Emerging Copper-Based Semiconducting Materials for Photocathodic Applications in Solar Driven Water Splitting
Abstract
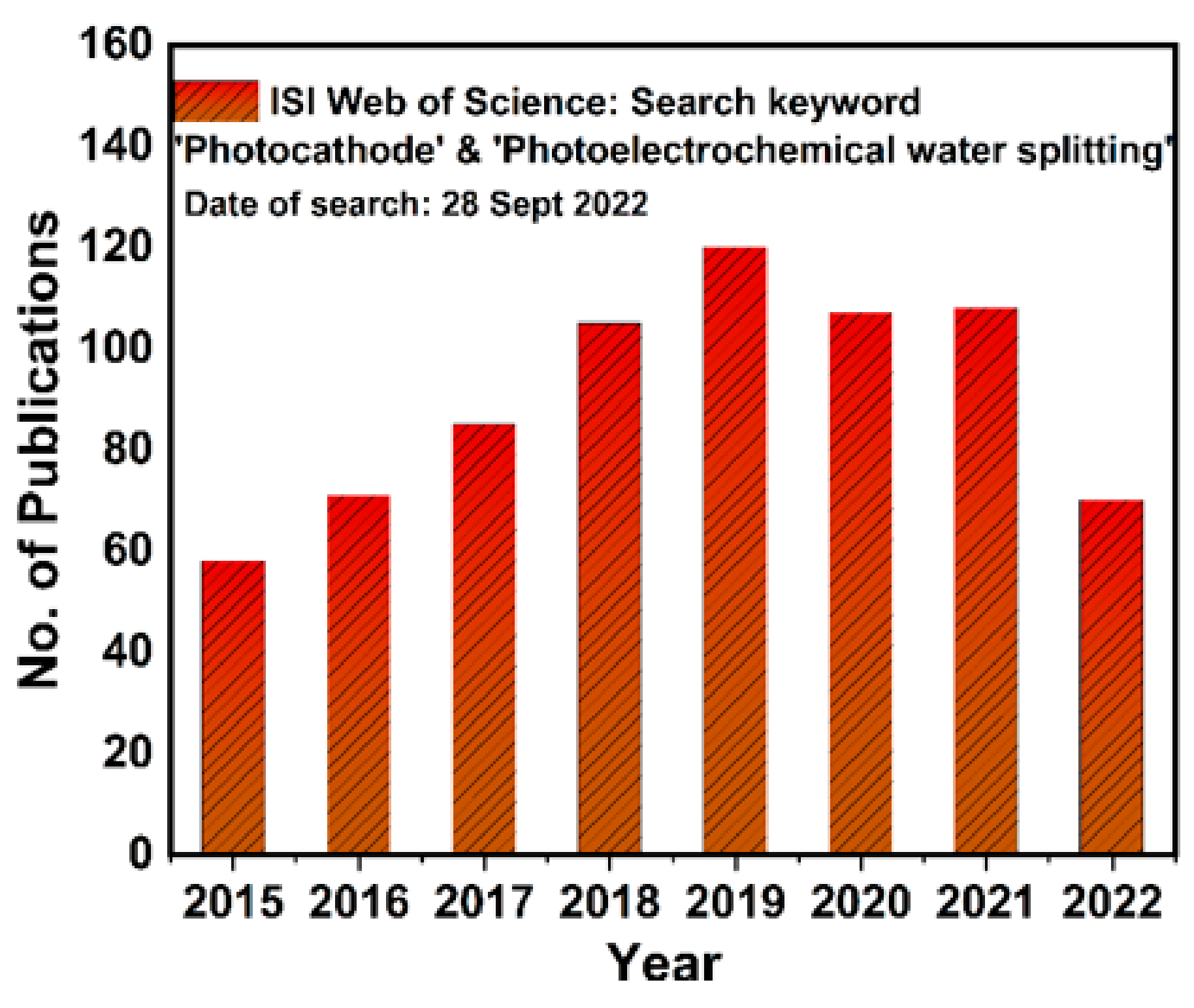
1. Introduction
2. PEC Water Splitting
2.1. Understanding PEC Water Splitting
2.2. Working Principle of Water Splitting
3. Photocathode in a PEC Cell
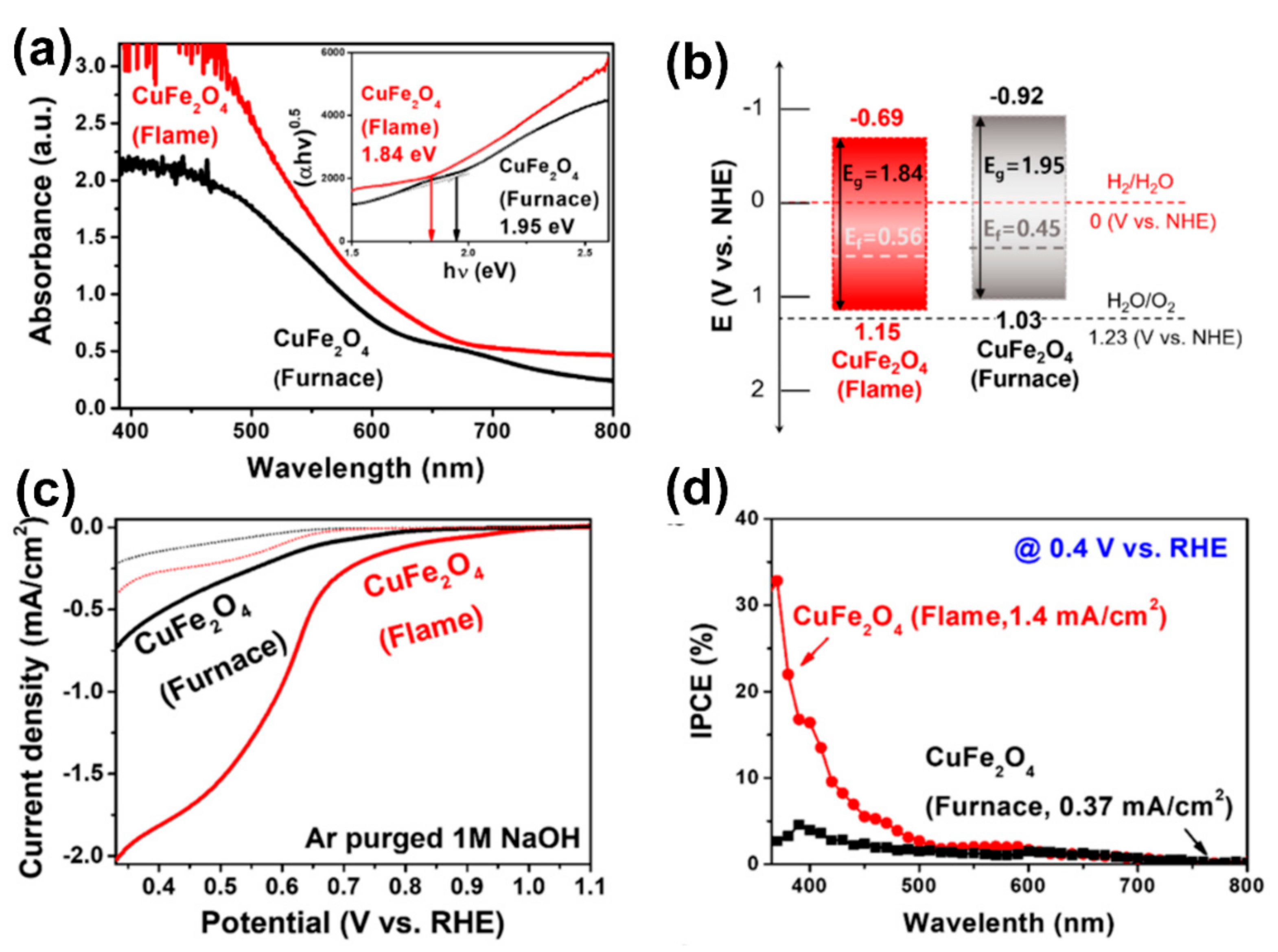
4. Photocathode Materials
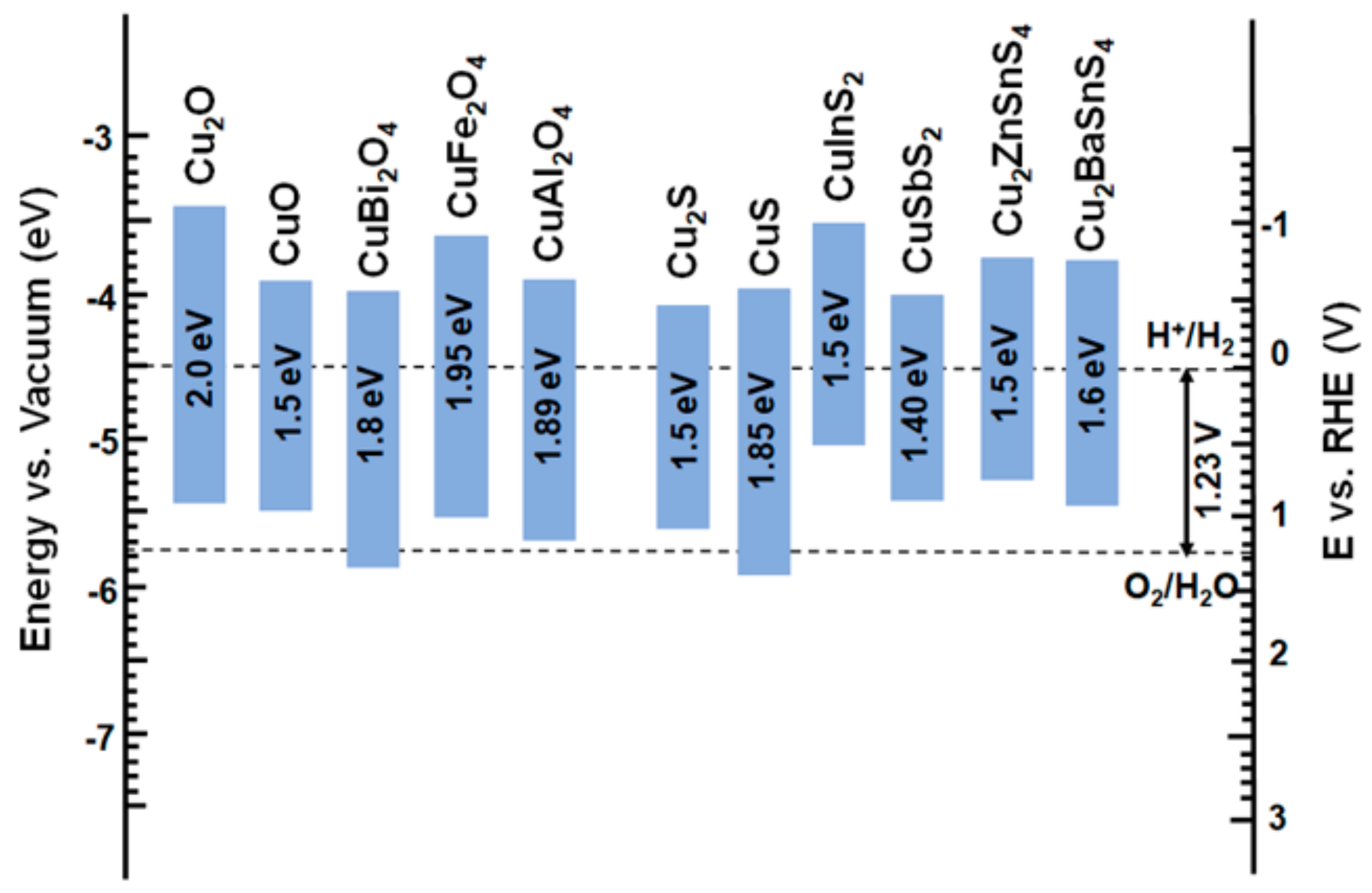
4.1. Cu-Based Metal Oxides for the Photocathode
4.2. Cu-Based Sulfides for Photocathode
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waheed, R.; Sarwar, S.; Wei, C. The Survey of Economic Growth, Energy Consumption and Carbon Emission. Energy Rep. 2019, 5, 1103–1115. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The Role of Hydrogen and Fuel Cells in the Global Energy System. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Sharif, A.; Raza, S.A.; Ozturk, I.; Afshan, S. The Dynamic Relationship of Renewable and Nonrenewable Energy Consumption with Carbon Emission: A Global Study with the Application of Heterogeneous Panel Estimations. Renew. Energy 2019, 133, 685–691. [Google Scholar] [CrossRef]
- Jacobsson, T.J. Photoelectrochemical Water Splitting: An Idea Heading towards Obsolescence? Energy Environ. Sci. 2018, 11, 1977–1979. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Sivula, K.; van de Krol, R. Semiconducting Materials for Photoelectrochemical Energy Conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent Advances in Semiconductors for Photocatalytic and Photoelectrochemical Water Splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Whang, D.R.; Apaydin, D.H. Artificial Photosynthesis: Learning from Nature. ChemPhotoChem 2018, 2, 148–160. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, S.; Han, D.; Liu, T.; Wang, G.; Li, Y. Progress in Developing Metal Oxide Nanomaterials for Photoelectrochemical Water Splitting. Adv. Energy Mater. 2017, 7, 1–26. [Google Scholar] [CrossRef]
- Chaves, A.; Azadani, J.G.; Alsalman, H.; da Costa, D.R.; Frisenda, R.; Chaves, A.J.; Song, S.H.; Kim, Y.D.; He, D.; Zhou, J.; et al. Bandgap Engineering of Two-Dimensional Semiconductor Materials. NPJ 2D Mater. Appl. 2020, 4, 29. [Google Scholar] [CrossRef]
- Su, J.; Vayssieres, L. A Place in the Sun for Artificial Photosynthesis? ACS Energy Lett. 2016, 1, 121–135. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Subramanyam, P.; Kumar, P.N.; Deepa, M.; Subrahmanyam, C.; Ghosal, P. Bismuth Sulfide Nanocrystals and Gold Nanorods Increase the Photovoltaic Response of a TiO2/CdS Based Cell. Sol. Energy Mater. Sol. Cells 2017, 159, 296–306. [Google Scholar] [CrossRef]
- Subramanyam, P.; Vinodkumar, T.; Nepak, D.; Deepa, M.; Subrahmanyam, C. Mo-Doped BiVO4@reduced Graphene Oxide Composite as an Efficient Photoanode for Photoelectrochemical Water Splitting. Catal. Today 2019, 325, 73–80. [Google Scholar] [CrossRef]
- Subramanyam, P.; Meena, B.; Sinha, G.N.; Deepa, M.; Subrahmanyam, C. Decoration of Plasmonic Cu Nanoparticles on WO3/Bi2S3 QDs Heterojunction for Enhanced Photoelectrochemical Water Splitting. Int. J. Hydrogen Energy 2020, 45, 7706–7715. [Google Scholar] [CrossRef]
- Subramanyam, P.; Meena, B.; Suryakala, D.; Deepa, M.; Subrahmanyam, C. Plasmonic Nanometal Decorated Photoanodes for Efficient Photoelectrochemical Water Splitting. Catal. Today 2020, 379, 1–6. [Google Scholar] [CrossRef]
- Li, J.; Cushing, S.K.; Zheng, P.; Senty, T.; Meng, F.; Bristow, A.D.; Manivannan, A.; Wu, N. Solar Hydrogen Generation by a CdS-Au-TiO2 Sandwich Nanorod Array Enhanced with Au Nanoparticle as Electron Relay and Plasmonic Photosensitizer. J. Am. Chem. Soc. 2014, 136, 8438–8449. [Google Scholar] [CrossRef]
- Shi, H.; Guo, H.; Wang, S.; Zhang, G.; Hu, Y.; Jiang, W.; Liu, G. Visible Light Photoanode Material for Photoelectrochemical Water Splitting: A Review of Bismuth Vanadate. Energy Fuels 2022, 36, 11404–11427. [Google Scholar] [CrossRef]
- Yang, W.; Moon, J. Recent Advances in Earth-Abundant Photocathodes for Photoelectrochemical Water Splitting. ChemSusChem 2019, 12, 1889–1899. [Google Scholar] [CrossRef]
- Toe, C.Y.; Zhou, S.; Gunawan, M.; Lu, X.; Ng, Y.H.; Amal, R. Recent Advances and the Design Criteria of Metal Sulfide Photocathodes and Photoanodes for Photoelectrocatalysis. J. Mater. Chem. A 2021, 9, 20277–20319. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering Heterogeneous Semiconductors for Solar Water Splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Chen, Q.; Fan, G.; Fu, H.; Li, Z.; Zou, Z. Tandem Photoelectrochemical Cells for Solar Water Splitting. Adv. Phys. X 2018, 3, 1487267. [Google Scholar] [CrossRef]
- Prévot, M.S.; Sivula, K. Photoelectrochemical Tandem Cells for Solar Water Splitting. J. Phys. Chem. C 2013, 117, 17879–17893. [Google Scholar] [CrossRef]
- Chen, Z.; Jaramillo, T.F.; Deutsch, T.G.; Kleiman-Shwarsctein, A.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating Materials Development for Photoelectrochemical Hydrogen Production: Standards for Methods, Definitions, and Reporting Protocols. J. Mater. Res. 2010, 25, 3–16. [Google Scholar] [CrossRef]
- Lin, Y.; Battaglia, C.; Boccard, M.; Hettick, M.; Yu, Z.; Ballif, C.; Ager, J.W.; Javey, A. Amorphous Si Thin Film Based Photocathodes with High Photovoltage for Efficient Hydrogen Production. Nano Lett. 2013, 13, 5615–5618. [Google Scholar] [CrossRef]
- Lee, M.H.; Takei, K.; Zhang, J.; Kapadia, R.; Zheng, M.; Chen, Y.-Z.; Nah, J.; Matthews, T.S.; Chueh, Y.-L.; Ager, J.W.; et al. P-Type InP Nanopillar Photocathodes for Efficient Solar-Driven Hydrogen Production. Angew. Chem. Int. Ed. 2012, 51, 10760–10764. [Google Scholar] [CrossRef]
- Liu, C.; Sun, J.; Tang, J.; Yang, P. Zn-Doped p-Type Gallium Phosphide Nanowire Photocathodes from a Surfactant-Free Solution Synthesis. Nano Lett. 2012, 12, 5407–5411. [Google Scholar] [CrossRef]
- Yang, W.; Kim, J.H.; Hutter, O.S.; Phillips, L.J.; Tan, J.; Park, J.; Lee, H.; Major, J.D.; Lee, J.S.; Moon, J. Benchmark Performance of Low-Cost Sb2Se3 Photocathodes for Unassisted Solar Overall Water Splitting. Nat. Commun. 2020, 11, 861. [Google Scholar] [CrossRef]
- Meena, B.; Kumar, M.; Gupta, S.; Sinha, L.; Subramanyam, P.; Subrahmanyam, C. Rational Design of TiO2/BiSbS3 Heterojunction for Efficient Solar Water Splitting. Sustain. Energy Technol. Assessments 2022, 49, 101775. [Google Scholar] [CrossRef]
- Li, C.; He, J.; Xiao, Y.; Li, Y.; Delaunay, J.-J. Earth-Abundant Cu-Based Metal Oxide Photocathodes for Photoelectrochemical Water Splitting. Energy Environ. Sci. 2020, 13, 3269–3306. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly Active Oxide Photocathode for Photoelectrochemical Water Reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Hara, M. Cu2O as a Photocatalyst for Overall Water Splitting under Visible Light Irradiation. Chem. Commun. 1998, 2, 357–358. [Google Scholar] [CrossRef]
- Ding, C.; Shi, J.; Wang, Z.; Li, C. Photoelectrocatalytic Water Splitting: Significance of Cocatalysts, Electrolyte, and Interfaces. ACS Catal. 2017, 7, 675–688. [Google Scholar] [CrossRef]
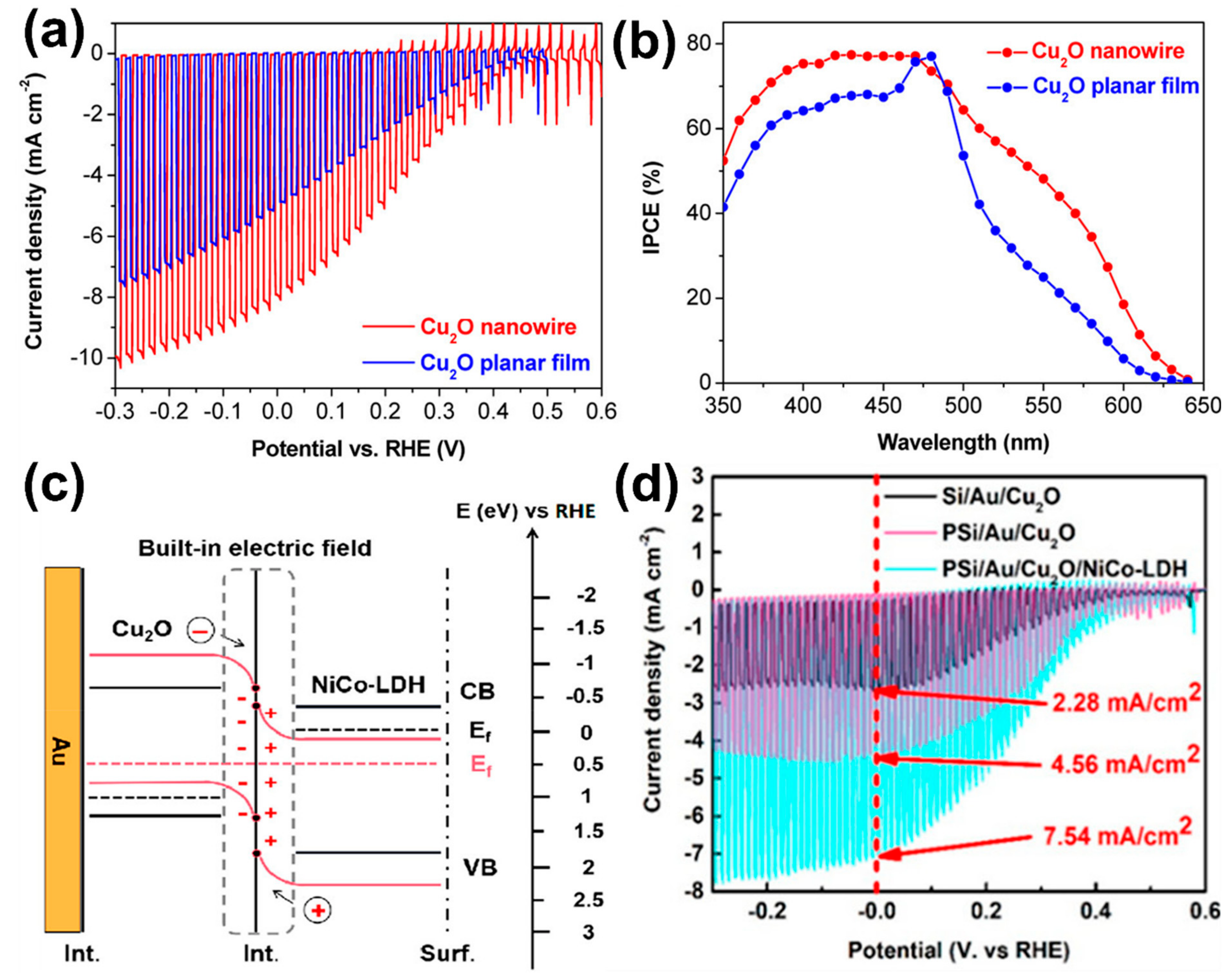
- Luo, J.; Steier, L.; Son, M.-K.; Schreier, M.; Mayer, M.T.; Grätzel, M. Cu2O Nanowire Photocathodes for Efficient and Durable Solar Water Splitting. Nano Lett. 2016, 16, 1848–1857. [Google Scholar] [CrossRef]
- Son, M.-K.; Pan, L.; Mayer, M.T.; Hagfeldt, A.; Grätzel, M.; Luo, J. Structural and Compositional Investigations on the Stability of Cuprous Oxide Nanowire Photocathodes for Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 55080–55091. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, W.; Wang, D.; Chen, H.; Zhou, G. Enhanced Light Trapping and Charge Separation via Pyramidal Cu2O/NiCo-LDH Photocathode for Efficient Water Splitting. ACS Appl. Energy Mater. 2022, 5, 992–1001. [Google Scholar] [CrossRef]
- Dubale, A.A.; Tamirat, A.G.; Chen, H.-M.; Berhe, T.A.; Pan, C.-J.; Su, W.-N.; Hwang, B.-J. A Highly Stable CuS and CuS–Pt Modified Cu2O/CuO Heterostructure as an Efficient Photocathode for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2016, 4, 2205–2216. [Google Scholar] [CrossRef]
- Son, M.K.; Steier, L.; Schreier, M.; Mayer, M.T.; Luo, J.; Grätzel, M. A Copper Nickel Mixed Oxide Hole Selective Layer for Au-Free Transparent Cuprous Oxide Photocathodes. Energy Environ. Sci. 2017, 10, 912–918. [Google Scholar] [CrossRef]
- Subramanyam, P.; Meena, B.; Biju, V.; Misawa, H.; Challapalli, S. Emerging Materials for Plasmon-Assisted Photoelectrochemical Water Splitting. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100472. [Google Scholar] [CrossRef]
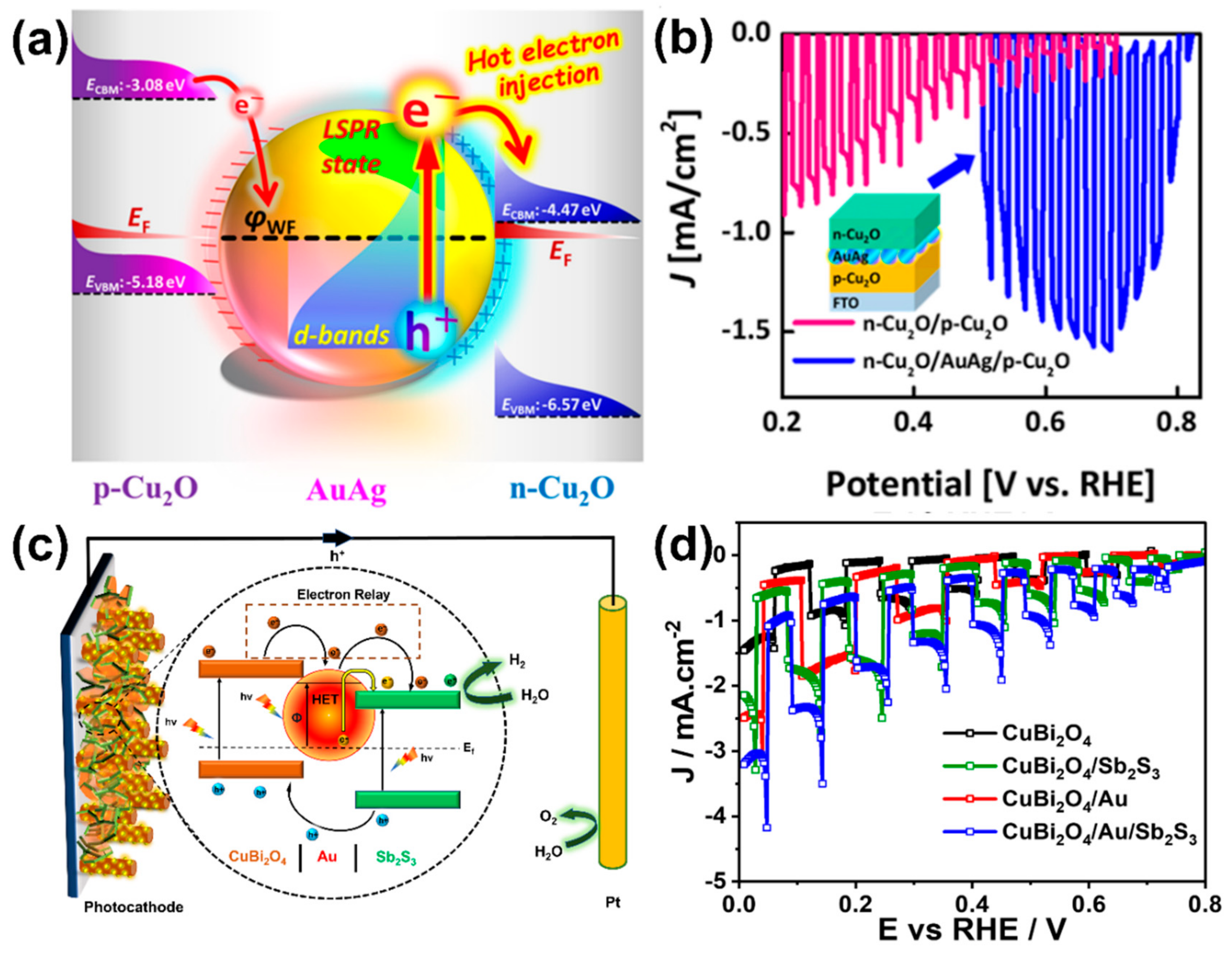
- Lin, Y.-C.; Hsu, L.-C.; Lin, C.-Y.; Chiang, C.-L.; Chou, C.-M.; Wu, W.-W.; Chen, S.-Y.; Lin, Y.-G. Sandwich-Nanostructured n-Cu2O/AuAg/p-Cu2O Photocathode with Highly Positive Onset Potential for Improved Water Reduction. ACS Appl. Mater. Interfaces 2019, 11, 38625–38632. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ghosh, C.C.; Meena, B.; Ma, T.; Subrahmanyam, C. Plasmonic Au Nanoparticle Sandwiched CuBi2O4/S2S3 Photocathode with Multi-Mediated Electron Transfer for Efficient Solar Water Splitting. Sustain. Energy Fuels 2022, 6, 3961–3974. [Google Scholar] [CrossRef]
- Septina, W.; Prabhakar, R.R.; Wick, R.; Moehl, T.; Tilley, S.D. Stabilized Solar Hydrogen Production with CuO/CdS Heterojunction Thin Film Photocathodes. Chem. Mater. 2017, 29, 1735–1743. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Moakhar, R.S.; Chua, C.S.; Tan, H.R.; Wong, T.I.; Chi, D.; Dalapati, G.K. Nanocrystal Engineering of Sputter-Grown CuO Photocathode for Visible-Light-Driven Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2016, 8, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Cots, A.; Bonete, P.; Gómez, R. Improving the Stability and Efficiency of CuO Photocathodes for Solar Hydrogen Production through Modification with Iron. ACS Appl. Mater. Interfaces 2018, 10, 26348–26356. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, L.; Schülli, T.U.; Bai, Y.; Monny, S.A.; Du, A.; Wang, L. Identifying Copper Vacancies and Their Role in the CuO Based Photocathode for Water Splitting. Angew. Chem. 2019, 131, 17768–17773. [Google Scholar] [CrossRef]
- Nakabayashi, Y.; Nishikawa, M.; Nosaka, Y. Fabrication of CuBi2O4 Photocathode through Novel Anodic Electrodeposition for Solar Hydrogen Production. Electrochim. Acta 2014, 125, 191–198. [Google Scholar] [CrossRef]
- Park, S.; Baek, J.H.; Zhang, L.; Lee, J.M.; Stone, K.H.; Cho, I.S.; Guo, J.; Jung, H.S.; Zheng, X. Rapid Flame-Annealed CuFe2O4 as Efficient Photocathode for Photoelectrochemical Hydrogen Production. ACS Sustain. Chem. Eng. 2019, 7, 5867–5874. [Google Scholar] [CrossRef]
- Tan, R.; Hwang, S.W.; Sivanantham, A.; Cho, I.S. Solution Synthesis and Activation of Spinel CuAl2O4 Film for Solar Water-Splitting. J. Catal. 2021, 400, 218–227. [Google Scholar] [CrossRef]
- Crespo, C.T. CuNbO3 as a Solar Energy Converter to Fuel and Electricity. Sol. Energy Mater. Sol. Cells 2018, 179, 305–311. [Google Scholar] [CrossRef]
- Creissen, C.E.; Warnan, J.; Reisner, E. Solar H2 Generation in Water with a CuCrO2 Photocathode Modified with an Organic Dye and Molecular Ni Catalyst. Chem. Sci. 2018, 9, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Monny, S.A.; Zhang, L.; Wang, Z.; Luo, B.; Konarova, M.; Du, A.; Wang, L. Fabricating Highly Efficient Heterostructured CuBi2O4 Photocathodes for Unbiased Water Splitting. J. Mater. Chem. A 2020, 8, 2498–2504. [Google Scholar] [CrossRef]
- Kang, D.; Hill, J.C.; Park, Y.; Choi, K.-S. Photoelectrochemical Properties and Photostabilities of High Surface Area CuBi2O4 and Ag-Doped CuBi2O4 Photocathodes. Chem. Mater. 2016, 28, 4331–4340. [Google Scholar] [CrossRef]
- Xu, N.; Li, F.; Gao, L.; Hu, H.; Hu, Y.; Long, X.; Ma, J.; Jin, J. N,Cu-Codoped Carbon Nanosheet/Au/CuBi2O4 Photocathodes for Efficient Photoelectrochemical Water Splitting. ACS Sustain. Chem. Eng. 2018, 6, 7257–7264. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.E.; Kim, J.H.; Lee, J.S. Ferrites: Emerging Light Absorbers for Solar Water Splitting. J. Mater. Chem. A 2020, 8, 9447–9482. [Google Scholar] [CrossRef]
- Maitra, S.; Pal, S.; Maitra, T.; Halder, S.; Roy, S. Solvothermal Etching-Assisted Phase and Morphology Tailoring in Highly Porous CuFe2O4 Nanoflake Photocathodes for Solar Water Splitting. Energy Fuels 2021, 35, 14087–14100. [Google Scholar] [CrossRef]
- Atacan, K.; Topaloğlu, B.; Özacar, M. New CuFe2O4/Amorphous Manganese Oxide Nanocomposites Used as Photocatalysts in Photoelectrochemical Water Splitting. Appl. Catal. A Gen. 2018, 564, 33–42. [Google Scholar] [CrossRef]
- Oh, Y.; Yang, W.; Kim, J.; Jeong, S.; Moon, J. Enhanced Photocurrent of Transparent CuFeO2 Photocathodes by Self-Light-Harvesting Architecture. ACS Appl. Mater. Interfaces 2017, 9, 14078–14087. [Google Scholar] [CrossRef]
- Siripala, W.; Ivanovskaya, A.; Jaramillo, T.F.; Baeck, S.H.; McFarland, E.W. A Cu2O/TiO2 Heterojunction Thin Film Cathode for Photoelectrocatalysis. Sol. Energy Mater. Sol. Cells 2003, 77, 229–237. [Google Scholar] [CrossRef]
- Zhang, Z.; Dua, R.; Zhang, L.; Zhu, H.; Zhang, H.; Wang, P. Carbon-Layer-Protected Cuprous Oxide Nanowire Arrays for Efficient Water Reduction. ACS Nano 2013, 7, 1709–1717. [Google Scholar] [CrossRef]
- Paracchino, A.; Mathews, N.; Hisatomi, T.; Stefik, M.; Tilley, S.D.; Grätzel, M. Ultrathin Films on Copper(i) Oxide Water Splitting Photocathodes: A Study on Performance and Stability. Energy Environ. Sci. 2012, 5, 8673. [Google Scholar] [CrossRef]
- Tilley, S.D.; Schreier, M.; Azevedo, J.; Stefik, M.; Graetzel, M. Ruthenium Oxide Hydrogen Evolution Catalysis on Composite Cuprous Oxide Water-Splitting Photocathodes. Adv. Funct. Mater. 2014, 24, 303–311. [Google Scholar] [CrossRef]
- Azevedo, J.; Steier, L.; Dias, P.; Stefik, M.; Sousa, C.T.; Araújo, J.P.; Mendes, A.; Graetzel, M.; Tilley, S.D. On the Stability Enhancement of Cuprous Oxide Water Splitting Photocathodes by Low Temperature Steam Annealing. Energy Environ. Sci. 2014, 7, 4044–4052. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Tilley, S.D.; Vrubel, H.; Grätzel, M.; Hu, X. Hydrogen Evolution from a Copper(I) Oxide Photocathode Coated with an Amorphous Molybdenum Sulphide Catalyst. Nat. Commun. 2014, 5, 3059. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Liardet, L.; Mayer, M.T.; Tilley, S.D.; Grätzel, M.; Hu, X. Photoelectrochemical Hydrogen Production in Alkaline Solutions Using Cu2O Coated with Earth-Abundant Hydrogen Evolution Catalysts. Angew. Chem. Int. Ed. 2014, 54, 664–667. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Guo, Z.; Yan, W.; Ruan, M. Decorating Cu2O Photocathode with Noble-Metal-Free Al and NiS Cocatalysts for Efficient Photoelectrochemical Water Splitting by Light Harvesting Management and Charge Separation Design. Chem. Eng. J. 2020, 381, 122655. [Google Scholar] [CrossRef]
- Kim, J.S.; Cho, S.W.; Deshpande, N.G.; Kim, Y.B.; Yun, Y.D.; Jung, S.H.; Kim, D.S.; Cho, H.K. Toward Robust Photoelectrochemical Operation of Cuprous Oxide Nanowire Photocathodes Using a Strategically Designed Solution-Processed Titanium Oxide Passivation Coating. ACS Appl. Mater. Interfaces 2019, 11, 14840–14847. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, Z.; Liu, Z. FeOOH as Hole Transfer Layer to Retard the Photocorrosion of Cu2O for Enhanced Photoelctrochemical Performance. Appl. Catal. B Environ. 2020, 260, 118213. [Google Scholar] [CrossRef]
- Pan, L.; Liu, Y.; Yao, L.; Ren, D.; Sivula, K.; Grätzel, M.; Hagfeldt, A. Cu2O Photocathodes with Band-Tail States Assisted Hole Transport for Standalone Solar Water Splitting. Nat. Commun. 2020, 11, 318. [Google Scholar] [CrossRef]
- Fu, X.; Chang, H.; Shang, Z.; Liu, P.; Liu, J.; Luo, H. Three-Dimensional Cu2O Nanorods Modified by Hydrogen Treated Ti3C2TX MXene with Enriched Oxygen Vacancies as a Photocathode and a Tandem Cell for Unassisted Solar Water Splitting. Chem. Eng. J. 2020, 381, 122001. [Google Scholar] [CrossRef]
- Tawfik, W.Z.; Hassan, M.A.; Johar, M.A.; Ryu, S.-W.; Lee, J.K. Highly Conversion Efficiency of Solar Water Splitting over P-Cu2O/ZnO Photocatalyst Grown on a Metallic Substrate. J. Catal. 2019, 374, 276–283. [Google Scholar] [CrossRef]
- Caretti, M.; Lazouni, L.; Xia, M.; Wells, R.A.; Nussbaum, S.; Ren, D.; Grätzel, M.; Sivula, K. Transparency and Morphology Control of Cu2O Photocathodes via an in Situ Electroconversion. ACS Energy Lett. 2022, 7, 1618–1625. [Google Scholar] [CrossRef]
- Varunkumar, K.; Sellappan, R. Photoelectrochemical Behaviour of CuBi2O4@MoS2 Photocathode for Solar Water Splitting. Mater. Chem. Phys. 2021, 261, 124245. [Google Scholar] [CrossRef]
- Wang, F.; Chemseddine, A.; Abdi, F.F.; van de Krol, R.; Berglund, S.P. Spray Pyrolysis of CuBi2O4 Photocathodes: Improved Solution Chemistry for Highly Homogeneous Thin Films. J. Mater. Chem. A 2017, 5, 12838–12847. [Google Scholar] [CrossRef]
- Berglund, S.P.; Abdi, F.F.; Bogdanoff, P.; Chemseddine, A.; Friedrich, D.; van de Krol, R. Comprehensive Evaluation of CuBi2O4 as a Photocathode Material for Photoelectrochemical Water Splitting. Chem. Mater. 2016, 28, 4231–4242. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, C.Y.; Reisner, E. Photoelectrochemical Reduction of Aqueous Protons with a CuO|CuBi2O4 Heterojunction under Visible Light Irradiation. Phys. Chem. Chem. Phys. 2014, 16, 22462–22465. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, H.; Choi, K.S.; Kim, S.; Seo, S.; Song, J.; Choi, B.; Ryu, J.; Ryu, S.; Oh, J.; et al. Template Engineering of CuBi2O4 Single-Crystal Thin Film Photocathodes. Small 2020, 16, 2002429. [Google Scholar] [CrossRef]
- Lee, J.; Yoon, H.; Kim, S.; Seo, S.; Song, J.; Choi, B.-U.; Choi, S.Y.; Park, H.; Ryu, S.; Oh, J.; et al. Long-Term Stabilized High-Density CuBi2O4/NiO Heterostructure Thin Film Photocathode Grown by Pulsed Laser Deposition. Chem. Commun. 2019, 55, 12447–12450. [Google Scholar] [CrossRef]
- Wei, S.; Xu, N.; Li, F.; Long, X.; Hu, Y.; Gao, L.; Wang, C.; Li, S.; Ma, J.; Jin, J. Rationally Designed Heterojunction on a CuBi2O4 Photocathode for Improved Activity and Stability during Photoelectrochemical Water Reduction. ChemElectroChem 2019, 6, 3367–3374. [Google Scholar] [CrossRef]
- Wei, S.; Wang, C.; Long, X.; Wang, T.; Wang, P.; Zhang, M.; Li, S.; Ma, J.; Jin, J.; Wu, L. A Oxygen Vacancy-Modulated Homojunction Structural CuBi2O4 photocathodes for Efficient Solar Water Reduction. Nanoscale 2020, 12, 15193–15200. [Google Scholar] [CrossRef]
- Cao, D.; Nasori, N.; Wang, Z.; Mi, Y.; Wen, L.; Yang, Y.; Qu, S.; Wang, Z.; Lei, Y. P-Type CuBi2O4: An Easily Accessible Photocathodic Material for High-Efficiency Water Splitting. J. Mater. Chem. A 2016, 4, 8995–9001. [Google Scholar] [CrossRef]
- Pulipaka, S.; Boni, N.; Ummethala, G.; Meduri, P. CuO/CuBi2O4 Heterojunction Photocathode: High Stability and Current Densities for Solar Water Splitting. J. Catal. 2020, 387, 17–27. [Google Scholar] [CrossRef]
- Wang, F.; Septina, W.; Chemseddine, A.; Abdi, F.F.; Friedrich, D.; Bogdanoff, P.; van de Krol, R.; Tilley, S.D.; Berglund, S.P. Gradient Self-Doped CuBi2O4 with Highly Improved Charge Separation Efficiency. J. Am. Chem. Soc. 2017, 139, 15094–15103. [Google Scholar] [CrossRef] [PubMed]
- Prévot, M.S.; Guijarro, N.; Sivula, K. Enhancing the Performance of a Robust Sol-Gel-Processed p-Type Delafossite CuFeO2 Photocathode for Solar Water Reduction. ChemSusChem 2015, 8, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Park, Y.B.; Kim, H.E.; Choi, Y.H.; Choi, S.H.; Lee, J.S. Oxygen-Intercalated CuFeO2 Photocathode Fabricated by Hybrid Microwave Annealing for Efficient Solar Hydrogen Production. Chem. Mater. 2016, 28, 6054–6061. [Google Scholar] [CrossRef]
- Prévot, M.S.; Li, Y.; Guijarro, N.; Sivula, K. Improving Charge Collection with Delafossite Photocathodes: A Host–Guest CuAlO2/CuFeO2 Approach. J. Mater. Chem. A 2016, 4, 3018–3026. [Google Scholar] [CrossRef]
- Li, M.; Zhao, R.; Su, Y.; Yang, Z.; Zhang, Y. Carbon Quantum Dots Decorated Cu2S Nanowire Arrays for Enhanced Photoelectrochemical Performance. Nanoscale 2016, 8, 8559–8567. [Google Scholar] [CrossRef]
- Chen, L.; Hu, H.; Chen, Y.; Gao, J.; Li, G. Plasmonic Cu2-XS Nanoparticles: A Brief Introduction of Optical Properties and Applications. Mater. Adv. 2021, 2, 907–926. [Google Scholar] [CrossRef]
- Yu, Y.-X.; Pan, L.; Son, M.-K.; Mayer, M.T.; Zhang, W.-D.; Hagfeldt, A.; Luo, J.; Grätzel, M. Solution-Processed Cu2S Photocathodes for Photoelectrochemical Water Splitting. ACS Energy Lett. 2018, 3, 760–766. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, X.; Chen, D. Photoelectrochemical Water Splitting of CuInS2 Photocathode Collaborative Modified with Separated Catalysts Based on Efficient Photogenerated Electron–Hole Separation. ACS Sustain. Chem. Eng. 2018, 6, 10289–10294. [Google Scholar] [CrossRef]
- Feng, X.; Li, R.; Wang, M.; Chen, Y. Switchable Synthesis of P- and n-Type Cu–In–S Grooved Pyramid-like Microcrystals for Unassisted Photoelectrochemical Water Splitting. J. Mater. Chem. A 2018, 6, 11180–11188. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, M. Co-Modification with Cost-Effective Nickel Oxides and Nickel Sulfides on CuInS2 Nanosheets Photocathode for Enhanced Photoelectrochemical Performance. ACS Sustain. Chem. Eng. 2020, 8, 512–519. [Google Scholar] [CrossRef]
- Gunawan; Septina, W.; Harada, T.; Nose, Y.; Ikeda, S. Investigation of the Electric Structures of Heterointerfaces in Pt- and In2S3 -Modified CuInS2 Photocathodes Used for Sunlight-Induced Hydrogen Evolution. ACS Appl. Mater. Interfaces 2015, 7, 16086–16092. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.Y.; Kim, Y.; Park, E.D.; Im, S.H.; Joo, O.-S. CuInS2 Photocathodes with Atomic Gradation-Controlled (Ta,Mo) x (O,S) y Passivation Layers for Efficient Photoelectrochemical H2 Production. ACS Appl. Mater. Interfaces 2021, 13, 58447–58457. [Google Scholar] [CrossRef] [PubMed]
- Connor, S.T.; Weil, B.D.; Misra, S.; Cui, Y.; Toney, M.F. Behaviors of Fe, Zn, and Ga Substitution in CuInS2 Nanoparticles Probed with Anomalous X-Ray Diffraction. Chem. Mater. 2013, 25, 320–325. [Google Scholar] [CrossRef]
- Vahidshad, Y.; Tahir, M.N.; Zad, A.I.; Mirkazemi, S.M.; Ghasemzadeh, R.; Huesmann, H.; Tremel, W. Structural and Optical Study of Ga3+ Substitution in CuInS2 Nanoparticles Synthesized by a One-Pot Facile Method. J. Phys. Chem. C 2014, 118, 24670–24679. [Google Scholar] [CrossRef]
- Septina, W.; Gunawan; Ikeda, S.; Harada, T.; Higashi, M.; Abe, R.; Matsumura, M. Photosplitting of Water from Wide-Gap Cu(In,Ga)S2 Thin Films Modified with a CdS Layer and Pt Nanoparticles for a High-Onset-Potential Photocathode. J. Phys. Chem. C 2015, 119, 8576–8583. [Google Scholar] [CrossRef]
- Kaga, H.; Tsutsui, Y.; Nagane, A.; Iwase, A.; Kudo, A. An Effect of Ag(i)-Substitution at Cu Sites in CuGaS2 on Photocatalytic and Photoelectrochemical Properties for Solar Hydrogen Evolution. J. Mater. Chem. A 2015, 3, 21815–21823. [Google Scholar] [CrossRef]
- Teimouri, R.; Mohammadpour, R. Potential Application of CuSbS2 as the Hole Transport Material in Perovskite Solar Cell: A Simulation Study. Superlattices Microstruct. 2018, 118, 116–122. [Google Scholar] [CrossRef]
- Ramasamy, K.; Tien, B.; Archana, P.S.; Gupta, A. Copper Antimony Sulfide (CuSbS2) Mesocrystals: A Potential Counter Electrode Material for Dye-Sensitized Solar Cells. Mater. Lett. 2014, 124, 227–230. [Google Scholar] [CrossRef]
- Macías, C.; Lugo, S.; Benítez, Á.; López, I.; Kharissov, B.; Vázquez, A.; Peña, Y. Thin Film Solar Cell Based on CuSbS2 Absorber Prepared by Chemical Bath Deposition (CBD). Mater. Res. Bull. 2017, 87, 161–166. [Google Scholar] [CrossRef]
- Medina-Montes, M.I.; Campos-González, E.; Morales-Luna, M.; Sánchez, T.G.; Becerril-Silva, M.; Mayén-Hernández, S.A.; de Moure-Flores, F.; Santos-Cruz, J. Development of Phase-Pure CuSbS2 Thin Films by Annealing Thermally Evaporated CuS/Sb2S3 Stacking Layer for Solar Cell Applications. Mater. Sci. Semicond. Process. 2018, 80, 74–84. [Google Scholar] [CrossRef]
- Rastogi, A.C.; Janardhana, N.R. Properties of CuSbS2 Thin Films Electrodeposited from Ionic Liquids as P-Type Absorber for Photovoltaic Solar Cells. Thin Solid Films 2014, 565, 285–292. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Yang, Z.; Ng, B.K.; Jiang, L.; Lai, Y.; Jia, M. CuSbS2 Solar Cells Using CdS, In2S3 and the In/Cd-Based Hybrid Buffers. J. Electron. Mater. 2021, 50, 3283–3287. [Google Scholar] [CrossRef]
- Aquino, J.A.R.; Vela, D.L.R.; Shaji, S.; Avellaneda, D.A.; Krishnan, B. Spray Pyrolysed Thin Films of Copper Antimony Sulfide as Photovoltaic Absorber. Phys. Status Solidi C 2016, 13, 24–29. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Li, X.; Li, C.; Zhang, R.; Delaunay, J.-J.; Zhu, H. Solution-Processed CuSbS2 Thin Film: A Promising Earth-Abundant Photocathode for Efficient Visible-Light-Driven Hydrogen Evolution. Nano Energy 2016, 28, 135–142. [Google Scholar] [CrossRef]
- Kumar, M.; Dubey, A.; Adhikari, N.; Venkatesan, S.; Qiao, Q. Strategic Review of Secondary Phases, Defects and Defect-Complexes in Kesterite CZTS-Se Solar Cells. Energy Environ. Sci. 2015, 8, 3134–3159. [Google Scholar] [CrossRef]
- Rovelli, L.; Tilley, S.D.; Sivula, K. Optimization and Stabilization of Electrodeposited Cu2ZnSnS4 Photocathodes for Solar Water Reduction. ACS Appl. Mater. Interfaces 2013, 5, 8018–8024. [Google Scholar] [CrossRef]
- Jiang, F.; Gunawan; Harada, T.; Kuang, Y.; Minegishi, T.; Domen, K.; Ikeda, S. Pt/In2S3/CdS/Cu2ZnSnS4 Thin Film as an Efficient and Stable Photocathode for Water Reduction under Sunlight Radiation. J. Am. Chem. Soc. 2015, 137, 13691–13697. [Google Scholar] [CrossRef]
- Huang, D.; Wang, K.; Yu, L.; Nguyen, T.H.; Ikeda, S.; Jiang, F. Over 1% Efficient Unbiased Stable Solar Water Splitting Based on a Sprayed Cu2ZnSnS4 Photocathode Protected by a HfO2 Photocorrosion-Resistant Film. ACS Energy Lett. 2018, 3, 1875–1881. [Google Scholar] [CrossRef]
- Yang, W.; Oh, Y.; Kim, J.; Jeong, M.J.; Park, J.H.; Moon, J. Molecular Chemistry-Controlled Hybrid Ink-Derived Efficient Cu2ZnSnS4 Photocathodes for Photoelectrochemical Water Splitting. ACS Energy Lett. 2016, 1, 1127–1136. [Google Scholar] [CrossRef]
- Tay, Y.F.; Kaneko, H.; Chiam, S.Y.; Lie, S.; Zheng, Q.; Wu, B.; Hadke, S.S.; Su, Z.; Bassi, P.S.; Bishop, D.; et al. Solution-Processed Cd-Substituted CZTS Photocathode for Efficient Solar Hydrogen Evolution from Neutral Water. Joule 2018, 2, 537–548. [Google Scholar] [CrossRef]
- Tay, Y.F.; Hadke, S.S.; Zhang, M.; Lim, N.; Chiam, S.Y.; Wong, L.H. Improving the Interfacial Properties of CZTS Photocathodes by Ag Substitution. J. Mater. Chem. A 2020, 8, 8862–8867. [Google Scholar] [CrossRef]
- Kumar, S.M.; Madhusudanan, S.P.; Rajamani, A.R.; Siaj, M.; Batabyal, S.K. Barium Substitution in Kesterite Cu2ZnSnS4: Cu2Zn1−xBaxSnS4 Quinary Alloy Thin Films for Efficient Solar Energy Harvesting. Cryst. Growth Des. 2020, 20, 4387–4394. [Google Scholar] [CrossRef]
- Todorov, T.; Gunawan, O.; Guha, S. A Road towards 25% Efficiency and beyond: Perovskite Tandem Solar Cells. Mol. Syst. Des. Eng. 2016, 1, 370–376. [Google Scholar] [CrossRef]
- Márquez, J.A.; Sun, J.-P.; Stange, H.; Ali, H.; Choubrac, L.; Schäfer, S.; Hages, C.J.; Leifer, K.; Unold, T.; Mitzi, D.B.; et al. High-Temperature Decomposition of Cu2BaSnS4 with Sn Loss Reveals Newly Identified Compound Cu2Ba3SnS8. J. Mater. Chem. A 2020, 8, 11346–11353. [Google Scholar] [CrossRef]
- Xie, J.; Yi, Q.; Zhang, F.; Bagheri, R.; Zheng, F.; Zou, G. Large-Grained Cu2BaSnS4 Films for Photocathodes. ACS Appl. Mater. Interfaces 2019, 11, 33102–33108. [Google Scholar] [CrossRef]
- Ge, J.; Roland, P.J.; Koirala, P.; Meng, W.; Young, J.L.; Petersen, R.; Deutsch, T.G.; Teeter, G.; Ellingson, R.J.; Collins, R.W.; et al. Employing Overlayers To Improve the Performance of Cu2BaSnS4 Thin Film Based Photoelectrochemical Water Reduction Devices. Chem. Mater. 2017, 29, 916–920. [Google Scholar] [CrossRef]
- Zhou, Y.; Shin, D.; Ngaboyamahina, E.; Han, Q.; Parker, C.B.; Mitzi, D.B.; Glass, J.T. Efficient and Stable Pt/TiO2/CdS/Cu2BaSn(S,Se)4 Photocathode for Water Electrolysis Applications. ACS Energy Lett. 2018, 3, 177–183. [Google Scholar] [CrossRef]
- Song, J.; Teymur, B.; Zhou, Y.; Ngaboyamahina, E.; Mitzi, D.B. Porous Cu2BaSn(S,Se)4 Film as a Photocathode Using Non-Toxic Solvent and a Ball-Milling Approach. ACS Appl. Energy Mater. 2021, 4, 81–87. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Niu, W.; Adams, P.; Siol, S.; Wang, Z.; Tilley, S.D. Thiol-Amine-Based Solution Processing of Cu2S Thin Films for Photoelectrochemical Water Splitting. ChemSusChem 2021, 14, 3967–3974. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z. Tetrafunctional Cu2S Thin Layers on Cu2O Nanowires for Efficient Photoelectrochemical Water Splitting. Nano Res. 2018, 11, 1530–1540. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Y. A Cu2O/CuS-ZnO/CdS Tandem Photoelectrochemical Cell for Self-Driven Solar Water Splitting. J. Alloys Compd. 2017, 698, 133–140. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, R.; Yin, Z.; Wang, X.; Wang, Z.; Fan, F.; Ma, Y. Surface Assistant Charge Separation in PEC Cu2S–Ni/Cu2O Cathode. ACS Appl. Mater. Interfaces 2019, 11, 34000–34009. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, L.; Su, Y.; Yin, H.; Hu, K. Hexagonally Ordered Microbowl Arrays Decorated with Ultrathin CuInS2 Nanosheets for Enhanced Photoelectrochemical Performance. J. Energy Chem. 2020, 51, 134–142. [Google Scholar] [CrossRef]
- Cai, Q.; Liu, Z.; Han, C.; Tong, Z.; Ma, C. CuInS2/Sb2S3 Heterostructure Modified with Noble Metal Co-Catalyst for Efficient Photoelectrochemical Water Splitting. J. Alloys Compd. 2019, 795, 319–326. [Google Scholar] [CrossRef]
- Li, M.; Zhao, R.; Su, Y.; Hu, J.; Yang, Z.; Zhang, Y. Synthesis of CuInS2 Nanowire Arrays via Solution Transformation of Cu2S Self-Template for Enhanced Photoelectrochemical Performance. Appl. Catal. B Environ. 2017, 203, 715–724. [Google Scholar] [CrossRef]
- Matoba, K.; Matsuda, Y.; Takahashi, M.; Sakata, Y.; Zhang, J.; Higashimoto, S. Fabrication of Pt/In2S3/CuInS2 Thin Film as Stable Photoelectrode for Water Splitting under Solar Light Irradiation. Catal. Today 2021, 375, 87–93. [Google Scholar] [CrossRef]
- Luo, J.; Tilley, S.D.; Steier, L.; Schreier, M.; Mayer, M.T.; Fan, H.J.; Grätzel, M. Solution Transformation of Cu2O into CuInS2 for Solar Water Splitting. Nano Lett. 2015, 15, 1395–1402. [Google Scholar] [CrossRef]
- Yang, W.; Oh, Y.; Kim, J.; Kim, H.; Shin, H.; Moon, J. Photoelectrochemical Properties of Vertically Aligned CuInS2 Nanorod Arrays Prepared via Template-Assisted Growth and Transfer. ACS Appl. Mater. Interfaces 2016, 8, 425–431. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.; Zhou, W.; Ren, C.; Gao, H.; Tian, G. Hierarchical SnS2/CuInS2 Nanosheet Heterostructure Films Decorated with C60 for Remarkable Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2019, 11, 9093–9101. [Google Scholar] [CrossRef]
- Patra, B.K.; Khilari, S.; Pradhan, D.; Pradhan, N. Hybrid Dot–Disk Au-CuInS2 Nanostructures as Active Photocathode for Efficient Evolution of Hydrogen from Water. Chem. Mater. 2016, 28, 4358–4366. [Google Scholar] [CrossRef]
- Ran, F.; Li, P.; Yuan, X.; Zhang, J.; Zhang, D.; Chen, S. Fabrication of a Sb2Se3/CuSbS2 Heterojunction Photocathode for Photoelectrochemical Water Splitting. J. Phys. Chem. C 2022, 126, 8581–8587. [Google Scholar] [CrossRef]
- Yokoyama, D.; Minegishi, T.; Jimbo, K.; Hisatomi, T.; Ma, G.; Katayama, M.; Kubota, J.; Katagiri, H.; Domen, K. H2 Evolution from Water on Modified Cu2ZnSnS4 Photoelectrode under Solar Light. Appl. Phys. Express 2010, 3, 101202. [Google Scholar] [CrossRef]
- Wang, K.; Huang, D.; Yu, L.; Gu, H.; Ikeda, S.; Jiang, F. Environmentally Friendly Cu2ZnSnS4-Based Photocathode Modified with a ZnS Protection Layer for Efficient Solar Water Splitting. J. Coll. Interface Sci. 2019, 536, 9–16. [Google Scholar] [CrossRef]
- Huang, D.; Wang, K.; Li, L.; Feng, K.; An, N.; Ikeda, S.; Kuang, Y.; Ng, Y.; Jiang, F. 3.17% Efficient Cu2ZnSnS4—BiVO4 Integrated Tandem Cell for Standalone Overall Solar Water Splitting. Energy Environ. Sci. 2021, 14, 1480–1489. [Google Scholar] [CrossRef]
- Xu, Z.; Guan, Z.; Yang, J.; Li, Q. Band Positions and Photoelectrochemical Properties of Solution-Processed Silver-Substituted Cu2ZnSnS4 Photocathode. ACS Appl. Energy Mater. 2019, 2, 2779–2785. [Google Scholar] [CrossRef]
- Ikeda, S.; Nguyen, T.H.; Okamoto, R.; Remeika, M.; Abdellaoui, I.; Islam, M.M.; Harada, T.; Abe, R.; Sakurai, T. Effects of Incorporation of Ag into a Kesterite Cu2ZnSnS4 Thin Film on Its Photoelectrochemical Properties for Water Reduction. Phys. Chem. Chem. Phys. 2022, 24, 468–476. [Google Scholar] [CrossRef]
- Wen, X.; Luo, W.; Guan, Z.; Huang, W.; Zou, Z. Boosting Efficiency and Stability of a Cu2ZnSnS4 Photocathode by Alloying Ge and Increasing Sulfur Pressure Simultaneously. Nano Energy 2017, 41, 18–26. [Google Scholar] [CrossRef]



| Device Structure | Photocurrent (mA/cm2); Applied Bias | Onset Potential (VRHE) | Stability (J/J0); Time; Applied Bias (VRHE) | Maximum STH or IPCE (%) | Faradaic Efficiency | Electrolyte; Light Source | Ref. |
|---|---|---|---|---|---|---|---|
| Cu2O/TiO2 | −0.7; −1 VAg/Agcl | ~ 0 VAg/Agcl | - | - | - | 0.1 M Sodium Acetate, Xe Lamp (700 mW/cm2) | [59] |
| Cu2O/Carbon | −3.95; 0 VRHE | ~ 0.6 | ~80%; 20 min; 0 | 0.56 | - | 1 M Na2SO4, AM 1.5G | [60] |
| FTO/Au/Cu2O/AZO/TiO2/Pt | −4.5; 0 VRHE | 0.4 | 100%;1 h; 0 | 0.66 | - | 1 M Na2SO4/0.1 M potassium phosphate (pH = 4.9); AM 1.5G | [61] |
| FTO/Au/Cu2O/AZO/TiO2/Pt | −6.0; 0 VRHE | 0.55 | - | ~1.5 | ~100% | 0.5 M Na2SO4/0.1 M potassium phosphate (pH = 5); AM 1.5G | [62] |
| FTO/Au/Cu2O/AZO/TiO2/RuOx | −5.0; 0 VRHE | 0.5 | ~100%; 4 h; 0 | ~1.1 | - | 0.5 M Na2SO4/0.1 M potassium phosphate (pH = 5); AM 1.5G | [62] |
| FTO/Au/Cu2O/AZO/TiO2/RuOx | −5.2; 0 VRHE | 0.55 | ~100%; 25 h; 0 | - | - | 1 M Na2SO4/0.1 M potassium phosphate (pH = 4.9); AM 1.5G | [63] |
| FTO/Au/Cu2O/AZO/TiO2/MoSx | −4.8; 0 VRHE | 0.45 | ~100%; 10 h; 0 | - | 100% | 0.5 M Na2SO4/0.2 M potassium phosphate (pH = 4); AM 1.5G | [64] |
| FTO/Au/Cu2O/AZO/TiO2/Ni-Mo | −6.3; 0 VRHE | 0.53 | ~25%; 10 h; 0 | - | ~100% | 1 M KOH (pH = 13.6); AM 1.5G | [65] |
| FTO/Al/Cu2O/NiS | −5.16; 0 VRHE | 0.6 | - | 1.12 | - | 0.1 M Na2SO4; AM 1.5G | [66] |
| ITO/Cu/Cu2O/TiO2 | −1.5; 0 VRHE | 0.55 | - | 0.28 | 98% | 1 M Na2SO4; AM 1.5G | [67] |
| FTO/FeOOH/Cu2O/Pt | −1.5; 0 VRHE | 0.6 | 66%, 1 h, 0 | 20% | 0.1 M Na2SO4; AM 1.5G | [68] | |
| FTO/Au/CuSCN/Cu2O/Ga2O3/TiO2/RuOx | −6.4; 0 VRHE | 1.0 | ~100%; 10 h | 4.2 | 100% | 0.5 M Na2SO4/0.1 M NaH2PO4 (pH = 5); AM 1.5G | [69] |
| FTO/H:Ti3C2TX/Cu2O | −5.41; 0 VRHE | 0.4 | - | 0.55 | - | 1 M Na2SO4; AM 1.5G | [70] |
| Ti/Cu2O/ZnO | −7.23; 0 VRHE | 0.83 | - | 1.77 | - | 0.5 M Na2SO4; AM 1.5G | [71] |
| Cu2O/Ga2O3/TiO2/RuOx | −4.0; 0 VRHE | 0.8 | - | 60% @ 450 nm at 0 VRHE | - | 0.5 M Na2SO4/0.1 M Sodium phosphate (pH = 5); AM 1.5G | [72] |
| FTO/CuBi2O4/MoS2 | −0.182; 0.6 VRHE | 0.9 | 100%; 200 s; 0 | - | - | 0.1 M NaOH (pH = 12.5) | [73] |
| FTO/CuBi2O4 | −0.3; 0.6 VRHE | ~0.8 | 20%; 15 min; 0.6 | ~14% @ 550 nm, 0.6 VRHE | - | Ar-purged 0.3 M K2SO4/0.2 M phosphate buffer (pH = 6.65); AM 1.5G | [74] |
| FTO/CuBi2O4/Pt | −0.5; 0.4 VRHE | ~1 | ~10%; 3 min; 0.6 | ~10% @ 400 nm, 0.6 V vs. RHE | - | Ar-purged 0.3 M K2SO4/0.2 M phosphate buffer (pH = 6.65); AM 1.5G | [75] |
| FTO/CuO/CuBi2O4/Pt | −0.72; 0 VRHE | - | 100%; 600s; 0 | - | - | 0.3 M K2SO4/0.1 M Phosphate buffer pH = 6.8; AM 1.5G | [76] |
| SrTiO3/SrRuO3/NiO/CuBi2O4 | −0.4 at 0 VRHE | 1.22 | ~100%; 3 h; 0 | ~11% @ 345 nm, 0.2 VRHE~ | - | 0.1kPi Buffer solution (pH = 8.55); AM 1.5G | [77] |
| FTO/NiO/CuBi2O4 | −0.5; 0.4 VRHE | ~1.0 | ~50%; 3 h; 0.4 | Ar-purged 0.1 M potassium phosphate (KPi) buffered solution (pH = 8.55); AM 1.5G | [78] | ||
| FTO/CBO/ZnSe/P25 | −0.43; 0.3 VRHE | ~1.0 | ~50%; 5000s; 0.3 | - | - | 0.3 M K2SO4/0.2 M phosphate buffer (pH = 6.65); 300 W Xe lamp | [79] |
| Ov/CBO/Zn-CBO | −0.6; 0.3 VRHE | ~1.0 | ~50%; 300s; 0.3 | - | - | 0.3 M K2SO4/0.2 M phosphate buffer (pH = 6.65); 300 W Xe lamp | [80] |
| FTO/Au/CBO/Pt | −1.24; 0.1 VRHE | ~1.0 | ~50%; 3000 s; 0 | 84.49% | 0.1 M Na2SO4 (pH = 6.8); 300 W Xe lamp | [81] | |
| FTO/CuO/CuBi2O4 | −0.9 at 0.1 VRHE | ~1.0 | 75%; 2500 s; 0.1 | 0.19 | - | 0.5 M Na2SO4 solution (pH = 7); 250 W Xe lamp | [82] |
| FTO/CuBi2O4/CdS/TiO2/Pt | −1; 0 VRHE | ~0.6 | ~60%; 3 h; 0 | ~0.13 | ∼91% | Ar-purged 0.3 M K2SO4/0.2 M phosphate buffer (pH = 6.65); AM 1.5G | [83] |
| FTO/CuFeO2/AZO/TiO2/Pt | −1.25; 0.4 VRHE | ~0.9 | 100%; 600 s; 0.4 VRHE | - | - | Ar purged 0.5 M Na2SO4; AM 1.5G | [84] |
| FTO/CuFeO2/NiFe-LDH/rGO | −2.4; 0.4 VRHE | ~0.65 | 100%; 1200 s; 0.4 VRHE | - | 94% | 1 M NaOH; AM 1.5G | [85] |
| FTO/CuAlO2/CuFeO2 | −2.6; 0.4 VRHE | ~0.75 | - | - | - | 1 M NaOH purged with O2 | [86] |
| Device Structure | Photocurrent (mA/cm2); Applied Bias | Onset Potential (VRHE) | Stability (J/J0); Time; Applied Bias (VRHE) | Maximum STH or IPCE (%) | Faradaic Efficiency | Electrolyte; Light Source | Ref. |
|---|---|---|---|---|---|---|---|
| FTO/Au/Cu2S/CdS/TiO2/RuOx | −2.5; −0.3 VRHE | 0.42 | 76%; 12 h; 0 | - | - | 1 M kPi buffer solution (pH = 7); AM 1.5G | [121] |
| FTO/Cu2S/Cu2O/Cu foam Au/Cu2S/CdS/TiO2/RuOx | −5.05; 0 VRHE | 0.35 | 80%; 1 h ; 0 | 40% @ 450 nm at 0 VRHE | - | 1 M Na2SO4/0.1 M KH2PO4 at (pH 4.9); AM 1.5G | [122] |
| FTO/Cu2O/Cu2S | −4.1; −0.6 VAg/AgCl | −0.29 VAg/AgCl | - | 0.38 | - | 0.5 M Na2SO4; AM 1.5G | [123] |
| FTO/Cu2O/Cu2S-Ni | −1.70; 0 VRHE | 0.5 | 45%; 500 s; 0 | - | - | 0.5 M Na2SO4; 300 W Xe lamp with AM 1.5G filter | [124] |
| FTO/CuInS2/CdS@MBAs | −0.487; −0.15 VRHE | - | ~100%; 400 s; 0 | 10% @ 400 nm at 0 VRHE | - | 1 M KCl Solution (pH = 5.97); AM 1.5G | [125] |
| FTO/CuInS2/Sb2S3/Pt | −2.48; −0.6 VRHE | 0.6 | ~88%; 180 s; −0.6 | 21.41% @ 550 nm at −0.6 VRHE | - | 0.1 M Na2SO4 (pH = 7.1); AM 1.5G | [126] |
| FTO/CuInS2/CdS | −0.71; −0.2 VRHE | 0.25 | ~100%; 1500 s; 0 | 9% @ 425 nm at 0 VRHE | - | 1 M KCl Solution (pH = 5.97); 500 W Xe lamp with AM 1.5G filter | [127] |
| Mo/CuInS2/In2S3/Pt | −5.6; 0 VRHE | 0.7 | ~100%;80 min; 0.1 | 0.7 | 100% | 0.1 M Na2SO4 (pH = 10); AM 1.5G | [128] |
| FTO/CuInS2/CdS/AZO/TiO2/Pt | −3.5; −0.3 VRHE | 0.6 | 80%; 2 h; 0 | ~20% @ 500 nm at 0 VRHE | - | 0.5 M Na2SO4/0.1 M KH2PO4 (pH = 5.0); 300 W Xe lamp with AM 1.5G filter | [129] |
| FTO/CIS NR/CdS/ZnS | −2.0; 0.3 VRHE | 1.06 | ~100%; 3000 h; 0.3 | - | - | 0.5 M Na2SO4 (adjusted to pH 10 by adding NaOH).; AM 1.5G | [130] |
| FTO/CuInS2/SnS2–1.6/C60 | −4.51; −0.45 VRHE | - | - | 8% @ 450 nm at −0.45 VRHE | - | 0.5 M Na2SO4; AM 1.5 G | [131] |
| FTO/Au-CuInS2 | −15.2; 0 VRHE | 0.3 VSCE | ~100%; 400 s; −0.5 VSCE | 4.29 | - | 0.5 M Na2SO4; AM 1.5G | [132] |
| FTO/CuSbS2/Sb2Se3/TiO2/Pt | −18.0; 0 VRHE | 0.2 | - | - | - | 1 M H2SO4 (pH = 0); AM 1.5G | [133] |
| FTO/CZTS/CdS/TiO2-Pt | −9.0; 0 VRHE | 0.6 | - | 1.2 | - | 0.1 M Na2SO4 (pH = 9.5); 300 Xe lamp with AM 1.5G filter | [134] |
| FTO/CZTS/CdS/ZnO/Pt | −8.0; 0 VRHE | 0.63 | ~100%; 2 h; 0 | 2.1 | - | 0.2 M Na2HPO4/NaH2PO4 (pH 6.5); 300 W Xe lamp with AM 1.5G filter | [135] |
| FTO/CZTS/HfO2/CdS/HfO2/Pt | −28.0; 0 VRHE | 0.7 | ~100%; 24 h; 0 | 2.4 | - | 0.2 M Na2HPO4/NaH2PO4 (pH 6.5); AM 1.5G filter | [136] |
| FTO/ACZTS/CdS/Pt | −3.78; 0 VRHE | 0.33 | ~100%; 1 h; 0 | 0.32 | 95% | 0.2 M Na2HPO4 (pH = 10); AM 1.5G | [137] |
| FTO/ACZTS/CdS/In2S3/Pt | −15.0; 0 VRHE | 0.7 | ~50%; 3 h; 0 | 2.4 | 98% | 0.2 M K2HPO4/KH2PO4 (pH = 6.85); AM 1.5G | [138] |
| FTO/CGZTS/CdS/In2S3/Pt | −11.1; | 0.6 | 90%; 7000 s; 0 | 1.7 | - | 0.2 M K2HPO4/KH2PO4 (pH = 6.85); AM 1.5G | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Meena, B.; Subramanyam, P.; Suryakala, D.; Subrahmanyam, C. Emerging Copper-Based Semiconducting Materials for Photocathodic Applications in Solar Driven Water Splitting. Catalysts 2022, 12, 1198. https://doi.org/10.3390/catal12101198
Kumar M, Meena B, Subramanyam P, Suryakala D, Subrahmanyam C. Emerging Copper-Based Semiconducting Materials for Photocathodic Applications in Solar Driven Water Splitting. Catalysts. 2022; 12(10):1198. https://doi.org/10.3390/catal12101198
Chicago/Turabian StyleKumar, Mohit, Bhagatram Meena, Palyam Subramanyam, Duvvuri Suryakala, and Challapalli Subrahmanyam. 2022. "Emerging Copper-Based Semiconducting Materials for Photocathodic Applications in Solar Driven Water Splitting" Catalysts 12, no. 10: 1198. https://doi.org/10.3390/catal12101198
APA StyleKumar, M., Meena, B., Subramanyam, P., Suryakala, D., & Subrahmanyam, C. (2022). Emerging Copper-Based Semiconducting Materials for Photocathodic Applications in Solar Driven Water Splitting. Catalysts, 12(10), 1198. https://doi.org/10.3390/catal12101198






