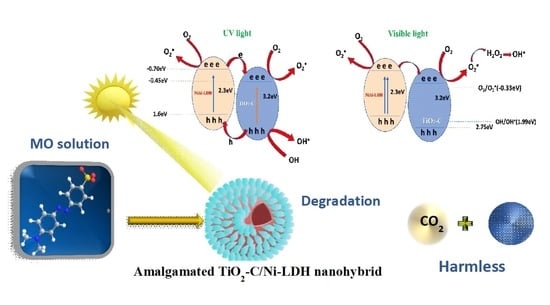
Amalgamated Titanium Oxide-Carbon Hollow Sphere/Nickel-Layered Double Hydroxide as an Efficient Photocatalyst for the Degradation of Methyl Orange
Abstract
1. Introduction
2. Results
2.1. Characterization of Catalysts
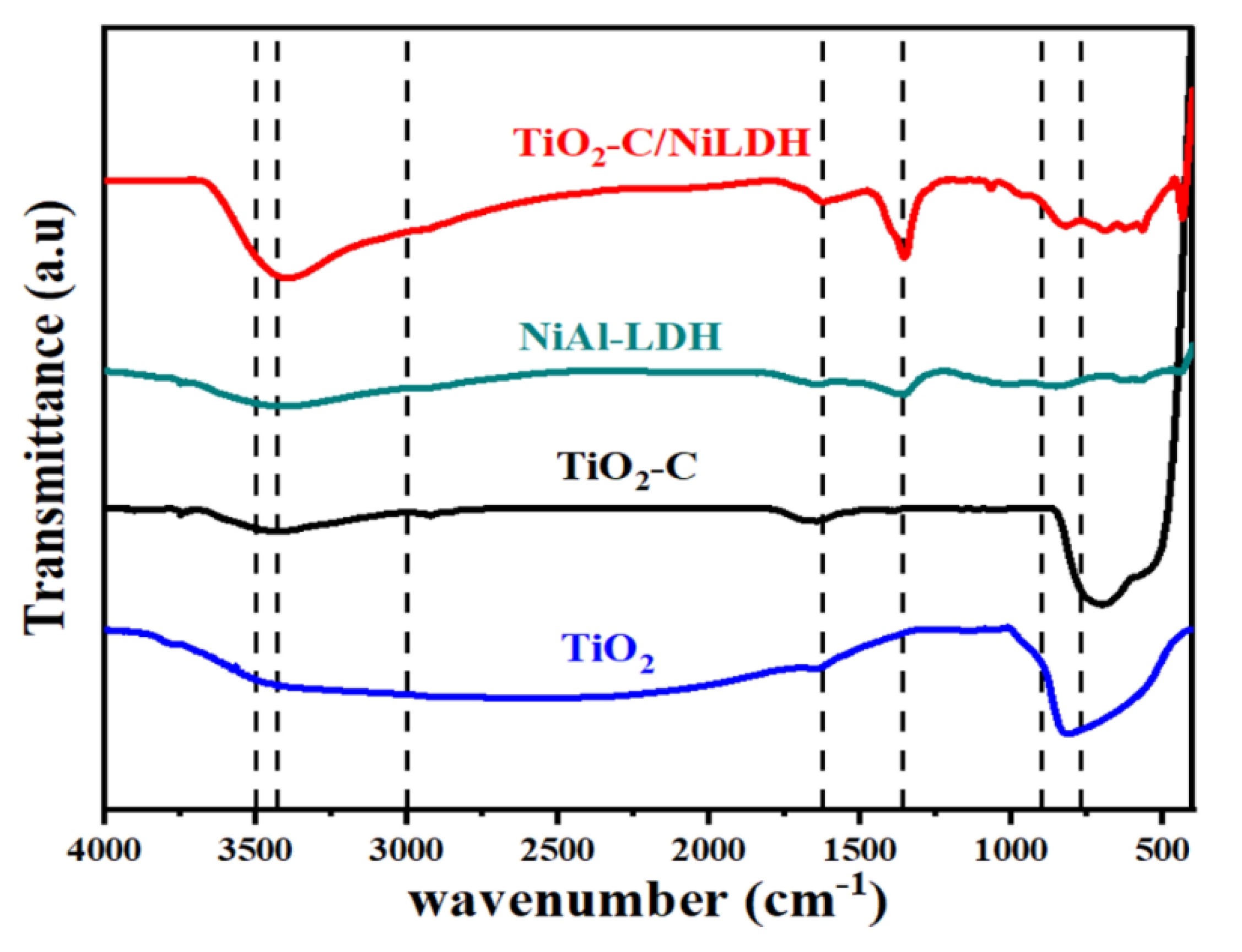
2.1.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
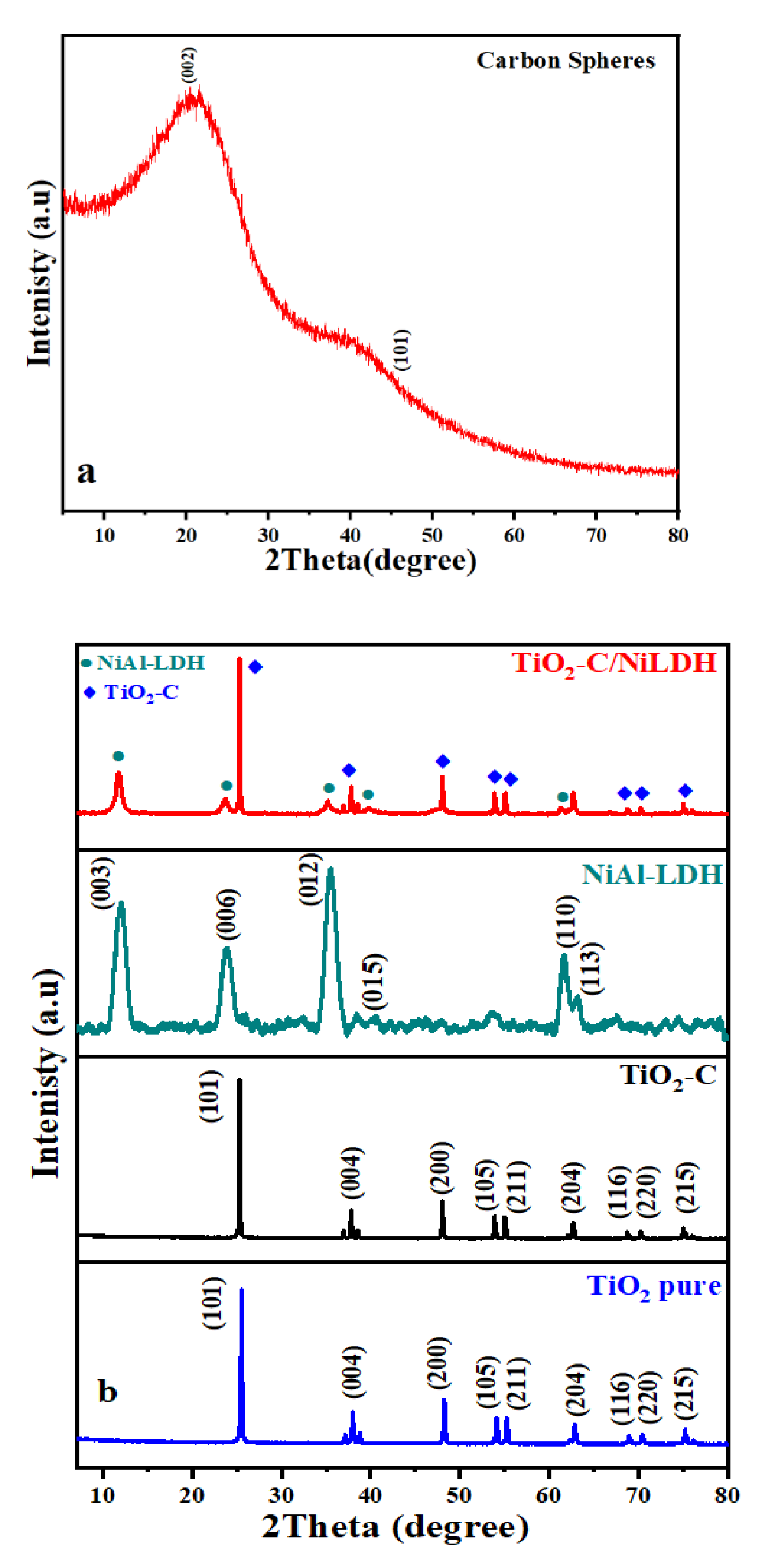
2.1.2. X-ray Diffraction (XRD)
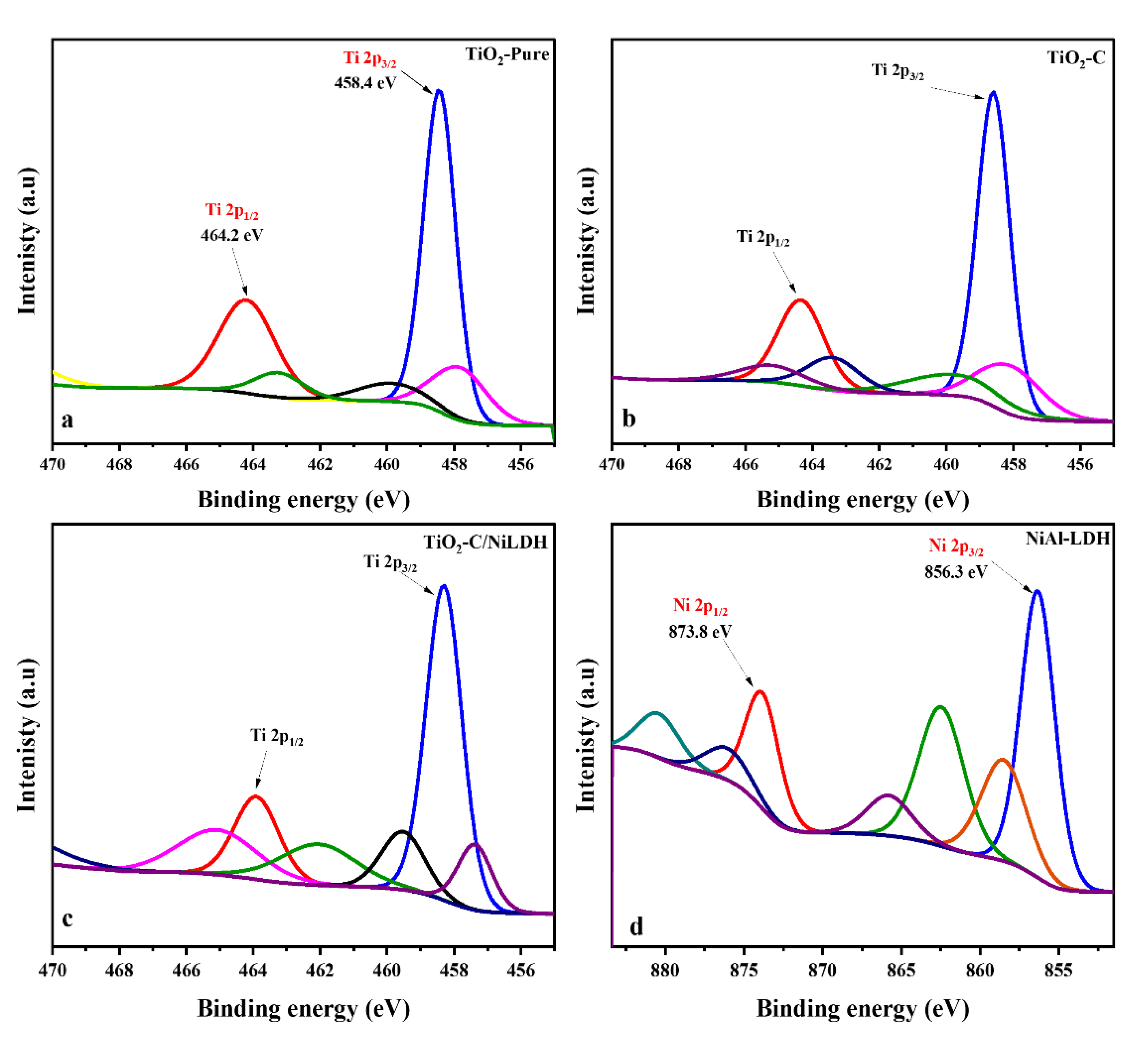
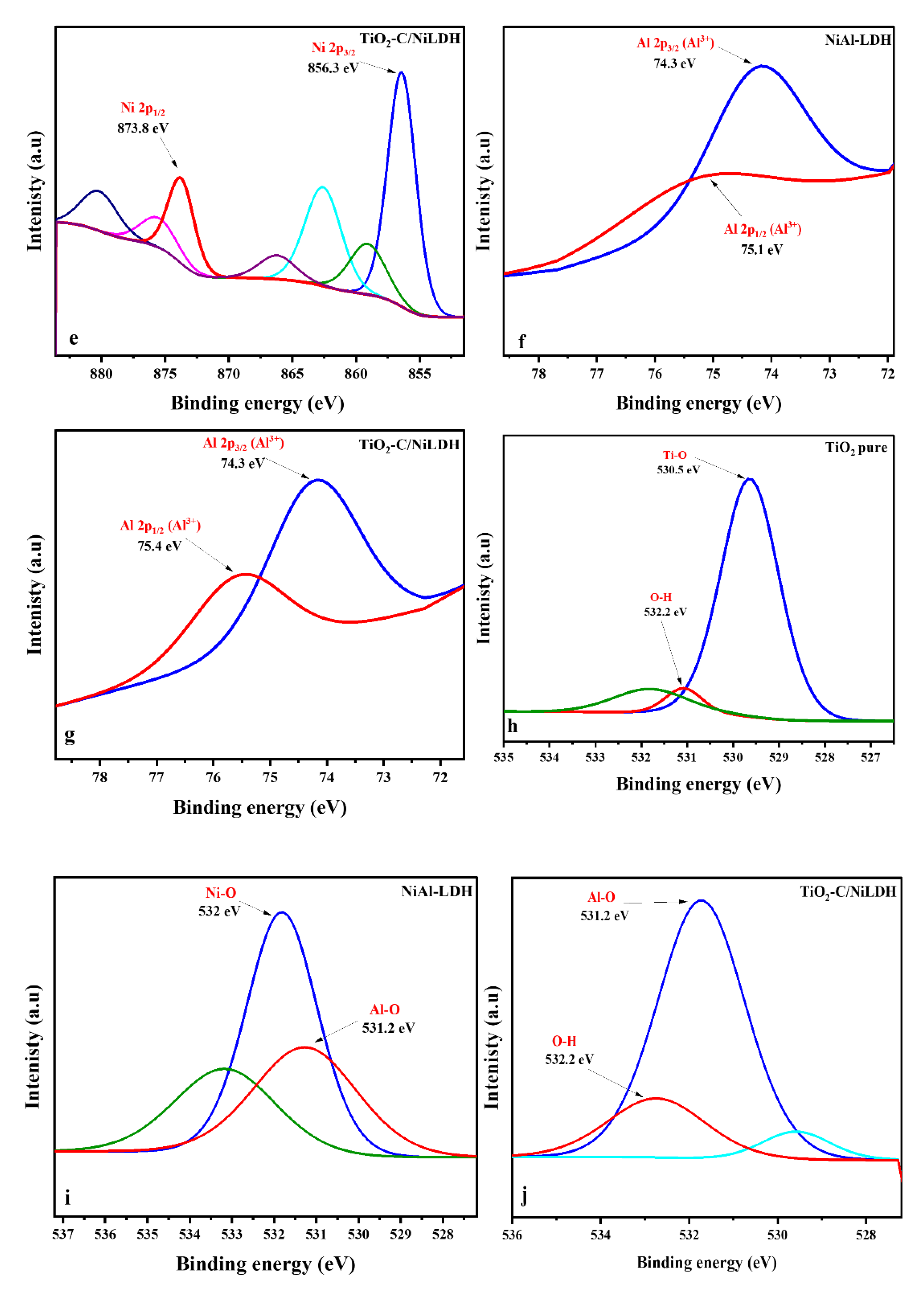
2.1.3. X-ray Photoelectron Microscopy (XPS)
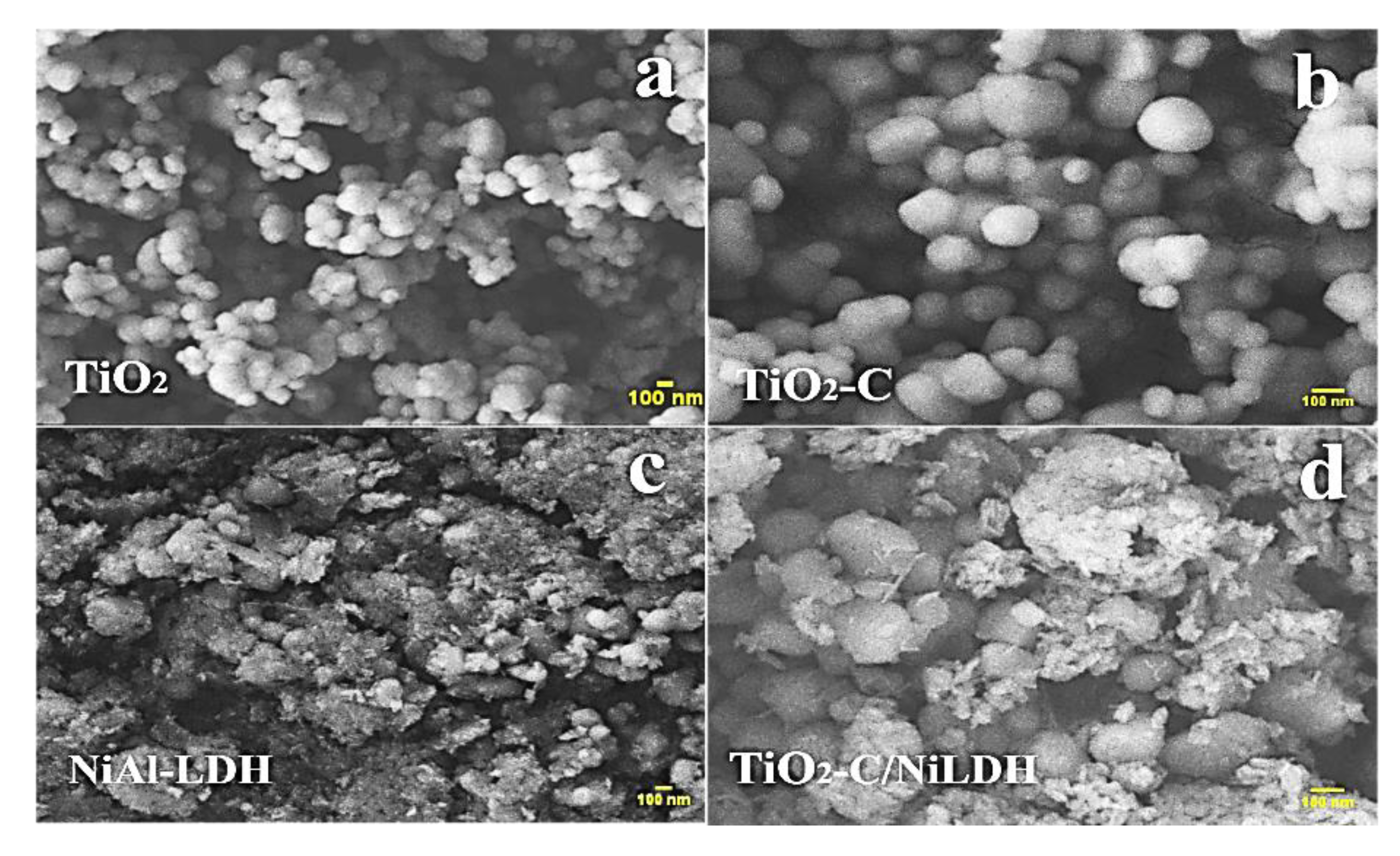
2.1.4. Scanning Electron Microscopy (SEM)
2.1.5. Energy Dispersive Spectroscopy (EDS)
2.1.6. Transmission Electron Microscopy (TEM)
2.1.7. Diffuse Reflectance Spectroscopy (UV-DRS)
2.1.8. N2 Physisorption Measurement
2.2. Evaluation of Photocatalytic Activity
2.2.1. Photocatalytic Studies
2.2.2. Recycling Test
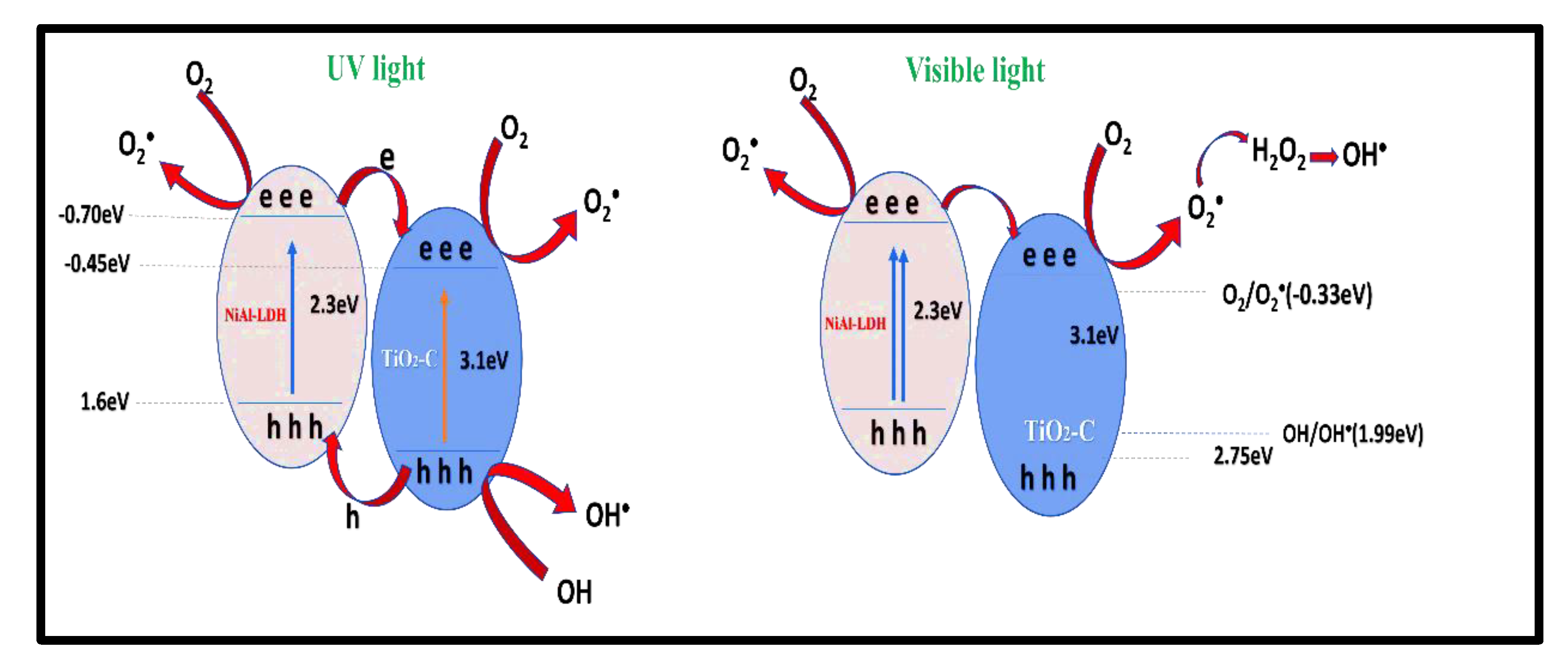
2.2.3. Photocatalytic Mechanism
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Carbon Spheres
4.3. Preparation of TiO2-Carbon Hollow Spheres
4.4. Preparation of Amalgamated TiO2-C/Ni-LDH
4.5. Experimental Techniques
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, B.; Liu, G.; Wang, L. Recent advances in 2D materials for photocatalysis. Nanoscale 2016, 8, 6904–6920. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Prouty, M.D.; Guo, Z.; Golub, V.; Kumar, C.S.S.R.; Lvov, Y.M. Magnetic switch of permeability for polyelectrolyte microcapsules embedded with Co@Aunanoparticles. Langmuir 2005, 21, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mao, S.; Shi, M.; Hong, X.; Wang, D.; Wang, F.; Xia, M.; Chen, Q. Enhanced photocatalytic degradation performance of BiVO4/BiOBr through combining Fermi level alteration and oxygen defect engineering. Chem. Eng. J. 2022, 449, 137757. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Tu, W.; Chen, G.; Xu, R. Metal-free photocatalysts for various applications in energy conversion and environmental purification. Green Chem. 2016, 19, 882–899. [Google Scholar] [CrossRef]
- Lai, C.; An, N.; Li, B.; Zhang, M.; Yi, H.; Liu, S.; Qin, L.; Liu, X.; Li, L.; Fu, Y.; et al. Future roadmap on nonmetal-based 2D ultrathin nanomaterials for photocatalysis. Chem. Eng. J. 2020, 406, 126780. [Google Scholar] [CrossRef]
- Li, Y.; Gao, C.; Long, R.; Xiong, Y. Photocatalyst design based on two-dimensional materials. Mater. Today Chem. 2019, 11, 197–216. [Google Scholar] [CrossRef]
- Yang, X.; Singh, D.; Ahuja, R. Recent advancements and future prospects in ultrathin 2d semiconductor-based photocatalysts for water splitting. Catalysts 2020, 10, 1111. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Xie, Y. Recent progress in ultrathin two-dimensional semiconductors for photocatalysis. Mater. Sci. Eng. R Rep. 2018, 130, 1–39. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Ali, S.L.; Salama, R.S. Promotional Synergistic Effect of Cs–Au NPs on the Performance of Cs–Au/MgFe2O4 Catalysts in Catalysis 3,4-Dihydropyrimidin-2(1H)-Ones and Degradation of RhB Dye. J. Inorg. Organomet. Polym. Mater. 2022, 2, 1–12. [Google Scholar] [CrossRef]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B Environ. 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Alswat, A.A.; Salama, R.S. Gold-selenide quantum dots supported onto cesium ferrite nanocomposites for the efficient degradation of rhodamine B. Heliyon 2022, 8, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Alshorifi, F.T.; Alswat, A.A.; Mannaa, M.A.; Alotaibi, M.T.; El-Bahy, S.M.; Salama, R.S. Facile and Green Synthesis of Silver Quantum Dots Immobilized onto a Polymeric CTS–PEO Blend for the Photocatalytic Degradation of p-Nitrophenol. ACS Omega 2021, 6, 30432–30441. [Google Scholar] [CrossRef] [PubMed]
- El-Hakam, S.A.; Alshorifi, F.T.; Salama, R.S.; Gamal, S.; El-Yazeed, W.A.; Ibrahim, A.A.; Ahmed, A.I. Application of nanostructured mesoporous silica/ bismuth vanadate composite catalysts for the degradation of methylene blue and brilliant green. J. Mater. Res. Technol. 2022, 18, 1963–1976. [Google Scholar] [CrossRef]
- Jovanov, V.; Rudic, O.; Ranogajec, J.; Fidanchevska, E. Synthesis of nanocomposite coating based on TiO2/ZnAl layer double hydroxides. Mater. Constr. 2017, 67, 112. [Google Scholar] [CrossRef]
- Balu, S.; Chen, Y.-L.; Yang, T.C.-K.; Chen, J.-N.; Chen, S.-W. Effect of ultrasound-induced hydroxylation and exfoliation on P90–TiO2/g-C3N4 hybrids with enhanced optoelectronic properties for visible-light photocatalysis and electrochemical sensing. Ceram. Int. 2020, 46, 18002–18018. [Google Scholar] [CrossRef]
- Seftel, E.M.; Popovici, E.; Beyers, E.; Mertens, M.; Zhu, H.Y.; Vansant, E.F.; Cool, P. New TiO 2/MgAl-LDH nanocomposites for the photocatalytic degradation of dyes. J. Nanosci. Nanotechnol. 2010, 10, 8227–8233. [Google Scholar] [CrossRef]
- Korošec, R.C.; Miljević, B.; Umek, P.; van der Bergh, J.M.; Vučetić, S.; Ranogajec, J. Photocatalytic self-cleaning properties of Mo:TiO2 loaded Zn–Al layered double hydroxide synthesised at optimised pH value for the application on mineral substrates. Ceram. Int. 2019, 46, 6756–6766. [Google Scholar] [CrossRef]
- Al-Ghafri, B.; Lau, W.-J.; Al-Abri, M.; Goh, P.-S.; Ismail, A.F. Titanium dioxide-modified polyetherimide nanofiber membrane for water treatment. J. Water Process Eng. 2019, 32, 100970. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, S.; Pan, T.; Xu, S.; Zhou, A.; Pu, M.; Yan, H.; Han, J.; Wei, M.; Evans, D.G.; et al. TiO2@layered double hydroxide core-shell nanospheres with largely enhanced photocatalytic activity toward O2 generation. Adv. Funct. Mater. 2015, 25, 2243–2249. [Google Scholar] [CrossRef]
- Pai, S.; Kini, S.M.; Selvaraj, R.; Pugazhendhi, A. A review on the synthesis of hydroxyapatite, its composites and adsorptive removal of pollutants from wastewater. J. Water Process Eng. 2020, 38, 101574. [Google Scholar] [CrossRef]
- Jo, W.-K.; Moru, S.; Tonda, S. A green approach to the fabrication of a TiO2/NiAl-LDH core–shell hybrid photocatalyst for efficient and selective solar-powered reduction of CO2 into value-added fuels. J. Mater. Chem. A 2020, 8, 8020–8032. [Google Scholar] [CrossRef]
- Saber, O.; Kotb, H.M. Designing dual-function nanostructures for water purification in sunlight. Appl. Sci. 2020, 10, 1786. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.; Popov, A. Extraction–pyrolytic method for TiO2 polymorphs production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Singh, T.; Pal, D.B.; Bhatiya, A.K.; Mishra, P.K.; Hashem, A.; Alqarawi, A.A.; AbdAllah, E.F.; Gupta, V.K.; Srivastava, N. Integrated process approach for degradation of p-cresol pollutant under photocatalytic reactor using activated carbon/TiO2 nanocomposite: Application in wastewater treatment. Environ. Sci. Pollut. Res. 2021, 29. [Google Scholar] [CrossRef] [PubMed]
- Tonda, S.; Kumar, S.; Bhardwaj, M.; Yadav, P.; Ogale, S. g-C3N4/NiAl-LDH 2D/2D Hybrid Heterojunction for High-Performance Photocatalytic Reduction of CO2 into Renewable Fuels. ACS Appl. Mater. Interfaces 2018, 10, 2667–2678. [Google Scholar] [CrossRef]
- Zhang, H.; Du, G.; Lu, W.; Cheng, L.; Zhu, X.; Jiao, Z. Porous TiO2 hollow nanospheres: Synthesis, characterization and enhanced photocatalytic properties. CrystEngComm 2012, 14, 3793–3801. [Google Scholar] [CrossRef]
- Yang, H.; Wang, G.; Ding, N.; Yin, C.; Chen, Y. Size-controllable synthesis of carbon spheres with assistance of metal ions. Synth. Met. 2016, 214, 1–4. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Xu, Y.; Feng, Z.; Huang, J. Defect-assisted surface modification enhances the visible light photocatalytic performance of g-C3N4@C-TiO2 direct Z-scheme heterojunctions. Chin. J. Catal. 2019, 40, 424–433. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Thommes, M. Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 2018, 4, 559–566. [Google Scholar] [CrossRef]
- Lai, C.W.; Sreekantan, S. Single step formation of C-TiO2 nanotubes: Influence of applied voltage and their photocatalytic activity under solar illumination. Int. J. Photoenergy 2013, 2013, 11–15. [Google Scholar] [CrossRef]
- Claydon, R.; Wood, J. A Mechanistic Study of Layered-Double Hydroxide (LDH)-Derived Nickel-Enriched Mixed Oxide (Ni-MMO) in Ultradispersed Catalytic Pyrolysis of Heavy Oil and Related Petroleum Coke Formation. Energy Fuels 2019, 33, 10820–10832. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Xue, Z.; Yang, C.; Qin, J.; Zhang, L.; Yu, P.; Wang, S.; Zhao, Y.; Zhang, X.; Liu, R. Insights into the Li+ storage mechanism of TiC@C-TiO2 core-shell nanostructures as high-performance anodes. Nano Energy 2018, 50, 25–34. [Google Scholar] [CrossRef]
- Jeong, I.R.; Lee, J.H.; Song, J.; Oh, Y.S.; Cho, S. Control of structural disorder in spinel ceramics derived from layered double hydroxides. Ceram. Int. 2019, 46, 6594–6599. [Google Scholar] [CrossRef]
- Kumar, S.; Isaacs, M.A.; Trofimovaite, R.; Durndell, L.; Parlett, C.M.; Douthwaite, R.E.; Coulson, B.; Cockett, M.C.; Wilson, K.; Lee, A.F. P25@CoAl layered double hydroxide heterojunction nanocomposites for CO2 photocatalytic reduction. Appl. Catal. B Environ. 2017, 209, 394–404. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, L.; Zhu, S. Synthesis of core-shell Fe 3O 4@SiO 2@TiO 2 microspheres and their application as recyclable photocatalysts. Int. J. Photoenergy 2012, 2012, 202519. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Vu, A.T.; Dang, V.H.; Wu, J.C.-S.; Le, M.T. Photocatalytic Degradation of Phenol and Methyl Orange with Titania-Based Photocatalysts Synthesized by Various Methods in Comparison with ZnO–Graphene Oxide Composite. Top. Catal. 2020, 63, 1215–1226. [Google Scholar] [CrossRef]
- Baig, M.M.; Pervaiz, E.P.E.; Afzal, M.J.A.M.J. Catalytic Activity and Kinetic Studies of Core@Shell Nanostructure NiFe2O4@TiO2 for Photocatalytic Degradation of Methyl Orange Dye. J. Chem. Soc. Pak. 2020, 42, 531. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Shi, M.; Wang, F.; Xia, M.; Chen, Q.; Ju, X. Peroxymonosulfate activation through 2D/2D Z-scheme CoAl-LDH/BiOBr photocatalyst under visible light for ciprofloxacin degradation. J. Hazard. Mater. 2021, 420, 126613. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Wang, H.; Wu, Y.; Wang, F.; Xia, M.; Chen, Q. Peroxymonosulfate-assisted for facilitating photocatalytic degradation performance of 2D/2D WO3/BiOBr S-scheme heterojunction. Chem. Eng. J. 2021, 430, 132806. [Google Scholar] [CrossRef]
- Ali, T.T.; Narasimharao, K.; Basahel, S.N.; Mokhtar, M.; Alsharaeh, E.H.; Mahmoud, H.A. Template Assisted Microwave Synthesis of rGO-ZrO2 Composites: Efficient Photocatalysts Under Visible Light. J. Nanosci. Nanotechnol. 2019, 19, 5177–5188. [Google Scholar] [CrossRef]
- Kowsari, E. Carbon-Based Nanocomposites for Visible Light-Induced Photocatalysis. In Nanocomposites for Visible Light-Induced Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 203–249. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: A review. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Seftel, E.; Niarchos, M.; Mitropoulos, C.; Mertens, M.; Vansant, E.; Cool, P. Photocatalytic removal of phenol and methylene-blue in aqueous media using TiO2@LDH clay nanocomposites. Catal. Today 2015, 252, 120–127. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.K. Theory of Optical Processes in Semiconductors. Theory Opt. Process. Semicond. 2010. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol-gel and commercial TiO 2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
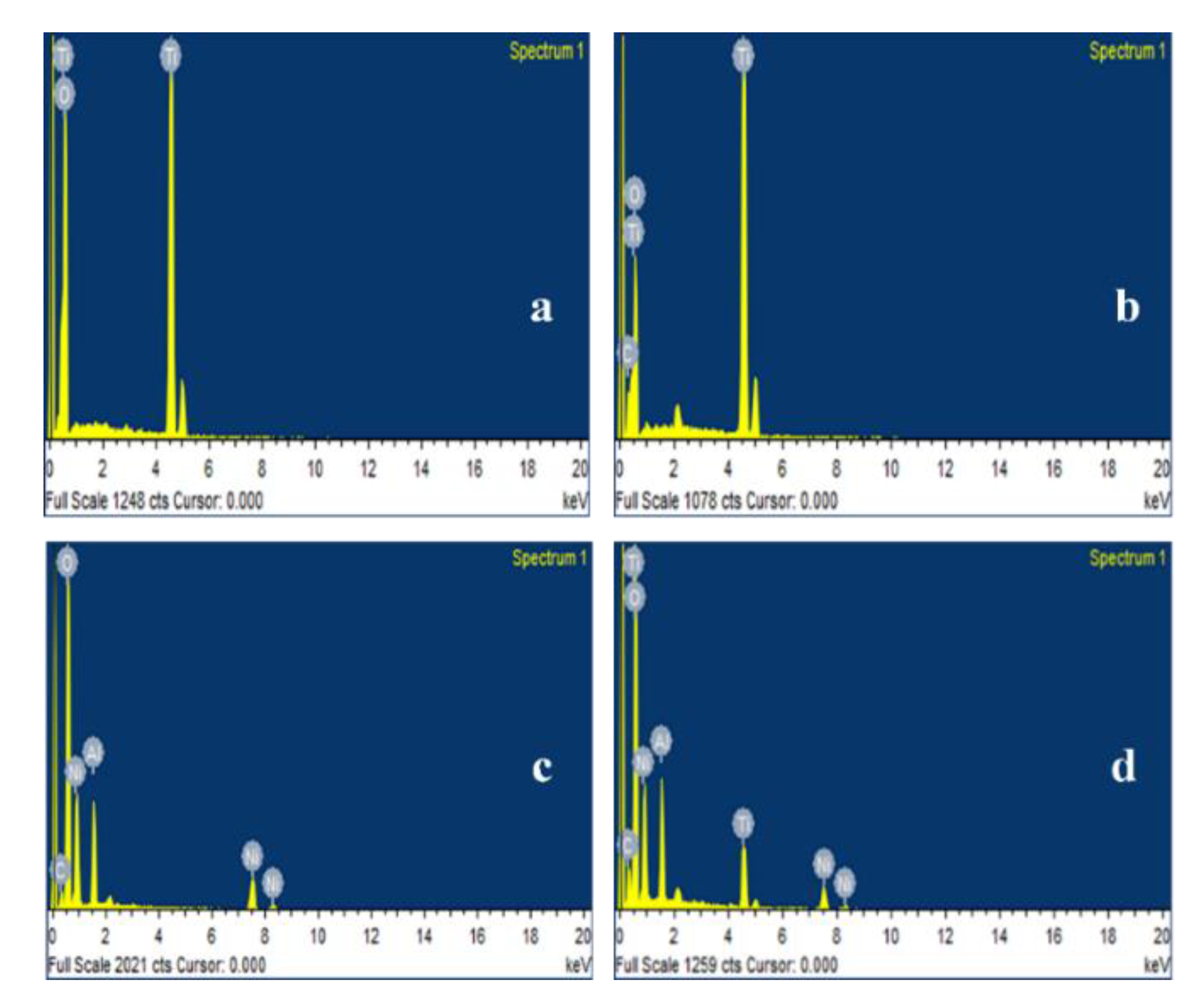


| Element (Atomic %) | C | Ti | O | Ni | Al |
|---|---|---|---|---|---|
| TiO2 | - | 42.12 | 57.88 | - | - |
| TiO2-C | 5.26 | 50.30 | 44.44 | - | - |
| NiAl-LDH | 8.48 | - | 52.19 | 29.77 | 9.56 |
| CTL-Ni | 10.21 | 11.97 | 56.02 | 14.78 | 7.02 |
| Catalyst | SBET m2 g−1 | Vp cm3 g−1 | Vmic. cm3 g−1 | Av. Pore Diameter nm | Cconstant |
|---|---|---|---|---|---|
| NiAl-LDH | 61 | 0.046 | 13.943 | 2.8591 | 85.449 |
| TiO2-C/NiLDH | 72 | 0.01259 | 16.536 | 4.919 | 90.725 |
| Catalyst | Process Operating Condition | % Photocatalytic Degradation | Reference |
|---|---|---|---|
| TiO2 (anatase) | UV irradiation, A 100 W Hg lamp (Toshiba BLB 20 W), pH = 4 | about 51% | In this study |
| GO–TiO2 | UV irradiation, (λ = 254 nm), 100 W | about 18% | [38] |
| NiFe2O4@TiO2 | UV lamp, (λ = 400 nm), 300 W Xenon | about 90.06% | [39] |
| Fe3O4@SiO2@TiO2 | UV light 300 W, pH = 3 | about 91% | [37] |
| TiO2-C/NiLDH amalgam | UV irradiation, A 100 W Hg lamp (Toshiba BLB 20 W), pH = 4 | 100% | In this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Soihi, A.S.; Alsulami, Q.A.; Mostafa, M.M.M. Amalgamated Titanium Oxide-Carbon Hollow Sphere/Nickel-Layered Double Hydroxide as an Efficient Photocatalyst for the Degradation of Methyl Orange. Catalysts 2022, 12, 1200. https://doi.org/10.3390/catal12101200
Al-Soihi AS, Alsulami QA, Mostafa MMM. Amalgamated Titanium Oxide-Carbon Hollow Sphere/Nickel-Layered Double Hydroxide as an Efficient Photocatalyst for the Degradation of Methyl Orange. Catalysts. 2022; 12(10):1200. https://doi.org/10.3390/catal12101200
Chicago/Turabian StyleAl-Soihi, Auhood S., Qana A. Alsulami, and Mohamed Mokhtar M. Mostafa. 2022. "Amalgamated Titanium Oxide-Carbon Hollow Sphere/Nickel-Layered Double Hydroxide as an Efficient Photocatalyst for the Degradation of Methyl Orange" Catalysts 12, no. 10: 1200. https://doi.org/10.3390/catal12101200
APA StyleAl-Soihi, A. S., Alsulami, Q. A., & Mostafa, M. M. M. (2022). Amalgamated Titanium Oxide-Carbon Hollow Sphere/Nickel-Layered Double Hydroxide as an Efficient Photocatalyst for the Degradation of Methyl Orange. Catalysts, 12(10), 1200. https://doi.org/10.3390/catal12101200