10,000 cycles): negligible. CA @ 100 mA/cm2, 100 h: slight decline in current density. |
| NiSe2/Ti3C2Tx [190] | Ti3C2Tx | Few-layer | 0.5 M H2SO4 | - | 200 | 37.7 | LSV loss (2000 cycles): negligible. CA @ 10 mA/cm2, 10 h: slight decline in current density. |
| S-M-5Pt [178] | Ti3C2Tx | Few-layer | 0.5 M H2SO4 | - | 62 | 78 | CP @ 10 mA/cm2, 800 h: slight potential loss. |
| Porous MXenes | |||||||
| P-MoO2 FCL/MXene/NF [191] | Ti3C2Tx | Multilayer on porous scaffold (NF) | 1 M KOH | 5.96 (mesopores of P-MoO2 FCL/MXene/NF) | 179 | 40.44 | CA @ 10 mA/cm2, 42 h: 99.73% maintained current density. |
| NiFe-LDH/MXene/NF [181] | Ti3C2Tx | Few-layer on porous scaffold (NF) | 1 M KOH | - | 132 | 70 | CP @ 10 mA/cm2, 280 h: steady potential. |
| Pt3Ni/Ti3C2Tx [184] | Ti3C2Tx | In-plane porous | 1 M KOH and 0.5 M H2SO4 | - | 46.8 (1 M KOH), 30 (0.5 M H2SO4) | 44.15 (1 M KOH), 26.51 (0.5 M H2SO4) | CA @ 10 mA/cm2, 10 h: steady current density (1 M KOH and 0.5 M H2SO4). |
| IrCo@ac-Ti3C2 [182] | Ti3C2 | In-plane porous | 1 M KOH | 175 (IrCo@ac-Ti3C2) 189.1 (ac-Ti3C2) 6.5 (pristine multilayer Ti3C2) 19.6 (delaminated Ti3C2) | 135 | 56 | CA @ 10 mA/cm2, 30 h: 98% maintained current density. |
| 3D MX/NG [187] | Ti3C2Tx | MXene-graphene porous network | 0.5 M H2SO4 | 148.2 (MX/NG) 12.2 (pristine few-layer Ti3C2Tx) | 354 | 84 | LSV loss (2000 cycles): negligible. CA @ 10 mA/cm2, 5000 s: slight decline in current density |
| 3D MX/CN/RGO [158] | Ti3C2Tx | MXene-g-C3N4-RGO porous network | 0.5 M H2SO4 | 345.6 (3D MX/CN/RGO) 11.2 (GO) 4 (g-C3N4) 12.2 (pristine few-layer Ti3C2Tx) | 38 | 76 | LSV loss (2000 cycles): negligible. CA @ 10 mA/cm2, 20,000 s: steady current density. |
| NiFe LDH/MX-rGO [192] | Ti3C2Tx | MXene-rGO porous network | 0.5 M H2SO4 | 254.7 (NiFe LDH/MX-rGO) 116.4 (bare LDH) 12.2 (pristine few-layer Ti3C2Tx) | 326 | 100 | CA@ 20 mA/cm2, 40 h: steady current density. |
| Pt-Porous Ti3C2Tx/Ti3AlC2 monolith [193] | Ti3C2Tx | In-plane porous | 0.5 M H2SO4 | - | 37 | 89 | CP @ 10 mA/cm2, 10 h: slight potential increase. |
| P-Ti3C2Tx@NiCoP [183] | Ti3C2Tx | In-plane porous | 1 M KOH and 0.5 M H2SO4 | 80.09 (P-Ti3C2Tx@NiCoP) 9.06 (pristine multilayer Ti3C2Tx) 30.97 (porous Ti3C2Tx) | 101 (1 M KOH), 115 (0.5 M H2SO4) | 69 (1 M KOH), 76 (0.5 M H2SO4) | CA @ 10 mA/cm2, 1 M KOH and 0.5 M H2SO4, 60 h: slight decline in current density. |
| IrSA-2NS-Ti3C2Tx [185] | Ti3C2Tx | Self-assembled porous framework | 1 M KOH and 0.5 M H2SO4 | 107 (IrSA-2NS-Ti3C2Tx) 24.795 (pristine few-layer Ti3C2Tx) | 40.9 (1 M KOH), 57.7 (0.5 M H2SO4) | 50.5 (1 M KOH), 25 (0.5 M H2SO4) | LSV loss (10,000 cycles, 0.5 M H2SO4): slight increase overpotential @ 10 mA/cm2. |
| Pt SA-PNPM [186] | Ti3C2Tx | Self-assembled porous framework | 1 M KOH and 0.5 M H2SO4 | 121 (Pt SA-PNPM) 38.79 (freeze-dried obtained pristine Ti3C2Tx) | 36 (1 M KOH). 35 (0.5 M H2SO4) | 33 (1 M KOH), 31 (0.5 M H2SO4) | LSV loss (5000 cycles, 0.5 M H2SO4): negligible. CA @ 10 mA/cm2, 60 h: negligible loss. |
| Special structures MXene | |||||||
| Pt/Crumpled MXene [171] | Ti3C2Tx | Crumpled | 0.5 M H2SO4 | 7.2 (crumpled spray-dried Ti3C2Tx) 1.9 (pristine freeze-dried Ti3C2Tx) | 34 | 29.7 | CP @ 10 mA/cm2, 10,000 s: 9 mV potential drop. |
| MoS2/Ti3C2Tx nanoroll [110] | Ti3C2Tx | Nanoroll | 0.5 M H2SO4 | - | 152 | 70 | LSV loss (3000 cycles): negligible. CA @ 10 mA/cm2, 12 h: steady current density. |
| Synthesis method | Morphology | Advantages | Limitations |
|---|---|---|---|
| HF-etching of MAX phase to MXene [194] | Multi-layered MXenes |
|
|
| LiF + HCl-etching of MAX phase to MXene [22,194] | Few-layered MXenes, multi-layered MXenes |
|
|
| Microwave-assisted LiF + HCl-etching of MAX phase to MXene [197] | Few-layered MXenes |
|
|
| Coating MXene on porous scaffold (i.e.: NF) through dip-coating [181,201] | MXene on porous scaffold |
|
|
| Chemical etching of MXene in the presence of acid and H2O2 [170,182,183] | In-plane porous |
|
|
| Poring agents/templating [170,184] | In-plane porous |
|
|
| Gelation-pyrolysis for self-assembled porous structure [170,205] | Self-assembled porous structure |
|
|
| Co-assembly for multicomponent porous structure [158,192,205] | Multicomponent self-assembled porous structure |
|
|
| Spray drying of colloidal few-layer MXene [171] | Crumpled MXene |
|
|
| Rapid freeze drying [110,204] | Rolled MXene |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raja Sulaiman, R.R.; Hanan, A.; Wong, W.Y.; Mohamad Yunus, R.; Shyuan Loh, K.; Walvekar, R.; Chaudhary, V.; Khalid, M. Structurally Modified MXenes-Based Catalysts for Application in Hydrogen Evolution Reaction: A Review. Catalysts 2022, 12, 1576. https://doi.org/10.3390/catal12121576
Raja Sulaiman RR, Hanan A, Wong WY, Mohamad Yunus R, Shyuan Loh K, Walvekar R, Chaudhary V, Khalid M. Structurally Modified MXenes-Based Catalysts for Application in Hydrogen Evolution Reaction: A Review. Catalysts. 2022; 12(12):1576. https://doi.org/10.3390/catal12121576
Chicago/Turabian StyleRaja Sulaiman, Raja Rafidah, Abdul Hanan, Wai Yin Wong, Rozan Mohamad Yunus, Kee Shyuan Loh, Rashmi Walvekar, Vishal Chaudhary, and Mohammad Khalid. 2022. "Structurally Modified MXenes-Based Catalysts for Application in Hydrogen Evolution Reaction: A Review" Catalysts 12, no. 12: 1576. https://doi.org/10.3390/catal12121576
APA StyleRaja Sulaiman, R. R., Hanan, A., Wong, W. Y., Mohamad Yunus, R., Shyuan Loh, K., Walvekar, R., Chaudhary, V., & Khalid, M. (2022). Structurally Modified MXenes-Based Catalysts for Application in Hydrogen Evolution Reaction: A Review. Catalysts, 12(12), 1576. https://doi.org/10.3390/catal12121576









