Substrate-Dependent Selectivity in Sc(OTf)3-Catalyzed Cyclization of Alkenoic Acids and N-Protected Alkenamides
Abstract
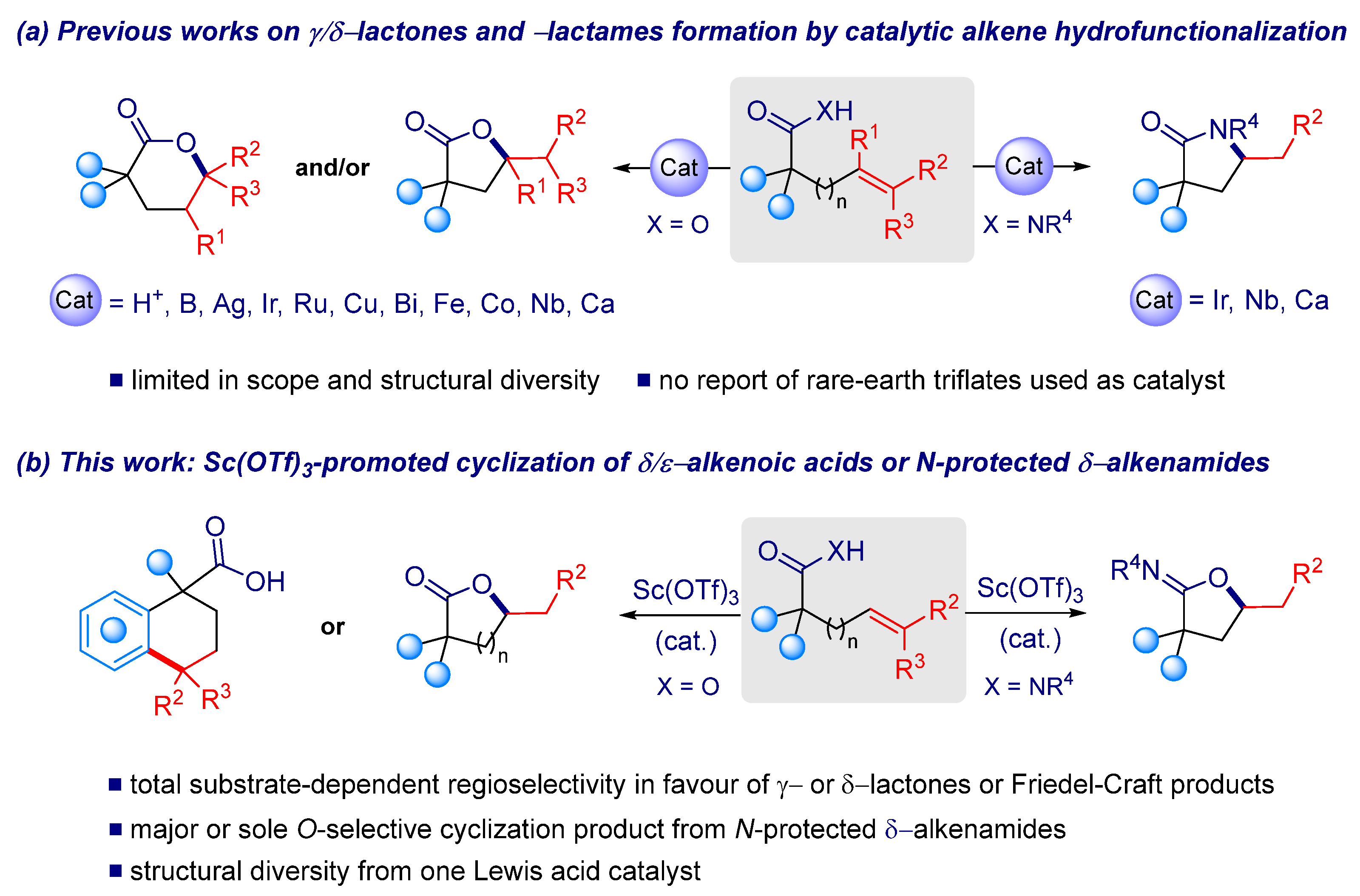
1. Introduction
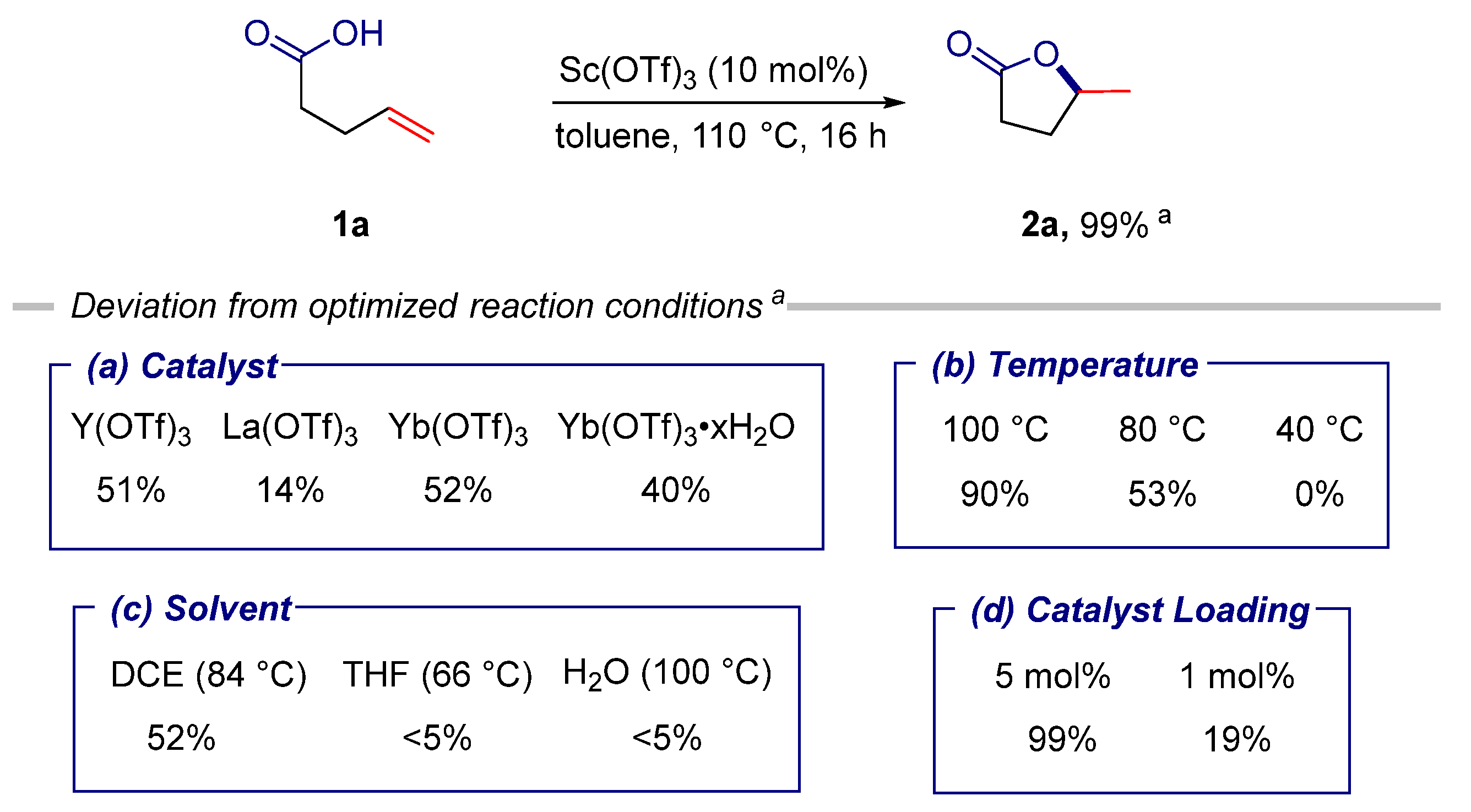
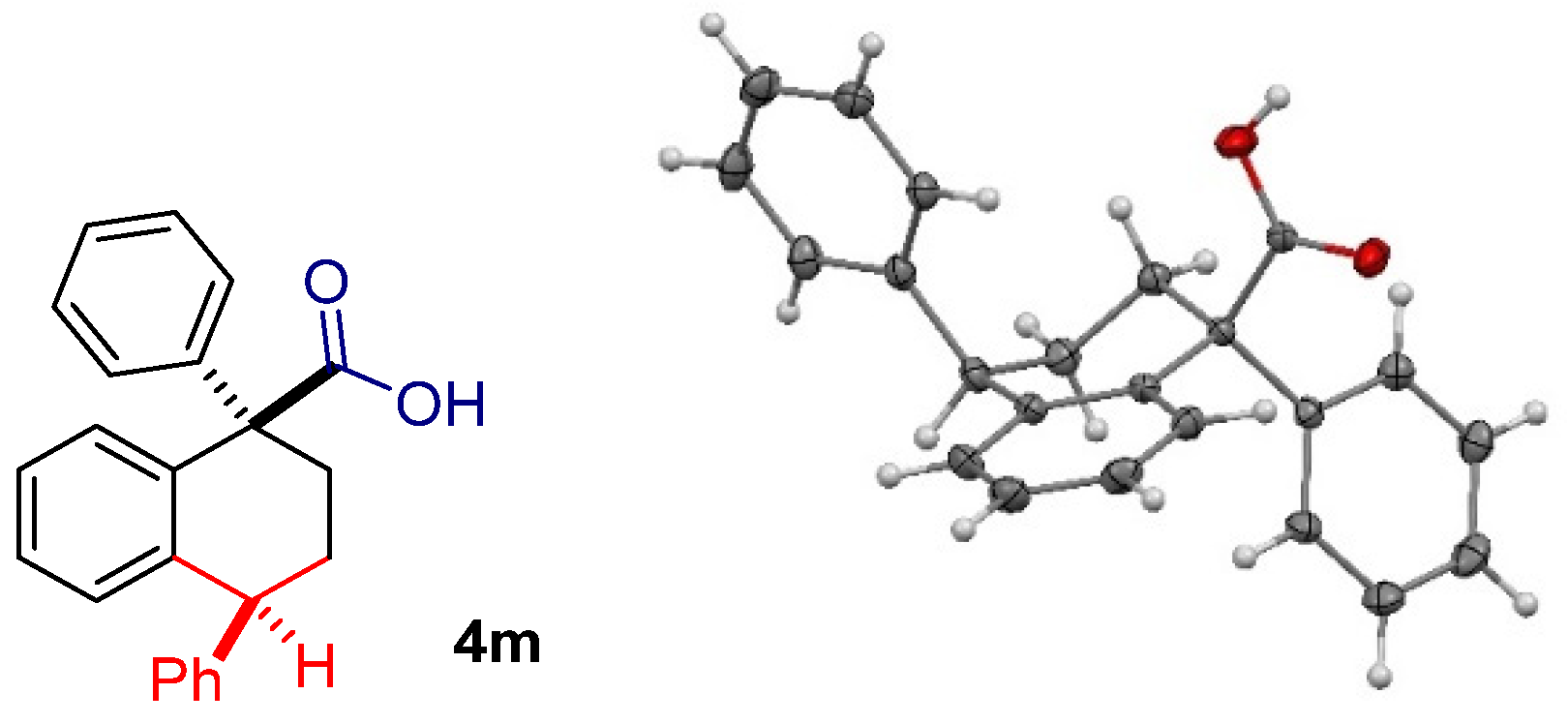
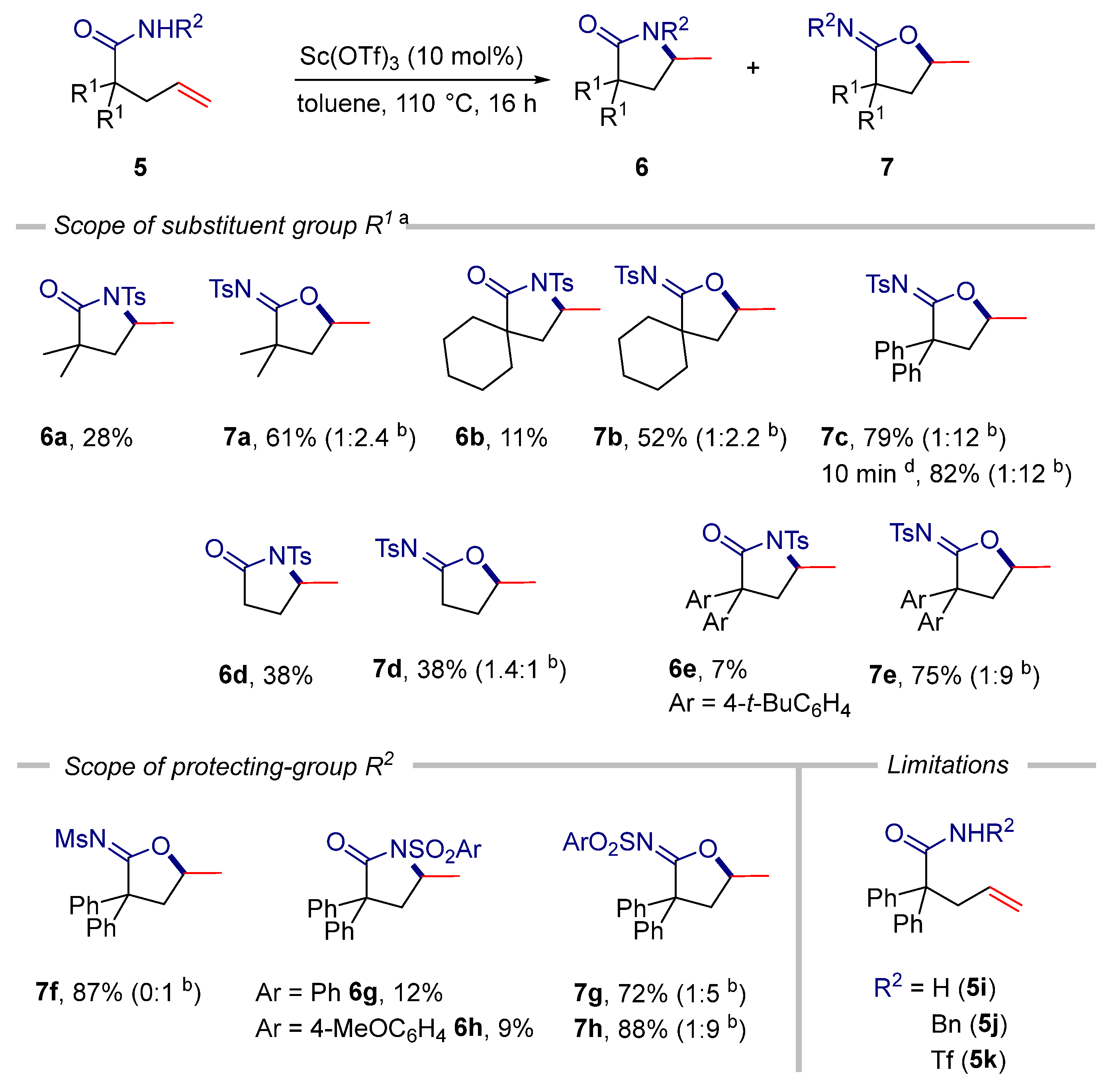
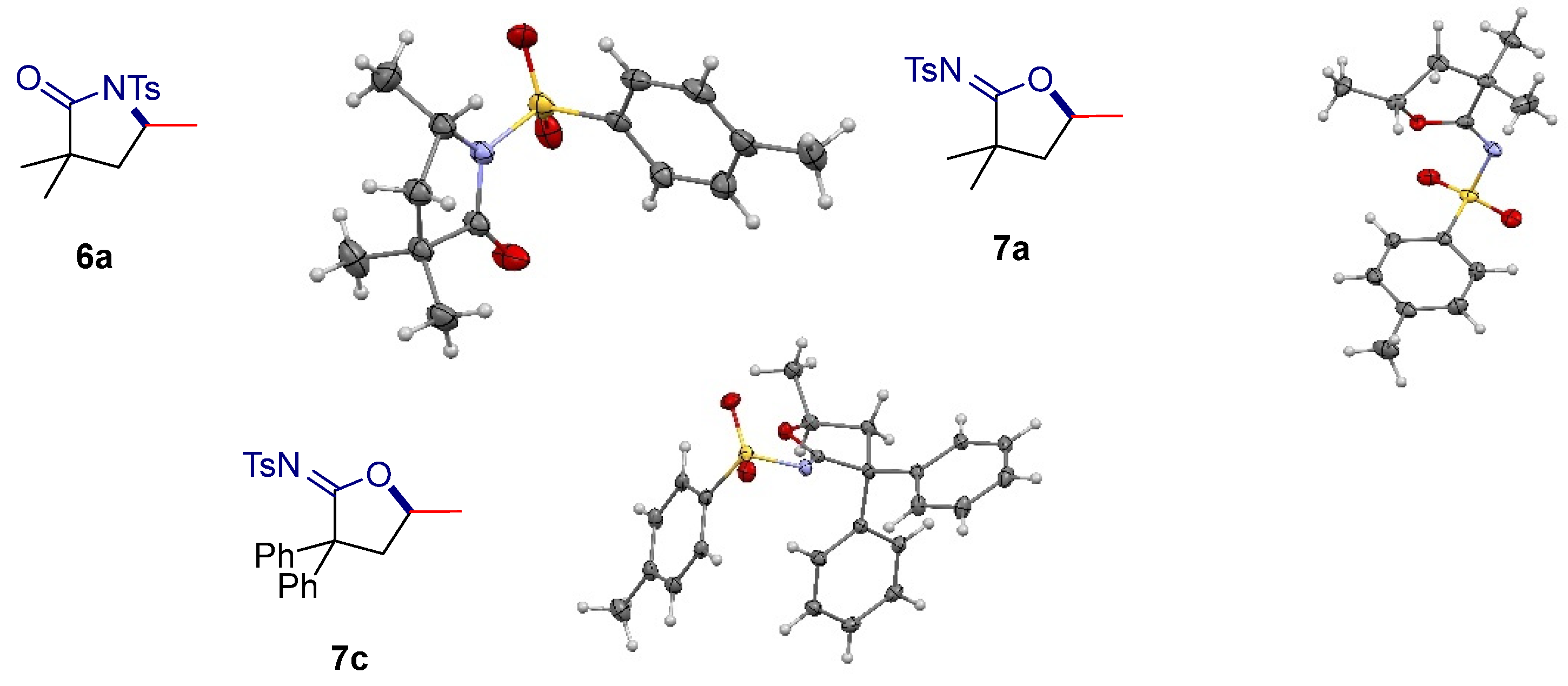
2. Results
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Experimental Methods
3.2.1. General Procedure for the Sc(OTf)3-Catalyzed Cyclization of Alkenoic Acids and Alkenamides under Thermal Conditions
3.2.2. General Procedure for the Sc(OTf)3-Catalyzed Cyclization of Alkenoic Acids or Alkenamide 5c under Microwave Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Natural Lactones and Lactams: Synthesis, Occurrence and Biological Activity; Janecki, T., Ed.; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Caruano, J.; Muccioli, G.G.; Robiette, R. Biologically Active γ-Lactams: Synthesis and Natural Sources. Org. Biomol. Chem. 2016, 14, 10134–10156. [Google Scholar] [CrossRef]
- Kamath, A.; Ojima, I. Advances in the Chemistry of β-Lactam and Its Medicinal Applications. Tetrahedron 2012, 68, 10640–10664. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.V.; Cox, L.R.; Meek, G. (π-Allyl)Tricarbonyliron Lactone Complexes in Organic Synthesis: A Useful and Conceptually Unusual Route to Lactones and Lactams. Chem. Rev. 1996, 96, 423–442. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.E.; Alper, H. The Application of Transition Metal Catalysis for Selective Cyclocarbonylation Reactions. Synthesis of Lactones and Lactams. Synlett 2000, 2000, 161–171. [Google Scholar] [CrossRef]
- Edlin, C.D.; Faulkner, J.; Fengas, D.; Helliwell, M.; Knight, C.K.; House, D.; Parker, J.; Preece, I.; Quayle, P.; Raftery, J.; et al. Toward Catalyst Economy: A Programmed Approach to the Synthesis of Bicyclic Lactones and Lactams. J. Organomet. Chem. 2006, 691, 5375–5382. [Google Scholar] [CrossRef]
- Kitson, R.R.A.; Millemaggi, A.; Taylor, R.J.K. The Renaissance of α-Methylene-γ-Butyrolactones: New Synthetic Approaches. Angew. Chem. Int. Ed. 2009, 48, 9426–9451. [Google Scholar] [CrossRef]
- Albrecht, A.; Albrecht, Ł.; Janecki, T. Recent Advances in the Synthesis of α-Alkylidene-Substituted δ-Lactones, γ-Lactams and δ-Lactams. Eur. J. Org. Chem. 2011, 2011, 2747–2766. [Google Scholar] [CrossRef]
- Kammerer, C.; Prestat, G.; Madec, D.; Poli, G. Synthesis of γ-Lactams and γ-Lactones via Intramolecular Pd-Catalyzed Allylic Alkylations. Acc. Chem. Res. 2014, 47, 3439–3447. [Google Scholar] [CrossRef]
- Pitts, C.R.; Lectka, T. Chemical Synthesis of β-Lactams: Asymmetric Catalysis and Other Recent Advances. Chem. Rev. 2014, 114, 7930–7953. [Google Scholar] [CrossRef]
- Ye, L.-W.; Shu, C.; Gagosz, F. Recent Progress towards Transition Metal-Catalyzed Synthesis of γ-Lactams. Org. Biomol. Chem. 2014, 12, 1833–1845. [Google Scholar] [CrossRef]
- Rivas, F.; Ling, T. Advances toward the Synthesis of Functionalized γ-Lactams. Org. Prep. Proced. Int. 2016, 48, 254–295. [Google Scholar] [CrossRef]
- Lawer, A.; Rossi-Ashton, J.A.; Stephens, T.C.; Challis, B.J.; Epton, R.G.; Lynam, J.M.; Unsworth, W.P. Internal Nucleophilic Catalyst Mediated Cyclisation/Ring Expansion Cascades for the Synthesis of Medium-Sized Lactones and Lactams. Angew. Chem. Int. Ed 2019, 58, 13942–13947. [Google Scholar] [CrossRef] [PubMed]
- Bernoud, E.; Lepori, C.; Mellah, M.; Schulz, E.; Hannedouche, J. Recent Advances in Metal Free- and Late Transition Metal-Catalysed Hydroamination of Unactivated Alkenes. Catal. Sci. Technol. 2015, 5, 2017–2037. [Google Scholar] [CrossRef]
- Bezzenine-Lafollée, S.; Gil, R.; Prim, D.; Hannedouche, J. First-Row Late Transition Metals for Catalytic Alkene Hydrofunctionalisation: Recent Advances in C-N, C-O and C-P Bond Formation. Molecules 2017, 22, 1901. [Google Scholar] [CrossRef] [PubMed]
- Rocard, L.; Chen, D.; Stadler, A.; Zhang, H.; Gil, R.; Bezzenine, S.; Hannedouche, J. Earth-Abundant 3d Transition Metal Catalysts for Hydroalkoxylation and Hydroamination of Unactivated Alkenes. Catalysts 2021, 11, 674. [Google Scholar] [CrossRef]
- Coulombel, L.; Duñach, E. Cycloisomerization of Olefinic Carboxylic Acids Catalyzed by Trifluoromethanesulfonic Acid. Synth. Commun. 2005, 35, 153–160. [Google Scholar] [CrossRef]
- Yang, C.-G.; Reich, N.W.; Shi, Z.; He, C. Intramolecular Additions of Alcohols and Carboxylic Acids to Inert Olefins Catalyzed by Silver(I) Triflate. Org. Lett. 2005, 7, 4553–4556. [Google Scholar] [CrossRef]
- Coulombel, L.; Rajzmann, M.; Pons, J.-M.; Olivero, S.; Duñach, E. Aluminium(III) Trifluoromethanesulfonate as an Efficient Catalyst for the Intramolecular Hydroalkoxylation of Unactivated Olefins: Experimental and Theoretical Approaches. Chem. Eur. J. 2006, 12, 6356–6365. [Google Scholar] [CrossRef]
- Ohta, T.; Kataoka, Y.; Miyoshi, A.; Oe, Y.; Furukawa, I.; Ito, Y. Ruthenium-Catalyzed Intramolecular Cyclization of Hetero-Functionalized Allylbenzenes. J. Organomet. Chem. 2007, 692, 671–677. [Google Scholar] [CrossRef]
- Adrio, L.A.; Quek, L.S.; Taylor, J.G.; Kuok (Mimi) Hii, K. Copper-Catalysed Intramolecular O–H Addition to Unactivated Alkenes. Tetrahedron 2009, 65, 10334–10338. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Ohlmann, D.M.; Dierker, M. Silver Triflate-Catalysed Synthesis of γ-Lactones from Fatty Acids. Green Chem. 2010, 12, 197–200. [Google Scholar] [CrossRef]
- Adrio, L.A.; Hii, K.K. An Expedient Synthesis of Olfactory Lactones by Intramolecular Hydroacylalkoxylation Reactions. Eur. J. Org. Chem. 2011, 2011, 1852–1857. [Google Scholar] [CrossRef]
- Sakuma, M.; Sakakura, A.; Ishihara, K. Kinetic Resolution of Racemic Carboxylic Acids through Asymmetric Protolactonization Promoted by Chiral Phosphonous Acid Diester. Org. Lett. 2013, 15, 2838–2841. [Google Scholar] [CrossRef] [PubMed]
- Nagamoto, M.; Nishimura, T. Iridium-Catalyzed Asymmetric Cyclization of Alkenoic Acids Leading to γ-Lactones. Chem. Commun. 2015, 51, 13466–13469. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Fan, W.-W.; Liu, G.-Q.; Duan, L.; Li, L.; Li, Y.-M. On the Understanding of BF3·Et2O-Promoted Intra- and Intermolecular Amination and Oxygenation of Unfunctionalized Olefins. RSC Adv. 2015, 5, 61081–61093. [Google Scholar] [CrossRef]
- Shigehisa, H.; Hayashi, M.; Ohkawa, H.; Suzuki, T.; Okayasu, H.; Mukai, M.; Yamazaki, A.; Kawai, R.; Kikuchi, H.; Satoh, Y.; et al. Catalytic Synthesis of Saturated Oxygen Heterocycles by Hydrofunctionalization of Unactivated Olefins: Unprotected and Protected Strategies. J. Am. Chem. Soc. 2016, 138, 10597–10604. [Google Scholar] [CrossRef]
- Ferrand, L.; Tang, Y.; Aubert, C.; Fensterbank, L.; Mouriès-Mansuy, V.; Petit, M.; Amatore, M. Niobium-Catalyzed Intramolecular Addition of O–H and N–H Bonds to Alkenes: A Tool for Hydrofunctionalization. Org. Lett. 2017, 19, 2062–2065. [Google Scholar] [CrossRef]
- Nagamoto, M.; Nishimura, T.; Yorimitsu, H. Iridium-Catalyzed Intramolecular Oxidative Cyclization of Alkenyl Amides and Alkenoic Acids. Synthesis 2017, 49, 4272–4282. [Google Scholar] [CrossRef]
- Chou, T.-H.; Yu, B.-H.; Chein, R.-J. ZnI2/Zn(OTf)2-TsOH: A Versatile Combined-Acid System for Catalytic Intramolecular Hydrofunctionalization and Polyene Cyclization. Chem. Commun. 2019, 55, 13522–13525. [Google Scholar] [CrossRef]
- Qi, C.; Yang, S.; Gandon, V.; Lebœuf, D. Calcium(II)- and Triflimide-Catalyzed Intramolecular Hydroacyloxylation of Unactivated Alkenes in Hexafluoroisopropanol. Org. Lett. 2019, 21, 7405–7409. [Google Scholar] [CrossRef]
- Kobayashi, S. Rare Earth Metal Trifluoromethanesulfonates as Water-Tolerant Lewis Acid Catalysts in Organic Synthesis. Synlett 1994, 1994, 689–701. [Google Scholar] [CrossRef]
- Ladziata, V. Recent Applications of Rare-Earth Metal(III) Triflates in Cycloaddition and Cyclization Reactions. Arkivoc 2014, 2014, 307–336. [Google Scholar] [CrossRef]
- Luo, S.; Zhu, L.; Talukdar, A.; Zhang, G.; Mi, X.; Cheng, J.-P.; Wang, P.G. Recent Advances in Rare Earth-Metal Triflate Catalyzed Organic Synthesis in Green Media. Mini-Rev. Org. Chem. 2005; 2, 177–202. [Google Scholar]
- Kobayashi, S.; Sugiura, M.; Kitagawa, H.; Lam, W.W.-L. Rare-Earth Metal Triflates in Organic Synthesis. Chem. Rev. 2002, 102, 2227–2302. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Zhang, L.-Y.; Wang, N.-X.; Xing, Y. Recent Advances in the Rare-Earth Metal Triflates-Catalyzed Organic Reactions. Catal. Rev. 2022, 64, 679–715. [Google Scholar] [CrossRef]
- Kobayashi, S. Scandium Triflate in Organic Synthesis. Eur. J. Org. Chem 1999, 1999, 15–27. [Google Scholar] [CrossRef]
- Pellissier, H. Recent Developments in Enantioselective Scandium–Catalyzed Transformations. Coord. Chem. Rev. 2016, 313, 1–37. [Google Scholar] [CrossRef]
- Longo Jr, L.S.; Siqueira, F.A.; Anjos, N.S.; Santos, G.F.D. Scandium(III)-Triflate-Catalyzed Multicomponent Reactions for the Synthesis of Nitrogen Heterocycles. ChemistrySelect 2021, 6, 5097–5109. [Google Scholar] [CrossRef]
- Combettes, L.E.; Schuler, M.; Patel, R.; Bonillo, B.; Odell, B.; Thompson, A.L.; Claridge, T.D.W.; Gouverneur, V. Synthesis of 3-Fluoropyrrolidines and 4-Fluoropyrrolidin-2-Ones from Allylic Fluorides. Chem.—A Eur. J. 2012, 18, 13126–13132. [Google Scholar] [CrossRef]
- Fischer, D.M.; Balkenhohl, M.; Carreira, E.M. Cobalt-Catalyzed Cyclization of Unsaturated N-Acyl Sulfonamides: A Diverted Mukaiyama Hydration Reaction. JACS Au. 2022, 2, 1071–1077. [Google Scholar] [CrossRef]
- Craig, P.N. Cyclic Quaternary Immonium Compounds Derived from 2,2-Diphenyl-4-Pentenoic Acid1. J. Am. Chem. Soc. 1952, 74, 129–131. [Google Scholar] [CrossRef]
- Biloski, A.J.; Wood, R.D.; Ganem, B. A New .Beta.-Lactam Synthesis. J. Am. Chem. Soc. 1982, 104, 3233–3235. [Google Scholar] [CrossRef]
- Hirama, M.; Iwashita, M.; Yamazaki, Y.; Itô, S. Carbamate Mediated Functionalization of Unsaturated Alcohols II. Regio- and Stereo-Selective Synthesis of 1,3-Syn and 1,2-Anti Amino Alcohol Derivatives via Iodocarbamation. Tetrahedron Lett. 1984, 25, 4963–4964. [Google Scholar] [CrossRef]
- Knapp, S.; Levorse, A.T. Synthesis and Reactions of Iodo Lactams. J. Org. Chem. 1988, 53, 4006–4014. [Google Scholar] [CrossRef]
- Fujita, M.; Kitagawa, O.; Suzuki, T.; Taguchi, T. Regiocontrolled Iodoaminocyclization Reaction of an Ambident Nucleophile Mediated by Basic Metallic Reagent. J. Org. Chem. 1997, 62, 7330–7335. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, O.; Fujita, M.; Li, H.; Taguchi, T. Regio-Controlled Iodoaminocyclization Reaction of an Ambident Nucleophile Mediated by LiAl(Ot-Bu)4. Tetrahedron Lett. 1997, 38, 615–618. [Google Scholar] [CrossRef]
- Liu, G.-Q.; Li, L.; Duan, L.; Li, Y.-M. MCPBA-Mediated Metal-Free Intramolecular Aminohydroxylation and Dioxygenation of Unfunctionalized Olefins. RSC Adv. 2015, 5, 61137–61143. [Google Scholar] [CrossRef]
- Nagamoto, M.; Yanagi, T.; Nishimura, T.; Yorimitsu, H. Asymmetric Cyclization of N-Sulfonyl Alkenyl Amides Catalyzed by Iridium/Chiral Diene Complexes. Org. Lett. 2016, 18, 4474–4477. [Google Scholar] [CrossRef]
- Lorion, M.M.; Duarte, F.J.S.; Calhorda, M.J.; Oble, J.; Poli, G. Opening the Way to Catalytic Aminopalladation/Proxicyclic Dehydropalladation: Access to Methylidene γ-Lactams. Org. Lett. 2016, 18, 1020–1023. [Google Scholar] [CrossRef]
- Qi, C.; Hasenmaile, F.; Gandon, V.; Lebœuf, D. Calcium(II)-Catalyzed Intra- and Intermolecular Hydroamidation of Unactivated Alkenes in Hexafluoroisopropanol. ACS Catal. 2018, 8, 1734–1739. [Google Scholar] [CrossRef]
- Shigehisa, H.; Koseki, N.; Shimizu, N.; Fujisawa, M.; Niitsu, M.; Hiroya, K. Catalytic Hydroamination of Unactivated Olefins Using a Co Catalyst for Complex Molecule Synthesis. J. Am. Chem. Soc. 2014, 136, 13534–13537. [Google Scholar] [CrossRef]
- Thakur, R.; Jaiswal, Y.; Kumar, A. Imidates: An Emerging Synthon for N-Heterocycles. Org. Biomol. Chem. 2019, 17, 9829–9843. [Google Scholar] [CrossRef]
- Co, K. Controlled Microwave Heating in Modern Organic Synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Henary, M.; Kananda, C.; Rotolo, L.; Savino, B.; Owens, E.A.; Cravotto, G. Benefits and Applications of Microwave-Assisted Synthesis of Nitrogen Containing Heterocycles in Medicinal Chemistry. RSC Adv. 2020, 10, 14170–14197. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Carlino, R.; Guillot, R.; Gil, R.; Bezzenine, S.; Hannedouche, J. Substrate-Dependent Selectivity in Sc(OTf)3-Catalyzed Cyclization of Alkenoic Acids and N-Protected Alkenamides. Catalysts 2022, 12, 1481. https://doi.org/10.3390/catal12111481
Zhang H, Carlino R, Guillot R, Gil R, Bezzenine S, Hannedouche J. Substrate-Dependent Selectivity in Sc(OTf)3-Catalyzed Cyclization of Alkenoic Acids and N-Protected Alkenamides. Catalysts. 2022; 12(11):1481. https://doi.org/10.3390/catal12111481
Chicago/Turabian StyleZhang, Hailong, Romain Carlino, Régis Guillot, Richard Gil, Sophie Bezzenine, and Jérôme Hannedouche. 2022. "Substrate-Dependent Selectivity in Sc(OTf)3-Catalyzed Cyclization of Alkenoic Acids and N-Protected Alkenamides" Catalysts 12, no. 11: 1481. https://doi.org/10.3390/catal12111481
APA StyleZhang, H., Carlino, R., Guillot, R., Gil, R., Bezzenine, S., & Hannedouche, J. (2022). Substrate-Dependent Selectivity in Sc(OTf)3-Catalyzed Cyclization of Alkenoic Acids and N-Protected Alkenamides. Catalysts, 12(11), 1481. https://doi.org/10.3390/catal12111481





