Engineering Heterostructures of Layered Double Hydroxides and Metal Nanoparticles for Plasmon-Enhanced Catalysis
Abstract
1. Introduction
Hybrid Plasmonic toward Optimized Photothermal-Photocatalytic Performances
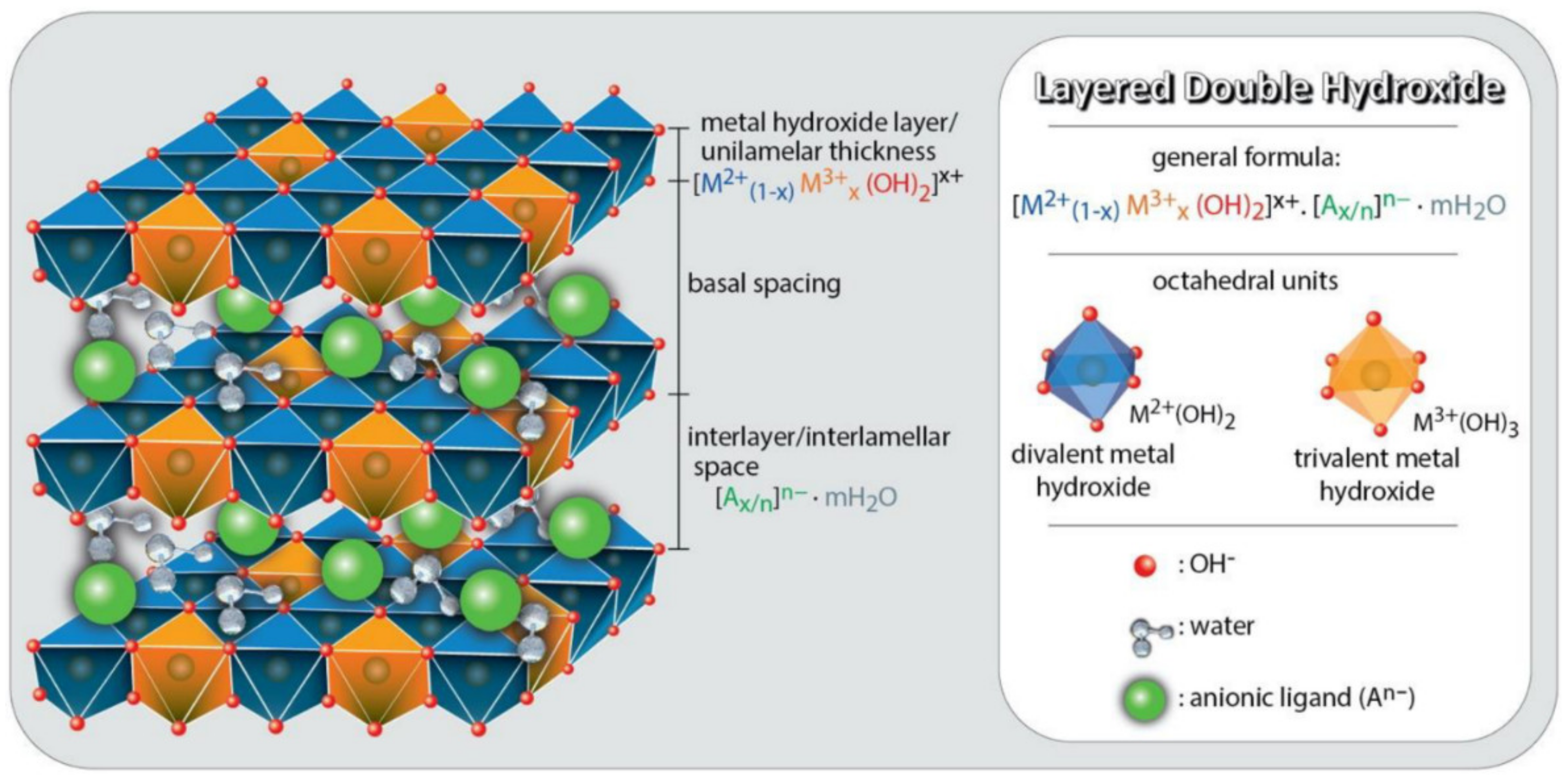
2. 2-D Nanoarchitectures of Layered Double Hydroxides as Light Absorbers
3. Heterostructures of Plasmonic Metal Nanoparticles/Support
3.1. Plasmon Excitation in Metal Nanoparticles
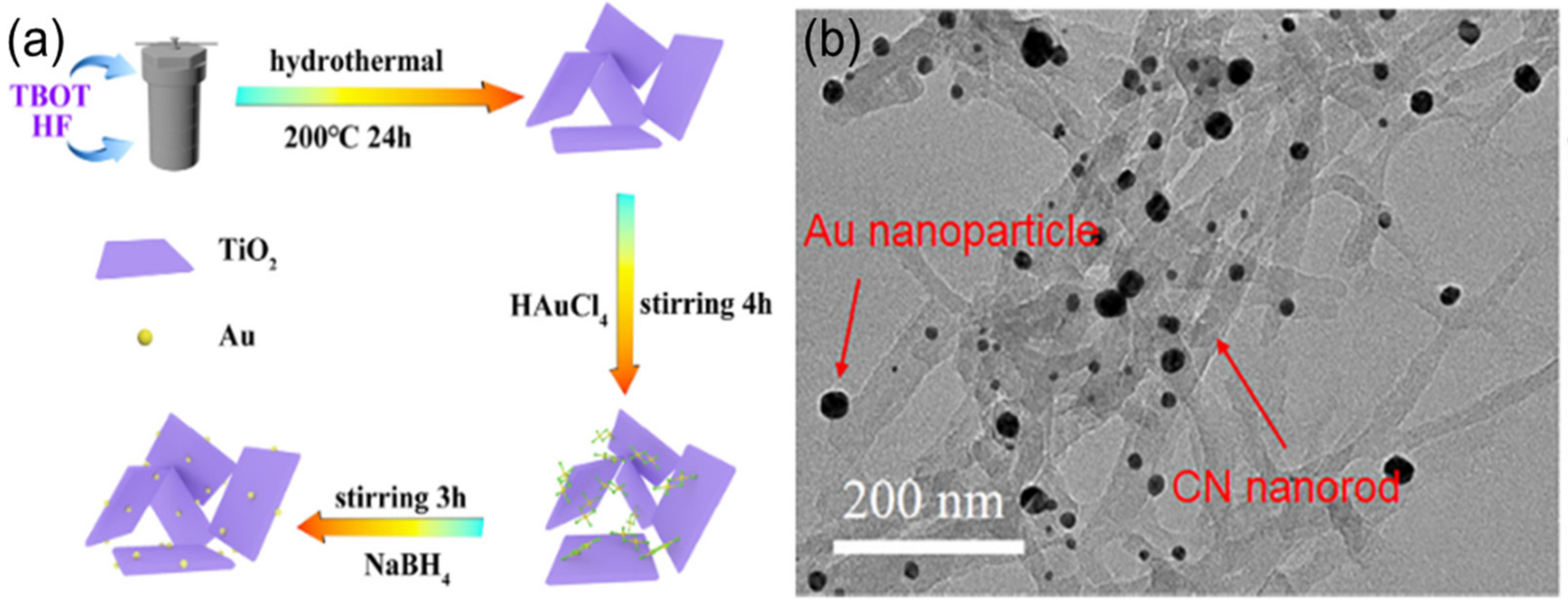
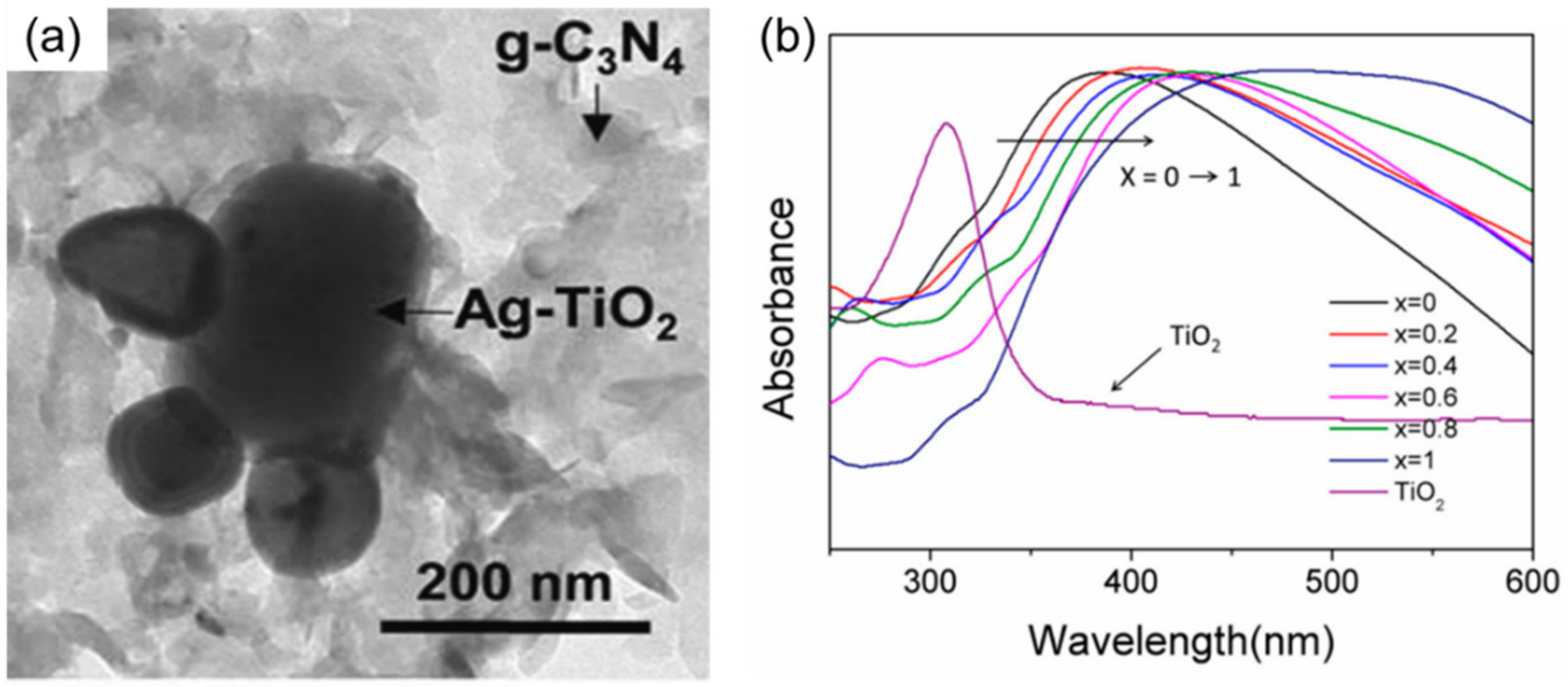
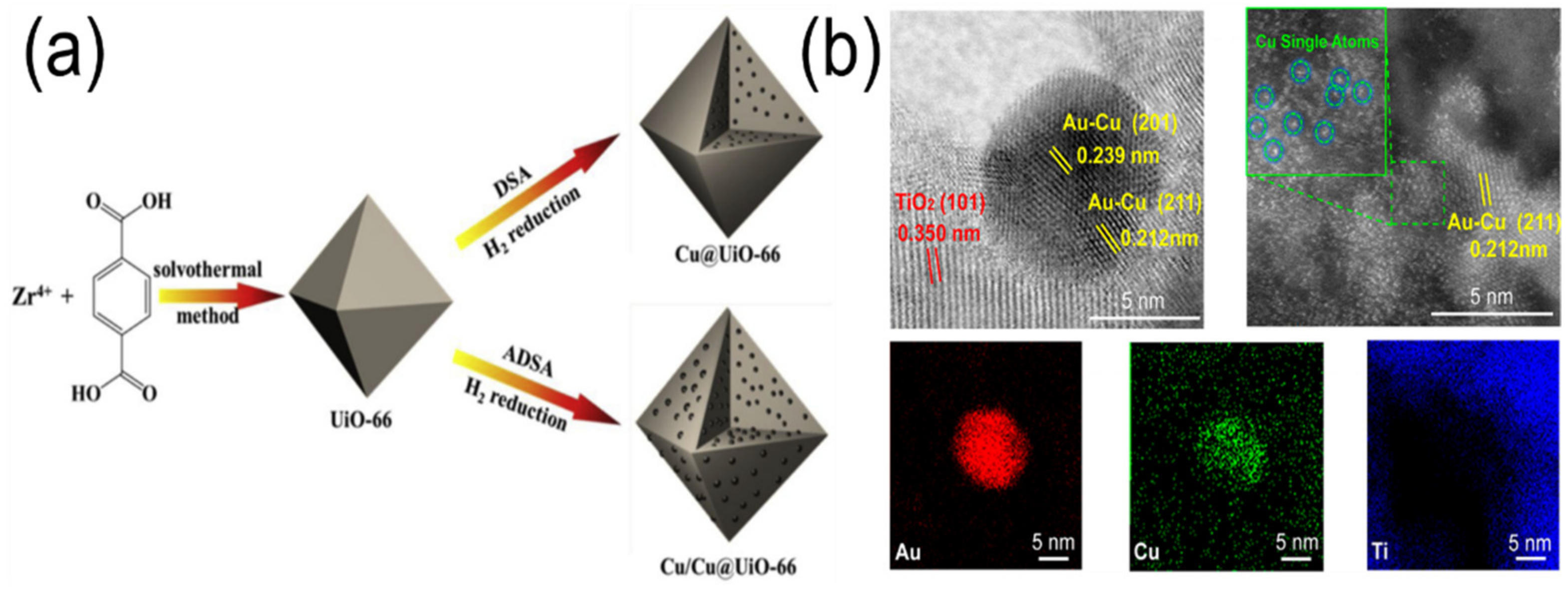
3.2. Heterostructures of Noble Plasmonic Metals/Support
3.3. Heterostructures of Non-Noble Plasmonic Metals/Support
4. Hybrid Plasmonics in Metal Nanoparticles/Layered Double Hydroxides Heterostructures
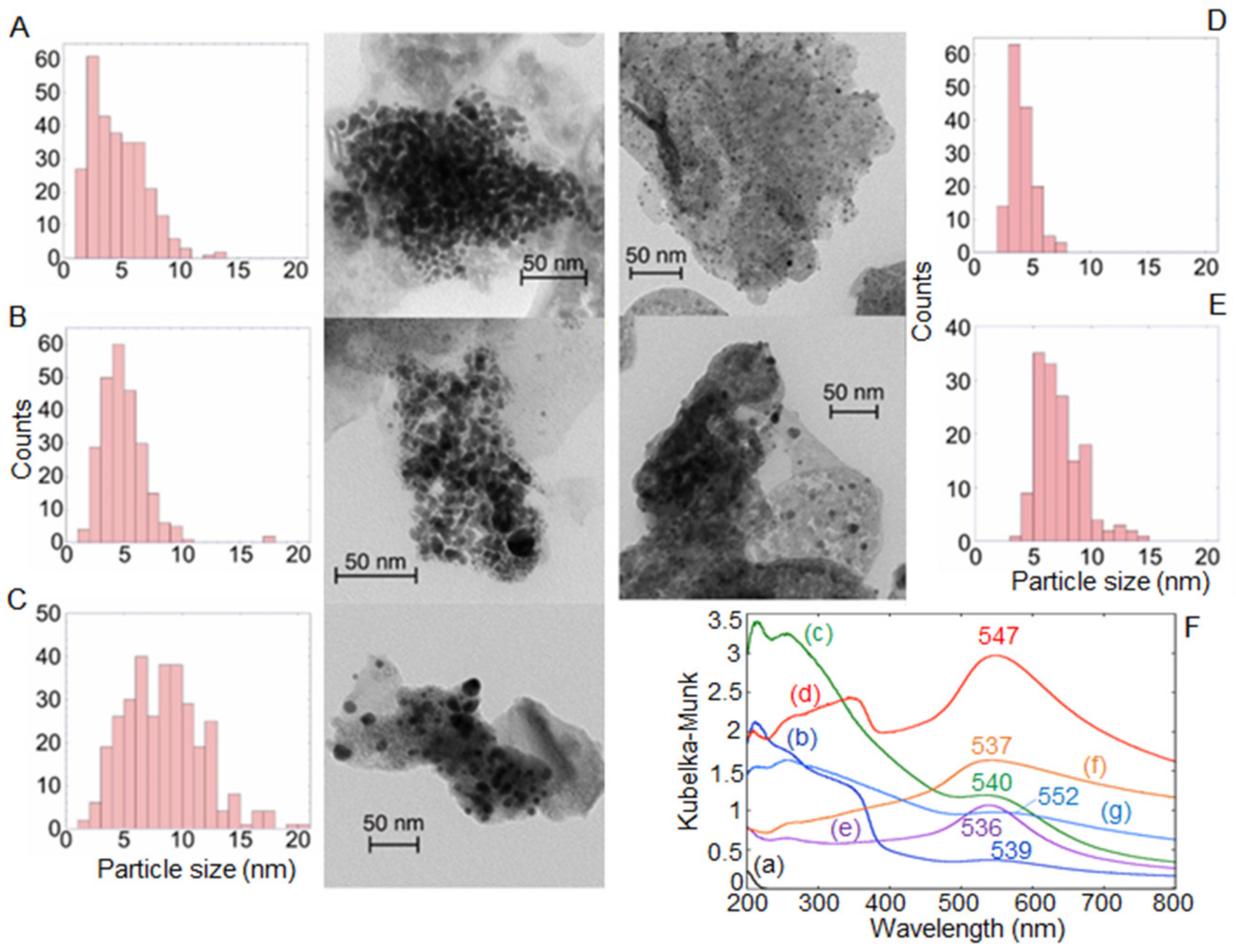
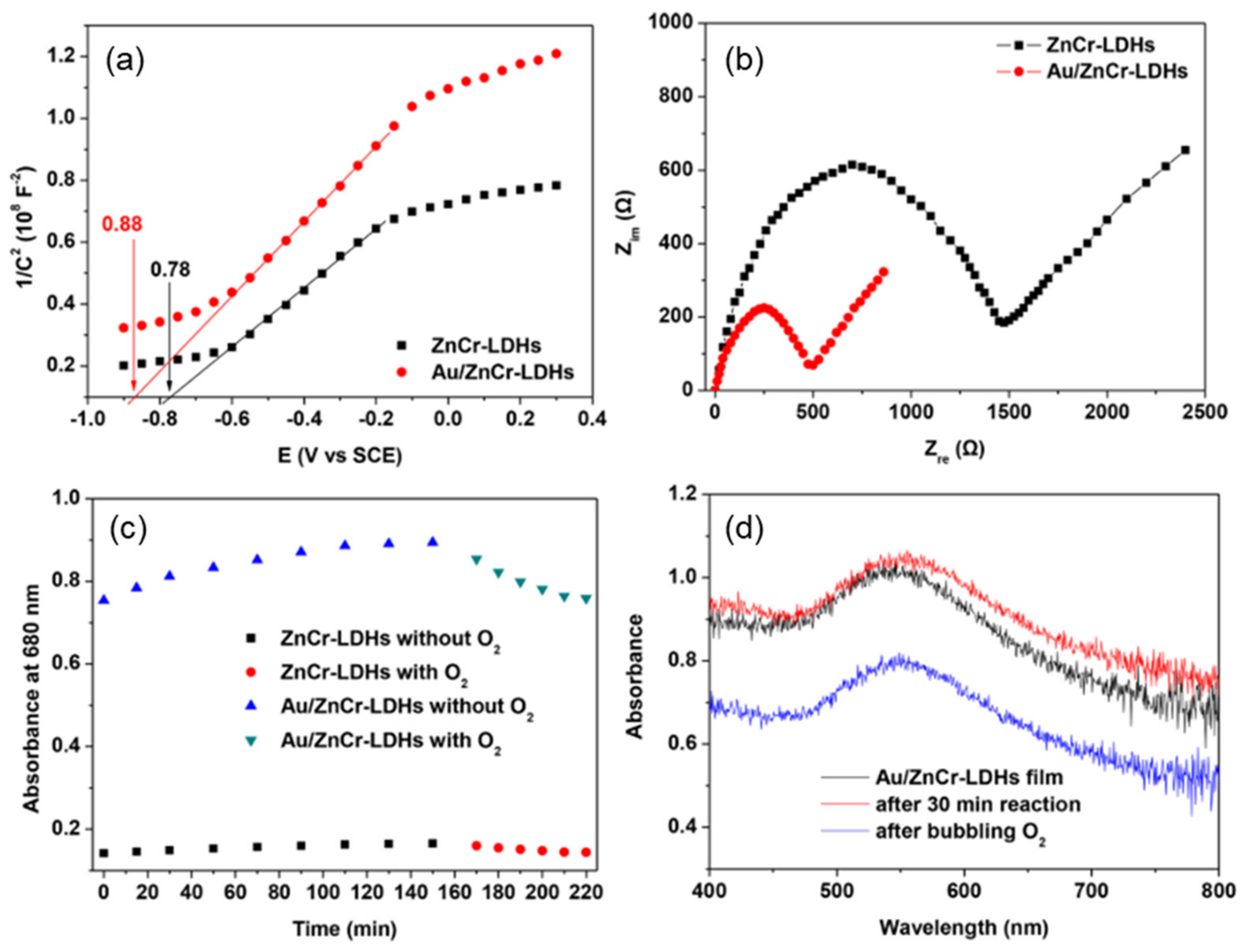
4.1. Heterostructures of Noble Plasmonic Metals/ Layered Double Hydroxides
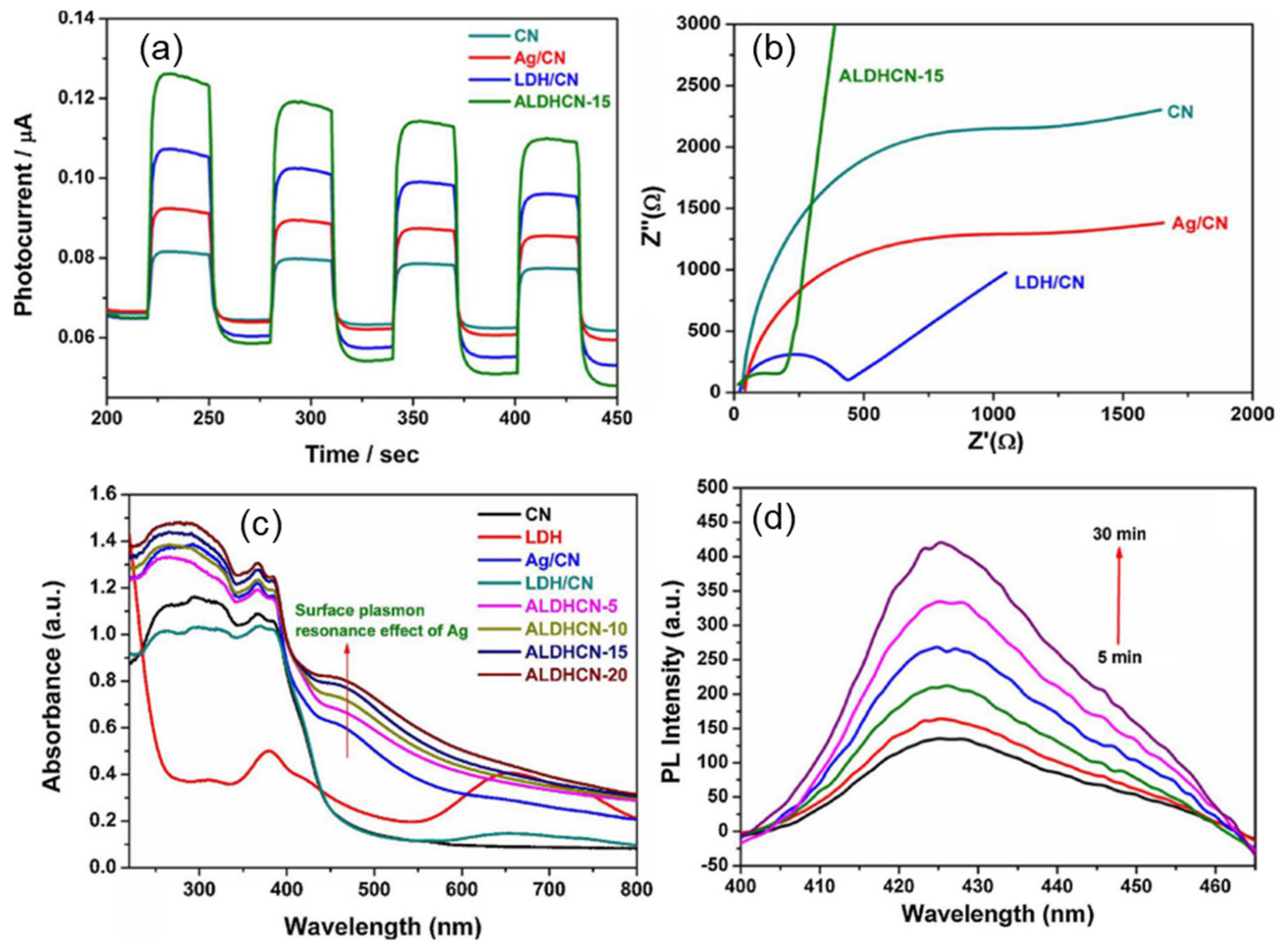
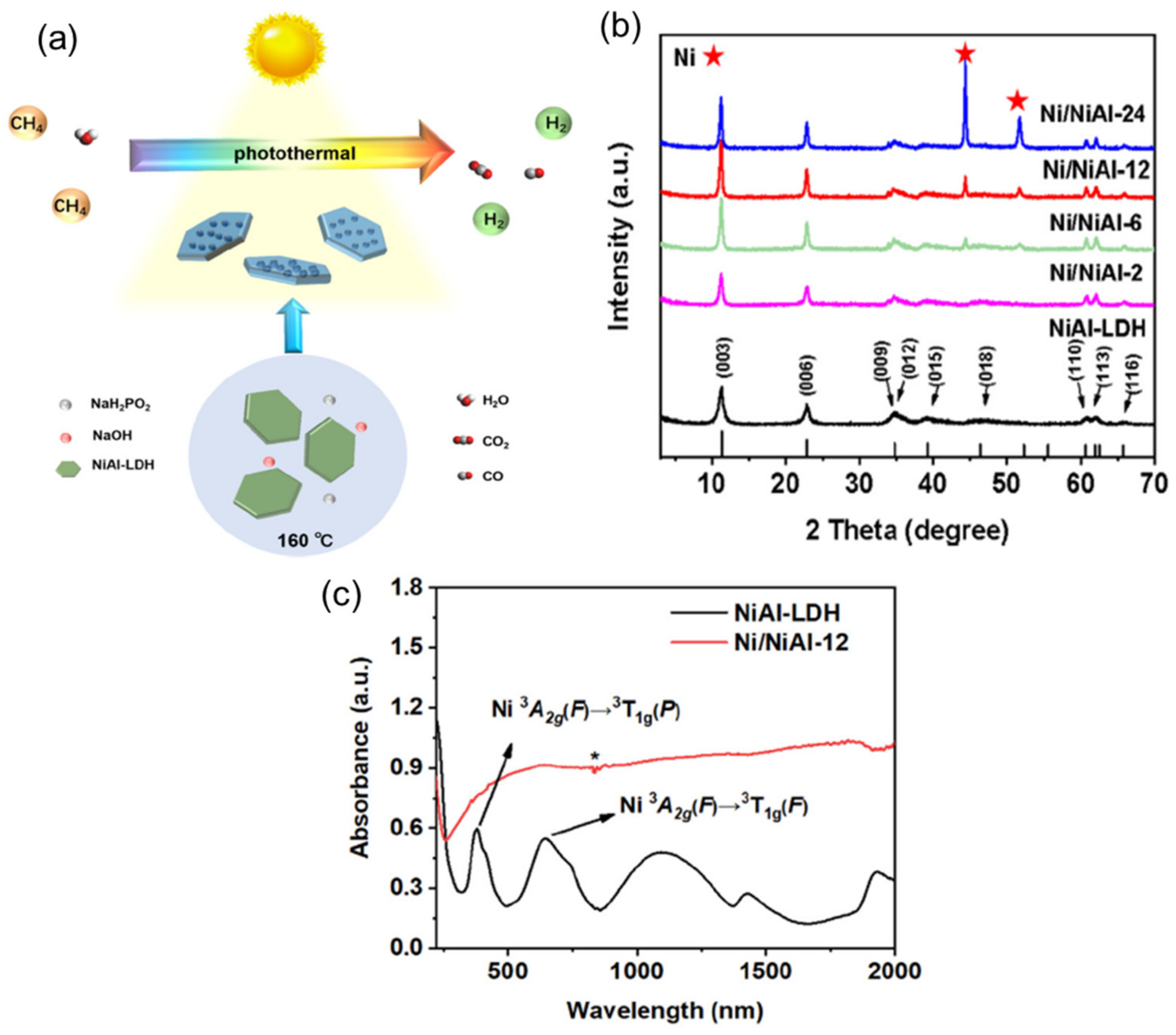
4.2. Heterostructures of Non-Noble Plasmonic Metals/Layered Double Hydroxides
5. Metals/Layered Double Hydroxides Heterostructures for Applications in Plasmonic Catalysis
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Brongersma, M.L.; Shalaev, V.M. Applied physics. The case for plasmonics. Science 2010, 328, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- King, M.E.; Fonseca Guzman, M.V.; Ross, M.B. Material strategies for function enhancement in plasmonic architectures. Nanoscale 2022, 14, 602–611. [Google Scholar] [CrossRef]
- Van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Han, B.; Guo, Y.; Huang, Y.; Xi, W.; Xu, J.; Luo, J.; Qi, H.; Ren, Y.; Liu, X.; Qiao, B.; et al. Strong Metal-Support Interactions between Pt Single Atoms and TiO2. Angew. Chem. Int. Ed. Engl. 2020, 59, 11824–11829. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, F.S.; Grillo, F.; Ravikumar, V.R.; Benz, D.; Shekhar, A.; Griffiths, M.B.; Barry, S.T.; Van Ommen, J.R. Thermal Atomic Layer Deposition of Gold Nanoparticles: Controlled Growth and Size Selection for Photocatalysis. Nanoscale 2020, 12, 9005–9013. [Google Scholar] [CrossRef]
- Sayed, M.; Yu, J.; Liu, G.; Jaroniec, M. Non-Noble Plasmonic Metal-Based Photocatalysts. Chem. Rev. 2022, 122, 10484–10537. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Chavez, S.; Aslam, U.; Linic, S. Design Principles for Directing Energy and Energetic Charge Flow in Multicomponent Plasmonic Nanostructures. ACS Energy Lett. 2018, 3, 1590–1596. [Google Scholar] [CrossRef]
- Carja, G.; Birsanu, M.; Okada, K.; Garcia, H. Composite plasmonic gold/layered double hydroxides and derived mixed oxides as novel photocatalysts for hydrogen generation under solar irradiation. J. Mater. Chem. A 2013, 1, 9092–9098. [Google Scholar] [CrossRef]
- Ukhtary, M.S.; Saito, R. Surface plasmons in graphene and carbon nanotubes. Carbon 2020, 167, 455–474. [Google Scholar] [CrossRef]
- Xiao, J.D.; Han, L.; Luo, J.; Yu, S.H.; Jiang, H.L. Integration of Plasmonic Effects and Schottky Junctions into Metal-Organic Framework Composites: Steering Charge Flow for Enhanced Visible-Light Photocatalysis. Angew. Chem. Int. Ed. Engl. 2018, 57, 1103–1107. [Google Scholar] [CrossRef]
- Kale, M.J.; Avanesian, T.; Christopher, P. Direct Photocatalysis by Plasmonic Nanostructures. ACS Catal. 2013, 4, 116–128. [Google Scholar] [CrossRef]
- Cushing, S.K.; Li, J.; Meng, F.; Senty, T.R.; Suri, S.; Zhi, M.; Li, M.; Bristow, A.D.; Wu, N. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041. [Google Scholar] [CrossRef]
- Rossi, T.P.; Shegai, T.; Erhart, P.; Antosiewicz, T.J. Strong plasmon-molecule coupling at the nanoscale revealed by first-principles modeling. Nat. Commun. 2019, 10, 3336. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.J.; Wu, J.Z.; Zhang, J.; Chen, H.; Zhou, J.Z.; Qian, G.R.; Xu, Z.P.; Du, Z.; Rao, Q.L. A review on fabricating heterostructures from layered double hydroxides for enhanced photocatalytic activities. Catal. Sci. Technol. 2018, 8, 1207–1228. [Google Scholar] [CrossRef]
- Naldoni, A.; Riboni, F.; Guler, U.; Boltasseva, A.; Shalaev, V.M.; Kildishev, A.V. Solar-Powered Plasmon-Enhanced Heterogeneous Catalysis. Nanophotonics 2016, 5, 112–133. [Google Scholar] [CrossRef]
- Tatsuma, T.; Nishi, H.; Ishida, T. Plasmon-induced charge separation: Chemistry and wide applications. Chem. Sci. 2017, 8, 3325–3337. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Mao, F.; Hao, P.; Zhu, Y.; Kong, X.; Duan, X. Layered double hydroxides: Scale production and application in soil remediation as super-stable mineralizer. Chin. J. Chem. Eng. 2022, 41, 42–48. [Google Scholar] [CrossRef]
- Kong, X.; Ge, R.; Liu, T.; Xu, S.; Hao, P.; Zhao, X.; Li, Z.; Lei, X.; Duan, H. Super-stable mineralization of cadmium by calcium-aluminum layered double hydroxide and its large-scale application in agriculture soil remediation. Chem. Eng. J. 2021, 407, 127178. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Layered Materials; CRC Press: New York, NY, USA, 2004; p. 664. [Google Scholar]
- Britto, S.; Kamath, P.V. Polytypism in the lithium-aluminum layered double hydroxides: The [LiAl2(OH)6]+ layer as a structural synthon. Inorg. Chem. 2011, 50, 5619–5627. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Xiao, H.; Lu, H.; Zhou, Y. Synthesis of Li–Al Layered Double Hydroxides (LDHs) for Efficient Fluoride Removal. Ind. Eng. Chem. Res. 2012, 51, 11490–11498. [Google Scholar] [CrossRef]
- Seftel, E.M.; Popovici, E.; Mertens, M.; Van Tendeloo, G.; Cool, P.; Vansant, E.F. The influence of the cationic ratio on the incorporation of Ti4+ in the brucite-like sheets of layered double hydroxides. Microporous Mesoporous Mater. 2008, 111, 12–17. [Google Scholar] [CrossRef]
- Zhang, W.H.; Guo, X.D.; He, J.; Qian, Z.Y. Preparation of Ni(II)/Ti(IV) layered double hydroxide at high supersaturation. J. Eur. Ceram. Soc. 2008, 28, 1623–1629. [Google Scholar] [CrossRef]
- Seftel, E.M.; Popovici, E.; Mertens, M.; Stefaniak, E.A.; Van Grieken, R.; Cool, P.; Vansant, E.F. SnIV-containing layered double hydroxides as precursors for nano-sized ZnO/SnO2 photocatalysts. Appl. Catal. B Environ. 2008, 84, 699–705. [Google Scholar] [CrossRef]
- Feng-Xian, L.I.U.; Zhe-Ming, N.I.; Sheng-Jie, X.I.A.; Jun-Hui, J.; Meng-Meng, S. Visible-light Photocatalytic Degradation Effect of Sn-Contained Layered Double Hydroxides on4-Chlorophenol. J. Inorg. Mater. 2016, 31, 7–13. [Google Scholar] [CrossRef][Green Version]
- Gabriel, R.; Carvalho, S.H.V.d.; Duarte, J.L.d.S.; Oliveira, L.M.T.M.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; Soletti, J.I.; Meili, L. Mixed metal oxides derived from layered double hydroxide as catalysts for biodiesel production. Appl. Catal. A Gen. 2022, 630, 118470. [Google Scholar] [CrossRef]
- Razzaq, A.; Ali, S.; Asif, M.; In, S.-I. Layered Double Hydroxide (LDH) Based Photocatalysts: An Outstanding Strategy for Efficient Photocatalytic CO2 Conversion. Catalysts 2020, 10, 1185. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, X.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; O’Hare, D.; Zhang, T. Layered Double Hydroxide Nanostructured Photocatalysts for Renewable Energy Production. Adv. Energy Mater. 2016, 6, 1501974. [Google Scholar] [CrossRef]
- Silva, C.G.; Bouizi, Y.; Fornés, V.; García, H. Layered Double Hydroxides as Highly Efficient Photocatalysts for Visible Light Oxygen Generation from Water. J. Am. Chem. Soc. 2009, 131, 13833–13839. [Google Scholar] [CrossRef]
- Lestari, P.R.; Takei, T.; Yanagida, S.; Kumada, N. Facile and controllable synthesis of Zn-Al layered double hydroxide/silver hybrid by exfoliation process and its plasmonic photocatalytic activity of phenol degradation. Mater. Chem. Phys. 2020, 250, 122988. [Google Scholar] [CrossRef]
- Gilea, D.; Radu, T.; Muresanu, M.; Carja, G. Plasmonic photocatalysts based on silver nanoparticles—layered double hydroxides for efficient removal of toxic compounds using solar light. Appl. Surf. Sci. 2018, 444, 407–413. [Google Scholar] [CrossRef]
- Chen, C.R.; Zeng, H.Y.; Yi, M.Y.; Xiao, G.F.; Zhu, R.L.; Cao, X.J.; Shen, S.G.; Peng, J.W. Fabrication of Ag2O/Ag decorated ZnAl-layered double hydroxide with enhanced visible light photocatalytic activity for tetracycline degradation. Ecotoxicol. Environ. Saf. 2019, 172, 423–431. [Google Scholar] [CrossRef]
- Sobhana, L.; Sarakha, M.; Prevot, V.; Fardim, P. Layered double hydroxides decorated with Au-Pd nanoparticles to photodegradate Orange II from water. Appl. Clay Sci. 2016, 134, 120–127. [Google Scholar] [CrossRef]
- Darie, M.; Seftel, E.M.; Mertens, M.; Ciocarlan, R.G.; Cool, P.; Carja, G. Harvesting solar light on a tandem of Pt or Pt-Ag nanoparticles on layered double hydroxides photocatalysts for p-nitrophenol degradation in water. Appl. Clay Sci. 2019, 182, 105250. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Zhu, G.; Wang, M.; Chen, Y.; Zhu, J.; Xi, Y.; He, H. Plasmonic Ag coated Zn/Ti-LDH with excellent photocatalytic activity. Appl. Surf. Sci. 2018, 433, 458–467. [Google Scholar] [CrossRef]
- Fu, S.; Zheng, Y.; Zhou, X.; Ni, Z.; Xia, S. Visible light promoted degradation of gaseous volatile organic compounds catalyzed by Au supported layered double hydroxides: Influencing factors, kinetics and mechanism. J. Hazard. Mater. 2019, 363, 41–54. [Google Scholar] [CrossRef]
- Mikami, G.; Grosu, F.; Kawamura, S.; Yoshida, Y.; Carja, G.; Izumi, Y. Harnessing self-supported Au nanoparticles on layered double hydroxides comprising Zn and Al for enhanced phenol decomposition under solar light. Appl. Catal. B Environ. 2016, 199, 260–271. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, Y.; He, S.; Wei, M.; Duan, X. Immobilized Cu–Cr layered double hydroxide films with visible-light responsive photocatalysis for organic pollutants. Chem. Eng. J. 2012, 184, 261–267. [Google Scholar] [CrossRef]
- Seftel, E.M.; Puscasu, M.; Mertens, M.; Cool, P.; Carja, G. Photo-responsive behavior of γ-Fe2O3 NPs embedded into ZnAlFe-LDH matrices and their catalytic efficiency in wastewater remediation. Catal. Today 2015, 252, 7–13. [Google Scholar] [CrossRef]
- Seftel, E.M.; Niarchos, M.; Mitropoulos, C.; Mertens, M.; Vansant, E.F.; Cool, P. Photocatalytic removal of phenol and methylene-blue in aqueous media using TiO2@LDH clay nanocomposites. Catal. Today 2015, 252, 120–127. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Zhang, C.; Zeng, G.-M.; Tan, X.-F.; Huang, D.-L.; Zhou, J.-W.; Fang, Q.-Z.; Yang, K.-H.; Wang, H.; Wei, J.; et al. State-of-the-art progress in the rational design of layered double hydroxide based photocatalysts for photocatalytic and photoelectrochemical H2/O2 production. Coord. Chem. Rev. 2021, 446, 214103. [Google Scholar] [CrossRef]
- Parida, K.M.; Mohapatra, L. Carbonate intercalated Zn/Fe layered double hydroxide: A novel photocatalyst for the enhanced photo degradation of azo dyes. Chem. Eng. J. 2012, 179, 131–139. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K.M. Zn–Cr layered double hydroxide: Visible light responsive photocatalyst for photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2012, 91, 73–80. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, L.; Su, Y.; Shao, D.; Zeng, S.; Wang, W. Photoreduction of carbon dioxide of atmospheric concentration to methane with water over CoAl-layered double hydroxide nanosheets. J. Mater. Chem. A 2018, 6, 8366–8373. [Google Scholar] [CrossRef]
- Bai, S.; Wang, Z.; Tan, L.; Waterhouse, G.I.N.; Zhao, Y.; Song, Y.-F. 600 nm Irradiation-Induced Efficient Photocatalytic CO2 Reduction by Ultrathin Layered Double Hydroxide Nanosheets. Ind. Eng. Chem. Res. 2020, 59, 5848–5857. [Google Scholar] [CrossRef]
- Low, T.; Chaves, A.; Caldwell, J.D.; Kumar, A.; Fang, N.X.; Avouris, P.; Heinz, T.F.; Guinea, F.; Martin-Moreno, L.; Koppens, F. Polaritons in layered two-dimensional materials. Nat. Mater. 2017, 16, 182–194. [Google Scholar] [CrossRef]
- Cortes, E.; Besteiro, L.V.; Alabastri, A.; Baldi, A.; Tagliabue, G.; Demetriadou, A.; Narang, P. Challenges in Plasmonic Catalysis. ACS Nano 2020, 14, 16202–16219. [Google Scholar] [CrossRef]
- Ahmed, N.; Shibata, Y.; Taniguchi, T.; Izumi, Y. Photocatalytic conversion of carbon dioxide into methanol using zinc–copper–M(III) (M = aluminum, gallium) layered double hydroxides. J. Catal. 2011, 279, 123–135. [Google Scholar] [CrossRef]
- Iguchi, S.; Teramura, K.; Hosokawa, S.; Tanaka, T. Effect of the chloride ion as a hole scavenger on the photocatalytic conversion of CO2 in an aqueous solution over Ni-Al layered double hydroxides. Phys. Chem. Chem. Phys. 2015, 17, 17995–18003. [Google Scholar] [CrossRef]
- Flores-Flores, M.; Luévano-Hipólito, E.; Martínez, L.M.T.; Morales-Mendoza, G.; Gómez, R. Photocatalytic CO2 conversion by MgAl layered double hydroxides: Effect of Mg2+ precursor and microwave irradiation time. J. Photochem. Photobiol. A Chem. 2018, 363, 68–73. [Google Scholar] [CrossRef]
- Puscasu, C.-M.; Seftel, E.M.; Mertens, M.; Cool, P.; Carja, G. ZnTiLDH and the Derived Mixed Oxides as Mesoporous Nanoarchitectonics with Photocatalytic Capabilities. J. Inorg. Organomet. Polym. Mater. 2014, 25, 259–266. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Ha, M.; Kim, J.H.; You, M.; Li, Q.; Fan, C.; Nam, J.M. Multicomponent Plasmonic Nanoparticles: From Heterostructured Nanoparticles to Colloidal Composite Nanostructures. Chem. Rev. 2019, 119, 12208–12278. [Google Scholar] [CrossRef]
- Cobley, C.M.; Skrabalak, S.E.; Campbell, D.J.; Xia, Y. Shape-Controlled Synthesis of Silver Nanoparticles for Plasmonic and Sensing Applications. Plasmonics 2009, 4, 171–179. [Google Scholar] [CrossRef]
- Koya, A.N.; Zhu, X.; Ohannesian, N.; Yanik, A.A.; Alabastri, A.; Proietti Zaccaria, R.; Krahne, R.; Shih, W.C.; Garoli, D. Nanoporous Metals: From Plasmonic Properties to Applications in Enhanced Spectroscopy and Photocatalysis. ACS Nano 2021, 15, 6038–6060. [Google Scholar] [CrossRef]
- Cortie, M.B.; McDonagh, A.M. Synthesis and optical properties of hybrid and alloy plasmonic nanoparticles. Chem. Rev. 2011, 111, 3713–3735. [Google Scholar] [CrossRef]
- Vinci, G.; Rapa, M. Noble Metal Nanoparticles Applications: Recent Trends in Food Control. Bioengineering 2019, 6, 10. [Google Scholar] [CrossRef]
- Wang, R.; Shen, J.; Sun, K.; Tang, H.; Liu, Q. Enhancement in photocatalytic activity of CO2 reduction to CH4 by 0D/2D Au/TiO2 plasmon heterojunction. Appl. Surf. Sci. 2019, 493, 1142–1149. [Google Scholar] [CrossRef]
- Kunthakudee, N.; Puangpetch, T.; Ramakul, P.; Serivalsatit, K.; Hunsom, M. Light-assisted synthesis of Au/TiO2 nanoparticles for H2 production by photocatalytic water splitting. Int. J. Hydrog. Energy 2022, 47, 23570–23582. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, Z.; Shen, X.; Zhu, B.; Macharia, D.K.; Chen, Z.; Zhang, L. Synthesis of Au nanoparticle-decorated carbon nitride nanorods with plasmon-enhanced photoabsorption and photocatalytic activity for removing various pollutants from water. J. Hazard. Mater. 2018, 344, 1188–1197. [Google Scholar] [CrossRef]
- Kashyap, T.; Biswasi, S.; Pal, A.R.; Choudhury, B. Unraveling the Catalytic and Plasmonic Roles of g-C3N4 Supported Ag and Au Nanoparticles Under Selective Photoexcitation. ACS Sustain. Chem. Eng. 2019, 7, 19295–19302. [Google Scholar] [CrossRef]
- Yang, F.; Du, M.; Yin, K.; Qiu, Z.; Zhao, J.; Liu, C.; Zhang, G.; Gao, Y.; Pang, H. Applications of Metal-Organic Frameworks in Water Treatment: A Review. Small 2022, 18, 2105715. [Google Scholar] [CrossRef]
- Liang, R.; Jing, F.; Shen, L.; Qin, N.; Wu, L. M@MIL-100(Fe) (M = Au, Pd, Pt) nanocomposites fabricated by a facile photodeposition process: Efficient visible-light photocatalysts for redox reactions in water. Nano Res. 2015, 8, 3237–3249. [Google Scholar] [CrossRef]
- Cure, J.; Mattson, E.; Cocq, K.; Assi, H.; Jensen, S.; Tan, K.; Catalano, M.; Yuan, S.; Wang, H.; Feng, L.; et al. High stability of ultra-small and isolated gold nanoparticles in metal–organic framework materials. J. Mater. Chem. A 2019, 7, 17536–17546. [Google Scholar] [CrossRef]
- Stucchi, M.; Bianchi, C.L.; Argirusis, C.; Pifferi, V.; Neppolian, B.; Cerrato, G.; Boffito, D.C. Ultrasound assisted synthesis of Ag-decorated TiO2 active in visible light. Ultrason. Sonochem 2018, 40, 282–288. [Google Scholar] [CrossRef]
- Rabhi, S.; Mahtout, L.; Bououdina, M.; Khezami, L.; Belkacemi, H.; Kerrami, A.; Sakher, E. Tuning the photocatalytic activity of TiO2 by Ag loading: Experimental and modelling studies for the degradation of amlodipine besylate drug. Ceram. Int. 2021, 47, 21509–21521. [Google Scholar] [CrossRef]
- Cruz, D.; Ortiz-Oliveros, H.B.; Flores-Espinosa, R.M.; Ávila Pérez, P.; Ruiz-López, I.I.; Quiroz-Estrada, K.F. Synthesis of Ag/TiO2 composites by combustion modified and subsequent use in the photocatalytic degradation of dyes. J. King Saud Univ.—Sci. 2022, 34, 101966. [Google Scholar] [CrossRef]
- Chen, M.; Guo, C.; Hou, S.; Wu, L.; Lv, J.; Hu, C.; Zhang, Y.; Xu, J. In-situ fabrication of Ag/P-g-C3N4 composites with enhanced photocatalytic activity for sulfamethoxazole degradation. J. Hazard. Mater. 2019, 366, 219–228. [Google Scholar] [CrossRef]
- Narkbuakaew, T.; Sattayaporn, S.; Saito, N.; Sujaridworakun, P. Investigation of the Ag species and synergy of Ag-TiO2 and g-C3N4 for the enhancement of photocatalytic activity under UV–Visible light irradiation. Appl. Surf. Sci. 2022, 573, 151617. [Google Scholar] [CrossRef]
- Guo, F.; Yang, S.; Liu, Y.; Wang, P.; Huang, J.; Sun, W.-Y. Size Engineering of Metal–Organic Framework MIL-101(Cr)–Ag Hybrids for Photocatalytic CO2 Reduction. ACS Catal. 2019, 9, 8464–8470. [Google Scholar] [CrossRef]
- Hayati, P.; Mehrabadi, Z.; Karimi, M.; Janczak, J.; Mohammadi, K.; Mahmoudi, G.; Dadi, F.; Fard, M.J.S.; Hasanzadeh, A.; Rostamnia, S. Photocatalytic activity of new nanostructures of an Ag(i) metal–organic framework (Ag-MOF) for the efficient degradation of MCPA and 2,4-D herbicides under sunlight irradiation. New J. Chem. 2021, 45, 3408–3417. [Google Scholar] [CrossRef]
- Caswell, T.; Dlamini, M.W.; Miedziak, P.J.; Pattisson, S.; Davies, P.R.; Taylor, S.H.; Hutchings, G.J. Enhancement in the rate of nitrate degradation on Au- and Ag-decorated TiO2 photocatalysts. Catal. Sci. Technol. 2020, 10, 2082–2091. [Google Scholar] [CrossRef]
- Kashyap, T.; Biswas, S.; Ahmed, S.; Kalita, D.; Nath, P.; Choudhury, B. Plasmon activation versus plasmon quenching on the overall photocatalytic performance of Ag/Au bimetal decorated g-C3N4 nanosheets under selective photoexcitation: A mechanistic understanding with experiment and theory. Appl. Catal. B Environ. 2021, 298, 120614. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhang, L.; Fu, H.; He, P.; Han, D.; Lawson, T.; An, X. The Use of Tunable Optical Absorption Plasmonic Au and Ag Decorated TiO2 Structures as Efficient Visible Light Photocatalysts. Catalysts 2020, 10, 139. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.M.; Park, J.E.; Nam, J.M. Nonnoble-Metal-Based Plasmonic Nanomaterials: Recent Advances and Future Perspectives. Adv. Mater. 2018, 30, e1704528. [Google Scholar] [CrossRef]
- Wei, D.; Tan, Y.; Wang, Y.; Kong, T.; Shen, S.; Mao, S.S. Function-switchable metal/semiconductor junction enables efficient photocatalytic overall water splitting with selective water oxidation products. Sci. Bull. 2020, 65, 1389–1395. [Google Scholar] [CrossRef]
- DeSario, P.A.; Gordon, W.O.; Balboa, A.; Pennington, A.M.; Pitman, C.L.; McEntee, M.; Pietron, J.J. Photoenhanced Degradation of Sarin at Cu/TiO2 Composite Aerogels: Roles of Bandgap Excitation and Surface Plasmon Excitation. ACS Appl. Mater. Interfaces 2021, 13, 12550–12561. [Google Scholar] [CrossRef]
- Dai, B.; Zhao, W.; Huang, H.; Li, S.; Yang, G.; Wu, H.; Sun, C.; Leung, D.Y.C. Constructing an ohmic junction of copper@ cuprous oxide nanocomposite with plasmonic enhancement for photocatalysis. J. Colloid Interface Sci. 2022, 616, 163–176. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, J.; Yu, J.; Wang, R.; He, B.; Jin, J.; Wang, H.; Gong, Y. Rational design of all-solid-state TiO2-x/Cu/ZnO Z-scheme heterojunction via ALD-assistance for enhanced photocatalytic activity. J. Colloid Interface Sci. 2022, 607, 760–768. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, H.; Baran, P.; Matras-Postolek, K.; Yang, P. Cu nanocrystals enhanced charge separation and transfer in superior thin g-C3N4 nanosheets. J. Alloy. Compd. 2022, 919, 165892. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Q.; Chen, P.; Chen, L.; Ding, F.; Tang, J.; Li, Y.-J.; Au, C.-T.; Yin, S.-F. Copper-mediated metal-organic framework as efficient photocatalyst for the partial oxidation of aromatic alcohols under visible-light irradiation: Synergism of plasmonic effect and schottky junction. Appl. Catal. B Environ. 2019, 248, 380–387. [Google Scholar] [CrossRef]
- Yu, Y.; Dong, X.; Chen, P.; Geng, Q.; Wang, H.; Li, J.; Zhou, Y.; Dong, F. Synergistic Effect of Cu Single Atoms and Au-Cu Alloy Nanoparticles on TiO2 for Efficient CO2 Photoreduction. ACS Nano 2021, 15, 14453–14464. [Google Scholar] [CrossRef]
- Babu, P.; Dash, S.R.; Behera, A.; Vijayaraghavan, T.; Ashok, A.; Parida, K. Prominence of Cu in a plasmonic Cu–Ag alloy decorated SiO2@S-doped C3N4 core–shell nanostructured photocatalyst towards enhanced visible light activity. Nanoscale Adv. 2022, 4, 150–162. [Google Scholar] [CrossRef]
- He, S.; Huang, J.; Goodsell, J.L.; Angerhofer, A.; Wei, W.D. Plasmonic Nickel-TiO2 Heterostructures for Visible-Light-Driven Photochemical Reactions. Angew. Chem. Int. Ed. Engl. 2019, 58, 6038–6041. [Google Scholar] [CrossRef]
- Wang, C.-C.; Chou, P.-H.; Yu, Y.-H.; Kei, C.-C. Deposition of Ni nanoparticles on black TiO2 nanowire arrays for photoelectrochemical water splitting by atomic layer deposition. Electrochim. Acta 2018, 284, 211–219. [Google Scholar] [CrossRef]
- Tudu, B.; Nalajala, N.; Saikia, P.; Gopinath, C.S. Cu–Ni Bimetal Integrated TiO2 Thin Film for Enhanced Solar Hydrogen Generation. Solar RRL 2020, 4, 1900557. [Google Scholar] [CrossRef]
- Naresh, G.; Kumar, V.; Sasikumar, B.; Venugopal, A. Improved H2 yields over Cu-Ni-TiO2 under solar light irradiation: Behaviour of alloy nano particles on photocatalytic H2O splitting. Appl. Catal. B Environ. 2021, 299, 120654. [Google Scholar] [CrossRef]
- Xu, M.; Wei, M. Layered Double Hydroxide-Based Catalysts: Recent Advances in Preparation, Structure, and Applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Zecevic, J.; Vanbutsele, G.; de Jong, K.P.; Martens, J.A. Nanoscale intimacy in bifunctional catalysts for selective conversion of hydrocarbons. Nature 2015, 528, 245–248. [Google Scholar] [CrossRef]
- Cadars, S.; Layrac, G.; Gérardin, C.; Deschamps, M.; Yates, J.R.; Tichit, D.; Massiot, D. Identification and Quantification of Defects in the Cation Ordering in Mg/Al Layered Double Hydroxides. Chem. Mater. 2011, 23, 2821–2831. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, Z.; Zhou, S.; Wei, M. Perspectives on Multifunctional Catalysts Derived from Layered Double Hydroxides toward Upgrading Reactions of Biomass Resources. ACS Catal. 2021, 11, 6440–6454. [Google Scholar] [CrossRef]
- Aslam, U.; Rao, V.G.; Chavez, S.; Linic, S. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures. Nat. Catal. 2018, 1, 656–665. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, X.; Feng, C.; Li, B.; Chen, T.; Lu, W.; Lei, X.; Xu, S. Crystal-Face-Selective Supporting of Gold Nanoparticles on Layered Double Hydroxide as Efficient Catalyst for Epoxidation of Styrene. ACS Catal. 2011, 1, 232–237. [Google Scholar] [CrossRef]
- Varade, D.; Haraguchi, K. Efficient approach for preparing gold nanoparticles in layered double hydroxide: Synthesis, structure, and properties. J. Mater. Chem. 2012, 22, 17649–17655. [Google Scholar] [CrossRef]
- Mitsudome, T.; Noujima, A.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Supported gold nanoparticles as a reusable catalyst for synthesis of lactones from diols using molecular oxygen as an oxidant under mild conditions. Green Chem. 2009, 11, 793–797. [Google Scholar] [CrossRef]
- Mitsudome, T.; Noujima, A.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Efficient Aerobic Oxidation of Alcohols using a Hydrotalcite-Supported Gold Nanoparticle Catalyst. Adv. Synth. Catal. 2009, 351, 1890–1896. [Google Scholar] [CrossRef]
- Liu, P.; Degirmenci, V.; Hensen, E.J.M. Unraveling the synergy between gold nanoparticles and chromium-hydrotalcites in aerobic oxidation of alcohols. J. Catal. 2014, 313, 80–91. [Google Scholar] [CrossRef]
- Gupta, N.K.; Nishimura, S.; Takagaki, A.; Ebitani, K. Hydrotalcite-supported gold-nanoparticle-catalyzed highly efficient base-free aqueous oxidation of 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid under atmospheric oxygen pressure. Green Chem. 2011, 13, 824–827. [Google Scholar] [CrossRef]
- Noujima, A.; Mitsudome, T.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Selective deoxygenation of epoxides to alkenes with molecular hydrogen using a hydrotalcite-supported gold catalyst: A concerted effect between gold nanoparticles and basic sites on a support. Angew. Chem. Int. Ed. Engl. 2011, 50, 2986–2989. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Zhang, C.; Zeng, G.-M.; Tan, X.-F.; Wang, H.; Huang, D.-L.; Yang, K.-H.; Wei, J.-J.; Ma, C.; Nie, K. Design and engineering of layered double hydroxide based catalysts for water depollution by advanced oxidation processes: A review. J. Mater. Chem. A 2020, 8, 4141–4173. [Google Scholar] [CrossRef]
- Xiao, Q.; Liu, Z.; Wang, F.; Sarina, S.; Zhu, H. Tuning the reduction power of visible-light photocatalysts of gold nanoparticles for selective reduction of nitroaromatics to azoxy-compounds—Tailoring the catalyst support. Appl. Catal. B Environ. 2017, 209, 69–79. [Google Scholar] [CrossRef]
- Arnob, M.M.P.; Artur, C.; Misbah, I.; Mubeen, S.; Shih, W.C. 10x-Enhanced Heterogeneous Nanocatalysis on a Nanoporous Gold Disk Array with High-Density Hot Spots. ACS Appl. Mater. Interfaces 2019, 11, 13499–13506. [Google Scholar] [CrossRef]
- Ao, Y.; Wang, D.; Wang, P.; Wang, C.; Hou, J.; Qian, J. Enhanced photocatalytic properties of the 3D flower-like Mg-Al layered double hydroxides decorated with Ag2CO3 under visible light illumination. Mater. Res. Bull. 2016, 80, 23–29. [Google Scholar] [CrossRef]
- Tonda, S.; Jo, W.-K. Plasmonic Ag nanoparticles decorated NiAl-layered double hydroxide/graphitic carbon nitride nanocomposites for efficient visible-light-driven photocatalytic removal of aqueous organic pollutants. Catal. Today 2018, 315, 213–222. [Google Scholar] [CrossRef]
- Fujiwara, K.; Okuyama, K.; Pratsinis, S.E. Metal–support interactions in catalysts for environmental remediation. Environ. Sci. Nano 2017, 4, 2076–2092. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Patnaik, S.; Rath, D.; Mohapatra, P.; Mohanty, A.; Parida, K. Influence of Au/Pd alloy on an amine functionalised ZnCr LDH–MCM-41 nanocomposite: A visible light sensitive photocatalyst towards one-pot imine synthesis. Catal. Sci. Technol. 2019, 9, 2493–2513. [Google Scholar] [CrossRef]
- Park, J.-E.; Kim, M.; Hwang, J.-H.; Nam, J.-M. Golden Opportunities: Plasmonic Gold Nanostructures for Biomedical Applications based on the Second Near-Infrared Window. Small Methods 2017, 1, 1600032. [Google Scholar] [CrossRef]
- Ma, J.; Ding, J.; Li, L.; Zou, J.; Kong, Y.; Komarneni, S. In situ reduction for synthesis of nano-sized Cu2O particles on MgCuAl-LDH layers for degradation of orange II under visible light. Ceram. Int. 2015, 41, 3191–3196. [Google Scholar] [CrossRef]
- Carja, G.; Dartu, L.; Okada, K.; Fortunato, E. Nanoparticles of copper oxide on layered double hydroxides and the derived solid solutions as wide spectrum active nano-photocatalysts. Chem. Eng. J. 2013, 222, 60–66. [Google Scholar] [CrossRef]
- Wang, X.; Wang, R.; Wang, J.; Fan, C.; Zheng, Z. The synergistic role of the support surface and Au-Cu alloys in a plasmonic Au-Cu@LDH photocatalyst for the oxidative esterification of benzyl alcohol with methanol. Phys. Chem. Chem. Phys. 2020, 22, 1655–1664. [Google Scholar] [CrossRef]
- Liu, H.; Dao, T.D.; Liu, L.; Meng, X.; Nagao, T.; Ye, J. Light assisted CO2 reduction with methane over group VIII metals: Universality of metal localized surface plasmon resonance in reactant activation. Appl. Catal. B Environ. 2017, 209, 183–189. [Google Scholar] [CrossRef]
- Carja, G.; Husanu, E.; Gherasim, C.; Iovu, H. Layered double hydroxides reconstructed in NiSO4 aqueous solution as highly efficient photocatalysts for degrading two industrial dyes. Appl. Catal. B Environ. 2011, 107, 253–259. [Google Scholar] [CrossRef]
- Li, T.; Tan, L.; Zhao, Y.; Song, Y.-F. Solar-driven hydrogen production from steam methane reforming using highly dispersed metallic Ni catalysts supported on layered double hydroxide nanosheets. Chem. Eng. Sci. 2021, 245, 116839. [Google Scholar] [CrossRef]
- Wang, J.; Wei, X.; Song, W.; Shi, X.; Wang, X.; Zhong, W.; Wang, M.; Ju, J.; Tang, Y. Plasmonic Enhancement in Water Splitting Performance for NiFe Layered Double Hydroxide-N10 TC MXene Heterojunction. ChemSusChem 2021, 14, 1948–1954. [Google Scholar] [CrossRef]


| LDH | PNPs | Photocatalytic Application | Reference |
|---|---|---|---|
| ZnAlLDH and ZnCeAlLDH | Au | H2 production from by water splitting | [12] |
| ZnCrLDH | Au | Degradation of VOCs | [42] |
| ZnAlLDH | Au | Phenol degradation | [43] |
| MgAlLDH | Au | 4-nitrophenol degradation | [94,95] |
| MgAlCr-HT | Au | Aerobic oxidation of alcohols | [98] |
| MgAlLDH | Au | Aqueous oxidation of 5-hydroxymethylfurfural | [99] |
| MgAlLDH | Au | Deoxygenation of epoxides to alkenes | [100] |
| M-MgAlLDH (M = Ga3+, Fe3+, Zn2+) | Au | Selective reduction of nitroaromatics to azoxy-compounds | [102] |
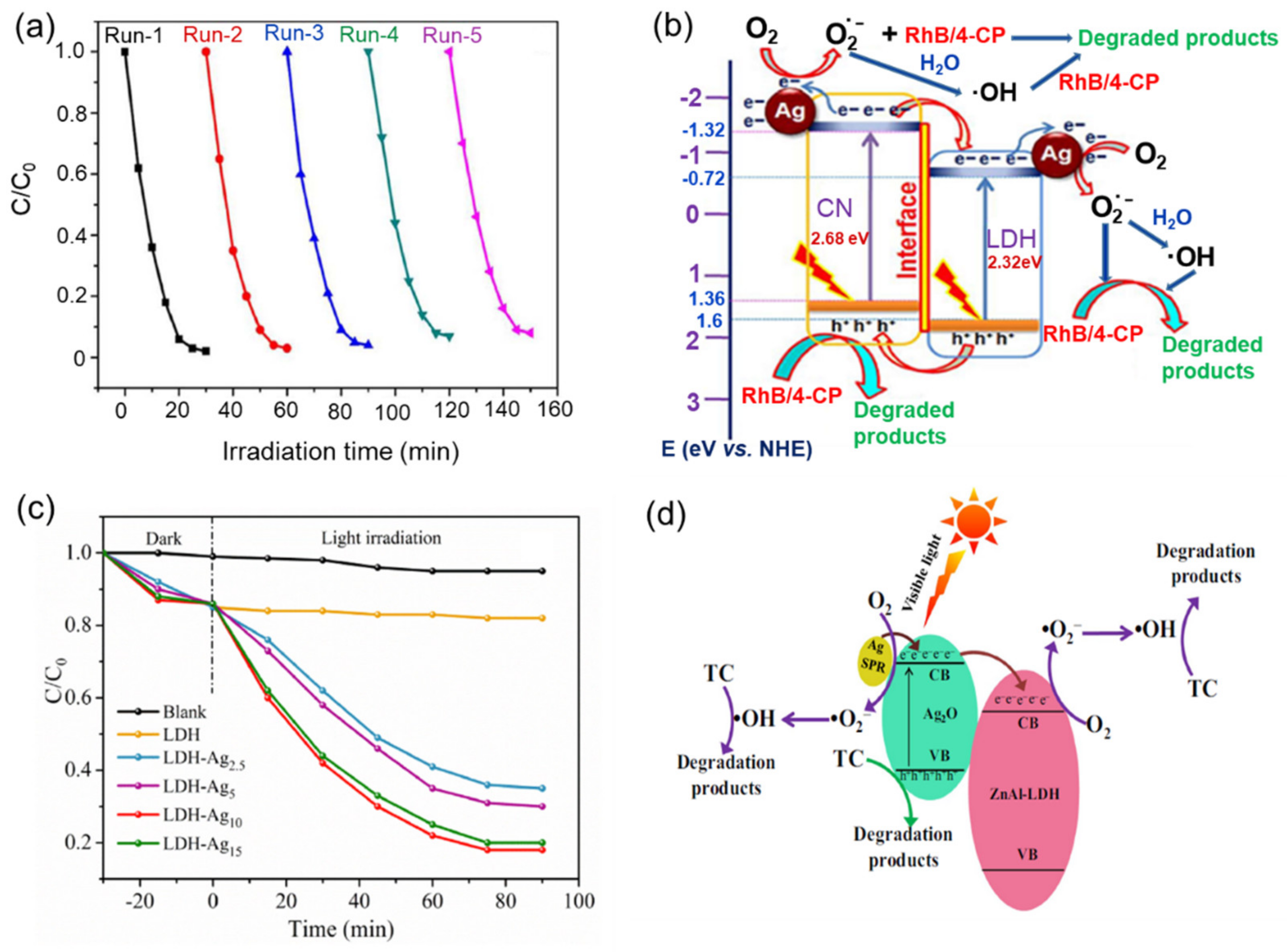
| ZnAlLDH | Ag/Ag2O | Tetracycline degradation | [38] |
| NiAlLDH/CN | Ag | RhB and 4-CP degradation | [105] |
| ZnAlLDH and MgAlLDH | Ag | Phenol degradation | [37] |
| ZnAlLDH | Ag | Phenol degradation | [36] |
| ZnTiLDH | Ag | Degradation of Rhodamine-B (RhB) and NOx | [41] |
| ZnAlLDH and ZnFeAlLDH | Pt, Pt-Ag | p-nitrophenol degradation | [40] |
| ZnCr LDH–MCM-41 | Pd, Au-Pd | One pot synthesis of imines | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilea, D.; Ciocarlan, R.G.; Seftel, E.M.; Cool, P.; Carja, G. Engineering Heterostructures of Layered Double Hydroxides and Metal Nanoparticles for Plasmon-Enhanced Catalysis. Catalysts 2022, 12, 1210. https://doi.org/10.3390/catal12101210
Gilea D, Ciocarlan RG, Seftel EM, Cool P, Carja G. Engineering Heterostructures of Layered Double Hydroxides and Metal Nanoparticles for Plasmon-Enhanced Catalysis. Catalysts. 2022; 12(10):1210. https://doi.org/10.3390/catal12101210
Chicago/Turabian StyleGilea, Diana, Radu G. Ciocarlan, Elena M. Seftel, Pegie Cool, and Gabriela Carja. 2022. "Engineering Heterostructures of Layered Double Hydroxides and Metal Nanoparticles for Plasmon-Enhanced Catalysis" Catalysts 12, no. 10: 1210. https://doi.org/10.3390/catal12101210
APA StyleGilea, D., Ciocarlan, R. G., Seftel, E. M., Cool, P., & Carja, G. (2022). Engineering Heterostructures of Layered Double Hydroxides and Metal Nanoparticles for Plasmon-Enhanced Catalysis. Catalysts, 12(10), 1210. https://doi.org/10.3390/catal12101210








