Cu/CuOx@C Composite as a High-Efficiency Electrocatalyst for Oxygen Reduction Reactions
Abstract
1. Introduction
2. Results
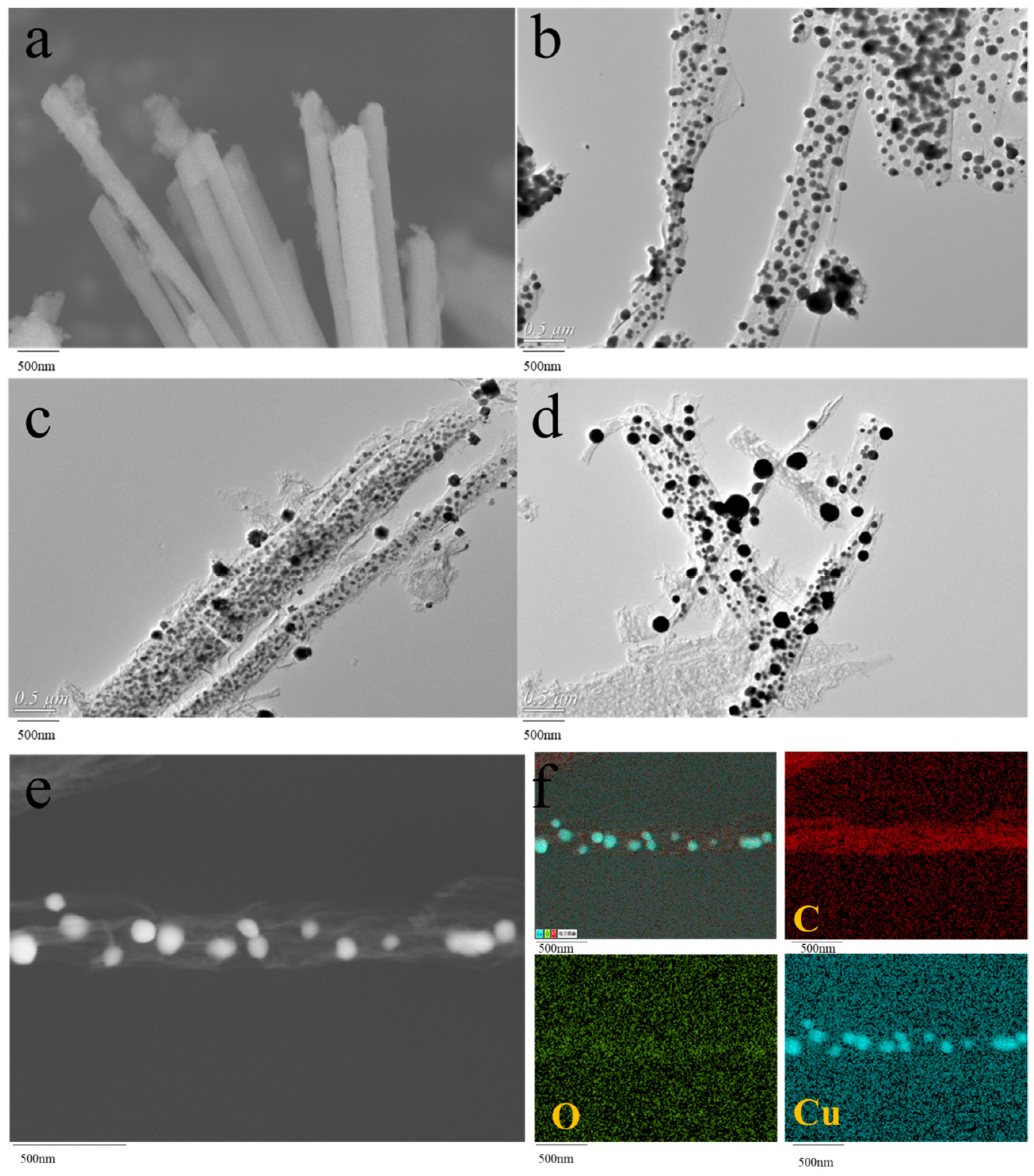
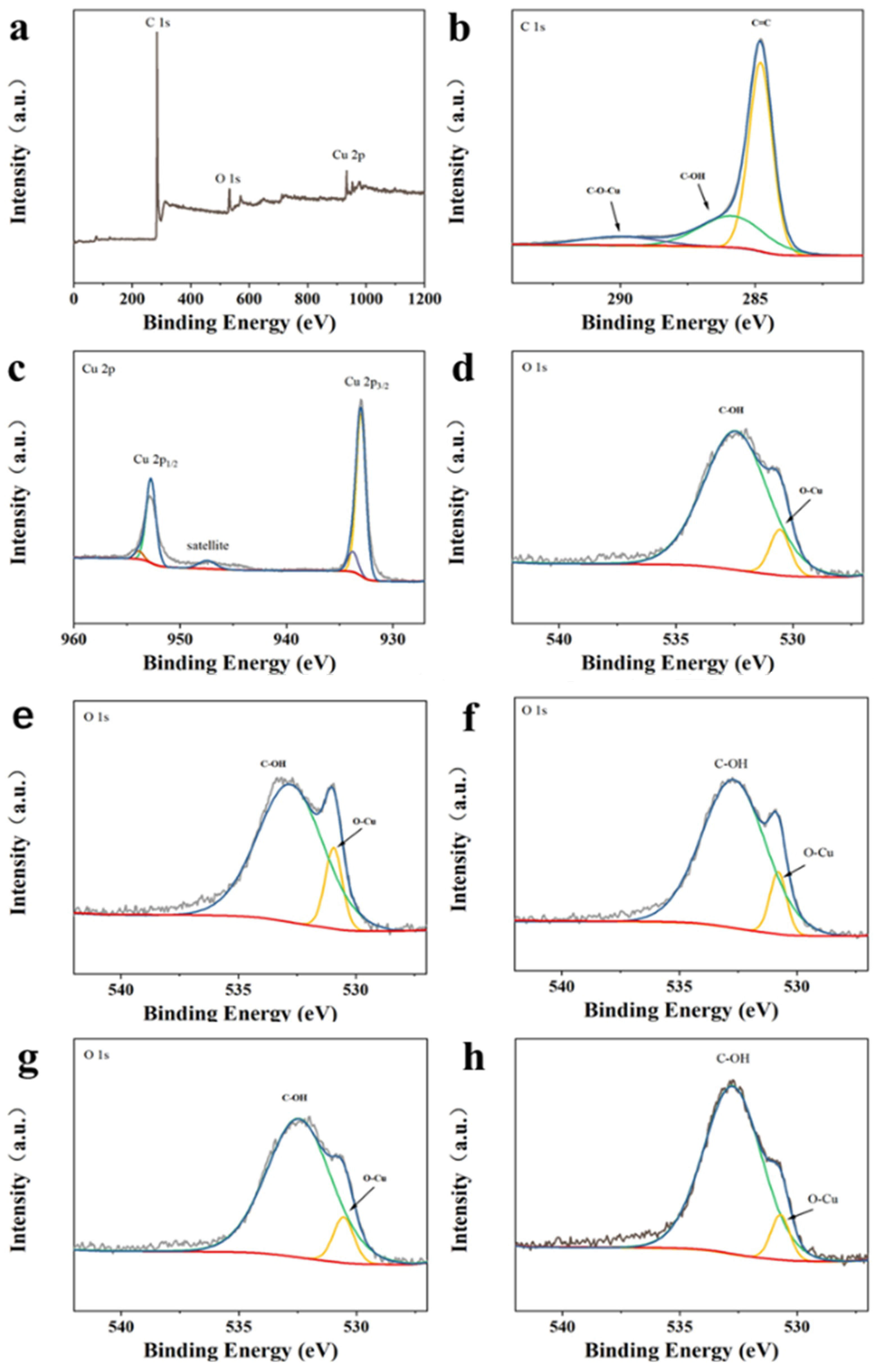
2.1. Synthesis and Characterization
2.2. Electrocatalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Electrocatalysts
3.2.1. Synthesis of Cu(OH)(Hsal)·H2O Precursor
3.2.2. Synthesis of Cu@C Nanofibers
3.2.3. Synthesis of Cu/CuOx@C Nanofibers
3.3. Characterization
3.4. Electrochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lewis, N.; Nocera, D. Powering the planet chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shao, Z.; Jiang, S.P. High-Entropy Materials for Water Electrolysis. Energy Technol. 2022, 10, 1–17. [Google Scholar] [CrossRef]
- Hamrock, S.; Herring, A.; Zawodzinski, T. Fuel Cell Chemistry and Operation. ACS Symposium. 2010, 172, 1. [Google Scholar] [CrossRef]
- Xu, X.; Su, C.; Shao, Z. Fundamental Understanding and Application of Ba0.5Sr0.5Co0.8Fe0.2O3−δPerovskite in Energy Storage and Conversion: Past, Present, and Future. Energy Fuels. 2021, 35, 13585–13609. [Google Scholar] [CrossRef]
- Sumboja, A.; Ge, X.; Goh, T.; Li, B.; Geng, D.; Hor, T.; Zong, Y.; Liu, Z. Manganese Oxide Catalyst Grown on Carbon Paper as an Air Cathode for High-Performance Rechargeable Zinc-Air Batteries. ChemPlusChem 2015, 80, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zaman, S.; Tian, X.; Wang, Z.; Fang, W.; Xia, B. Advanced Platinum-Based Oxygen Reduction Electrocatalysts for Fuel Cells. Accounts Chem. Res. 2021, 54, 311–322. [Google Scholar] [CrossRef]
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, 1804799. [Google Scholar] [CrossRef]
- Paul, R.; Du, F.; Dai, L.; Ding, Y.; Wang, Z.; Wei, F.; Roy, A. 3D Heteroatom-Doped Carbon Nanomaterials as Multifunctional Metal-Free Catalysts for Integrated Energy Devices. Adv. Mater. 2019, 31, 1805598. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef]
- Chen, G.; Liu, P.; Liao, Z.; Sun, F.; He, Y.; Zhong, H.; Zhang, T.; Zschech, E.; Chen, M.; Wu, G.; et al. Zinc-Mediated Template Synthesis of Fe-N-C Electrocatalysts with Densely Accessible Fe-Nx Active Sites for Efficient Oxygen Reduction. Adv. Mater. 2020, 32, 1907399. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Yao, S.; Hao, C.; Pan, C.; Xiang, X.; Tian, Z.; Shen, P.; Shao, Z.; Jiang, S.; et al. Boosting Electrocatalytic Activity of Single Atom Catalysts Supported on Nitrogen-Doped Carbon through N Coordination Environment Engineering. Small 2022, 18, 2105329. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yin, H.; Wang, Y.; Chuang, C.; Xing, L.; Dong, M.; Lu, Y.; Casillas, G.; Zheng, Y.; Chen, S.; et al. Coexisting Single-Atomic Fe and Ni Sites on Hierarchically Ordered Porous Carbon as a Highly Efficient ORR Electrocatalyst. Adv. Mater. 2020, 32, 2004670. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Liu, F.; Liu, X.; Liao, S.; You, C.; Tian, X.; Nan, H.; Luo, F.; Song, H.; Fu, Z.; et al. Effect of Transition Metals on the Structure and Performance of the Doped Carbon Catalysts Derived From Polyaniline and Melamine for ORR Application. ACS Catal. 2014, 4, 3797–3805. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, D.; Xu, H.; Zeng, X.; Wan, X.; Shui, J.; Xiang, Z.; Cao, D. Unveiling the high-activity origin of single-atom iron catalysts for oxygen reduction reaction. Proc. Natl. Acad. Sci. USA 2018, 115, 6626–6631. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Yao, T.; Wu, Y.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Deng, Z.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. 2016, 128, 10958–10963. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, Y.; Disalvo, F.J.; Abruna, H.D. Metal-Organic-Framework-Derived Co-Fe Bimetallic Oxygen Reduction Electrocatalysts for Alkaline Fuel Cells. J. Am. Chem. Soc. 2019, 141, 10744–10750. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Luo, G.; Li, Z.; Zhao, C.; Zhang, H.; Zhu, M.; Xu, Q.; Wang, X.; Zhao, C.; et al. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energ. Environ. Sci. 2019, 11, 3375–3379. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, R.; Ji, S.; Li, H.; Tang, K.; Jiang, P.; Hu, H.; Zhang, Z.; Hao, H.; Qu, Q.; et al. Atomic-Level Modulation of Electronic Density at Cobalt Single-Atom Sites Derived from Metal-Organic Frameworks: Enhanced Oxygen Reduction Performance. Angew. Chem. Int. Edit. 2022, 61, 3212–3221. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Mao, X.; Liu, D.; He, W.; Li, J.; Liu, J.; Yan, R.; Chen, J.; Song, L.; et al. Edge-Rich FeN4 Active Sites in Defective Carbon for Oxygen Reduction Catalysis. Adv. Mater. 2020, 32, 2000966. [Google Scholar] [CrossRef]
- Shao, Y.; Dodelet, J.P.; Wu, G.; Zelenay, P. PGM-Free Cathode Catalysts for PEM Fuel Cells: A Mini-Review on Stability Challenges. Adv. Mater. 2019, 31, 1807615. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Cullen, D.A.; Hwang, S.; Wang, M.; Li, B.; Liu, K.; Karakalos, S.; Lucero, M.; Zhang, H.; et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945. [Google Scholar] [CrossRef]
- Yu, H.; Fisher, A.C.; Cheng, D.; Cao, D. Cu, N-codoped Hierarchical Porous Carbons as Electrocatalysts for Oxygen Reduction Reaction. ACS App. Mater. Inter. 2016, 8, 21431–21439. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Sun, F.; Xie, Y.; Wang, Y.; Yang, Y.; Tian, C.; Wang, L.; Fu, H. Operando Cooperated Catalytic Mechanism of Atomically Dispersed Cu-N4 and Zn-N4 for Promoting Oxygen Reduction Reaction. Angew. Chem. 2021, 133, 14124–14131. [Google Scholar] [CrossRef]
- Shang, H.; Zhou, X.; Dong, J.; Li, A.; Zhao, X.; Liu, Q.; Lin, Y.; Pei, J.; Li, Z.; Jiang, Z.; et al. Engineering unsymmetrically coordinated Cu-S1N3 single atom sites with enhanced oxygen reduction activity. Nat. Commun. 2020, 11, 3049. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.; Wang, Q.; Han, P.; Zheng, G. Cu, Co-Embedded N-Enriched Mesoporous Carbon for Efficient Oxygen Reduction and Hydrogen Evolution Reactions. Adv. Energy Mater. 2017, 7, 1700193. [Google Scholar] [CrossRef]
- Yan, X.; Tong, X.; Zhang, Y.; Han, X.; Wang, Y.; Jin, G.; Qin, Y.; Guo, X. Cuprous oxide nanoparticles dispersed on reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction. Chem. Commun. 2012, 48, 1892–1894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cheng, R.; Li, B.; Wang, C.; Guo, Y.; Liu, J.; Wang, L. Novel one-dimensional Cu@C nanofibers: Direct solid-state synthesis and applications in electrocatalytic water splitting. Chem. Commun. 2021, 57, 769–772. [Google Scholar] [CrossRef]
- Dai, L.; Qin, Q.; Wang, P.; Zhao, X.; Hu, C.; Liu, P.; Qin, R.; Chen, M.; Ou, D.; Xu, C.; et al. Ultrastable atomic copper nanosheets for selective electrochemical reduction of carbon dioxide. Sci. Adv. 2017, 3, 1701069. [Google Scholar] [CrossRef]
- Xie, Y.; Li, C.; Castillo, E.; Fang, J.; Dimitrov, N. Nanoporous Pd-Cu Thin Films as Highly Active and Durable Catalysts for Oxygen Reduction in Alkaline Media. Electrochim. Acta 2021, 385, 138306. [Google Scholar] [CrossRef]
- Yang, L.; Liu, D.; Cui, G.; Xie, Y. Cu2+1O/graphene nanosheets supported on three dimensional copper foam for sensitive and efficient non-enzymatic detection of glucose. RSC Adv. 2017, 7, 19312–19317. [Google Scholar] [CrossRef]
- Cao, X.; Cui, L.; Liu, B.; Liu, Y.; Jia, D.; Yang, W.; Razal, J.M.; Liu, J. Reverse synthesis of star anise-like cobalt doped Cu-MOF/Cu2+1O hybrid materials based on a Cu(OH)2 precursor for high performance supercapacitors. J. Mater. Chem. A 2019, 7, 3815–3827. [Google Scholar] [CrossRef]
- Wan, C.; Duan, X.; Huang, Y. Molecular Design of Single-Atom Catalysts for Oxygen Reduction Reaction. Adv. Energy Mater. 2020, 10, 1903815. [Google Scholar] [CrossRef]
- Hong, J.; Jin, C.; Yuan, J.; Zhang, Z. Atomic Defects in Two-Dimensional Materials: From Single-Atom Spectroscopy to Functionalities in Opto-/Electronics, Nanomagnetism, and Catalysis. Adv. Mater. 2017, 29, 1606434. [Google Scholar] [CrossRef]
- Shi, Z.; Sun, G.; Yuan, R.; Chen, W.; Wang, Z.; Zhang, L.; Zhan, K.; Zhu, M.; Yang, J.; Zhao, B. Scalable fabrication of NiCo2O4/reduced graphene oxide composites by ultrasonic spray as binder-free electrodes for supercapacitors with ultralong lifetime. J. Mater. Sci. Technol. 2022, 99, 260–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Yin, X.; Yan, Y.; Zhan, K.; Yang, J.; Zhao, B. Cobalt sulfide supported on nitrogen and sulfur dual-doped reduced graphene oxide for highly active oxygen reduction reaction. RSC Adv. 2017, 7, 5024. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Xiong, D.; Qiao, Y.; Tang, Y.; Gao, F. Hybridized phosphate with ultrathin nanoslices and single crystal microplatelets for high performance supercapacitors. SCI. REP-UK 2016, 6, 17613. [Google Scholar] [CrossRef]
- Reichardt, W.; Gompf, F.; Aïn, M.; Wanklyn, B.M. Lattice dynamics of cupric oxide. Zeitschrift für Physik B Condensed Matter 1990, 81, 19–24. [Google Scholar] [CrossRef]
- Chen, X.; Irwin, J.C.; Franck, J.P. Evidence for a strong spin-phonon interaction in cupric oxide. Phys. Rev. B 1995, 52, 13130–13133. [Google Scholar] [CrossRef]
- Kliche, G.; Popovic, Z. Far-infrared spectroscopic investigations on CuO. Phys. Rev. B 1991, 42, 10060–10066. [Google Scholar] [CrossRef]
- Debbichi, L.; Lucas, M.C.M.D.; Pierson, J.F.; Krüger, P. Vibrational Properties of CuO and Cu4O3 from First-Principles Calculations, and Raman and Infrared Spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Petroff, Y.; Yu, P.Y.; Shen, Y.R. Study of photoluminescence in Cu2O. Phys. Rev. B 1975, 12, 2488–2495. [Google Scholar] [CrossRef]
- Ivanda, M.; Waasmaier, D.; Endriss, A. Low-temperature anomalies of cuprite observed by Raman spectroscopy and x-ray powder diffraction. J. Raman. Spectrosc. 1997, 28, 487–493. [Google Scholar] [CrossRef]
- Guo, Y.; Dai, M.; Zhu, Z.; Chen, Y.; He, H.; Qin, T. Chitosan modified Cu2O nanoparticles with high catalytic activity for p-nitrophenol reduction. Appl. Surf. Sci. 2019, 480, 601–610. [Google Scholar] [CrossRef]
- Guo, D.; Wang, L.; Du, Y.; Ma, Z.; Shen, L. Preparation of octahedral Cu2O nanoparticles by a green route. Mater. Lett. 2015, 160, 541–543. [Google Scholar] [CrossRef]
- Sahoo, R.; Dutta, S.; Pradhan, M.; Ray, C.; Roy, A.; Pal, T.; Pal, A. Arsenate stabilized Cu2O nanoparticle catalyst for one-electron transfer reversible reaction. Dalton Trans. 2014, 43, 6677–6683. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface. Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Tran, D.T.; Le, H.T.; Doan, T.L.L.; Kim, N.H.; Lee, J.H. Pt Nanodots Monolayer Modified Mesoporous Cu@CuxO Nanowires for Improved Overall Water Splitting Reactivity. Nano Energy 2019, 59, 216–228. [Google Scholar] [CrossRef]
- He, H.; Wang, M.; Zhao, J.; Zhang, Y. Poly (10,12-bis(4-hexylthiophen-2-yl)thieno[3’,4’:5,6]pyrazino[2,3-f][1,10]-phenanthroline)-copper(II) complex as an efficient electrocatalyst for oxygen reduction. Chem. Eng. J. 2017, 316, 680–691. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Y.; Wei, P.; Zhang, Q.; Liu, J. Efficient electrocatalytic O2 reduction at copper complexes grafted onto polyvinylimidazole coated carbon nanotubes. Chem. Commun. 2017, 53, 1514–1517. [Google Scholar] [CrossRef]
- Yang, L.; Yu, J.; Wei, Z.; Li, G.; Cao, L.; Zhou, W.; Chen, S. Co-N-doped MoO2 Nanowires as Efficient Electrocatalysts for the Oxygen Reduction Reaction and Hydrogen Evolution Reaction. Nano Energy 2017, 41, 772–779. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Preuss, K.; Marinovic, A.; Jorge, A.B.; Mansor, N.; Brett, D.J.L.; Fuertes, A.B.; Sevilla, M.; Titirici, M. Fe-N-Doped Carbon Capsules with Outstanding Electrochemical Performance and Stability for the Oxygen Reduction Reaction in Both Acid and Alkaline Conditions. ACS Nano 2016, 10, 5922–5932. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Min, C.; Tan, F.; Li, Z.; Zhang, B.; Si, R.; Xu, M.; Liu, W.; Zhou, L.; Wei, Q.; et al. Bottom-Up Construction of Active Sites in a Cu-N4-C Catalyst for Highly Efficient Oxygen Reduction Reaction. ACS Nano 2019, 13, 3177–3187. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, Y.; Cui, T.; Xu, L.; Wang, Y.; Chen, W.; Zhang, P.; Zheng, T.; Fu, X.; Zhang, S.; et al. Engineering of Coordination Environment and Multiscale Structure in Single-Site Copper Catalyst for Superior Electrocatalytic Oxygen Reduction. Nano Lett. 2020, 20, 6206–6214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, W.; Shang, H.; Chen, W.; Sun, T.; Li, H.; Dong, J.; Zhou, J.; Li, Z.; Wang, Y.; et al. Atomic interface effect of a single atom copper catalyst for enhanced oxygen reduction reactions. Energ. Environ. Sci. 2019, 12, 3508–3514. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, B.; Peng, Q.; Zhou, J.; Sun, Z. Mo2 B2 MBene-supported single-atom catalysts as bifunctional HER/OER and OER/ORR electrocatalysts. J. Mater. Chem. A 2021, 9, 433–441. [Google Scholar] [CrossRef]
- Nilekar, A.U.; Mavrikakis, M. Improved oxygen reduction reactivity of platinum monolayers on transition metal surfaces. Surf. Sci. 2008, 602, 89–94. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, S.; Zuo, J.; Zhang, B. Photocatalytic CO2 Reduction Coupled with Alcohol Oxidation over Porous Carbon Nitride. Catalysts 2022, 12, 672. [Google Scholar] [CrossRef]
- Zhang, B.; Qiu, C.; Wang, S.; Gao, H.; Yu, K.; Zhang, Z.; Ling, X.; Ou, W.; Su, C. Electrocatalytic water-splitting for the controllable and sustainable synthesis of deuterated chemicals. Sci. Bull. 2021, 66, 562–569. [Google Scholar] [CrossRef]
- Li, P.; Jin, Z.; Qian, Y.; Fang, Z.; Xiao, D.; Yu, G. Supramolecular confinement of single Cu atoms in hydrogel frameworks for oxygen reduction electrocatalysis with high atom utilization. Mater. Today. 2020, 35, 78–86. [Google Scholar] [CrossRef]
- Meng, Y.; Yin, J.; Jiao, T.; Bai, J.; Zhang, L.; Su, J.; Liu, S.; Bai, Z.; Cao, M.; Peng, Q. Self-assembled copper/cobalt-containing polypyrrole hydrogels for highly efficient ORR electrocatalysts. J. Mol. Liq. 2019, 298, 112010. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, C.; He, X.; Su, J.W.; Parker, T.; White, T.A.; Griep, M.H.; Lin, J. Copper-promoted nitrogen-doped carbon derived from zeolitic imidazole frameworks for oxygen reduction reaction. Appl. Surf. Sci. 2019, 464, 344–350. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Gao, S.; Mao, K.; Xia, G.; Lin, Z.; Jiang, P.; Hu, L.; Chen, Q. Incorporation of Cu-Nx cofactors into graphene encapsulated Co as biomimetic electrocatalysts for efficient oxygen reduction. Nanoscale. 2018, 10, 21076–21086. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jin, H.; Meng, T.; Liao, K.; Meng, W.; Yang, J.; He, D.; Xiong, Y.; Mu, S. Fe, Cu-Coordinated ZIF-Derived Carbon Framework for Efficient Oxygen Reduction Reaction and Zinc-Air Batteries. Adv. Funct. Mater. 2018, 28, 1802596. [Google Scholar] [CrossRef]
- Saianand, G.; Gopalan, A.; Lee, J.C.; Sathish, C.I.; Gopalakrishnan, K.; Unni, G.; Shanbhag, D.; Dasireddy, V.D.B.C.; Yi, J.; Xi, S.; et al. Mixed Copper/Copper-Oxide Anchored Mesoporous Fullerene Nanohybrids as Superior Electrocatalysts toward Oxygen Reduction Reaction. Small 2020, 16, 1903937. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, R.; Shi, N.; Yang, J.; Yan, H.; Wang, J.; Ding, Z.; Huang, W.; Luo, Q.; Lin, Y.; et al. Cu, N-Codoped Carbon Nanodisks with Biomimic Stomata-Like Interconnected Hierarchical Porous Topology as Efficient Electrocatalyst for Oxygen Reduction Reaction. Small 2019, 15, 1902410. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Li, Y.-F.; Liu, L.-X.; Duan, L.; Ren, Z.-L.; Xu, S.-D.; Chen, L.; Guo, H.-J.; Huang, Y.; Shi, L.-J.; et al. Cu/CuOx@C Composite as a High-Efficiency Electrocatalyst for Oxygen Reduction Reactions. Catalysts 2022, 12, 1515. https://doi.org/10.3390/catal12121515
Zhang D, Li Y-F, Liu L-X, Duan L, Ren Z-L, Xu S-D, Chen L, Guo H-J, Huang Y, Shi L-J, et al. Cu/CuOx@C Composite as a High-Efficiency Electrocatalyst for Oxygen Reduction Reactions. Catalysts. 2022; 12(12):1515. https://doi.org/10.3390/catal12121515
Chicago/Turabian StyleZhang, Ding, Yun-Fei Li, Li-Xue Liu, Lei Duan, Zhi-Li Ren, Shou-Dong Xu, Liang Chen, Hui-Juan Guo, Yi Huang, Li-Juan Shi, and et al. 2022. "Cu/CuOx@C Composite as a High-Efficiency Electrocatalyst for Oxygen Reduction Reactions" Catalysts 12, no. 12: 1515. https://doi.org/10.3390/catal12121515
APA StyleZhang, D., Li, Y.-F., Liu, L.-X., Duan, L., Ren, Z.-L., Xu, S.-D., Chen, L., Guo, H.-J., Huang, Y., Shi, L.-J., & Yi, Q. (2022). Cu/CuOx@C Composite as a High-Efficiency Electrocatalyst for Oxygen Reduction Reactions. Catalysts, 12(12), 1515. https://doi.org/10.3390/catal12121515






