High Surface Area ZnO-Nanorods Catalyze the Clean Thermal Methane Oxidation to CO2
Abstract
:1. Introduction
2. Results
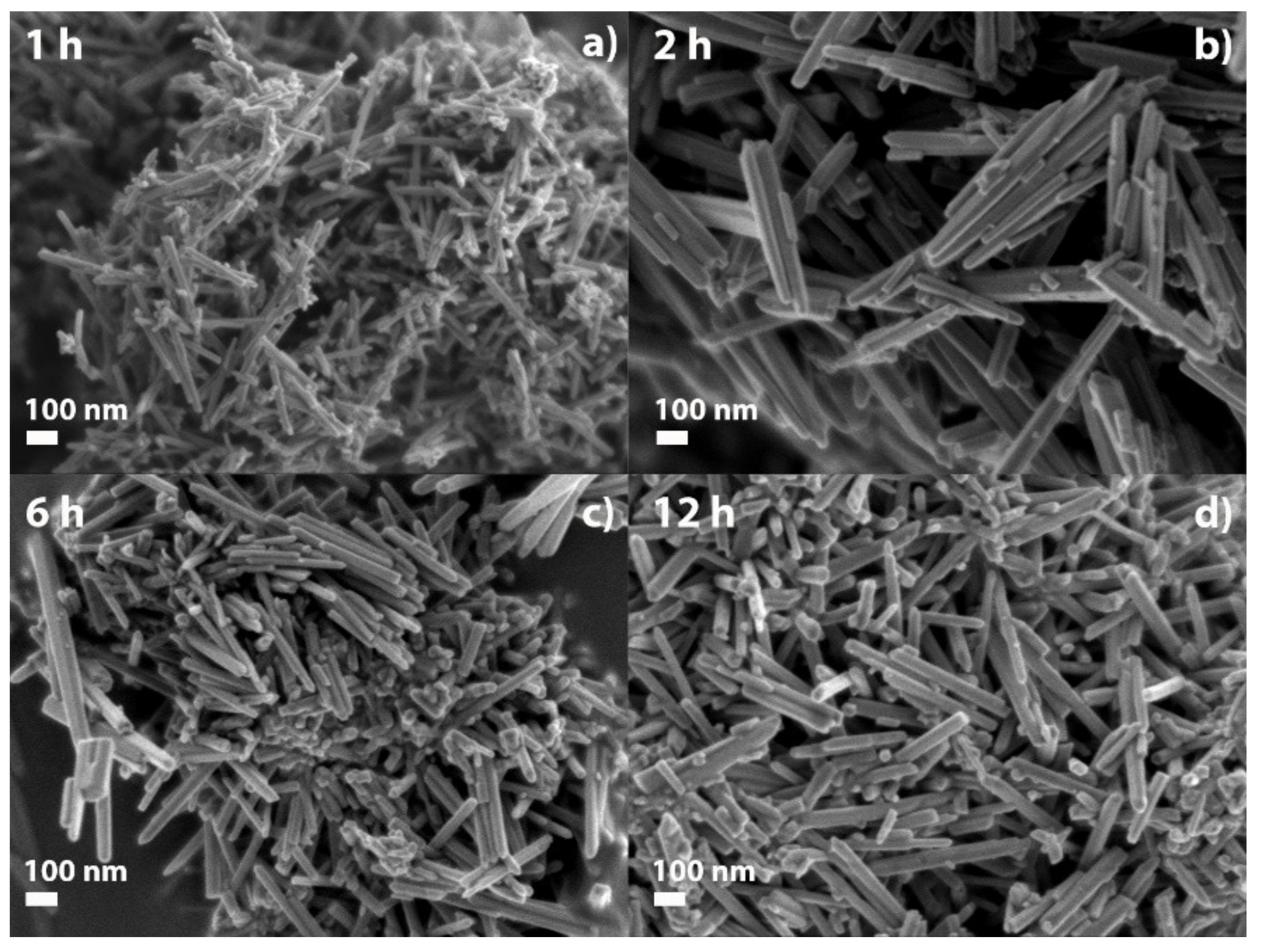
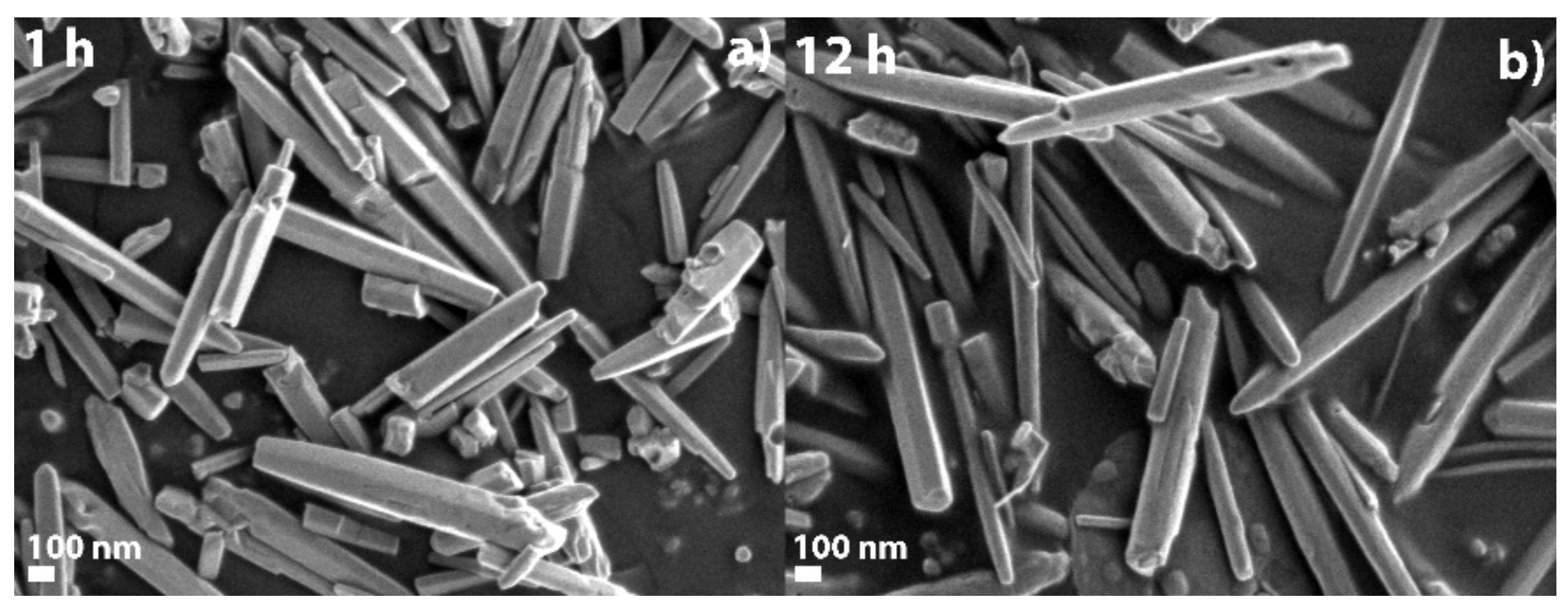
2.1. SEM Characterization
2.2. BET Characterization
2.3. X-ray Diffraction Characterization
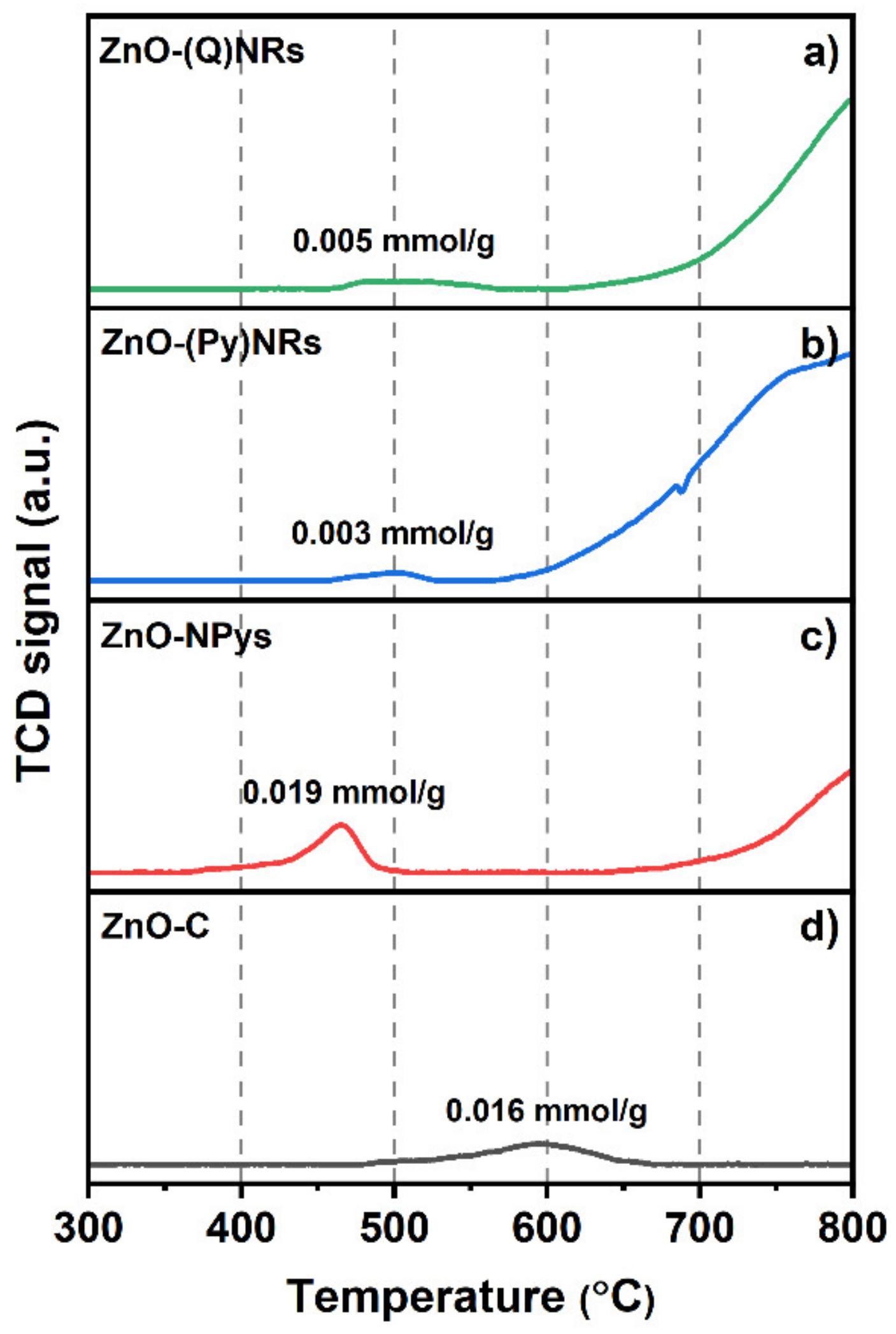
2.4. H2-TPR Characterization
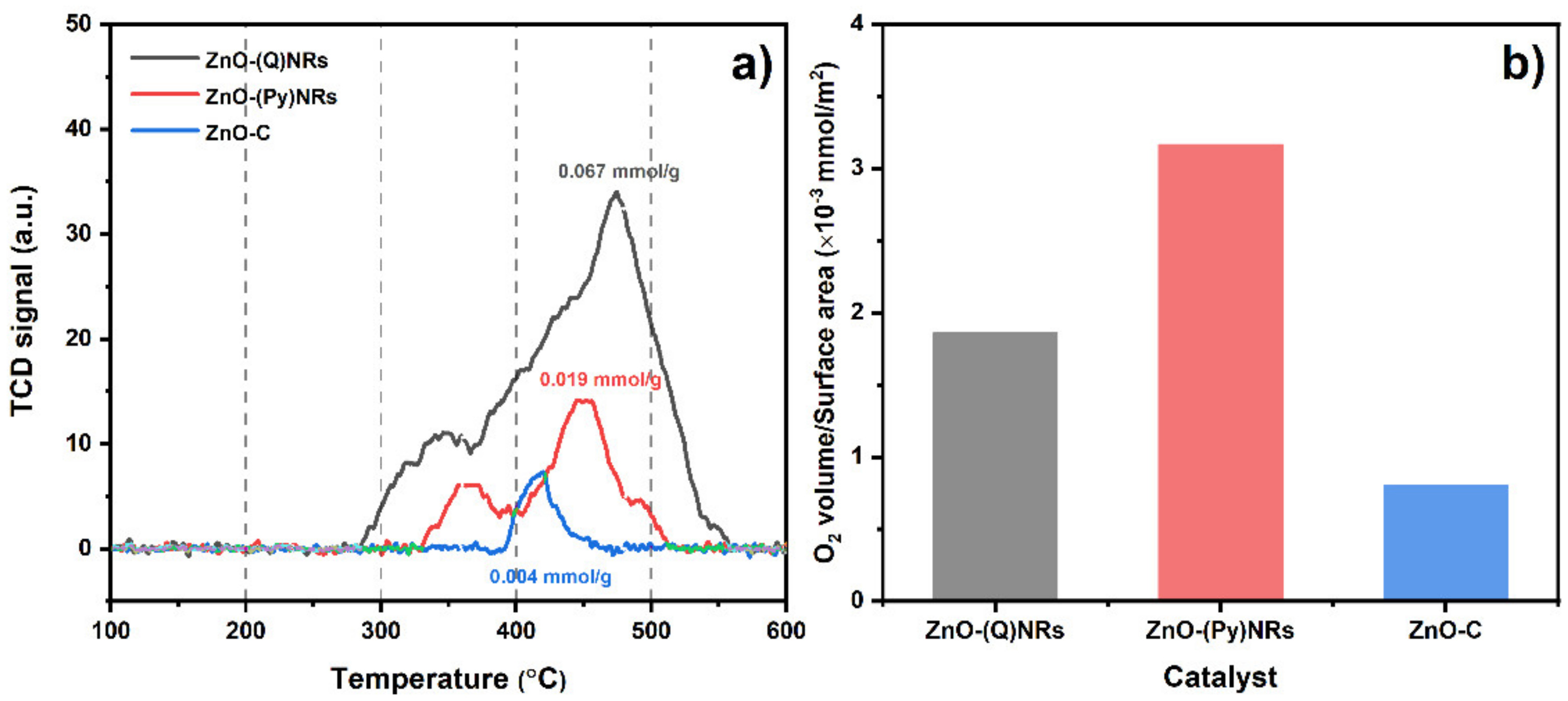
2.5. O2-TPD Characterization
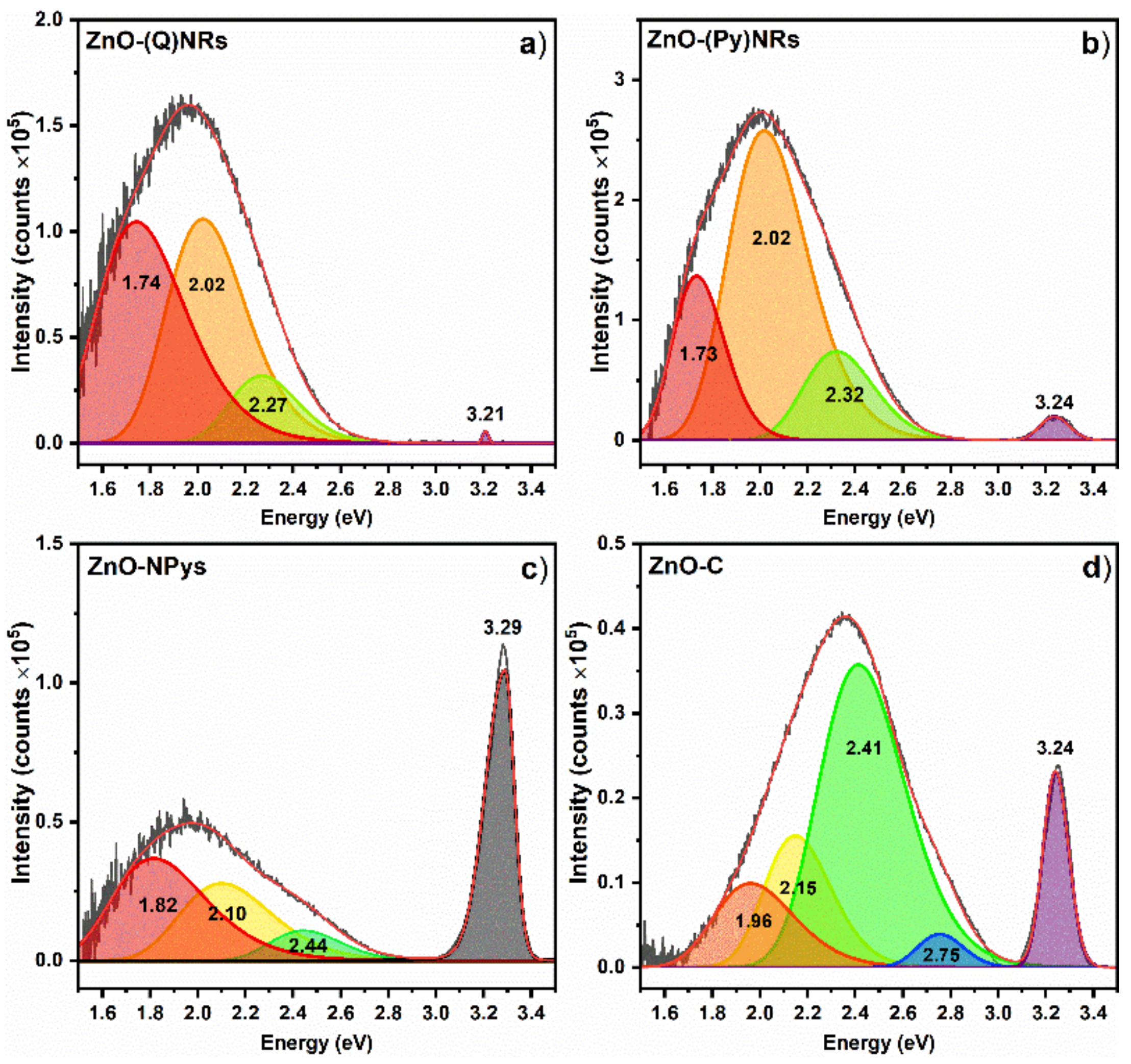
2.6. Photoluminescence Spectroscopy Characterization
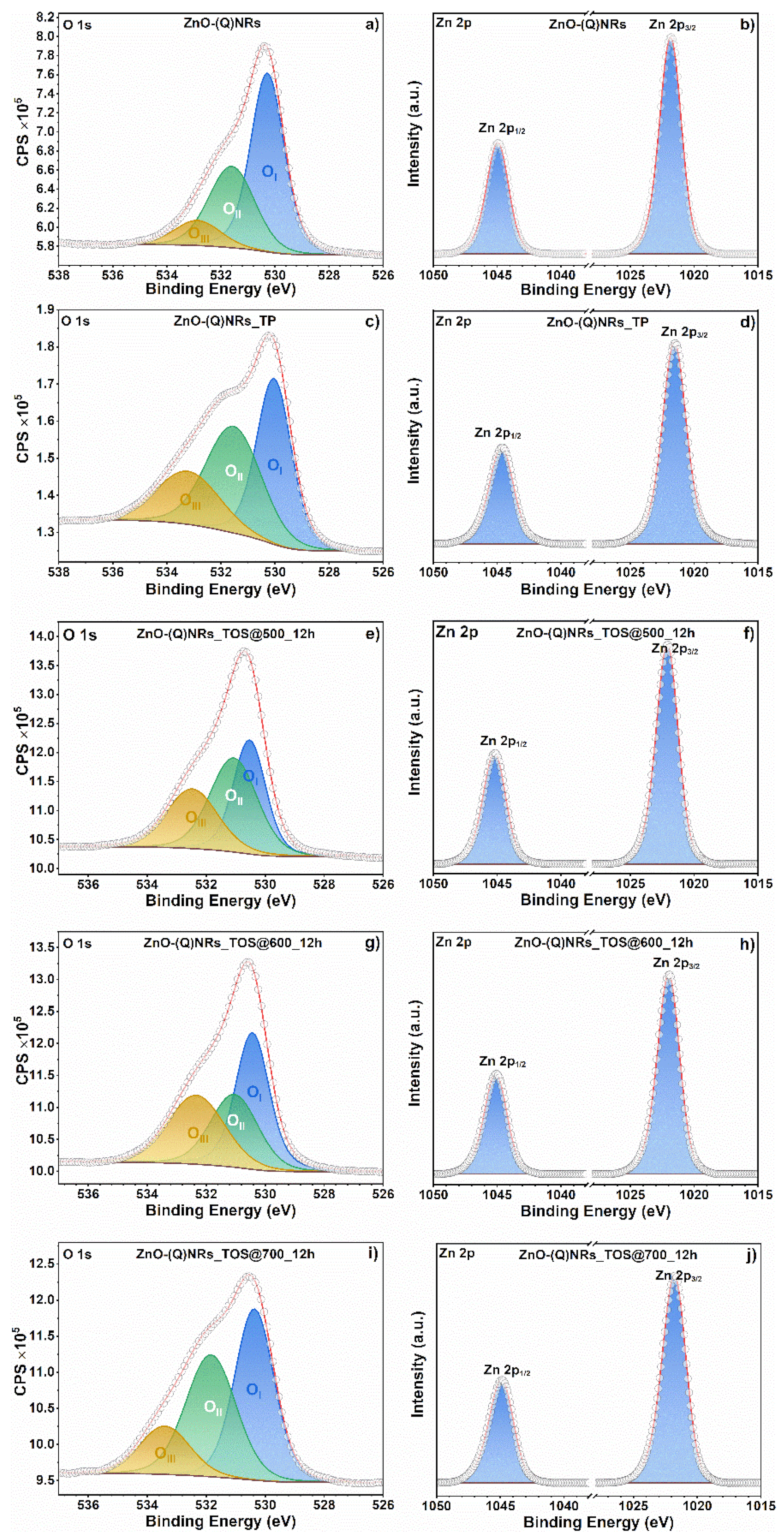
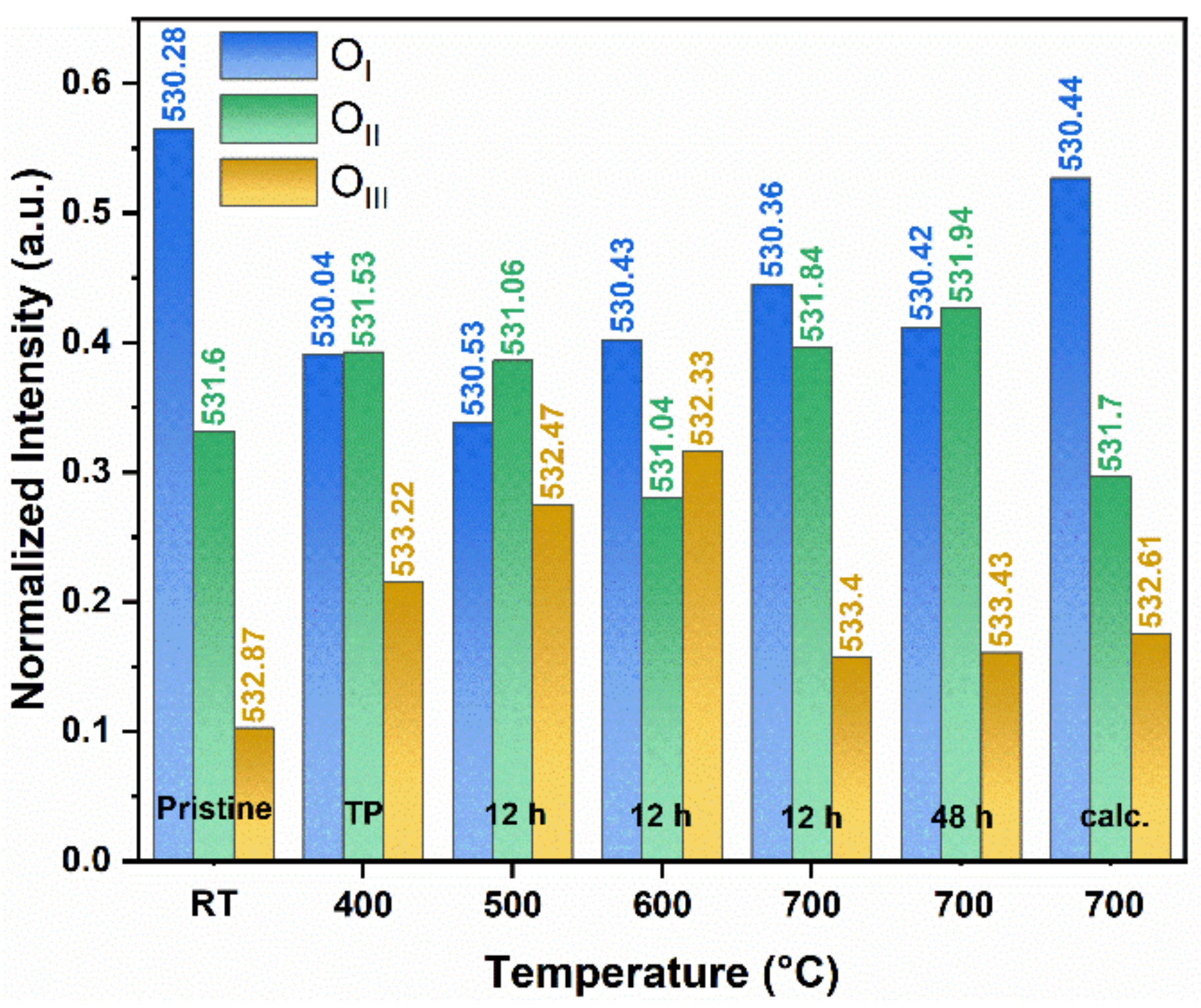
2.7. XPS Investigation
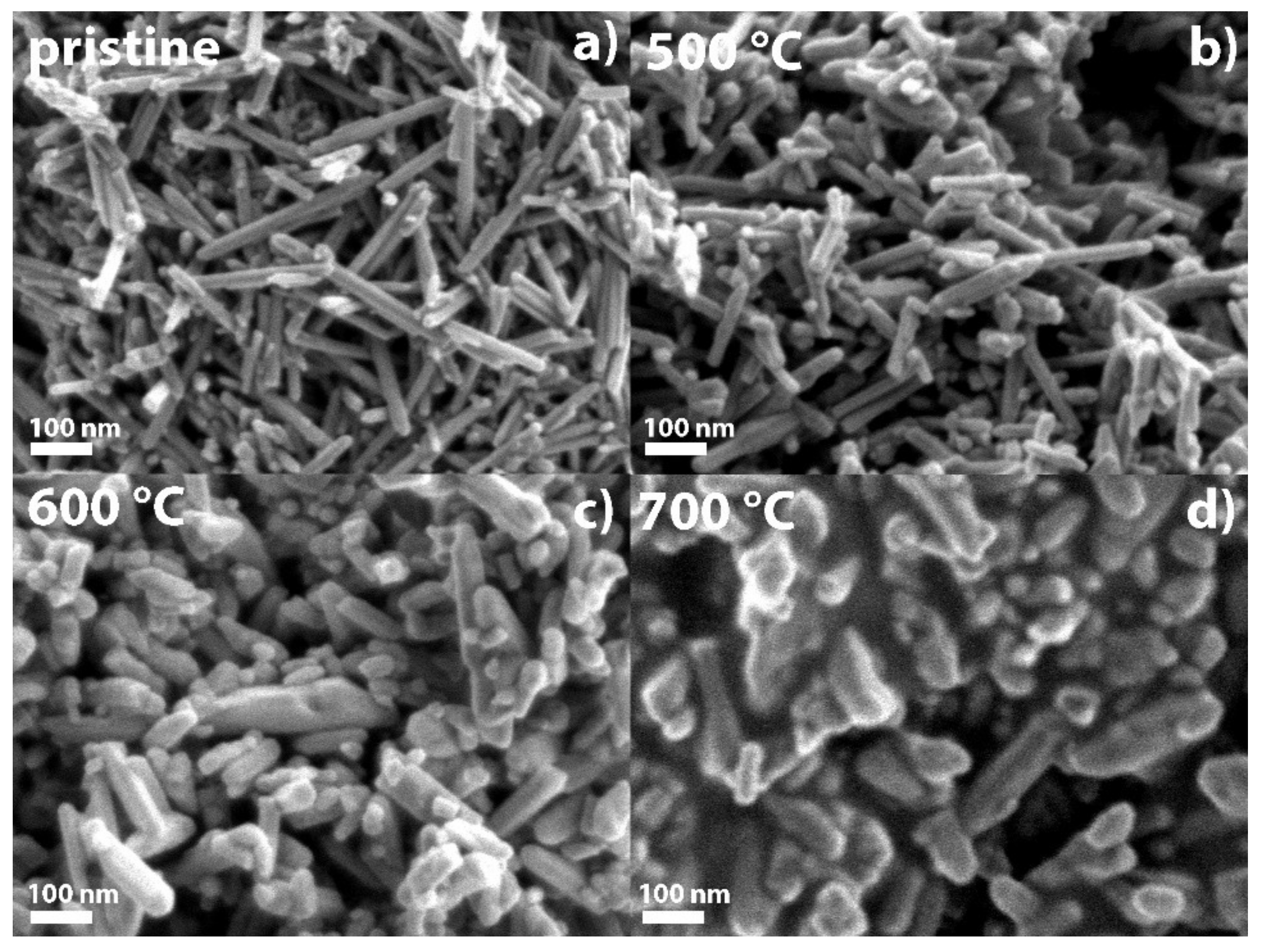
2.8. Morphological Characterization of Spent Catalysts
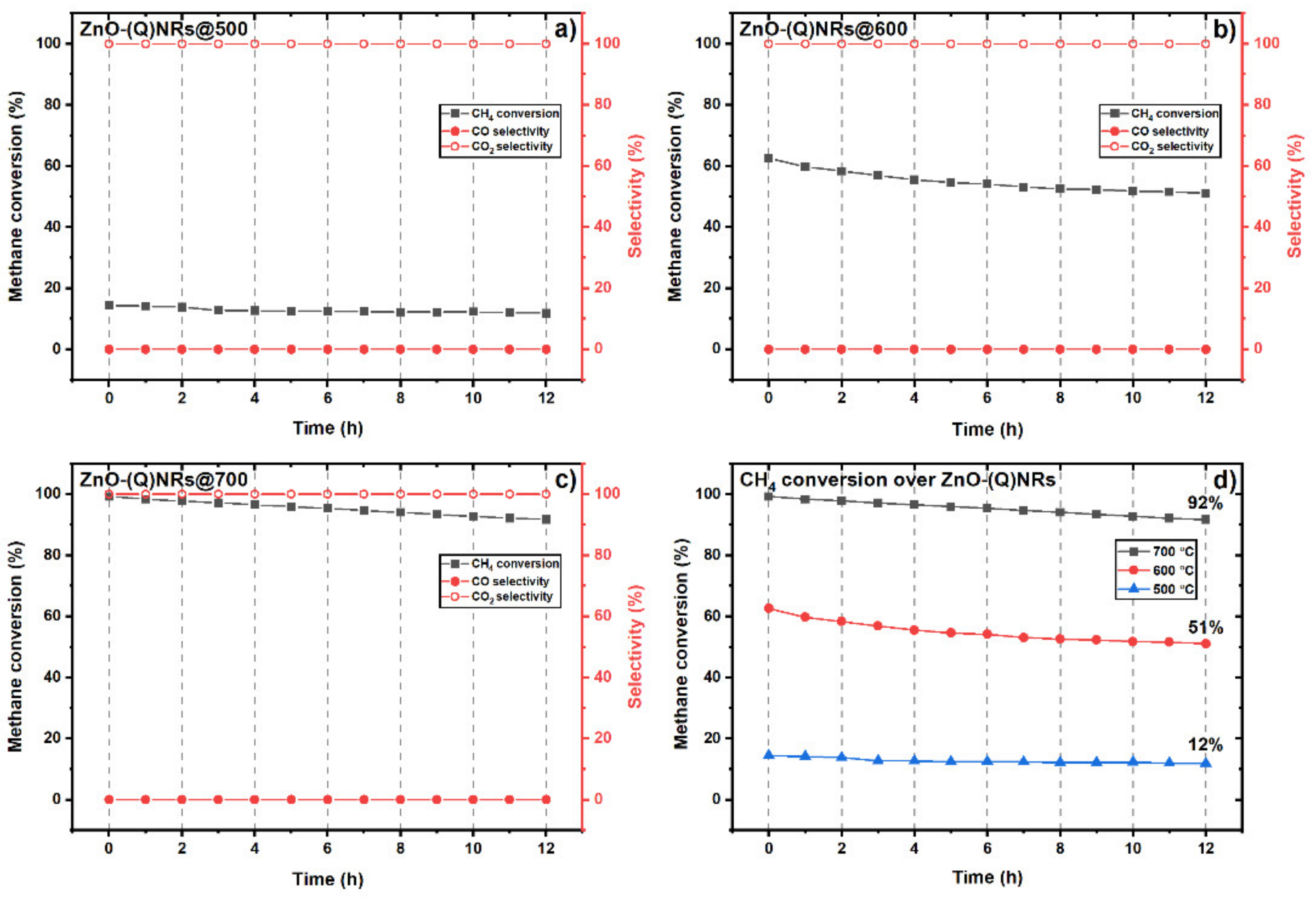
2.9. Catalytic Methane Combustion
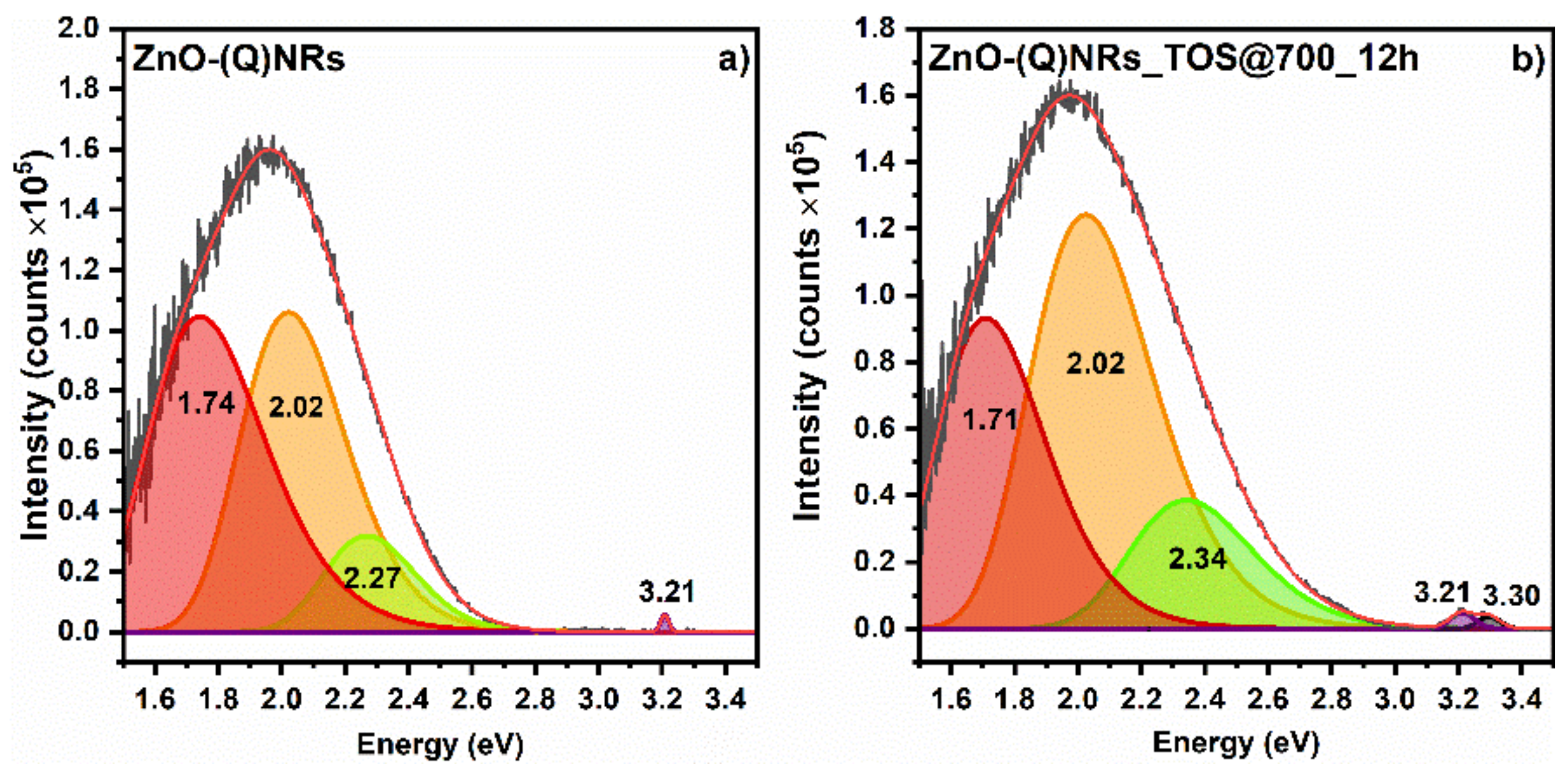
3. PL and XPS Investigation of Spent ZnO-(Q)NRs
4. Materials and Methods
4.1. Materials
4.2. Catalysts Preparation
4.2.1. Synthesis of ZnO Quantum Dots (ZnO-QDs)
4.2.2. Synthesis of ZnO Nanopyramids (ZnO-NPys)
4.2.3. Synthesis of ZnO Nanorods
Synthesis of ZnO Nanorods from ZnO-QDs (ZnO-(Q)NRs)
Synthesis of ZnO Nanorods from ZnO-NPys (ZnO-(Py)NRs)
4.3. Catalyst Characterizations
4.3.1. Scanning Electron Microscopy (SEM)
4.3.2. Powder X-ray Diffraction (PXRD)
4.3.3. X-ray Photoelectron Spectroscopy (XPS)
4.3.4. Photoluminescence Measurements (PL)
4.3.5. BET Surface Area Determination
4.3.6. H2-Temperature Programmed Reduction (H2-TPR)
4.3.7. O2-Temperature Programmed Desorption (O2-TPD)
4.4. Catalytic Activity Tests
4.4.1. Catalytic Activity Test Setup
4.4.2. Light-Off Curve Determination with Temperature Program
4.4.3. Time on Stream Test (TOS)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Chen, L.; Bao, Y.; Zhang, Y.; Wang, J.; Fu, M.; Wu, J.; Ye, D. The Applications of Morphology Controlled ZnO in Catalysis. Catalysts 2016, 6, 188. [Google Scholar] [CrossRef] [Green Version]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Teh, S.J.; Lai, C.W. Photocatalytic Water Oxidation on ZnO: A Review. Catalysts 2017, 7, 93. [Google Scholar] [CrossRef] [Green Version]
- Goktas, S.; Goktas, A. A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: A review. J. Alloys Compd. 2021, 863, 158734. [Google Scholar] [CrossRef]
- Pandey, R.K.; Dutta, J.; Brahma, S.; Rao, B.; Liu, C.-P. Review on ZnO-based piezotronics and piezoelectric nanogenerators: Aspects of piezopotential and screening effect. J. Phys. Mater. 2021, 4, 44011. [Google Scholar] [CrossRef]
- Puspasari, V.; Ridhova, A.; Hermawan, A.; Amal, M.I.; Khan, M.M. ZnO-based antimicrobial coatings for biomedical applications. Bioprocess Biosyst. Eng. 2022, 45, 1421–1445. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Rahman, F. Zinc oxide light-emitting diodes: A review. Opt. Eng. 2019, 58, 10901. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Ha, M.; Song, I.; Lee, J.H.; Won, Y.; Lim, S.; Ko, H.; Oh, J.H. High-Performance Hybrid Photovoltaics with Efficient Interfacial Contacts between Vertically Aligned ZnO Nanowire Arrays and Organic Semiconductors. ACS Omega 2019, 4, 9996–10002. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.; Marsudi, M.A.; Amal, M.I.; Ananda, M.B.; Stephanie, R.; Ardy, H.; Diguna, L.J. ZnO nanostructured materials for emerging solar cell applications. RSC Adv. 2020, 10, 42838–42859. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Chen, J.; Shi, Z.; Fang, X. Self-powered UV photodetectors based on ZnO nanomaterials. Appl. Phys. Rev. 2021, 8, 31315. [Google Scholar] [CrossRef]
- Chen, H.; Cui, H.; Lv, Y.; Liu, P.; Hao, F.; Xiong, W.; Luo, H.a. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts: Effects of ZnO morphology and oxygen vacancy. Fuel 2022, 314, 123035. [Google Scholar] [CrossRef]
- Li, D.; Xu, F.; Tang, X.; Dai, S.; Pu, T.; Liu, X.; Tian, P.; Xuan, F.; Xu, Z.; Wachs, I.E.; et al. Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol. Nat. Catal. 2022, 5, 99–108. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Bhonsle, A.K.; Trivedi, J.; Bangwal, D.P.; Singh, L.P.; Atray, N. Synthesis and characterization of coal fly ash supported zinc oxide catalyst for biodiesel production using used cooking oil as feed. Renew. Energy 2021, 170, 302–314. [Google Scholar] [CrossRef]
- Del Gobbo, S.; Poolwong, J.; D’Elia, V.; Ogawa, M. Simultaneous Controlled Seeded-Growth and Doping of ZnO Nanorods with Aluminum and Cerium: Feasibility Assessment and Effect on Photocatalytic Activity. Cryst. Growth Des. 2020, 20, 5508–5525. [Google Scholar] [CrossRef]
- Sanakousar, F.M.; Vidyasagar, C.C.; Jiménez-Pérez, V.M.; Prakash, K. Recent progress on visible-light-driven metal and non-metal doped ZnO nanostructures for photocatalytic degradation of organic pollutants. Mater. Sci. Semicond. Process. 2022, 140, 106390. [Google Scholar] [CrossRef]
- Sofianos, V.M.; Lee, J.; Silvester, D.S.; Samanta, P.K.; Paskevicius, M.; English, N.J.; Buckley, C.E. Diverse morphologies of zinc oxide nanoparticles and their electrocatalytic performance in hydrogen production. J. Energy Chem. 2021, 56, 162–170. [Google Scholar] [CrossRef]
- Yu, D.; Jia, Y.; Yang, Z.; Zhang, H.; Zhao, J.; Zhao, Y.; Weng, B.; Dai, W.; Li, Z.; Wang, P.; et al. Solar Photocatalytic Oxidation of Methane to Methanol with Water over RuOx/ZnO/CeO2 Nanorods. ACS Sustain. Chem. Eng. 2021, 10, 16–22. [Google Scholar] [CrossRef]
- Wolf, J.; Sandstead, H.H.; Rink, L. Zinc. In Handbook on the Toxicology of Metals; Volume II: Specific Metals; Academic Press: Cambridge, MA, USA, 2022; pp. 963–984. [Google Scholar]
- Hessien, M. Recent progress in zinc oxide nanomaterials and nanocomposites: From synthesis to applications. Ceram. Int. 2022, 48, 22609–22628. [Google Scholar] [CrossRef]
- Primc, G.; Brenčič, K.; Mozetič, M.; Gorjanc, M. Recent Advances in the Plasma-Assisted Synthesis of Zinc Oxide Nanoparticles. Nanomaterials 2021, 11, 1191. [Google Scholar] [CrossRef] [PubMed]
- Del Gobbo, S.; Poolwong, J.; D’Elia, V. In-Suspension Growth of ZnO Nanorods with Tunable Length and Diameter Using Polymorphic Seeds. Cryst. Growth Des. 2019, 19, 6792–6800. [Google Scholar] [CrossRef]
- Aspoukeh, P.K.; Barzinjy, A.A.; Hamad, S.M. Synthesis, properties and uses of ZnO nanorods: A mini review. Int. Nano Lett. 2021, 12, 153–168. [Google Scholar] [CrossRef]
- Saputra, I.S.; Apriandanu, D.O.B.; Yulizar, Y.; Maryanti, E.; Permana, Y.N.; Suhartati, S.; Sudirman. A facile preparation of ZnO/Au nano-needles: Optical, morphological and structural properties. Opt. Mater. 2021, 121, 111628. [Google Scholar] [CrossRef]
- Noh, Y.; Jeong, H.; Lee, D. Enhanced ultraviolet photodetector using zinc oxide nanowires with intense pulsed light post-treatment. J. Alloys Compd. 2021, 871, 159537. [Google Scholar] [CrossRef]
- Ali, M.; Tit, N.; Yamani, Z.H. Role of defects and dopants in zinc oxide nanotubes for gas sensing and energy storage applications. Int. J. Energy Res. 2020, 44, 10926–10936. [Google Scholar] [CrossRef]
- Naderi, S.; Javaheri, S.; Shahrokhi, M.; Nia, B.A.; Shahmoradi, S. Optical properties of zigzag and armchair ZnO nanoribbons. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 124, 114218. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, X. Ultra-High Sensitivity Zinc Oxide Nanocombs for On-Chip Room Temperature Carbon Monoxide Sensing. Sensors 2015, 15, 8919–8930. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Xu, A.-W.; Deng, B.; Antonietti, M.; Cölfen, H. Polymer-Controlled Crystallization of Zinc Oxide Hexagonal Nanorings and Disks. J. Phys. Chem. B 2006, 110, 2988–2993. [Google Scholar] [CrossRef] [PubMed]
- Ha, L.P.P.; Vinh, T.H.T.; Thuy, N.T.B.; Thi, C.M.; Viet, P.V. Visible-light-driven photocatalysis of anisotropic silver nanoparticles decorated on ZnO nanorods: Synthesis and characterizations. J. Environ. Chem. Eng. 2021, 9, 105103. [Google Scholar] [CrossRef]
- Moon, D.-B.; Bag, A.; Lee, H.-B.; Meeseepong, M.; Lee, D.-H.; Lee, N.-E. A stretchable, room-temperature operable, chemiresistive gas sensor using nanohybrids of reduced graphene oxide and zinc oxide nanorods. Sens. Actuators B Chem. 2021, 345, 130373. [Google Scholar] [CrossRef]
- Patzke, G.R.; Krumeich, F.; Nesper, R. Oxidic Nanotubes and Nanorods—Anisotropic Modules for a Future Nanotechnology. Angew. Chem. Int. Ed. 2002, 41, 2446–2461. [Google Scholar] [CrossRef]
- McLaren, A.; Valdes-Solis, T.; Li, G.; Tsang, S.C. Shape and Size Effects of ZnO Nanocrystals on Photocatalytic Activity. J. Am. Chem. Soc. 2009, 131, 12540–12541. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Jin, W.; Duan, X.; Ma, X.; Han, H.; Zhang, Z.; Yu, J.; Wu, Y. From the absolute surface energy to the stabilization mechanism of high index polar surface in wurtzite structure: The case of ZnO. J. Alloys Compd. 2019, 772, 482–488. [Google Scholar] [CrossRef]
- Ayoub, I.; Kumar, V.; Abolhassani, R.; Sehgal, R.; Sharma, V.; Sehgal, R.; Swart, H.C.; Mishra, Y.K. Advances in ZnO: Manipulation of defects for enhancing their technological potentials. Nanotechnol. Rev. 2022, 11, 575–619. [Google Scholar] [CrossRef]
- Bernard, P.; Stelmachowski, P.; Broś, P.; Makowski, W.; Kotarba, A. Demonstration of the Influence of Specific Surface Area on Reaction Rate in Heterogeneous Catalysis. J. Chem. Educ. 2021, 98, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Poolwong, J.; Del Gobbo, S.; D’Elia, V. Transesterification of dimethyl carbonate with glycerol by perovskite-based mixed metal oxide nanoparticles for the atom-efficient production of glycerol carbonate. J. Ind. Eng. Chem. 2021, 104, 43–60. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C. Mxene enhanced the photocatalytic activity of ZnO nanorods under visible light. Mater. Lett. 2020, 261, 127127. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, Y.; Wang, L.; Chen, X.; Lin, W.; Wang, X. Remarkable oxygen evolution by Co-doped ZnO nanorods and visible light. Appl. Catal. B Environ. 2021, 296, 120369. [Google Scholar] [CrossRef]
- Wang, H.; Dai, M.; Li, Y.; Bai, J.; Liu, Y.; Li, Y.; Wang, C.; Liu, F.; Lu, G. The influence of different ZnO nanostructures on NO2 sensing performance. Sens. Actuators B Chem. 2021, 329, 129145. [Google Scholar] [CrossRef]
- Verma, S.; Tirumala Rao, B.; Singh, R.; Kaul, R. Photocatalytic degradation kinetics of cationic and anionic dyes using Au–ZnO nanorods: Role of pH for selective and simultaneous degradation of binary dye mixtures. Ceram. Int. 2021, 47, 34751–34764. [Google Scholar] [CrossRef]
- Lim, S.K.; Hwang, S.-H.; Kim, S.; Park, H. Preparation of ZnO nanorods by microemulsion synthesis and their application as a CO gas sensor. Sens. Actuators B Chem. 2011, 160, 94–98. [Google Scholar] [CrossRef]
- Chen, M.-H.; Lu, Q.-Y.; Li, Y.-M.; Chu, M.-M.; Cao, X.-B. ZnO@ZIF-8 core–shell heterostructures with improved photocatalytic activity. CrystEngComm 2021, 23, 4327–4335. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.; Wang, Y.; Zhang, F.; Liu, X.; Guo, Y.; Lu, G. Nanocasted Synthesis of Mesoporous LaCoO3 Perovskite with Extremely High Surface Area and Excellent Activity in Methane Combustion. J. Phys. Chem. C 2008, 112, 15293–15298. [Google Scholar] [CrossRef]
- Arandiyan, H.; Scott, J.; Wang, Y.; Dai, H.; Sun, H.; Amal, R. Meso-Molding Three-Dimensional Macroporous Perovskites: A New Approach to Generate High-Performance Nanohybrid Catalysts. ACS Appl. Mater. Interfaces 2016, 8, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, B.; Okal, J.; Tylus, W.; Winiarski, J.; Szczygieł, B. The effect of the calcination temperature of LaFeO3 precursors on the properties and catalytic activity of perovskite in methane oxidation. Ceram. Int. 2019, 45, 2779–2788. [Google Scholar] [CrossRef]
- Fan, X.; Li, L.; Yang, X.; Guo, Z.; Jing, F.; Chu, W. High-performance CoxM3-xAlOy (M=Ni, Mn) catalysts derived from microwave-assisted synthesis of hydrotalcite precursors for methane catalytic combustion. Catal. Today 2020, 347, 23–30. [Google Scholar] [CrossRef]
- Tao, F.F.; Shan, J.-j.; Nguyen, L.; Wang, Z.; Zhang, S.; Zhang, L.; Wu, Z.; Huang, W.; Zeng, S.; Hu, P. Understanding complete oxidation of methane on spinel oxides at a molecular level. Nat. Commun. 2015, 6, 7798. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Hu, S.; Shi, L.; Hoang, S.; Yang, W.; Fang, Y.; Liang, Z.; Pan, C.; Zhu, Y.; Li, L.; et al. Oxygen Vacancies and Lewis Acid Sites Synergistically Promoted Catalytic Methane Combustion over Perovskite Oxides. Environ. Sci. Technol. 2021, 55, 9243–9254. [Google Scholar] [CrossRef]
- Campagnoli, E.; Tavares, A.C.; Fabbrini, L.; Rossetti, I.; Dubitsky, Y.A.; Zaopo, A.; Forni, L. La1−xA′xCo1−yFeyO3±δ (A′=Ce,Sr) catalysts for the flameless combustion of methane. J. Mater. Sci. 2006, 41, 4713–4719. [Google Scholar] [CrossRef]
- Bashan, V.; Ust, Y. Perovskite catalysts for methane combustion: Applications, design, effects for reactivity and partial oxidation. Int. J. Energy Res. 2019, 43, 7755–7789. [Google Scholar] [CrossRef]
- Chang-Ke, W.; Xin-Zheng, L.; Hua, Z. Shares Differences of Greenhouse Gas Emissions Calculated with GTP and GWP for Major Countries. Adv. Clim. Change Res. 2013, 4, 127–132. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- D’Elia, V.; Kleij, A.W. Surface science approach to the heterogeneous cycloaddition of CO2 to epoxides catalyzed by site-isolated metal complexes and single atoms: A review. Green Chem. Eng. 2022, 3, 210–227. [Google Scholar] [CrossRef]
- Sodpiban, O.; Phungpanya, C.; Del Gobbo, S.; Arayachukiat, S.; Piromchart, T.; D’Elia, V. Rational engineering of single-component heterogeneous catalysts based on abundant metal centers for the mild conversion of pure and impure CO2 to cyclic carbonates. Chem. Eng. J. 2021, 422, 129930. [Google Scholar] [CrossRef]
- Xie, W.-H.; Li, H.; Yang, M.; He, L.-N.; Li, H.-R. CO2 capture and utilization with solid waste. Green Chem. Eng. 2022, 3, 199–209. [Google Scholar] [CrossRef]
- He, L.; Fan, Y.; Bellettre, J.; Yue, J.; Luo, L. A review on catalytic methane combustion at low temperatures: Catalysts, mechanisms, reaction conditions and reactor designs. Renew. Sustain. Energy Rev. 2020, 119, 109589. [Google Scholar] [CrossRef]
- Tian, M.; Wang, X.D.; Zhang, T. Hexaaluminates: A review of the structure, synthesis and catalytic performance. Catal. Sci. Technol. 2016, 6, 1984–2004. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y. Nanostructured perovskite oxides as promising substitutes of noble metals catalysts for catalytic combustion of methane. Chin. Chem. Lett. 2018, 29, 252–260. [Google Scholar] [CrossRef]
- Cargnello, M.; Delgado Jaén, J.J.; Hernández Garrido, J.C.; Bakhmutsky, K.; Montini, T.; Calvino Gámez, J.J.; Gorte, R.J.; Fornasiero, P. Exceptional activity for methane combustion over modular Pd@CeO2 subunits on functionalized Al2O3. Science 2012, 337, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Florén, C.-R.; Demirci, C.; Carlsson, P.-A.; Creaser, D.; Skoglundh, M. Total oxidation of methane over Pd/Al2O3 at pressures from 1 to 10 atm. Catal. Sci. Technol. 2020, 10, 5480–5486. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Scott, J.; Akia, M.; Dai, H.; Deng, J.; Aguey-Zinsou, K.-F.; Amal, R. High Performance Au–Pd Supported on 3D Hybrid Strontium-Substituted Lanthanum Manganite Perovskite Catalyst for Methane Combustion. ACS Catal. 2016, 6, 6935–6947. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Deng, J.; Zhang, Y.; Xie, S.; Zhao, X.; Wang, Z.; Guo, G.; Dai, H. 3DOM LaMnAl11O19-supported AuPd alloy nanoparticles: Highly active catalysts for methane combustion in a continuous-flow microreactor. Catal. Today 2018, 308, 71–80. [Google Scholar] [CrossRef]
- Arandiyan, H.; Dai, H.; Ji, K.; Sun, H.; Li, J. Pt Nanoparticles Embedded in Colloidal Crystal Template Derived 3D Ordered Macroporous Ce0.6Zr0.3Y0.1O2: Highly Efficient Catalysts for Methane Combustion. ACS Catal. 2015, 5, 1781–1793. [Google Scholar] [CrossRef]
- Jones, J.M.; Dupont, V.A.; Brydson, R.; Fullerton, D.J.; Nasri, N.S.; Ross, A.B.; Westwood, A.V.K. Sulphur poisoning and regeneration of precious metal catalysed methane combustion. Catal. Today 2003, 81, 589–601. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, H.; Liu, J.; Zhang, T. Preparation of high surface area La1−xAxMnO3 (A=Ba, Sr or Ca) ultra-fine particles used for CH4 oxidation. Chem. Eng. J. 2002, 89, 213–221. [Google Scholar] [CrossRef]
- Rossetti, I.; Buchneva, O.; Biffi, C.; Rizza, R. Effect of sulphur poisoning on perovskite catalysts prepared by flame-pyrolysis. Appl. Catal. B Environ. 2009, 89, 383–390. [Google Scholar] [CrossRef]
- Du, X.; Zou, G.; Zhang, Y.; Wang, X. A novel strategy for low-temperature synthesis of Ruddlesden–Popper type layered perovskite La3Mn2O7+δ for methane combustion. J. Mater. Chem. A 2013, 1, 8411–8416. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Tahini, H.A.; Scott, J.; Tan, X.; Dai, H.; Gale, J.D.; Rohl, A.L.; Smith, S.C.; Amal, R. The controlled disassembly of mesostructured perovskites as an avenue to fabricating high performance nanohybrid catalysts. Nat. Commun. 2017, 8, 15553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.; Botu, V.; Wang, S.; Meng, Y.; Song, W.; Guo, Y.; Ramprasad, R.; Suib, S.L.; Gao, P.X. Monolithically Integrated Spinel MxCo3−xO4 (M=Co, Ni, Zn) Nanoarray Catalysts: Scalable Synthesis and Cation Manipulation for Tunable Low-Temperature CH4 and CO Oxidation. Angew. Chem. Int. Ed. 2014, 53, 7223–7227. [Google Scholar] [CrossRef] [PubMed]
- Shan, W. Reduction property and catalytic activity of Ce1−XNiXO2 mixed oxide catalysts for CH4 oxidation. Appl. Catal. A Gen. 2003, 246, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Liang, X.; Xu, S.; Hao, J. Catalytic performance of manganese cobalt oxides on methane combustion at low temperature. Appl. Catal. B Environ. 2009, 90, 307–312. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Oxidation of residual methane from VNG vehicles over Co3O4-based catalysts: Comparison among bulk, Al2O3-supported and Ce-doped catalysts. Appl. Catal. B Environ. 2018, 237, 844–854. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Z.; Wang, G.; Zhu, H.; Dong, M.; Li, S.; Wu, Z.; Li, Z.; Wu, Z.; Zhang, J.; et al. Catalytic performance of MnOx–NiO composite oxide in lean methane combustion at low temperature. Appl. Catal. B Environ. 2013, 129, 172–181. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, C.; Li, X.; Li, Z.; Wang, L.; Zhang, Q.; Wu, G.; Li, Z. Engineering an effective MnO2 catalyst from LaMnO3 for catalytic methane combustion. Fuel 2019, 239, 1240–1245. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhang, Y.; Zhang, T.; Chang, H.; Zhang, Y.; Jiang, L. Structural requirements of manganese oxides for methane oxidation: XAS spectroscopy and transition-state studies. Appl. Catal. B Environ. 2018, 229, 52–62. [Google Scholar] [CrossRef]
- Rhodes, C.J. Endangered elements, critical raw materials and conflict minerals. Sci. Prog. 2019, 102, 304–350. [Google Scholar] [CrossRef] [PubMed]
- Biswal, A.; Chandra Tripathy, B.; Sanjay, K.; Subbaiah, T.; Minakshi, M. Electrolytic manganese dioxide (EMD): A perspective on worldwide production, reserves and its role in electrochemistry. RSC Adv. 2015, 5, 58255–58283. [Google Scholar] [CrossRef]
- Ji, X.; Xia, Q.; Xu, Y.; Feng, H.; Wang, P.; Tan, Q. A review on progress of lithium-rich manganese-based cathodes for lithium ion batteries. J. Power Sources 2021, 487, 229362. [Google Scholar] [CrossRef]
- Euro Manganese Sees Spike in EV Battery Maker Demand by 2030. Available online: https://www.marketindex.com.au/news/euro-manganese-sees-spike-in-ev-battery-maker-demand-by-2030 (accessed on 30 September 2022).
- Zhanpeisov, N. Cluster quantum-chemical study of the chemisorption of methane on zinc oxide surface. J. Mol. Catal. A Chem. 1995, 99, 35–39. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, W.; Chi, X.; Lu, X.; Hu, S.; Wu, Z.; Tang, W.; Ren, Z.; Wang, S.; Yu, X.; et al. Solar-driven efficient methane catalytic oxidation over epitaxial ZnO/La0.8Sr0.2CoO3 heterojunctions. Appl. Catal. B Environ. 2020, 265, 118469. [Google Scholar] [CrossRef]
- Wang, S.; Ren, Z.; Song, W.; Guo, Y.; Zhang, M.; Suib, S.L.; Gao, P.-X. ZnO/perovskite core–shell nanorod array based monolithic catalysts with enhanced propane oxidation and material utilization efficiency at low temperature. Catal. Today 2015, 258, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-J.; Chuang, Y.-J. Hydrothermal synthesis and characterization of hexagonal zinc oxide nanorods with a hexamethylenetetramine (HMTA) template-assisted at a low temperature. Mater. Lett. 2012, 68, 460–462. [Google Scholar] [CrossRef]
- Biswas, K.; Das, B.; Rao, C.N.R. Growth Kinetics of ZnO Nanorods: Capping-Dependent Mechanism and Other Interesting Features. J. Phys. Chem. C 2008, 112, 2404–2411. [Google Scholar] [CrossRef]
- Rayathulhan, R.; Sodipo, B.K.; Aziz, A.A. Nucleation and growth of zinc oxide nanorods directly on metal wire by sonochemical method. Ultrason. Sonochem. 2017, 35, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Leontiou, A.A.; Ladavos, A.K.; Giannakas, A.E.; Bakas, T.V.; Pomonis, P.J. A comparative study of substituted perovskite-type solids of oxidic La1−xSrxFeO3±δ and chlorinated La1−xSrxFeO3±δClσ form: Catalytic performance for CH4 oxidation by O2 or N2O. J. Catal. 2007, 251, 103–112. [Google Scholar] [CrossRef]
- Najjar, H.; Batis, H.; Lamonier, J.-F.; Mentré, O.; Giraudon, J.-M. Effect of praseodymium and europium doping in La1−xLnxMnO3±δ (Ln: Pr or Eu, 0 ≤ x ≤ 1) perosvkite catalysts for total methane oxidation. Appl. Catal. A Gen. 2014, 469, 98–107. [Google Scholar] [CrossRef]
- Valdez, C.N.; Delley, M.F.; Mayer, J.M. Cation Effects on the Reduction of Colloidal ZnO Nanocrystals. J. Am. Chem. Soc. 2018, 140, 8924–8933. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Na, W.; Gao, W.; Wang, H. Carbon-Modified CuO/ZnO Catalyst with High Oxygen Vacancy for CO2 Hydrogenation to Methanol. Energy Technol. 2020, 8, 2000194. [Google Scholar] [CrossRef]
- Wang, W.; Xie, Y.; Zhang, S.; Liu, X.; Haruta, M.; Huang, J. Selective Hydrogenation of Cinnamaldehyde Catalyzed by ZnO-Fe2O3 Mixed Oxide Supported Gold Nanocatalysts. Catalysts 2018, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, X.; Zhang, Y.; Zhang, R.; Ge, H.; Bi, J.; Tang, M. Strong metal–support interactions between Ni and ZnO particles and their effect on the methanation performance of Ni/ZnO. Catal. Sci. Technol. 2017, 7, 4413–4421. [Google Scholar] [CrossRef]
- Lv, Y.; Yao, W.; Ma, X.; Pan, C.; Zong, R.; Zhu, Y. The surface oxygen vacancy induced visible activity and enhanced UV activity of a ZnO1−x photocatalyst. Catal. Sci. Technol. 2013, 3, 3136–3146. [Google Scholar] [CrossRef]
- Han, X.-G.; He, H.-Z.; Kuang, Q.; Zhou, X.; Zhang, X.-H.; Xu, T.; Xie, Z.-X.; Zheng, L.-S. Controlling Morphologies and Tuning the Related Properties of Nano/Microstructured ZnO Crystallites. J. Phys. Chem. C 2008, 113, 584–589. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Bi, S.; Liu, Q. Metal–organic framework-derived CeO2–ZnO catalysts for C3H6-SCR of NO: An in situ DRIFTS study. RSC Adv. 2019, 9, 19236–19242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Siqueira, R.N.C.; de Albuquerque Brocchi, E.; de Oliveira, P.F.; Motta, M.S. Hydrogen Reduction of Zinc and Iron Oxides Containing Mixtures. Metall. Mater. Trans. B 2013, 45, 66–75. [Google Scholar] [CrossRef]
- Maity, N.; Barman, S.; Minenkov, Y.; Ould-Chikh, S.; Abou-Hamad, E.; Ma, T.; Qureshi, Z.S.; Cavallo, L.; D’Elia, V.; Gates, B.C.; et al. A Silica-Supported Monoalkylated Tungsten Dioxo Complex Catalyst for Olefin Metathesis. ACS Catal. 2018, 8, 2715–2729. [Google Scholar] [CrossRef]
- Song, S.; Song, H.; Li, L.; Wang, S.; Chu, W.; Peng, K.; Meng, X.; Wang, Q.; Deng, B.; Liu, Q.; et al. A selective Au-ZnO/TiO2 hybrid photocatalyst for oxidative coupling of methane to ethane with dioxygen. Nat. Catal. 2021, 4, 1032–1042. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, J.; Xu, X.; Fang, X.; Guo, Y.; Liu, R.; Zhong, W.; Wang, X. Tailoring Active O2– and O22– Anions on a ZnO Surface with the Addition of Different Alkali Metals Probed by CO Oxidation. Ind. Eng. Chem. Res. 2020, 59, 9382–9392. [Google Scholar] [CrossRef]
- Najjar, H.; Lamonier, J.-F.; Mentré, O.; Giraudon, J.-M.; Batis, H. Combustion synthesis of LaMn1−xAlxO3+δ (0 ≤ x ≤ 1): Tuning catalytic properties for methane deep oxidation. Catal. Sci. Technol. 2013, 3, 1002–1016. [Google Scholar] [CrossRef]
- Ferri, D.; Forni, L. Methane combustion on some perovskite-like mixed oxides. Appl. Catal. B Environ. 1998, 16, 119–126. [Google Scholar] [CrossRef]
- Zhou, J.; Nomenyo, K.; Cesar, C.C.; Lusson, A.; Schwartzberg, A.; Yen, C.-C.; Woon, W.-Y.; Lerondel, G. Giant defect emission enhancement from ZnO nanowires through desulfurization process. Sci. Rep. 2020, 10, 4237. [Google Scholar] [CrossRef] [PubMed]
- Uklein, A.V.; Multian, V.V.; Kuz’micheva, G.M.; Linnik, R.P.; Lisnyak, V.V.; Popov, A.I.; Gayvoronsky, V.Y. Nonlinear optical response of bulk ZnO crystals with different content of intrinsic defects. Opt. Mater. 2018, 84, 738–747. [Google Scholar] [CrossRef]
- Brik, M.G.; Srivastava, A.M.; Popov, A.I. A few common misconceptions in the interpretation of experimental spectroscopic data. Opt. Mater. 2022, 127, 112276. [Google Scholar] [CrossRef]
- Panigrahy, B.; Aslam, M.; Misra, D.S.; Ghosh, M.; Bahadur, D. Defect-Related Emissions and Magnetization Properties of ZnO Nanorods. Adv. Funct. Mater. 2010, 20, 1161–1165. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Z.; Ren, T.; Ding, H.; Yao, W.; Zong, R.; Zhu, Y. Influence of Defects on the Photocatalytic Activity of ZnO. J. Phys. Chem. C 2014, 118, 15300–15307. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, Y.; Naschitzki, M.; Gewinner, S.; Schöllkopf, W.; Kuhlenbeck, H.; Pentcheva, R.; Roldan Cuenya, B. Surface oxygen Vacancies on Reduced Co3O4 (100): Superoxide Formation and Ultra-Low-Temperature CO Oxidation. Angew. Chem. Int. Ed. 2021, 60, 16514–16520. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, J.; Song, J.; Huang, S.; Yang, N.; Zhang, J.; Sun, Y.; Zhu, Y. Exploring the Effect of Co3O4 Nanocatalysts with Different Dimensional Architectures on Methane Combustion. ChemCatChem 2016, 8, 540–545. [Google Scholar] [CrossRef]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen Ionosorption and Spectroscopic Evidence for Adsorbed Oxygen. ChemPhysChem 2006, 7, 2041–2052. [Google Scholar] [CrossRef]
- Anpo, M.; Costentin, G.; Giamello, E.; Lauron-Pernot, H.; Sojka, Z. Characterisation and reactivity of oxygen species at the surface of metal oxides. J. Catal. 2021, 393, 259–280. [Google Scholar] [CrossRef]
- Liang, S.; Teng, F.; Bulgan, G.; Zhu, Y. Effect of Jahn−Teller Distortion in La0.5Sr0.5MnO3 Cubes and Nanoparticles on the Catalytic Oxidation of CO and CH4. J. Phys. Chem. C 2007, 111, 16742–16749. [Google Scholar] [CrossRef]
- Gandla, S.; Gollu, S.R.; Sharma, R.; Sarangi, V.; Gupta, D. Dual role of boron in improving electrical performance and device stability of low temperature solution processed ZnO thin film transistors. Appl. Phys. Lett. 2015, 107, 152012. [Google Scholar] [CrossRef]
- Fan, X.; Li, L.; Jing, F.; Li, J.; Chu, W. Effects of preparation methods on CoAlOx/CeO2 catalysts for methane catalytic combustion. Fuel 2018, 225, 588–595. [Google Scholar] [CrossRef]
- Zhong, L.; Fang, Q.; Li, X.; Li, Q.; Zhang, C.; Chen, G. Influence of preparation methods on the physicochemical properties and catalytic performance of Mn-Ce catalysts for lean methane combustion. Appl. Catal. A Gen. 2019, 579, 151–158. [Google Scholar] [CrossRef]
- Arandiyan, H.; Chang, H.; Liu, C.; Peng, Y.; Li, J. Dextrose-aided hydrothermal preparation with large surface area on 1D single-crystalline perovskite La0.5Sr0.5CoO3 nanowires without template: Highly catalytic activity for methane combustion. J. Mol. Catal. A Chem. 2013, 378, 299–306. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, S.; Zhang, L.; Chen, Z.; Wang, M.; Wang, S. A facile method to promote LaMnO3 perovskite catalyst for combustion of methane. Catal. Commun. 2017, 97, 88–92. [Google Scholar] [CrossRef]
- Pecchi, G.; Jiliberto, M.G.; Buljan, A.; Delgado, E.J. Relation between defects and catalytic activity of calcium doped LaFeO3 perovskite. Solid State Ion. 2011, 187, 27–32. [Google Scholar] [CrossRef]
- Arandiyan, H.; Dai, H.; Deng, J.; Liu, Y.; Bai, B.; Wang, Y.; Li, X.; Xie, S.; Li, J. Three-dimensionally ordered macroporous La0.6Sr0.4MnO3 with high surface areas: Active catalysts for the combustion of methane. J. Catal. 2013, 307, 327–339. [Google Scholar] [CrossRef]
- Arandiyan, H.; Dai, H.; Deng, J.; Wang, Y.; Sun, H.; Xie, S.; Bai, B.; Liu, Y.; Ji, K.; Li, J. Three-Dimensionally Ordered Macroporous La0.6Sr0.4MnO3 Supported Ag Nanoparticles for the Combustion of Methane. J. Phys. Chem. C 2014, 118, 14913–14928. [Google Scholar] [CrossRef]
- Taguchi, H.; Nakade, K.; Yosinaga, M.; Kato, M.; Hirota, K. Methane Oxidation on Perovskite-Type Ca(Mn1−xTix)O3−δ. J. Am. Ceram. Soc. 2007, 91, 308–310. [Google Scholar] [CrossRef]
- Li, H.; Lu, G.; Qiao, D.; Wang, Y.; Guo, Y.; Guo, Y. Catalytic Methane Combustion over Co3O4/CeO2 Composite Oxides Prepared by Modified Citrate Sol–Gel Method. Catal. Lett. 2010, 141, 452–458. [Google Scholar] [CrossRef]
- Gao, Q.-X.; Wang, X.-F.; Di, J.-L.; Wu, X.-C.; Tao, Y.-R. Enhanced catalytic activity of α-Fe2O3 nanorods enclosed with {110} and {001} planes for methane combustion and CO oxidation. Catal. Sci. Technol. 2011, 1, 574–577. [Google Scholar] [CrossRef]
- Gao, Q.-X.; Wang, X.-F.; Wu, X.-C.; Tao, Y.-R.; Zhu, J.-J. Mesoporous zirconia nanobelts: Preparation, characterization and applications in catalytical methane combustion. Microporous Mesoporous Mater. 2011, 143, 333–340. [Google Scholar] [CrossRef]
- Chen, J.; Shi, W.; Zhang, X.; Arandiyan, H.; Li, D.; Li, J. Roles of Li+ and Zr4+ Cations in the Catalytic Performances of Co1–xMxCr2O4 (M = Li, Zr; x = 0–0.2) for Methane Combustion. Environ. Sci. Technol. 2011, 45, 8491–8497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Hartlaub, S.; Petrovic, I.; Yilmaz, B. Raman Spectroscopy Characterization of Amorphous Coke Generated in Industrial Processes. ACS Omega 2022, 7, 2565–2570. [Google Scholar] [CrossRef] [PubMed]
- Vogelaar, B.M.; van Langeveld, A.D.; Eijsbouts, S.; Moulijn, J.A. Analysis of coke deposition profiles in commercial spent hydroprocessing catalysts using Raman spectroscopy. Fuel 2007, 86, 1122–1129. [Google Scholar] [CrossRef]
- Daumann, S.; Andrzejewski, D.; Di Marcantonio, M.; Hagemann, U.; Wepfer, S.; Vollkommer, F.; Bacher, G.; Epple, M.; Nannen, E. Water-free synthesis of ZnO quantum dots for application as an electron injection layer in light-emitting electrochemical cells. J. Mater. Chem. C 2017, 5, 2344–2351. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Chang, J.; Ahmad, M.S.; Waclawik, E.R.; Wlodarski, W. Non-aqueous synthesis of hexagonal ZnO nanopyramids: Gas sensing properties. Sens. Actuators B Chem. 2013, 177, 286–294. [Google Scholar] [CrossRef]
- Li, Y.; Luo, C.; Liu, Z.; Lin, F. Experimental Study on Catalytic Combustion of Methane in a Microcombustor with Metal Foam Monolithic Catalyst. Catalysts 2018, 8, 536. [Google Scholar] [CrossRef] [Green Version]
- Wierzbicki, T.A.; Lee, I.C.; Gupta, A.K. Rh assisted catalytic oxidation of jet fuel surrogates in a meso-scale combustor. Appl. Energy 2015, 145, 1–7. [Google Scholar] [CrossRef]






| Material | Synthesis Time (h) | Length (nm) | Diameter (nm) |
|---|---|---|---|
| ZnO-(Q)NRs | 1 | 230 ± 69 | 25 ± 1 |
| 2 | 574 ± 17, (825 ± 5) | 65 ± 1 | |
| 6 | 679 ± 33 | 58 ± 2 | |
| 12 | 559 ± 3, (920 ± 6) a, (1192 ± 7) a | 52 ± 2, (76 ± 1) a | |
| ZnO-(Py)NRs | 1 | 885 ± 11 | 98 ± 4 |
| 12 | 1106 ± 25 | 94 ± 3 |
| Material | Synthesis Time (h) | BET Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|---|
| ZnO-(Q)NRs | 1 | 36 | 0.47 |
| 2 | 15 | 0.089 | |
| 6 | 17 | 0.12 | |
| 12 | 17 | 0.14 | |
| ZnO-(Py)NRs | 1 | 6 | 0.033 |
| 12 | 6 | 0.033 | |
| ZnO-NPys | - | 14 | 0.21 |
| ZnO-C | - | 5 | 0.025 |
| Catalyst | OIII/Otot | OII/Otot | OI/Otot | (OIII+OII)/Otot |
|---|---|---|---|---|
| ZnO-(Q)NRs | 0.10 | 0.33 | 0.57 | 0.43 |
| ZnO-(Py)NRs | 0.15 | 0.45 | 0.40 | 0.60 |
| ZnO-NPys | 0.08 | 0.34 | 0.58 | 0.42 |
| ZnO-C | 0.10 | 0.33 | 0.57 | 0.43 |
| Catalyst | T50 (°C) | T90 (°C) | Conv. (%) (at 650 °C) | Surface Area (m2·g−1) |
|---|---|---|---|---|
| ZnO-(Q)NRs | 576 | 659 | 88 | 36 |
| ZnO-(Py)NRs | 609 | 689 | 74 | 6 |
| ZnO-NPys | 615 | 696 | 71 | 14 |
| ZnO-C | 665 | - | 41 | 5 |
| Entry | Catalyst | [CH4] (vol%) | GHSV (mL·gcat–1·h–1) | T50 (°C) | T90 (°C) | Ref. |
|---|---|---|---|---|---|---|
| 1 | La0.7Sr0.3FeO3 | 4.35 | 26,700 | 580 | - | [87] |
| 2 | La0.6Sr0.4MnO3 | 2 | 30,000 | 566 | 661 | [118] |
| 3 | ZnO/La0.8Sr0.2CoO3 | 1 | 40,000 | 568 | ~650 | [82] |
| 4 | 3DOM a La0.6Sr0.4MnO3 | 2 | 30,000 | 566 | 661 | [119] |
| 5 | La0.7Ce0.3CoO3 | 2 | 30,000 | 564 | 662 | [45] |
| 6 | CaMn0.6Ti0.4O3 | 3 | 30,000 | 585 | - | [120] |
| 7 | ZnO-(Q)NRs | 1 | 30,000 | 576 | 659 | This work |
| 8 | CeO2 b | 1 | 30,000 | 632 | - | [121] |
| 9 | α-Fe2O3 nanotubes | 2 | 25,500 | 610 | 750 | [122] |
| 10 | ZrO2 nanobelts | 2 | 25,000 | 650 | 700 | [123] |
| 11 | 3D-hm La0.6Sr0.4MnO3 c | 5 | 51,360 | 360 | 438 | [69] |
| 12 | Co0.95Zr0.05Cr2O4 | 0.2 | 36,000 | 376 | 448 | [124] |
| 13 | Co/Mn oxide (5:1) d | 1 | 36,000 | 293 | 324 | [72] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kessaratikoon, T.; Saengsaen, S.; Del Gobbo, S.; D’Elia, V.; Sooknoi, T. High Surface Area ZnO-Nanorods Catalyze the Clean Thermal Methane Oxidation to CO2. Catalysts 2022, 12, 1533. https://doi.org/10.3390/catal12121533
Kessaratikoon T, Saengsaen S, Del Gobbo S, D’Elia V, Sooknoi T. High Surface Area ZnO-Nanorods Catalyze the Clean Thermal Methane Oxidation to CO2. Catalysts. 2022; 12(12):1533. https://doi.org/10.3390/catal12121533
Chicago/Turabian StyleKessaratikoon, Tanika, Sawarin Saengsaen, Silvano Del Gobbo, Valerio D’Elia, and Tawan Sooknoi. 2022. "High Surface Area ZnO-Nanorods Catalyze the Clean Thermal Methane Oxidation to CO2" Catalysts 12, no. 12: 1533. https://doi.org/10.3390/catal12121533
APA StyleKessaratikoon, T., Saengsaen, S., Del Gobbo, S., D’Elia, V., & Sooknoi, T. (2022). High Surface Area ZnO-Nanorods Catalyze the Clean Thermal Methane Oxidation to CO2. Catalysts, 12(12), 1533. https://doi.org/10.3390/catal12121533






