Band-Gap Engineering of Layered Perovskites by Cu Spacer Insertion as Photocatalysts for Depollution Reaction
Abstract
:1. Introduction
2. Results and Discussion
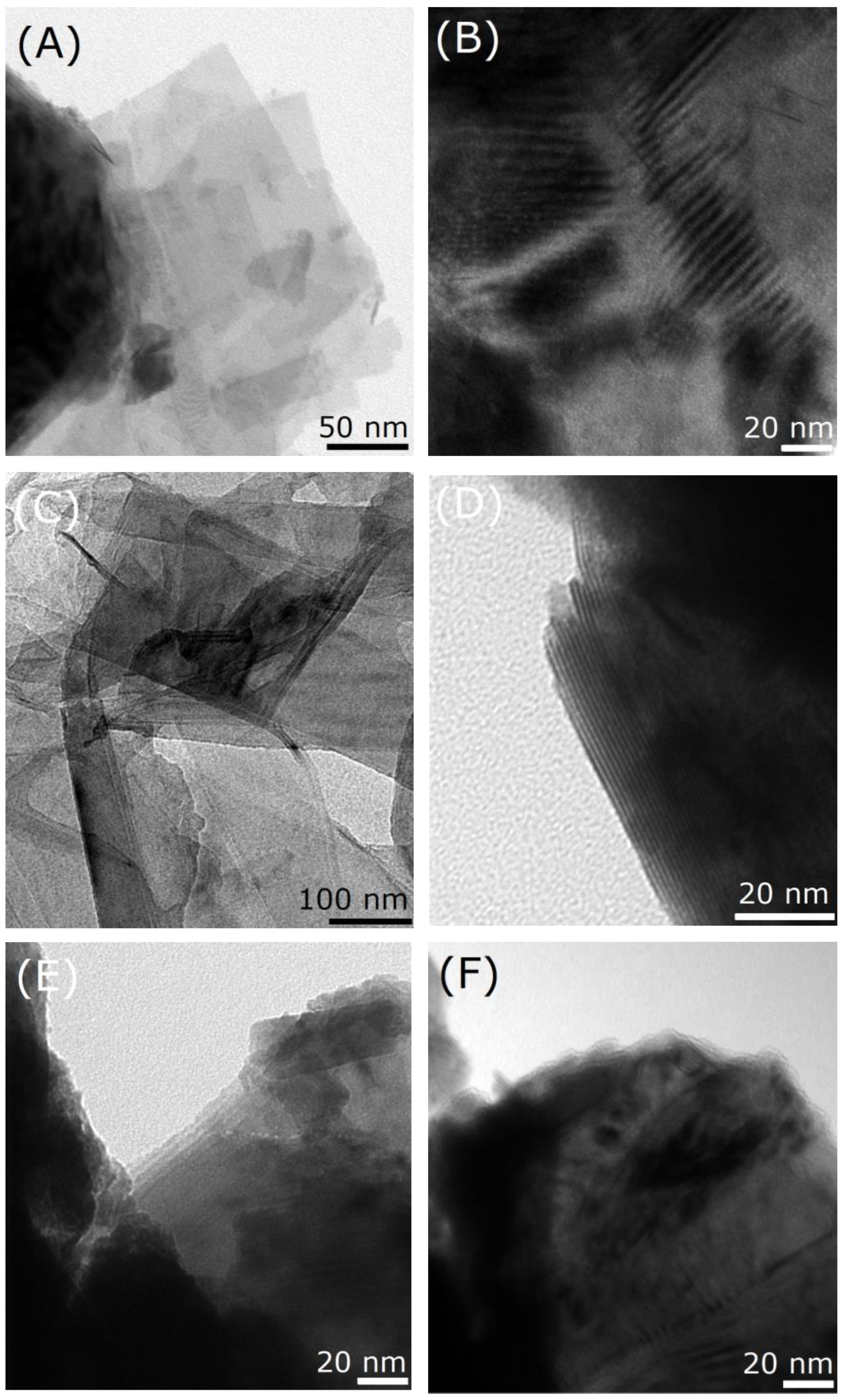
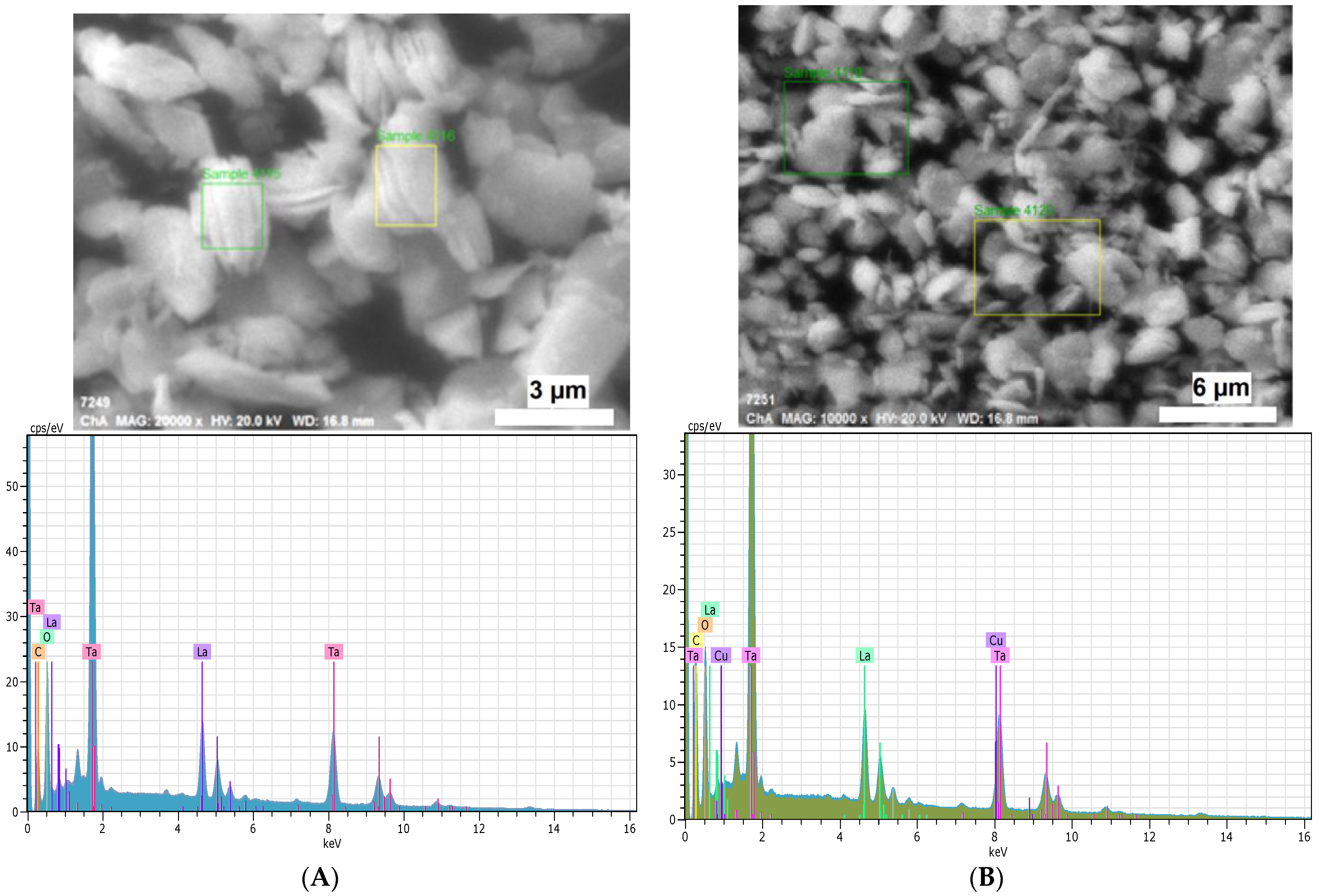
2.1. Crystal Structure and Morphology

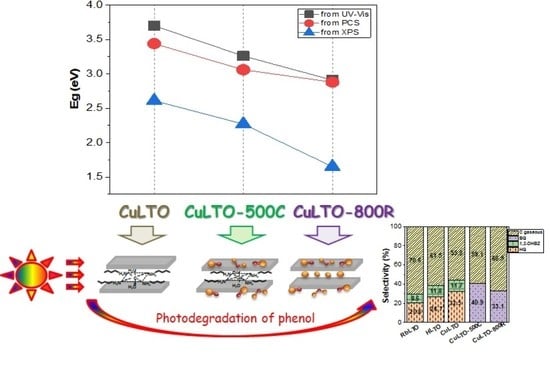
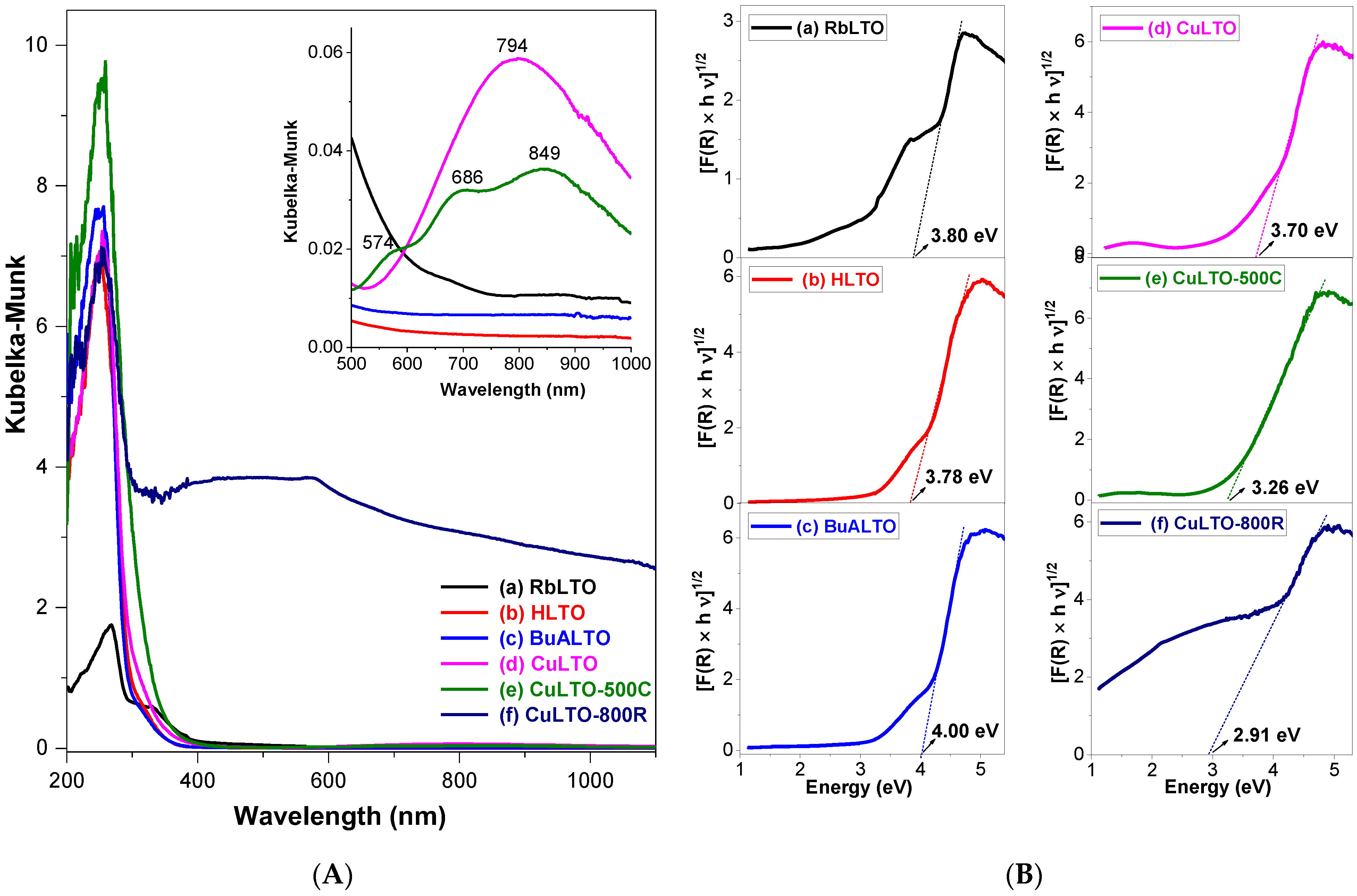
2.2. Optical Absorption of Samples by UV–Vis Spectroscopy
 pairs and the metallic copper [36,37].
pairs and the metallic copper [36,37].2.3. Tauc Plot Derived from Photocurrent Spectroscopy (PCS)
2.4. FTIR Absorption Spectra of the Layered Perovskites
2.5. Temperature-Programmed Reduction (TPR) Measurements
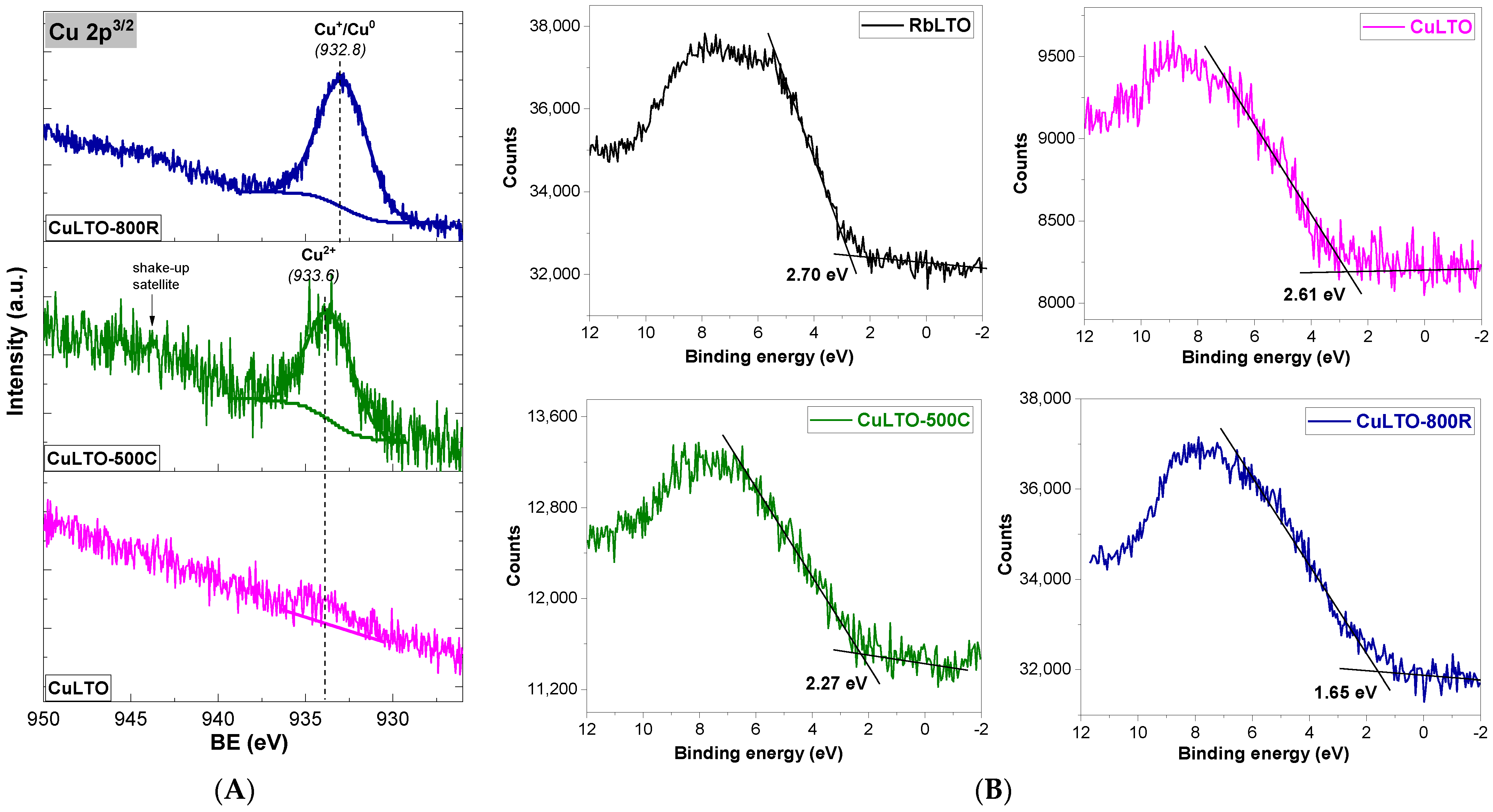
2.6. XPS Measurements
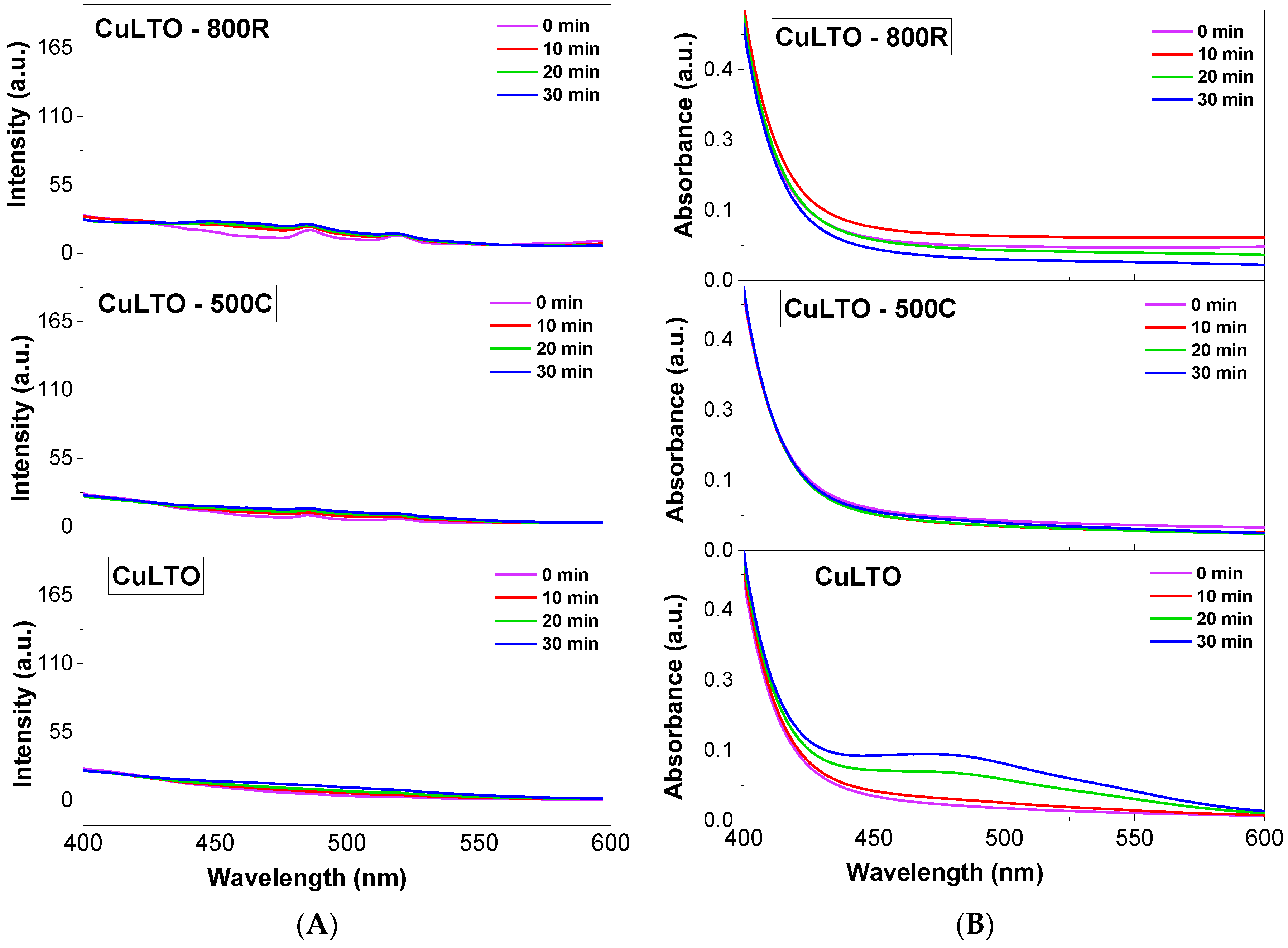
3. Evaluation of Photocatalytic Activity
4. Materials and Methods
4.1. Synthesis of Catalytic Materials
4.2. Characterization of Photocatalysts
4.3. Photocatalytic Degradation of Phenol under Simulated Solar Irradiation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fang, M.; Kim, C.H.; Mallouk, T.E. Dielectric Properties of the Lamellar Niobates and Titanoniobates AM2Nb3O10 and ATiNbO5 (A = H, K, M = Ca, Pb), and Their Condensation Products Ca4Nb6O19 and Ti2Nb2O9. Chem. Mater. 1999, 11, 1519–1525. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, Z.-Q. Unconventional superconductivity in Sr2RuO4. Phys. C Supercond. Appl. 2015, 514, 339–353. [Google Scholar] [CrossRef] [Green Version]
- Ida, S.; Ogata, C.; Eguchi, M.; Youngblood, W.J.; Mallouk, T.E.; Matsumoto, Y. Photoluminescence of Perovskite Nanosheets Prepared by Exfoliation of Layered Oxides, K2Ln2Ti3O10, KLnNb2O7, and RbLnTa2O7 (Ln: Lanthanide Ion). J. Am. Chem. Soc. 2008, 130, 7052–7059. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Mao, L.; Guan, X.; Tucker, K.A.; Xie, H.; Wu, X.; Shi, J. Layered perovskite oxides and their derivative nanosheets adopting different modification strategies towards better photocatalytic performance of water splitting. Renew. Sustain. Energy Rev. 2020, 119, 109527. [Google Scholar] [CrossRef]
- Oshima, T.; Yokoi, T.; Eguchi, M.; Maeda, K. Synthesis and photocatalytic activity of K2CaNaNb3O10, a new Ruddlesden-Popper phase layered perovskite. Dalton Trans. 2017, 46, 10594–10601. [Google Scholar] [CrossRef] [PubMed]
- Atri, S.; Tomar, R. A Review on the Synthesis and Modification of Functional Inorganic-Organic-Hybrid Materials via Microwave-Assisted Method. ChemistrySelect 2021, 6, 9351–9362. [Google Scholar] [CrossRef]
- Machida, M.; Yabunaka, J.-I.; Kijima, T. Synthesis and Photocatalytic Property of Layered Perovskite Tantalates, RbLnTa2O7 (Ln = La, Pr, Nd, and Sm). Chem. Mater. 2000, 12, 812–817. [Google Scholar] [CrossRef]
- Sanjaya Ranmohotti, K.G.; Josepha, E.; Choi, J.; Zhang, J.; Wiley, J.B. Topochemical Manipulation of Perovskites: Low-Temperature Reaction Strategies for Directing Structure and Properties. Adv. Mater. 2011, 23, 442–460. [Google Scholar] [CrossRef]
- Wang, B.; Dong, X.; Pan, Q.; Cheng, Z.; Yang, Y. Intercalation behavior of n-alkylamines into an A-site defective layered perovskite H2W2O7. J. Solid State Chem. 2007, 180, 1125–1129. [Google Scholar] [CrossRef]
- Uppuluri, R.; Gupta, A.S.; Rosas, A.S.; Mallouk, T.E. Soft chemistry of ion-exchangeable layered metal oxides. Chem. Soc. Rev. 2018, 47, 2401–2430. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, A.; Taniguchi, T.; Tokita, Y.; Murakami, T.; Nojiri, Y.; Matsumoto, Y. Nd3+-doped perovskite nanosheets with NIR luminescence. Mater. Lett. 2014, 114, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Timmerman, M.A.; Xia, R.; Le, P.T.P.; Wang, Y.; ten Elshof, J.E. Metal Oxide Nanosheets as 2D Building Blocks for the Design of Novel Materials. Chem. A Eur. J. 2020, 26, 9084–9098. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xie, Y.; Hu, Y.; Long, M.; Zhang, Y.; Xu, J.; Qin, M.; Lu, X.; Liu, M. Effects of alkyl chain length on crystal growth and oxidation process of two dimensional tin halide perovskites. ACS Energy Lett. 2020, 5, 1422–1429. [Google Scholar] [CrossRef]
- Minich, I.A.; Silyukov, O.I.; Gak, V.V.; Borisov, E.V.; Zvereva, I.A. Synthesis of organic-inorganic hybrids based on perovskite-like bismuth titanate H2K0.5Bi2.5Ti4O13·H2O and n-alkylamines. ACS Omega 2020, 5, 8158–8168. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Wei, Y.; Cheng, Y.; Huang, Y.; Hao, S.; Wu, J. Preparation and photocatalytic properties of HLaNb2O7/(Pt, TiO2) perovskite intercalated nanomaterial. Int. J. Hydrog. Energy 2014, 39, 7747–7752. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, Y.; Lin, J.; Huang, Y.; Huang, M.; Hao, S. Fabrication and photocatalytic properties of HLaNb2O7/(Pt, Fe2O3) pillared nanomaterial. J. Phys. Chem. C 2007, 111, 3624–3628. [Google Scholar] [CrossRef]
- Ebina, Y.; Sakai, N.; Nagasaki, T. Photocatalyst of lamellar aggregates of RuOx-loaded perovskite nanosheets for overall water splitting. J. Phys. Chem. B 2005, 109, 17212–17216. [Google Scholar] [CrossRef]
- Oshima, T.; Wang, Y.; Lu, D.; Yokoi, T.; Maeda, K. Photocatalytic overall water splitting on Pt nanocluster-intercalated, restacked KCa2Nb3O10 nanosheets: The promotional effect of co-existing ions. Nanoscale Adv. 2019, 1, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting-materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, D.; Ghosh, S. Recent advancements of copper oxide based nanomaterials for supercapacitor applications. J. Energy Storage 2021, 34, 101995. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate photocatalysts for light-driven water splitting: Mechanisms, challenges, and design strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Iwashina, K.; Iwase, A.; Nozawa, S.; Adachi, S.-I.; Kudo, A. New Visible-Light-Driven H2- and O2-Evolving Photocatalysts Developed by Ag(I) and Cu(I) Ion Exchange of Various Layered and Tunneling Metal Oxides Using Molten Salts Treatments. Chem. Mater. 2020, 32, 10524–10537. [Google Scholar] [CrossRef]
- Iwashina, K.; Iwase, A.; Kudo, A. Sensitization of wide band gap photocatalysts to visible light by molten CuCl treatment. Chem. Sci. 2015, 6, 687–692. [Google Scholar] [CrossRef] [Green Version]
- Wysocka, I.; Kowalska, E.; Trzciński, K.; Łapiński, M.; Nowaczyk, G.; Zielińska-Jurek, A. UV-Vis-Induced Degradation of Phenol over Magnetic Photocatalysts Modified with Pt, Pd, Cu and Au Nanoparticles. Nanomaterials 2018, 8, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordova Villegas, L.G.; Mashhadi, N.; Chen, M.; Mukherjee, D.; Taylor, K.E.; Biswas, N. A Short Review of Techniques for Phenol Removal from Wastewater. Curr. Pollut. Rep. 2016, 2, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Alonso, J.A.; Martínez-Lope, M.J.; Casais, M.T.; Fernández-Díaz, M.T. Evolution of the Jahn−Teller Distortion of MnO6 Octahedra in RMnO3 Perovskites (R = Pr, Nd, Dy, Tb, Ho, Er, Y): A Neutron Diffraction Study. Inorg. Chem. 2000, 39, 917–923. [Google Scholar] [CrossRef]
- Hyeon, K.-A.; Byeon, S.-H. Synthesis and Structure of New Layered Oxides, MIILa2Ti3O10 (M = Co, Cu, and Zn). Chem. Mater. 1998, 11, 352–357. [Google Scholar] [CrossRef]
- Ogawa, M.; Kuroda, K. Preparation of Inorganic-Organic Nanocomposites through Intercalation of Organoammonium Ions into Layered Silicates. Bull. Chem. Soc. Jpn. 1997, 70, 2593–2618. [Google Scholar] [CrossRef]
- Sasaki, T.; Kooli, F.; Iida, M.; Michiue, Y.; Takenouchi, S.; Yajima, Y.; Izumi, F.; Chakoumakos, B.C.; Watanabe, M. A Mixed Alkali Metal Titanate with the Lepidocrocite-like Layered Structure. Preparation, Crystal Structure, Protonic Form, and Acid−Base Intercalation Properties. Chem. Mater. 1998, 10, 4123–4128. [Google Scholar] [CrossRef]
- Sasaki, T.; Izumi, F.; Watanabe, M. Intercalation of Pyridine in Layered Titanates. Chem. Mater. 1996, 8, 777–782. [Google Scholar] [CrossRef]
- Sun, C.; Peng, P.; Zhu, L.; Zheng, W.; Zhao, Y. Designed Reversible Alkylamine Intercalation-Deintercalation in the Layered Perovskite-Type Oxide KCa2Nb3O10. Eur. J. Inorg. Chem. 2008, 24, 3864–3870. [Google Scholar] [CrossRef]
- Raciulete, M.; Papa, F.; Culita, D.C.; Munteanu, C.; Atkinson, I.; Bratan, V.; Pandele-Cusu, J.; State, R.; Balint, I. Impact of RbLaTa2O7 Layered Perovskite Synthesis Conditions on their Activity for Photocatalytic Abatement of Trichloroethylene. Rev. Roum. Chim. 2018, 63, 821–828. [Google Scholar]
- Geselbracht, M.J.; White, H.K.; Blaine, J.M.; Diaz, M.J.; Hubbs, J.L.; Adelstein, N.; Kurzman, J.A. New solid acids in the triple-layer Dion-Jacobson layered perovskite family. Mater. Res. Bull. 2011, 46, 398–406. [Google Scholar] [CrossRef]
- Cheng, S.; Hwang, H.-D.; Maciel, G.E. Synthesis and pillaring of a layered vanadium oxide from V2O5 at ambient temperature. J. Mol. Struct. 1998, 470, 135–149. [Google Scholar] [CrossRef]
- Castellini, E.; Bernini, F.; Sebastianelli, L.; Sainz-Díaz, C.I.; Serrano, A.; Castro, G.R.; Malferrari, D.; Brigatti, M.F.; Borsari, M. Interlayer-Confined Cu(II) Complex as an Efficient and Long-Lasting Catalyst for Oxidation of H2S on Montmorillonite. Minerals 2020, 10, 510. [Google Scholar] [CrossRef]
- Mokhtar, A.; Medjhouda, Z.A.K.; Djelad, A.; Boudia, A.; Bengueddach, A.; Sassi, M. Structure and intercalation behavior of copper II on the layered sodium silicate magadiite material. Chem. Pap. 2018, 72, 39–50. [Google Scholar] [CrossRef]
- Ding, Z.; Frost, R.L. Study of copper adsorption on montmorillonites using thermal analysis methods. J. Colloid Interface Sci. 2004, 269, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakiki, A.; Kerbadou, R.M.; Boukoussa, B.; Zahmani, H.H.; Launay, F.; Pailleret, A.; Pillier, F.; Hacini, S.; Bengueddach, A.; Hamacha, R. Catalytic behavior of copper-amine complex supported on mesoporous silica SBA-15 toward mono-Aza-Michael addition: Role of amine groups. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1773–1784. [Google Scholar] [CrossRef]
- Chen, Z.; Deutsch, T.G.; Dinh, H.N.; Domen, K.; Emery, K.; Forman, A.J.; Gaillard, N.; Garland, R.; Heske, C.; Jaramillo, T.F.; et al. Photoelectrochemical Water Splitting. Incident photon-to-current efficiency and photocurrent spectroscopy. In Photoelectrochemical Water Splitting; Chen, Z., Dinh, H.N., Miller, E., Eds.; Briefs in Energy; Springer: New York, NY, USA, 2013; pp. 87–97. [Google Scholar] [CrossRef]
- Ghosh, B.; Halder, S.; Sinha, T.P. Dielectric Relaxation and Collective Vibrational Modes of Double-Perovskites A2SmTaO6 (A = Ba, Sr and Ca). J. Am. Ceram. Soc. 2014, 97, 2564–2572. [Google Scholar] [CrossRef]
- Kumar, D.V.R.; Kim, I.; Zhong, Z.; Kim, K.; Lee, D.; Moon, J. Cu(II)-alkyl amine complex mediated hydrothermal synthesis of Cu nanowires: Exploring the dual role of alkyl amines. Phys. Chem. Chem. Phys. 2014, 16, 22107–22115. [Google Scholar] [CrossRef]
- Espeel, P.; Goethals, F.; Driessen, F.; Nguyen, L.-T.T.; Du Prez, F.E. One-pot, additive-free preparation of functionalized polyurethanes via amine-thiol-ene conjugation. Polym. Chem. 2013, 4, 2449–2456. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Zhao, Y.; Chen, J.; Zhang, P.; Gao, L.; Wang, M.; Cao, C.; Wen, W.; Zhu, C. Difunctional Cu-doped carbon dots: Catalytic activity and fluorescence indication for the reduction reaction of p-nitrophenol. RSC Adv. 2017, 7, 33929–33936. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Dong, S.; Shi, L.; Sun, Q. Modification of CuCl2∙2H2O by dielectric barrier discharge and its application in the hydroxylation of benzene. New J. Chem. 2019, 43, 12744–12753. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Ramesh, C.; Ragunathan, V.; Thamilselvan, M.; Thamilselvan, M. Antibacterial activity of Cu2O nanoparticles on E Coli synthesized from Tridax Procumbens leaf extract and surface coating with polyaniline. Dig. J. Nanomater. Biostruct. 2012, 7, 833–839. [Google Scholar]
- Morioka, T.; Takesue, M.; Hayashi, H.; Watanabe, M.; Smith, R.L. Antioxidation properties and surface interactions of polyvinylpyrrolidone-capped zerovalent copper nanoparticles synthesized in supercritical water. ACS Appl. Mater. Interfaces 2016, 8, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Torre-Abreu, C.; Ribeiro, M.F.; Henriques, C.; Delahay, G. The role of Si/Al ratio, copper content and co-cation. Appl. Catal. B Environ. 1997, 14, 261–272. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, X.; Bi, X.; Wang, L.; Zhang, Z.; Jiang, Z.; Xiao, T.; Umar, A.; Wang, Q. Lanthanum-promoted copper-based hydrotalcites derived mixed oxides for NOx adsorption, soot combustion and simultaneous NOx-soot removal. Mater. Res. Bull. 2014, 51, 119–127. [Google Scholar] [CrossRef]
- Gervasini, A.; Bennici, S. Dispersion and surface states of copper catalysts by temperature-programmed-reduction of oxidized surfaces (s-TPR). Appl. Catal. A Gen. 2005, 281, 199–205. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, T.; Zhang, S.; Wang, Y.; Zhong, Q. Sol-gel synthesis of CuO-TiO2 catalyst with high dispersion CuO species for selective catalytic oxidation of NO. Appl. Surf. Sci. 2017, 411, 227–234. [Google Scholar] [CrossRef]
- Nestroinaia, O.V.; Ryltsova, I.G.; Lebedeva, O.E. Effect of synthesis method on properties of layered double hydroxides containing Ni(III). Crystals 2021, 11, 1429. [Google Scholar] [CrossRef]
- Wachs, I.E.; Briand, L.E.; Jehng, J.-M.; Burcham, L.; Gao, X. Molecular structure and reactivity of the group V metal oxides. Catal. Today 2000, 57, 323–330. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Cojocaru, B.; Jurca, B.; Tiseanu, C.; Nedelec, J.-M.; Kessler, V.G.; Parvulescu, V.I. Mesoporous tantalum oxide photocatalyst: Structure and activity evaluation. ChemistrySelect 2017, 2, 421–427. [Google Scholar] [CrossRef]
- Liu, M.; Qiu, X.; Hashimoto, K.; Miyauchi, M. Cu(II) nanocluster-grafted, Nb-doped TiO2 as an efficient visible-light-sensitive photocatalyst based on energy-level matching between surface and bulk states. J. Mater. Chem. A 2014, 2, 13571–13579. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Pan, X.; Wang, G.; Yi, Z. Improved propane photooxidation activities upon nano Cu2O/TiO2 heterojunction semiconductors at room temperature. RSC Adv. 2015, 5, 22038–22043. [Google Scholar] [CrossRef]
- Li, J.; Zeng, J.; Jia, L.; Fang, W. Investigations on the effect of Cu2+/Cu1+ redox couples and oxygen vacancies on photocatalytic activity of treated LaNi1−xCuxO3 (x = 0.1, 0.4, 0.5). Int. J. Hydrog. Energy 2010, 35, 12733–12740. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Evans, D.G.; Duan, X. Structure and surface chemistry of manganese-doped copper-based mixed metal oxides derived from layered double hydroxides. Colloids Surf. A Physicochem. Eng. Asp. 2004, 244, 169–177. [Google Scholar] [CrossRef]
- Sandulescu, A.; Anastasescu, C.; Papa, F.; Raciulete, M.; Vasile, A.; Spataru, T.; Scarisoreanu, M.; Fleaca, C.; Mihailescu, C.N.; Teodorescu, V.S.; et al. Advancements on Basic Working Principles of Photo-Driven Oxidative Degradation of Organic Substrates over Pristine and Noble Metal-Modified TiO2. Model Case of Phenol Photo Oxidation. Catalysts 2021, 11, 487. [Google Scholar] [CrossRef]
- Sobczyński, A.; Duczmal, Ł.; Zmudziński, W. Phenol destruction by photocatalysis on TiO2: An attempt to solve the reaction mechanism. J. Molec. Catal. A Chem. 2004, 213, 225–230. [Google Scholar] [CrossRef]
- Rydosz, A. The use of copper oxide thin films in gas-sensing applications. Coatings 2018, 8, 425. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Bi, J.; Li, Z.; Wang, X.; Fu, X. Rapid preparation of Bi2WO6 photocatalyst with nanosheet morphology via microwave-assisted solvothermal synthesis. Catal. Today 2008, 131, 15–20. [Google Scholar] [CrossRef]
- Tang, J.; Zou, Z.; Ye, J. Photocatalytic Decomposition of Organic Contaminants by Bi2WO6 Under Visible Light Irradiation. Catal. Lett. 2004, 92, 53–56. [Google Scholar] [CrossRef]
- Yan, H.; Wang, X.; Yao, M.; Yao, X. Band structure design of semiconductors for enhanced photocatalytic activity: The case of TiO2. Prog. Nat. Sci. Mater. Int. 2013, 23, 402–407. [Google Scholar] [CrossRef]
- Dey, S.; Ricciardo, R.A.; Cuthbert, H.L.; Woodward, P.M. Metal-to-metal charge transfer in AWO4 (A = Mg, Mn, Co, Ni, Cu, or Zn) compounds with the wolframite structure. Inorg. Chem. 2014, 53, 4394–4399. [Google Scholar] [CrossRef]
- Palasyuk, O.; Palasyuk, A.; Maggard, P.A. Syntheses, optical properties and electronic structures of copper (I) tantalates: Cu5Ta11O30 and Cu3Ta7O19. J. Solid State Chem. 2010, 183, 814–822. [Google Scholar] [CrossRef]
- Mao, L.; Cai, X.; Gao, H.; Diao, X.; Zhang, J. A newly designed porous oxynitride photoanode with enhanced charge carrier mobility. Nano Energy 2017, 39, 172–182. [Google Scholar] [CrossRef]
- Jiang, C.-M.; Farmand, M.; Wu, C.H.; Liu, Y.-S.; Guo, J.; Drisdell, W.S.; Cooper, J.K.; Sharp, I.D. Electronic Structure, Optoelectronic Properties, and Photoelectrochemical Characteristics of γ-Cu3V2O8 Thin Films. Chem. Mater. 2017, 29, 3334–3345. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Sun, Y.; Fan, Z.; Zhao, D.; Xiong, S.; Zhang, B.; Zhou, S.; Liu, G. Mechanisms for O2− and OH production on flowerlike BiVO4 photocatalysis based on electron spin resonance. Front. Chem. 2018, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Dubale, A.A.; Ahmed, I.N.; Chen, X.-H.; Ding, C.; Hou, G.-H.; Guan, R.-F.; Meng, X.; Yang, X.-L.; Xie, M.-H. A highly stable metal-organic framework derived phosphorus doped carbon/Cu2O structure for efficient photocatalytic phenol degradation and hydrogen production. J. Mater. Chem. A 2019, 7, 6062–6079. [Google Scholar] [CrossRef]
- Raciulete, M.; Papa, F.; Kawamoto, D.; Munteanu, C.; Culita, D.C.; Negrila, C.; Atkinson, I.; Bratan, V.; Pandele-Cusu, J.; Balint, I. Particularities of trichloroethylene photocatalytic degradation over crystalline RbLaTa2O7 nanowire bundles grown by solid-state synthesis route. J. Environ. Chem. Eng. 2019, 7, 102789. [Google Scholar] [CrossRef]
- Raciulete, M.; Papa, F.; Negrila, C.; Bratan, V.; Munteanu, C.; Pandele-Cusu, J.; Culita, D.C.; Atkinson, I.; Balint, I. Strategy for Modifying Layered Perovskites toward Efficient Solar Light-Driven Photocatalysts for Removal of Chlorinated Pollutants. Catalysts 2020, 10, 637. [Google Scholar] [CrossRef]
- Li, T.F.; Liu, J.J.; Jin, X.M.; Wang, F.; Song, Y. Composition-dependent electro-catalytic activities of covalent carbon-LaMnO3 hybrids as synergistic catalysts for oxygen reduction reaction. Electrochim. Acta 2016, 198, 115–126. [Google Scholar] [CrossRef]

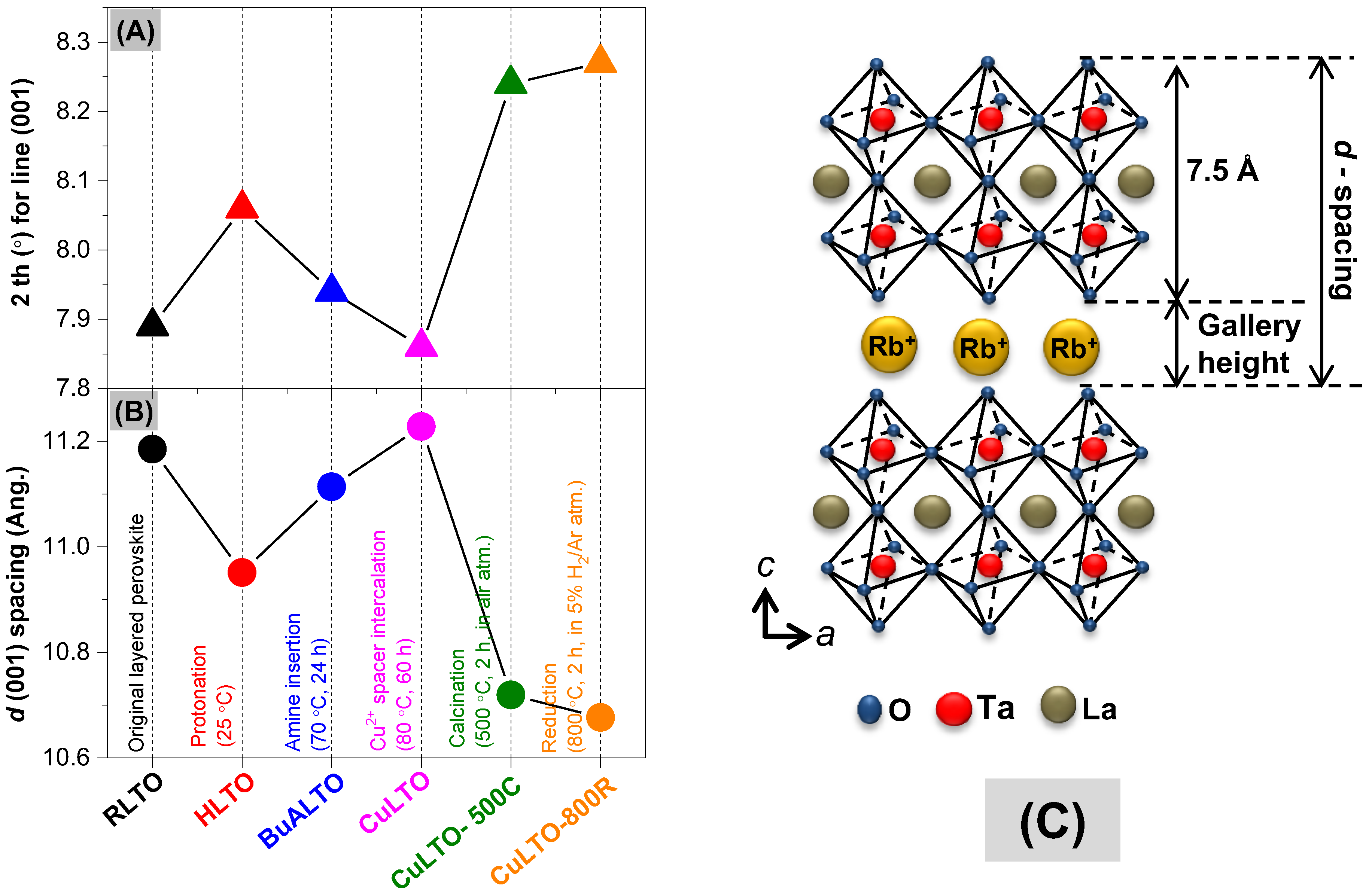











| Sample | Lattice Parameters | d-Spacing for l(001), (Å) | Gallery Height (Å) | Phase Composition | |
|---|---|---|---|---|---|
| a (Å) | c (Å) | ||||
| RbLTO | 3.881 | 11.121 | 11.185 | 3.69 | RbLaTa2O7 |
| HLTO | 3.883 | 10.924 | 10.951 | 3.45 | HLaTa2O7 |
| BuALTO | 3.889 | 11.113 | 11.113 | 3.61 | (C4H11N)LaTa2O7 |
| CuLTO | 3.888 | 11.023 | 11.228 | 3.73 | Cu(LaTa2O7)2 |
| CuLTO-500C | 3.880 | 10.718 | 10.719 | 3.22 | Cu(LaTa2O7)2, Cu2O, CuO, Ta2O5 |
| CuLTO-800R | 3.884 | 10.676 | 10.676 | 3.18 | Cu(LaTa2O7)2, Cu2O, CuO, Ta2O5 |
| Sample | Chemical Analysis of the Products (at. %) | Atomic Ratio | |||||
|---|---|---|---|---|---|---|---|
| Rb (%) | C (%) | O (%) | La (%) | Ta (%) | Cu (%) | Cu/(La + Ta) | |
| RbLTO | 10.0 | 39.6 | 36.5 | 5.4 | 8.5 | 0.0 | 0.00 |
| CuLTO | 0.0 | 67.0 | 32.9 | 0.0 | 0.0 | 0.1 | 0.00 |
| CuLTO-500C | 0.0 | 33.3 | 58.4 | 1.5 | 5.6 | 1.3 | 0.18 |
| CuLTO-800R | 0.0 | 39.3 | 45.1 | 3.1 | 8.9 | 3.5 | 0.29 |
| Catalyst | H2 Consumption (µmoles∙g−1) | Total H2 Consumption (µmoles∙g−1) | ||
|---|---|---|---|---|
| Cu from Surface | Cu from Interlayer | |||
| Cu2+/Cu+ | Cu+/Cu0 | Cu2+/Cu0 | ||
| CuLTO | 11.64 | 44.25 | 662.16 | 718.05 |
| CuLTO-500C | 149.92 | 140.88 | 466.61 | 757.41 |
| CuLTO-800R | 91.37 | 88.54 | 66.58 | 246.49 |
| Photocatalyst | Phenol Conversion (%) 1 | SSA (m2·g−1) |
|---|---|---|
| RbLTO | 7.6 | 1.5 |
| HLTO | 8.5 | 2.6 |
| CuLTO | 13.0 | 4.1 |
| CuLTO-500C | 14.9 | 4.9 |
| CuLTO-800R | 16.6 | 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raciulete, M.; Anastasescu, C.; Papa, F.; Atkinson, I.; Bradu, C.; Negrila, C.; Eftemie, D.-I.; Culita, D.C.; Miyazaki, A.; Bratan, V.; et al. Band-Gap Engineering of Layered Perovskites by Cu Spacer Insertion as Photocatalysts for Depollution Reaction. Catalysts 2022, 12, 1529. https://doi.org/10.3390/catal12121529
Raciulete M, Anastasescu C, Papa F, Atkinson I, Bradu C, Negrila C, Eftemie D-I, Culita DC, Miyazaki A, Bratan V, et al. Band-Gap Engineering of Layered Perovskites by Cu Spacer Insertion as Photocatalysts for Depollution Reaction. Catalysts. 2022; 12(12):1529. https://doi.org/10.3390/catal12121529
Chicago/Turabian StyleRaciulete, Monica, Crina Anastasescu, Florica Papa, Irina Atkinson, Corina Bradu, Catalin Negrila, Diana-Ioana Eftemie, Daniela C. Culita, Akane Miyazaki, Veronica Bratan, and et al. 2022. "Band-Gap Engineering of Layered Perovskites by Cu Spacer Insertion as Photocatalysts for Depollution Reaction" Catalysts 12, no. 12: 1529. https://doi.org/10.3390/catal12121529
APA StyleRaciulete, M., Anastasescu, C., Papa, F., Atkinson, I., Bradu, C., Negrila, C., Eftemie, D.-I., Culita, D. C., Miyazaki, A., Bratan, V., Pandele-Cusu, J., Munteanu, C., Dobrescu, G., Sandulescu, A., & Balint, I. (2022). Band-Gap Engineering of Layered Perovskites by Cu Spacer Insertion as Photocatalysts for Depollution Reaction. Catalysts, 12(12), 1529. https://doi.org/10.3390/catal12121529









.jpg)