Removal of Tetracycline Hydrochloride from Water by Visible-Light Photocatalysis Using BiFeO3/BC Materials
Abstract
1. Introduction
2. Results and Discussions
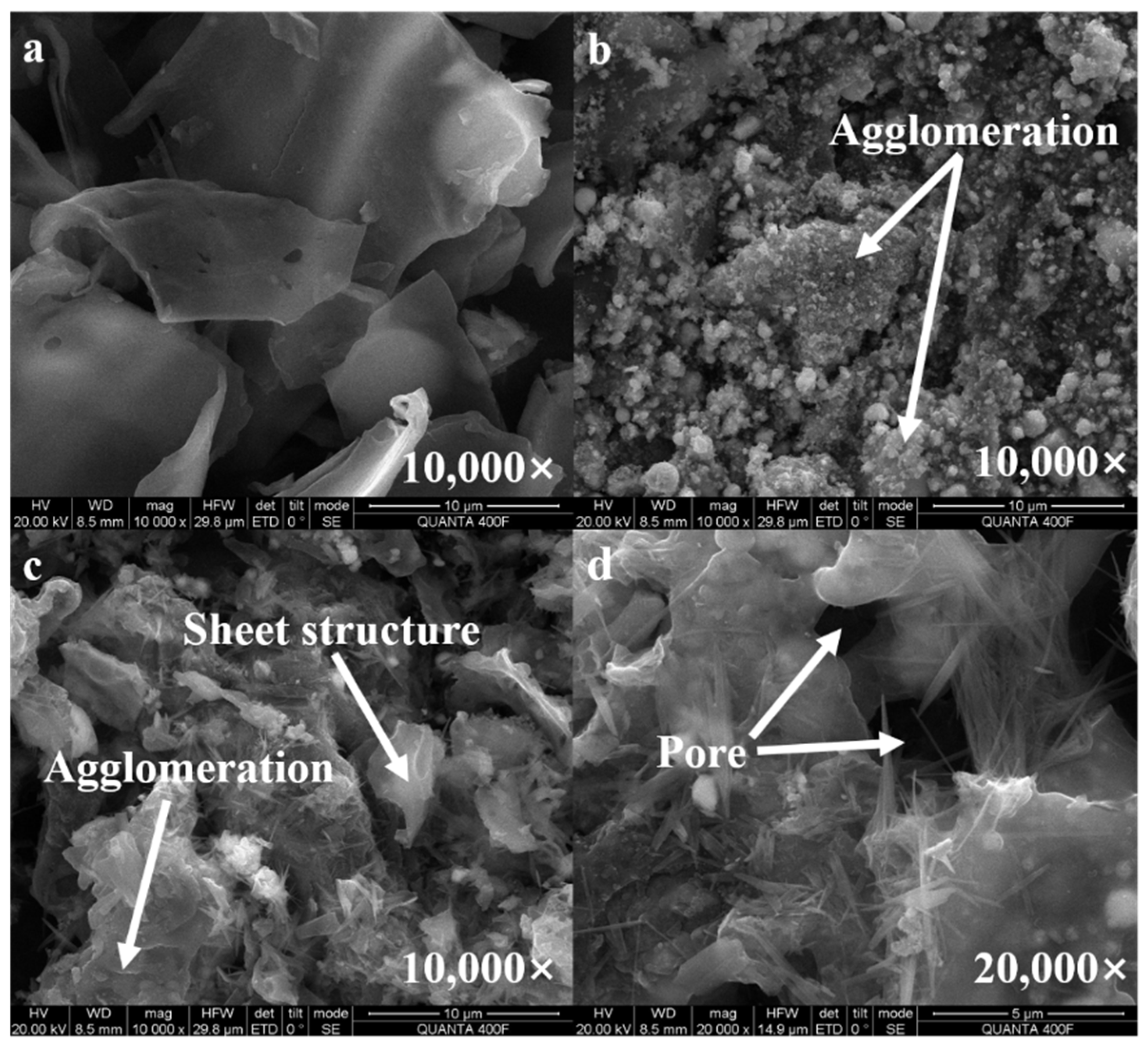
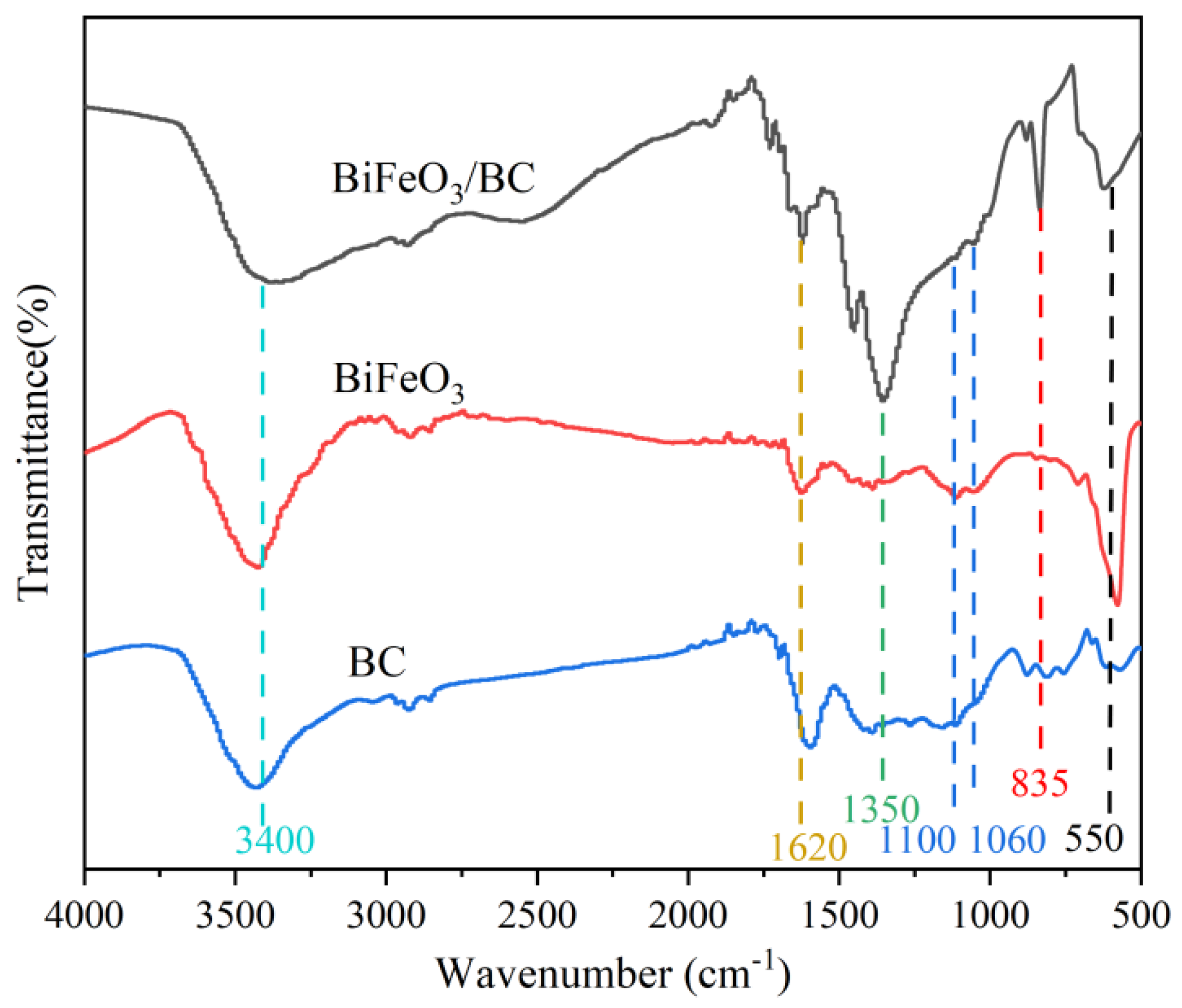
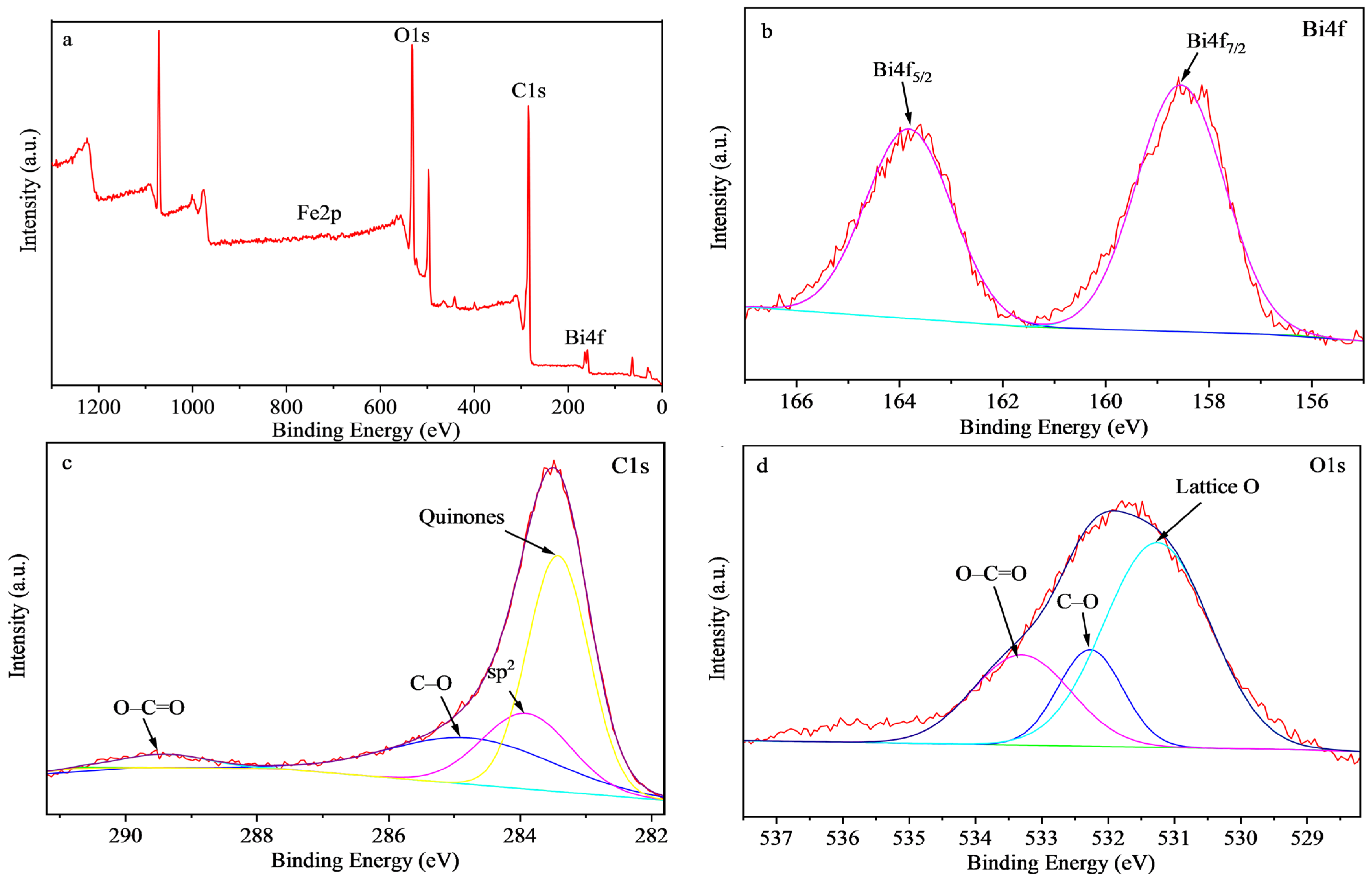
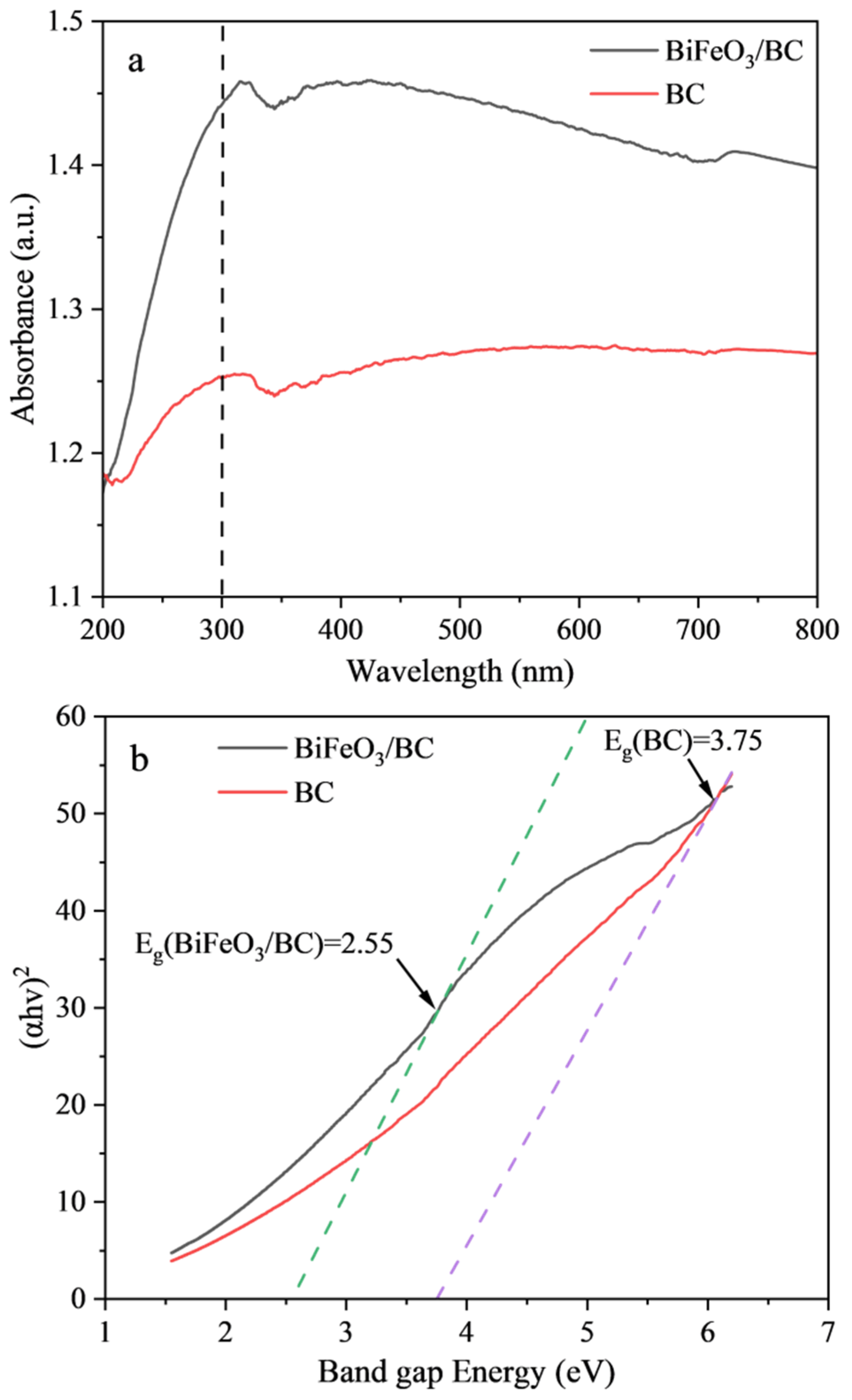
2.1. Characterization
2.2. Experimental Section
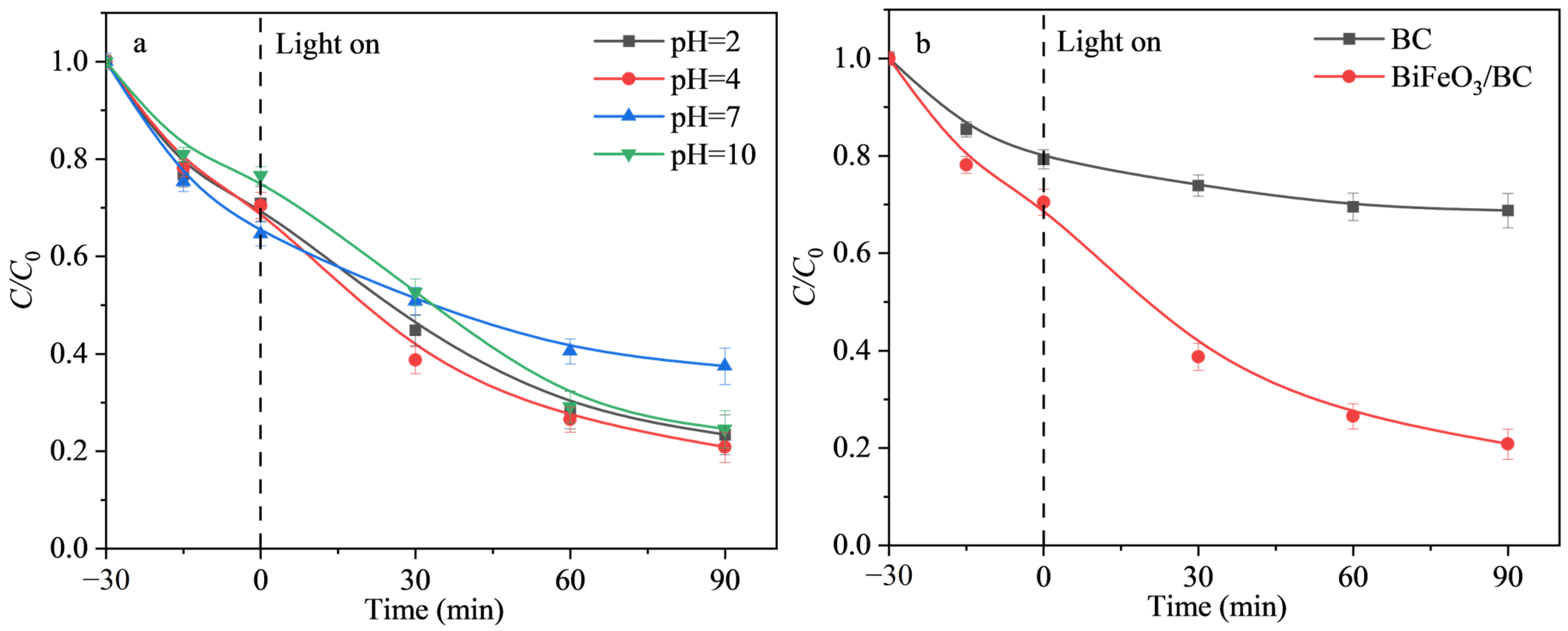
2.2.1. Effect of pH on Photocatalytic Efficiency
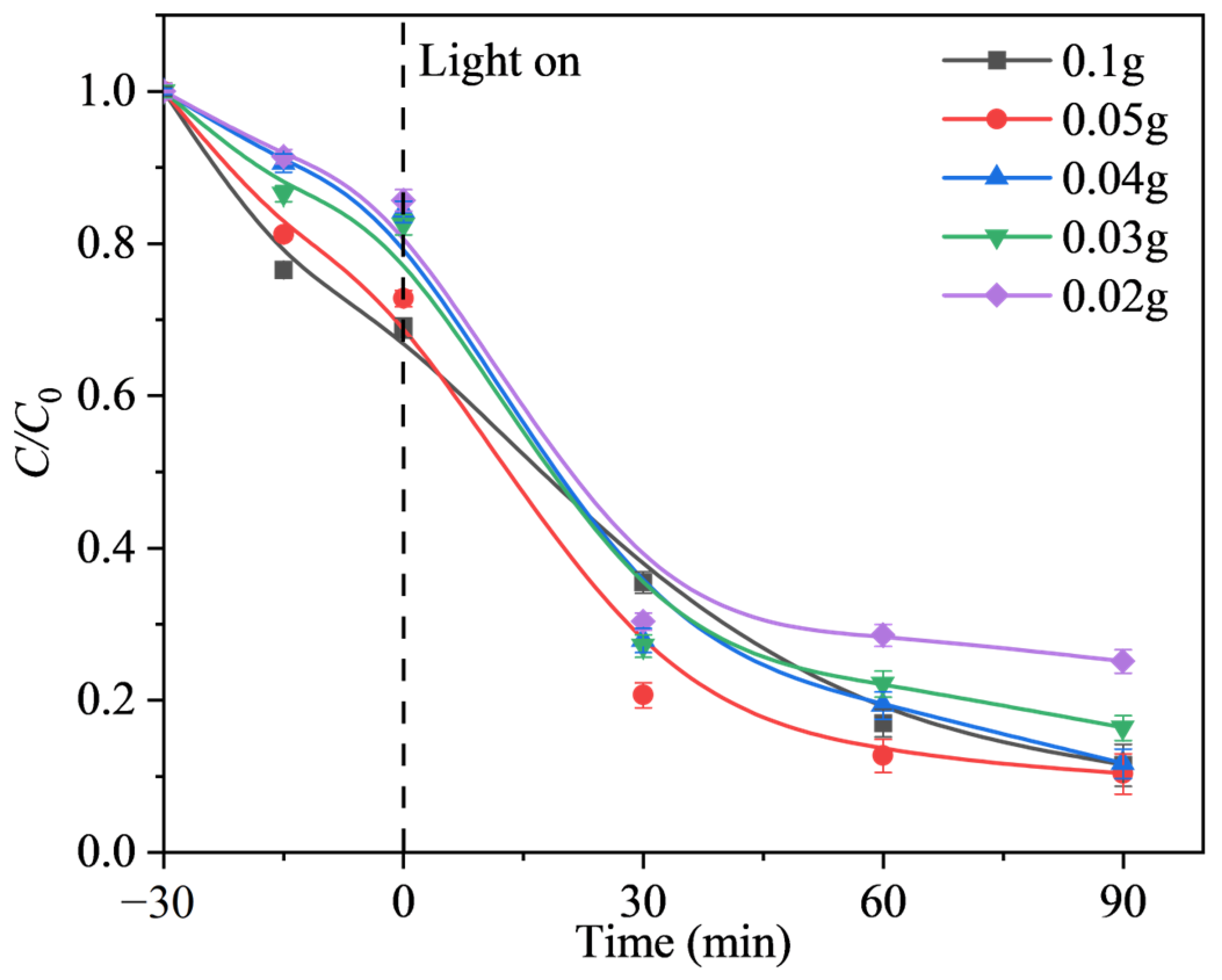
2.2.2. Effect of Photocatalysis Dosage
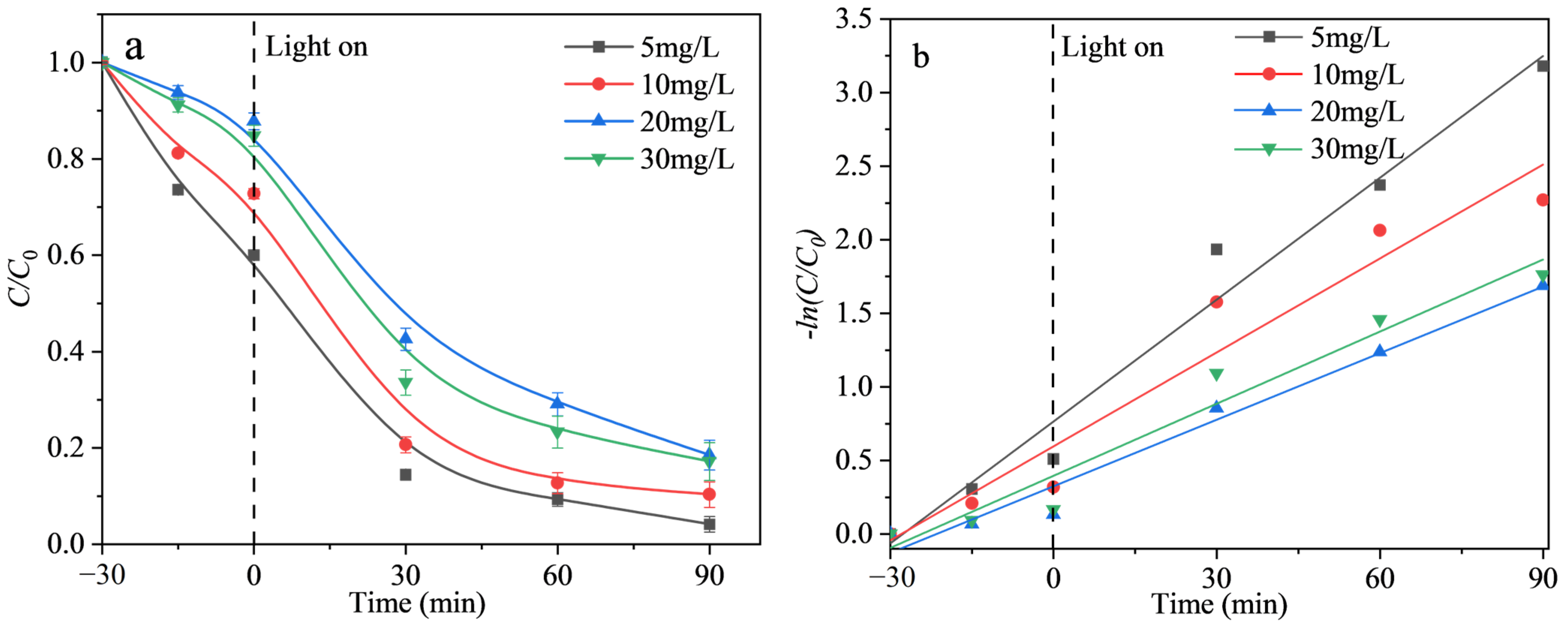
2.2.3. Effect of Initial TCH Concentration
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Bismuth Ferrite Coupled with Biochar Material (BiFeO3/BC)
3.3. Characterization
3.4. Photocatalytic Degradation of TCH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.; Chen, L.; Fang, Y.; Jia, X.; Chen, J. High temperatures can effectively degrade residual tetracyclines in chicken manure through composting. J. Hazard. Mater. 2019, 380, 120862. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.J.; Zhang, X.L.; Bai, X.M.; Fu, B.J.; Chen, D.L. Four steps to food security for swelling cities. Nature 2019, 566, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yang, Z.H.; Wang, Q.P.; Bai, Y.; Liu, J.B.; Zheng, Y.; Zhang, Y.R.; Xiong, W.P.; Ahmad, K.; Fan, C.Z. Rapid startup of thermophilic anaerobic digester to remove tetracycline and sulfonamides resistance genes from sewage sludge. Sci. Total Environ. 2018, 612, 788–798. [Google Scholar] [CrossRef]
- Granados-Chinchilla, F.; Rodríguez, C. Tetracyclines in Food and Feedingstuffs: From Regulation to Analytical Methods, Bacterial Resistance, and Environmental and Health Implications. J. Anal. Methods Chem. 2017, 2017, 1–24. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Xiang, Y.; Wang, P.; Zhang, J.; Zhang, F.; Wei, J.; Luo, L.; Lei, M.; Tang, L. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef]
- Bagheri, S.; TermehYousefi, A.; Do, T.-O. Photocatalytic pathway toward degradation of environmental pharmaceutical pollutants: Structure, kinetics and mechanism approach. Catal. Sci. Technol. 2017, 7, 4548–4569. [Google Scholar] [CrossRef]
- Wu, S.; Lin, Y.; Hu, Y.H. Strategies of tuning catalysts for efficient photodegradation of antibiotics in water environments: A review. J. Mater. Chem. A 2021, 9, 2592–2611. [Google Scholar] [CrossRef]
- Xie, G.; Hu, X.; Du, Y.; Jin, Q.; Liu, Y.; Tang, C.; Hu, X.; Li, G.; Chen, Z.; Zhou, D.; et al. Light-driven breakdown of microcystin-LR in water: A critical review. Chem. Eng. J. 2021, 417, 129244. [Google Scholar] [CrossRef]
- Ikhlef-Taguelmimt, T.; Hamiche, A.; Yahiaoui, I.; Bendellali, T.; Lebik-Elhadi, H.; Ait-Amar, H.; Aissani-Benissad, F. Tetracycline hydrochloride degradation by heterogeneous photocatalysis using TiO2(P25) immobilized in biopolymer (chitosan) under UV irradiation. Water Sci. Technol. 2020, 82, 1570–1578. [Google Scholar] [CrossRef]
- Yang, Y.; Lai, M.; Huang, J.; Li, J.; Gao, R.; Zhao, Z.; Song, H.; He, J.; Ma, Y. Bi5O7I/g-C3N4 Heterostructures with Enhanced Visible-Light Photocatalytic Performance for Degradation of Tetracycline Hydrochloride. Front. Chem. 2021, 9, 1107. [Google Scholar] [CrossRef]
- Jiang, Q.; Han, Z.; Qian, Y.; Yuan, Y.; Ren, Y.; Wang, M.; Cheng, Z. Enhanced visible-light photocatalytic performance of ZIF-8-derived ZnO/TiO2 nano-burst-tube by solvothermal system adjustment. J. Water Process Eng. 2022, 47, 102768. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Ali, S.I.; Amin, F.; Tariq, A.; Iqbal, M.Z.; Rizwan, S. La- and Mn-Codoped Bismuth Ferrite/Ti3C2 MXene Composites for Efficient Photocatalytic Degradation of Congo Red Dye. ACS Omega 2019, 4, 8661–8668. [Google Scholar] [CrossRef] [PubMed]
- Bharathkumar, S.; Sakar, M.; Balakumar, S. Experimental Evidence for the Carrier Transportation Enhanced Visible Light Driven Photocatalytic Process in Bismuth Ferrite (BiFeO3) One-Dimensional Fiber Nanostructures. J. Phys. Chem. C 2016, 120, 18811–18821. [Google Scholar] [CrossRef]
- Tang, J.; Wang, R.; Liu, M.; Zhang, Z.; Song, Y.; Xue, S.; Zhao, Z.; Dionysiou, D.D. Construction of novel Z-scheme Ag/FeTiO3/Ag/BiFeO3 photocatalyst with enhanced visible-light-driven photocatalytic performance for degradation of norfloxacin. Chem. Eng. J. 2018, 351, 1056–1066. [Google Scholar] [CrossRef]
- Niloy, N.R.; Chowdhury, M.I.; Shanto, M.A.H.; Islam, J.; Rhaman, M.M. Multiferroic Bismuth ferrite nanocomposites as a potential photovoltaic material. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1091, 012049. [Google Scholar] [CrossRef]
- Park, B.-G. Bismuth ferrite thin film coated on polycarbonate surface and its photocatalytic properties in visible light. Mater. Lett. 2021, 285, 129006. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, C.M. Rational design of a novel LaFeO3/g-C3N4/BiFeO3 double Z-scheme structure: Photocatalytic performance for antibiotic degradation and mechanistic insight. Chem. Eng. J. 2021, 423, 130076. [Google Scholar] [CrossRef]
- Mostafaloo, R.; Mahmoudian, M.H.; Asadi-Ghalhari, M. BiFeO3/Magnetic nanocomposites for the photocatalytic degradation of cefixime from aqueous solutions under visible light. J. Photochem. Photobiol. A Chem. 2019, 382, 111926. [Google Scholar] [CrossRef]
- Balta, Z.; Simsek, E.B. Uncovering the systematical charge separation effect of boron nitride quantum dots on photocatalytic performance of BiFeO3 perovskite towards degradation of tetracycline antibiotic. J. Environ. Chem. Eng. 2021, 9, 106567. [Google Scholar] [CrossRef]
- Jiang, Y.; Xing, C.; Chen, Y.; Shi, J.; Wang, S. Preparation of BiFeO3 and photodegradation of tetracycline pollutant in the UV-heterogeneous Fenton-like system. Environ. Sci. Pollut. Res. 2022, 29, 57656–57668. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Bollinger, J.-C.; Belkhiri, L.; Tiri, A.; Bouzaza, A.; El Jery, A.; Assadi, A.; Amrane, A.; Mouni, L. Zeolite Waste Characterization and Use as Low-Cost, Ecofriendly, and Sustainable Material for Malachite Green and Methylene Blue Dyes Removal: Box-Behnken Design, Kinetics, and Thermodynamics. Appl. Sci. 2022, 12, 7587. [Google Scholar] [CrossRef]
- Chedri Mammar, A.; Mouni, L.; Bollinger, J.-C.; Belkhiri, L.; Bouzaza, A.; Assadi, A.A.; Belkacemi, H. Modeling and optimization of process parameters in elucidating the adsorption mechanism of Gallic acid on activated carbon prepared from date stones. Sep. Sci. Technol. 2020, 55, 3113–3125. [Google Scholar] [CrossRef]
- Bouchelkia, N.; Mouni, L.; Belkhiri, L.; Bouzaza, A.; Bollinger, J.-C.; Madani, K.; Dahmoune, F. Removal of lead(II) from water using activated carbon developed from jujube stones, a low-cost sorbent. Sep. Sci. Technol. 2016, 51, 1645–1653. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, C.; Zhao, X.; Bu, X.; Liao, X.; Fan, H.; Gao, W.; Hu, H.; Zhang, Y.; Huang, Z. Malachite green degradation by persulfate activation with CuFe2O4@biochar composite: Efficiency, stability and mechanism. J. Environ. Chem. Eng. 2021, 9, 105800. [Google Scholar] [CrossRef]
- Yi, Y.; Luo, J.; Fang, Z. Magnetic biochar derived from Eichhornia crassipes for highly efficient Fenton-like degradation of antibiotics: Mechanism and contributions. J. Environ. Chem. Eng. 2021, 9, 106258. [Google Scholar] [CrossRef]
- Roy, A.; Chakraborty, S.; Kundu, S.P.; Adhikari, B.; Majumder, S.B. Adsorption of Anionic-Azo Dye from Aqueous Solution by Lignocellulose-Biomass Jute Fiber: Equilibrium, Kinetics, and Thermodynamics Study. Ind. Eng. Chem. Res. 2012, 51, 12095–12106. [Google Scholar] [CrossRef]
- Saeed, A.; Harun, N.; Sufian, S.; Afolabi, H.; Al-Qadami, E.; Roslan, F.; Rahim, S.; Ghaleb, A. Production and Characterization of Rice Husk Biochar and Kenaf Biochar for Value-Added Biochar Replacement for Potential Materials Adsorption. Ecol. Eng. Environ. Technol. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Park, H.; Byun, J.; Han, J. Economically feasible thermochemical process for methanol production from kenaf. Energy 2021, 230, 120729. [Google Scholar] [CrossRef]
- Yang, H.; Ye, S.; Zeng, Z.; Zeng, G.; Tan, X.; Xiao, R.; Wang, J.; Song, B.; Du, L.; Qin, M.; et al. Utilization of biochar for resource recovery from water: A review. Chem. Eng. J. 2020, 397, 125502. [Google Scholar] [CrossRef]
- Haromae, H.; Pattananuwat, P. Preparation of bismuth ferrite as photo-supercapacitive electrode. IOP Conf. Ser. Mater. Sci. Eng. 2019, 600, 012005. [Google Scholar] [CrossRef]
- Odinga, E.S.; Waigi, M.G.; Gudda, F.O.; Wang, J.; Yang, B.; Hu, X.; Li, S.; Gao, Y. Occurrence, formation, environmental fate and risks of environmentally persistent free radicals in biochars. Environ. Int. 2020, 134, 105172. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.-H.; Wang, J.; Yan, Y.; Peng, Y.; Wang, W.-L.; Xiao, H.-B.; Wang, C.-C. Enhanced As(III) transformation and removal with biochar/SnS2/phosphotungstic acid composites: Synergic effect of overcoming the electronic inertness of biochar and W2O3(AsO4)2 (As(V)-POMs) coprecipitation. J. Hazard. Mater. 2021, 408, 124961. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Tian, F.; Zou, H.; Ye, Z.; Peng, C.; Huang, J.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wei, X.; et al. ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J. Hazard. Mater. 2021, 415, 125511. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, F.; Chen, X.; Hoop, M.; Torlakcik, H.; Pellicer, E.; Sort, J.; Gattinoni, C.; Nelson, B.J.; Pané, S. Piezoelectrically Enhanced Photocatalysis with BiFeO3 Nanostructures for Efficient Water Remediation. iScience 2018, 4, 236–246. [Google Scholar] [CrossRef]
- Alikhanov, N.M.R.; Rabadanov, M.K.; Orudzhev, F.F.; Gadzhimagomedov, S.K.; Emirov, R.M.; Sadykov, S.A.; Kallaev, S.N.; Ramazanov, S.M.; Abdulvakhidov, K.G.; Sobola, D. Size-dependent structural parameters, optical, and magnetic properties of facile synthesized pure-phase BiFeO3. J. Mater. Sci. Mater. Electron. 2021, 32, 13323–13335. [Google Scholar] [CrossRef]
- Cai, X.; Li, J.; Liu, Y.; Hu, X.; Tan, X.; Liu, S.; Wang, H.; Gu, Y.; Luo, L. Design and Preparation of Chitosan-Crosslinked Bismuth Ferrite/Biochar Coupled Magnetic Material for Methylene Blue Removal. Int. J. Environ. Res. Public Health 2019, 17, 6. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Zheng, H.; Zheng, Y.; Zhang, G. Adsorption and one-step degradation-regeneration of 4-amino-5-hydroxynaphthalene-2,7-disulfonic acid using biochar-based BiFeO3 nanocomposites. Bioresour. Technol. 2017, 245, 1103–1109. [Google Scholar] [CrossRef]
- Zhou, D.; Xie, G.; Hu, X.; Cai, X.; Zhao, Y.; Hu, X.; Jin, Q.; Fu, X.; Tan, X.; Liang, C.; et al. Coupling of kenaf Biochar and Magnetic BiFeO3 onto Cross-Linked Chitosan for Enhancing Separation Performance and Cr(VI) Ions Removal Efficiency. Int. J. Environ. Res. Public Health 2020, 17, 788. [Google Scholar] [CrossRef]
- Liu, N.; Hu, Z.; Hao, L.; Bai, H.; He, P.; Niu, R.; Gong, J. Trash into treasure: Converting waste polyester into C3N4-based intramolecular donor-acceptor conjugated copolymer for efficient visible-light photocatalysis. J. Environ. Chem. Eng. 2022, 10, 106959. [Google Scholar] [CrossRef]
- Zhao, N.; Li, B.; Huang, H.; Lv, X.; Zhang, M.; Cao, L. Modification of kelp and sludge biochar by TMT-102 and NaOH for cadmium adsorption. J. Taiwan Inst. Chem. Eng. 2020, 116, 101–111. [Google Scholar] [CrossRef]
- Hou, L.; Li, X.; Yang, Q.; Chen, F.; Wang, S.; Ma, Y.; Wu, Y.; Zhu, X.; Huang, X.; Wang, D. Heterogeneous activation of peroxymonosulfate using Mn-Fe layered double hydroxide: Performance and mechanism for organic pollutant degradation. Sci. Total Environ. 2019, 663, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Xiong, H.; Zhang, Y.; Tong, Z.; Liao, A.; Qin, Z. Preparation magnetic cassava residue microspheres and its application for Cu(II) adsorption. J. Environ. Chem. Eng. 2017, 5, 2800–2806. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Liu, J.; Wang, Z.; Chen, S.; Fu, X. Photocatalytic degradation of benzene over different morphology BiPO4: Revealing the significant contribution of high–energy facets and oxygen vacancies. Appl. Catal. B Environ. 2019, 243, 780–789. [Google Scholar] [CrossRef]
- Gao, M.Y.; Chen, X.W.; Huang, W.X.; Wu, L.; Yu, Z.S.; Xiang, L.; Mo, C.H.; Li, Y.W.; Cai, Q.Y.; Wong, M.H.; et al. Cell wall modification induced by an arbuscular mycorrhizal fungus enhanced cadmium fixation in rice root. J. Hazard. Mater. 2021, 416, 125894. [Google Scholar] [CrossRef]
- Jadhav, V.V.; Zate, M.K.; Liu, S.; Naushad, M.; Mane, R.S.; Hui, K.N.; Han, S.-H. Mixed-phase bismuth ferrite nanoflake electrodes for supercapacitor application. Appl. Nanosci. 2015, 6, 511–519. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, L.; Tang, Y.; Zhou, J.; Shi, B. Novel g-C3N4/C/Fe2O3 Composite for Efficient Photocatalytic Reduction of Aqueous Cr(VI) under Light Irradiation. Ind. Eng. Chem. Res. 2021, 60, 13594–13603. [Google Scholar] [CrossRef]
- Hu, X.; Wang, W.; Xie, G.; Wang, H.; Tan, X.; Jin, Q.; Zhou, D.; Zhao, Y. Ternary assembly of g-C3N4/graphene oxide sheets /BiFeO3 heterojunction with enhanced photoreduction of Cr(VI) under visible-light irradiation. Chemosphere 2019, 216, 733–741. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, T.; Liu, J.; Li, D. Adsorption of tetracycline antibiotics from an aqueous solution onto graphene oxide/calcium alginate composite fibers. RSC Adv. 2018, 8, 2616–2621. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Fang, B.; Cui, Y.; Xing, Z.; Li, Z.; Zhou, W. Self-floating biomass charcoal supported flower-like plasmon silver/carbon, nitrogen co-doped defective TiO2 as robust visible light photocatalysts. J. Clean. Prod. 2021, 329, 129723. [Google Scholar] [CrossRef]
- Xu, R.; Li, M.; Zhang, Q. Collaborative optimization for the performance of ZnO/biochar composites on persulfate activation through plant enrichment-pyrolysis method. Chem. Eng. J. 2022, 429, 132294. [Google Scholar] [CrossRef]
- Ruan, X.; Sun, Y.; Du, W.; Tang, Y.; Liu, Q.; Zhang, Z.; Doherty, W.; Frost, R.L.; Qian, G.; Tsang, D.C.W. Formation, characteristics, and applications of environmentally persistent free radicals in biochars: A review. Bioresour. Technol. 2019, 281, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, S.K.; Sharma, G.; Al-Muhtaseb, A.A.H.; Naushad, M.; Ghfar, A.A.; Stadler, F.J. Wide spectral degradation of Norfloxacin by Ag@BiPO4/BiOBr/BiFeO3 nano-assembly: Elucidating the photocatalytic mechanism under different light sources. J. Hazard. Mater. 2019, 364, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.-T.; Cui, H.; Zhang, Q.-W.; Cui, C.-W. Facile synthesis of Co(Ⅱ)-BiOCl@biochar nanosheets for photocatalytic degradation of p-nitrophenol under vacuum ultraviolet (VUV) irradiation. Appl. Surf. Sci. 2021, 559, 149938. [Google Scholar] [CrossRef]
- Wang, T.; Bai, Y.; Si, W.; Mao, W.; Gao, Y.; Liu, S. Heterogeneous photo-Fenton system of novel ternary Bi2WO6/BiFeO3/g-C3N4 heterojunctions for highly efficient degrading persistent organic pollutants in wastewater. J. Photochem. Photobiol. A Chem. 2021, 404, 112856. [Google Scholar] [CrossRef]
- Gao, F.; Chen, X.Y.; Yin, K.B.; Dong, S.; Ren, Z.F.; Yuan, F.; Yu, T.; Zou, Z.; Liu, J.M. Visible-light photocatalytic properties of weak magnetic BiFeO3 nanoparticles. Adv. Mater. 2007, 19, 2889. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Naushad, M.; Stadler, F.J.; Ghfar, A.A.; Dhiman, P.; Saini, R.V. Sustainable nano-hybrids of magnetic biochar supported g-C3N4/FeVO4 for solar powered degradation of noxious pollutants- Synergism of adsorption, photocatalysis & photo-ozonation. J. Clean. Prod. 2017, 165, 431–451. [Google Scholar] [CrossRef]
- Yang, H.; Yu, H.; Wang, J.; Ning, T.; Chen, P.; Yu, J.; Di, S.; Zhu, S. Magnetic porous biochar as a renewable and highly effective adsorbent for the removal of tetracycline hydrochloride in water. Environ. Sci. Pollut. Res. 2021, 28, 61513–61525. [Google Scholar] [CrossRef]
| Photocatalyst | Initial Concentration (mg/L) | Photocatalyst Dosage | Degradation Rate; Time Duration | Note | Reference |
|---|---|---|---|---|---|
| Chitosan/TiO2 (P25) | 30–40 | 1.2 g/L | ~95%; 360 min | UV | [9] |
| Bi5O7I/g-C3N4 | 20 | 1.0 g/L | ~95%; 180 min | [10] | |
| ZnO/TiO2 NBT | 10 | 0.2 g/L | 100%; 20 min | Complexed material | [11] |
| BFO/BNQDs | 10 | 0.2 g/L | 88%; 120 min | H2O2 (10 mM) | [19] |
| BiFeO3 | 40 | 2.0 g/L | 100%; 150 min | UV, H2O2 (9.8 mM) | [20] |
| Bi2WO6/BiFeO3/g-C3N4 | 10 | 0.1 g/L | 83.68%; 45 min | H2O2 (8 M) | [55] |
| BiFeO3/BC | 10 | 0.02–0.12 g/L | 88.32%; 90 min | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Jiang, H.; Gong, J.; Zhang, H.; Hu, X.; Ouyang, K.; Guo, Y.; Hu, X.; Wang, H.; Wang, P. Removal of Tetracycline Hydrochloride from Water by Visible-Light Photocatalysis Using BiFeO3/BC Materials. Catalysts 2022, 12, 1461. https://doi.org/10.3390/catal12111461
Fang Z, Jiang H, Gong J, Zhang H, Hu X, Ouyang K, Guo Y, Hu X, Wang H, Wang P. Removal of Tetracycline Hydrochloride from Water by Visible-Light Photocatalysis Using BiFeO3/BC Materials. Catalysts. 2022; 12(11):1461. https://doi.org/10.3390/catal12111461
Chicago/Turabian StyleFang, Zhengyang, Honghui Jiang, Jiamin Gong, Hengrui Zhang, Xi Hu, Ke Ouyang, Yuan Guo, Xinjiang Hu, Hui Wang, and Ping Wang. 2022. "Removal of Tetracycline Hydrochloride from Water by Visible-Light Photocatalysis Using BiFeO3/BC Materials" Catalysts 12, no. 11: 1461. https://doi.org/10.3390/catal12111461
APA StyleFang, Z., Jiang, H., Gong, J., Zhang, H., Hu, X., Ouyang, K., Guo, Y., Hu, X., Wang, H., & Wang, P. (2022). Removal of Tetracycline Hydrochloride from Water by Visible-Light Photocatalysis Using BiFeO3/BC Materials. Catalysts, 12(11), 1461. https://doi.org/10.3390/catal12111461






