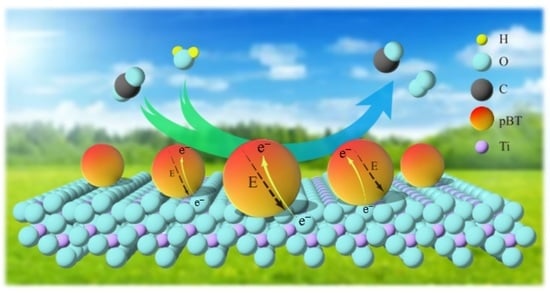
Enhanced CO2 Photoreduction over Bi2Te3/TiO2 Nanocomposite via a Seebeck Effect
Abstract
1. Introduction
2. Results and Discussion
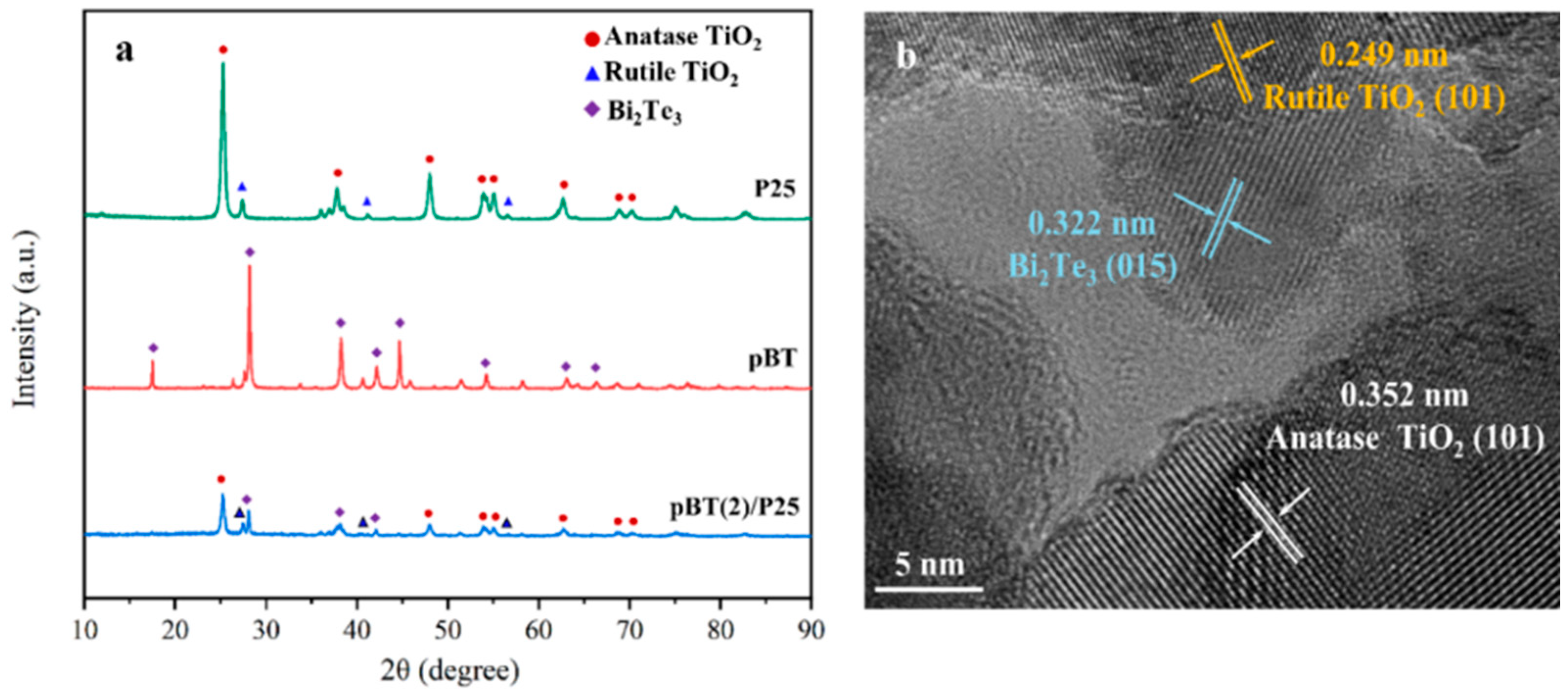
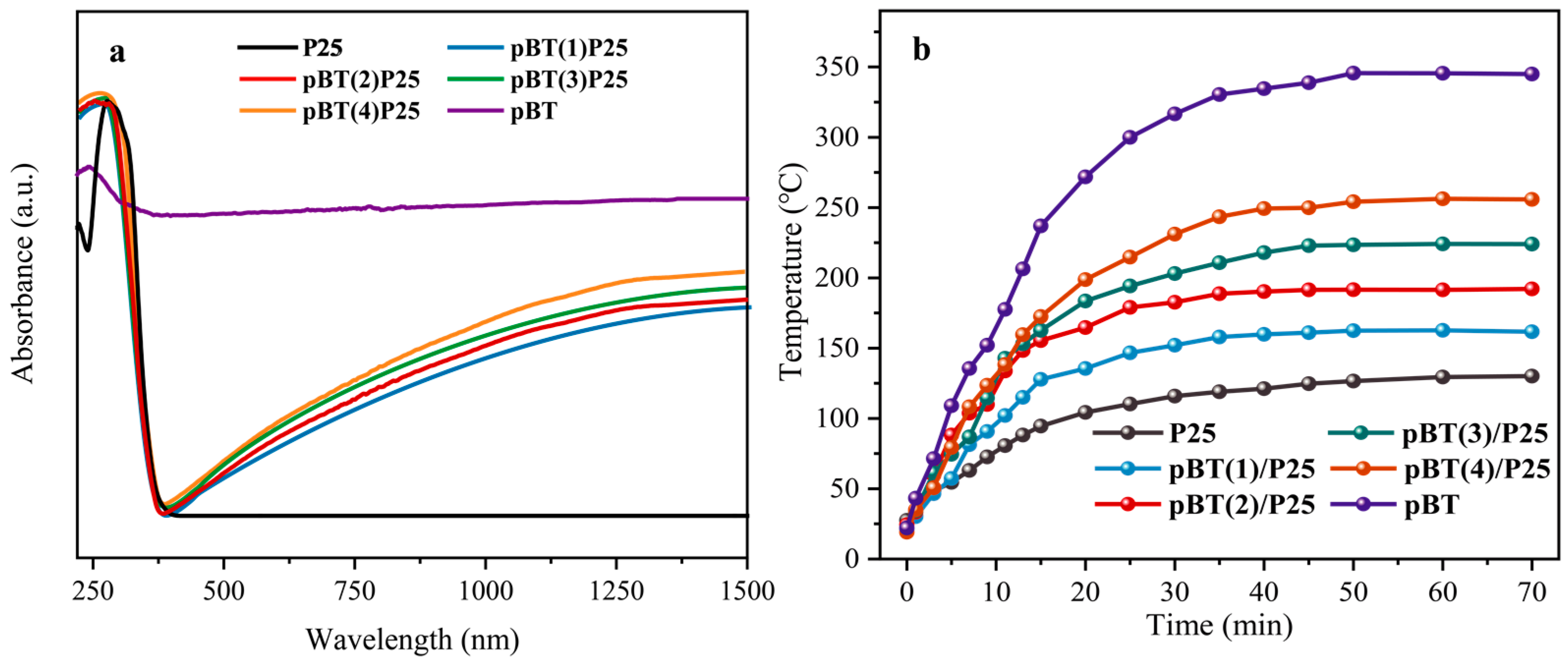
2.1. Structural, Morphological, and Optical Characterizations
2.2. Temperature Gradient and Electric Potential Simulation
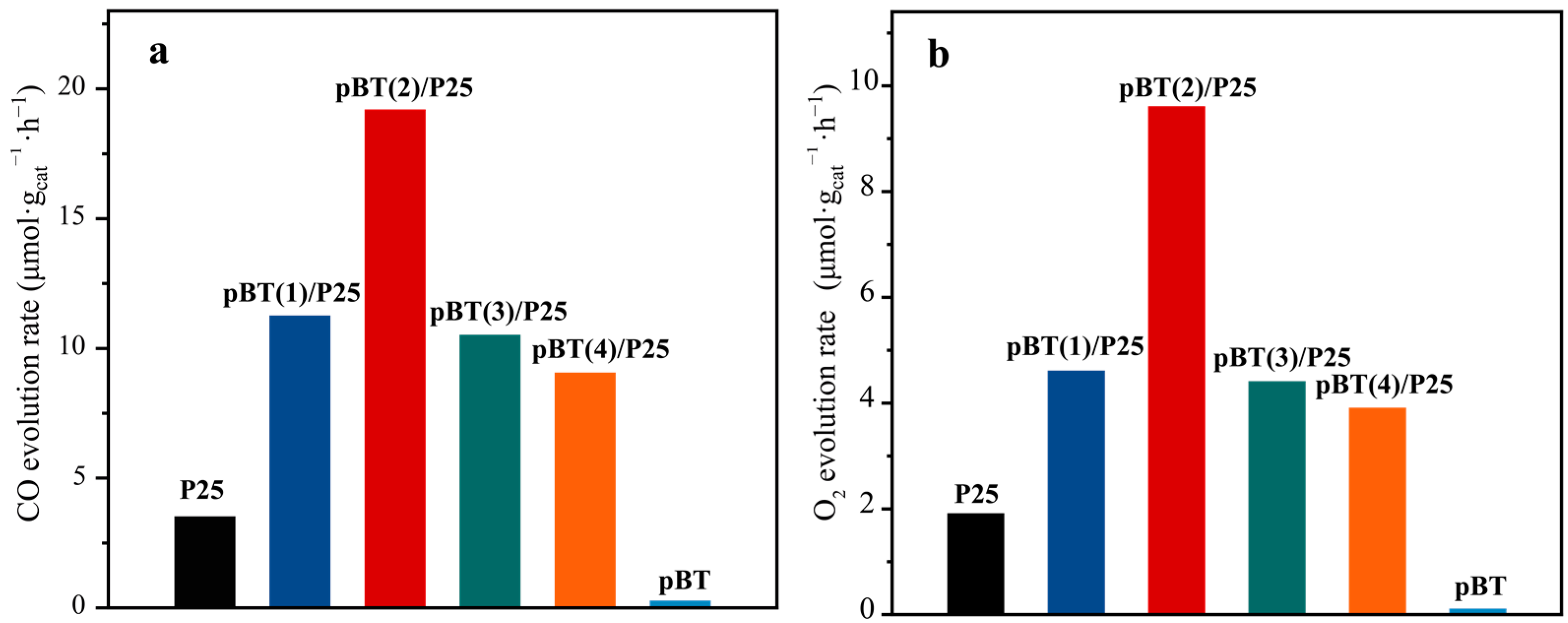
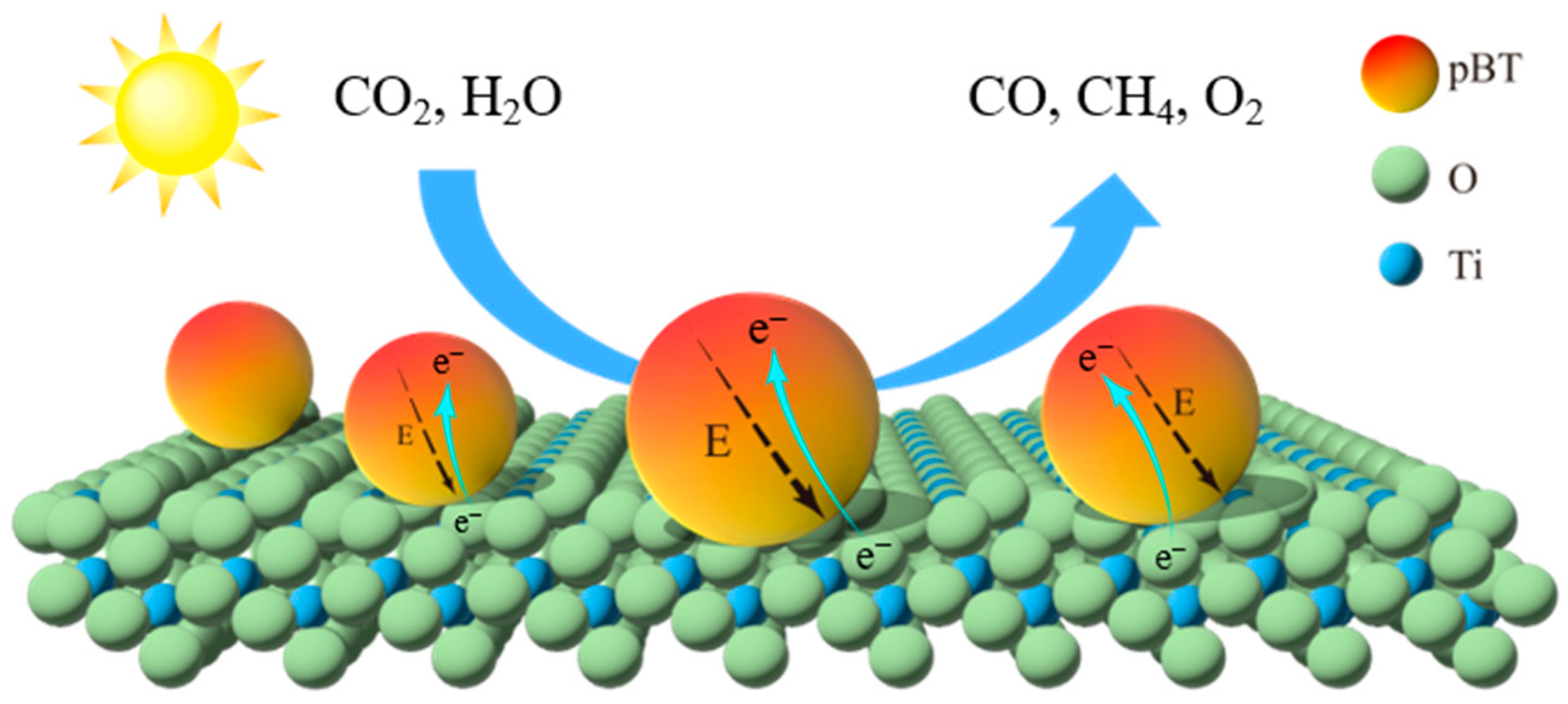
2.3. CO2 Photoreduction Performances
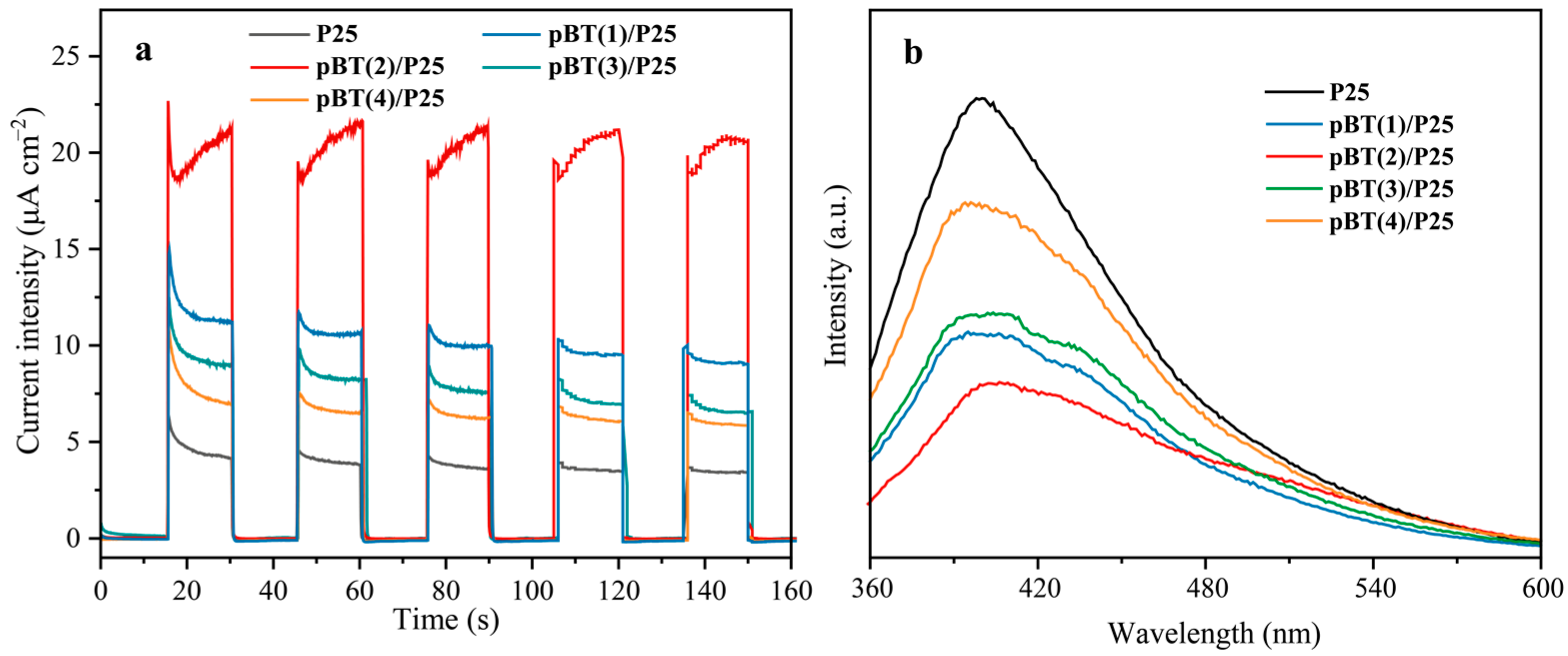
2.4. Photoelectrochemical and Photoluminescence Properties
3. Materials and Methods
3.1. Chemicals and Synthesis
3.2. Materials Characterization
3.3. CO2 Photoreduction Evaluation
3.4. Photoelectrochemical Measurement
3.5. COMSOL Multiphysics Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bibi, M.; Ullah, R.; Sadiq, M.; Sadiq, S.; Khan, I.; Saeed, K.; Zia, M.A.; Iqbal, Z.; Ullah, I.; Iqbal, Z.; et al. Catalytic hydrogenation of carbon dioxide over magnetic nanoparticles: Modification in fixed-bed reactor. Catalysts 2021, 11, 592. [Google Scholar] [CrossRef]
- Zahedi, G.; Elkamel, A.; Lohi, A. Dynamic optimization strategies of a heterogeneous reactor for CO2 conversion to methanol. Energy Fuels 2007, 21, 2977–2983. [Google Scholar] [CrossRef]
- Shi, L.; Liu, H.; Ning, S.; Ye, J. Localized surface plasmon resonance effect enhanced Cu/TiO2 core–shell catalyst for boosting CO2 hydrogenation reaction. Catal. Sci. Technol. 2022, 12, 6155–6162. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Mebrahtu, C.; Xi, X.; Liao, L.; Ren, J.; Xie, J.; Heeres, H.J.; Palkovits, R. Catalysts design for higher alcohols synthesis by CO2 hydrogenation: Trends and future perspectives. Appl. Catal. B Environ. 2021, 291, 120073. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Tan, L.L.; Ong, W.J.; Chai, S.P.; Mohamed, A.R. Photocatalytic reduction of CO2 with H2O over graphene oxide-supported oxygen-rich TiO2 hybrid photocatalyst under visible light irradiation: Process and kinetic studies. Chem. Eng. J. 2017, 308, 248–255. [Google Scholar] [CrossRef]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward solar fuels: Photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, X.; Zheng, Y. Enhanced photocatalytic reduction of CO2 to methanol by ZnO nanoparticles deposited on ZnSe nanosheet. Chem. Phys. Lett. 2018, 693, 170–175. [Google Scholar] [CrossRef]
- Ye, R.P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. Double-slit photoelectron interference in strong-field ionization of the neon dimer. Nat. Commun. 2019, 10, 1. [Google Scholar]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Shanmugaratnam, S.; Selvaratnam, B.; Baride, A.; Koodali, R.; Ravirajan, P.; Velauthapillai, D.; Shivatharsiny, Y. SnS2/TiO2 nanocomposites for hydrogen production and photodegradation under extended solar irradiation. Catalysts 2021, 11, 589. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, D.; Shen, S.; Wan, J.; Zheng, X.; Yu, L.; Phillips, D.L. The photocatalytic activity and degradation mechanism of methylene blue over copper (ii) tetra (4-carboxyphenyl) porphyrin sensitized TiO2 under visible light irradiation. RSC Adv. 2014, 4, 28978–28986. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Corma, A.; Garcia, H. Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 2012, 5, 9217. [Google Scholar] [CrossRef]
- Hao, L.; Kang, L.; Huang, H.; Ye, L.; Han, K.; Yang, S.; Yu, H.; Batmunkh, M.; Zhang, Y.; Ma, T. Surface-halogenation-induced atomic-site activation and local charge separation for superb CO2 photoreduction. Adv. Mater. 2019, 31, 1900546. [Google Scholar] [CrossRef]
- Chen, F.; Ma, Z.; Ye, L.; Ma, T.; Zhang, T.; Zhang, Y.; Huang, H. Macroscopic spontaneous polarization and surface oxygen vacancies collaboratively boosting CO2 photoreduction on BiOIO3 single crystals. Adv. Mater. 2020, 32, 1908350. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, W.; Wang, X.; Zhang, X.; Chen, H.; Hu, H.; Liu, L.; Ye, J.; Wang, D. Enhanced photocatalytic CO2 reduction over TiO2 using metalloporphyrin as the cocatalyst. Catalysts 2020, 10, 654. [Google Scholar] [CrossRef]
- Meng, X.; Wang, T.; Liu, L.; Ouyang, S.; Li, P.; Hu, H.; Kako, T.; Iwai, H.; Tanaka, A.; Ye, J. Photothermal conversion of CO2 into CH4 with H2 over group viii nanocatalysts: An alternative approach for solar fuel production. Angew. Chem. Int. Ed. 2014, 53, 11478–11482. [Google Scholar] [CrossRef]
- Liu, H.; Dao, T.; Liu, L.; Meng, X.; Nagao, T.; Ye, J. Light assisted CO2 reduction with methane over group VIII metals: Universality of metal localized surface plasmon resonance in reactant activation. Appl. Catal. B: Environ. 2017, 209, 183–189. [Google Scholar] [CrossRef]
- Meng, X.; Liu, L.; Ouyang, S.; Xu, H.; Wang, D.; Zhao, N.; Ye, J. Nanometals for solar-to-chemical energy conversion: From semiconductor-based photocatalysis to plasmon-mediated photocatalysis and photo-thermocatalysis. Adv. Mater. 2016, 28, 6781–6803. [Google Scholar] [CrossRef]
- Qi, Y.; Song, L.; Ouyang, S.; Liang, X.; Ning, S.; Zhang, Q.; Ye, J. Photoinduced defect engineering: Enhanced photothermal catalytic performance of 2D black In2O3−x nanosheets with bifunctional oxygen vacancies. Adv. Mater. 2020, 32, 1903915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Sun, J.; Chen, H.; Zhu, Y.; Zhao, X.; Zhang, L.; Wang, S.; Zhang, H.; Duan, X.; et al. Regulation of energetic hot carriers on Pt/TiO2 with thermal energy for photothermal catalysis. Appl. Catal. B Environ. 2022, 133, 121263. [Google Scholar] [CrossRef]
- Wang, W.; An, W.J.; Ramalingam, B.; Mukherjee, S.; Niedzwiedzki, D.M.; Gangopadhyay, S.; Biswas, P. Size and structure matter: Enhanced CO2 photoreduction efficiency by size-resolved ultrafine Pt nanoparticles on TiO2 single crystals. J. Am. Chem. Soc. 2012, 134, 11276–11281. [Google Scholar] [CrossRef]
- Liu, Z.; Niu, L.; Zong, X.; An, L.; Qu, D.; Wang, X.; Sun, Z. Ambient photothermal catalytic CO oxidation over a carbon-supported palladium catalyst. Appl. Catal. B Environ. 2022, 313, 121439. [Google Scholar] [CrossRef]
- Soltani, T.; Zhu, X.; Yamamoto, A.; Singh, S.P.; Fudo, E.; Tanaka, A.; Kominami, H.; Yoshida, H. Effect of transition metal oxide cocatalyst on the photocatalytic activity of Ag loaded CaTiO3 for CO2 reduction with water and water splitting. Appl. Catal. B Environ. 2021, 52, 119899. [Google Scholar] [CrossRef]
- Neatu, S.; Macia-Agullo, J.A.; Concepcion, P.; Garcia, H. Gold-copper nanoalloys supported on TiO2 as photocatalysts for CO2 reduction by water. J. Am. Chem. Soc. 2014, 136, 15969–15976. [Google Scholar] [CrossRef] [PubMed]
- AlOtaibi, B.; Fan, S.; Wang, D.; Ye, J.; Mi, Z. Wafer-level artificial photosynthesis for CO2 reduction into CH4 and Co using GaN nanowires. ACS Catal. 2015, 5, 5342–5348. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, T.; Li, P.; Liu, L.; Chang, K.; Li, M.; Ye, J. Photocatalytic reduction of carbon dioxide by hydrous hydrazine over Au–Cu alloy nanoparticles supported on SrTiO3/TiO2 coaxial nanotube arrays. Angew. Chem. Int. Ed. 2015, 54, 841–845. [Google Scholar] [CrossRef]
- Varghese, O.K.; Paulose, M.; LaTempa, T.J.; Grimes, C.A. High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano Lett. 2009, 9, 731–737. [Google Scholar] [CrossRef]
- Ye, M.; Wang, X.; Liu, E.; Ye, J.; Wang, D. Boosting the photocatalytic activity of P25 for carbon dioxide reduction by using a surface-alkalinized titanium carbide mxene as cocatalyst. ChemSusChem 2018, 11, 1606–1611. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Chen, H.; Zhang, Q.; Hu, H.; Liu, L.; Ye, J.; Wang, D. A surface-alkalinized Ti3C2 MXene as an efficient cocatalyst for enhanced photocatalytic CO2 reduction over ZnO. Catal. Sci. Technol. 2021, 11, 4953–4961. [Google Scholar] [CrossRef]
- Li, K.; Chen, T.; Yan, L.; Dai, Y.; Huang, Z.; Guo, H.; Jiang, L.; Gao, X.; Xiong, J.; Song, D. Synthesis of mesoporous graphene and tourmaline co-doped titania composites and their photocatalytic activity towards organic pollutant degradation and eutrophic water treatment. Catal. Commun. 2012, 28, 196–201. [Google Scholar] [CrossRef]
- Yin, L.; Zhao, M.; Hu, H.; Ye, J.; Wang, D. Synthesis of graphene/tourmaline/TiO2 composites with enhanced activity for photocatalytic degradation of 2-propanol. Chin. J. Catal. 2017, 38, 1307–1314. [Google Scholar] [CrossRef]
- Pang, F.; Zhang, R.; Lan, D.; Ge, J. Synthesis of magnetite-semiconductor-metal trimer nanoparticles through functional modular assembly: A magnetically separable photocatalyst with photothermic enhancement for water reduction. ACS Appl. Mater. Interfaces 2018, 10, 4929–4936. [Google Scholar] [CrossRef]
- Tan, J.; Wang, X.; Hou, W.; Zhang, X.; Liu, L.; Ye, J.; Wang, D. Fabrication of Fe3O4@graphene/TiO2 nanohybrid with enhanced photocatalytic activity for isopropanol degradation. J. Alloy Compd. 2019, 792, 918–927. [Google Scholar] [CrossRef]
- Li, F.; Wang, H.; Huang, R.; Chen, W.; Zhang, H. Recent advances in SnSe nanostructures beyond thermoelectricity. Adv. Funct. Mater. 2022, 32, 2200516. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, M.; Dong, H.; Wang, L.; Hou, T.; Li, Y. Monolayer group IVA monochalcogenides as potential and efficient catalysts for the oxygen reduction reaction from first-principles calculations. J. Mater. Chem. A 2017, 5, 1734. [Google Scholar] [CrossRef]
- Rajamathi, C.R.; Gupta, U.; Pal, K.; Kumar, N.; Yang, H.; Sun, Y.; Shekhar, C.; Yan, B.; Parkin, S.; Waghmare, U.V.; et al. Photochemical water splitting by bismuth chalcogenide topological insulators. ChemPhysChem 2017, 18, 2322–2327. [Google Scholar] [CrossRef]
- Li, X.; Zuo, X.; Jiang, X.; Li, D.; Cui, B.; Liu, D. Enhanced photocatalysis for water splitting in layered tin chalcogenides with high carrier mobility. Phys. Chem. Chem. Phys. 2019, 21, 7559. [Google Scholar] [CrossRef]
- Wu, Q.; Wei, W.; Lv, X.; Huang, B.; Dai, Y. Computational screening of defective group IVA monochalcogenides as efficient catalysts for hydrogen evolution reaction. J. Phys. Chem. C 2019, 123, 11791–11797. [Google Scholar] [CrossRef]
- Chen, P.; Dai, X.; Xing, P.; Zhao, X.; Zhang, Q.; Ge, S.; Si, J.; Zhao, L.; He, Y. Microwave heating assisted synthesis of novel SnSe/g-C3N4 composites for effective photocatalytic H2 production. J. Ind. Eng. Chem. 2019, 80, 74. [Google Scholar] [CrossRef]
- Wu, D.; Guo, J.; Ge, Z.; Feng, J. Facile Synthesis Bi2Te3 based nanocomposites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Nanomaterials 2021, 11, 3390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zheng, F.; Huang, B.; Ji, Y.; Shao, Q.; Li, Y.; Xiao, X.; Huang, X. Exploring Bi2Te3 nanoplates as versatile catalysts for electrochemical reduction of small molecules. Adv. Mater. 2020, 32, 1906477. [Google Scholar] [CrossRef]
- Shim, X.; Zoum, J.; Chenm, Z. Advanced thermoelectric design: From materials and structures to devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar]
- Ryu, B. Work function of bismuth telluride: First-principles approach. J. Korean Phys. Soc. 2018, 72, 122–128. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, G.; Li, S.J. The doping effect of Bi on TiO2 for photocatalytic hydrogen generation and photodecolorization of rhodamine B. J. Phys. Chem. C. 2009, 113, 9950–9955. [Google Scholar] [CrossRef]
- Patil, P.; Mali, S.; Kondalkar, V.; Mane, R.; Patil, P.S.; Hong, C.; Bhosale, P. Bismuth telluride quantum dot assisted titanium oxide microflowers for efficient photoelectrochemical performance. Mater. Lett. 2015, 159, 177–181. [Google Scholar] [CrossRef]
- Kim, S.; We, J.; Cho, B. A wearable thermoelectric generator fabricated on a glass fabric. Energy Environ. Sci. 2014, 7, 1959–1965. [Google Scholar] [CrossRef]
- Yang, L.; Wang, C.; Lin, S.; Chen, T.; Cao, Y.; Zhang, P.; Liu, X. Thermal conductivity of TiO2 nanotube: A molecular dynamics study. J. Phys.: Condens. Matter 2019, 31, 055302. [Google Scholar] [CrossRef]
- Muñoz Rojo, M.; Abad, B.; Manzano, C.; Torres, P.; Cartoixà, X.; Alvarezb, F.; Martín Gonzalez, M. Thermal conductivity of Bi2Te3 nanowires: How size affects phonon scattering. Nanoscale 2017, 9, 6741–6747. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jiang, P.; Bao, X. Phonon-enhanced photothermoelectric effect in SrTiO3 ultra-broadband photodetector. Nat. Commun. 2019, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Bell, L. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guai, G.H.; Gong, C.; Hu, W.; Zhu, J.; Yang, H.; Yan, Q.; Li, C. Thermoelectric Bi2Te3-improved charge collection for high-performance dye-sensitized solar cells. Energy Environ. Sci. 2012, 5, 6294–6298. [Google Scholar] [CrossRef]
- He, H.; Zhang, C.; Liu, T.; Cao, Y.; Wang, N.; Guo, Z. Thermoelectric–photoelectric composite nanocables induced a larger efficiency in dye-sensitized solar cells. J. Mater. Chem. A 2016, 4, 9362–9369. [Google Scholar] [CrossRef]
- Jia, Z.; Ning, S.; Tong, Y.; Chen, X.; Hu, H.; Liu, L.; Ye, J.; Wang, D. Selective photothermal reduction of CO2 to CO over Ni-nanoparticle/N-doped CeO2 nanocomposite catalysts. ACS Appl. Nano Mater. 2021, 4, 10485–10494. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Shi, R.; Waterhouse, G.I.N.; Wen, X.; Zhang, T. Fe-based catalysts for the direct photohydrogenation of CO2 to value-added hydrocarbons. Adv. Energy Mater. 2021, 11, 2002783. [Google Scholar] [CrossRef]
- Han, B.; Wei, W.; Chang, L.; Cheng, P.; Hu, Y. Efficient visible light photocatalytic CO2 reforming of CH4. ACS Catal. 2016, 6, 494–497. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wang, D.; Ye, J. Conformal BiVO4-layer/WO3-nanoplate-array heterojunction photoanode modified with cobalt phosphate cocatalyst for significantly enhanced photoelectrochemical performances. ACS Appl. Mater. Interfaces 2019, 11, 5623–5631. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Ku, S.; Luo, C.; Lin, J.; Wu, K.; Juang, J.; Kobayashi, T.; Cheng, C.; Tsuei, K.; et al. Manifestation of a second dirac surface state and bulk bands in THz radiation from topological insulators. Sci. Rep. 2015, 5, 14128. [Google Scholar]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/Gold nanocomposites. Effect of metal particle size on the fermi level equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Jakob, M.; Levanon, H.; Kamat, P.V. Charge distribution between UV-Irradiated TiO2 and gold nanoparticles: Determination of shift in the fermi level. Nano Lett. 2003, 3, 353–358. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Wang, Z.; Li, M.; Su, W.; Zhao, M.; Huang, S.; Xia, S.; Wang, C. Accurate measurement of Seebeck coefficient. Rev. Sci. Instrum. 2016, 87, 064701. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Walsh, A.G.; Li, D.; Do, D.; Ma, H.; Wang, C.; Zhang, P.; Zhang, X. W-Doped TiO2 for photothermocatalytic CO2 reduction. Nanoscale 2020, 12, 17245–17252. [Google Scholar] [CrossRef]
- Li, G.; Sun, Y.; Zhang, Q.; Gao, Z.; Sun, W.; Zhou, X. Ag quantum dots modified hierarchically porous and defective TiO2 nanoparticles for improved photocatalytic CO2 reduction. Chem. Eng. J. 2021, 410, 128397. [Google Scholar] [CrossRef]
- Sun, Y.; Li, G.; Gong, Y.; Sun, Z.; Yao, H.; Zhou, X. Ag and TiO2 nanoparticles co-modified defective zeolite TS-1 for improved photocatalytic CO2 reduction. J. Hazard. Mater. 2021, 403, 124019. [Google Scholar] [CrossRef]
- Xing, M.; Zhou, Y.; Dong, C.; Cai, L.; Zeng, L.; Shen, B.; Pan, L.; Dong, C.; Chai, Y.; Zhang, J.; et al. Modulation of the reduction potential of TiO2−x by fluorination for efficient and selective CH4 generation from CO2 photoreduction. Nano Lett. 2018, 18, 3384–3390. [Google Scholar] [CrossRef]
- Feng, S.; Zhao, J.; Bai, Y.; Liang, X.; Wang, T.; Wang, C. Facile synthesis of Mo-doped TiO2 for selective photocatalytic CO2 reduction to methane: Promoted H2O dissociation by Mo doping. J. CO2 Util. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Bian, J.; Qu, Y.; Zhang, X.; Sun, N.; Tang, D.; Jing, L. Dimension-matched plasmonic Au/TiO2/BiVO4 nanocomposites as efficient wide-visible-light photocatalysts to convert CO2 and mechanistic insights. J. Mater. Chem. A 2018, 6, 11838–11845. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 4613. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B 2020, 272, 119006. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Jia, Z.; Hu, H.; Liu, L.; Ye, J.; Wang, D. Enhanced CO2 Photoreduction over Bi2Te3/TiO2 Nanocomposite via a Seebeck Effect. Catalysts 2022, 12, 1323. https://doi.org/10.3390/catal12111323
Lei Y, Jia Z, Hu H, Liu L, Ye J, Wang D. Enhanced CO2 Photoreduction over Bi2Te3/TiO2 Nanocomposite via a Seebeck Effect. Catalysts. 2022; 12(11):1323. https://doi.org/10.3390/catal12111323
Chicago/Turabian StyleLei, Yiming, Zewei Jia, Huilin Hu, Lequan Liu, Jinhua Ye, and Defa Wang. 2022. "Enhanced CO2 Photoreduction over Bi2Te3/TiO2 Nanocomposite via a Seebeck Effect" Catalysts 12, no. 11: 1323. https://doi.org/10.3390/catal12111323
APA StyleLei, Y., Jia, Z., Hu, H., Liu, L., Ye, J., & Wang, D. (2022). Enhanced CO2 Photoreduction over Bi2Te3/TiO2 Nanocomposite via a Seebeck Effect. Catalysts, 12(11), 1323. https://doi.org/10.3390/catal12111323