Influence of Components Deposition Order on Silver Species Formation in Bimetallic Ag-Fe System Supported on Mordenite
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Samples Preparation
3.2. Characterization Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Corma, A. Confining isolated atoms and clusters in crystalline porous materials for catalysis. Nat. Rev. Mater. 2020, 6, 244–263. [Google Scholar] [CrossRef]
- Sánchez-López, P.; Kotolevich, Y.; Yocupicio-Gaxiola, R.I.; Antúnez-García, J.; Chowdari, R.K.; Petranovskii, V.; Fuentes-Moyado, S. Recent Advances in Catalysis Based on Transition Metals Supported on Zeolites. Front. Chem. 2021, 9, 716745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, S.; Yu, J. Metal Sites in Zeolites: Synthesis, Characterization, and Catalysis. Chem. Rev. 2022. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Mo, Y.; Long, B.; Wei, W.; Shan, C.; Guo, S.; Niu, L. Single-atom catalysts supported on ordered porous materials: Synthetic strategies and applications. InfoMat 2022, 4, e12296. [Google Scholar] [CrossRef]
- Luo, W.; Cao, W.; Bruijnincx, P.C.A.; Lin, L.; Wang, A.; Zhang, T. Zeolite-supported metal catalysts for selective hydrodeoxygenation of biomass-derived platform molecules. Green Chem. 2019, 21, 3744–3768. [Google Scholar] [CrossRef]
- Dalena, F.; Giglio, E.; Marino, A.; Aloise, A.; Giorgianni, G.; Migliori, M.; Giordano, G. Steam Reforming of Bioethanol Using Metallic Catalysts on Zeolitic Supports: An Overview. Catalysts 2022, 12, 617. [Google Scholar] [CrossRef]
- Liu, H.; You, C.; Wang, H. Experimental and Density Functional Theory Studies on the Zeolite-Based Fe–Ni–W Trimetallic Catalyst for High-Temperature NOx Selective Catalytic Reduction: Identification of Active Sites Suppressing Ammonia Over-oxidation. ACS Catal. 2021, 11, 1189–1201. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Z.; Lin, F.; Wang, H.; Wang, Y. Single-atom Automobile Exhaust Catalysts. ChemNanoMat 2020, 6, 1659–1682. [Google Scholar] [CrossRef]
- Pappas, D.K.; Kvande, K.; Kalyva, M.; Dyballa, M.; Lomachenko, K.A.; Arstad, B.; Borfecchia, E.; Bordiga, S.; Olsbye, U.; Beato, P.; et al. Influence of Cu-speciation in mordenite on direct methane to methanol conversion: Multi-Technique characterization and comparison with NH3 selective catalytic reduction of NOx. Catal. Today 2021, 369, 105–111. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H.; Yu, Y.; He, H. Role of silver species in H2-NH3-SCR of NOx over Ag/Al2O3 catalysts: Operando spectroscopy and DFT calculations. J. Catal. 2021, 395, 1–9. [Google Scholar] [CrossRef]
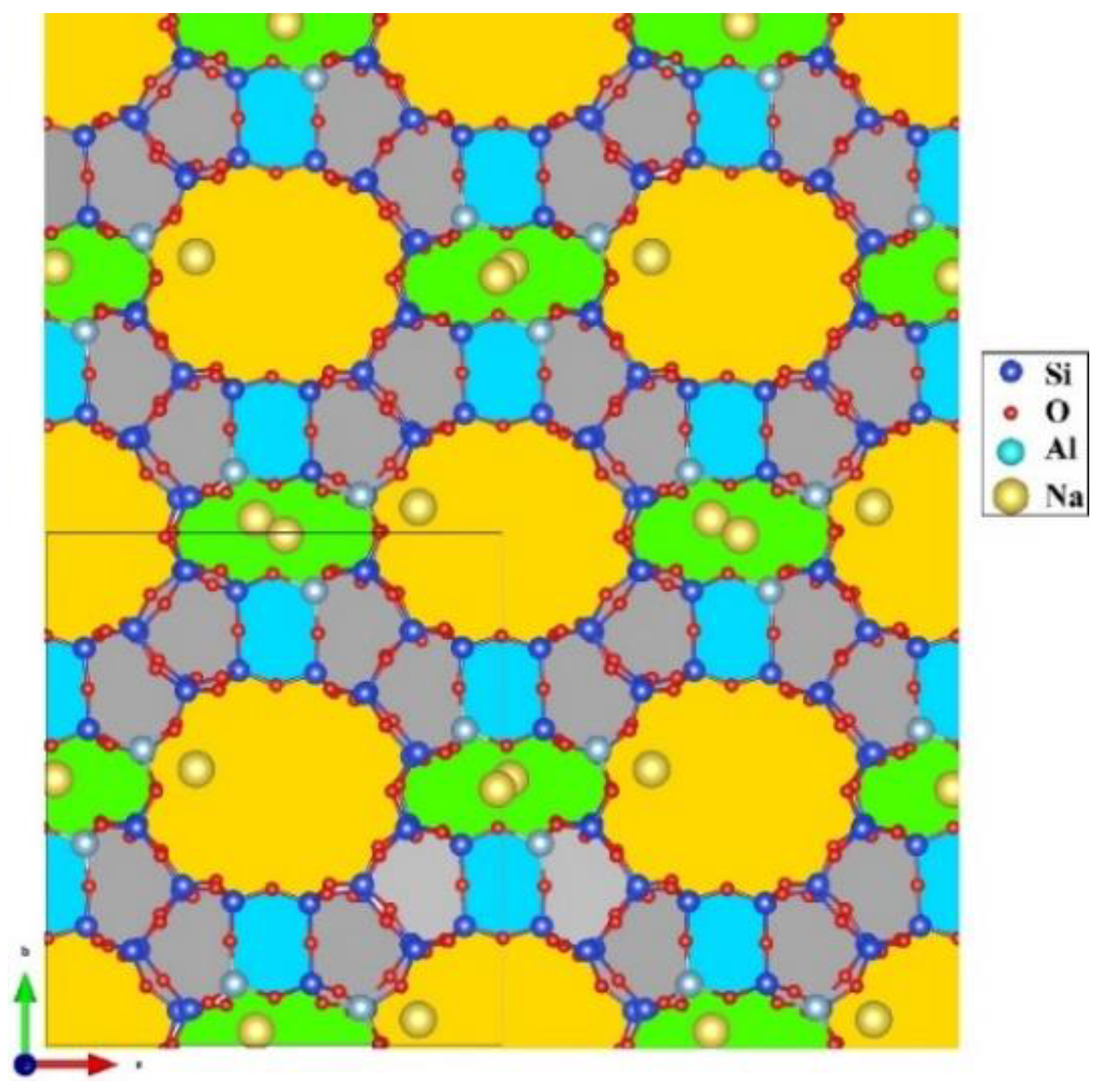
- Antúnez-García, J.; Galván, D.H.; Petranovskii, V.; Murrieta-Rico, F.N.; Yocupicio-Gaxiola, R.I.; Shelyapina, M.G.; Fuentes-Moyado, S. Aluminum distribution in mordenite-zeolite framework: A new outlook based on density functional theory calculations. J. Solid State Chem. 2022, 306, 122725. [Google Scholar] [CrossRef]
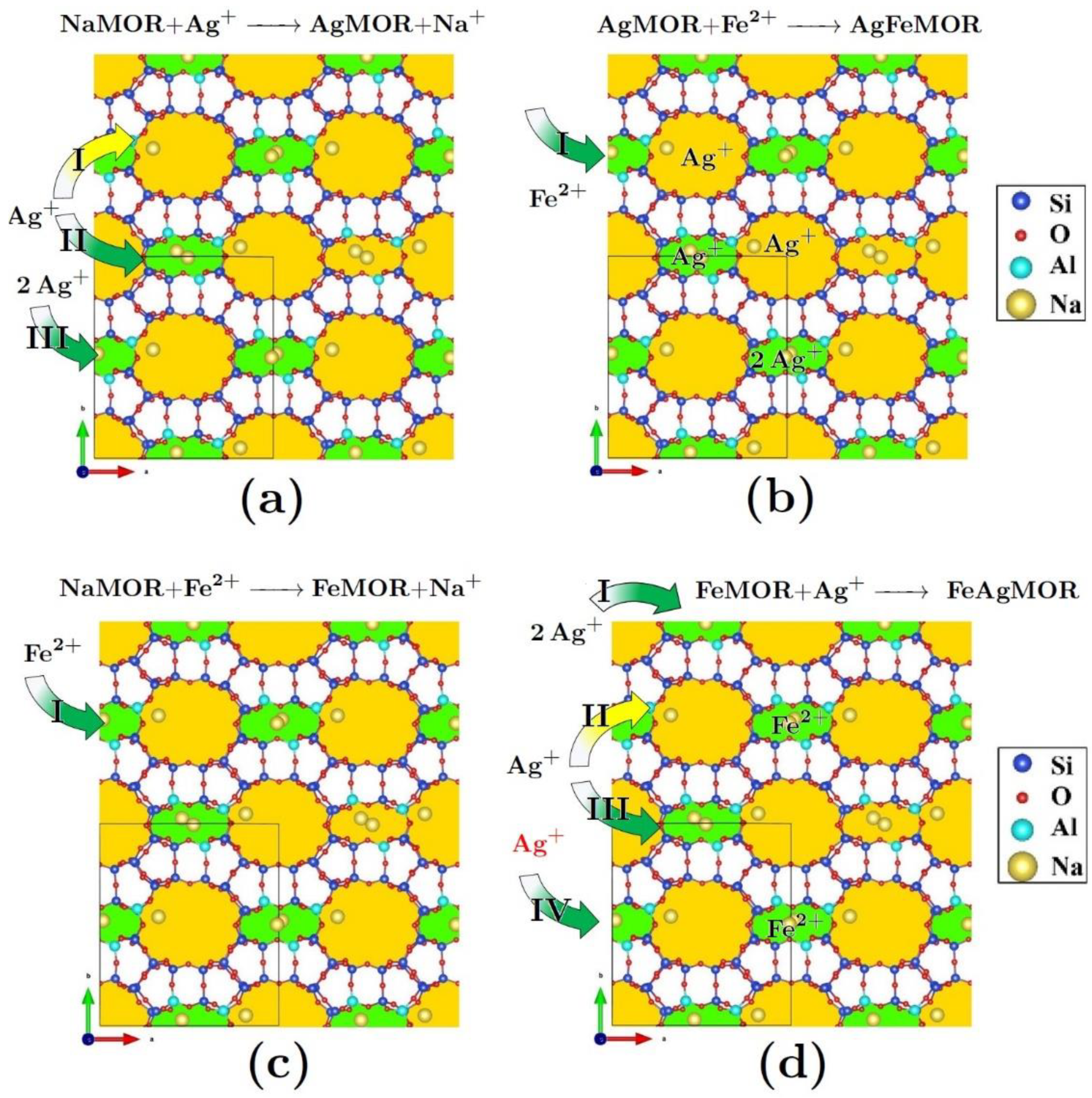
- Sánchez-López, P.; Kotolevich, Y.; Miridonov, S.; Chávez-Rivas, F.; Fuentes, S.; Petranovskii, V. Bimetallic AgFe Systems on Mordenite: Effect of Cation Deposition Order in the NO Reduction with C3H6/CO. Catalysts 2019, 9, 58. [Google Scholar] [CrossRef]
- Yeom, Y.; Li, M.; Sachtler, W.; Weitz, E.W. Low-temperature NOx reduction with ethanol over Ag/Y: A comparison with Ag/γ-Al2O3 and BaNa/Y. J. Catal. 2007, 246, 413–427. [Google Scholar] [CrossRef]
- Bartolomeu, R.; Mendes, A.N.; Fernandes, A.; Henriques, C.; da Costa, P.; Ribeiro, M.F. NOx SCR with decane using Ag–MFI catalysts: On the effect of silver content and co-cation presence. Catal. Sci. Technol. 2015, 6, 3038–3048. [Google Scholar] [CrossRef]
- Rodríguez-Iznaga, I.; Petranovskii, V.; Chávez-Rivas, F.; Shelyapina, M.G. Bimetallic Copper-Silver Systems Supported on Natural Clinoptilolite: Long-Term Changes in Nanospecies’ Composition and Stability. Inorganics 2022, 10, 34. [Google Scholar] [CrossRef]
- Liu, Q.; Bian, C.; Ming, S.; Guo, L.; Zhang, S.; Pang, L.; Liu, P.; Chen, Z.; Li, T. The opportunities and challenges of iron-zeolite as NH3-SCR catalyst in purification of vehicle exhaust. Appl. Catal. A Gen. 2020, 607, 117865. [Google Scholar] [CrossRef]
- Gao, F. Fe-Exchanged Small-Pore Zeolites as Ammonia Selective Catalytic Reduction (NH3-SCR) Catalysts. Catalysts 2020, 10, 1324. [Google Scholar] [CrossRef]
- Zhu, N.; Lian, Z.; Zhang, Y.; Shan, W.; He, H. Improvement of low-temperature catalytic activity over hierarchical Fe-Beta catalysts for selective catalytic reduction of NO with NH3. Chin. Chem. Lett. 2019, 30, 867–870. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Gurgul, J.; Łątka, K.; Sánchez-López, P.; Bogdanov, D.; Kotolevich, Y.; Petranovskii, V.; Fuentes, S. Mechanism of formation of framework Fe3+ in bimetallic Ag-Fe mordenites-Effective catalytic centers for deNOx reaction. Microporous Mesoporous Mater. 2020, 299, 109841. [Google Scholar] [CrossRef]
- Sánchez-López, P.; Kotolevich, Y.; Khramov, E.; Chowdari, R.K.; Estrada, M.A.; Berlier, G.; Zubavichus, Y.; Fuentes, S.; Petranovskii, V.; Chávez-Rivas, F. Properties of Iron-Modified-by-Silver Supported on Mordenite as Catalysts for NOx Reduction. Catalysts 2020, 10, 1156. [Google Scholar] [CrossRef]
- Fuentes, S.; Petranovskii, V.; Kotolevich, Y.; Miridonov, S.; Sánchez-López, P.; Chávez-Rivas, F.; Machorro, R. Self-assembling of ordered domains of silver nanoparticles into the mordenite channel system. In Proceedings of the 13th International Symposium on Nanophotonics and Metamaterials, St. Petersburg, Russia, 4–8 June 2018; p. 194. [Google Scholar]
- Sánchez-Lopez, P.; Miridonov, S.; Kotolevich, Y.; Chávez-Rivas, F.; Machorro, R.; Shelyapina, M.; Petranovskii, V.; Fuentes-Moyado, S. Domains of ordered monosized Ag clusters stabilized in mordenite channels formed in bimetallic Fe-Ag system supported on mordenite. In Proceedings of the XV Congreso Mexicano de Catálisis y VI Congreso Internacional, Monterrey, Mexico, 1–6 October 2017. [Google Scholar]
- Heo, N.H.; Kim, Y.; Kim, J.J.; Seff, K. Surprising Intrazeolitic Chemistry of Silver. J. Phys. Chem. C 2016, 120, 5277–5287. [Google Scholar] [CrossRef]
- Ogden, J.S.; Bogdanchikova, N.E.; Corker, J.M.; Petranovskii, V.P. Structure of silver clusters embedded in erionite channels. Eur. Phys. J. D 1999, 9, 605–608. [Google Scholar] [CrossRef]
- Fiddy, S.G.; Bogdanchikova, N.E.; Petranovskii, V.P.; Ogden, J.S.; Avalos-Borja, M. EXAFS and optical spectroscopy characterisation of silver within zeolite matrices. Stud. Surf. Sci. Catal. 2002, 142, 1939–1946. [Google Scholar] [CrossRef]
- Fiddy, S.G.; Ogden, J.S.; Petranovskii, V.P. EXAFS and optical spectroscopy characterisation of reduction products of binary silver-copper ion mixture in mordenite. Eur. Phys. J. D–At. Mol. Opt. Phys. 2003, 24, 253–256. [Google Scholar] [CrossRef]
- Antúnez-García, J.; Galván, D.H.; Petranovskii, V.; Posada-Amarillas, A. A DFT study of copper-oxide clusters embedded in dry and water-immersed siliceous mordenite. Comput. Mater. Sci. 2015, 106, 140–148. [Google Scholar] [CrossRef]
- Bogdanchikova, N.E.; Petranovskii, V.P.; Machorro, M.R.; Sugi, Y.; Soto, G.V.M.; Fuentes, S. Stability of silver clusters in mordenites with different SiO2/Al2O3 molar ratio. Appl. Surf. Sci. 1999, 150, 58–64. [Google Scholar] [CrossRef]
- Gurin, V.S.; Petranovskii, V.P.; Bogdanchikova, N.E. Metal clusters and nanoparticles assembled in zeolites: An example of stable materials with controllable particle size. Mater. Sci. Eng. C 2002, 19, 327–331. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface Plasmon Spectroscopy of Nanosized Metal Particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Gurin, V.S.; Petranovskii, V.P.; Hernandez, M.-A.; Bogdanchikova, N.E.; Alexeenko, A.A. Silver and copper clusters and small particles stabilized within nanoporous silicate-based materials. Mater. Sci. Eng. A 2005, 391, 71–76. [Google Scholar] [CrossRef]
- Mogensen, K.B.; Kneipp, K. Size-Dependent Shifts of Plasmon Resonance in Silver Nanoparticle Films Using Controlled Dissolution: Monitoring the Onset of Surface Screening Effects. J. Phys. Chem. C 2014, 118, 28075–28083. [Google Scholar] [CrossRef]
- Lysenko, V.S.; Mal’nev, A.F. Optical characteristics of metal blacks. J. Appl. Spectrosc. 1969, 10, 566–570. [Google Scholar] [CrossRef]
- Rodríguez-Iznaga, I.; Petranovskii, V.; Castillón-Barraza, F.; Concepción-Rosabal, B. Copper-Silver Bimetallic System on Natural Clinoptilolite: Thermal Reduction of Cu2+ and Ag+ Exchanged. J. Nanosci. Nanotechnol. 2011, 11, 5580–5586. [Google Scholar] [CrossRef]
- Santhosh Kumar, M.; Schwidder, M.; Grunert, W.; Bentrup, U.; Bruckner, A. Selective reduction of NO with Fe-ZSM-5 catalysts of low Fe content: Part II. Assessing the function of different Fe sites by spectroscopic in situ studies. J. Catal. 2006, 239, 173–186. [Google Scholar] [CrossRef]
- Bordiga, S.; Buzzoni, R.; Geobaldo, F.; Lamberti, C.; Giamello, E.; Zecchina, A.; Leofanti, G.; Petrini, G.; Tozzola, G.; Vlaic, G. Structure and Reactivity of Framework and Extraframework Iron in Fe-Silicalite as Investigated by Spectroscopic and Physicochemical Methods. J. Catal. 1996, 158, 486–501. [Google Scholar] [CrossRef]
- Setyawati, I.A.; Rettig, S.J.; Orvig, C. Cationic iron(III) complex with a hexadentate N2, N′2′, O2-aminopyridylphenolate ligand. Can. J. Chem. 1999, 77, 2033–2038. [Google Scholar] [CrossRef]
- Rtimi, S.; Baghriche, O.; Sanjines, R.; Pulgarin, C.; Bensimon, M.; Kiwi, J. TiON and TiON-Ag sputtered surfaces leading to bacterial inactivation under indoor actinic light. J. Photochem. Photobiol. A Chem. 2013, 256, 52–63. [Google Scholar] [CrossRef]
- Mejía, M.I.; Restrepo, G.; Marín, J.M.; Sanjines, R.; Pulgarín, C.; Mielczarski, E.; Mielczarski, J.; Kiwi, J. Magnetron-Sputtered Ag Surfaces. New Evidence for the Nature of the Ag Ions Intervening in Bacterial Inactivation. ACS Appl. Mater. Interfaces 2010, 2, 230–235. [Google Scholar] [CrossRef]
- Lopez-Salido, I.; Lim, D.C.; Kim, Y.D. Ag nanoparticles on highly ordered pyrolytic graphite (HOPG) surfaces studied using STM and XPS. Surf. Sci. 2005, 588, 6–18. [Google Scholar] [CrossRef]
- Lim, D.C.; Lopez-Salido, I.; Kim, Y.D. Size selectivity for CO-oxidation of Ag nanoparticles on highly ordered pyrolytic graphite (HOPG). Surf. Sci. 2005, 598, 96–103. [Google Scholar] [CrossRef]
- Suzuki, Y.; Miyanaga, T.; Hoshino, H.; Matsumoto, N.; Ainai, T. In-Situ XAFS Study of Ag Clusters in Zeolite 4A. Phys. Scr. 2005, 2005, 765. [Google Scholar] [CrossRef]
- Borgna, A.; Stagg, S.M.; Resasco, D.E. Interference Phenomena in the EXAFS Spectra of Pt−Sn Bimetallic Catalysts. J. Phys. Chem. B 1998, 102, 5077–5081. [Google Scholar] [CrossRef]
- Niggli, P. XII. Die Kristallstruktur einiger Oxyde I. Z. Für Krist.-Cryst. Mater. 1922, 57, 253–299. [Google Scholar] [CrossRef]
- Boix, A.V.; Aspromonte, S.G.; Miro, E.E. Deactivation studies of the SCR of NOx with hydrocarbons on Co-mordenite monolithic catalysts. Appl. Catal. A Gen. 2008, 341, 26–34. [Google Scholar] [CrossRef]
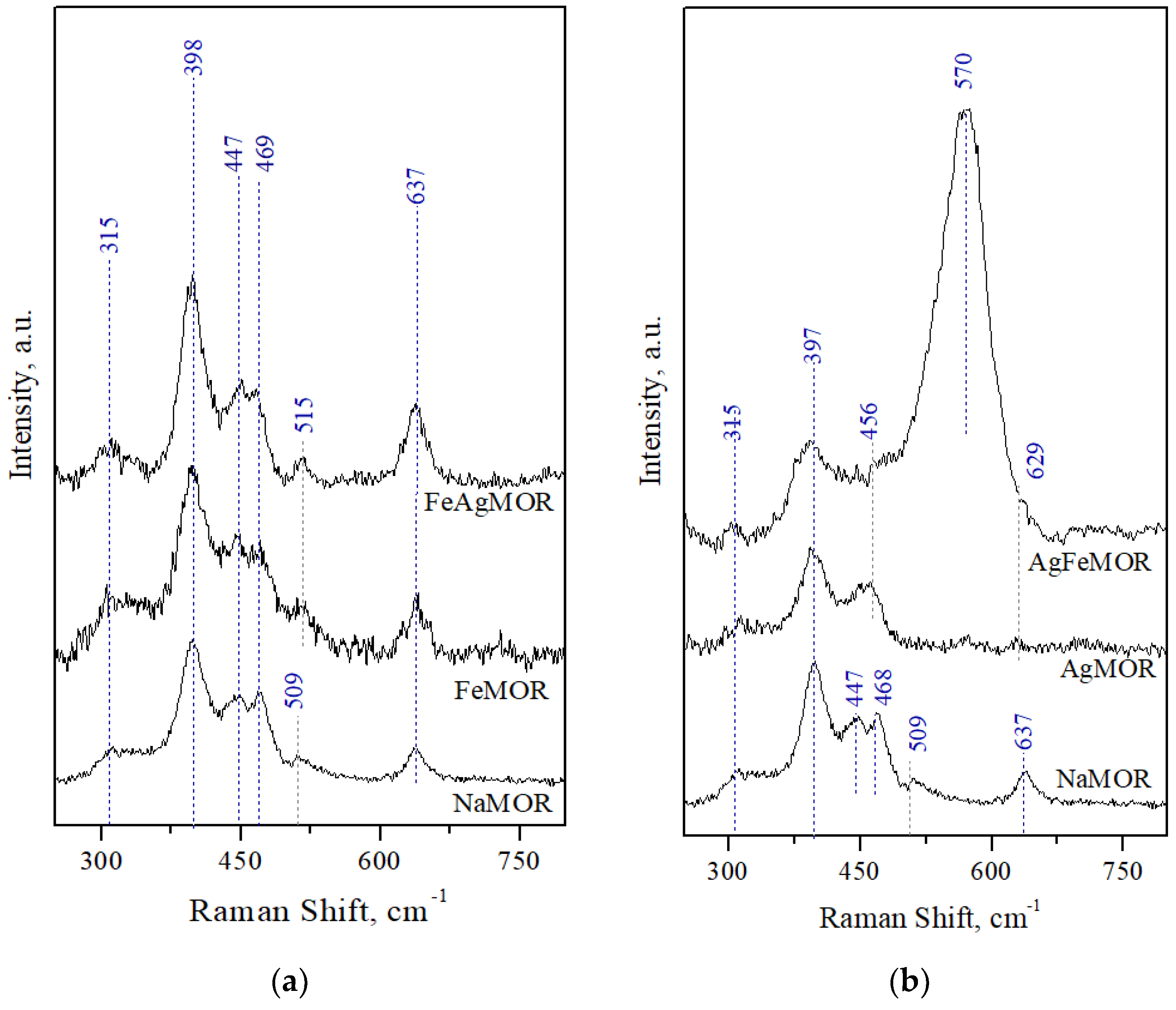
- Dutta, P.K.; Rao, K.M.; Park, J.Y. Correlation of Raman Spectra of Zeolites with Framework Architecture. J. Phys. Chem. 1991, 95, 6654–6656. [Google Scholar] [CrossRef]
- Dutta, P.K.; Puri, M. Synthesis and Structure of Zeolite ZSM-5: A Raman Spectroscopic Study. J. Phys. Chem. 1987, 91, 4329–4333. [Google Scholar] [CrossRef]
- Król, M.; Mozgawa, W.; Barczyk, K.; Bajda, T.; Kozanecki, M. Changes in the vibrational spectra of zeolites due to sorption of heavy metal cations. J. Appl. Spectrosc. 2013, 80, 644–650. [Google Scholar] [CrossRef]
- Jin, S.; Feng, Z.; Fan, F. UV Raman Spectroscopic Characterization of Catalysts and Catalytic Active Sites. Catal. Lett. 2015, 145, 468–481. [Google Scholar] [CrossRef]
- Yu, Y.; Xiong, G.; Li, C.; Xiao, F.-S. Characterization of Iron Atoms in the Framework of MFI-Type Zeolites by UV Resonance Raman Spectroscopy. J. Catal. 2000, 194, 487–490. [Google Scholar] [CrossRef]
- Sun, K.; Fan, F.; Xia, H.; Feng, Z.; Li, W.-X.; Li, C. Framework Fe Ions in Fe-ZSM-5 Zeolite Studied by UV Resonance Raman Spectroscopy and Density Functional Theory Calculations. J. Phys. Chem. C 2008, 112, 16036–16041. [Google Scholar] [CrossRef]
- Ju, X.; Tian, F.; Wang, Y.; Fan, F.; Feng, Z.; Li, C. A novel synthetic strategy of Fe-ZSM-35 with pure framework Fe species and its formation mechanism. Inorg. Chem. Front. 2018, 5, 2031–2037. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Z.; Xin, H.; Fan, F.; Zhang, J.; Magusin, P.C.M.M.; Hensen, E.J.M.; van Santen, R.A.; Yang, Q.; Li, C. Effect of Aluminum on the Nature of the Iron Species in Fe-SBA-15. J. Phys. Chem. B 2006, 110, 26114–26121. [Google Scholar] [CrossRef]
- Chlebda, D.K.; Stachurska, P.; Jedrzejczyk, R.J.; Kuterasinski, Ł.; Dziedzicka, A.; Górecka, S.; Chmielarz, L.; Łojewska, J.; Sitarz, M.; Jodłowski, P.J. DeNOx Abatement over Sonically Prepared Iron-Substituted Y, USY and MFI Zeolite Catalysts in Lean Exhaust Gas Conditions. Nanomaterials 2018, 8, 21. [Google Scholar] [CrossRef]
- Bremard, C.; Le Maire, M. Low-frequency Raman spectra of dehydrated faujasite zeolites. J. Phys. Chem. 1993, 97, 9695–9702. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The State of the Art in Selective Catalytic Reduction of NOx by Ammonia Using Metal-Exchanged Zeolite Catalysts. Catal. Rev. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Newville, M. IFEFFIT: Interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 2001, 8, 322–324. [Google Scholar] [CrossRef]
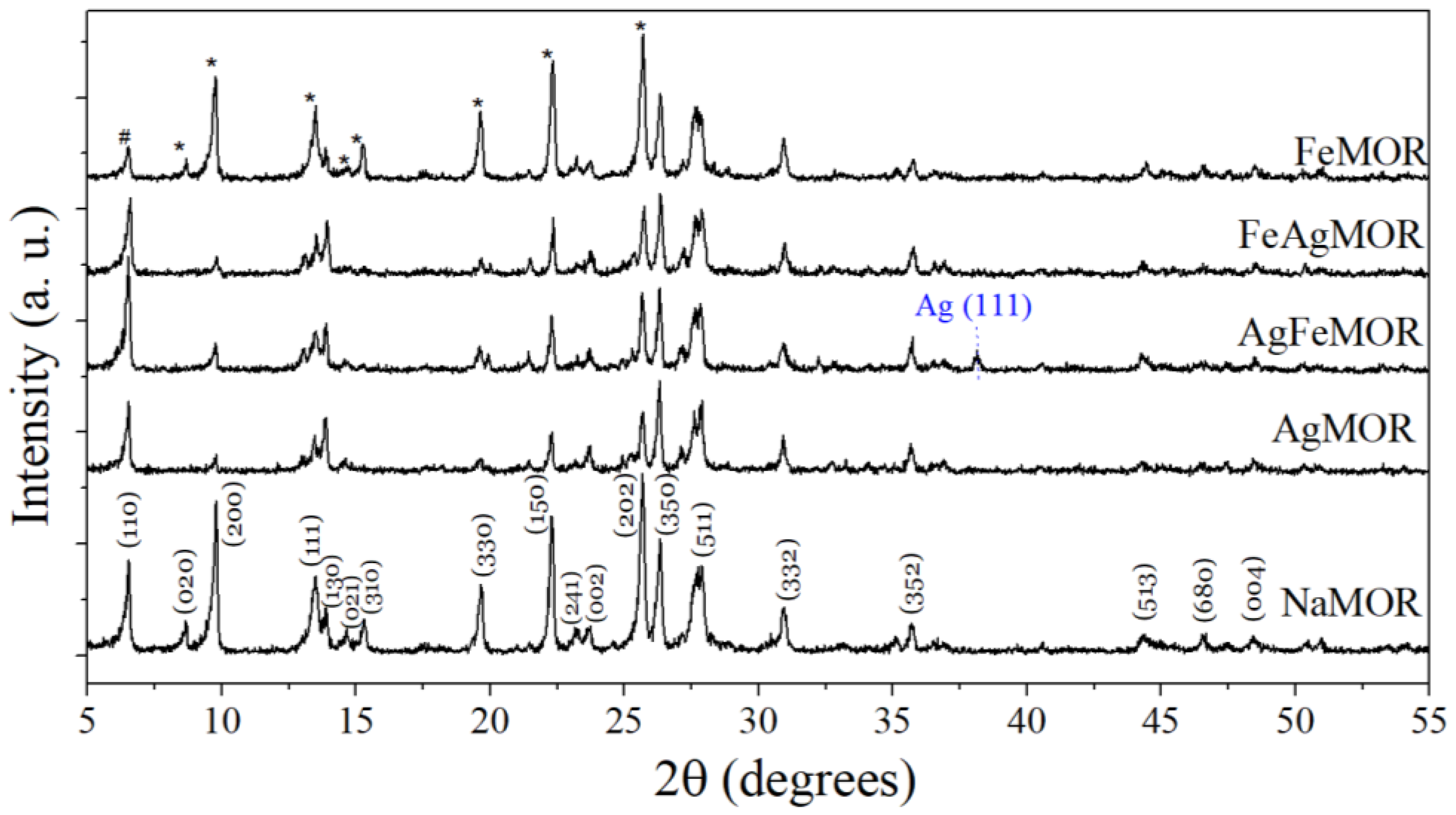
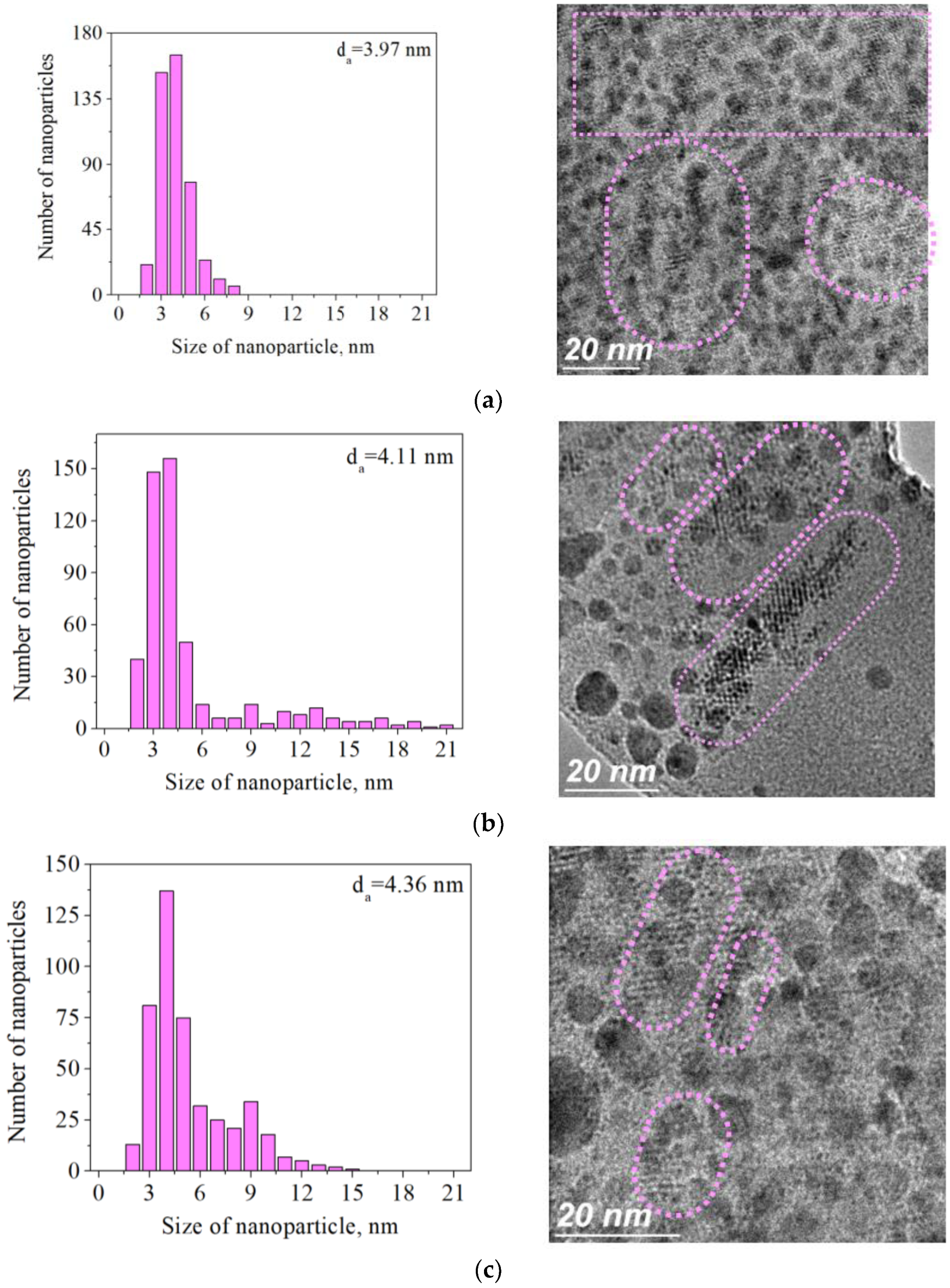
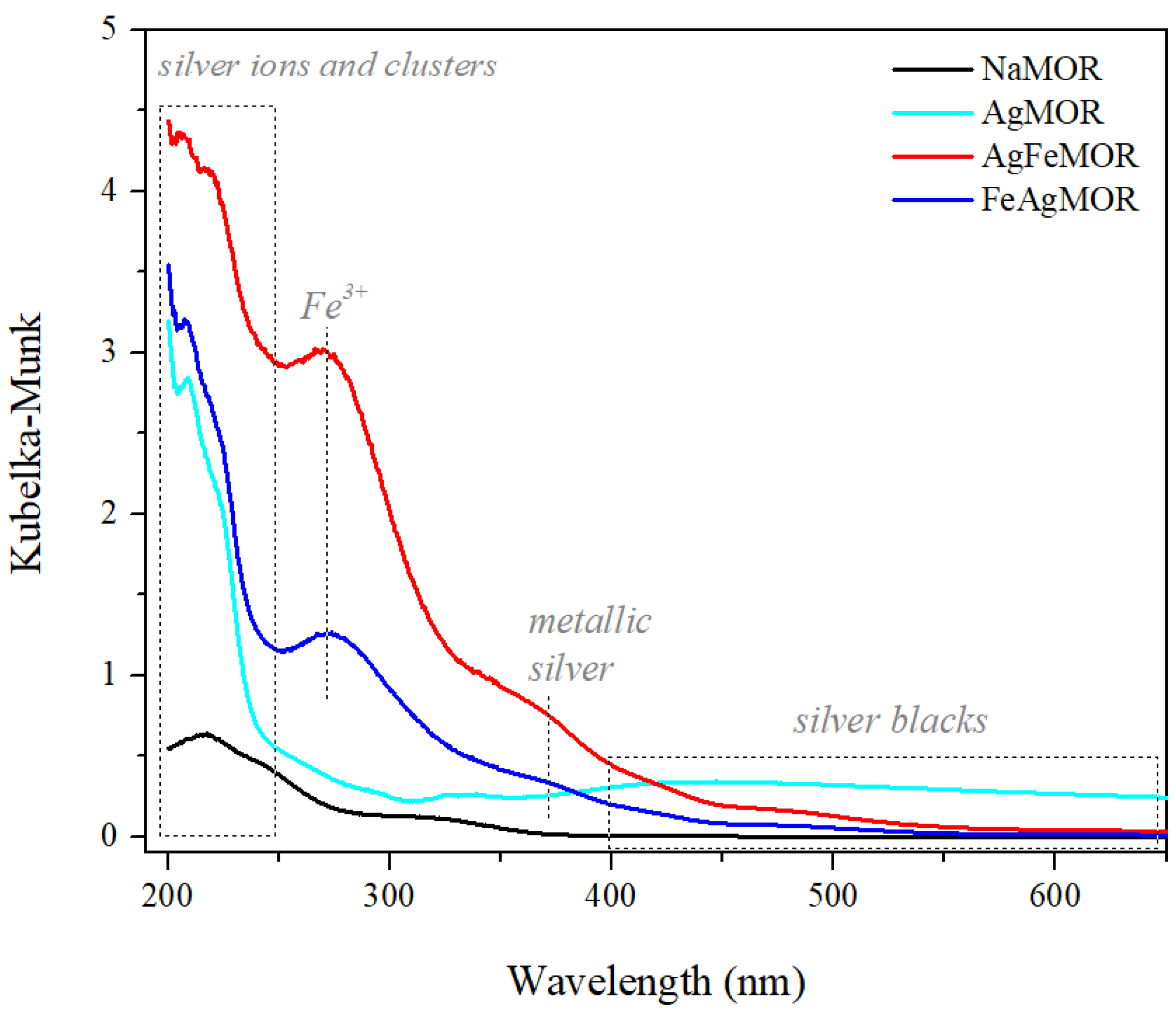
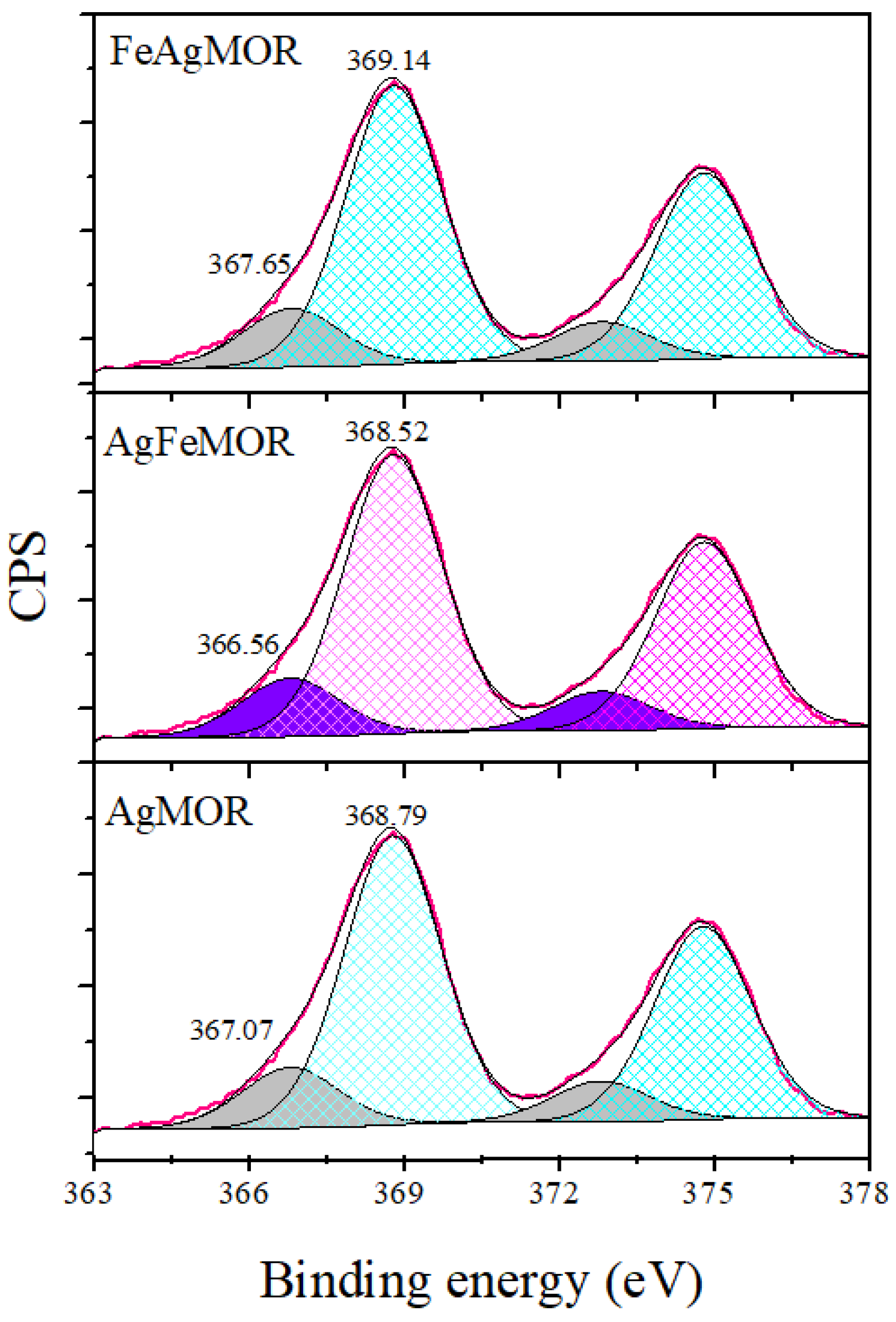
| Sample | SBET, m2∙g−1 | Vtotal, cm3∙g−1 | Vmicro, cm3∙g−1 | Pore Diameter, Å |
|---|---|---|---|---|
| NaMOR | 338 | 0.19 | 0.16 | 22.8 |
| AgMOR | 322 | 0.19 | 0.14 | 23.2 |
| FeMOR | 398 | 0.23 | 0.17 | 23.3 |
| AgFeMOR | 343 | 0.20 | 0.15 | 23.7 |
| FeAgMOR | 336 | 0.20 | 0.14 | 24.1 |
| Sample | Atomic % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Si | Al | O | Ag | Fe | Na | Si/Al | EIEM | ||
| EIEM-Fe2+ | EIEM–Fe3+ | ||||||||
| NaMOR | 48.9 | 7.5 | 33.9 | - | - | 9.7 | 6.5 | 1.29 | |
| AgMOR | 38.1 | 5.8 | 49.9 | 4.6 | - | 1.6 | 6.5 | 1.07 | |
| FeMOR | 44.0 | 6.4 | 45.4 | - | 0.8 | 3.4 | 6.8 | 0.78 | 0.91 |
| FeAgMOR | 37.2 | 5.4 | 51.9 | 3.6 | 0.4 | 1.5 | 6.9 | 1.09 | 1.17 |
| AgFeMOR | 39.7 | 5.9 | 49.7 | 3.1 | 0.9 | 0.7 | 6.7 | 0.95 | 1.10 |
| Samples | Ag ions | Ag-Support Interaction | Ag0 | Agn Clusters, Ø < 2 nm |
|---|---|---|---|---|
| 366.2 eV | 367.4–368 eV | 368.0–368.2 eV | ≥369 eV | |
| AgMOR | - | 367.07-(12%) | - | 368.79-(88%) |
| AgFeMOR | 366.56-(20%) | 368.52-(80%) | ||
| FeAgMOR | - | 367.65-(29%) | - | 369.14-(71%) |
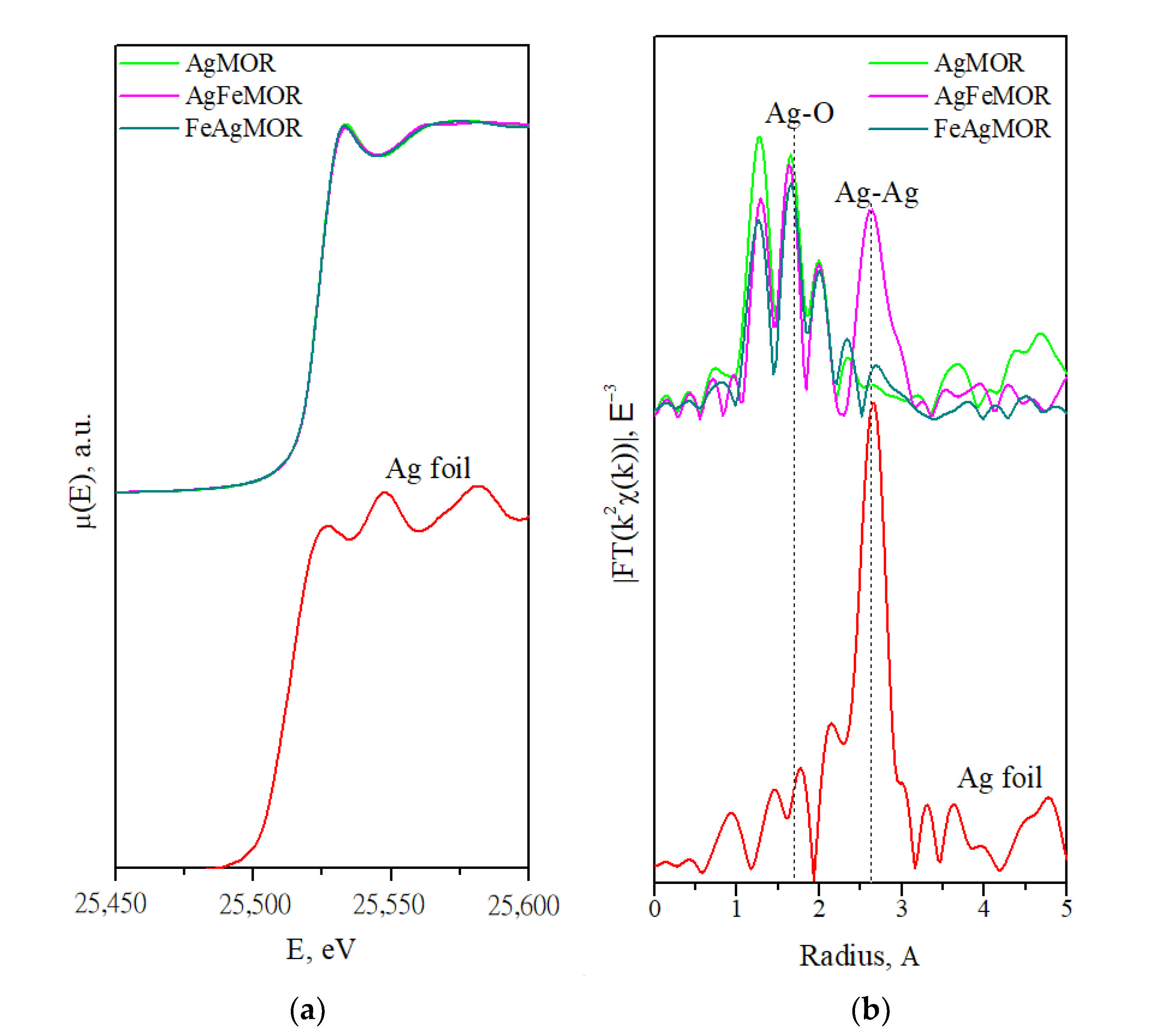
| Sample | Scattering Path | Coordination Number | Interatomic Distance, Å | Debye Factor, Å2 | R-Factor, % |
|---|---|---|---|---|---|
| Ref. Ag foil | Ag-Ag | 12 | 2.89 | - | - |
| Ref. Ag2O | Ag-O | 4 | 2.04 [44] | - | - |
| Ag-Ag | 8 | 3.34 | - | ||
| AgMOR | Ag-O | 1.4 | 1.91 | 0.0001 | 3.1 |
| 1.4 | 2.05 | ||||
| 1.4 | 2.12 | ||||
| 1.4 | 2.24 | ||||
| Ag-(Si or Al) | 1.3 | 2.82 | 0.0020 | ||
| 1.3 | 2.96 | ||||
| 1.3 | 3.02 | ||||
| 1.3 | 3.15 | ||||
| Ag-Ag | 0 | - | - | ||
| AgFeMOR | Ag-O | 1 | 1.90 | 0.0001 | 2.3 |
| 1 | 2.04 | ||||
| 1 | 2.10 | ||||
| 1 | 2.22 | ||||
| Ag-(Si or Al) | 1 | 2.92 | 0.0028 | ||
| 1 | 2.92 | ||||
| 1 | 3.12 | ||||
| 1 | 3.21 | ||||
| Ag-Ag | 1.6 | 2.82 | 0.0059 | ||
| FeAgMOR | Ag-O | 1 | 1.90 | 0.0009 | 1.3 |
| 1 | 2.04 | ||||
| 1 | 2.11 | ||||
| 1 | 2.22 | ||||
| Ag-(Si or Al) | 1 | 2.90 | 0.0030 | ||
| 1 | 2.90 | ||||
| 1 | 3.02 | ||||
| 1 | 3.16 | ||||
| Ag-Ag | 0.5 | 2.76 | 0.0043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-López, P.; Kotolevich, Y.; Antúnez-García, J.; Chávez-Rivas, F.; Khramov, E.; Berlier, G.; Moreno-Ruiz, L.; Zubavichus, Y.; Petranovskii, V.; Fuentes-Moyado, S.; et al. Influence of Components Deposition Order on Silver Species Formation in Bimetallic Ag-Fe System Supported on Mordenite. Catalysts 2022, 12, 1453. https://doi.org/10.3390/catal12111453
Sánchez-López P, Kotolevich Y, Antúnez-García J, Chávez-Rivas F, Khramov E, Berlier G, Moreno-Ruiz L, Zubavichus Y, Petranovskii V, Fuentes-Moyado S, et al. Influence of Components Deposition Order on Silver Species Formation in Bimetallic Ag-Fe System Supported on Mordenite. Catalysts. 2022; 12(11):1453. https://doi.org/10.3390/catal12111453
Chicago/Turabian StyleSánchez-López, Perla, Yulia Kotolevich, Joel Antúnez-García, Fernando Chávez-Rivas, Evgeny Khramov, Gloria Berlier, Luis Moreno-Ruiz, Yan Zubavichus, Vitalii Petranovskii, Sergio Fuentes-Moyado, and et al. 2022. "Influence of Components Deposition Order on Silver Species Formation in Bimetallic Ag-Fe System Supported on Mordenite" Catalysts 12, no. 11: 1453. https://doi.org/10.3390/catal12111453
APA StyleSánchez-López, P., Kotolevich, Y., Antúnez-García, J., Chávez-Rivas, F., Khramov, E., Berlier, G., Moreno-Ruiz, L., Zubavichus, Y., Petranovskii, V., Fuentes-Moyado, S., & Pestryakov, A. (2022). Influence of Components Deposition Order on Silver Species Formation in Bimetallic Ag-Fe System Supported on Mordenite. Catalysts, 12(11), 1453. https://doi.org/10.3390/catal12111453











