Crystal Structure and Properties of Gd1-xSrxCo1-yFeyO3-δ Oxides as Promising Materials for Catalytic and SOFC Application
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystal Structure of Gd1-xSrxCo1-yFeyO3-δ
2.2. Oxygen Content of Gd1-xSrxCo1-yFeyO3-δ
| at 298 K: | at 1373 K: |
2.3. Electrical Conductivity of Gd1-xSrxCo1-yFeyO3-δ
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vereshchagin, S.N.; Solovyov, L.A.; Rabchevskii, E.V.; Dudnikov, V.A.; Ovchinnikov, S.G.; Anshits, A.G. Methane oxidation over A-site ordered and disordered Sr0.8Gd0.2CoO3−δ perovskites. Chem. Commun. 2014, 50, 6112–6115. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, N.; Kuhn, J.N.; Rykov, S.A.; Millet, J.-M.M.; Ozkan, U.S. Doped LaFeO3 as SOFC catalysts: Control of oxygen mobility and oxidation activity. Catal. Today 2010, 157, 446–450. [Google Scholar] [CrossRef]
- Sheshko, T.F.; Kryuchkova, T.A.; Serov, Y.M.; Chislova, I.V.; Zvereva, I.A. New mixed perovskite-type Gd2–xSr1+xFe2O7 catalysts for dry reforming of methane, and production of light olefins. Catal. Ind. 2017, 9, 162–169. [Google Scholar] [CrossRef]
- Sheshko, T.F.; Serov, Y.M.; Kryuchkova, T.A.; Khayrullina, I.A.; Chislova, I.V.; Yafarova, L.V.; Zverev, I.A. Study of effect of preparation method and composition on the catalytic properties of complex oxides (Gd,Sr)n+1FenO3n+1 for dry reforming of methane. Nanotechnologies Russ. 2017, 12, 174–184. [Google Scholar] [CrossRef]
- Lenka, R.K.; Mahata, T.; Patro, P.K.; Tyagi, A.K.; Sinha, P.K. Synthesis and characterization of GdCoO3−δ as a potential SOFC cathode material. J. Alloys Compd. 2012, 537, 100–105. [Google Scholar] [CrossRef]
- Qiu, L.; Ichikawa, T.; Hirano, A.; Imanishi, N.; Takeda, Y. Ln1−xSrxCo1−yFeyO3−δ (Ln = Pr, Nd, Gd; x = 0.2, 0.3) for the electrodes of solid oxide fuel cells. Solid State Ion. 2003, 158, 55–65. [Google Scholar] [CrossRef]
- Takeda, Y.; Ueno, H.; Imanishi, N.; Yamamoto, O.; Sammes, N.; Philipps, M.B. Gd1−xSrxCoO3 for the electrode of solid oxide fuel cells. Solid State Ion. 1996, 86–88, 1187–1190. [Google Scholar] [CrossRef]
- Rossignol, C.; Ralph, J.M.; Bae, J.-M.; Vaughey, J.T. Ln1−xSrxCoO3 (Ln = Gd, Pr) as a cathode for intermediate-temperature solid oxide fuel cells. Solid State Ion. 2004, 175, 59–61. [Google Scholar] [CrossRef]
- Dyck, C.R.; Yu, Z.B.; Krstic, V.D. Thermal expansion matching of Gd1−xSrxCoO3−δ composite cathodes to Ce0.8Gd0.2O1.95 IT-SOFC electrolytes. Solid State Ion. 2004, 171, 17–23. [Google Scholar] [CrossRef]
- Dyck, C.R.; Yu, G.; Krstic, V.D. Synthesis and characterization of Gd1−xSrxCo1−yFeyO3−δ as a cathode material for intermediate temperature solid oxide fuel cells. Mater. Res. Soc. 2003, 801, 114–119. [Google Scholar] [CrossRef]
- Bedekar, V.; Jayakumar, O.D.; Manjanna, J.; Tyagi, A.K. Synthesis and magnetic studies of nano-crystalline GdFeO3. Mater. Lett. 2008, 62, 3793–3795. [Google Scholar] [CrossRef]
- Ryu, K.H.; Roh, K.S.; Lee, S.J.; Yo, C.H. Studies of nonstoichiometry and magnetic properties of the perovskite Gd1−xSrxCoO3−y system. J. Solid State Chem. 1993, 105, 550–560. [Google Scholar] [CrossRef]
- Gildo-Ortiz, L.; Rodríguez-Betancourtt, V.M.; Blanco-Alonso, O.; Guillén-Bonilla, A.; Guillén-Bonilla, J.T.; Guillén-Cervantes, A.; Santoyo-Salazar, J.; Guillén-Bonill, H. A simple route for the preparation of nanostructured GdCoO3 via the solution method, as well as its characterization and its response to certain gases. Results Phys. 2019, 12, 475–483. [Google Scholar] [CrossRef]
- Michel, C.R.; Martínez, A.H.; Huerta-Villalpando, F.; Morán-Lázaro, J.P. Carbon dioxide gas sensing behavior of nanostructured GdCoO3 prepared by a solution-polymerization method. J. Alloys Compd. 2009, 484, 605–611. [Google Scholar] [CrossRef]
- Balamurugana, C.; Song, S.-J.; Lee, D.-W. Porous nanostructured GdFeO3 perovskite oxides and their gas response performance to NOx. Sens. Actuators B Chem. 2018, 272, 400–414. [Google Scholar] [CrossRef]
- Yafarova, L.V.; Mamontov, G.V.; Chislova, I.V.; Silyukov, O.I.; Zvereva, I.A. The Effect of transition metal substitution in the perovskite-type oxides on the physicochemical properties and the catalytic Performance in Diesel Soot Oxidation. Catalysts 2021, 11, 1256. [Google Scholar] [CrossRef]
- Dreyer, M.; Krebs, M.; Najafishirtari, S.; Rabe, A.; Ortega, K.F.; Behrens, M. The effect of Co incorporation on the CO oxidation activity of LaFe1−xCoxO3 perovskites. Catalysts 2021, 11, 550. [Google Scholar] [CrossRef]
- Kryuchkova, T.A.; Sheshko, T.F.; Kost’, V.V.; Chislova, I.V.; Yafarov, L.V.; Zvereva, I.A.; Lyadov, A.S. Dry reforming of methane over GdFeO3-based catalysts. Pet. Chem. 2020, 60, 1052–1058. [Google Scholar] [CrossRef]
- Kryuchkova, T.A.; Kost’, V.V.; Sheshko, T.F.; Chislova, I.V.; Yafarova, L.V.; Zvereva, I.A. Effect of cobalt in GdFeO3 catalyst systems on their activity in the dry reforming of methane to synthesis Gas. Pet. Chem. 2020, 60, 609–615. [Google Scholar] [CrossRef]
- Petrov, A.N.; Kononchuk, O.F.; Andreev, A.V.; Cherepanov, V.A.; Kofstad, P. Crystal structure, electrical and magnetic properties of La1−xSrxCoO3−y. Solid State Ion. 1995, 80, 189–199. [Google Scholar] [CrossRef]
- Alhokbany, N.; Almotairi, S.; Ahmed, J.; Al-Saeedi, S.I.; Ahamad, T.; Alshehri, S.M. Investigation of structural and electrical properties of synthesized Sr-doped lanthanum cobaltite (La1−xSrxCoO3) perovskite oxide. J. King Saud Univ. Sci. 2021, 33, 101419. [Google Scholar] [CrossRef]
- Cherepanov, V.A.; Gavrilova, L.Y.; Barkhatova, L.Y.; Voronin, V.I.; Trifonova, M.V.; Bukhner, O.A. The phase equilibria in the La-Me-Co-O (Me=Ca, Sr and Ba) systems. Ionics 1998, 4, 309–315. [Google Scholar] [CrossRef]
- Park, S.; Choi, S.; Shin, J.; Kim, G. Electrochemical investigation of strontium doping effect on high performance Pr1−xSrxCoO3−δ (x = 0.1, 0.3, 0.5, and 0.7) cathode for intermediate-temperature solid oxide fuel cells. J. Power Sources 2012, 210, 172–177. [Google Scholar] [CrossRef]
- Aksenova, T.V.; Efimova, T.G.; Elkalashy, S.I.; Urusova, A.S.; Cherepanov, V.A. Phase equilibria, crystal structure and properties of complex oxides in the Nd2O3–SrO–CoO system. J. Solid State Chem. 2017, 248, 183–191. [Google Scholar] [CrossRef]
- Lee, K.T.; Manthiram, A. Characterization of Nd1−xSrxCoO3−δ (0 ≤ x ≤ 0.5) cathode materials for intermediate temperature SOFCs. J. Electrochem. Soc. 2005, 152, A197–A204. [Google Scholar] [CrossRef]
- James, M.; Tedesco, T.; Cassidy, D.J.; Withers, R.L. Oxygen vacancy ordering in strontium doped rare earth cobaltate perovskites Ln1−xSrxCoO3−δ (Ln = La, Pr and Nd; x > 0.60). Mater. Res. Bull. 2005, 40, 990–1000. [Google Scholar] [CrossRef]
- James, M.; Cassidy, D.; Goossens, D.J.; Withers, R.L. The phase diagram and tetragonal superstructures of the rare earth cobaltate phases Ln1−xSrxCoO3−δ (Ln = La3+, Pr3+, Nd3+, Sm3+, Gd3+, Y3+, Ho3+, Dy3+, Er3+, Tm3+ and Yb3+). J. Solid State Chem. 2004, 177, 1886–1895. [Google Scholar] [CrossRef]
- Volkova, N.E.; Maklakova, A.V.; Gavrilova, L.Y.; Cherepanov, V.A. Phase equilibria, crystal structure, and properties of intermediate oxides in the Sm2O3–SrO–CoO system. Eur. J. Inorg. Chem. 2017, 26, 3285–3292. [Google Scholar] [CrossRef]
- Maklakova, A.V.; Vlasova, M.A.; Volkova, N.E.; Gavrilova, L.Y.; Cherepanov, V.A. Oxygen content in oxides and subsolidus phase diagram of the Gd2O3–SrO–CoO system. J. Alloys Compd. 2021, 883, 160794. [Google Scholar] [CrossRef]
- Maklakova, A.V.; Baten’kova, A.S.; Vlasova, M.A.; Volkova, N.E.; Gavrilova, L.Y.; Cherepanov, V.A. Crystal structure, oxygen content and conductivity of Sr1−xGdxCoO3−δ. Solid State Sci. 2020, 110, 106453. [Google Scholar] [CrossRef]
- Istomin, S.Y.; Drozhzhin, O.A.; Svensson, G.; Antipov, E.V. Synthesis and characterization of Sr1−xLnxCoO3−δ, Ln = Y, Sm-Tm, 0.1 ≤ x ≤ 0.5. Solid State Sci. 2004, 6, 539–546. [Google Scholar] [CrossRef]
- Dudnikov, V.A.; Orlova, Y.S.; Kazak, N.V.; Fedorova, A.S.; Solov′yov, L.A.; Vereshchagin, S.N.; Burkov, A.T.; Novikov, S.V.; Gavrilkin, S.Y.; Ovchinnikov, S.G. Effect of A-site cation ordering on the thermoelectric properties of the complex cobalt oxides Gd1−xSrxCoO3−δ (x = 0.8 and 0.9). Ceram. Int. 2018, 44, 10299–10305. [Google Scholar] [CrossRef]
- Vereshchagin, S.N.; Dudnikov, V.A.; Shishkina, N.N.; Solovyov, L.A. Phase transformation behavior of Sr0.8Gd0.2CoO3−δ perovskite in the vicinity of order-disorder transition. Thermochim. Acta 2017, 655, 34–41. [Google Scholar] [CrossRef]
- Dudnikov, V.A.; Orlova, Y.S.; Kazak, N.V.; Fedorova, A.S.; Solov’yov, L.A.; Vereshchagin, S.N.; Burkov, A.T.; Novikov, S.V.; Ovchinnikov, S.G. Thermoelectric properties and stability of the Re0.2Sr0.8CoO3−δ (Re = Gd, Dy) complex cobalt oxides in the temperature range of 300–800 K. Ceram. Int. 2019, 45, 5553–5558. [Google Scholar] [CrossRef]
- Dudnikov, V.A.; Kazak, N.V.; Orlov, Y.S.; Vereshchagin, S.N.; Gavrilkin, S.Y.; Tsvetkov, A.Y.; Gorev, M.V.; Veligzhanin, A.A.; Trigub, A.L.; Troyanchuk, I.O.; et al. Structural, magnetic, and thermodynamic properties of ordered and disordered cobaltite Gd0.1Sr0.9CoO3–δ. J. Exp. Theor. Phys. 2019, 128, 630–640. [Google Scholar] [CrossRef]
- Tugova, E.A.; Krasilin, A.A.; Panchuk, V.V.; Semenov, V.G.; Gusarov, V.V. Subsolidus phase equilibria in the GdFeO3-SrFeO3−δ system in air. Ceram. Int. 2020, 46, 24526–24533. [Google Scholar] [CrossRef]
- Khvostova, L.V.; Volkova, N.E.; Gavrilova, L.Y.; Maignan, A.; Cherepanov, V.A. Gd2O3–SrO–Fe2O3 system: The phase diagram and oxygen content in oxides. Mater. Today Commun. 2021, 29, 102885. [Google Scholar] [CrossRef]
- Lee, K.T.; Manthiram, A. Comparison of Ln0.6Sr0.4CoO3−δ (Ln = La, Pr, Nd, Sm, and Gd) as cathode materials for intermediate temperature solid oxide fuel cells. J. Electrochem. Soc. 2006, 153, A794–A798. [Google Scholar] [CrossRef]
- Kuhn, M.; Hashimoto, S.; Sato, K.; Yashiro, K.; Mizusaki, J. Oxygen nonstoichiometry and thermo-chemical stability of La0.6Sr0.4CoO3−δ. J. Solid State Chem. 2013, 197, 38–45. [Google Scholar] [CrossRef]
- Morin, F.; Trudel, G.; Denos, Y. The phase stability of La0.5Sr0.5CoO3−δ. Solid State Ion. 1997, 96, 129–139. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Huheey, J.I. Inorganic Chemistry; Harper & Row: Manhattan, NY, USA, 1983. [Google Scholar]
- Khvostova, L.V. Phase Equilibria, Crystal Structure and Properties of Oxides in the ½Ln2O3–SrO–½Fe2O3 (Ln = Sm, Gd) Systems. Ph.D. Thesis, Ural Federal University, Yekaterinburg, Russia, 2020. [Google Scholar]
- Bucher, E.; Sitte, W.; Caraman, G.B.; Cherepanov, V.A.; Aksenova, T.V.; Ananyev, M.V. Defect equilibria and partial molar properties of (La,Sr)(Co,Fe)O3−δ. Solid State Ion. 2006, 177, 3109–3115. [Google Scholar] [CrossRef]
- Miller, R.C.; Heikes, R.R.; Mazelsky, R. Model for the electronic transport properties of mixed valency semiconductors. J. Appl. Phys. 1961, 32, 2202–2206. [Google Scholar] [CrossRef]

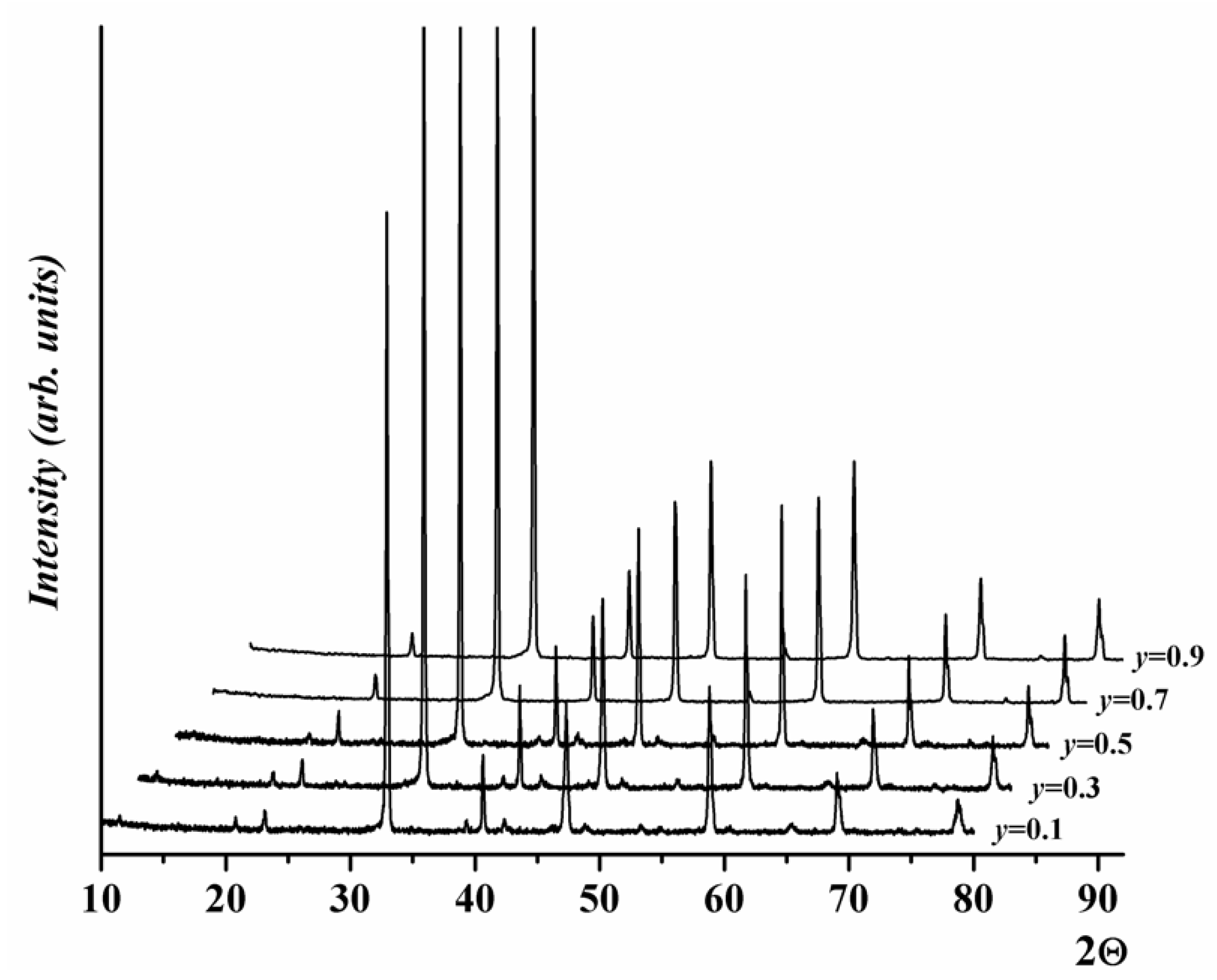
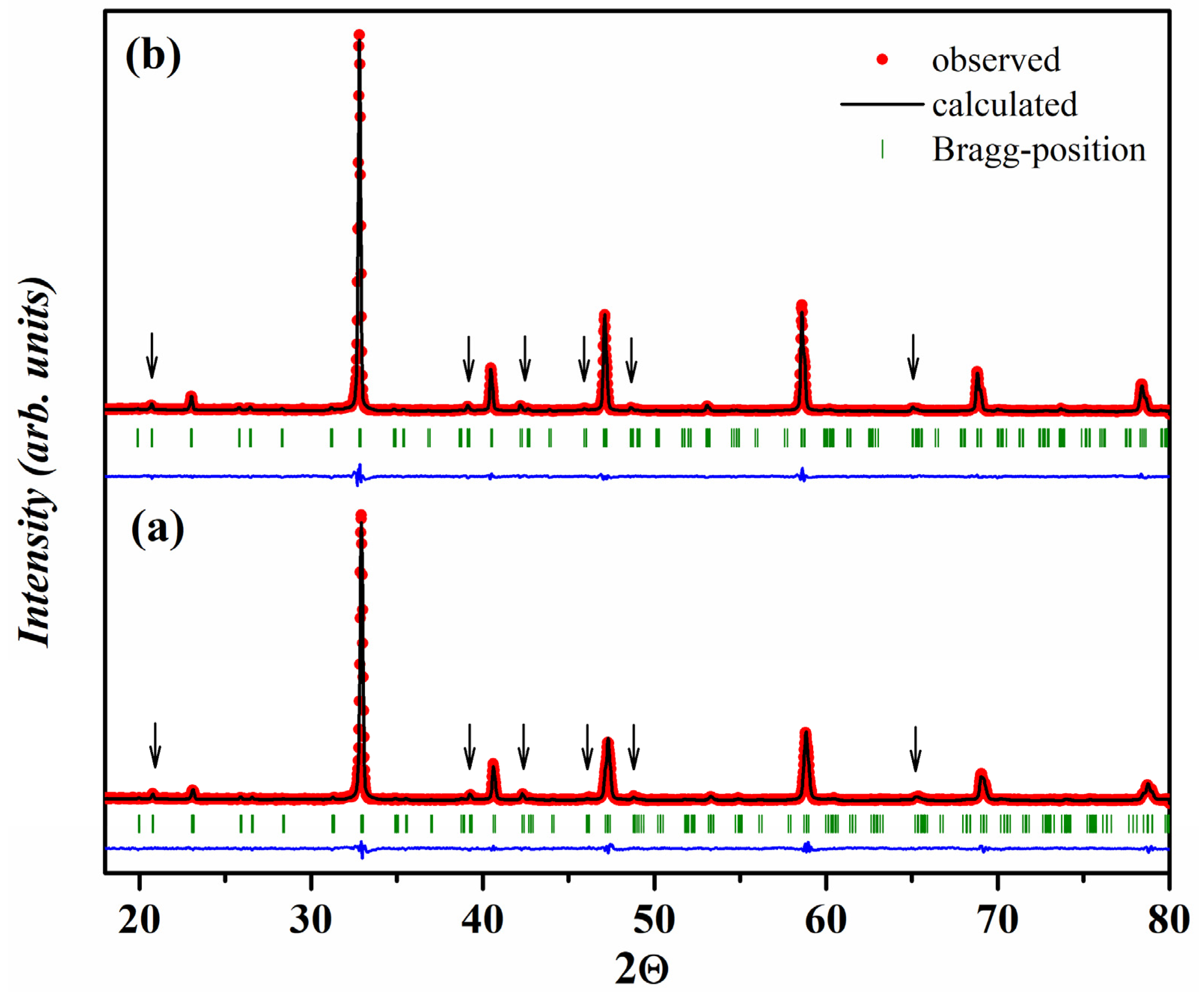
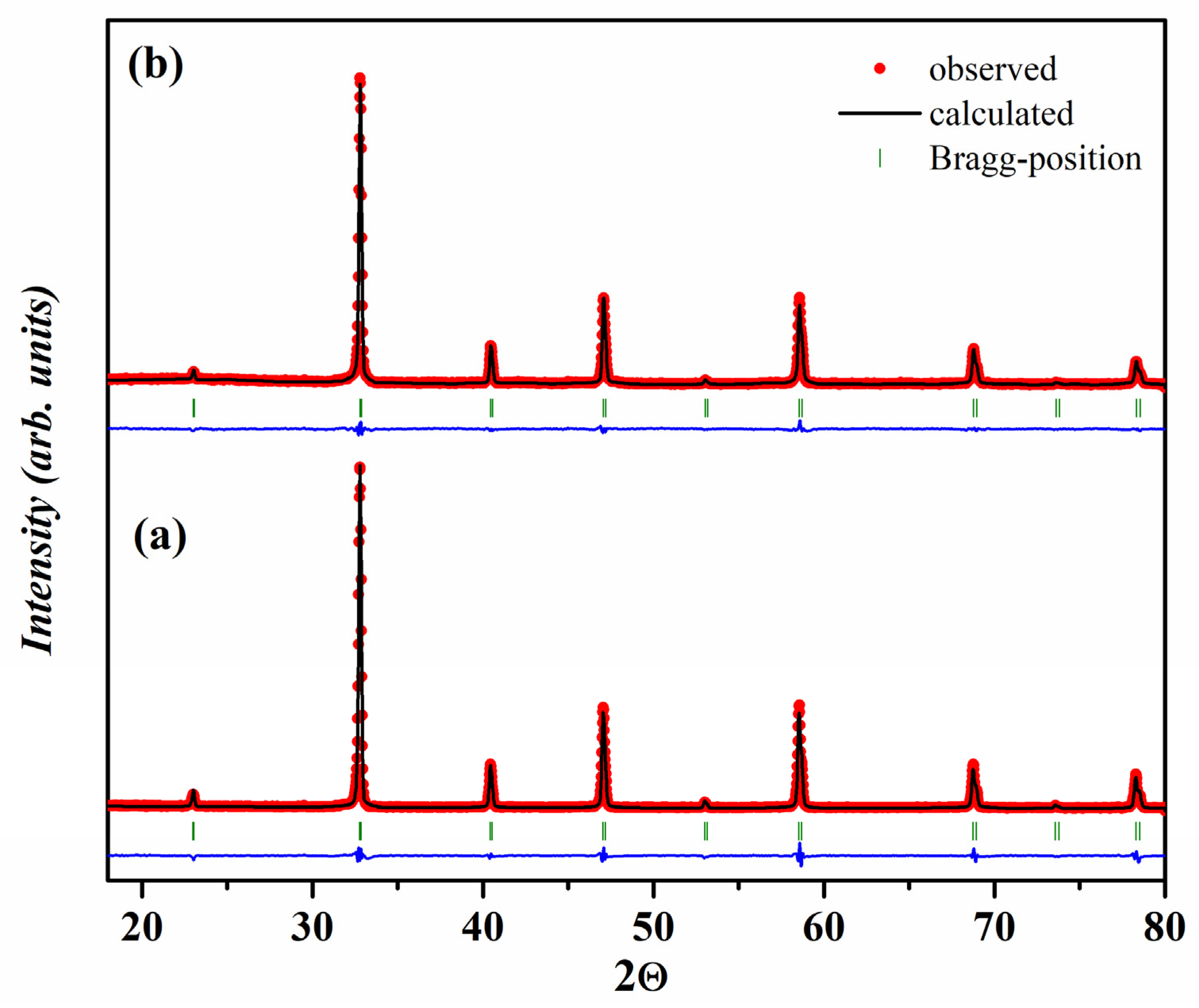
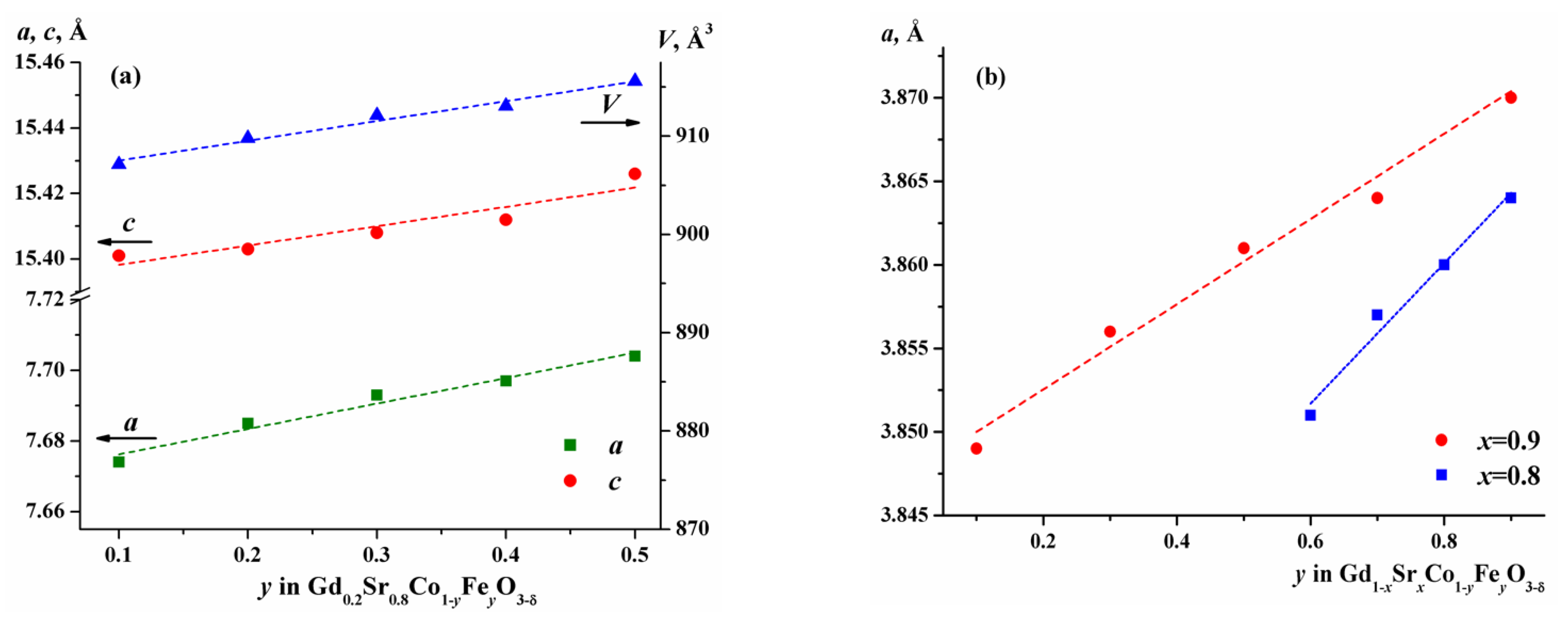
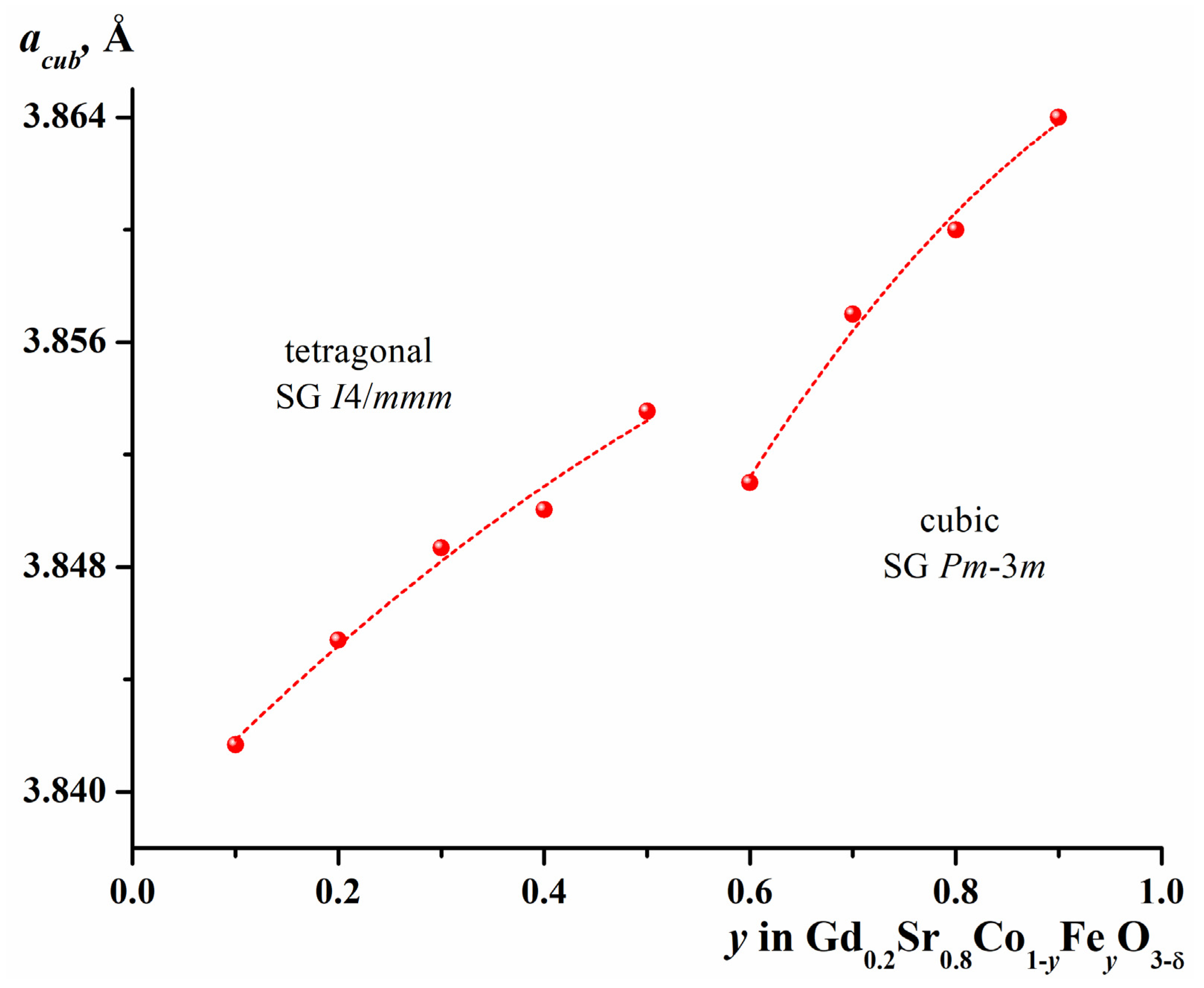
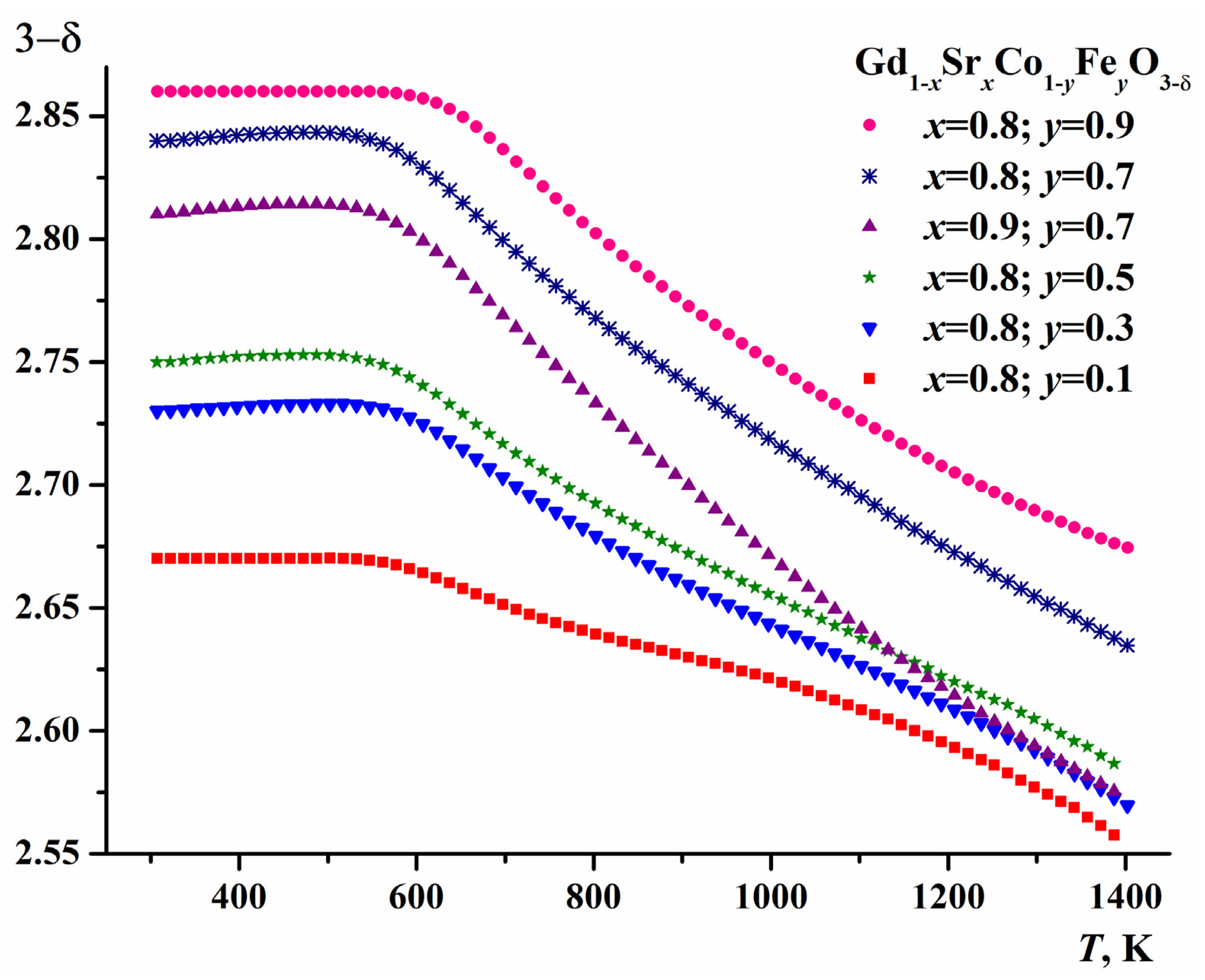
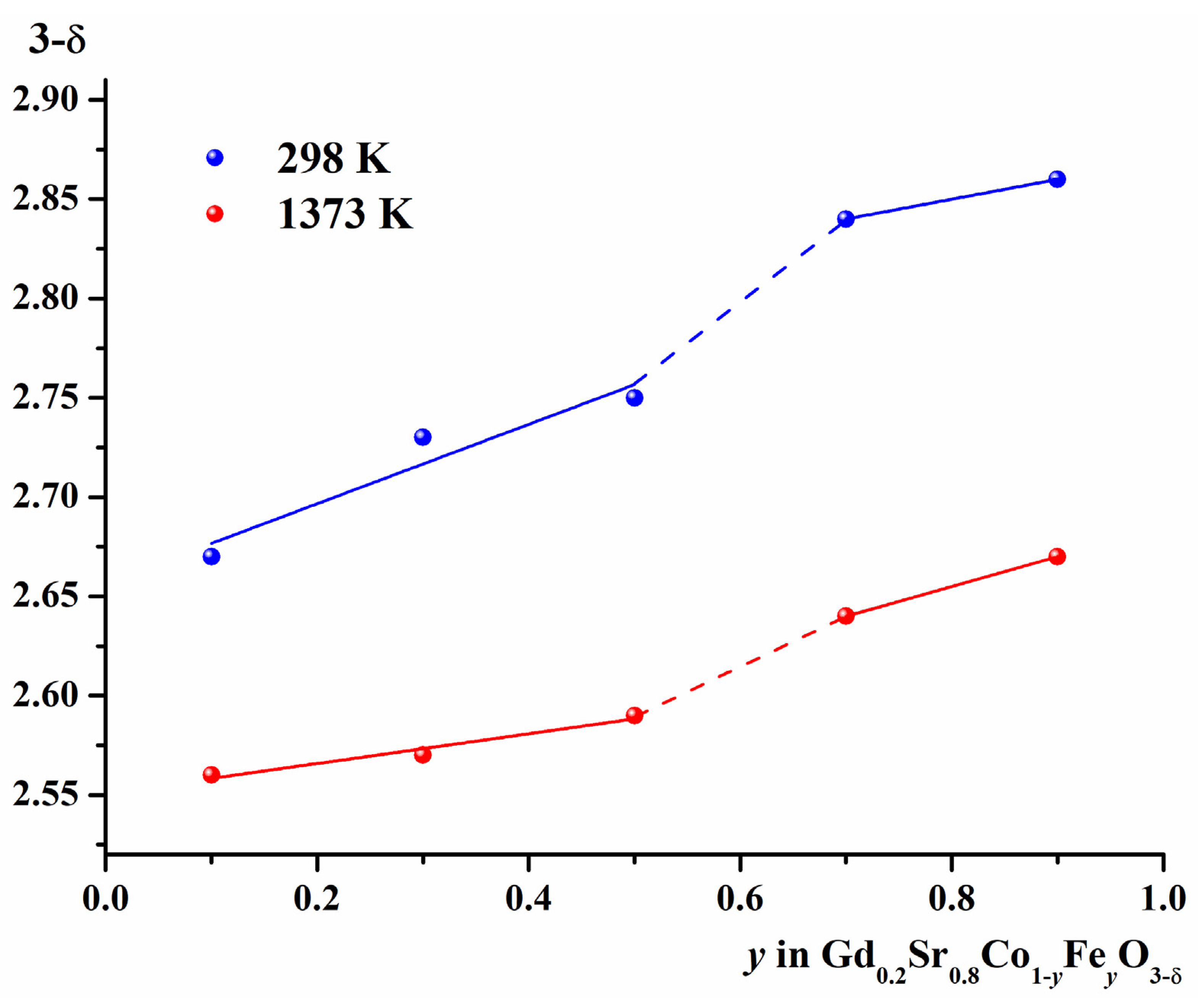
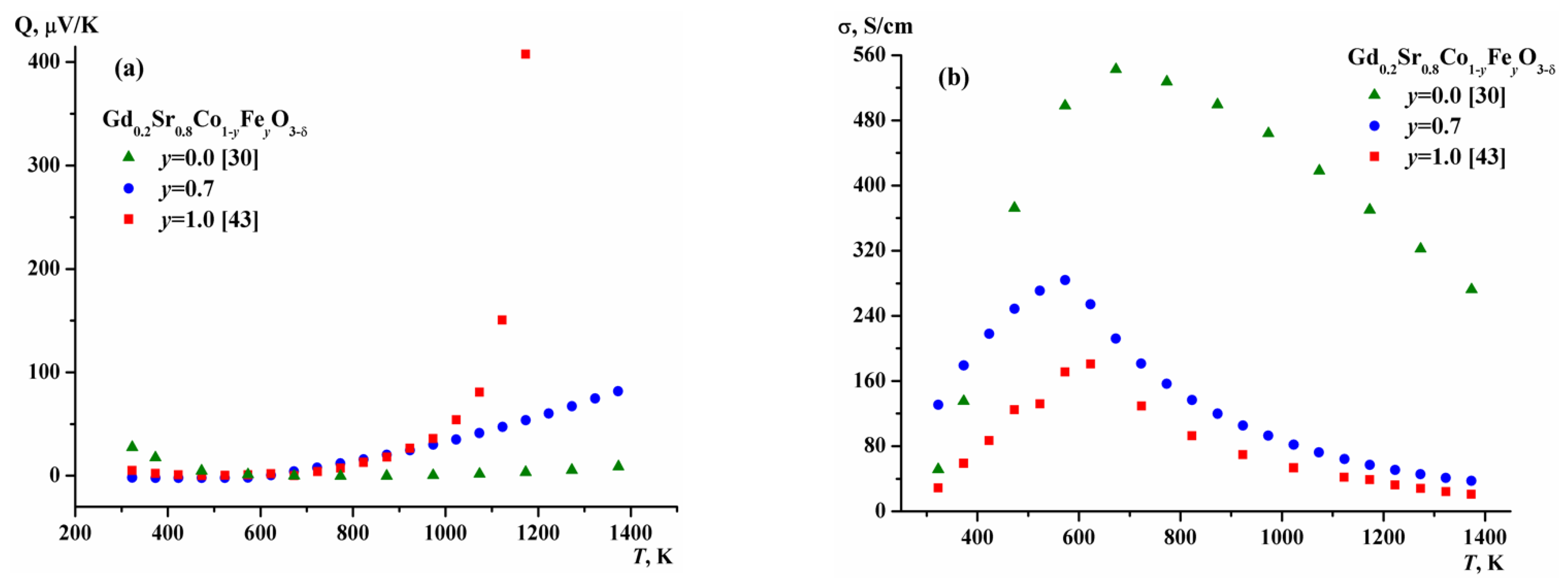
| y | a, Å | c, Å | V, (Å)3 | R-Factors, % | ||
|---|---|---|---|---|---|---|
| RBr | Rf | Rp | ||||
| 0.1 | 7.674(1) | 15.401(1) | 907.15(2) | 6.32 | 11.3 | 8.14 |
| 0.2 | 7.685(1) | 15.403(1) | 909.79(2) | 5.61 | 9.66 | 7.53 |
| 0.3 | 7.693(1) | 15.408(1) | 912.13(1) | 5.85 | 11.6 | 9.67 |
| 0.4 | 7.697(1) | 15.412(1) | 913.09(3) | 6.24 | 11.5 | 7.19 |
| 0.5 | 7.704(1) | 15.426(1) | 915.59(3) | 6.13 | 9.25 | 7.82 |
| Atom | x | y | z |
|---|---|---|---|
| Co1/Fe1 | 0.245(1) | 0.245(1) | 0 |
| Co2/Fe2 | 0.250 | 0.250 | 0.250 |
| Sr1/Gd1 | 0 | 0 | 0.138(1) |
| Sr2 | 0 | 0 | 0.627(1) |
| Sr3 | 0 | 0.5 | 0.130(1) |
| O1 | 0.221(2) | 0.221(2) | 0.117(1) |
| O2 | 0.179(1) | 0 | 0 |
| O3 | 0.211(3) | 0.5 | 0 |
| O4 | 0 | 0.238(4) | 0.251(1) |
| SG Pm-3m: Gd/Sr (0.5;0.5;0.5); Fe/Co (0; 0; 0); O (0.5; 0; 0) | R-Factors, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | a, Å | V, (Å)3 | dFe/Co-O, Å | dGd/Sr-O, Å | dGd/Sr-Fe/Co, Å | RBr | Rf | Rp |
| 0.8 | 0.6 | 3.851(1) | 57.11(1) | 1.925(1) | 2.723(1) | 3.335(1) | 5.06 | 4.84 | 8.46 |
| 0.7 | 3.857(1) | 57.40(1) | 1.928(1) | 2.727(1) | 3.340(1) | 3.79 | 4.85 | 7.04 | |
| 0.8 | 3.860(1) | 57.52(1) | 1.930(1) | 2.729(1) | 3.343(1) | 5.35 | 4.19 | 11.6 | |
| 0.9 | 3.864(1) | 57.72(1) | 1.932(1) | 2.732(1) | 3.346(1) | 4.52 | 3.93 | 9.60 | |
| 0.9 | 0.1 | 3.849(1) | 57.03(1) | 1.924(1) | 2.721(1) | 3.333(1) | 5.46 | 4.24 | 9.58 |
| 0.3 | 3.856(1) | 57.36(1) | 1.928(1) | 2.727(1) | 3.340(1) | 2.49 | 2.21 | 7.24 | |
| 0.5 | 3.861(1) | 57.59(1) | 1.930(1) | 2.730(1) | 3.344(1) | 5.95 | 3.93 | 7.88 | |
| 0.7 | 3.864(1) | 57.70(1) | 1.932(1) | 2.732(1) | 3.346(1) | 3.33 | 2.41 | 9.59 | |
| 0.9 | 3.870(1) | 57.99(1) | 1.934(1) | 2.734(1) | 3.348(1) | 1.11 | 2.98 | 7.90 | |
| Composition | T, K | Oxygen Content 3-δ | Mean Oxidation State of 3d-Transition Metals |
|---|---|---|---|
| Gd0.2Sr0.8Co0.9Fe0.1O3-δ | 298 1373 | 2.67 ± 0.01 2.56 ± 0.01 | 3.14 2.92 |
| Gd0.2Sr0.8Co0.7Fe0.3O3-δ | 298 1373 | 2.73 ± 0.01 2.57 ± 0.01 | 3.26 2.94 |
| Gd0.2Sr0.8Co0.5Fe0.5O3-δ | 298 1373 | 2.75 ± 0.01 2.59 ± 0.01 | 3.30 2.98 |
| Gd0.2Sr0.8Co0.3Fe0.7O3-δ | 298 1373 | 2.84 ± 0.01 2.64 ± 0.01 | 3.48 3.08 |
| Gd0.2Sr0.8Co0.1Fe0.9O3-δ | 298 1373 | 2.86 ± 0.01 2.67 ± 0.01 | 3.52 3.14 |
| Gd0.1Sr0.9Co0.3Fe0.7O3-δ | 298 1373 | 2.81 ± 0.01 2.58 ± 0.01 | 3.52 3.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aksenova, T.V.; Mysik, D.K.; Cherepanov, V.A. Crystal Structure and Properties of Gd1-xSrxCo1-yFeyO3-δ Oxides as Promising Materials for Catalytic and SOFC Application. Catalysts 2022, 12, 1344. https://doi.org/10.3390/catal12111344
Aksenova TV, Mysik DK, Cherepanov VA. Crystal Structure and Properties of Gd1-xSrxCo1-yFeyO3-δ Oxides as Promising Materials for Catalytic and SOFC Application. Catalysts. 2022; 12(11):1344. https://doi.org/10.3390/catal12111344
Chicago/Turabian StyleAksenova, Tatiana V., Darya K. Mysik, and Vladimir A. Cherepanov. 2022. "Crystal Structure and Properties of Gd1-xSrxCo1-yFeyO3-δ Oxides as Promising Materials for Catalytic and SOFC Application" Catalysts 12, no. 11: 1344. https://doi.org/10.3390/catal12111344
APA StyleAksenova, T. V., Mysik, D. K., & Cherepanov, V. A. (2022). Crystal Structure and Properties of Gd1-xSrxCo1-yFeyO3-δ Oxides as Promising Materials for Catalytic and SOFC Application. Catalysts, 12(11), 1344. https://doi.org/10.3390/catal12111344








