Peroxymonosulfate Activation by BaTiO3 Piezocatalyst
Abstract
1. Introduction
2. Results and Discussion
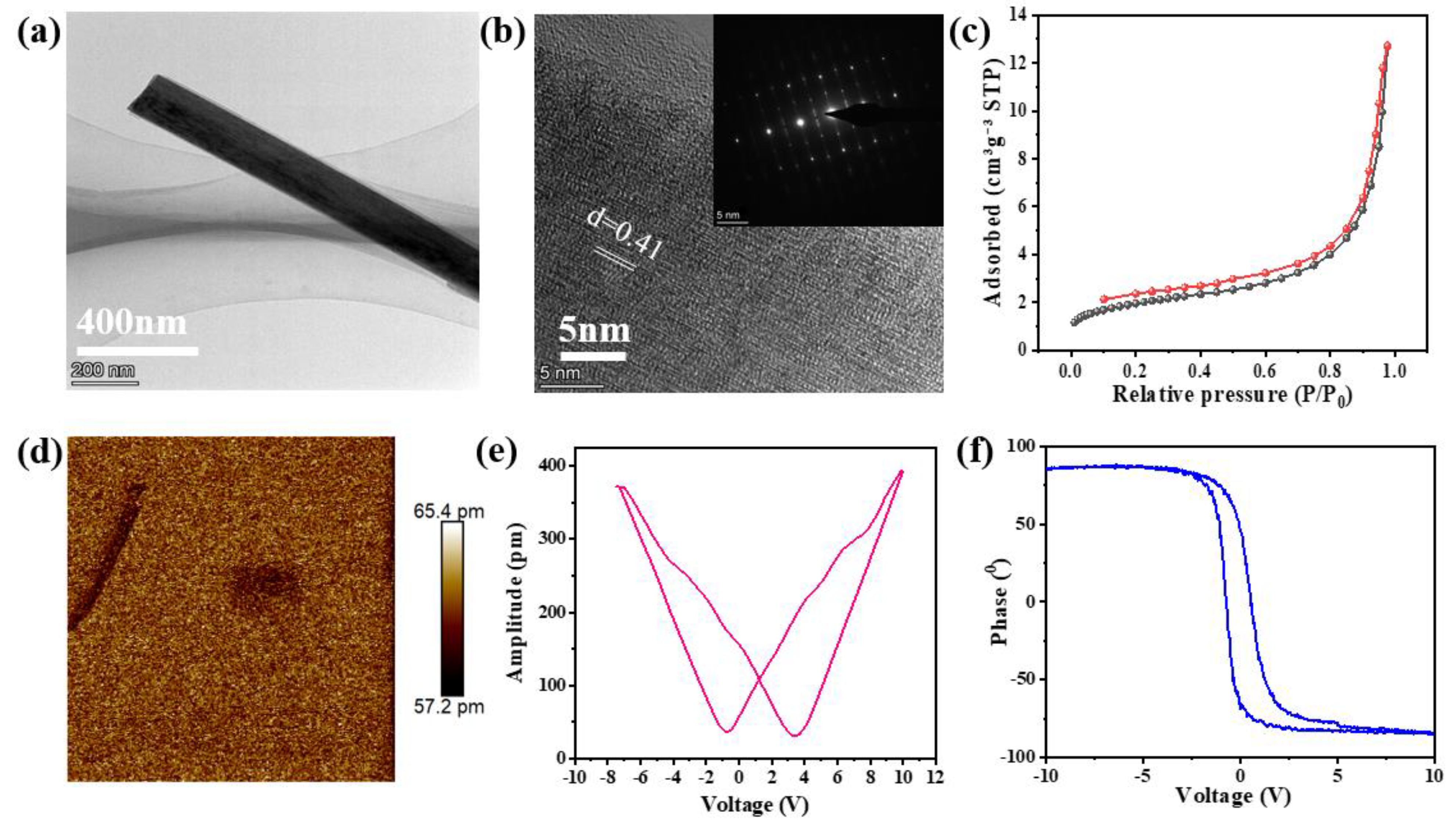
2.1. Characterizations
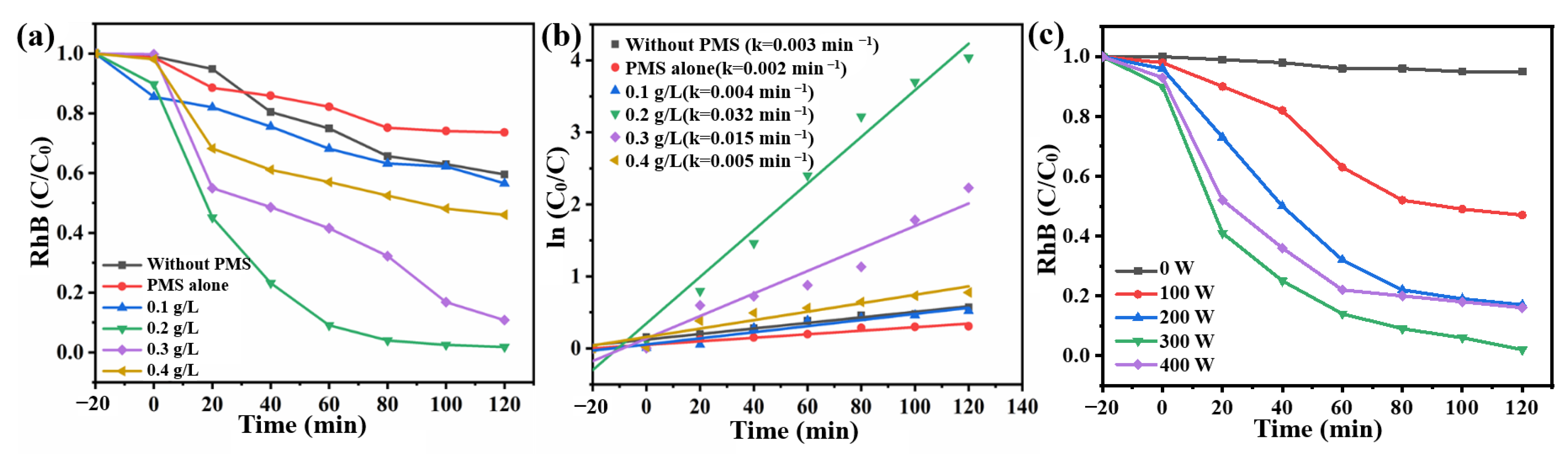
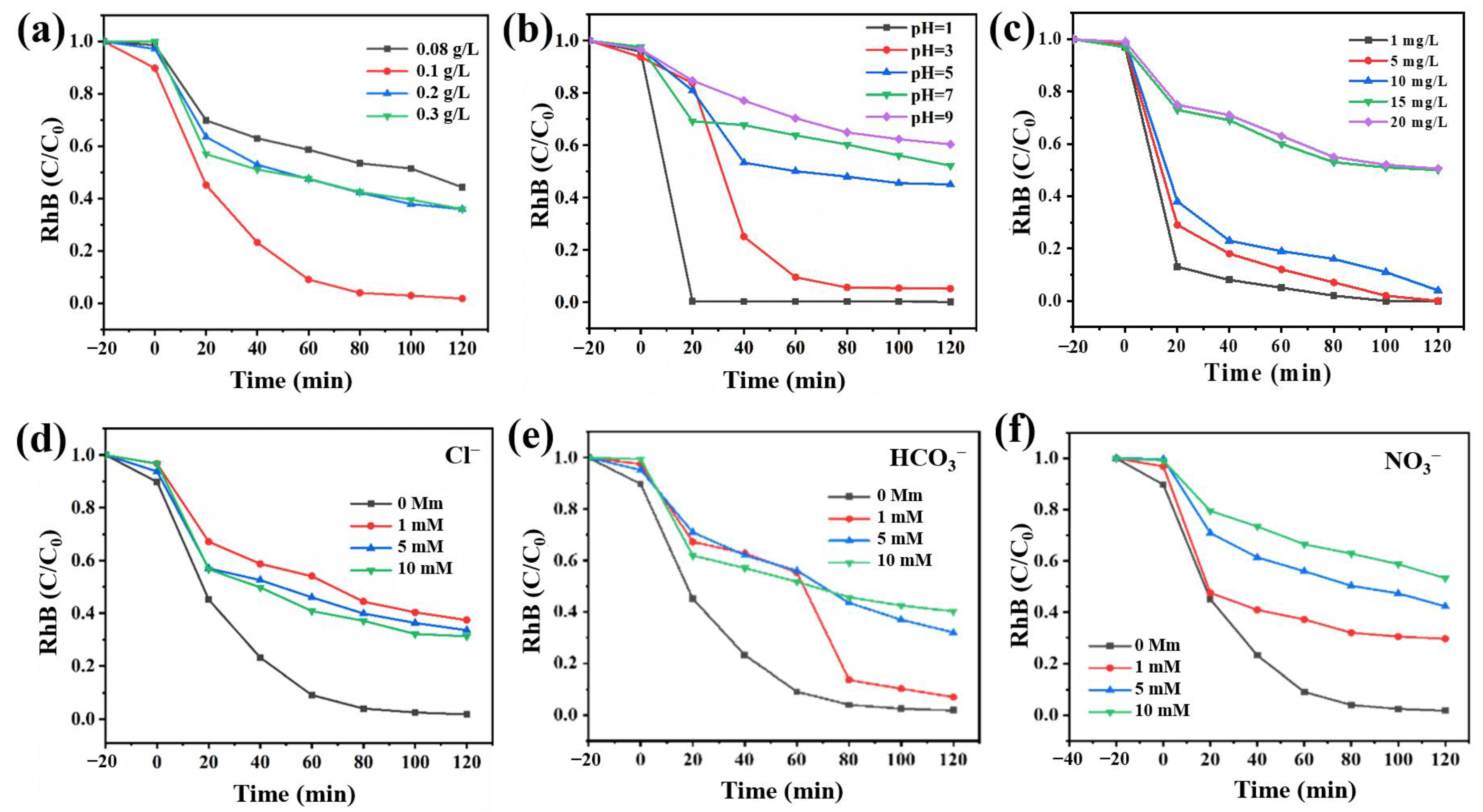
2.2. Performances
2.3. Mechanism Discussion
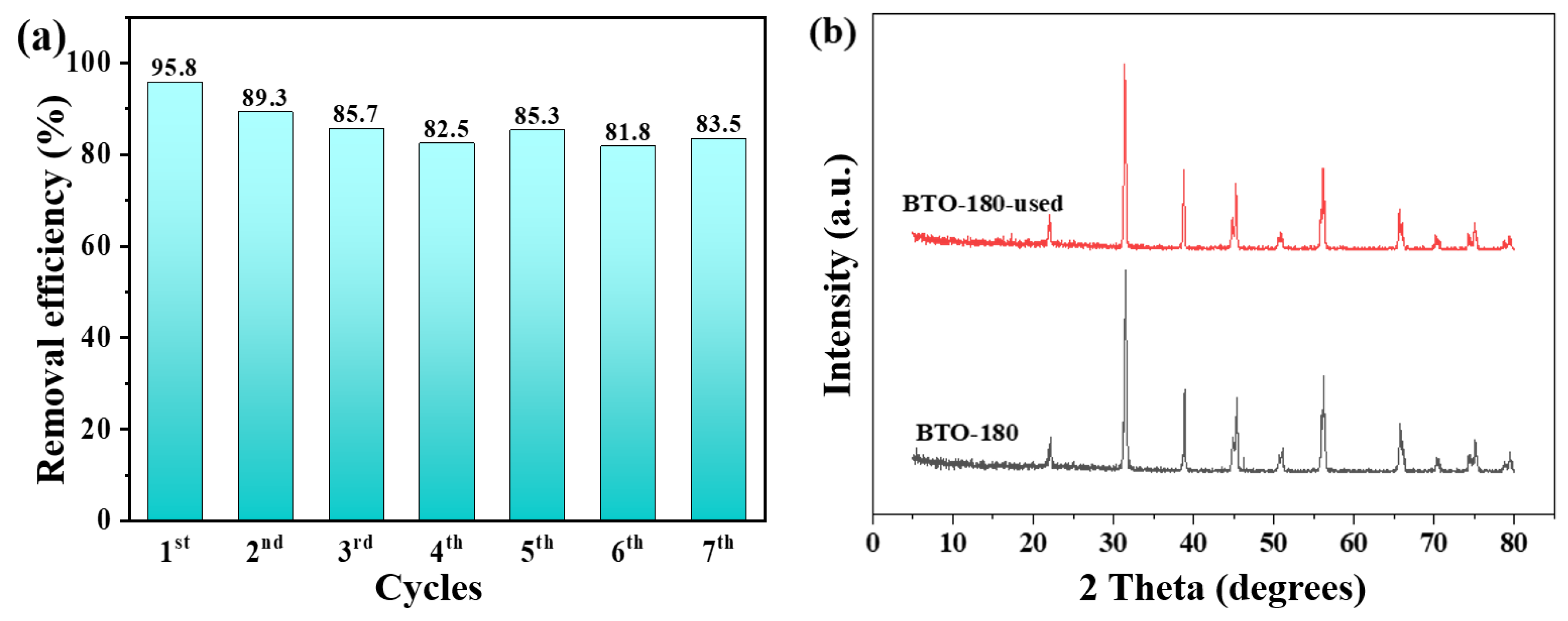
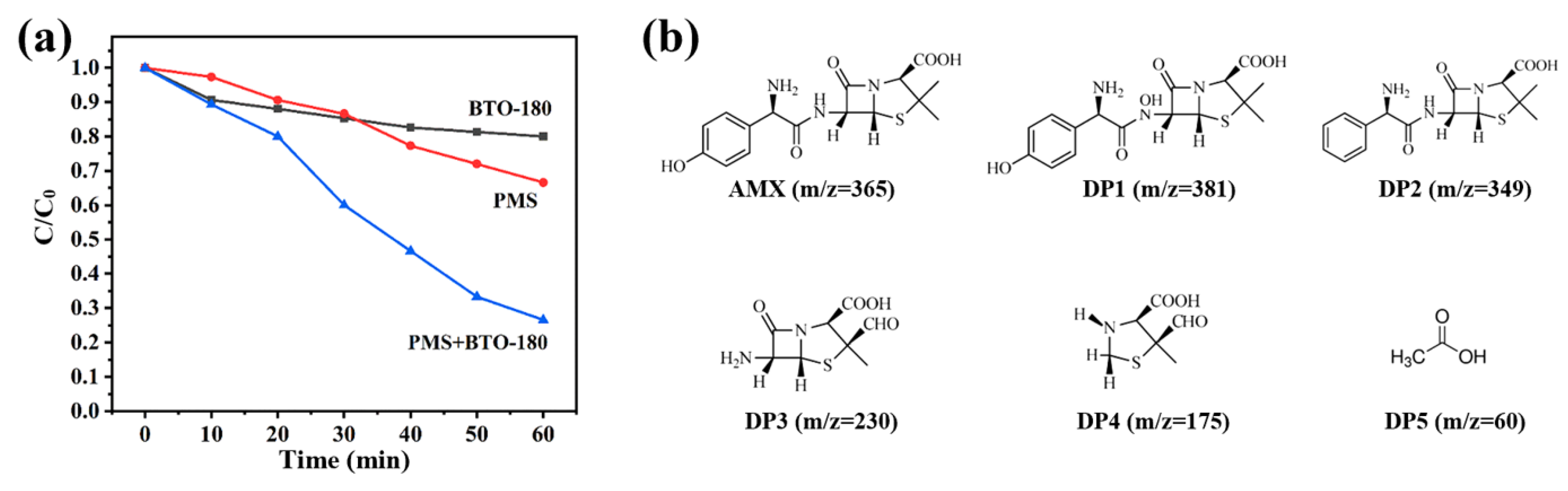
2.4. Environmental Implication
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterizations
3.3. Experimental Conditions and Analysis Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, X.; Zhou, T.; Mao, J.; Wu, X. Synergistic degradation of antibiotic sulfadiazine in a heterogeneous ultrasound-enhanced Fe0/Peroxymonosulfate Fenton-like system. Chem. Eng. J. 2014, 257, 36–44. [Google Scholar] [CrossRef]
- Soumia, F.; Petrier, C. Effect of potassium monopersulfate (oxone) and operating parameters on sonochemical degradation of cationic dye in an aqueous solution. Ultrason. Sonochem. 2016, 32, 343–347. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Ding, Z.; Zhao, Z.; Xu, X.; Fang, Z. Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye. Ultrason. Sonochem. 2017, 34, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, M.; Yao, G.; Lai, B. Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: Kinetic, degradation intermediates, and toxicity evaluation. Chem. Eng. J. 2018, 348, 1012–1024. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Huang, F.; Liu, Y.; Feng, L.; Jiang, L.; Qi, F.; Liu, C. Carbonized polyaniline activated peroxymonosulfate (PMS) for phenol degradation: Role of PMS adsorption and singlet oxygen generation. Appl. Catal. B Environ. 2021, 286, 119921. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal-and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: A review with emphasis on cost estimation. Ultrason. Sonochem. 2010, 17, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, Z.; Wang, S.; Liu, J.; Zhang, Y.; Wang, B.; Gong, Z.; Wang, M.; Dong, H.; Shi, J.; et al. Enhanced removal of methylparaben mediated by cobalt/carbon nanotubes (Co/CNTs) activated peroxymonosulfate in chloride-containing water: Reaction kinetics, mechanisms and pathways. Chem. Eng. J. 2021, 409, 128176. [Google Scholar] [CrossRef]
- Qi, C.; Wen, Y.; Zhao, Y.; Dai, Y.; Li, Y.; Xu, C.; Yang, S.; He, H. Enhanced degradation of organic contaminants by Fe (III)/peroxymonosulfate process with L-cysteine. Chin. Chem. Lett. 2022, 33, 2125–2128. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Ma, J.; Jiang, J.; Liu, Y.; Song, Y.; Yang, Y.; Guan, Y.; Wu, D. Simulation and comparative study on the oxidation kinetics of atrazine by UV/H2O2, UV/HSO5− and UV/S2O82−. Water Res. 2015, 80, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.-D.; Dionysiou, D.D.; Wang, Y.; Al-Abed, S.R.; Zhou, D.-M. Sulfate radical-based degradation of polychlorinated biphenyls: Effects of chloride ion and reaction kinetics. J. Hazard. Mater. 2012, 227, 394–401. [Google Scholar] [CrossRef]
- Song, Y.; Fang, G.; Zhu, C.; Zhu, F.; Wu, S.; Chen, N.; Wu, T.; Wang, Y.; Gao, J.; Zhou, D. Zero-valent iron activated persulfate remediation of polycyclic aromatic hydrocarbon-contaminated soils: An in situ pilot-scale study. Chem. Eng. J. 2019, 355, 65–75. [Google Scholar] [CrossRef]
- Li, Y.-T.; Zhang, J.-J.; Li, Y.-H.; Chen, J.-L.; Du, W.-Y. Treatment of soil contaminated with petroleum hydrocarbons using activated persulfate oxidation, ultrasound, and heat: A kinetic and thermodynamic study. Chem. Eng. J. 2022, 428, 131336. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Liang, L.; Duan, X.; Li, R.; Chen, G.; Yu, Y.; Liang, L.; Duan, X.; Li, R.; et al. Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. J. Hazard. Mater. 2021, 408, 124461. [Google Scholar] [CrossRef]
- Zhang, P.; Song, D.; Hao, Y.; Shang, X.; Wang, C.; Tang, J.; Sun, H. Sulfidated zero valent iron as a persulfate activator for oxidizing organophosphorus pesticides (OPPs) in aqueous solution and aged contaminated soil columns. Chemosphere 2021, 281, 130760. [Google Scholar] [CrossRef]
- Hussain, A.; Sinha, N.; Bhandari, S.; Yadav, H.; Kumar, B. Synthesis of 0.64 Pb (Mg1/3Nb2/3) O3–0.36 PbTiO3 ceramic near morphotropic phase boundary for high performance piezoelectric, ferroelectric and pyroelectric applications. J. Asian. Ceram. Soc. 2016, 4, 337–343. [Google Scholar] [CrossRef]
- Bi, L.; Gao, X.; Zhang, L.; Wang, D.; Zou, X.; Xie, T. Enhanced photocatalytic hydrogen evolution of NiCoP/g-C3N4 with improved separation efficiency and charge transfer efficiency. Chem. Sus. Chem. 2018, 11, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Li, S.; Wu, Q.; Lin, Y.; Wang, D.; Zou, X.; Xie, T. In situ synthesis of FeP-decorated Ti–Fe2O3: An effective strategy to improve the interfacial charge transfer in the photoelectrochemical water oxidation reaction. Catal. Sci. Technol. 2019, 9, 5812–5818. [Google Scholar] [CrossRef]
- Han, B.; Zhu, P.; Liu, Y.; Qiu, Q.; Li, J.; Liang, T.; Xie, T. Enhanced photocatalytic degradation activity via a stable perovskite-type LaFeO3/In2S3 Z-scheme heterostructured photocatalyst: Unobstructed photoexcited charge behavior of Z scheme photocatalytic system exploration. J. Alloy. Compd. 2022, 901, 163628. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Chen, Y.; Zhang, R.; Li, C.; Zhang, K.; Li, M.; Lin, Y.; Wang, D.; Zou, X.; et al. Interface engineering Z-scheme Ti-Fe2O3/In2O3 photoanode for highly efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2021, 290, 120058. [Google Scholar] [CrossRef]
- Bu, Q.; Li, S.; Zhang, K.; Lin, Y.; Wang, D.; Zou, X.; Xie, T. Hole transfer channel of ferrihydrite designed between Ti–Fe2O3 and copi as an efficient and durable photoanode. ACS Sustain. Chem. Eng. 2019, 7, 10971–10978. [Google Scholar] [CrossRef]
- Bi, L.; Zhang, R.; Zhang, K.; Lin, Y.; Wang, D.; Zou, X.; Xie, T. Sulfidization of platinum nickel bimetal-decorated g-C3N4 for photocatalytic hydrogen production: Photogenerated charge behavior study. ACS Sustain. Chem. Eng. 2019, 7, 15137–15145. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhu, P.; Liu, Y.; Liang, T.; Xie, T.; Lin, Y. Highly efficient In2S3/WO3 photocatalysts: Z-scheme photocatalytic mechanism for enhanced photocatalytic water pollutant degradation under visible light irradiation. RSC Adv. 2021, 11, 3333–3341. [Google Scholar] [CrossRef]
- Li, K.; Zhou, W.; Li, X.; Li, Q.; Carabineiro, S.; Zhang, S.; Fan, J.; Lv, K. Synergistic effect of cyano defects and CaCO3 in graphitic carbon nitride nanosheets for efficient visible-light-driven photocatalytic NO removal. J. Hazard. Mater. 2023, 442, 130040. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Z.; Zhao, J.; Zhang, Z.; Li, X.; Zhang, J. Recent advances of ferro-, piezo-, and pyroelectric nanomaterials for catalytic applications. ACS Appl. Nano Mater. 2020, 3, 1063–1079. [Google Scholar] [CrossRef]
- Nie, Q.; Xie, Y.; Ma, J.; Wang, L.; Zhang, G. High piezo–catalytic activity of ZnO/Al2O3 nanosheets utilizing ultrasonic energy for wastewater treatment. J. Clean. Prod. 2020, 242, 118532. [Google Scholar] [CrossRef]
- Hao, L.; Huang, H.; Guo, Y.; Zhang, Y. Multifunctional Bi2O2 (OH)(NO3) nanosheets with {001} active exposing facets: Efficient photocatalysis, dye-sensitization, and piezoelectric-catalysis. ACS Sustain. Chem. Eng. 2018, 6, 1848–1862. [Google Scholar] [CrossRef]
- Liang, Z.; Yan, C.F.; Rtimi, S.; Bandara, J. Piezoelectric materials for catalytic/photocatalytic removal of pollutants: Recent advances and outlook. Appl. Catal. B Environ. 2019, 241, 256–269. [Google Scholar] [CrossRef]
- Ling, J.; Wang, K.; Wang, Z.; Huang, H.; Zhang, G. Enhanced piezoelectric-induced catalysis of SrTiO3 nanocrystal with well-defined facets under ultrasonic vibration. Ultrason. Sonochem. 2020, 61, 104819. [Google Scholar] [CrossRef]
- Feng, Y.; Ling, L.; Wang, Y.; Xu, Z.; Cao, F.; Li, H.; Bian, Z. Engineering spherical lead zirconate titanate to explore the essence of piezo-catalysis. Nano Energy 2017, 40, 481–486. [Google Scholar] [CrossRef]
- Wang, M.; Wang, B.; Huang, F.; Lin, Z. Enabling PIEZO potential in PIEZO electric semiconductors for enhanced catalytic activities. Angew. Chem. Int. Edit. 2019, 58, 7526–7536. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.; Fang, J.; Gao, L.; Cai, W.; Li, X.; Xu, A.; Ruan, X. The photocatalytic performance of g-C3N4 from melamine hydrochloride for dyes degradation with peroxymonosulfate. J. Photoch. Photobio. A 2017, 336, 54–62. [Google Scholar] [CrossRef]
- Cao, F.; Yang, L.; Zhang, Y.; Zhao, X.; Lu, H.; Wang, J. Peroxymonosulfate activation in ultrasound-driven molybdenum disulfide piezocatalysis: The effect of sulfur vacancy. J. Clean. Prod. 2022, 380, 135002. [Google Scholar] [CrossRef]
- Lin, E.; Wu, J.; Qin, N.; Yuan, B.; Bao, D. Silver modified barium titanate as a highly efficient piezocatalyst. Catal. Sci. Technol. 2018, 8, 4788–4796. [Google Scholar] [CrossRef]
- Hong, K.; Xu, H.; Konishi, H.; Li, X. Piezoelectrochemical effect: A new mechanism for azo dye decolorization in aqueous solution through vibrating piezoelectric microfibers. J. Physi. Chem. C 2012, 116, 13045–13051. [Google Scholar] [CrossRef]
- Lan, S.; Feng, J.; Xiong, Y.; Tian, S.; Liu, S.; Kong, L. Performance and mechanism of piezo-catalytic degradation of 4-chlorophenol: Finding of effective piezo-dechlorination. Environ. Sci. Technol. 2017, 51, 6560–6569. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qin, N.; Bao, D. Effective enhancement of piezocatalytic activity of BaTiO3 nanowires under ultrasonic vibration. Nano Energy 2018, 45, 44–51. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Z.; Xiao, L.; Jia, Y.; Ma, J.; Wang, F.; Wang, L.; Wang, M.; Huang, H. Strong piezo-electro-chemical effect of piezoelectric BaTiO3 nanofibers for vibration-catalysis. J. Alloy. Compd. 2018, 762, 915–921. [Google Scholar] [CrossRef]
- Mukasa, S.; Nomura, S.; Toyota, H. Measurement of temperature in sonoplasma. Jpn. J. Appl. Phys. 2004, 43, 2833. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, W.; Huang, Y.; Zhang, G. A mechanistic study of amorphous CoSx cages as advanced oxidation catalysts for excellent peroxymonosulfate activation towards antibiotics degradation. Chem. Eng. J. 2020, 381, 122768. [Google Scholar] [CrossRef]
- Chen, F.; Ren, Z.; Gong, S.; Li, X.; Shen, G.; Han, G. Selective deposition of silver oxide on single-domain ferroelectric nanoplates and their efficient visible-light photoactivity. Chem. Eur. J. 2016, 22, 12160–12165. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Guo, X.; Yang, C.; Ouyang, Z. Synthesis of g-C3N4 by different precursors under burning explosion effect and its photocatalytic degradation for tylosin. Appl. Catal. B Environ. 2018, 230, 65–76. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Li, X.; Ji, M.; Xu, Z.; Chen, Z.; Li, H. Constructing confined surface carbon defects in ultrathin graphitic carbon nitride for photocatalytic free radical manipulation. Carbon 2016, 107, 1–10. [Google Scholar] [CrossRef]
- Ma, J.; Cao, R.; Dang, Y.; Wang, J. A recent progress of room–temperature airborne ozone decomposition catalysts. Chinese Chem. Lett. 2021, 32, 2985–2993. [Google Scholar] [CrossRef]
- Rehman, F.; Sayed, M.; Khan, J.A.; Shah, N.S.; Khan, H.M.; Dionysiou, D.D. Oxidative removal of brilliant green by UV/S2O82−, UV/HSO5−and UV/H2O2 processes in aqueous media: A comparative study. J. Hazard. Mater. 2018, 357, 506–514. [Google Scholar] [CrossRef]
- Shah, N.; Khan, J.; Sayed, M.; Khan, Z.; Ali, H.; Murtaza, B.; Khan, H.; Imran, M.; Muhammad, N. Hydroxyl and sulfate radical mediated degradation of ciprofloxacin using nano zerovalent manganese catalyzed S2O82−. Chem. Eng. J. 2019, 356, 199–209. [Google Scholar] [CrossRef]
- Aryee, A.; Han, R.; Qu, L. Occurrence, detection and removal of amoxicillin in wastewater: A review. J. Clean. Prod. 2022, 368, 133140. [Google Scholar] [CrossRef]
- Sodhi, K.; Kumar, M.; Singh, D. Insight into the amoxicillin resistance, ecotoxicity, and remediation strategies. J. Water Process. Eng. 2021, 39, 101858. [Google Scholar] [CrossRef]
- El-Nahhal, Y.; El-Dahdouh, N. Toxicity of amoxicillin and erythromycin to fish and mosquito. Ecotox Environ. 2015, 10, 13–21. [Google Scholar]
- Shah, N.; Khan, J.; Sayed, M.; Khan, Z.; Rizwan, A.; Muhammad, N.; Boczkaj, G.; Murtaza, B.; Imran, M.; Khan, H.; et al. Solar light driven degradation of norfloxacin using as-synthesized Bi3+ and Fe2+ co-doped ZnO with the addition of HSO5−: Toxicities and degradation pathways investigation. Chem. Eng. J. 2018, 351, 841–855. [Google Scholar] [CrossRef]


| Compound | Acute Toxicity | Chronic Toxicity | ||||
|---|---|---|---|---|---|---|
| Fish (LC50) Time 96 h | Daphnia (LC50) Time 48 h | Green Algae (EC50) Time 96 h | Fish (ChV) | Daphnia (ChV) | Green Algae (ChV) | |
| AMX | 184 | 15.3 | 30.3 | 1.73 | 3.19 | 5.12 |
| DP1 | 281 | 19.0 | 42.9 | 2.43 | 4.04 | 7.03 |
| DP2 | 3.73 × 103 | 4.05 × 103 | 1.73 × 103 | 2.02 × 103 | 26.2 | 430 |
| DP3 | 1.63 × 104 | 116 | 1.13 × 103 | 58.3 | 31.1 | 129 |
| DP4 | 5.39 × 104 | 8.45 × 104 | 2.64 × 104 | 1.28 × 105 | 320 | 4.23 × 103 |
| DP5 | 2.58 × 104 | 1.23 × 104 | 4.40 × 103 | 2.05 × 103 | 732 | 778 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Ni, C.; Hou, T.; Guo, W.; Wang, J. Peroxymonosulfate Activation by BaTiO3 Piezocatalyst. Catalysts 2022, 12, 1452. https://doi.org/10.3390/catal12111452
Yu M, Ni C, Hou T, Guo W, Wang J. Peroxymonosulfate Activation by BaTiO3 Piezocatalyst. Catalysts. 2022; 12(11):1452. https://doi.org/10.3390/catal12111452
Chicago/Turabian StyleYu, Maogen, Cheng Ni, Tian Hou, Weihong Guo, and Jinlong Wang. 2022. "Peroxymonosulfate Activation by BaTiO3 Piezocatalyst" Catalysts 12, no. 11: 1452. https://doi.org/10.3390/catal12111452
APA StyleYu, M., Ni, C., Hou, T., Guo, W., & Wang, J. (2022). Peroxymonosulfate Activation by BaTiO3 Piezocatalyst. Catalysts, 12(11), 1452. https://doi.org/10.3390/catal12111452







