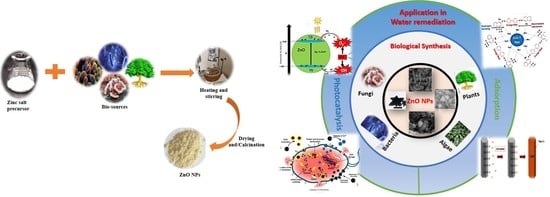
An Evaluation of the Biocatalyst for the Synthesis and Application of Zinc Oxide Nanoparticles for Water Remediation—A Review
Abstract
1. Introduction
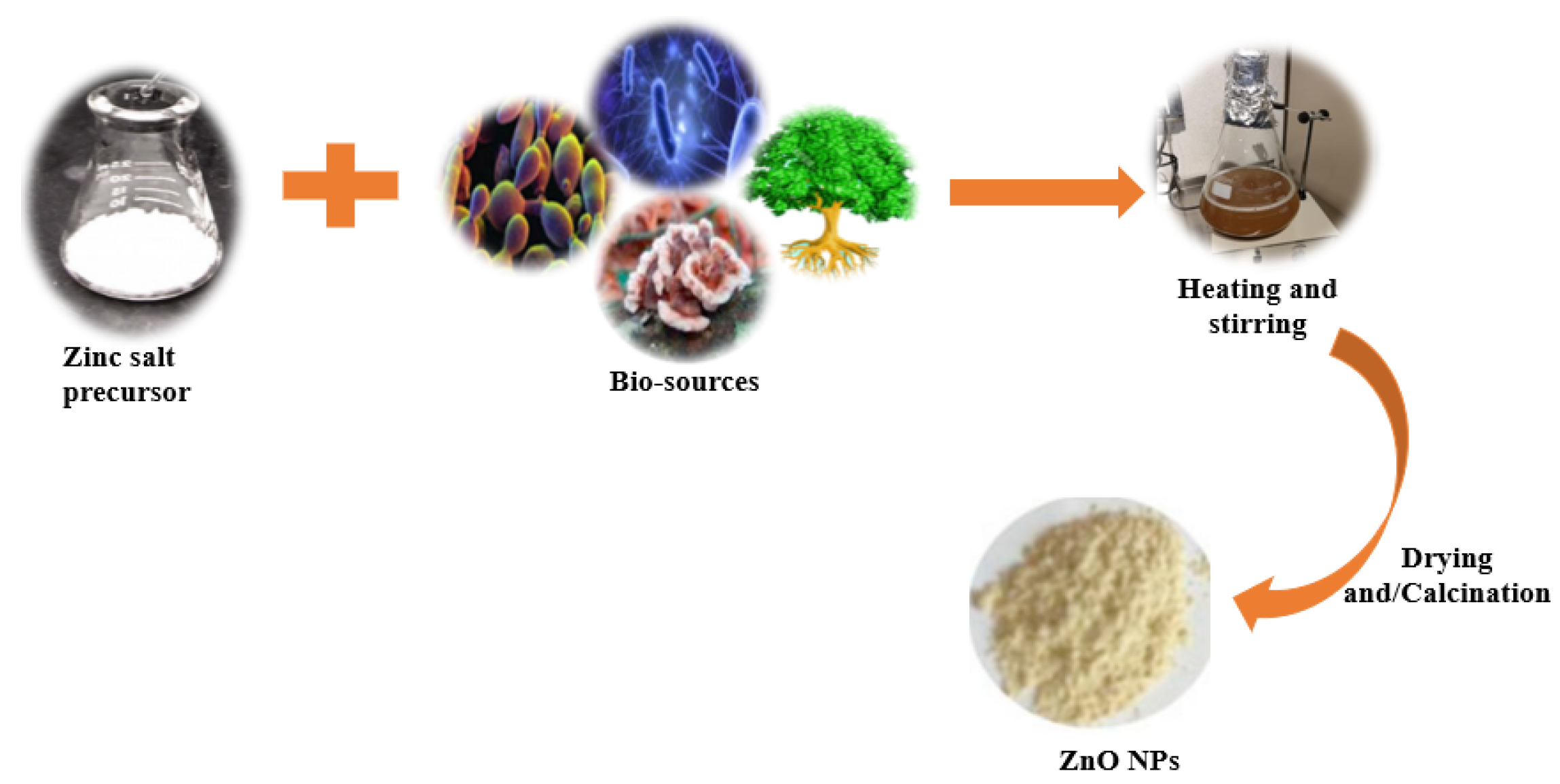
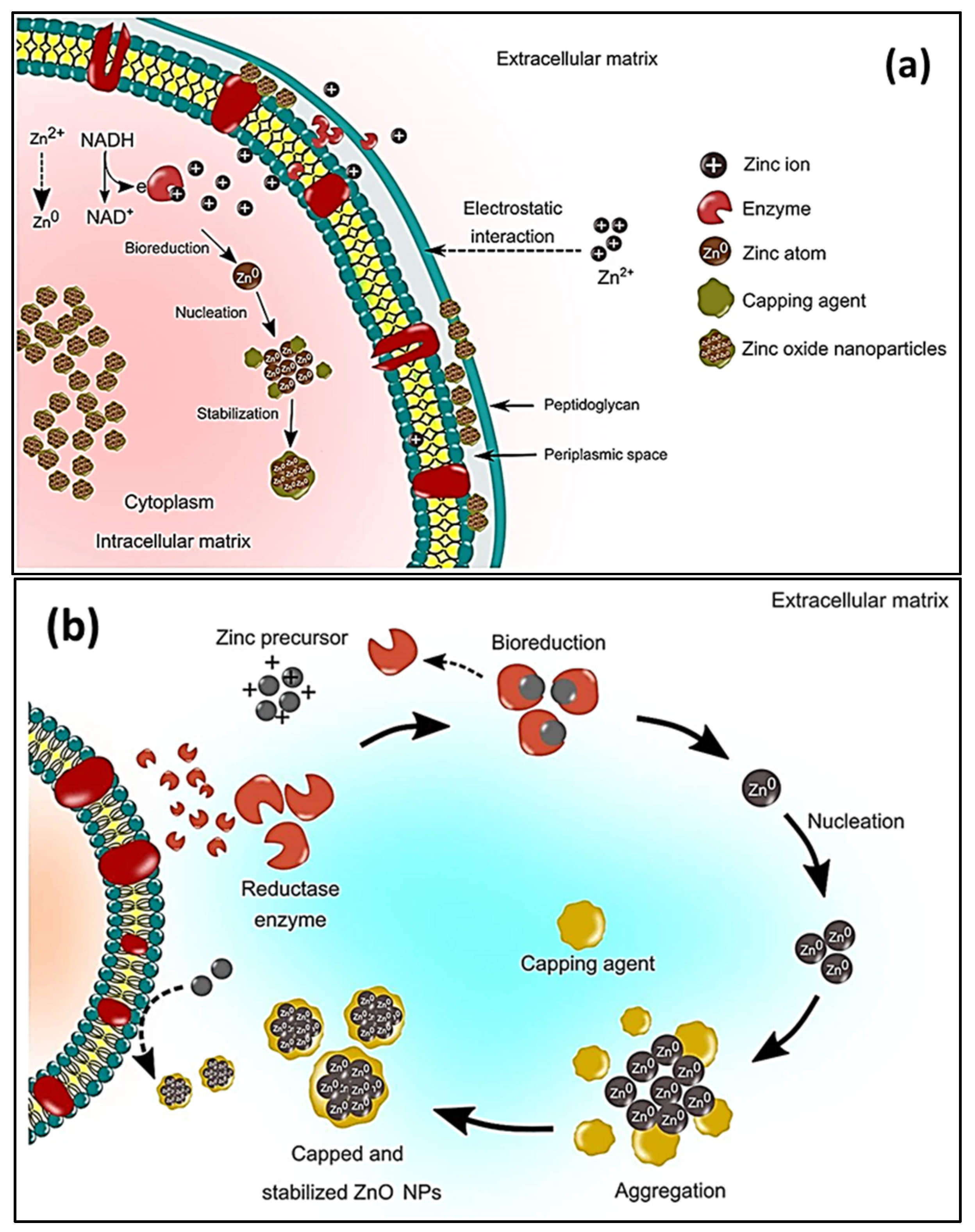
2. Green Synthesis
2.1. Plant-Mediated/Phytosynthesis of ZnO NPs
2.1.1. Phytosynthesis Synthesis of Undoped ZnO NPs
| Serial No. | Plant Name | Plant Part | Extraction Solvent Temp (°C) Time | Zinc Precursor | Biosynthesis Time (h)/Temp (°C) | Calcination Temp (°C)/Time (h) | Shape | Size (nm) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pithecellobium dulce | Peel | Water, 70 °C for 50 min | Zn(CH₃CO₂)₂ | 400/2 | Spherical | 30 | [83] | |
| 2 | Ananas comosus | Pericarp | Water, 80 °C for 20 min | Zn(CH3COO)2·2H2O, | Sonicated 1/40 | − | Spherical | 10–60 | [82] |
| 3 | Punica granatum | Fruit peel | Water, 65 °C for 1 h | Zn(NO3)2·6H2O | –/90 | 600/1 | Spherical and hexagonal | 38.98 | [84] |
| 4 | Zizyphus jujuba | Fruit | Refluxed with water, 80 °C for 30 min | Zn(NO3)2·6H2O | 4/80 | 550/3 | Spherical | 29 | [85] |
| 5 | Myristica fragrans | Fruit | Water, 150 °C for 20 min | Zn(NO3)2·2H2O | 2/60 | 500/2 | Semispherical | 41.23 | [86] |
| 6 | Hibiscus sabdariffa | Flower | Water, 60 °C, for 1 h after 2 h stirring at RT | Zn(NO3)2 | 1/RT. | 400/1 | Semi spherical | 8.7–88.6 | [87] |
| 7 | Peltophorum pterocarpum | Flower | Water, 80 °C for 1 h | Zn(NO3)2 | –/80 | 400/2 | Spherical and irregular | 69.45 | [88] |
| 8 | Aspalathus linearis | Flower | Water, 25 °C for 48 h | Zn(NO3)2·6H2O | −/− | 300/2 | Quasi-spherical | 1–8.5 | [89] |
| 9 | Eucalyptus spp. | Leaves | Water, 90 °C for 90 min | Zn(NO3)2·6H2O | 2/110 | 250/6 | Irregular shapes | 32.71 | [90] |
| 10 | Capparis zeylanica | Leaves | Water, 60 °C for 20 min | Zn(OOCCH3)2·2(H2O), | 2/80 | 400/2 | Spherical | 20–40 | [91] |
| 11 | Ocimum tenuiflorum | Leaves | Powder | ZnSO4 | 0.5/RT | 600/0.5 | Spherical and granular | 50–63 | [92] |
| 12 | Euphorbia sanguinea | Leaves | Water, 80 °C for 45 min | ZnCl2 | 2/90 | – | Flower-like | 34 | [93] |
| 13 | Moringa oleifera | Leaves | Water, 50 °C for 105 min | Zn(NO3)2·6H2O | 18/RT then 100 | 500/1 | Spherical | 16–31.9 | [97] |
| Gathosma betulina | Leaves | Water, 100 °C for 60 min | Zn(NO3)2·6H2O | 2/100 | 500/2 | Quasi-spherical | 15.8 | [98] | |
| 14 | Quince seeds | Mucilage | Water, 60 °C for 4 h | Zn(NO3)2·6H2O | 2/80 after keeping for 4 h at RT | 600/2 | Spherical | 25 | [94] |
| 15 | Abelmoschus esculentus | Mucilage | Soaked for 24 h at RT | Zn(OOCCH3)2·2(H2O), | 4/RT | 700/2 | Spherical Rods | 29 70 | [95] |
| 16 | Panax spp. | Roots and stems | Water, 85 °C for 8 h | Zn(NO3)2 | 2.5/85 | 500/2 | Leafy flower | 480 | [96] |
| 17 | Salvadora persica | Roots | Water and methanol, 80 °C for 3 h | Zn(CH3COO)2·2H2O | 2–3/80 | 400/2 | Hexagonal and rods | 24–25 | [68] |
| 18 | Rubus fairholmianus | Roots | Acetone using Soxhlet apparatus | Zn(NO3)2 | 48/80 | − | Spherical | 1–100 clusters | [100] |
2.1.2. Phytosynthesis of Doped ZnO Nanoparticles
2.2. Microbial Synthesis of ZnO Nanoparticles
2.2.1. Bacterial Synthesis
2.2.2. Fungal Synthesis
2.2.3. Algal Synthesis
2.2.4. Preparation of ZnO NPs Using Biological Derivatives Synthesis
2.3. Factors Influencing ZnO Nanoparticle Biosynthesis
2.3.1. Temperature
2.3.2. Effect of pH
2.3.3. Effect of the Concentration of the Precursor
2.3.4. Effect of Reaction Time
2.3.5. Effect of Different Biological Species
3. Characterization Techniques for ZnO
3.1. UV-Vis Spectroscopy
3.2. X-ray Diffraction (XRD)
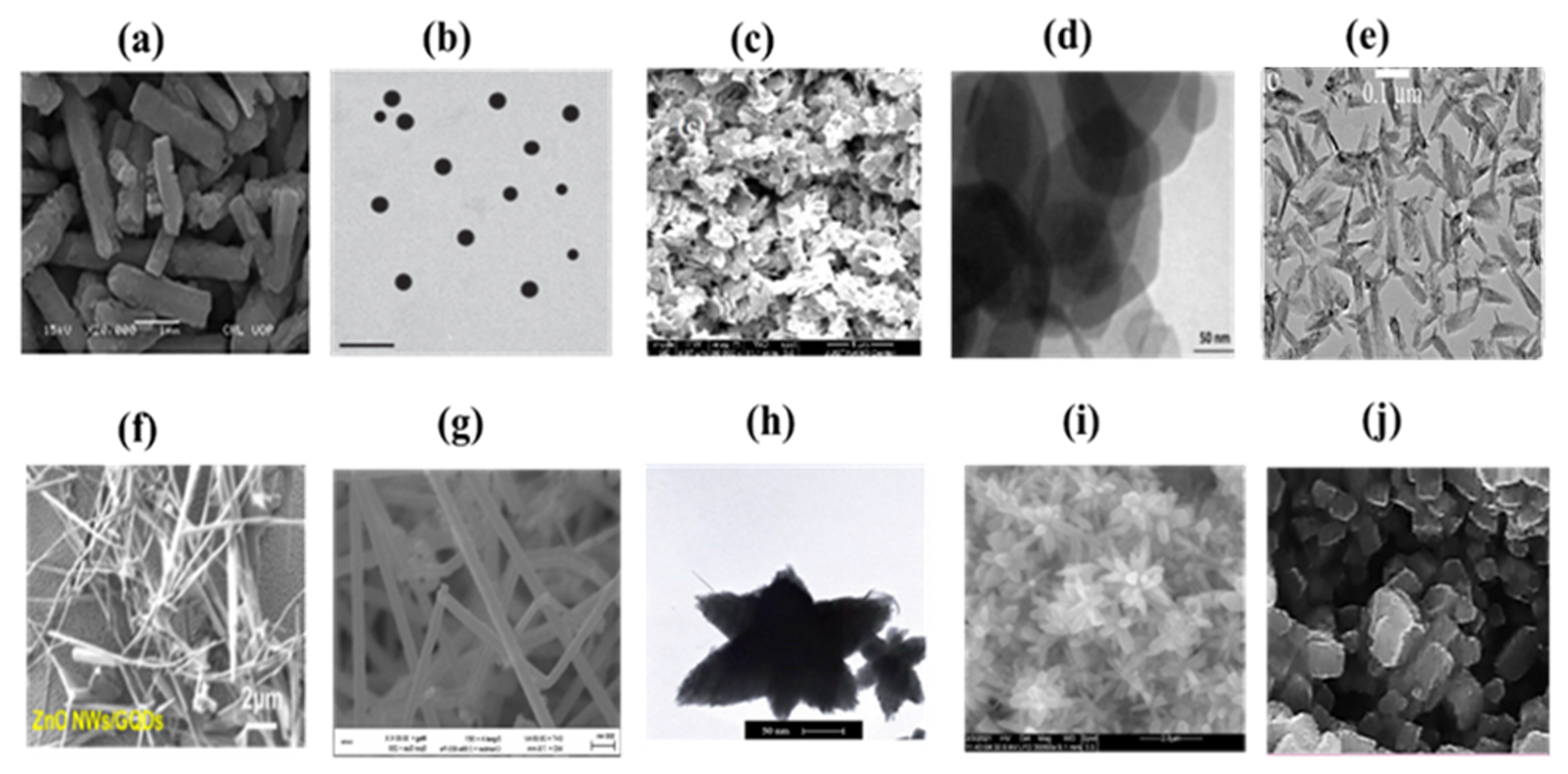
3.3. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
3.4. Energy-Dispersive X-ray Spectroscopy (EDX)
3.5. Fourier-Transform Infrared Spectroscopy (FTIR)
3.6. Other Techniques for ZnO Analysis
4. Application of Green ZnO NPs in Water Remediation
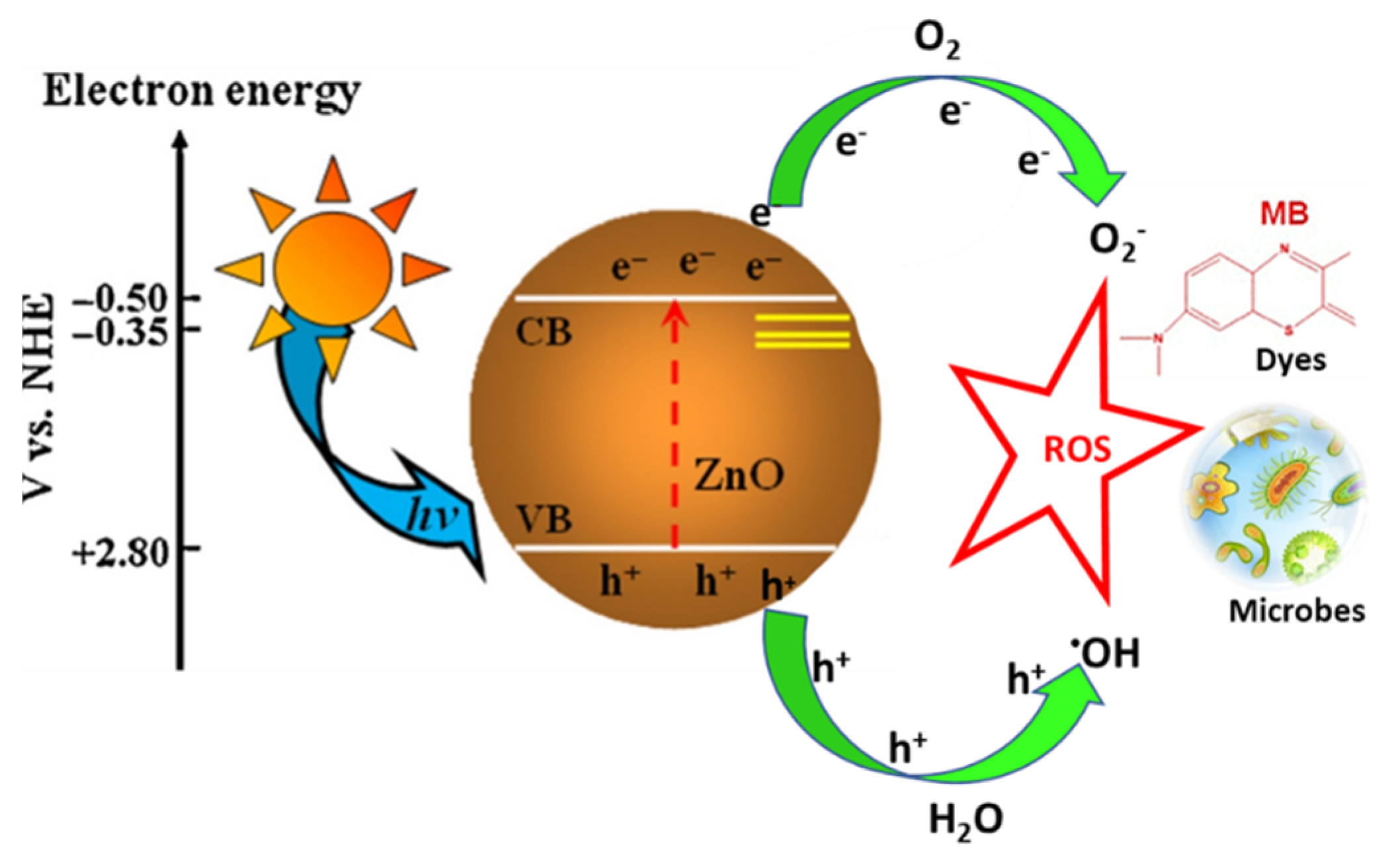
4.1. Photocatalytic Degradation of Pollutants
| Name of Biological Source | ZnO Morphology | Pollutant | Catalyst Concentration | Pollutant Concentration | Radiation | Exposure Time (min) | pH | Efficiency (%) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Plant based | |||||||||
| Lycopersicon esculentum, | Polyhedral | Methylene blue (MB) | _ | 15 mg/L | 10 W UV light | 180 | 6 | 97 | [186] |
| Calliandra haematocephala-L. | Nanoflower-like | MB | 50 mg/100 mL | 20 mg/L | Sunlight | 270 | 6 | 88 | [187] |
| Abelmoschus esculentus L. | Spherical | MB and methyl orange (MO) | 15 mg/mL | 10 mg/10 mL | UV light | 270 | 4 | 96 | [188] |
| Camellia Sinensis | Spherical | MB | 15 mg/L | 200 mg/L | 10 W UV light | 120 | 6 | 84.37 | [189] |
| Artocarpus Heterophyllus | Spherical | Congo red (CR) | 24 g/L | 20 mg/L | 30 W UV light = 2 | 60 | 9 | 90 | [190] |
| Eucalyptus globulus | Spherical | Free cyanide | 0.04 g/100 mL | 0.3 g/L | UV light | 20 | unspecified | 98 | [191] |
| Azadirachta indica | Spherical and flower-like | MB | 10 mg/mL | 10 mg/L | Sunlight | 120 | 6 | 97 | [192] |
| Rosmarinus officinalis | Quasi-hexagonal | Industrial dye effluent | 50 mg/50 mL TE | − | 20 W Vis light | 100 | 5.28 | 63 | [193] |
| Synadium grantii | Spherical | MB Indigo carmine RhB | 100 mg/L in 100 mL | 25 mg/L | 30 W UV light | 75 75 75 | unspecified | 79.2% 80.8% 75% | [194] |
| Algae | |||||||||
| Chlorella | Spherical | DBT | 0.01 g/20 mL | 10 mgL/20 mL | UV light | 180 | 7 | 97 | [140] |
| Sargassum specie | Irregular | Malachite green (MG) | 0.06 g | 5 mg/L | 150 W Vis light | 60 | 8 | 92.5 | [143] |
| Fungi | |||||||||
| Cordyceps militaris | Flower-like | MB | 0.25 mg/mL | 10 mg/L | UV light | 180 | 6 | 97 | [195] |
| Saccharomyces cerevisiae | Spherical | MB | 12 mg/mL | 5 mg/L | UV light | 200 | 3 | 67 | [138] |
| Saccharomyces cerevisiae | Spherical | 4-nitrophenol | 50 mg/L | 1.5 × 10−4 M | UV-Vis light | 100 | 6 | 78 | [196] |
| Aspergillus niger. | Near spherical | Bismarck Brown | 1 × 10−4 M | Vis light | 72 h | unspecified | 89 | [197] | |
| Biological derivatives | |||||||||
| Pullulan | Spherical and hexagonal | Amoxicillin Paracetamol | 50 mg/100 mL | 30 mg/L | 9 W Mercury lamp | 120 | 9 5 | 85.7 96.8 | [147] |
| Artemia eggshell | Spherical or ellipsoidal | RhB MB Neutral red | 10 mg/100 mL | 4 mg/10 mg/mL (10 mg/mL) | − | 120 | 3–11 | 88.75 93.3 86.6 | [150] |
| Chitosan | Flower-like | hexavalent chromium | 25–100 mg | 50 mg/L (50 mL) | 32 W UV light 365 | 60 | 5.2 | 99.2 | [198] |
| Cellulose Fibers | Flower-like | Methyl orange Phenol | 0.3 g/15 mL | 0.06 mmol/L (15 mL) 60 mg/L (15 mL) | 6.6 W UV light 365 | 9 h | Dye | 100 | [199] |
4.2. Adsorption of Heavy Metals and Organic Dyes
4.2.1. Heavy Metal Adsorption Using ZnO NPs
4.2.2. Adsorption of Organic Dyes Using ZnO NPs
| Name of Biological Source | Morphology of ZnO | Pollutant | Concentration of Adsorbent | Concentration of Pollutant | Time (min) | pH | Removal Efficiency (%) | Isotherm Model | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Heavy metals | ||||||||||
| Guar gum | Spherical | Cr (VI) | 1.0 g | 25 mg/L | 50 | 7 | 98.63 | Freundlich | 55.56 | [211] |
| Parthenium hysterophorus L. | Hexagonal and spherical | Cr (VI) MB | 50 mg/L | 100 mg/L | 180 150 | 4 6 | 98.8 | − | [212] | |
| Ceisen | Quasi-spherical | Cd Co Pb | (2 g/L)/100 mL | 50–500 mg/L | 120 | 7 8 | 85.63 71.23 95.33 | Langmuir | 156.74 67.93 194.93 | [215] |
| Mangifera indica (leaves) | Semi-spherical | Cu2+ Pb2+ Cd2+ | 1.0 g | 50 mg/L | 50 | 6 | 89.1 92.1 84.6 | Freundlich | 2.35 3.41 2.65 | [208] |
| Shorea robusta (Leaf) | Smaller clusters | Pb2+ Cd2+ | 0.8 g | 1000 mg/L | 50 | 6 | 92.9 89.9 | [216] | ||
| Organic dyes | ||||||||||
| Hibiscus rosa-sinensis. | Spherical, cubic, and hexagonal | CR | 0.05 g | 4 mg/L | 20 | 4 | 95.55 | Langmuir | [218] | |
| Ocimum sanctum (leaf) | Spherical | CR | 0.2 g | 40 mg/l | 30 | 4 | 97 | Langmuir | 74.07 | [205] |
| Cucumin | Spherical | CR | 0.15 g | 11.15 mg/L | 24 h | − | − | 89.85 | [219] | |
| Aloe vera (Leaf) | Nanosheets | MG | 10 mg/500 mL | 10 mg/L | 180 | − | 76.23 | [220] | ||
| Gum Arabic | Flower-like | MG | 0.4 g/L | 50–800 mg/L | 60 | 7 | 99 | 766.52 | [221] | |
| Becium grandiflorum (Leaf) | Spherical | MB | 25 mg/L | 25 mg/L, | 180 | − | 71.53 | 143.6 | [222] | |
| Cellulose | Spherical | MB | 0.15 | 100 mg/L | 1440 | 4 | 97.5 | Langmuir | 64.93 | [223] |
| Ananas comosus | Irregular | Celestine blue (CEB) | 0.15 | 50 mg/L | 120 | 9 | 70.2 | Langmuir | 6.52 | [224] |
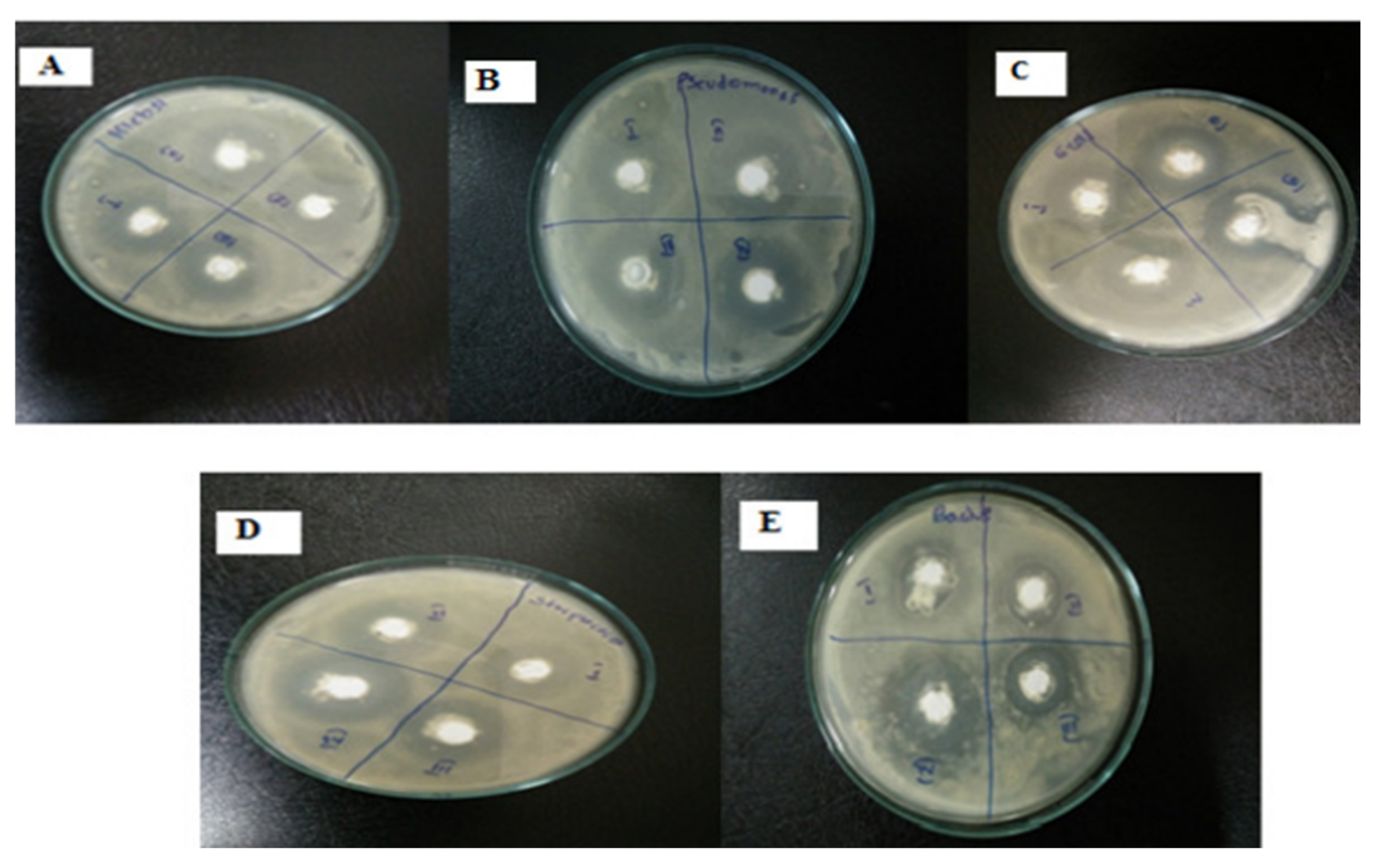
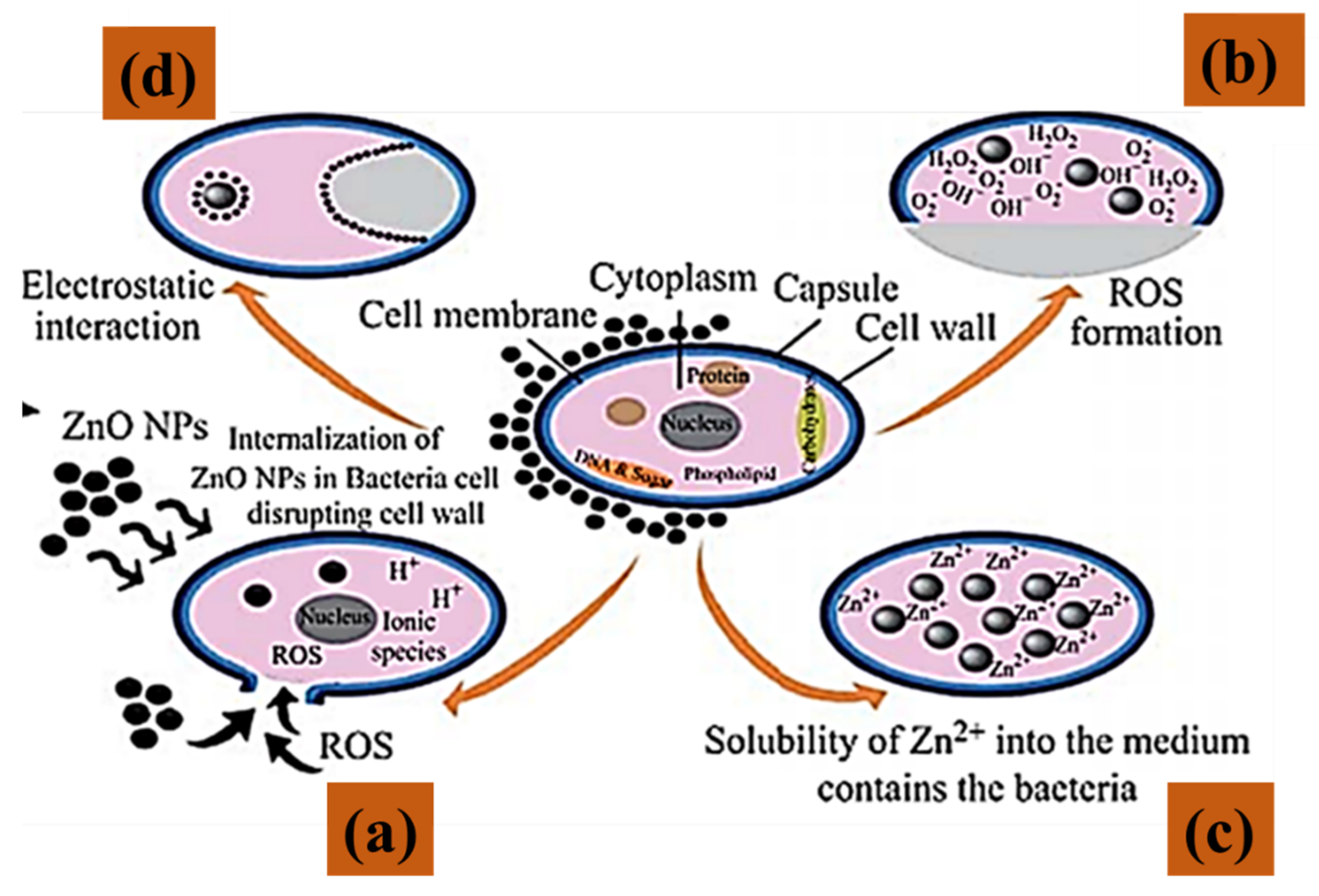
4.3. Antimicrobial Activity of ZnO NPs
4.3.1. Antibacterial Activity of Biologically Prepared ZnO NPs
4.3.2. Antifungal Activity of ZnO NPs
5. Conclusions
6. Future Perspectives
- The likelihood of integrating cheap, readily available biological sources with one another, and gaining control of the parameters concerning environmentally benign syntheses, justifies a future focus on the sustainable and scalable production of ZnO NPs; however, to completely maximize and make use of the advantages that biogenic ZnONPs provide, several areas need more research to enable a better understanding of the topic.
- As there are few studies on ZnO nanomaterial synthesis using microorganisms, biopolymers, marine fungi, algae, and waste materials, there exists a need for further exploration into biogenic synthesis protocols, largely because it offers more advantages over conventional chemical and physical synthesis.
- It is difficult to use ZnO NPs for biosynthesis on an industrial level as the underlying reaction mechanisms and kinetics of the process are not yet fully understood, especially microbial synthesis processes. Currently, reproducing bio-prepared NPs is difficult and may be time consuming because the mechanisms are insufficiently understood. The development of predictable synthesis protocols thus remains a challenge.
- A thorough understanding of the manner in which the most active of the bioprecursors that cap and reduce the nanomaterials are isolated and traced, in addition to an understanding of their structure, is necessary. Understanding the structure and chemistry of the reducing agents will speed up the commercialization of the green synthesis protocol. Moreover, the green protocol method must be explored in greater depth in order to understand the effects of the various biological extracts on the surface of ZnO, and to understand the deep level defects that occur, especially with respect to the photocatalytic break down of pollutants. Understanding this critical information may assist in eliminating the need to anneal the resultant samples, which consumes a great deal of energy.
- The manner in which the yield of the ZnO NPs corresponds with the precursors has not been reported, and process cost comparisons between biologically synthesized NPs and traditionally produced NPs have not been reported on as the former has not yet been upscaled. Understanding how the yield corresponds with the precursors should be a priority in order to obtain realistic expectations regarding this synthesis protocol. This requires a full understanding of the life cycle of specific bioprecursors in order to ensure continuous availability. This should prevent the production of the material from stopping or from being significantly reduced once the commercialization of green synthesis has been fully adopted. Such studies would also enable biosynthesis protocols to improve the atom economy in synthetic processes, thus meeting another pillar of green synthesis.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrmann, S.; De Matteis, L.; de la Fuente, J.M.; Mitchell, S.G.; Streb, C. Removal of Multiple Contaminants from Water by Polyoxometalate Supported Ionic Liquid Phases (POM-SILPs). Angew. Chem.-Int. Ed. 2017, 56, 1667–1670. [Google Scholar] [CrossRef]
- Sun, H.; Cao, L.; Lu, L. Magnetite/Reduced Graphene Oxide Nanocomposites: One Step Solvothermal Synthesis and Use as a Novel Platform for Removal of Dye Pollutants. Nano Res. 2011, 4, 550–562. [Google Scholar] [CrossRef]
- Mok, C.F.; Ching, Y.C.; Muhamad, F.; Abu Osman, N.A.; Hai, N.D.; Che Hassan, C.R. Adsorption of Dyes Using Poly(Vinyl Alcohol) (PVA) and PVA-Based Polymer Composite Adsorbents: A Review. J. Polym. Environ. 2020, 28, 775–793. [Google Scholar] [CrossRef]
- Dhiman, V.; Kondal, N. ZnO Nanoadsorbents: A Potent Material for Removal of Heavy Metal Ions from Wastewater. Colloids Interface Sci. Commun. 2021, 41, 100380. [Google Scholar] [CrossRef]
- Ashbolt, N.J. Microbial Contamination of Drinking Water and Human Health from Community Water Systems. Curr. Environ. Heal. Rep. 2015, 2, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.; Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Walter, P.; Edition, F.; Roberts, K.; Alberts, B.; Johnson, A.; et al. Molecular Biology of the Cell, Fourth Edition Description, 6th ed.; W.W. Norton & Company: Boca Raton, FL, USA, 2017; ISBN 9781315735368. [Google Scholar]
- Prasad, A.R.; Williams, L.; Garvasis, J.; Shamsheera, K.O.; Basheer, S.M.; Kuruvilla, M.; Joseph, A. Applications of Phytogenic ZnO Nanoparticles: A Review on Recent Advancements. J. Mol. Liq. 2021, 331, 115805. [Google Scholar] [CrossRef]
- Oluwafemi, O.S.; Anyik, J.L.; Zikalala, N.E.; Sakho, E.H.M. Biosynthesis of Silver Nanoparticles from Water Hyacinth Plant Leaves Extract for Colourimetric Sensing of Heavy Metals. Nano-Struct. Nano-Objects 2019, 20, 7–9. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, O.N. One-Step Green Synthesis of Gold Nanoparticles Using Black Cardamom and Effect of pH on Its Synthesis. Nanoscale Res. Lett. 2015, 10, 1–12. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S.; Folorunso, F.A.; Oyebamiji, A.K. Green Synthesis of Copper Oxide Nanoparticles for Biomedical Application and Environmental Remediation. Heliyon 2020, 6, e04508. [Google Scholar] [CrossRef]
- Zikalala, S.A.; Kuvarega, A.T.; Mamba, B.B.; Mhlanga, S.D.; Nxumalo, E.N. The Effect of Synthetic Routes on the Physicochemical Properties and Optical Response of N-Doped Titania–Oxidized Carbon Nanotube Nanohybrids. Mater. Today Chem. 2018, 10, 1–18. [Google Scholar] [CrossRef]
- Ling, D.; Lee, N.; Hyeon, T. Chemical Synthesis and Assembly of Uniformly Sized Iron Oxide Nanoparticles for Medical Applications. Acc. Chem. Res. 2015, 48, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Kuriganova, A.B.; Vlaic, C.A.; Ivanov, S.; Leontyeva, D.V.; Bund, A.; Smirnova, N.V. Electrochemical Dispersion Method for the Synthesis of SnO2 as Anode Material for Lithium Ion Batteries. J. Appl. Electrochem. 2016, 46, 527–538. [Google Scholar] [CrossRef]
- Sobhani-Nasab, A.; Behpour, M. Synthesis and Characterization of AgO Nanostructures by Precipitation Method and Its Photocatalyst Application. J. Mater. Sci. Mater. Electron. 2016, 27, 1191–1196. [Google Scholar] [CrossRef]
- Siddiqui, H.; Qureshi, M.S.; Haque, F.Z. Surfactant Assisted Wet Chemical Synthesis of Copper Oxide (CuO) Nanostructures and Their Spectroscopic Analysis. Optik 2016, 127, 2740–2747. [Google Scholar] [CrossRef]
- Sahu, K.; Choudhary, S.; Singh, J.; Kuriakose, S.; Singhal, R.; Mohapatra, S. Facile Wet Chemical Synthesis of ZnO Nanosheets: Effects of Counter Ions on the Morphological, Structural, Optical and Photocatalytic Properties. Ceram. Int. 2018, 44, 23094–23101. [Google Scholar] [CrossRef]
- Champi, A.; Marques, F.C. Structural Changes in Amorphous Carbon Nitride Films Due to Bias Voltage. Thin Solid Film. 2006, 501, 362–365. [Google Scholar] [CrossRef]
- Aminuzzaman, M.; Ying, L.P.; Goh, W.S.; Watanabe, A. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Garcinia mangostana Fruit Pericarp and Their Photocatalytic Activity. Bull. Mater. Sci. 2018, 41, 1–10. [Google Scholar] [CrossRef]
- Janotti, A.; Van De Walle, C.G. Fundamentals of Zinc Oxide as a Semiconductor. Rep. Prog. Phys. 2009, 72, 12–15. [Google Scholar] [CrossRef]
- Xu, S.; Weintraub, B.; Wang, Z.L. Zinc Oxide Nanowire Arrays on Flexible Substrates. Wet Chemical Growth and Applications in Energy Conversion, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; ISBN 9781437778236. [Google Scholar]
- Ayoub, I.; Kumar, V.; Abolhassani, R.; Sehgal, R.; Sharma, V.; Sehgal, R.; Swart, H.C.; Mishra, Y.K. Advances in ZnO: Manipulation of Defects for Enhancing Their Technological Potentials. Nanotechnol. Rev. 2022, 11, 575–619. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Elemike, E.E.; Onwudiwe, D.C. ZnO Nanoparticles Mediated by Aqueous Extracts of Dovyalis Caffra Fruits and the Photocatalytic Evaluations. Mater. Res. Express 2019, 6, 11–14. [Google Scholar] [CrossRef]
- Narendra Kumar, H.K.; Chandra Mohana, N.; Nuthan, B.R.; Ramesha, K.P.; Rakshith, D.; Geetha, N.; Satish, S. Phyto-Mediated Synthesis of Zinc Oxide Nanoparticles Using Aqueous Plant Extract of Ocimum americanum and Evaluation of Its Bioactivity. SN Appl. Sci. 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Awwad, A.M.; Albiss, B.; Ahmad, A.L. Green Synthesis, Characterization and Optical Properties of Zinc Oxide Nanosheets Using Olea europea Leaf Extract. Adv. Mater. Lett. 2014, 5, 520–524. [Google Scholar] [CrossRef]
- Shabaani, M.; Rahaiee, S.; Zare, M.; Jafari, S.M. Green Synthesis of ZnO Nanoparticles Using Loquat Seed Extract; Biological Functions and Photocatalytic Degradation Properties. LWT 2020, 134, 110133. [Google Scholar] [CrossRef]
- Rajabairavi, N.; Raju, C.S.; Karthikeyan, C.; Varutharaju, K.; Nethaji, S.; Hameed, A.S.H.; Shajahan, A. Biosynthesis of Novel Zinc Oxide Nanoparticles (ZnO NPs) Using Endophytic Bacteria Sphingobacterium thalpophilum. Springer Proc. Phys. 2017, 189, 245–254. [Google Scholar] [CrossRef]
- Wu, J.; Yin, C.; Zhou, J.; Li, H.; Liu, Y.; Shen, Y.; Garner, S.; Fu, Y.; Duan, H. Ultrathin Glass-Based Flexible, Transparent, and Ultrasensitive Surface Acoustic Wave Humidity Sensor with ZnO Nanowires and Graphene Quantum Dots. ACS Appl. Mater. Interfaces 2020, 12, 39817–39825. [Google Scholar] [CrossRef]
- Timerkaev, B.A.; Felsinger, V.S.; Kaleeva, A.A.; Erlingayte, E.A.; Uktamov, J.A.; Nuriddinov, H.S. Plasma-Chemical Synthesis of Zinc Oxide Nanotubes. J. Phys. Conf. Ser. 2021, 1870, 012003. [Google Scholar] [CrossRef]
- Ebadi, M.; Zolfaghari, M.R.; Aghaei, S.S.; Zargar, M.; Shafiei, M.; Zahiri, H.S.; Noghabi, K.A. A Bio-Inspired Strategy for the Synthesis of Zinc Oxide Nanoparticles (ZnO NPs) Using the Cell Extract of Cyanobacterium: Nostoc Sp. EA03: From Biological Function to Toxicity Evaluation. RSC Adv. 2019, 9, 23508–23525. [Google Scholar] [CrossRef]
- Mahdi, Z.S.; Talebnia Roshan, F.; Nikzad, M.; Ezoji, H. Biosynthesis of Zinc Oxide Nanoparticles Using Bacteria: A Study on the Characterization and Application for Electrochemical Determination of Bisphenol A. Inorg. Nano-Met. Chem. 2021, 51, 1249–1257. [Google Scholar] [CrossRef]
- Kiani, A.; Dastafkan, K. Zinc Oxide Nanocubes as a Destructive Nanoadsorbent for the Neutralization Chemistry of 2-Chloroethyl Phenyl Sulfide: A Sulfur Mustard Simulant. J. Colloid Interface Sci. 2016, 478, 271–279. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Ajala, A.O.; Mohammed, A.K. Application of TiO2 and ZnO Nanoparticles Immobilized on Clay in Wastewater Treatment: A Review. Appl. Water Sci. 2020, 10, 1–36. [Google Scholar] [CrossRef]
- Saeed, M.; Siddique, M.; Ibrahim, M.; Akram, N.; Usman, M.; Aleem, M.A.; Baig, A. Calotropis Gigantea Leaves Assisted Biosynthesis of ZnO and Ag@ZnO Catalysts for Degradation of Rhodamine B Dye in Aqueous Medium. Environ. Prog. Sustain. Energy 2020, 39, e13408. [Google Scholar] [CrossRef]
- Abdelhakim, H.K.; El-Sayed, E.R.; Rashidi, F.B. Biosynthesis of Zinc Oxide Nanoparticles with Antimicrobial, Anticancer, Antioxidant and Photocatalytic Activities by the Endophytic Alternaria tenuissima. J. Appl. Microbiol. 2020, 128, 1634–1646. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Zhang, Q.; Li, T. Adjusting the Proportions of {0001} Facets and High-Index Facets of ZnO Hexagonal Prisms and Their Photocatalytic Activity. RSC Adv. 2017, 7, 3515–3520. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, B.; Pan, Z.; Gu, M.; Punnoose, A. Synthesis of ZnO Nanoparticles with Controlled Shapes, Sizes, Aggregations, and Surface Complex Compounds for Tuning or Switching the Photoluminescence. Cryst. Growth Des. 2015, 15, 3144–3149. [Google Scholar] [CrossRef]
- Tănase, M.A.; Marinescu, M.; Oancea, P.; Răducan, A.; Mihaescu, C.I.; Alexandrescu, E.; Nistor, C.L.; Jinga, L.I.; Diţu, L.M.; Petcu, C.; et al. Antibacterial and Photocatalytic Properties of ZnO Nanoparticles Obtained from Chemical versus Saponaria Officinalis Extract-Mediated Synthesis. Molecules 2021, 26, 2072. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Vick, J.E. Nanostructured ZnO for Electrochemical Biosensors. J. Biosens. Bioelectron. 2012, 3, 1. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green Synthesis of Zinc Oxide Nanoparticles Using Phoenix dactylifera Waste as Bioreductant for Effective Dye Degradation and Antibacterial Performance in Wastewater Treatment. J. Hazard. Mater. 2021, 402, 123560. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, K.; Thakur, N.; Chauhan, M.S. Ocimum tenuiflorum Leaf Extract as a Green Mediator for the Synthesis of ZnO Nanocapsules Inactivating Bacterial Pathogens. Chem. Pap. 2020, 74, 3431–3444. [Google Scholar] [CrossRef]
- Fazlzadeh, M.; Khosravi, R.; Zarei, A. Green Synthesis of Zinc Oxide Nanoparticles Using Peganum harmala Seed Extract, and Loaded on Peganum harmala Seed Powdered Activated Carbon as New Adsorbent for Removal of Cr(VI) from Aqueous Solution. Ecol. Eng. 2017, 103, 180–190. [Google Scholar] [CrossRef]
- Bao, S.; Hou, T.; Tan, Q.; Kong, X.; Cao, H.; He, M.; Wu, G.; Xu, B. Immobilization of Zinc Oxide Nanoparticles on Graphene Sheets for Lithium Ion Storage and Electromagnetic Microwave Absorption. Mater. Chem. Phys. 2020, 245, 1–3. [Google Scholar] [CrossRef]
- Ren, Q.; Cao, Y.-Q.; Arulraj, D.; Liu, C.; Wu, D.; Li, W.-M.; Li, A.-D. Review—Resistive-Type Hydrogen Sensors Based on Zinc Oxide Nanostructures. J. Electrochem. Soc. 2020, 167, 067528. [Google Scholar] [CrossRef]
- Kim, K.B.; Kim, Y.W.; Lim, S.K.; Roh, T.H.; Bang, D.Y.; Choi, S.M.; Lim, D.S.; Kim, Y.J.; Baek, S.H.; Kim, M.K.; et al. Risk Assessment of Zinc Oxide, a Cosmetic Ingredient Used as a UV Filter of Sunscreens. J. Toxicol. Environ. Heal.-Part B Crit. Rev. 2017, 20, 155–182. [Google Scholar] [CrossRef] [PubMed]
- Khashan, K.S.; Sulaiman, G.M.; Hussain, S.A.; Marzoog, T.R.; Jabir, M.S. Synthesis, Characterization and Evaluation of Anti-Bacterial, Anti-Parasitic and Anti-Cancer Activities of Aluminum-Doped Zinc Oxide Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3677–3693. [Google Scholar] [CrossRef]
- Nakano, M.; Nakagawa, S.; Sato, F.; Shahiduzzaman, M.; Karakawa, M.; Taima, T.; Takahashi, K. Study on Properties of Low-Temperature-Prepared Zinc Oxide-Based Inverted Organic Solar Cells and Improvement of Their Photodurability. ACS Appl. Energy Mater. 2021, 4, 6385–6390. [Google Scholar] [CrossRef]
- Rafique, M.; Tahir, R.; Gillani, S.S.A.; Tahir, M.B.; Shakil, M.; Iqbal, T.; Abdellahi, M.O. Plant-Mediated Green Synthesis of Zinc Oxide Nanoparticles from Syzygium Cumini for Seed Germination and Wastewater Purification. Int. J. Environ. Anal. Chem. 2020, 102, 23–28. [Google Scholar] [CrossRef]
- Bandeira, M.; Chee, B.S.; Frassini, R.; Nugent, M.; Giovanela, M.; Roeschely, M.; Crespo, J. da S.; Devine, D.M. Antimicrobial Paa/Pah Electrospun Fiber Containing Green Synthesized Zinc Oxide Nanoparticles for Wound Healing. Matreials 2021, 14, 2889. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Srujana, S.; Bhagat, D. Chemical—Based Synthesis of ZnO Nanoparticles and Their Applications in Agriculture. Nanotechnol. Environ. Eng. 2022, 7, 269–275. [Google Scholar] [CrossRef]
- Naveed Ul Haq, A.; Nadhman, A.; Ullah, I.; Mustafa, G.; Yasinzai, M.; Khan, I. Synthesis Approaches of Zinc Oxide Nanoparticles: The Dilemma of Ecotoxicity. J. Nanomater. 2017, 1–14. [Google Scholar] [CrossRef]
- Jose Varghese, R.; Zikalala, N.; Sakho, E.H.M.; Oluwafemi, O.S. Green Synthesis Protocol on Metal Oxide Nanoparticles Using Plant Extracts. In Colloidal Metal Oxide Nanoparticles-Synthesis, Characterization and Applications; Thomas, S., Sani, A.T., Velayudhan, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–82. ISBN 978-0-12-813357-6. [Google Scholar]
- Jessop, P.G.; Trakhtenberg, S.; Warner, J. The Twelve Principles of Green Chemistry. ACS Symp. Ser. 2009, 1000, 401–436. [Google Scholar] [CrossRef]
- Rubab, L.; Anum, A.; Al-hussain, S.A.; Irfan, A.; Ahmad, S.; Ullah, S.; Al-mutairi, A.A.; Zaki, M.E.A. Green Chemistry in Organic Synthesis: Recent Update on Green Catalytic Approaches in Synthesis of 1, 2, 4-Thiadiazoles. Catalysts 2022, 12, 1329. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Liz-Marzán, L.M. Oleylamine in Nanoparticle Synthesis. Chem. Mater. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green Synthesis of Zinc Oxide Nanoparticles: A Review of the Synthesis Methodology and Mechanism of Formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Swati; Verma, R.; Chauhan, A.; Shandilya, M.; Li, X.; Kumar, R.; Kulshrestha, S. Antimicrobial Potential of Ag-Doped ZnO Nanostructure Synthesized by the Green Method Using Moringa oleifera Extract. J. Environ. Chem. Eng. 2020, 8, 103730. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, K.V.B. Novel Microbial Route to Synthesize ZnO Nanoparticles Using Aeromonas hydrophila and Their Activity against Pathogenic Bacteria and Fungi. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2012, 90, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Bhargava, A.; Tarafdar, J.C.; Singh, S.K.; Panwar, J. A Biomimetic Approach towards Synthesis of Zinc Oxide Nanoparticles. Appl. Microbiol. Biotechnol. 2013, 97, 859–869. [Google Scholar] [CrossRef]
- Rao, M.D.; Gautam, D.P. Nanoflowers Using Chlamydomonas reinhardtii: A Green Approach. Environ. Prog. Sustain. Energy 2016, 35, 1020–1026. [Google Scholar] [CrossRef]
- Divya, M.; Vaseeharan, B.; Abinaya, M.; Vijayakumar, S.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Biopolymer Gelatin-Coated Zinc Oxide Nanoparticles Showed High Antibacterial, Antibiofilm and Anti-Angiogenic Activity. J. Photochem. Photobiol. B Biol. 2018, 178, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zikalala, N.; Matshetshe, K.; Parani, S.; Oluwafemi, O.S. Biosynthesis Protocols for Colloidal Metal Oxide Nanoparticles. Nano-Struct. Nano-Objects 2018, 16, 288–299. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-Mediated Biosynthesis of Metallic Nanoparticles: A Review of Literature, Factors Affecting Synthesis, Characterization Techniques and Applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Chaudhry, S.A.; Ikram, S. A Review on Biogenic Synthesis of ZnO Nanoparticles Using Plant Extracts and Microbes: A Prospect towards Green Chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Akbar, S.; Tauseef, I.; Subhan, F.; Sultana, N.; Khan, I.; Ahmed, U.; Haleem, K.S. An Overview of the Plant-Mediated Synthesis of Zinc Oxide Nanoparticles and Their Antimicrobial Potential. Inorg. Nano-Met. Chem. 2020, 50, 257–271. [Google Scholar] [CrossRef]
- Prasad, A.R.; Anagha, M.; Shamsheera, K.O.; Joseph, A. Bio-Fabricated ZnO Nanoparticles: Direct Sunlight-Driven Selective Photodegradation, Antibacterial Activity, and Thermoluminescence-Emission Characteristics. New J. Chem. 2020, 44, 8273–8279. [Google Scholar] [CrossRef]
- Verma, P.R.; Khan, F.; Banerjee, S. Salvadora persica Root Extract-Mediated Fabrication of ZnO Nanoparticles and Characterization. Inorg. Nano-Met. Chem. 2020, 51, 427–433. [Google Scholar] [CrossRef]
- Fagier, M.A. Plant-Mediated Biosynthesis and Photocatalysis Activities of Zinc Oxide Nanoparticles: A Prospect towards Dyes Mineralization. J. Nanotechnol. 2021, 2021. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S.; Ravi, S.; Kathiravan, V.; Adaikala Raj, G. Bio-Approach: Plant Mediated Synthesis of ZnO Nanoparticles and Their Catalytic Reduction of Methylene Blue and Antimicrobial Activity. Adv. Powder Technol. 2015, 26, 1639–1651. [Google Scholar] [CrossRef]
- Gebre, S.H.; Sendeku, M.G. New Frontiers in the Biosynthesis of Metal Oxide Nanoparticles and Their Environmental Applications: An Overview. SN Appl. Sci. 2019, 1, 1–28. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Green-Synthesized Nanocatalysts and Nanomaterials for Water Treatment: Current Challenges and Future Perspectives. J. Hazard. Mater. 2021, 401, 123401. [Google Scholar] [CrossRef]
- Sharma, A.; Nagraik, R.; Sharma, S.; Sharma, G.; Pandey, S.; Azizov, S.; Chauhan, P.K.; Kumar, D. Green Synthesis of ZnO Nanoparticles Using Ficus palmata: Antioxidant, Antibacterial and Antidiabetic Studies. Results Chem. 2022, 4, 100509. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green Synthesis of Zinc Oxide Nanoparticles Using Hibiscus subdariffa Leaf Extract: Effect of Temperature on Synthesis, Anti-Bacterial Activity and Anti-Diabetic Activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Nandhakumar, E.; Priya, P.; Soni, D.; Vimalan, M.; Vetha Potheher, I. Synthesis of ZnO Nanoparticles Using Leaf Extract of Tectona grandis (L.) and Their Anti-Bacterial, Anti-Arthritic, Anti-Oxidant and in Vitro Cytotoxicity Activities. New J. Chem. 2017, 41, 10347–10356. [Google Scholar]
- Bowles, D.; Lim, E.K.; Poppenberger, B.; Vaistij, F.E. Glycosyltransferases of Lipophilic Small Molecules. Annu. Rev. Plant Biol. 2006, 57, 567–597. [Google Scholar] [CrossRef] [PubMed]
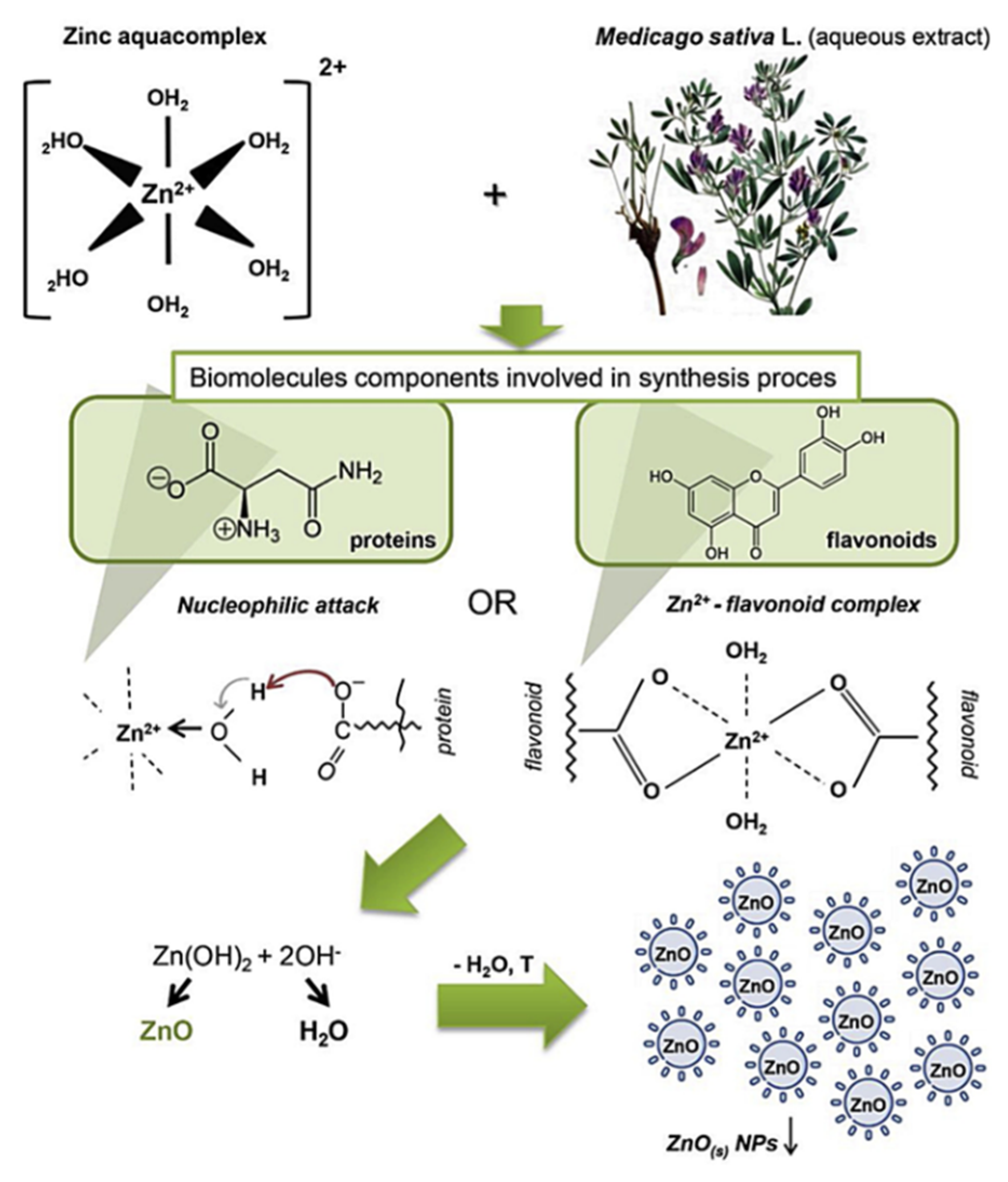
- Król, A.; Railean-Plugaru, V.; Pomastowski, P.; Buszewski, B. Phytochemical Investigation of Medicago sativa L. Extract and Its Potential as a Safe Source for the Synthesis of ZnO Nanoparticles: The Proposed Mechanism of Formation and Antimicrobial Activity. Phytochem. Lett. 2019, 31, 170–180. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The Biological Inorganic Chemistry of Zinc Ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green Approach for Fabrication and Applications of Zinc Oxide Nanoparticles. Bioinorg. Chem. Appl. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- De Souza, R.F.V.; Giovani, W.F. De Synthesis, Spectral and Electrochemical Properties of Al (III) and Zn (II) Complexes with Flavonoids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 1985–1990. [Google Scholar] [CrossRef]
- Mirgane, N.A.; Shivankar, V.S.; Kotwal, S.B.; Wadhawa, G.C.; Sonawale, M.C. Waste Pericarp of Ananas comosus in Green Synthesis Zinc Oxide Nanoparticles and Their Application in Waste Water Treatment. Mater. Today Proc. 2020, 37, 886–889. [Google Scholar] [CrossRef]
- Madhumitha, G.; Fowsiya, J.; Gupta, N.; Kumar, A.; Singh, M. Green Synthesis, Characterization and Antifungal and Photocatalytic Activity of Pithecellobium dulce Peel–Mediated ZnO Nanoparticles. J. Phys. Chem. Solids 2019, 127, 43–51. [Google Scholar] [CrossRef]
- Mohamad Sukri, S.N.A.; Shameli, K.; Mei-Theng Wong, M.; Teow, S.Y.; Chew, J.; Ismail, N.A. Cytotoxicity and Antibacterial Activities of Plant-Mediated Synthesized Zinc Oxide (ZnO) Nanoparticles Using Punica granatum (Pomegranate) Fruit Peels Extract. J. Mol. Struct. 2019, 1189, 57–65. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Honarmand, M.; Ghanbari, S. A Green Approach to Synthesis of ZnO Nanoparticles Using Jujube Fruit Extract and Their Application in Photocatalytic Degradation of Organic Dyes. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 229, 117961. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Soto-Robles, C.A.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Nava, O.; Vilchis-Nestor, A.R.; Lugo-Medina, E.; Ranjithkumar, R.; Castro-Beltrán, A. Study on the Effect of the Concentration of Hibiscus sabdariffa Extract on the Green Synthesis of ZnO Nanoparticles. Results Phys. 2019, 15, 102807. [Google Scholar] [CrossRef]
- Khara, G.; Padalia, H.; Moteriya, P.; Chanda, S. Peltophorum pterocarpum Flower-Mediated Synthesis, Characterization, Antimicrobial and Cytotoxic Activities of ZnO Nanoparticles. Arab. J. Sci. Eng. 2018, 43, 3393–3401. [Google Scholar] [CrossRef]
- Diallo, A.; Ngom, B.D.; Park, E.; Maaza, M. Green Synthesis of ZnO Nanoparticles by Aspalathus linearis: Structural & Optical Properties. J. Alloys Compd. 2015, 646, 425–430. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Kataria, N.; Garg, V.K. Green Fabrication of ZnO Nanoparticles Using Eucalyptus Spp. Leaves Extract and Their Application in Wastewater Remediation. Chemosphere 2020, 247, 125803. [Google Scholar] [CrossRef]
- Nilavukkarasi, M.; Vijayakumar, S.; Prathipkumar, S. Capparis Zeylanica Mediated Bio-Synthesized ZnO Nanoparticles as Antimicrobial, Photocatalytic and Anti-Cancer Applications. Mater. Sci. Energy Technol. 2020, 3, 335–343. [Google Scholar] [CrossRef]
- Bala Chennaiah, M.; Kumar, K.D.; Kumar, B.S.; Tanneeru, S.R. Characterisation of Zinc Oxide Nanoparticles–Herbal Synthesised Coated with Ocimum tenuiflorum. Adv. Mater. Process. Technol. 2021, 1–12. [Google Scholar] [CrossRef]
- Ekennia, A.C.; Uduagwu, D.N.; Nwaji, N.N.; Oje, O.O.; Emma-Uba, C.O.; Mgbii, S.I.; Olowo, O.J.; Nwanji, O.L. Green Synthesis of Biogenic Zinc Oxide Nanoflower as Dual Agent for Photodegradation of an Organic Dye and Tyrosinase Inhibitor. J. Inorg. Organomet. Polym. Mater. 2021, 31, 886–897. [Google Scholar] [CrossRef]
- Tabrizi Hafez Moghaddas, S.M.; Elahi, B.; Javanbakht, V. Biosynthesis of Pure Zinc Oxide Nanoparticles Using Quince Seed Mucilage for Photocatalytic Dye Degradation. J. Alloys Compd. 2020, 821, 153519. [Google Scholar] [CrossRef]
- Prasad, A.R.; Garvasis, J.; Oruvil, S.K.; Joseph, A. Bio-Inspired Green Synthesis of Zinc Oxide Nanoparticles Using Abelmoschus esculentus Mucilage and Selective Degradation of Cationic Dye Pollutants. J. Phys. Chem. Solids 2019, 127, 265–274. [Google Scholar] [CrossRef]
- Kaliraj, L.; Ahn, J.C.; Rupa, E.J.; Abid, S.; Lu, J.; Yang, D.C. Synthesis of Panos Extract Mediated ZnO Nano-Flowers as Photocatalyst for Industrial Dye Degradation by UV Illumination. J. Photochem. Photobiol. B Biol. 2019, 199, 111588. [Google Scholar] [CrossRef] [PubMed]
- Matinise, N.; Fuku, X.G.; Kaviyarasu, K.; Mayedwa, N.; Maaza, M. ZnO Nanoparticles via Moringa Oleifera Green Synthesis: Physical Properties & Mechanism of Formation. Appl. Surf. Sci. 2017, 406, 339–347. [Google Scholar] [CrossRef]
- Thema, F.T.; Manikandan, E.; Dhlamini, M.S.; Maaza, M. Green Synthesis of ZnO Nanoparticles via Agathosma betulina Natural Extract. Mater. Lett. 2015, 161, 124–127. [Google Scholar] [CrossRef]
- Ngom, I.; Ndiayea, N.M.; Bakayoko, A.F.M.; Ngom, B.D.; Maaza, M. On the Use of Moringa oleifera Leaves Extract for the Biosynthesis of NiO and ZnO Nanoparticles. MRS Adv. 2020, 5, 1145–1155. [Google Scholar] [CrossRef]
- Rajendran, N.K.; George, B.P.; Houreld, N.N.; Abrahamse, H. Synthesis of Zinc Oxide Nanoparticles Using Rubus Fairholmianus Root Extract and Their Activity against Pathogenic Bacteria. Molecules 2021, 26, 3029. [Google Scholar] [CrossRef]
- Zafar, Z.; Yi, S.; Li, J.; Li, C.; Zhu, Y.; Zada, A.; Yao, W.; Liu, Z.; Yue, X. Recent Development in Defects Engineered Photocatalysts: An Overview of the Experimental and Theoretical Strategies. Energy Environ. Mater. 2022, 5, 68–114. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Petru, M.; Mahmood, A.; Kejzlar, P. Photocatalytic Behaviour of Zinc Oxide Nanostructures on Surface Activation of Polymeric Fibres. Polymers 2021, 13, 1227. [Google Scholar] [CrossRef] [PubMed]
- Shelar, S.G.; Mahajan, V.K.; Patil, S.P.; Sonawane, G.H. Effect of Doping Parameters on Photocatalytic Degradation of Methylene Blue Using Ag Doped ZnO Nanocatalyst. SN Appl. Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Gupta, V.K.; Lal, B.; Roy, S.; Yadav, S.K.; Gurjar, M.S.; Bag, T.K.; Pandey, N.K.; Singh, B.P. Assessment of the Level of Knowledge and Training Needs of Potato Growing Tribal Farmers of Meghalaya. Int. J. Agric. Environ. Biotechnol. 2012, 5, 483–487. [Google Scholar]
- Jain, D.; Shivani; Bhojiya, A.A.; Singh, H.; Daima, H.K.; Singh, M.; Mohanty, S.R.; Stephen, B.J.; Singh, A. Microbial Fabrication of Zinc Oxide Nanoparticles and Evaluation of Their Antimicrobial and Photocatalytic Properties. Front. Chem. 2020, 8, 778. [Google Scholar] [CrossRef] [PubMed]
- Panchal, P.; Paul, D.R.; Sharma, A.; Choudhary, P.; Meena, P.; Nehra, S.P. Biogenic Mediated Ag/ZnO Nanocomposites for Photocatalytic and Antibacterial Activities towards Disinfection of Water. J. Colloid Interface Sci. 2020, 563, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Harunsani, M.H.; Tan, A.L.; Hojamberdiev, M.; Azamay, S.; Ahmad, N. Antibacterial Activities of Zinc Oxide and Mn-Doped Zinc Oxide Synthesized Using Melastoma malabathricum (L.) Leaf Extract. Bioprocess Biosyst. Eng. 2020, 43, 1499–1508. [Google Scholar] [CrossRef]
- Akbar Jan, F.; Wajidullah; Ullah, R.; Ullah, N.; Salman; Usman, M. Exploring the Environmental and Potential Therapeutic Applications of Myrtus communis L. Assisted Synthesized Zinc Oxide (ZnO) and Iron Doped Zinc Oxide (Fe-ZnO) Nanoparticles. J. Saudi Chem. Soc. 2021, 25, 101278. [Google Scholar] [CrossRef]
- Okeke, I.S.; Agwu, K.K.; Ubachukwu, A.A.; Madiba, I.G.; Maaza, M.; Whyte, G.M.; Ezema, F.I. Impact of Particle Size and Surface Defects on Antibacterial and Photocatalytic Activities of Undoped and Mg-Doped ZnO Nanoparticles, Biosynthesized Using One-Step Simple Process. Vacuum 2021, 187, 110110. [Google Scholar] [CrossRef]
- Rahman, A.; Harunsani, M.H.; Tan, A.L.; Ahmad, N.; Min, B.K.; Khan, M.M. Influence of Mg and Cu Dual-Doping on Phytogenic Synthesized ZnO for Light Induced Antibacterial and Radical Scavenging Activities. Mater. Sci. Semicond. Process. 2021, 128, 105761. [Google Scholar] [CrossRef]
- Nguyen, T.H.A.; Le, V.T.; Doan, V.D.; Tran, A.V.; Nguyen, V.C.; Nguyen, A.T.; Vasseghian, Y. Green Synthesis of Nb-Doped ZnO Nanocomposite for Photocatalytic Degradation of Tetracycline Antibiotic under Visible Light. Mater. Lett. 2022, 308, 131129. [Google Scholar] [CrossRef]
- Kitching, M.; Ramani, M.; Marsili, E. Fungal Biosynthesis of Gold Nanoparticles: Mechanism and Scale Up. Microb. Biotechnol. 2015, 8, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M.R.; Ando, R.A.; Oller Do Nascimento, C.A.; Corrêa, B. Intracellular Biosynthesis and Removal of Copper Nanoparticles by Dead Biomass of Yeast Isolated from the Wastewater of a Mine in the Brazilian Amazonia. PLoS ONE 2014, 9, e87968. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological Synthesis of Metallic Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, P.; Kumar, G.V.; Jeyanthi, V.; Das, J.; Pachaiappan, R. Bio-Inspired Green Nanoparticles: Synthesis, Mechanism, and Antibacterial Application. Toxicol. Res. 2016, 32, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.M.; Bhadwal, A.S.; Gupta, R.K.; Singh, P.; Shrivastav, A.; Shrivastav, B.R. ZnO Nanoflowers: Novel Biogenic Synthesis and Enhanced Photocatalytic Activity. J. Photochem. Photobiol. B Biol. 2014, 141, 288–295. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sengani, M.; Grumezescu, A.M.; Rajeswari, V.D. Recent Trends and Methodologies in Gold Nanoparticle Synthesis – A Prospective Review on Drug Delivery Aspect. OpenNano 2017, 2, 37–46. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial Synthesis of Zinc Oxide Nanoparticles and Their Potential Application as an Antimicrobial Agent and a Feed Supplement in Animal Industry: A Review. J. Anim. Sci. Biotechnol. 2019, 10, 1–22. [Google Scholar] [CrossRef]
- Shedbalkar, U.; Singh, R.; Wadhwani, S.; Gaidhani, S.; Chopade, B.A. Microbial Synthesis of Gold Nanoparticles: Current Status and Future Prospects. Adv. Colloid Interface Sci. 2014, 209, 40–48. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis of Metal and Metal Oxide Nanoparticles. ChemBioEng Rev. 2016, 3, 55–67. [Google Scholar] [CrossRef]
- Molnár, Z.; Bódai, V.; Szakacs, G.; Erdélyi, B.; Fogarassy, Z.; Sáfrán, G.; Varga, T.; Kónya, Z.; Tóth-Szeles, E.; Szucs, R.; et al. Green Synthesis of Gold Nanoparticles by Thermophilic Filamentous Fungi. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Hulkoti, N.I.; Taranath, T.C. Biosynthesis of Nanoparticles Using Microbes-A Review. Colloids Surf. B Biointerfaces 2014, 121, 474–483. [Google Scholar] [CrossRef]
- Dhandapani, P.; Prakash, A.A.; AlSalhi, M.S.; Maruthamuthu, S.; Devanesan, S.; Rajasekar, A. Ureolytic Bacteria Mediated Synthesis of Hairy ZnO Nanostructure as Photocatalyst for Decolorization of Dyes. Mater. Chem. Phys. 2020, 243, 122619. [Google Scholar] [CrossRef]
- Rehman, S.; Jermy, B.R.; Akhtar, S.; Borgio, J.F.; Abdul Azeez, S.; Ravinayagam, V.; Al Jindan, R.; Alsalem, Z.H.; Buhameid, A.; Gani, A. Isolation and Characterization of a Novel Thermophile; Bacillus haynesii, Applied for the Green Synthesis of ZnO Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2072–2082. [Google Scholar] [CrossRef] [PubMed]
- Al-Kordy, H.M.H.; Sabry, S.A.; Mabrouk, M.E.M. Statistical Optimization of Experimental Parameters for Extracellular Synthesis of Zinc Oxide Nanoparticles by a Novel Haloalaliphilic alkalibacillus sp. W7. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Jayabalan, J.; Mani, G.; Krishnan, N.; Pernabas, J.; Devadoss, J.M.; Jang, H.T. Green Biogenic Synthesis of Zinc Oxide Nanoparticles Using Pseudomonas putida Culture and Its In Vitro Antibacterial and Anti-Biofilm Activity. Biocatal. Agric. Biotechnol. 2019, 21, 101327. [Google Scholar] [CrossRef]
- Taran, M.; Rad, M.; Alavi, M. Biosynthesis of TiO2 and ZnO Nanoparticles by Halomonas Elongata IBRC-M 10214 in Different Conditions of Medium. BioImpacts 2018, 8, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yusof, H.; Abdul Rahman, N.A.; Mohamad, R.; Zaidan, U.H.; Samsudin, A.A. Biosynthesis of Zinc Oxide Nanoparticles by Cell-Biomass and Supernatant of Lactobacillus plantarum TA4 and Its Antibacterial and Biocompatibility Properties. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Rajivgandhi, G.; Mythili Gnanamangai, B.; Heela Prabha, T.; Poornima, S.; Maruthupandy, M.; Alharbi, N.S.; Kadaikunnan, S.; Li, W.-J. Biosynthesized Zinc Oxide Nanoparticles (ZnO NPs) Using Actinomycetes Enhance the Anti-Bacterial Efficacy against K. pneumoniae. J. King Saud Univ.-Sci. 2022, 34, 101731. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO Nanoparticle Biosynthesis and Its Effect on Phosphorous-Mobilizing Enzyme Secretion and Gum Contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Rajan, A.; Cherian, E.; Baskar, G. Biosynthesis of Zinc Oxide Nanoparticles Using Aspergillus fumigatus JCF and Its Antibacterial Activity. Int. J. Mod. Sci. Technol. 2016, 1, 52–57. [Google Scholar]
- Kalpana, V.N.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Devi Rajeswari, V. Biosynthesis of Zinc Oxide Nanoparticles Using Culture Filtrates of Aspergillus niger: Antimicrobial Textiles and Dye Degradation Studies. OpenNano 2018, 3, 48–55. [Google Scholar] [CrossRef]
- Mohana, S.; Sumathi, S. Synthesis of Zinc Oxide Using Agaricus Bisporus and Its In-Vitro Biological Activities. J. Environ. Chem. Eng. 2020, 8, 104192. [Google Scholar] [CrossRef]
- Sumanth, B.; Lakshmeesha, T.R.; Ansari, M.A.; Alzohairy, M.A.; Udayashankar, A.C.; Shobha, B.; Niranjana, S.R.; Srinivas, C.; Almatroudi, A. Mycogenic Synthesis of Extracellular Zinc Oxide Nanoparticles from Xylaria acuta and Its Nanoantibiotic Potential. Int. J. Nanomed. 2020, 15, 8519–8536. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Moniri, M.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Saad, W.Z.; Namvar, F.; Navaderi, M.; Mohamad, R. Biosynthesis of ZnO Nanoparticles by a New Pichia kudriavzevii Yeast Strain and Evaluation of Their Antimicrobial and Antioxidant Activities. Molecules 2017, 22, 872. [Google Scholar] [CrossRef] [PubMed]
- Kadam, V.V.; Ettiyappan, J.P.; Mohan Balakrishnan, R. Mechanistic Insight into the Endophytic Fungus Mediated Synthesis of Protein Capped ZnO Nanoparticles. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2019, 243, 214–221. [Google Scholar] [CrossRef]
- Motazedi, R.; Rahaiee, S.; Zare, M. Efficient Biogenesis of ZnO Nanoparticles Using Extracellular Extract of Saccharomyces cerevisiae: Evaluation of Photocatalytic, Cytotoxic and Other Biological Activities. Bioorg. Chem. 2020, 101, 103998. [Google Scholar] [CrossRef]
- Mani, V.M.; Nivetha, S.; Sabarathinam, S.; Barath, S.; Das, M.P.A.; Basha, S.; Elfasakhany, A.; Pugazhendhi, A. Multifunctionalities of Mycosynthesized Zinc Oxide Nanoparticles (ZnONPs) from Cladosporium tenuissimum FCBGr: Antimicrobial Additives for Paints Coating, Functionalized Fabrics and Biomedical Properties. Prog. Org. Coat. 2022, 163, 106650. [Google Scholar] [CrossRef]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and Enhanced Photocatalytic Activity of Zinc Oxide Nanoparticles Toward Organosulfur Pollutants. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- El-Belely, E.F.; Farag, M.M.S.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green Synthesis of Zinc Oxide Nanoparticles (Zno-Nps) Using Arthrospira platensis (Class: Cyanophyceae) and Evaluation of Their Biomedical Activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Rajaboopathi, S.; Thambidurai, S. Enhanced Photocatalytic Activity of Ag-ZnO Nanoparticles Synthesized by Using Padina gymnospora Seaweed Extract. J. Mol. Liq. 2018, 262, 148–160. [Google Scholar] [CrossRef]
- Rabie, A.M.; Abukhadra, M.R.; Rady, A.M.; Ahmed, S.A.; Labena, A.; Mohamed, H.S.H.; Betiha, M.A.; Shim, J.J. Instantaneous Photocatalytic Degradation of Malachite Green Dye under Visible Light Using Novel Green Co–ZnO/Algae Composites. Res. Chem. Intermed. 2020, 46, 1955–1973. [Google Scholar] [CrossRef]
- Syed, A.; Ahmad, A. Extracellular Biosynthesis of Platinum Nanoparticles Using the Fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2012, 97, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, A.; Klimek-Ochab, M. Fungal Synthesis of Size-Defined Nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 043001. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green Biosynthesis and Characterization of Zinc Oxide Nanoparticles Using Brown Marine Macroalga Sargassum muticum Aqueous Extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Mohamed Isa, E.D.; Shameli, K.; Ch’ng, H.J.; Che Jusoh, N.W.; Hazan, R. Photocatalytic Degradation of Selected Pharmaceuticals Using Green Fabricated Zinc Oxide Nanoparticles. Adv. Powder Technol. 2021, 32, 2398–2409. [Google Scholar] [CrossRef]
- El-Saied, H.A.A.; Ibrahim, A.M. Effective Fabrication and Characterization of Eco-Friendly Nano Chitosan Capped Zinc Oxide Nanoparticles for Effective Marine Fouling Inhibition. J. Environ. Chem. Eng. 2020, 8, 103949. [Google Scholar] [CrossRef]
- Vijayakumar, T.S.; Mahboob, S.; Bupesh, G.; Vasanth, S.; Al-Ghanim, K.A.; Al-Misned, F.; Govindarajan, M. Facile Synthesis and Biophysical Characterization of Egg Albumen-Wrapped Zinc Oxide Nanoparticles: A Potential Drug Delivery Vehicles for Anticancer Therapy. J. Drug Deliv. Sci. Technol. 2020, 60, 102015. [Google Scholar] [CrossRef]
- Qian, C.; Yin, J.; Zhao, J.; Li, X.; Wang, S.; Bai, Z.; Jiao, T. Facile Preparation and Highly Efficient Photodegradation Performances of Self-Assembled Artemia Eggshell-ZnO Nanocomposites for Wastewater Treatment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125752. [Google Scholar] [CrossRef]
- Rekha, R.; Mahboob, S.; Ramya, A.K.; Kerthekeyan, S.; Govindarajan, M.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Vaseeharan, B. Synthesis and Bio-Physical Characterization of Crustin Capped Zinc Oxide Nanoparticles, and Their Photocatalytic, Antibacterial, Antifungal and Antibiofilm Activity. J. Clust. Sci. 2021, 32, 843–855. [Google Scholar] [CrossRef]
- Almehizia, A.A.; Al-Omar, M.A.; Naglah, A.M.; Bhat, M.A.; Al-Shakliah, N.S. Facile Synthesis and Characterization of ZnO Nanoparticles for Studying Their Biological Activities and Photocatalytic Degradation Properties toward Methylene Blue Dye. Alex. Eng. J. 2021, 61, 2386–2395. [Google Scholar] [CrossRef]
- Kamaruzaman, A.; Lah, N.A.C. Formation of ZnO Nanoparticles in the Presence of Tannic Acid. Mater. Today Proc. 2020, 41, 61–64. [Google Scholar] [CrossRef]
- Ranjithkumar, B.; Ramalingam, H.B.; Kumar, E.R.; Srinivas, C.; Magesh, G.; Rahale, C.S.; El-Metwaly, N.M.; Shekar, B.C. Natural Fuels (Honey and Cow Urine) Assisted Combustion Synthesis of Zinc Oxide Nanoparticles for Antimicrobial Activities. Ceram. Int. 2021, 47, 14475–14481. [Google Scholar] [CrossRef]
- Motshekga, S.C.; Sinha Ray, S.; Maity, A. Synthesis and Characterization of Alginate Beads Encapsulated Zinc Oxide Nanoparticles for Bacteria Disinfection in Water. J. Colloid Interface Sci. 2018, 512, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Peychev, B.; Vasileva, P. Novel Starch-Mediated Synthesis of Au/ZnO Nanocrystals and Their Photocatalytic Properties. Heliyon 2021, 7, e07402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Li, Z.; Yang, D.; Qiu, X.; Liu, Y.; Yan, M.; Li, Q. Fabrication of Litchi-like Lignin/Zinc Oxide Composites with Enhanced Antibacterial Activity and Their Application in Polyurethane Films. J. Colloid Interface Sci. 2021, 594, 316–325. [Google Scholar] [CrossRef]
- Das, R.K.; Gogoi, N.; Babu, P.J.; Sharma, P.; Mahanta, C.; Bora, U. The Synthesis of Gold Nanoparticles Using Amaranthus spinosus Leaf Extract and Study of Their Optical Properties. Adv. Mater. Phys. Chem. 2012, 2, 275–281. [Google Scholar] [CrossRef]
- Umar, H.; Kavaz, D.; Rizaner, N. Biosynthesis of Zinc Oxide Nanoparticles Using Albizia Lebbeck Stem Bark, and Evaluation of Its Antimicrobial, Antioxidant, and Cytotoxic Activities on Human Breast Cancer Cell Lines. Int. J. Nanomed. 2019, 14, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Honary, S.; Ebrahimi, P.; Mohammadi, M.A.; Alizadeh, A.; Naghibi, F. Microbial Mediated Preparation, Characterization and Optimization of Gold Nanoparticles. Braz. J. Microbiol. 2014, 45, 1493–1501. [Google Scholar] [CrossRef]
- Guan, Y.; Yu, H.Y.; Abdalkarim, S.Y.H.; Wang, C.; Tang, F.; Marek, J.; Chen, W.L.; Militky, J.; Yao, J.M. Green One-Step Synthesis of ZnO/Cellulose Nanocrystal Hybrids with Modulated Morphologies and Superfast Absorption of Cationic Dyes. Int. J. Biol. Macromol. 2019, 132, 51–62. [Google Scholar] [CrossRef]
- Zheng, B.; Kong, T.; Jing, X.; Odoom-Wubah, T.; Li, X.; Sun, D.; Lu, F.; Zheng, Y.; Huang, J.; Li, Q. Plant-Mediated Synthesis of Platinum Nanoparticles and Its Bioreductive Mechanism. J. Colloid Interface Sci. 2013, 396, 138–145. [Google Scholar] [CrossRef]
- Vanaja, M. Kinetic Study on Green Synthesis of Silver Nanoparticles Using Coleus aromaticus Leaf Extract. Adv. Appl. Sci. Res. 2013, 4, 50–55. [Google Scholar]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green Synthesis of Zinc Oxide Nanoparticles Using Flower Extract of Nyctanthes arbor-tristis and Their Antifungal Activity. J. King Saud Univ.-Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef]
- Dhandapani, P.; Siddarth, A.S.; Kamalasekaran, S.; Maruthamuthu, S.; Rajagopal, G. Bio-Approach: Ureolytic Bacteria Mediated Synthesis of ZnO Nanocrystals on Cotton Fabric and Evaluation of Their Antibacterial Properties. Carbohydr. Polym. 2014, 103, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Shaziman, S.; Ismailrosdi, A.S.; Mamat, M.H.; Zoolfakar, A.S. Influence of Growth Time and Temperature on the Morphology of ZnO Nanorods via Hydrothermal. IOP Conf. Ser. Mater. Sci. Eng. 2015, 99, 1–2. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Fouda, A.; Abdel-Rahman, M.A.; Hassan, S.E.D.; El-Gamal, M.S.; Salem, S.S.; Shaheen, T.I. Fungal Strain Impacts the Shape, Bioactivity and Multifunctional Properties of Green Synthesized Zinc Oxide Nanoparticles. Biocatal. Agric. Biotechnol. 2019, 19, 101103. [Google Scholar] [CrossRef]
- Zak, K.; Razali, R.; Majid, W.A.; Darround, M. Synthesis and Characterization of a Narrow Size Distribution of Zinc Oxide Nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar] [CrossRef]
- Aldeen, T.S.; Ahmed Mohamed, H.E.; Maaza, M. ZnO Nanoparticles Prepared via a Green Synthesis Approach: Physical Properties, Photocatalytic and Antibacterial Activity. J. Phys. Chem. Solids 2022, 160, 110313. [Google Scholar] [CrossRef]
- Gabriel Kaningini, A.; Azizi, S.; Sintwa, N.; Mokalane, K.; Cecilia Mohale, K.; Nixwell Mudau, F.; Maaza, M. Effect of Optimized Precursor Concentration, Temperature, and Doping on Optical Properties of ZnO Nanoparticles Synthesized via a Green Route Using Bush Tea (Athrixia phylicoides DC.) Leaf Extracts. ACS Omega 2022, 7, 31658–31666. [Google Scholar] [CrossRef]
- Bharathi, D.; Ranjithkumar, R.; Chandarshekar, B.; Bhuvaneshwari, V. Preparation of Chitosan Coated Zinc Oxide Nanocomposite for Enhanced Antibacterial and Photocatalytic Activity: As a Bionanocomposite. Int. J. Biol. Macromol. 2019, 129, 989–996. [Google Scholar] [CrossRef]
- Ayoub, I.; Kumar, V.; Abolhassani, R.; Sehgal, R.R.; Sharma, V.; Sehgal, R.R.; Swart, H.C.; Mishra, Y.K.; Chen, D.; Wang, Z.; et al. Enhanced Photocatalytic Performance for the BiPO4-x Nanorod Induced by Surface Oxygen Vacancy. J. Phys. Chem. C 2022, 5, 18520–18528. [Google Scholar] [CrossRef]
- Hafiz, M.; 1ã, M.; Khusaimi, Z.; Zahidi, M.M.; Abu Bakar, S.; Siran, Y.M.; Anuar, S.; Rejab, M.; Asis, A.J.; Tahiruddin, S.; et al. Controllable Growth of Vertically Aligned Aluminum-Doped Zinc Oxide Nanorod Arrays by Sonicated Sol-Gel Immersion Method Depending on Precursor Solution Volumes. Jpn. J. Appl. Phys. 2011, 50, 06GH04. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Q.; Tian, S.; Zhao, X. Exposed Facet Dependent Stability of ZnO Micro/Nano Crystals as a Photocatalyst. Appl. Surf. Sci. 2019, 470, 807–816. [Google Scholar] [CrossRef]
- Debroye, E.; Van Loon, J.; Yuan, H.; Janssen, K.P.F.; Lou, Z.; Kim, S.; Majima, T.; Roeffaers, M.B.J. Facet-Dependent Photoreduction on Single ZnO Crystals. J. Phys. Chem. Lett. 2017, 8, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Sarkar, D.; Madras, G. Synthesis and Photocatalytic Performance of Quasi-Fibrous ZnO. RSC Adv. 2014, 4, 55807–55814. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, T.; Wu, T.; Jin, C.; Qiao, R.; Qian, Y.; Tong, G. Polymorphous ZnO Nanostructures: Zn Polar Surface-Guided Size and Shape Evolution Mechanism and Enhanced Photocatalytic Activity. ChemCatChem 2017, 9, 3180–3190. [Google Scholar] [CrossRef]
- Naderi, M. Surface Area: Brunauer–Emmett–Teller (BET). In Progress in Filtration and Separation; Tarleton, S., Ed.; Elsevier& Academic Press: London, UK, 2015; pp. 585–608. ISBN 9780123983077. [Google Scholar]
- Salvadori, M.R.; Ando, R.A.; Nascimento, C.A.O.; Corrêa, B. Extra and Intracellular Synthesis of Nickel Oxide Nanoparticles Mediated by Dead Fungal Biomass. PLoS ONE 2015, 10, e0129799. [Google Scholar] [CrossRef] [PubMed]
- Dimapilis, E.A.S.; Hsu, C.S.; Mendoza, R.M.O.; Lu, M.C. Zinc Oxide Nanoparticles for Water Disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Gnanaprakasam, A.; Sivakumar, V.M.; Thirumarimurugan, M. Influencing Parameters in the Photocatalytic Degradation of Organic Effluent via Nanometal Oxide Catalyst: A Review. Indian J. Mater. Sci. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Rathnasamy, R.; Thangasamy, P.; Thangamuthu, R.; Sampath, S.; Alagan, V. Green Synthesis of ZnO Nanoparticles Using Carica papaya Leaf Extracts for Photocatalytic and Photovoltaic Applications. J. Mater. Sci. Mater. Electron. 2017, 28, 10374–10381. [Google Scholar] [CrossRef]
- Ahmed, W.; Chantes, P.; Jarusutthirak, C.; Danwittayakul, S. A Comparison Study of Photocatalytic Activity of TiO2 and ZnO on the Degradation of Real Batik Wastewater. Int. Conf. Biol. Environ. Food Eng. 2015, 16. [Google Scholar] [CrossRef][Green Version]
- Gonçalves, R.A.; Toledo, R.P.; Joshi, N.; Berengue, O.M. Green Synthesis and Applications of Zno and TiO2 Nanostructures. Molecules 2021, 26, 2236. [Google Scholar] [CrossRef]
- Sin, J.C.; Lam, S.M.; Satoshi, I.; Lee, K.T.; Mohamed, A.R. Sunlight Photocatalytic Activity Enhancement and Mechanism of Novel Europium-Doped ZnO Hierarchical Micro/Nanospheres for Degradation of Phenol. Appl. Catal. B Environ. 2014, 148–149, 258–268. [Google Scholar] [CrossRef]
- Nava, O.J.; Soto-Robles, C.A.; Gómez-Gutiérrez, C.M.; Vilchis-Nestor, A.R.; Castro-Beltrán, A.; Olivas, A.; Luque, P.A. Fruit Peel Extract Mediated Green Synthesis of Zinc Oxide Nanoparticles. J. Mol. Struct. 2017, 1147, 1–6. [Google Scholar] [CrossRef]
- Vinayagam, R.; Selvaraj, R.; Arivalagan, P.; Varadavenkatesan, T. Synthesis, Characterization and Photocatalytic Dye Degradation Capability of Calliandra haematocephala-Mediated Zinc Oxide Nanoflowers. J. Photochem. Photobiol. B Biol. 2020, 203, 111760. [Google Scholar] [CrossRef] [PubMed]
- Mirgane, N.A.; Shivankar, V.S.; Kotwal, S.B.; Wadhawa, G.C.; Sonawale, M.C. Degradation of Dyes Using Biologically Synthesized Zinc Oxide Nanoparticles. Mater. Today Proc. 2020, 37, 849–853. [Google Scholar] [CrossRef]
- Nava, O.J.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Vilchis-Nestor, A.R.; Castro-Beltrán, A.; Mota-González, M.L.; Olivas, A. Influence of Camellia sinensis Extract on Zinc Oxide Nanoparticle Green Synthesis. J. Mol. Struct. 2017, 1134, 121–125. [Google Scholar] [CrossRef]
- Vidya, C.; Manjunatha, C.; Chandraprabha, M.N.; Rajshekar, M.; Antony Raj, M.A.L. Hazard Free Green Synthesis of ZnO Nano-Photo-Catalyst Using Artocarpus heterophyllus Leaf Extract for the Degradation of Congo Red Dye in Water Treatment Applications. J. Environ. Chem. Eng. 2017, 5, 3172–3180. [Google Scholar] [CrossRef]
- Razanamahandry, L.C.; Sackey, J.; Furqan, C.M.; Ntwampe, S.K.O.; Fosso-Kankeu, E.; Manikandan, E.; Maaza, M. Removal of Free Cyanide by a Green Photocatalyst ZnO Nanoparticle Synthesized via Eucalyptus globulus Leaves. In Photocatalysts in Advanced Oxidation Processes for Wastewater Treatment; Elvis Fosso-Kankeu, Pandey, S., Ray, S.S., Eds.; Srinevener Publishing, Wiley: Beverley, UK, 2020; pp. 271–288. ISBN 9781119631422. [Google Scholar]
- Patil, S.S.; Mali, M.G.; Tamboli, M.S.; Patil, D.R.; Kulkarni, M.V.; Yoon, H.; Kim, H.; Al-Deyab, S.S.; Yoon, S.S.; Kolekar, S.S.; et al. Green Approach for Hierarchical Nanostructured Ag-ZnO and Their Photocatalytic Performance under Sunlight. Catal. Today 2016, 260, 126–134. [Google Scholar] [CrossRef]
- Noukelag, S.K.; Razanamahandry, L.C.; Ntwampe, S.K.O.; Arendse, C.J.; Maaza, M. Industrial Dye Removal Using Bio-Synthesized Ag-Doped ZnO Nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100463. [Google Scholar] [CrossRef]
- Karthik, K.V.; Raghu, A.V.; Reddy, K.R.; Ravishankar, R.; Sangeeta, M.; Shetti, N.P.; Reddy, C.V. Green Synthesis of Cu-Doped ZnO Nanoparticles and Its Application for the Photocatalytic Degradation of Hazardous Organic Pollutants. Chemosphere 2022, 287, 132081. [Google Scholar] [CrossRef]
- Li, J.F.; Rupa, E.J.; Hurh, J.; Huo, Y.; Chen, L.; Han, Y.; Ahn, J.C.; Park, J.K.; Lee, H.A.; Mathiyalagan, R.; et al. Cordyceps militaris Fungus Mediated Zinc Oxide Nanoparticles for the Photocatalytic Degradation of Methylene Blue Dye. Optik 2019, 183, 691–697. [Google Scholar] [CrossRef]
- Kumar, N.; Bhadwal, A.S.; Garg, M.; Sharma, R.; Singh, S.; Mizaikoff, B. Photocatalytic and Antibacterial Biomimetic ZnO Nanoparticles. Anal. Methods 2017, 9, 4776–4782. [Google Scholar] [CrossRef]
- Deyab, M.; Elkatony, T.; Ward, F. Qualitative and Quantitative Analysis of Phytochemical Studies on Brown Seaweed, Dictyota dichotoma. Int. J. Eng. Dev. Res. 2016, 4, 2321–9939. [Google Scholar]
- Preethi, J.; Farzana, M.H.; Meenakshi, S. Photo-Reduction of Cr(VI) Using Chitosan Supported Zinc Oxide Materials. Int. J. Biol. Macromol. 2017, 104, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Wang, Z.; Wu, S.; Ma, J. Cellulose Controlled Zinc Oxide Nanoparticles with Adjustable Morphology and Their Photocatalytic Performances. Carbohydr. Polym. 2021, 259, 117752. [Google Scholar] [CrossRef] [PubMed]
- Rashtbari, Y.; Hazrati, S.; Azari, A.; Afshin, S.; Fazlzadeh, M.; Vosoughi, M. A Novel, Eco-Friendly and Green Synthesis of PPAC-ZnO and PPAC-NZVI Nanocomposite Using Pomegranate Peel: Cephalexin Adsorption Experiments, Mechanisms, Isotherms and Kinetics. Adv. Powder Technol. 2020, 31, 1612–1623. [Google Scholar] [CrossRef]
- Yokwana, K.; Kuvarega, A.T.; Mhlanga, S.D.; Nxumalo, E.N. Mechanistic Aspects for the Removal of Congo Red Dye from Aqueous Media through Adsorption over N-Doped Graphene Oxide Nanoadsorbents Prepared from Graphite Flakes and Powders. Phys. Chem. Earth 2018, 107, 58–70. [Google Scholar] [CrossRef]
- Nagar, N.; Devra, V. A Kinetic Study on the Degradation and Biodegradability of Silver Nanoparticles Catalyzed Methyl Orange and Textile Effluents. Heliyon 2019, 5, e01356. [Google Scholar] [CrossRef]
- Mahdavi, S.; Jalali, M.; Afkhami, A. Removal of Heavy Metals from Aqueous Solutions Using Fe3O4, ZnO, and CuO Nanoparticles. Nanotechnol. Sustain. Dev. 2014, 846, 171–188. [Google Scholar] [CrossRef]
- Kim, K.M.; Choi, M.H.; Lee, J.K.; Jeong, J.; Kim, Y.R.; Kim, M.K.; Paek, S.M.; Oh, J.M. Physicochemical Properties of Surface Charge-Modified ZnO Nanoparticles with Different Particle Sizes. Int. J. Nanomed. 2014, 9, 41–56. [Google Scholar] [CrossRef]
- Nayak, A.; Sahoo, J.K.; Sahoo, S.K.; Sahu, D. Removal of Congo Red Dye from Aqueous Solution Using Zinc Oxide Nanoparticles Synthesised from Ocimum sanctum (Tulsi Leaf): A Green Approach. Int. J. Environ. Anal. Chem. 2020, 1–22. [Google Scholar] [CrossRef]
- Muthukumaran, P.; Babu, P.S.; Shyamalagowri, S.; Kamaraj, M.; Manikandan, A.; Aravind, J. Nanotechnological Approaches as a Promising Way for Heavy Metal Mitigation in an Aqueous System. J. Basic Microbiol. 2021, 62, 376–394. [Google Scholar] [CrossRef] [PubMed]
- Le, A.T.; Pung, S.Y.; Sreekantan, S.; Matsuda, A.; Huynh, D.P. Mechanisms of Removal of Heavy Metal Ions by ZnO Particles. Heliyon 2019, 5, e01440. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Singh, A. Adsorptive Performances and Characterisations of Biologically Synthesised Zinc Oxide Based Nanosorbent (ZOBN). Groundw. Sustain. Dev. 2020, 10, 100325. [Google Scholar] [CrossRef]
- Tshangana, C.; Chabalala, M.; Muleja, A.; Nxumalo, E.; Mamba, B. Shape-Dependant Photocatalytic and Antimicrobial Activity of ZnO Nanostructures When Conjugated to Graphene Quantum Dots. J. Environ. Chem. Eng. 2020, 8, 103930. [Google Scholar] [CrossRef]
- Sun, H.; Brocato, J.; Costa, M. Oral Chromium Exposure and Toxicity. Curr. Environ. Health Rep. 2015, 2, 295–303. [Google Scholar] [CrossRef]
- Khan, T.A.; Nazir, M.; Ali, I.; Kumar, A. Removal of Chromium(VI) from Aqueous Solution Using Guar Gum–Nano Zinc Oxide Biocomposite Adsorbent. Arab. J. Chem. 2017, 10, S2388–S2398. [Google Scholar] [CrossRef]
- Kamaraj, M.; Srinivasan, N.R.; Assefa, G.; Adugna, A.T.; Kebede, M. Facile Development of Sunlit ZnO Nanoparticles-Activated Carbon Hybrid from Pernicious Weed as an Operative Nano-Adsorbent for Removal of Methylene Blue and Chromium from Aqueous Solution: Extended Application in Tannery Industrial Wastewater. Environ. Technol. Innov. 2020, 17, 100540. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead Toxicity: A Review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Gordon, B.; Callan, P.; Vickers, C. WHO Guidelines for Drinking-Water Quality. WHO Chron. 2008, 38, 564. [Google Scholar] [CrossRef]
- Somu, P.; Paul, S. Casein Based Biogenic-Synthesized Zinc Oxide Nanoparticles Simultaneously Decontaminate Heavy Metals, Dyes, and Pathogenic Microbes: A Rational Strategy for Wastewater Treatment. J. Chem. Technol. Biotechnol. 2018, 93, 2962–2976. [Google Scholar] [CrossRef]
- Joshi, N.; Malik, S.; Gururani, P. Utilization of Polypyrrole/ZnO Nanocomposite in the Adsorptive Removal of Cu2+, Pb2+ and Cd2+ Ions from Wastewater. Lett. Appl. NanoBioScience 2020, 10, 2339–2351. [Google Scholar] [CrossRef]
- Nel, L.; Strydom, N.A.; Bouwman, H. Preliminary Assessment of Contaminants in the Sediment and Organisms of the Swartkops Estuary, South Africa. Mar. Pollut. Bull. 2015, 101, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Debnath, P.; Mondal, N.K. Effective Removal of Congo Red Dye from Aqueous Solution Using Biosynthesized Zinc Oxide Nanoparticles. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100320. [Google Scholar] [CrossRef]
- Arab, C.; El Kurdi, R.; Patra, D. Efficient Removal of Congo Red Using Curcumin Conjugated Zinc Oxide Nanoparticles as New Adsorbent Complex. Chemosphere 2021, 276, 1–3. [Google Scholar] [CrossRef]
- Jose, L.M.; Raj, R.S.A.; Sajan, D.; Aravind, A. Adsorption and Photocatalytic Activity of Biosynthesised ZnO Nanoparticles Using Aloe vera Leaf Extract. Nano Express 2021, 2, 010039. [Google Scholar] [CrossRef]
- Mittal, H.; Morajkar, P.P.; Al Alili, A.; Alhassan, S.M. In-Situ Synthesis of ZnO Nanoparticles Using Gum Arabic Based Hydrogels as a Self-Template for Effective Malachite Green Dye Adsorption. J. Polym. Environ. 2020, 28, 1637–1653. [Google Scholar] [CrossRef]
- Kahsay, M.H. Synthesis and Characterization of ZnO Nanoparticles Using Aqueous Extract of Becium grandiflorum for Antimicrobial Activity and Adsorption of Methylene Blue. Appl. Water Sci. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Adeniyi, A.; Sithole, B.B.; Onyango, M.S. Sawdust-Based Cellulose Nanocrystals Incorporated with ZnO Nanoparticles as Efficient Adsorption Media in the Removal of Methylene Blue Dye. ACS Omega 2020, 5, 18798–18807. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Synthesis, Characterization, and Regeneration of an Inorganic–Organic Nanocomposite (ZnO@biomass) and Its Application in the Capture of Cationic Dye. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Singh, J.; Vishwakarma, K.; Ramawat, N.; Rai, P.; Singh, V.K.; Mishra, R.K.; Kumar, V.; Tripathi, D.K.; Sharma, S. Nanomaterials and Microbes’ Interactions: A Contemporary Overview. 3 Biotech 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Soren, S.; Kumar, S.; Mishra, S.; Jena, P.K.; Verma, S.K.; Parhi, P. Evaluation of Antibacterial and Antioxidant Potential of the Zinc Oxide Nanoparticles Synthesized by Aqueous and Polyol Method. Microb. Pathog. 2018, 119, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced Bioactivity of ZnO Nanoparticles—An Antimicrobial Study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Mahendra, C.; Murali, M.; Manasa, G.; Ponnamma, P.; Abhilash, M.R.; Lakshmeesha, T.R.; Satish, A.; Amruthesh, K.N.; Sudarshana, M.S. Antibacterial and Antimitotic Potential of Bio-Fabricated Zinc Oxide Nanoparticles of Cochlospermum religiosum (L.). Microb. Pathog. 2017, 110, 620–629. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Shanmugam, V.K. Functionalization of Zinc Oxide Nanoparticles Using Mucuna pruriens and Its Antibacterial Activity. Surf. Interfaces 2020, 19, 100521. [Google Scholar] [CrossRef]
- Theophil Anand, G.; Renuka, D.; Ramesh, R.; Anandaraj, L.; John Sundaram, S.; Ramalingam, G.; Magdalane, C.M.; Bashir, A.K.H.; Maaza, M.; Kaviyarasu, K. Green Synthesis of ZnO Nanoparticle Using Prunus dulcis (Almond Gum) for Antimicrobial and Supercapacitor Applications. Surf. Interfaces 2019, 17, 100376. [Google Scholar] [CrossRef]
- Kokabi, M.; Yousefzadi, M.; Nejad Ebrahimi, S.; Zarei, M. Green Synthesis of Zinc Oxide Nanoparticles Using Seaweed Aqueous Extract and Evaluation of Antibacterial and Ecotoxicological Activity. J. Persian Gulf (Mar. Sci.) 2017, 8, 61–72. [Google Scholar] [CrossRef]
- Jamil, Z.; Naqvi, S.T.Q.; Rasul, S.; Hussain, S.B.; Fatima, N.; Qadir, M.I.; Muhammad, S.A. Antibacterial Activity and Characterization of Zinc Oxide Nanoparticles Synthesized by Microalgae. Pak. J. Pharm. Sci. 2020, 33, 2497–2504. [Google Scholar] [CrossRef]
- Subramanian, H.; Krishnan, M.; Mahalingam, A. Photocatalytic Dye Degradation and Photoexcited Anti-Microbial Activities of Green Zinc Oxide Nanoparticles Synthesized via Sargassum muticum Extracts. RSC Adv. 2022, 12, 985–997. [Google Scholar] [CrossRef]
- Gharpure, S.; Yadwade, R.; Ankamwar, B. Non-Antimicrobial and Non-Anticancer Properties of ZnO Nanoparticles Biosynthesized Using Different Plant Parts of Bixa orellana. ACS Omega 2022, 7, 1914–1933. [Google Scholar] [CrossRef]
- Mocanu, A.; Isopencu, G.; Busuioc, C.; Popa, O.M.; Dietrich, P.; Socaciu-Siebert, L. Bacterial Cellulose Films with ZnO Nanoparticles and Propolis Extracts: Synergistic Antimicrobial Effect. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]

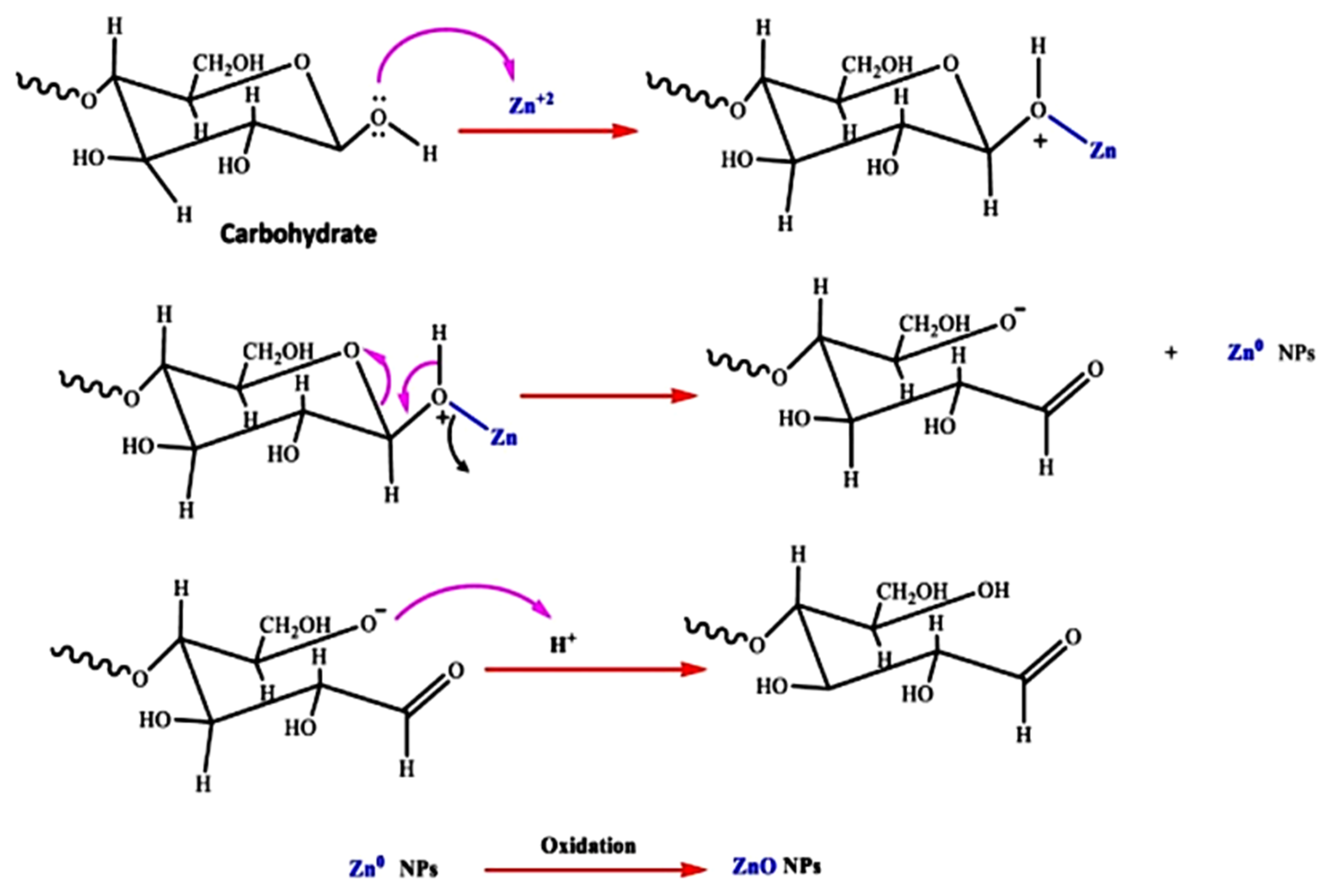
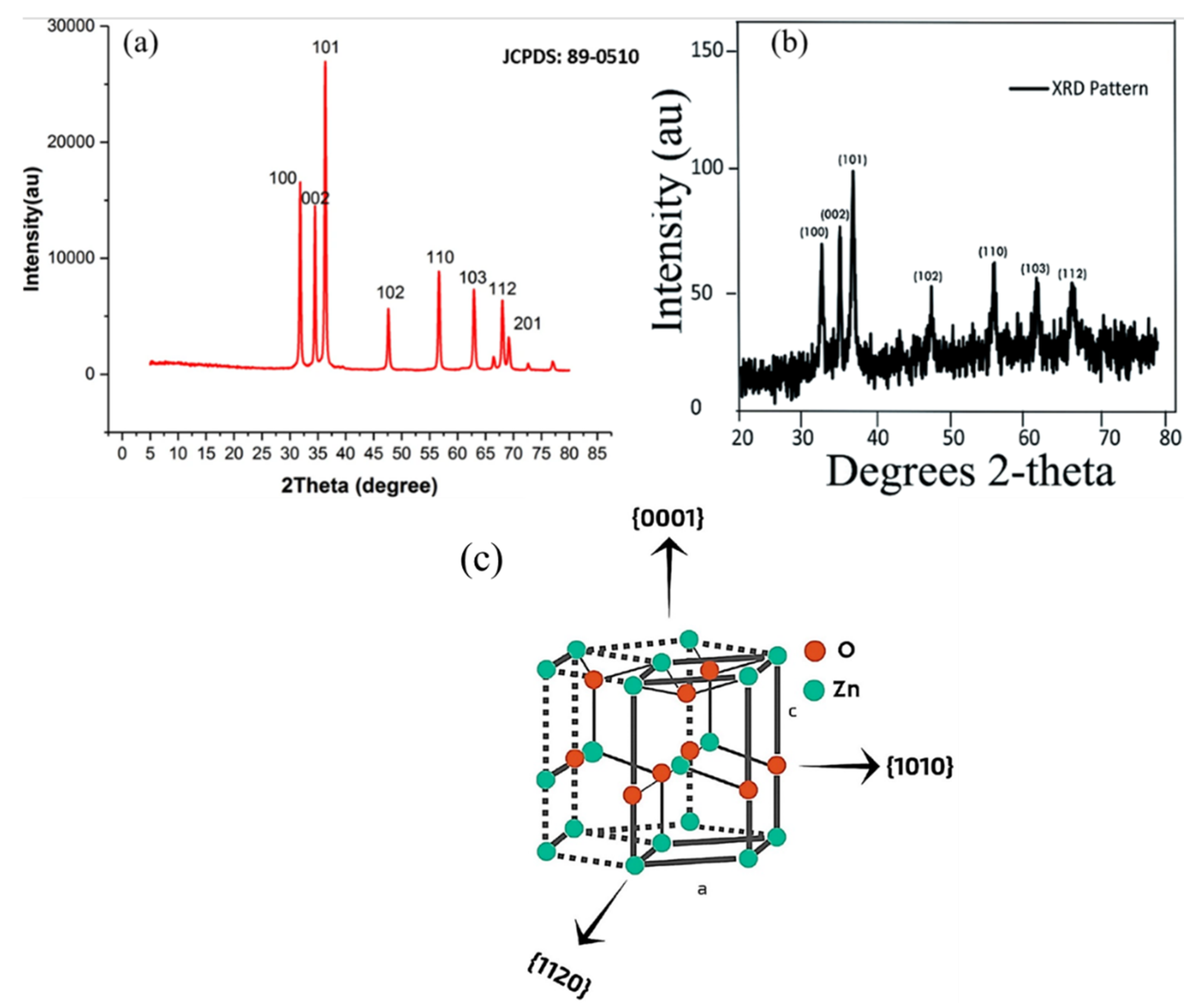
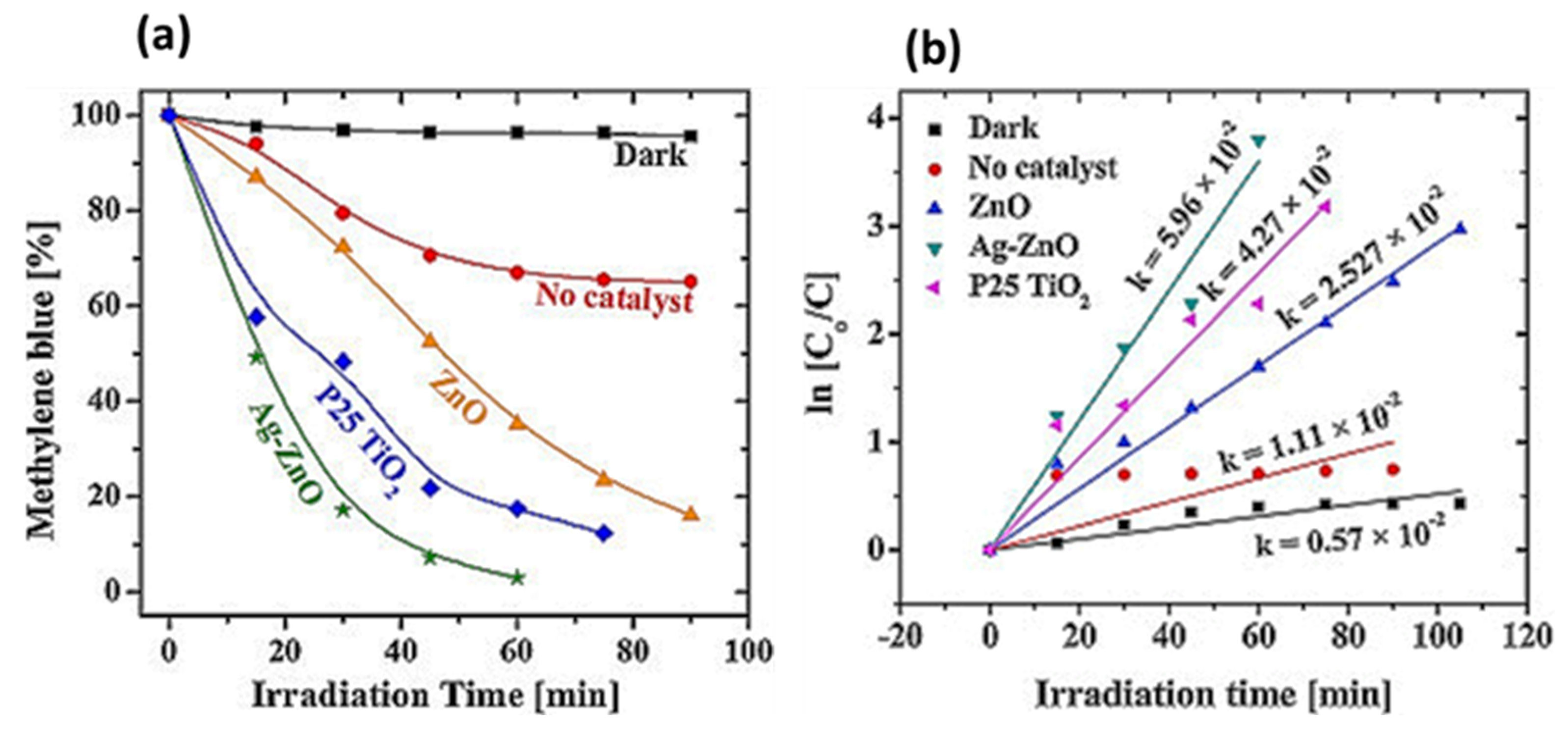
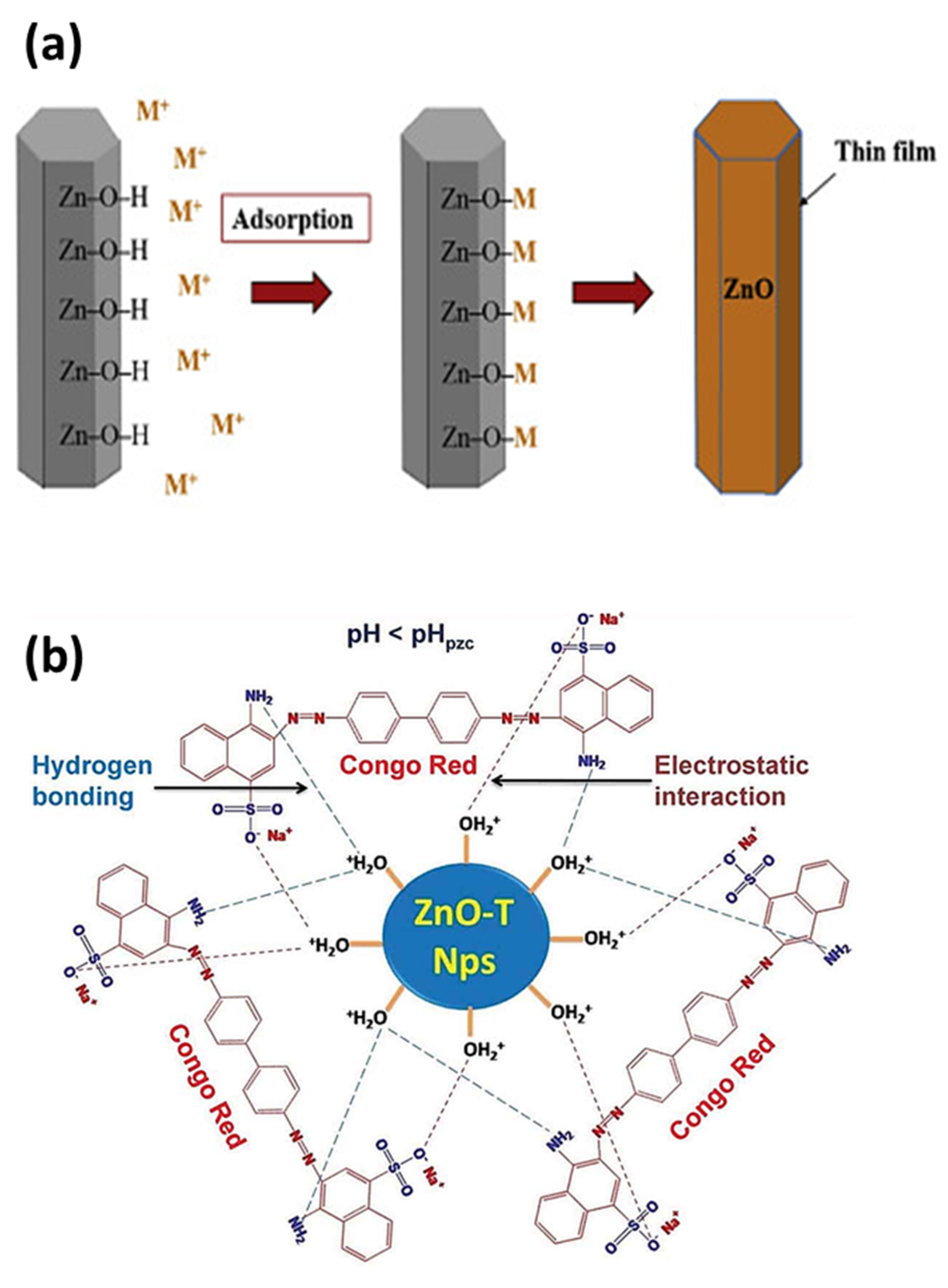
| Serial No. | Plant Name | Dopant | Extraction Solvent, Temp and Time | Precursors | Biosynthesis Time (h) and Temp (°C) | Calcination Temp (°C)/Time (h) | Shape | Size (nm) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Calotropis gigantea (leaves) | Ag | Refluxing | Zn(NO3)2·6H2O AgNO3 | 3/RT | 400/3 | Rods | None | [33] |
| 2 | Moringa oleifera (seeds) | Ag | Aqueous powder, RT | Zn(OOCCH3)2 AgNO3 | 50 min/RT | - | Flower-like | 54.1 | [58] |
| 3 | Ocimum tenuiflorum (seeds) | Ag | Water, boiled | Zn(NO3)2·6H2O AgNO3 | 2/RT | 450/3 | Hexagonal and spherical | 50–60 | [106] |
| 4 | Melastoma malabathricum (leaves) | Mn | Water, 60 °C | Zn(NO3)2·6H2O Mn(CH3CO2)2·4H2O | –/60 | 400/2 | Spherical | 222 | [107] |
| 5 | Myrtus communis L. | Fe | Water, 40 °C for 1 h | Zn(NO3)2·6H2O Fe(NO)3·9H2O | 3/60 | 400/3 | Pseudo spherical | 17 | [108] |
| 6 | Piper guineense (leaves) | Mg | Water, 60 °C for 20 min | Zn(NO3)2 Mg(NO3)2 | 0.25/80 | 350/2 | Spherical | 364 | [109] |
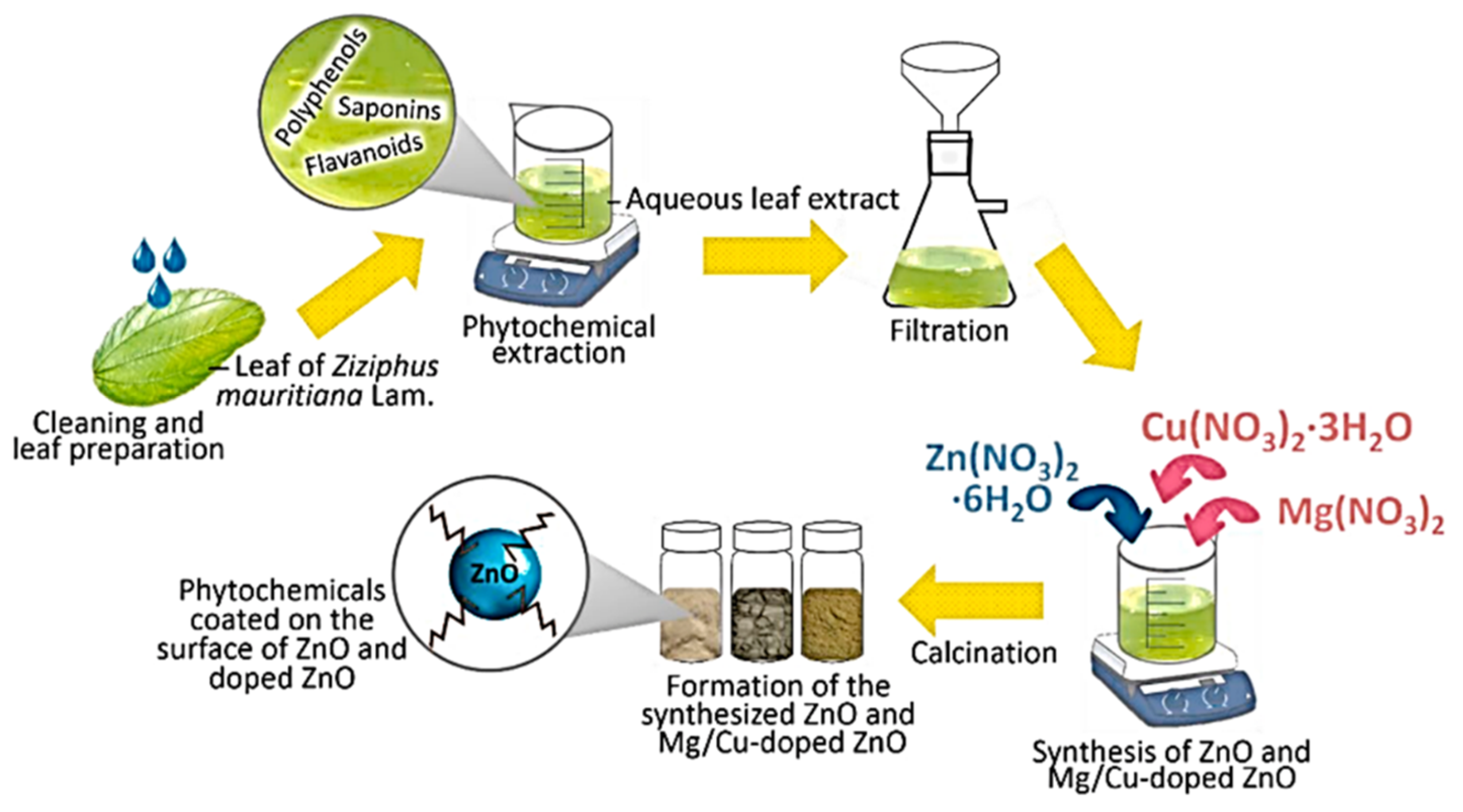
| 7 | Ziziphus mauritiana Lam. (leaves) | Mg and Cu | Water, RT for 1 h | Zn(NO3)2·6H2O Cu(NO3)2·3H2O Mg(NO3)2 | –/60 | 400/2 | Hexagonal disc shaped | 100–1000 | [110] |
| 8 | Vernonia amygdalina | Nb | Not available | Not mentioned | 1/60 | 400/1 | Spherical | 1000 | [111] |
| Serial No. | Microbe Name | Extraction Method | Salt | Biosynthesis Time (h)/Temp (°C) | Calcination Temp (°C) Time (h) | Shape | Size (nm) | Ref |
|---|---|---|---|---|---|---|---|---|
| Bacteria | ||||||||
| 1 | Cyanobacterium Nostoc sp. | Water, 60 °C for 15 min | Zn(CH3COO)2·2H2O | 2/RT | − | Star-shaped | 60 | [29] |
| 2 | Aeromonas hydrophila | Cell culture grew for 24 h and was diluted with water | ZnO | 24/30 | − | [59] | ||
| 3 | Serratia nematodiphila. | Inoculated in Luria-Bertani (LB) broth | ZnSO4 | 0.17/80 Then stored for 24 h | − | Near spherical | 15–30 | [105] |
| 4 | Bacillus subtillis | Cell culture in ammonium carbonate | Zn(CH3COO)2 | 3/80 | 250/3 | Spheres with hair-like appendages | 10–15 and 1.2–1.3 um aggregates | [124] |
| 5 | Bacillus haynesii | Cell culture grown on agar | ZnSO4 | 24/55 | 700/5 | Spherical | 20–200 | [125] |
| 6 | Alkalibacillus sp. | Cell-free supernatant collected by centrifugation | ZnSO4·7H2O | 48/35 | − | Quasi-spherical | 1–30 | [126] |
| 7 | Pseudomonas putida | Cell culture solution | Zn (NO3)2 | 24/37 | 400/2 | Spherical agglomerates | 25–45 | [127] |
| 8 | Halomonas elongata | Centrifugation | ZnCl2 | Taguchi | − | Multiform shapes | 18.11 | [128] |
| 9 | Lactobacillus plantarum | Cell biomass collected by centrifugation | Zn(NO3)2·6H2O, | 24/37 | − | Flower-like | 152.8–613.5 | [129] |
| 10 | Streptomyces spp. | Broth dilution then centrifugation | ZnSO4 | 0.25/40 | 400/8 | Needle-like | 12–35 | [130] |
| Fungi | ||||||||
| 11 | Aspergillus aeneus | Cell-free filtrate | Zn (NO3)2 | 48/28 | − | Spherical | 100–140 | [60] |
| 12 | Aspergillus fumigatus | Plating the inoculum on Martin Rose-Bengal agar medium | Zn (NO3)2 | 72/28 | − | Oblate spherical | 1.2–6.8 | [131] |
| 13 | Aspergillus fumigatus | Cell biomass | ZnSO4 | 72/32 | − | Spherical | 60–80 | [132] |
| 14 | Aspergillus niger. | Culture filtrate | Zn(NO3)2 | 48/32 | − | Near spherical | 53–69 | [133] |
| 15 | Agaricus bisporus | Purchased | Zn(NO3)2·6H2O | 2/90 | 400/4 | Spheroids | 32 | [134] |
| 16 | Xylaria acuta | Cultured then centrifuged | Zn(NO3)2·6H2O | –/400 | 700/2 | Hexagonal | 34–55 | [135] |
| 17 | Pichia kudriavzevii | Mycelia separated from the culture | Zn(CH3COO)2·2H2O | 36/35 | − | Hexagonal | 10–61 (TEM, XRD) | [136] |
| 18 | Cochliobolus geniculatus | Mycelial-free filtrate | Zn(CH3COO)2 | 72/28 | − | Quasi-spherical | 2–6 (TEM) | [137] |
| 19 | Saccharomyces cerevisiae | Cell-free filtrate | Zn(CH3COO)2·2H2O | 24/30 | − | Spherical | 20–30 | [138] |
| 20 | Cladosporium tenuissimum FCBGr | Extracellular culture | Zn(NO3)2 | 36/RT | − | Bouquet-like | 57 | [139] |
| Algae | ||||||||
| 21 | Chlorella | Soaking method | Zn(CH3COO)2·2H2O | 1/58 | − | Hexagonal | 20 | [140] |
| 22 | Arthrospira platensis | Biomass mixed with supernatant | Zn(CH3COO)2·2H2O | 24/30 | − | Spherical | 30–55 | [141] |
| 23 | Padina gymnospora | Millipore water stirred for 2 h at 100 °C | Zn(NO3)2 AgNO3 | 2/60 | − | Spherical | 20–40 | [142] |
| 24 | Sargassum species | Ball-milled | Zn (NO3)2·6H2O Co(NO3)2·6H2O | 2/RT | − | Nearly spherical | 5.4–6.8 | [143] |
| Serial No. | Bio-Product | Precursors | Biosynthesis Temp (°C)/Duration | Thermal Treatment Temp (°C)/Time (h) | Shape | Size (nm) | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Alginate | Purchased ZnO | − | Irregular spheres | 20–90 | [155] | |
| 2 | Starch | Zn(NO3)2·6H2O HAuCl4·3H2O | Stirred at 90–100 °C | Oven dried at 80 °C Calcined at 600 °C for 4 h | Spherical | 18.9 | [156] |
| 3 | Lignin | Zn(NO3)2·6H2O | Oven at 120 °C for 4 h | Oven dried at 120 °C for 4 h | Rods | 5–10 | [157] |
| 4 | Pullulan | Zn(NO3)2·6H2O, | Stirred at RT for 5 h | Oven dried at 60 °C for 24 h Calcined at 400 °C for 1 h | Spherical and hexagonal | 58.13 | [130] |
| 5 | Chitosan | Zn(NO3)2·6H2O | Stirred at 80 °C for 2 h | Oven dried at 80 °C for 24 h | Rods | 50–70 | [148] |
| 6 | Egg albumen | Zn(CH3COO)2 | Hydrothermal, at 85 °C for 3 h | − | Spherical and diagonal platelet | 20–60 | [149] |
| 7 | Artemia eggshell | Zn(CH3COO)2 | Hydrothermal, 180 °C for 20 h | − | Ellipsoidal | 50 | [150] |
| 8 | Crustin | Zn(CH3COO)2 | Co-precipitation, magnetically stirred for 2 h | Vacuum dried at 30 °C | 50 | [151] | |
| 9 | Amino acids | Zn(NO3)2·6H2O | Stirred at 22 °C for 30 min prior to increasing the temperature to 150 °C | Calcined at 550 °C for 3 h 20 min | Sheet-like particles | 22.46–40.29 | [152] |
| 10 | Tannic acid | Zn powder | Stirred at 70 °C for 1 h and 30 min | − | Spherical | 26–34 | [153] |
| 11 | Honey and cow urine | Zn(NO3)2·6H2O | Combustion method, further stirred at 100 °C | − | Hexagonal, leaf-like structure (cow urine) Spherical (honey) | 27 44 | [154] |
| Source | Name/Part | ZnO Morphology | Method | Concentration of ZnO NP and Microbe | MIC (μg/mL) | Test Organism and Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Antibacterial | |||||||
| Plant | Rubus fairholmianus root | Spherical | Agar well diffusion | N/A 1 × 105 CFU/mL bacteria | 157.22 | S. aureus | [100] |
| Ficus palmata. | Spherical | Agar well diffusion | N/A/ N/A | 390.6 | S. aureus (19.6 mm) B. subtilus (21.3 mm) E coli (18.6 mm) S. typhi (20.6 mm) K. pneumoniae (21.6 mm) | [73] | |
| Cochlospermum religiosum (L.) | Hexagonal | Disc diffusion | 50 μL of (100 μg) N/A N/A/ 100 μL of 1.5 × 108 CFU/mL bacteria | 4.8 to 625 | B. subtilis S. aureus E. coli P. aeruginosa | [229] | |
| Vitex trifolia L. | Spherical | Disc diffusion | 200 µg/mL N/A | 6.25–50 | ZOI= S. aureus (22.5 mm) B. subtilis (21.5 mm) P. aeruginosa (19.8 mm) P. mirabilis (19.3 mm) E. coli (20.1 mm) | [70] | |
| Mucuna pruriens | Nano-flower-like | Broth dilution assay | 100 µg/mL/ N/A | 20 | B. subtilis | [230] | |
| Phoenix roebelenii | Spherical | Agar well diffusion | 0.4 mg/mL/ N/A | - | S. typhi (21.4 mm) E. coli (15.8 mm) S. pneumoniae (15.1 mm) S. aureus (15 mm) | [169] | |
| Prunus dulcis | Near spherical | Well diffusion method | N/A/ Standard inoculum | 25 | S. aureus (18 mm) E. coli (32 mm) S. paratyphi (25 mm) K. pneumoniae (0 nm) P. mirabilius (0 nm) | [231] | |
| Algae | Hypnea musciformis | Spherical | Serial dilution | 32 mg/mL | S. aureus E. coli | [232] | |
| Phormidium, Viridiplanta, and Cosmarium sp. | Varying shapes | Well diffusion | 60 μL of | − | E. coli (20 mm) S. aureus (14.5 mm) K. pneumoniae (11 mm), C. freundii (20 mm) M. luteus (12 mm) | [233] | |
| Sargassum muticum | Agar well diffusion | 1.5 × 108 CFU/mL | 200 mg/mL | P. stutzeri A. baumannii (18 mm) B. filamentous Bacillus flexus (12 mm) | [234] | ||
| Fungi | Aspergillus fumigatus | Well diffusion | 1000 μg/mL | − | K. pneumoniae (30 mm) P. aeruginosa (27 mm) E. coli (25 mm) S. aureus (30 mm) B. subtilis (20 mm) | [132] | |
| Pichia kudriavzevii | Hexagonal | Disc diffusion | 100 μg/mL | − | B. subtilis (19 mm S. epidermidis (9 mm) S. aurous (10 mm) E. coli (10 mm) S. marcescens (mm) | [136] | |
| Saccharomyces cerevisiae | Spherical | Colony forming units | 100 μg/mL 108 CFU/mL E. coli | E. coli | [196] | ||
| Biological derivatives | Lignin | Litchi-like | Optical density | 200 μg/mL | 100 lg/mL | Inactivation of E. coli (97.54%) S. aureus (99.55%) | [157] |
| Crustin | Spherical | Agar well diffusion | 50 μg/mL | − | The inhibition zone for S. mutans (9.5 mm) L. fusiformis (4.5 mm) Proteus vulgaris 6.1 mm, A. hydrophila (7.2 mm) | [151] | |
| Honey and cow urine | Hexagonal and leaf-like | Dilution and plate count | 1 × 108 CFU/mL | − | E. coli B. subtilis | [154] | |
| Chitosan | Rods | Disc diffusion | 40 μg/mL 105 CFU/mL bacteria | − | E. coli (25.5 mm) K.pneumoniae (24.5 mm) S. aureus (22.5 mm) B. subtilis (21 mm) | [171] | |
| Antifungal | |||||||
| Plant | Pithecellobium dulce (peel) | Spherical | Broth dilution | 1000 mg/L ZnO 10 mL of nutrient potato dextrose agar/1 mL of fungal stains. | − | Growth inhibition A. flavus (63.57%) A. niger (57.17) | [83] |
| Capparis zeylanica | Spherical | Well diffusion | 100 μg/mL | − | ZOI= C. albicans (30 mm) A. niger (12 mm) | [91] | |
| Bixa orellana | Spherical Almond-shaped | Well diffusion | 10 mg/mL 104–105 CFU/mL (100 μL fungal spores) | − | Penicillium sp. Aspergillus flavus Fusarium oxysporum, Rhizoctonia solani | [235] | |
| Gelatin | Spherical | Light microscopy | 50 μg/mL | − | C. albicans | [62] | |
| Bacterial cellulose with propolis | Irregular | Disc diffusion | 0.4987/143 mL | 0.438 mg/mL | C. albicans | [236] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zikalala, N.E.; Azizi, S.; Zikalala, S.A.; Kamika, I.; Maaza, M.; Zinatizadeh, A.A.; Mokrani, T.; Kaviyarasu, K. An Evaluation of the Biocatalyst for the Synthesis and Application of Zinc Oxide Nanoparticles for Water Remediation—A Review. Catalysts 2022, 12, 1442. https://doi.org/10.3390/catal12111442
Zikalala NE, Azizi S, Zikalala SA, Kamika I, Maaza M, Zinatizadeh AA, Mokrani T, Kaviyarasu K. An Evaluation of the Biocatalyst for the Synthesis and Application of Zinc Oxide Nanoparticles for Water Remediation—A Review. Catalysts. 2022; 12(11):1442. https://doi.org/10.3390/catal12111442
Chicago/Turabian StyleZikalala, Nkosingiphile E., Shohreh Azizi, Sithembela A. Zikalala, Ilunga Kamika, Malik Maaza, Ali Akbar Zinatizadeh, Touhami Mokrani, and Kasinathan Kaviyarasu. 2022. "An Evaluation of the Biocatalyst for the Synthesis and Application of Zinc Oxide Nanoparticles for Water Remediation—A Review" Catalysts 12, no. 11: 1442. https://doi.org/10.3390/catal12111442
APA StyleZikalala, N. E., Azizi, S., Zikalala, S. A., Kamika, I., Maaza, M., Zinatizadeh, A. A., Mokrani, T., & Kaviyarasu, K. (2022). An Evaluation of the Biocatalyst for the Synthesis and Application of Zinc Oxide Nanoparticles for Water Remediation—A Review. Catalysts, 12(11), 1442. https://doi.org/10.3390/catal12111442