Formulation of Bismuth (Bi2O3) and Cerium Oxides (CeO2) Nanosheets for Boosted Visible Light Degradation of Methyl Orange and Methylene Blue Dyes in Water
Abstract
1. Introduction
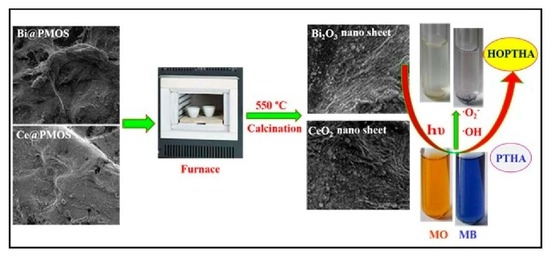
2. Results and Discussion
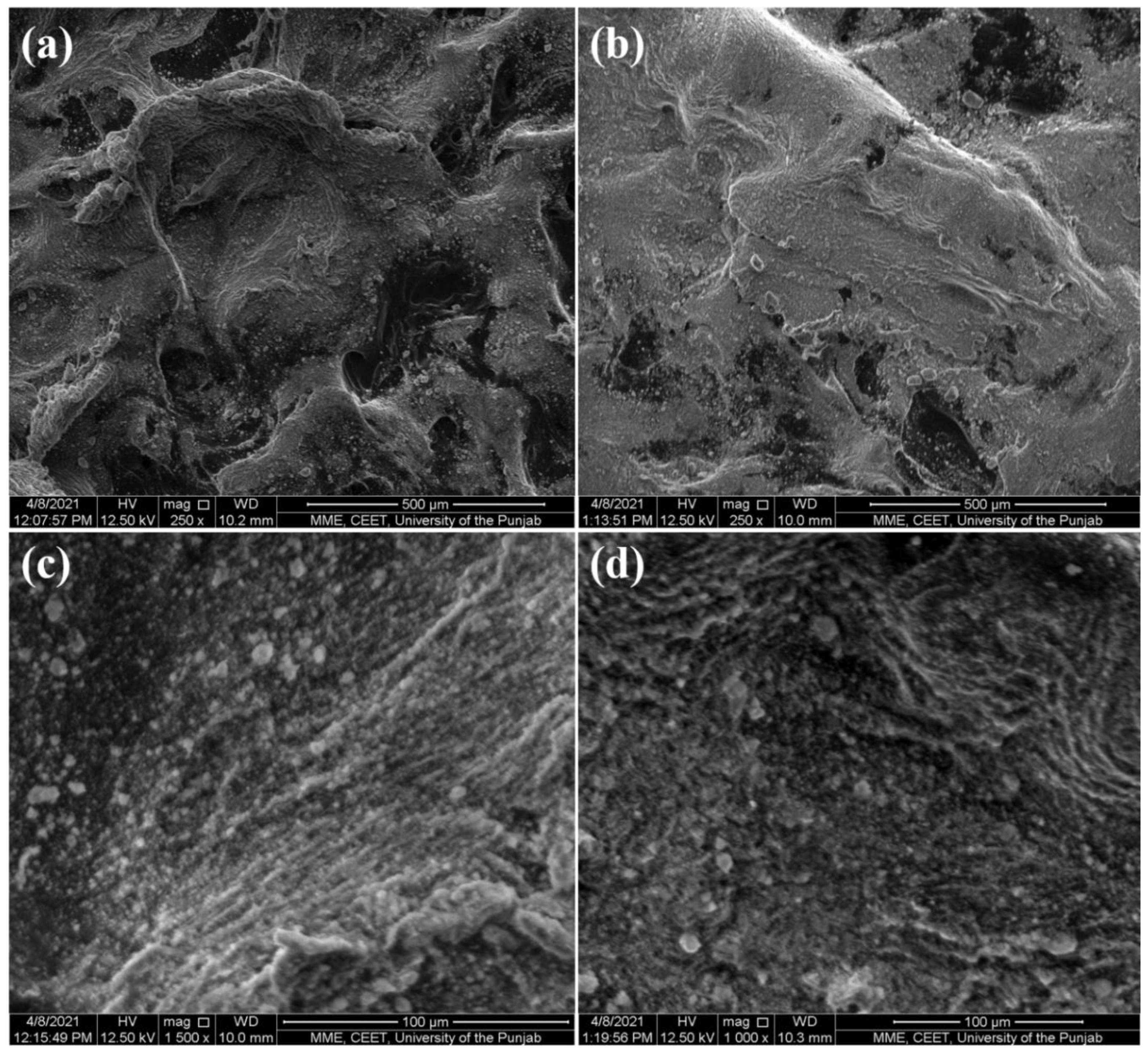
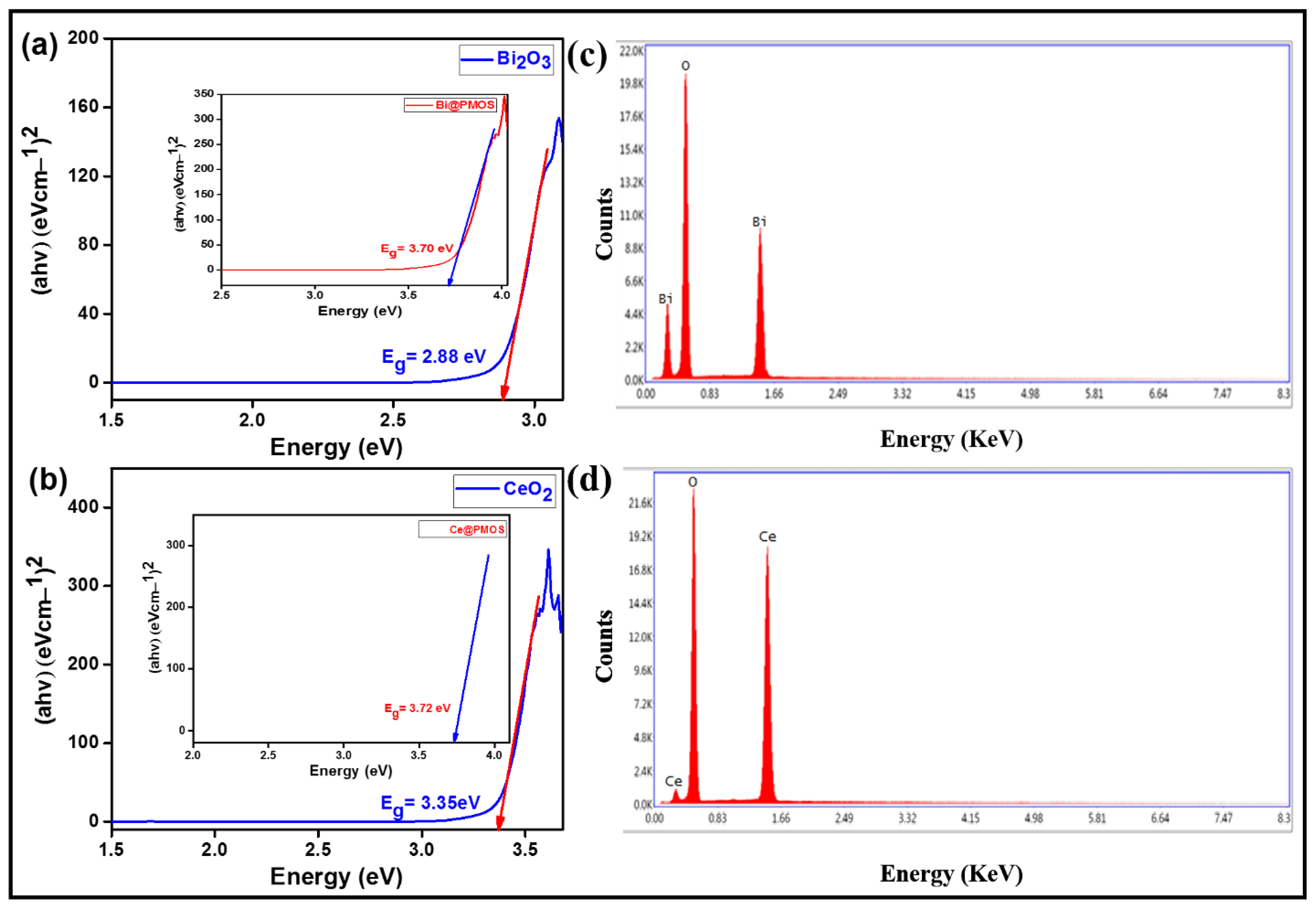
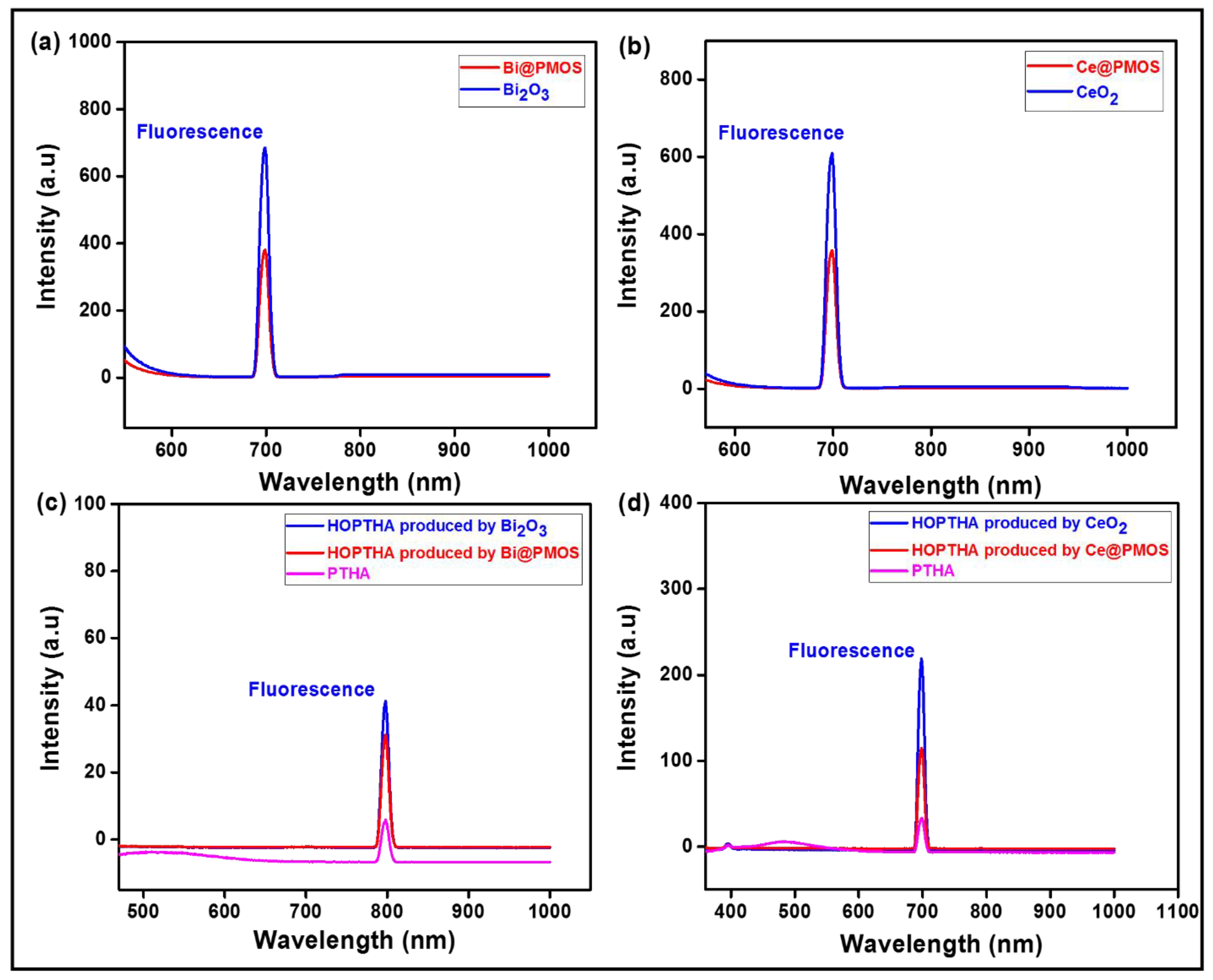
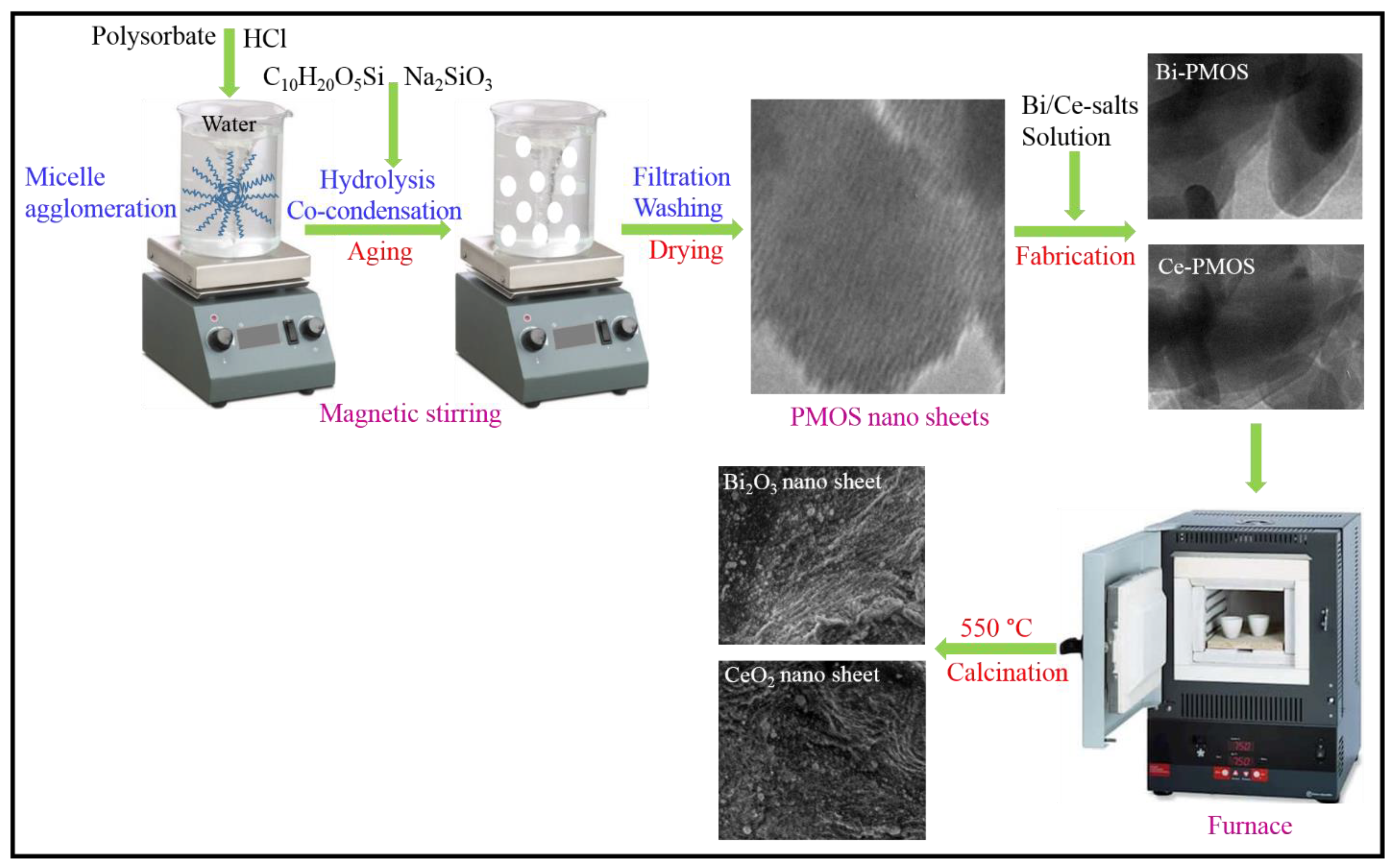
2.1. Materials Synthesis and Characterization
2.2. Kinetic Study
| Catalyst (Amount) | MB/MO (Amount) | % Reduction | Time (Hour) | [K] (Min−1) | Reference |
|---|---|---|---|---|---|
| PAM-TiO2/Porous TiO2 beads (5 mg/10 cm3) | (25 mg/L) | 16.2/32.8 | 01 | 0.00016/0.0024 | [36] |
| Ag-PMOS (10 mg/30 cm3) | (20 mg/L) | 81/48 | 01 | 0.025/0.0046 | [39] |
| ZnO-PMOS (10 mg/30 cm3) | (20 mg/L) | 47/57 | 01 | 0.0020/0.0062 | [39] |
| GF/TiO2 NTA 1 × 4 cm2-electrode | (5 mg/L) | 65.9 | 02 | 0.0088 | [40] |
| Bi@PMOS (10 mg/30 cm3) | (20 mg/L) | 74.7/53.2 | 01 | 0.0233/0.0101 | [41] |
| Ce@PMOS (10 mg/30 cm3) | (20 mg/L) | 41.4/39.4 | 01 | 0.0079/0.0085 | [41] |
| Bi2O3 (10 mg/30 cm3) | (20 mg/L) | 91.3/61 | 01 | 0.0375/0.0166 | Present study |
| CeO2 (10 mg/30 cm3) | (20 mg/L) | 76.2/85 | 01 | 0.0234/0.0303 | Present study |
2.3. Projected Photodegradation Mechanism
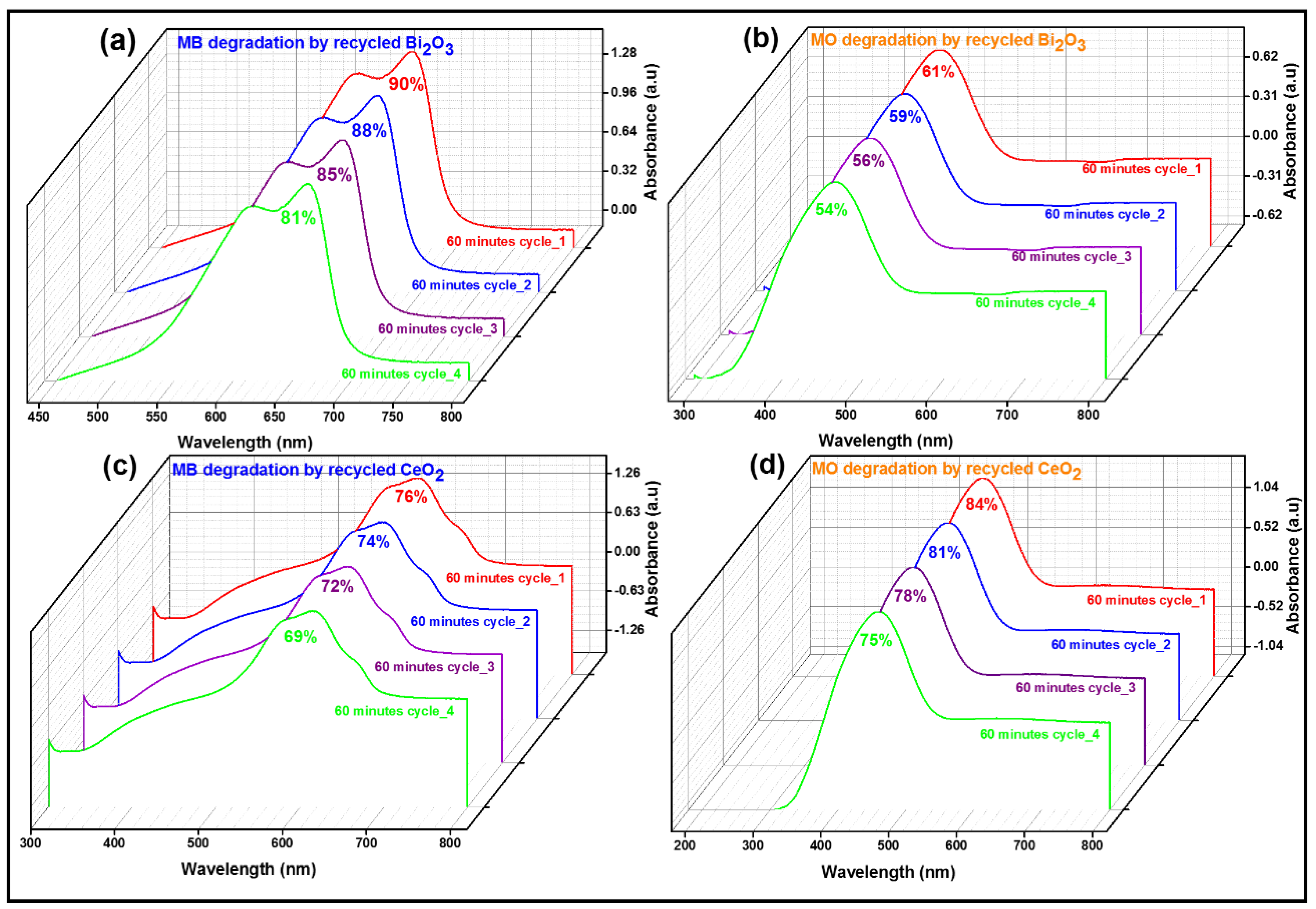
2.4. Recycling of Catalysts
2.4.1. UV/Visible Absorption Study
2.4.2. Photoreduction of Methylene Blue and Methyl Orange (%)
3. Experimental Section
3.1. Materials
3.2. Synthesis of Bismuth (Bi2O3) and Cerium (CeO2) Nanosheets
3.3. Photocatalytic Efficiencies of Bi@PMOS, Ce@PMOS, Bi2O3 and CeO2 for the Degradation of Methylene Blue and Methyl Orange Dyes
3.4. Examining the Generation of Hydroxyl Free Radicals (.OH)
3.5. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batool, M.; Nazar, M.F.; Awan, A.; Tahir, M.B.; Rahdar, A.; Shalan, A.E.; Lanceros-Méndez, S.; Zafar, M.N. Bismuth-based heterojunction nanocomposites for photocatalysis and heavy metal detection applications. Nano-Struct. Nano-Objects 2021, 27, 100762. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, N.; Fan, Z.; Hu, Z.-T.; Fan, L.; Zhou, J.; Huang, X. On-site H2O2 electro-generation process combined with ultraviolet: A promising approach for odorous compounds purification in drinking water system. Chem. Eng. J. 2022, 430, 132829. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, L.; Cao, Z.; Hong, F.F. Enhanced decolourization efficiency of textile dye Reactive Blue 19 in a horizontal rotating reactor using strips of BNC-immobilized laccase: Optimization of conditions and comparison of decolourization efficiency. Biochem. Eng. J. 2020, 156, 107501. [Google Scholar] [CrossRef]
- Kusuma, K.; Manju, M.; Ravikumar, C.; Dileepkumar, V.; Kumar, A.N.; Santosh, M.S.; Murthy, H.; Gurushantha, K. Probe sonicated synthesis of bismuth oxide (Bi2O3): Photocatalytic application and electrochemical sensing of ascorbic acid and lead. J. Nanomater. 2022, 2022, 3256611. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Haq, F.; Farid, A.; Ullah, N.; Kiran, M.; Khan, R.U.; Aziz, T.; Mehmood, S.; Haroon, M.; Mubashir, M.; Bokhari, A. A study on the uptake of methylene blue by biodegradable and eco-friendly carboxylated starch grafted polyvinyl pyrrolidone. Environ. Res. 2022, 215, 114241. [Google Scholar] [CrossRef]
- Kumar, O.P.; Ashiq, M.N.; Shah, S.S.A.; Akhtar, S.; Obaidi, M.A.A.; Mujtaba, I.M.; ur Rehman, A. Nanoscale ZrRGOCuFe layered double hydroxide composites for enhanced photocatalytic degradation of dye contaminant. Mater. Sci. Semicond. Process. 2021, 128, 105748. [Google Scholar] [CrossRef]
- Nazir, M.A.; Najam, T.; Zarin, K.; Shahzad, K.; Javed, M.S.; Jamshaid, M.; Bashir, M.A.; Shah, S.S.A.; Rehman, A.U. Enhanced adsorption removal of methyl orange from water by porous bimetallic Ni/Co MOF composite: A systematic study of adsorption kinetics. Int. J. Environ. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Balcha, A.; Yadav, O.P.; Dey, T. Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res. 2016, 23, 25485–25493. [Google Scholar] [CrossRef]
- Oh, S.; Shim, J.; Seo, D.; Shim, M.J.; Han, S.C.; Lee, C.; Nam, Y. Organic/inorganic hybrid cerium oxide-based superhydrophobic surface with enhanced weather resistance and self-recovery. Prog. Org. Coat. 2022, 170, 106998. [Google Scholar] [CrossRef]
- Qu, Z.; Jing, Z.; Chen, X.; Wang, Z.; Ren, H.; Huang, L. Preparation and photocatalytic performance study of dual Z-scheme Bi2Zr2O7/g-C3N4/Ag3PO4 for removal of antibiotics by visible-light. J. Environ. Sci. 2023, 125, 349–361. [Google Scholar] [CrossRef]
- Rizwan, K.; Bilal, M.; Slimani, Y.; Show, P.L.; Rtimi, S.; Roy, A.; Iqbal, H.M. Hydrogen-based sono-hybrid catalytic degradation and mitigation of industrially-originated dye-based pollutants. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Jun, S.C. Challenges and Strategies toward Cathode Materials for Rechargeable Potassium-Ion Batteries. Adv. Mater. 2021, 33, 2004689. [Google Scholar] [CrossRef]
- Rui, J.; Deng, N.; Zhao, Y.; Tao, C.; Zhou, J.; Zhao, Z.; Huang, X. Activation of persulfate via Mn doped Mg/Al layered double hydroxide for effective degradation of organics: Insights from chemical and structural variability of catalyst. Chemosphere 2022, 302, 134849. [Google Scholar] [CrossRef]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.; Kumar, M.S. Detoxification of water and wastewater by advanced oxidation processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Dong, B.; Li, L.; Dong, Z.; Xu, R.; Wu, Y. Fabrication of CeO2 nanorods for enhanced solar photocatalysts. Int. J. Hydrogen Energy 2018, 43, 5275–5282. [Google Scholar] [CrossRef]
- Singh, S.; Lo, S.-L. Single-phase cerium oxide nanospheres: An efficient photocatalyst for the abatement of rhodamine B dye. Environ. Sci. Pollut. Res. 2018, 25, 6532–6544. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, S.; Wen, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F.; Huang, Q.; Tang, Z.; Wang, X. A critical review on visible-light-response CeO2-based photocatalysts with enhanced photooxidation of organic pollutants. Catal. Today 2019, 335, 20–30. [Google Scholar] [CrossRef]
- Tian, N.; Hu, C.; Wang, J.; Zhang, Y.; Ma, T.; Huang, H. Layered bismuth-based photocatalysts. Coord. Chem. Rev. 2022, 463, 214515. [Google Scholar]
- Ma, R.; Zhang, S.; Li, L.; Gu, P.; Wen, T.; Khan, A.; Li, S.; Li, B.; Wang, S.; Wang, X. Enhanced visible-light-induced photoactivity of type-II CeO2/g-C3N4 nanosheet toward organic pollutants degradation. ACS Sustain. Chem. Eng. 2019, 7, 9699–9708. [Google Scholar] [CrossRef]
- Song, S.; Xing, Z.; Zhao, H.; Li, Z.; Zhou, W. Recent Advances in Bismuth-based Photocatalysts: Environment and Energy Applications. Green Energy Environ. 2022. [Google Scholar] [CrossRef]
- Gayathri, R.C.; Elakkiya, V.; Sumathi, S. Synthesis of cerium and bismuth doped nickel aluminate for the photodegradation of methylene blue, methyl orange and rhodamine B dyes. Chemosphere 2022, 303, 135056. [Google Scholar] [CrossRef]
- Murali, A.; Lan, Y.-P.; Sohn, H.Y. Effect of oxygen vacancies in non-stoichiometric ceria on its photocatalytic properties. Nano-Struct. Nano-Objects 2019, 18, 100257. [Google Scholar] [CrossRef]
- Назаренкo, О.; Іванченкo, А.; Кoлєснікoва, О. Дoслідження прoцесу нітратнoкислoтнoї перерoбки фoсфoгіпсу з oдержанням кoнцентрату рідкіснoземельних елементів. Вісник Київськoгo націoнальнoгo університету технoлoгій та дизайну. Серія Технічні науки 2020, 146, 186–194. [Google Scholar]
- Hsieh, S.-H.; Manivel, A.; Lee, G.-J.; Wu, J.J. Synthesis of mesoporous Bi2O3/CeO2 microsphere for photocatalytic degradation of Orange II dye. Mater. Res. Bull. 2013, 48, 4174–4180. [Google Scholar] [CrossRef]
- Siao, C.-W.; Lee, W.-L.W.; Dai, Y.-M.; Chung, W.-H.; Hung, J.-T.; Huang, P.-H.; Lin, W.-Y.; Chen, C.-C. BiOxCly/BiOmBrn/BiOpIq/GO quaternary composites: Syntheses and application of visible-light-driven photocatalytic activities. J. Colloid Interface Sci. 2019, 544, 25–36. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Pan, J.; Chen, A.; Chen, Y. Design and fabrication of carbon spheres-supported cerium oxide heterostructured composites for enhanced photocatalytic activity. Ceram. Int. 2022, 48, 17714–17722. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Zhou, G.; Zhao, P.; Li, R.; Mao, G.; Zhang, L.; Li, F.; Yin, J. Synthesis of CeO2 nanoparticles by sacrificial Mg powder with abundant oxygen vacancies and efficiently photocatalytic activity. Mater. Lett. 2022, 307, 131075. [Google Scholar] [CrossRef]
- Riaz, N.; Hassan, M.; Siddique, M.; Mahmood, Q.; Farooq, U.; Sarwar, R.; Khan, M.S. Photocatalytic degradation and kinetic modeling of azo dye using bimetallic photocatalysts: Effect of synthesis and operational parameters. Environ. Sci. Pollut. Res. 2020, 27, 2992–3006. [Google Scholar] [CrossRef]
- Habib, I.Y.; Burhan, J.; Jaladi, F.; Lim, C.M.; Usman, A.; Kumara, N.; Tsang, S.C.E.; Mahadi, A.H. Effect of Cr doping in CeO2 nanostructures on photocatalysis and H2O2 assisted methylene blue dye degradation. Catal. Today 2020, 375, 506–513. [Google Scholar] [CrossRef]
- Shahzad, K.; Imran Khan, M.; Elboughdiri, N.; Ghernaout, D.; ur Rehman, A. Energizing periodic mesoporous organosilica (PMOS) with Bismuth and Cerium for photo-degrading methylene blue and methyl orange in water. Water Environ. Res. 2021, 93, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Arshad, H.; Tahir, M.U.; Rehman, F.; Wang, L.; Wang, J.; Su, X.; Yang, C. Facile synthesis of bismuth oxide nanostructures derived from solvent-mediated oxalates and their visible-light-driven photocatalytic removal of organic pollutants. Appl. Surf. Sci. 2022, 574, 151678. [Google Scholar] [CrossRef]
- Inagaki, S.; Guan, S.; Fukushima, Y.; Ohsuna, T.; Terasaki, O. Novel mesoporous materials with a uniform distribution of organic groups and inorganic oxide in their frameworks. J. Am. Chem. Soc. 1999, 121, 9611–9614. [Google Scholar] [CrossRef]
- Nasreen, S.; Urooj, A.; Rafique, U.; Ehrman, S. Functionalized mesoporous silica: Absorbents for water purification. Desalination Water Treat. 2016, 57, 29352–29362. [Google Scholar] [CrossRef]
- Lee, B.; Kim, Y.; Lee, H.; Yi, J. Synthesis of functionalized porous silicas via templating method as heavy metal ion adsorbents: The introduction of surface hydrophilicity onto the surface of adsorbents. Microporous Mesoporous Mater. 2001, 50, 77–90. [Google Scholar] [CrossRef]
- Mudassir, M.A.; Hussain, S.Z.; Khan, M.; Asma, S.T.; Iqbal, Z.; Huma, Z.; Ullah, N.; Zhang, H.; Ansari, T.M.; Hussain, I. Polyacrylamide exotemplate-assisted synthesis of hierarchically porous nanostructured TiO2 macrobeads for efficient photodegradation of organic dyes and microbes. RSC Adv. 2018, 8, 29628–29636. [Google Scholar] [CrossRef]
- Lou, W.; Wang, L.; Dong, S.; Sun, J.; Zhang, Y. A facility synthesis of bismuth-iron bimetal MOF composite silver vanadate applied to visible light photocatalysis. Opt. Mater. 2022, 126, 112168. [Google Scholar] [CrossRef]
- Zhang, M.; Ke, J.; Xu, D.; Zhang, X.; Liu, H.; Wang, Y.; Yu, J. Construction of plasmonic Bi/Bismuth oxycarbonate/Zinc bismuth oxide ternary heterojunction for enhanced charge carrier separation and photocatalytic performances. J. Colloid Interface Sci. 2022, 615, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Roy, P.; Paramasivam, I.; Schmuki, P. Voltage-Induced Payload Release and Wettability Control on TiO2 and TiO2 Nanotubes. Angew. Chem. Int. Ed. 2010, 49, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Najam, T.; Bashir, M.S.; Nazir, M.A.; ur Rehman, A.; Bashir, M.A.; Shah, S.S.A. Fabrication of Periodic Mesoporous Organo Silicate (PMOS) composites of Ag and ZnO: Photo-catalytic degradation of methylene blue and methyl orange. Inorg. Chem. Commun. 2021, 123, 108357. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Dong, W.; Wang, D.; Wang, H.; Li, M.; Chen, F.; Jia, F.; Yang, Q.; Li, X.; Yuan, X.; Gong, J. Facile synthesis of In2S3/UiO-66 composite with enhanced adsorption performance and photocatalytic activity for the removal of tetracycline under visible light irradiation. J. Colloid Interface Sci. 2019, 535, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.-L.; Zhu, Z.-S.; Qu, J.; Jing, Y.-Q.; Yu, X.-J.; Abdelkrim, Y.; Hao, S.-M.; Yu, Z.-Z. BiOBr/Ag6Si2O7 heterojunctions for enhancing visible light catalytic degradation performances with a sequential selectivity enabled by dual synergistic effects. J. Colloid Interface Sci. 2020, 561, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.-S.; Yu, X.-J.; Qu, J.; Jing, Y.-Q.; Abdelkrim, Y.; Yu, Z.-Z. Preforming abundant surface cobalt hydroxyl groups on low crystalline flowerlike Co3(Si2O5)2(OH)2 for enhancing catalytic degradation performances with a critical nonradical reaction. Appl. Catal. B: Environ. 2020, 261, 118238. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, H.; Yang, H.; Lin, Y.; Liu, H.; Tong, Y. Efficient charges separation using advanced BiOI-based hollow spheres decorated with palladium and manganese dioxide nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 2751–2757. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Liu, W.; Zhang, S. Highly enhanced photocatalytic degradation of methylene blue over the indirect all-solid-state Z-scheme g-C3N4-RGO-TiO2 nanoheterojunctions. Appl. Surf. Sci. 2017, 405, 60–70. [Google Scholar] [CrossRef]
- Liu, F.-Y.; Jiang, Y.-R.; Chen, C.-C.; Lee, W.W. Novel synthesis of PbBiO2Cl/BiOCl nanocomposite with enhanced visible-driven-light photocatalytic activity. Catal. Today 2018, 300, 112–123. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzad, K.; Fernandez-Garcia, J.; Khan, M.I.; Shanableh, A.; Khan, N.A.; ur Rehman, A. Formulation of Bismuth (Bi2O3) and Cerium Oxides (CeO2) Nanosheets for Boosted Visible Light Degradation of Methyl Orange and Methylene Blue Dyes in Water. Catalysts 2022, 12, 1197. https://doi.org/10.3390/catal12101197
Shahzad K, Fernandez-Garcia J, Khan MI, Shanableh A, Khan NA, ur Rehman A. Formulation of Bismuth (Bi2O3) and Cerium Oxides (CeO2) Nanosheets for Boosted Visible Light Degradation of Methyl Orange and Methylene Blue Dyes in Water. Catalysts. 2022; 12(10):1197. https://doi.org/10.3390/catal12101197
Chicago/Turabian StyleShahzad, Khurram, Javier Fernandez-Garcia, Muhammad Imran Khan, Abdallah Shanableh, Naseem Ahmad Khan, and Aziz ur Rehman. 2022. "Formulation of Bismuth (Bi2O3) and Cerium Oxides (CeO2) Nanosheets for Boosted Visible Light Degradation of Methyl Orange and Methylene Blue Dyes in Water" Catalysts 12, no. 10: 1197. https://doi.org/10.3390/catal12101197
APA StyleShahzad, K., Fernandez-Garcia, J., Khan, M. I., Shanableh, A., Khan, N. A., & ur Rehman, A. (2022). Formulation of Bismuth (Bi2O3) and Cerium Oxides (CeO2) Nanosheets for Boosted Visible Light Degradation of Methyl Orange and Methylene Blue Dyes in Water. Catalysts, 12(10), 1197. https://doi.org/10.3390/catal12101197