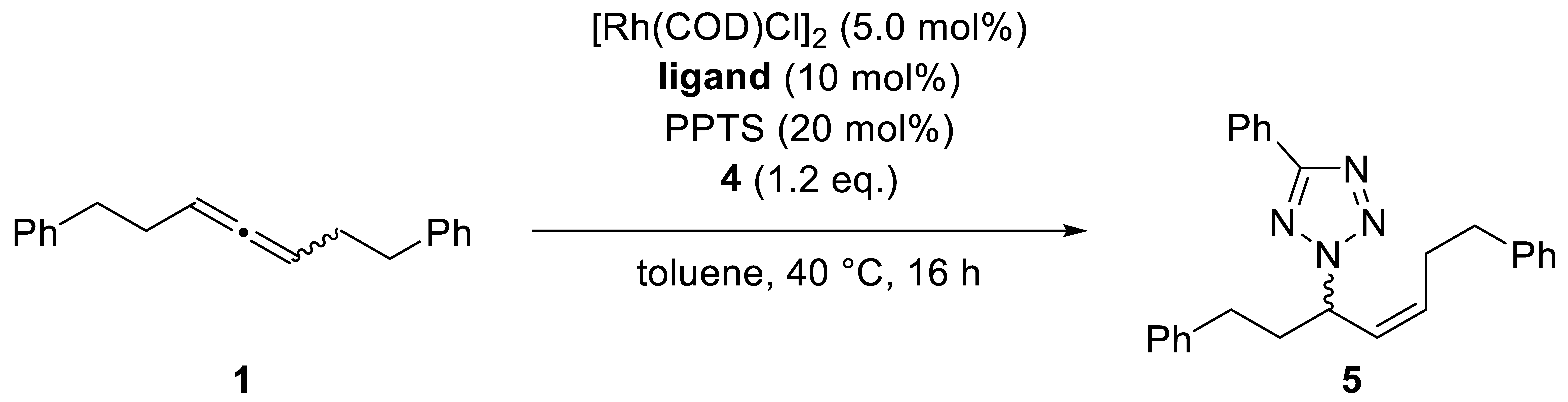Abstract
A general Rh-catalyzed addition reaction of nitrogen containing heterocycles to internal allenes is reported. Starting from racemic internal allenes a dynamic kinetic resolution (DKR) provides N-allylated triazoles and tetrazoles. Simultaneous control of N1/Nx-position selectivity, enantioselectivity and olefin geometry gives access to important building blocks of target-oriented synthesis. The synthetic utility is demonstrated by a gram-scale reaction and a broad substrate scope tolerating multiple functional groups. Deuterium labeling experiments and experiments with enantioenriched allenes as starting material support a plausible reaction mechanism.
1. Introduction
5-membered nitrogen containing heterocycles are found in a variety of bioactive compounds. Molecules containing triazoles exhibit antifungal [1], anxiolytic [2], antibacterial [3], and anticancerogenic [4] activity. Tetrazoles display antifungal [5], immunosuppressive [6], and antiviral [7] properties. In addition, the isosterism of tetrazoles to carboxylic acids makes them a suitable motif in drug design [8]. In particular, the subclass of α-chiral N-alkylated triazoles and tetrazoles features a broad range of biological activity (Figure 1) [5,9,10,11,12].
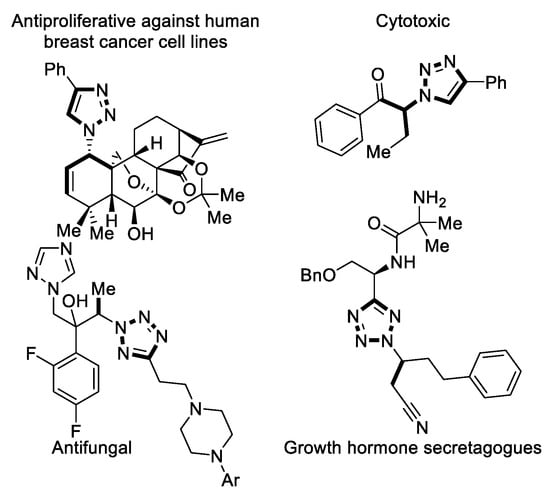
Figure 1.
Bioactive compounds possessing an α-chiral triazole or tetrazole scaffold [5,9,10,11].
Due to the importance of these moieties, different strategies for obtaining them have been developed in the past (Scheme 1). Zhao and coworkers published the transition metal catalyzed allylic substitution with sodium benzotriazolide [13,14]. Despite the excellent enantioselectivities in some cases, the method lacks regioselectivity and requires deprotonation of the triazole in a previous step. Khan recently reported the allylic substitution of cyclic carbonates with tetrazoles [15]. However, allylic substitutions in general are not in accordance with the principle of atom economy by releasing stoichiometric amounts of waste [16]. A series of organocatalyzed aza-Michael additions overcomes this shortcoming and in some cases convinces with high selectivities, but the scope of these transformations is limited [17,18,19,20,21,22]. Furthermore, a three-component reaction of tetrazoles, aldehydes, and acid anhydrides should be mentioned, which uses a Dynamic Kinetic Resolution to synthesize α-chiral tetrazoles as hemiaminals [23,24].
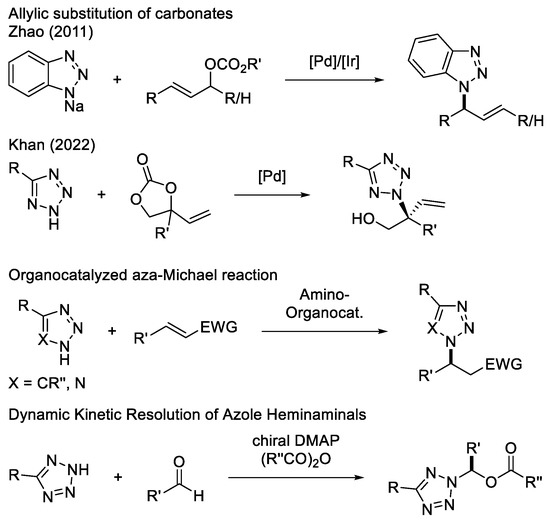
Scheme 1.
Selected methods for the synthesis of α-chiral triazoles and tetrazoles [13,14,15,16,17,18,19,20,21,22,23,24].
In the last decade, our group developed the transition metal-catalyzed addition of a variety of pronucleophiles to allenes and alkynes [25,26]. C- [27,28,29], N- [30,31,32], O- [33,34,35], and S-pronucleophiles [36,37] can thus be allylated enantioselectively, which creates an atom economic analogon to the Tsuji-Trost allylation [38,39,40,41,42,43,44,45] and allylic oxidations [46,47,48]. Furthermore, the synthetic utility of these reactions has already been demonstrated in several total syntheses [49,50,51]. We recently reported on the rhodium-catalyzed addition of pyrazoles to internal allenes [52]. Due to the importance of triazoles and tetrazoles, we also wanted to extend the Rh-catalysis for these heterocycles. Hence an optimized catalytic protocol for the synthesis of such frameworks is presented using a DKR, a powerful and important method in asymmetric catalysis and synthesis [53,54,55,56,57].
2. Results and Discussion
Coupling benzotriazole (2) with our screening allene, 1, resulted in 96% yield and perfect enantio-, regio-, and E/Z-selectivity by using [Rh(COD)Cl]2, (R,R)-DIOP, and 50 mol% of PPTS at 60 °C. Phenyltetrazole (4) was coupled at 40 °C and with 20 mol% PPTS with ideal regio- and Z/E-selectivity and good enantiomeric excess (Scheme 2) (For optimization and screening tables, see the Supporting Information).
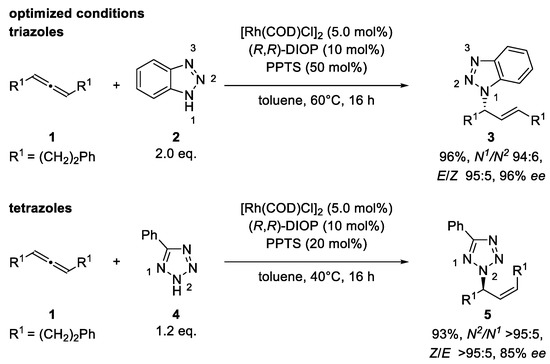
Scheme 2.
Optimized reaction conditions of the Rh-catalyzed hydroamination of racemic 1,3-disubstituted allenes. Scale: 0.25 mmol; 1.25 mL of toluene (0.2 m). Yields reported for isolated products. Enantioselectivities were determined by HPLC analysis using a chiral stationary phase. COD = cyclooctadien, PPTS = pyridinium p-toluenesulfonate. Gram-scale catalysis (4.03 mmol allene): benzotriazole: 96% yield, N1/N2 96:4, E/Z > 95:5, 95% ee; phenyltetrazole: 84% yield, Z/E > 95:5, 85% ee. For determination of absolute configuration, see the Supporting Information.
After identifying optimized reaction conditions, the broad applicability is demonstrated by several scopes. Initially we subjected a variety of triazoles to the screening allene 1 (Figure 2).
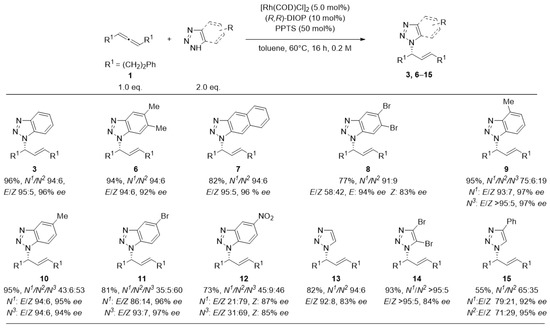
Figure 2.
Scope of the addition of Triazoles to rac-1,7-diphenyl-hepta-3,4-diene (1). Reactions were performed at 0.25 mmol scale. Cumulative yield for all isolated isomers. E/Z-selectivities determined by 1H-NMR. Enantioselectivities were determined by HPLC analysis using a chiral stationary phase. Unless specified, ee-values refer to main-stereoisomer of the main-regioisomer. Shown regioisomer is designated as an N1-product.
The benzotriazol-derived substrates (6–12) showed up to 96% yield and enantioselectivities up to 97% ee. While N1/N2-selectivity was consistently greater than 91:9 for symmetrically substituted triazoles (3, 6–8, 13, 14), asymmetric substrates added the challenge of N1/N3-regioselectivity. 5-substituted benzotriazoles (10–12) provided the corresponding products in an almost statistical N1/N3-distribution. Placing the methyl-group in 4- position (9) and thus closer to the reactive site increased N1/N3-selectivity to 4:1. Notably, there was a decrease of E/Z-selectivity for heterosubstituted benzotriazoles (8, 11, 12), whereas a nearly perfect E-selectivity was present in all other cases. The nitro-substituted benzotriazole (12) even led to an inversed olefine geometry, with the Z-product being preferred.
We subsequently tested several allenes for compatibility in the reaction with benzotriazole 2 (Figure 3). Dialkyl substituted allenes (16–18) yielded perfect regioselectivity and enantiomeric excess of up to 98% ee. Even a sterically more demanding (19) and a macrocyclic allene (20) were tolerated as well. The method has its limitations for sterically very demanding and aryl-substituted allenes (21, 22), as well as for a trisubstituted allene (23). For these substrates, yield and selectivity decreased. Next, we subjected several tetrazoles to the corresponding reaction conditions (Figure 4).
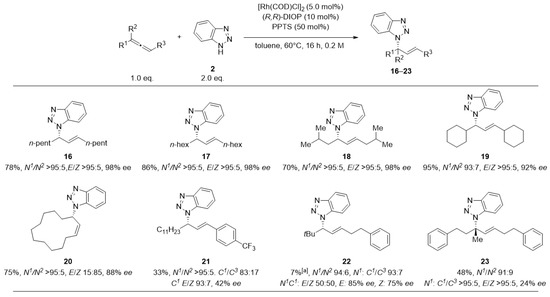
Figure 3.
Scope of the addition of Benzotriazole (2) to different racemic allenes. Reactions were performed at 0.25 mmol scale. Cumulative yield for all isolated isomers. E/Z-selectivities determined by 1H-NMR. Enantioselectivities were determined by HPLC analysis using a chiral stationary phase. Unless specified, ee-values refer to main-stereoisomer of the main-regioisomer. [a] Reaction was heated for 72 h.
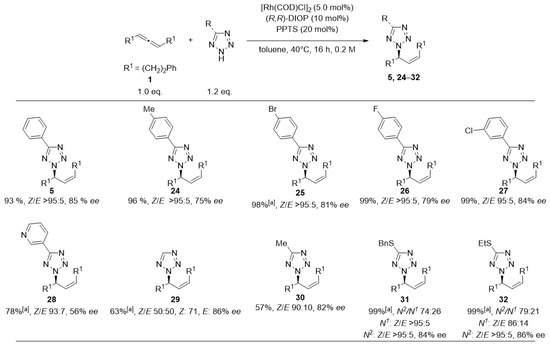
Figure 4.
Scope of the addition of Tetrazoles to rac-1,7-diphenyl-hepta-3,4-diene. (1) Reactions were performed at 0.25 mmol scale. Cumulative yield for all isolated isomers. Z/E-selectivities were determined by 1H-NMR. Enantioselectivities were determined by HPLC analysis using a chiral stationary phase. Unless specified, ee-values refer to main-stereoisomer of the main-regioisomer. Unless specified full N2-selectivity was obtained. [a] Reaction was performed at 60 °C.
Halogen-substituted phenyltetrazoles were coupled in nearly quantitative yields (25–27). Tetrazoles containing thioethers (31, 32) were the only ones to yield products with reduced N2/N1-regioselectivity (about 3:1); however, maintaining quantitative yield and consistently high Z/E- and enantioselectivity. Eventually, we subjected several allenes to the reaction with 5-phenyl-tetrazole (4) (Figure 5).
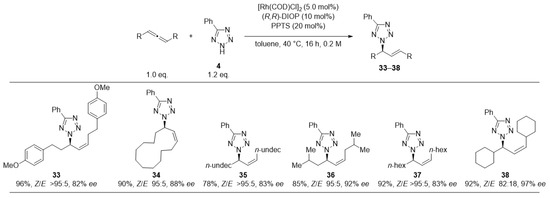
Figure 5.
Scope of the addition of 5-phenyl-2H-tetrazole to different racemic allenes. Reactions were performed at 0.25 mmol scale. Cumulative yield for all isolated isomers. E/Z-selectivities determined by 1H-NMR. Enantioselectivities were determined by HPLC analysis using a chiral stationary phase. Unless specified, ee-values refer to main-stereoisomer of the main-regioisomer. Unless specified full N2-selectivity was obtained.
Except for one sterically more challenging substrate (38), all products were obtained in perfect Z-selectivity. Only the di-cyclohexyl substituted substrate (38) showed shares of the E-product, indicating the limitation of the catalyst controlling the olefin geometry for sterically more demanding allenes. Some mechanistic control experiments were carried out (Scheme 3).
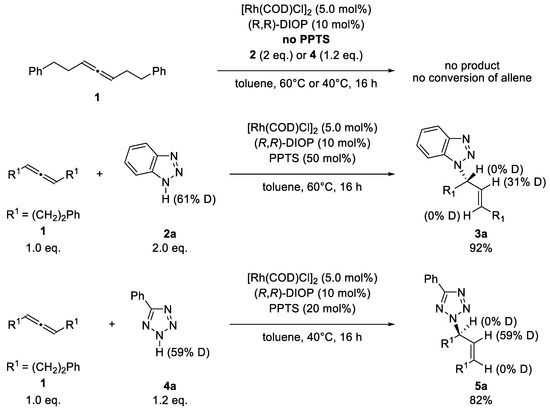
Scheme 3.
Control experiments: Without PPTS, only the allene could be reisolated. After reactions with deuterated pronucleophiles, the deuterium was observed exclusively at the former central atom of the allene.
If the catalysis was performed without PPTS, no reaction occurred, revealing the important role of the additive for the reaction. Furthermore, we prepared deuterated nucleophiles, which we subjected to the respective catalysis conditions. The deuterium atoms were found exclusively at the former central atom of the allene. This is in contrast to earlier results with terminal allenes, where deuterium was found at several positions [58,59,60]. Using enantiomerically enriched allenes in the catalysis, both enantiomers of allene yielded the (S)-product for the triazole and the (R)-product for the tetrazole regardless of the configuration of the allene used (Table 1).

Table 1.
Control experiments with enantioenriched allene.
When the achiral dppb ligand was used instead of the chiral DIOP ligand in the reactions with enantiomerically enriched allene, the racemic products were obtained, indicating a racemization step in the catalytic cycle. These findings lead us to propose the following reaction mechanism (Scheme 4).
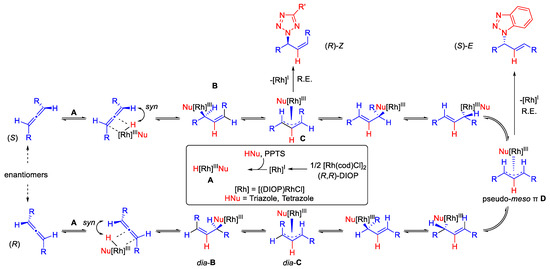
Scheme 4.
Proposed reaction mechanism of Rh-catalyzed hydroamination of internal allenes with triazoles and tetrazoles. R.E. = Reductive elimination.
A RhI-DIOP complex is first formed from [Rh(COD)Cl]2 and (R,R)-DIOP (inner box of Scheme 4). With the help of PPTS, the pronucleophile is then added by oxidative addition to form the Rh-hydride species A. Now, syn-hydrometallation from the sterically less demanding side occurs at the respective allene enantiomers. This occurs in such a way that the hydrogen atom results at the former central atom of the allene. Initially, two diastereomeric Z-configured σ-complexes B and dia-B are formed, which are in equilibrium with their corresponding syn, anti-configured π-complexes C and dia-C. Finally, a σ-π-σ-isomerization and a bond rotation lead to the identical pseudo-meso-π-allyl complex D for both diastereomers. Dynamic kinetic resolution is ensured via this syn, syn-configured π-complex, since the initial hydrometallation products B and dia-B can be converted into each other via D. If a triazole is the pronucleophile in the catalysis, reductive elimination occurs of pseudo-meso-π-allyl complex D, with the chiral ligand ensuring enantioselectivity. In contrast, when a tetrazole is used as a pronucleophile, reductive elimination preferentially occurs from syn-anti-π-complex C. This could explain why the (S)-E product is formed for the triazole and the (R)-Z product for the tetrazole.
3. Materials and Methods
3.1. Materials
Toluene was freshly distilled over Sodium/Benzophenone and degassed with argon prior to use. Solvents employed for work-up and column chromatography were purchased in technical grade quality and distilled by rotary evaporator before use. [Rh(COD)Cl]2 was purchased from Sigma-Aldrich (St. Louis, MO, USA). (R,R)-DIOP was prepared according to the procedure shown in the Supplementary Materials.
3.2. Methods
A 20 mL screw-cap Schlenk tube was dried under a vacuum, backfilled with argon (Argon 5.0 Sauerstoffwerke Friedrichshafen, Friedrichshafen, Germany), and cooled to room temperature using a standard Schlenk line apparatus. The tube was filled with [Rh(COD)Cl]2 (6.2 mg, 0.013 mmol, 5.0 mol%), (R,R)-DIOP (12.5 mg, 0.025 mmol, 10.0 mol%), PPTS (31.5 mg, 0.125 mmol, 50.0 mol% or 12.6 mg, 0.05 mmol, 20.0 mol%), and triazole (0.500 mmol, 2.0 equiv.) or tetrazole (0.30 mmol, 1.2 equiv.). The tube was put on a vacuum and backfilled with argon again. Freshly distilled toluene (1.25 mL) and allene (0.25 mmol, 1.0 equiv.) were added by syringe under a flow of argon, and then the tube was sealed by a screw cap. The mixture was stirred at 60 °C (for triazoles) or 40 °C (for tetrazoles) for 16 h. The tube was cooled to room temperature, the solvent was removed under reduced pressure and the residue was purified by flash column chromatography using AcOEt and hexanes as eluent on silica gel. In the case of the synthesis of racemic samples dppb was used as the catalyst.
4. Conclusions
To conclude, we developed a highly selective Rh-catalysis to add triazoles and tetrazoles to internal allenes. In only one step, allylated triazoles and tetrazoles were constructed in an enantio-, stereo-, and regioselective fashion. Mechanistic studies with deuterated pronucleophiles and enantioenriched substrates led us to propose a DKR mechanism. Further investigations in terms of compatible pronucleophiles that can be subjected to this catalytic system is the goal of future research in our laboratories.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12101209/s1, screening tables, preparation of ligand and substrates, analytical data, 1H and 13C NMR spectra, determination of absolute configuration, HPLC chromatograms of products and enantioenriched allenes, more detailed materials and methods [61,62,63,64,65,66].
Author Contributions
Conceptualization, B.B.; methodology, S.V.S. and B.B.; investigation, S.V.S. and I.L.; writing—original draft preparation, S.V.S.; writing—review and editing, S.V.S., I.L. and B.B.; supervision, B.B.; funding acquisition, B.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fonds der Chemischen Industrie. S.V.S. is grateful for a Ph.D. fellowship from the Fonds der Chemischen Industrie.
Data Availability Statement
All experimental data is contained in the article and supplementary material.
Acknowledgments
Jan Klauser, José Candia, and Julia Schmidt (Albert-Ludwigs Universität Freiburg) are acknowledged for their motivated technical assistance. Sincere thanks are given to Manfred Keller for NMR experiments, analysis of isomers and Joshua Emmerich for HPLC separations (Analytical Department of Albert-Ludwigs Universität Freiburg).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rezaei, Z.; Khabnadideh, S.; Pakshir, K.; Hossaini, Z.; Amiri, F.; Assadpour, E. Design, synthesis, and antifungal activity of triazole and benzotriazole derivatives. Eur. J. Med. Chem. 2009, 44, 3064–3067. [Google Scholar] [CrossRef] [PubMed]
- Paluchowska, M.H.; Bugno, R.; Charakchieva-Minol, S.; Bojarski, A.J.; Tatarczyńska, E.; Chojnacka-Wójcik, E. Conformational restriction in novel NAN-190 and MP3022 analogs and their 5-HT(1A) receptor activity. Arch. Pharm. 2006, 339, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Letavic, M.A.; Bronk, B.S.; Bertsche, C.D.; Casavant, J.M.; Cheng, H.; Daniel, K.L.; George, D.M.; Hayashi, S.F.; Kamicker, B.J.; Kolosko, N.L.; et al. Synthesis and Activity of a Novel Class of Tribasic Macrocyclic Antibiotics: The Triamilides. Bioorg. Med. Chem. Lett. 2002, 12, 2771–2774. [Google Scholar] [CrossRef]
- Sambasiva Rao, P.; Kurumurthy, C.; Veeraswamy, B.; Santhosh Kumar, G.; Poornachandra, Y.; Ganesh Kumar, C.; Vasamsetti, S.B.; Kotamraju, S.; Narsaiah, B. Synthesis of novel 1,2,3-triazole substituted-N-alkyl/aryl nitrone derivatives, their anti-inflammatory and anticancer activity. Eur. J. Med. Chem. 2014, 80, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Upadhayaya, R.S.; Jain, S.; Sinha, N.; Kishore, N.; Chandra, R.; Arora, S.K. Synthesis of novel substituted tetrazoles having antifungal activity. Eur. J. Med. Chem. 2004, 39, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Mollison, K.W.; Liu, L.; Henry, C.L.; Rosenberg, T.A.; Bamaung, N.; Tu, N.; Wiedeman, P.E.; Or, Y.; Luly, J.R.; et al. Rapamycin analogs with reduced systemic exposure. Bioorg. Med. Chem. Lett. 2005, 15, 5340–5343. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Poliakov, A.; Åkerblom, E.; Wiklund, K.; Lindeberg, G.; Winiwarter, S.; Danielson, U.; Samuelsson, B.; Hallberg, A. Acyl sulfonamides as potent protease inhibitors of the hepatitis C virus full-Length NS3 (Protease-Helicase/NTPase): A comparative study of different C-terminals. Bioorg. Med. Chem. 2003, 11, 2551–2568. [Google Scholar] [CrossRef]
- Herr, R. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: Medicinal chemistry and synthetic methods. Bioorg. Med. Chem. 2002, 10, 3379–3393. [Google Scholar] [CrossRef]
- Vantikommu, J.; Palle, S.; Reddy, P.S.; Ramanatham, V.; Khagga, M.; Pallapothula, V.R. Synthesis and cytotoxicity evaluation of novel 1,4-disubstituted 1,2,3-triazoles via CuI catalysed 1,3-dipolar cycloaddition. Eur. J. Med. Chem. 2010, 45, 5044–5050. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, Y.; Chen, H.; Wild, C.; Wang, T.; White, M.A.; Shen, Q.; Zhou, J. Overcoming synthetic challenges of oridonin A-ring structural diversification: Regio- and stereoselective installation of azides and 1,2,3-triazoles at the C-1, C-2, or C-3 position. Org. Lett. 2013, 15, 3718–3721. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.S.; Swartz, S.G.; Slusarchyk, D.; Yan, M.; Seethala, R.K.; Sleph, P.; Grover, G.; Dickinson, K.; Giupponi, L.; Harper, T.W.; et al. Optimization of 1H-tetrazole-1-alkanenitriles as potent orally bioavailable growth hormone secretagogues. Bioorg. Med. Chem. Lett. 2008, 18, 2067–2072. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Jiang, F.; Shi, F. Organocatalytic Asymmetric Synthesis of Indole-Based Chiral Heterocycles: Strategies, Reactions, and Outreach. Acc. Chem. Res. 2020, 53, 425–446. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, D.; Zheng, S.; Yue, Y.; Liu, D.; Zhao, X. Enantioselective Palladium-Catalyzed Allylic Substitution of Sodium Benzotriazolide. Eur. J. Org. Chem. 2011, 2011, 6288–6293. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, X.-W.; Zheng, S.-C.; Zhao, X.-M. Enantioselective iridium-catalyzed allylation of sodium benzotriazolide: An efficient way to chiral allylbenzotriazoles. Tetrahedron Lett. 2012, 53, 6995–6998. [Google Scholar] [CrossRef]
- Khan, S.; Wang, Y.; Zhang, M.-N.; Perveen, S.; Zhang, J.; Khan, A. Regio- and enantioselective formation of tetrazole-bearing quaternary stereocenters via palladium-catalyzed allylic amination. Org. Chem. Front. 2022, 9, 456–461. [Google Scholar] [CrossRef]
- Trost, B.M. The atom economy—A search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Dinér, P.; Nielsen, M.; Marigo, M.; Jørgensen, K.A. Enantioselective organocatalytic conjugate addition of N heterocycles to alpha, beta-unsaturated aldehydes. Angew. Chem. Int. Ed. 2007, 46, 1983–1987. [Google Scholar] [CrossRef]
- Gandelman, M.; Jacobsen, E.N. Highly enantioselective catalytic conjugate addition of N-heterocycles to alpha, beta-unsaturated ketones and imides. Angew. Chem. Int. Ed. 2005, 44, 2393–2397. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, S.; Duan, W.; Wang, W. Enantioselective Conjugate Addition of N-Heterocycles to α,β-Unsaturated Ketones Catalyzed by Chiral Primary Amines. Synthesis 2009, 2009, 1564–1572. [Google Scholar] [CrossRef]
- Uria, U.; Vicario, J.L.; Badía, D.; Carrillo, L. Organocatalytic enantioselective aza-Michael reaction of nitrogen heterocycles and alpha, beta-unsaturated aldehydes. Chem. Commun. 2007, 24, 2509–2511. [Google Scholar] [CrossRef]
- Wang, J.; Zu, L.; Li, H.; Xie, H.; Wang, W. Cinchona Alkaloid Based Thiourea Promoted Enantioselective Conjugate Addition of N-Heterocycles to Enones. Synthesis 2007, 2007, 2576–2580. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Zu, L.; Wang, W. Enantioselective organocatalytic Michael addition reactions between N-heterocycles and nitroolefins. Org. Lett. 2006, 8, 1391–1394. [Google Scholar] [CrossRef]
- Piotrowski, D.W.; Kamlet, A.S.; Dechert-Schmitt, A.-M.R.; Yan, J.; Brandt, T.A.; Xiao, J.; Wei, L.; Barrila, M.T. Regio- and Enantioselective Synthesis of Azole Hemiaminal Esters by Lewis Base Catalyzed Dynamic Kinetic Resolution. J. Am. Chem. Soc. 2016, 138, 4818–4823. [Google Scholar] [CrossRef]
- Kinens, A.; Sejejs, M.; Kamlet, A.S.; Piotrowski, D.W.; Vedejs, E.; Suna, E. Development of a Chiral DMAP Catalyst for the Dynamic Kinetic Resolution of Azole Hemiaminals. J. Org. Chem. 2017, 82, 869–886. [Google Scholar] [CrossRef]
- Koschker, P.; Breit, B. Branching Out: Rhodium-Catalyzed Allylation with Alkynes and Allenes. Acc. Chem. Res. 2016, 49, 1524–1536. [Google Scholar] [CrossRef]
- Haydl, A.M.; Breit, B.; Liang, T.; Krische, M.J. Alkynes as Electrophilic or Nucleophilic Allylmetal Precursors in Transition-Metal Catalysis. Angew. Chem. Int. Ed. 2017, 56, 11312–11325. [Google Scholar] [CrossRef]
- Berthold, D.; Klett, J.; Breit, B. Rhodium-catalyzed asymmetric intramolecular hydroarylation of allenes: Access to functionalized benzocycles. Chem. Sci. 2019, 10, 10048–10052. [Google Scholar] [CrossRef]
- Grugel, C.P.; Breit, B. Rhodium-Catalyzed Enantioselective Cyclization of 3-Allenyl-indoles: Access to Functionalized Tetrahydrocarbazoles. Org. Lett. 2019, 21, 5798–5802. [Google Scholar] [CrossRef]
- Hilpert, L.J.; Breit, B. Rhodium-Catalyzed Parallel Kinetic Resolution of Racemic Internal Allenes Towards Enantiopure Allylic 1,3-Diketones. Angew. Chem. Int. Ed. 2019, 58, 9939–9943. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Breit, B. Transition metal catalyzed stereodivergent synthesis of syn- and anti-δ-vinyl-lactams: Formal total synthesis of (-)-cermizine C and (-)-senepodine G. Chem. Sci. 2019, 10, 3074–3079. [Google Scholar] [CrossRef]
- Berthold, D.; Geissler, A.G.A.; Giofré, S.; Breit, B. Rhodium-Catalyzed Asymmetric Intramolecular Hydroamination of Allenes. Angew. Chem. 2019, 131, 10099–10102. [Google Scholar] [CrossRef]
- Berthold, D.; Breit, B. Asymmetric Total Syntheses of (-)-Angustureine and (-)-Cuspareine via Rhodium-Catalyzed Hydroamination. Org. Lett. 2020, 22, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Koschker, P.; Lumbroso, A.; Breit, B. Enantioselective synthesis of branched allylic esters via rhodium-catalyzed coupling of allenes with carboxylic acids. J. Am. Chem. Soc. 2011, 133, 20746–20749. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Breit, B. Rhodium-Catalyzed Enantioselective Intermolecular Hydroalkoxylation of Allenes and Alkynes with Alcohols: Synthesis of Branched Allylic Ethers. Angew. Chem. Int. Ed. 2016, 55, 8440–8443. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.P.; Breit, B. Rhodium-Catalyzed Cyclization of Terminal and Internal Allenols: An Atom Economic and Highly Stereoselective Access Towards Tetrahydropyrans. Angew. Chem. Int. Ed. 2020, 59, 23485–23490. [Google Scholar] [CrossRef] [PubMed]
- Pritzius, A.B.; Breit, B. Asymmetric rhodium-catalyzed addition of thiols to allenes: Synthesis of branched allylic thioethers and sulfones. Angew. Chem. 2015, 127, 3164–3168. [Google Scholar] [CrossRef]
- Khakyzadeh, V.; Wang, Y.-H.; Breit, B. Rhodium-catalyzed addition of sulfonyl hydrazides to allenes: Regioselective synthesis of branched allylic sulfones. Chem. Commun. 2017, 53, 4966–4968. [Google Scholar] [CrossRef]
- Evans, P.A.; Leahy, D.K. Regio- and enantiospecific rhodium-catalyzed allylic etherification reactions using copper(I) alkoxides: Influence of the copper halide salt on selectivity. J. Am. Chem. Soc. 2002, 124, 7882–7883. [Google Scholar] [CrossRef]
- Hayashi, T.; Okada, A.; Suzuka, T.; Kawatsura, M. High enantioselectivity in rhodium-catalyzed allylic alkylation of 1-substituted 2-propenyl acetates. Org. Lett. 2003, 5, 1713–1715. [Google Scholar] [CrossRef]
- Lu, Z.; Ma, S. Metal-catalyzed enantioselective allylation in asymmetric synthesis. Angew. Chem. 2008, 120, 264–303. [Google Scholar] [CrossRef]
- Madrahimov, S.T.; Li, Q.; Sharma, A.; Hartwig, J.F. Origins of Regioselectivity in Iridium Catalyzed Allylic Substitution. J. Am. Chem. Soc. 2015, 137, 14968–14981. [Google Scholar] [CrossRef] [PubMed]
- Petrone, D.A.; Isomura, M.; Franzoni, I.; Rössler, S.L.; Carreira, E.M. Allenylic Carbonates in Enantioselective Iridium-Catalyzed Alkylations. J. Am. Chem. Soc. 2018, 140, 4697–4704. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Helmchen, G. Applications of Iridium-Catalyzed Asymmetric Allylic Substitution Reactions in Target-Oriented Synthesis. Acc. Chem. Res. 2017, 50, 2539–2555. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Crawley, M.L. Asymmetric transition-metal-catalyzed allylic alkylations: Applications in total synthesis. Chem. Rev. 2003, 103, 2921–2944. [Google Scholar] [CrossRef]
- Trost, B.M.; van Vranken, D.L. Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev. 1996, 96, 395–422. [Google Scholar] [CrossRef]
- Liu, G.; Stahl, S.S. Two-faced reactivity of alkenes: Cis- versus trans-aminopalladation in aerobic Pd-catalyzed intramolecular aza-Wacker reactions. J. Am. Chem. Soc. 2007, 129, 6328–6335. [Google Scholar] [CrossRef]
- Ma, R.; Young, J.; Promontorio, R.; Dannheim, F.M.; Pattillo, C.C.; White, M.C. Synthesis of anti-1,3 Amino Alcohol Motifs via Pd(II)/SOX Catalysis with the Capacity for Stereodivergence. J. Am. Chem. Soc. 2019, 141, 9468–9473. [Google Scholar] [CrossRef]
- Liu, G.; Wu, Y. Palladium-catalyzed allylic C-H bond functionalization of olefins. Top. Curr. Chem. 2010, 292, 195–209. [Google Scholar] [CrossRef]
- Steib, P.; Breit, B. Concise Total Synthesis of (-)-Vermiculine through a Rhodium-Catalyzed C2 -Symmetric Dimerization Strategy. Chem. Eur. J. 2019, 25, 3532–3535. [Google Scholar] [CrossRef]
- Schotes, C.; Ostrovskyi, D.; Senger, J.; Schmidtkunz, K.; Jung, M.; Breit, B. Total synthesis of (18S)- and (18R)-homolargazole by rhodium-catalyzed hydrocarboxylation. Chem. Eur. J. 2014, 20, 2164–2168. [Google Scholar] [CrossRef]
- Brosowsky, J.; Lutterbeck, M.; Liebich, A.; Keller, M.; Herp, D.; Vogelmann, A.; Jung, M.; Breit, B. Syntheses of Thailandepsin B Pseudo-Natural Products: Access to New Highly Potent HDAC Inhibitors via Late-Stage Modification. Chem. Eur. J. 2020, 26, 16241–16245. [Google Scholar] [CrossRef] [PubMed]
- Hilpert, L.J.; Sieger, S.V.; Haydl, A.M.; Breit, B. Palladium- and Rhodium-Catalyzed Dynamic Kinetic Resolution of Racemic Internal Allenes Towards Chiral Pyrazoles. Angew. Chem. 2019, 131, 3416–3419. [Google Scholar] [CrossRef]
- Hang, Q.-Q.; Wu, S.-F.; Yang, S.; Wang, X.; Zhong, Z.; Zhang, Y.-C.; Shi, F. Design and catalytic atroposelective synthesis of axially chiral isochromenone-indoles. Sci. China Chem. 2022, 65, 1929–1937. [Google Scholar] [CrossRef]
- Wu, P.; Yu, L.; Gao, C.-H.; Cheng, Q.; Deng, S.; Jiao, Y.; Tan, W.; Shi, F. Design and synthesis of axially chiral aryl-pyrroloindoles via the strategy of organocatalytic asymmetric (2 + 3) cyclization. Fundam. Res. 2022; in press. [Google Scholar] [CrossRef]
- Deska, J.; Del Pozo Ochoa, C.; Bäckvall, J.-E. Chemoenzymatic dynamic kinetic resolution of axially chiral allenes. Chem. Eur. J. 2010, 16, 4447–4451. [Google Scholar] [CrossRef]
- Huerta, F.F.; Minidis, A.B.E.; Bäckvall, J.-E. Racemisation in asymmetric synthesis. Dynamic kinetic resolution and related processes in enzyme and metal catalysis. Chem. Soc. Rev. 2001, 30, 321–331. [Google Scholar] [CrossRef]
- Ward, R.S. Dynamic kinetic resolution. Tetrahedron Asymm. 1995, 6, 1475–1490. [Google Scholar] [CrossRef]
- Cooke, M.L.; Xu, K.; Breit, B. Enantioselective rhodium-catalyzed synthesis of branched allylic amines by intermolecular hydroamination of terminal allenes. Angew. Chem. Int. Ed. 2012, 51, 10876–10879. [Google Scholar] [CrossRef]
- Xu, K.; Thieme, N.; Breit, B. Atom-economic, regiodivergent, and stereoselective coupling of imidazole derivatives with terminal allenes. Angew. Chem. Int. Ed. 2014, 53, 2162–2165. [Google Scholar] [CrossRef]
- Haydl, A.M.; Xu, K.; Breit, B. Regio- and enantioselective synthesis of N-substituted pyrazoles by rhodium-catalyzed asymmetric addition to allenes. Angew. Chem. Int. Ed. 2015, 54, 7149–7153. [Google Scholar] [CrossRef]
- Fürstner, A.; Wuchrer, M. Concise approach to the “higher sugar” core of the nucleoside antibiotic hikizimycin. Chem. Eur. J. 2005, 12, 76–89. [Google Scholar] [CrossRef]
- Kielbasinski, P.; Albrycht, M.; Mikolajczyk, M.; Wieczorek, M.W.; Majzner, W.R.; Filipczak, A.; Ciolkiewicz, P. Synthesis of chiral hydroxythiolanes as potential catalysts for asymmetric organozinc additions to carbonyl compounds. Heteroatom Chem. 2005, 16, 93–103. [Google Scholar] [CrossRef]
- Hobbs, C.F.; Knowles, W.S. Asymmetric hydroformylation of vinyl acetate with DIOP-type ligands. J. Org. Chem. 1981, 46, 4422–4427. [Google Scholar] [CrossRef]
- Johansson, M.J.; Gorin, D.J.; Staben, S.T.; Toste, F.D. Gold(I)-catalyzed stereoselective olefin cyclopropanation. J. Am. Chem. Soc. 2005, 127, 18002–18003. [Google Scholar] [CrossRef] [PubMed]
- Berthold, D.; Breit, B. Chemo-, Regio-, and Enantioselective Rhodium-Catalyzed Allylation of Triazoles with Internal Alkynes and Terminal Allenes. Org. Lett. 2018, 20, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Raimondi, W.; Bury, T.; Breit, B. Enantioselective formation of tertiary and quaternary allylic C-N bonds via allylation of tetrazoles. Chem. Commun. 2015, 51, 10861–10863. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).