The Synthesis of Cu–Mn–Al Mixed-Oxide Combustion Catalysts by Co-Precipitation in the Presence of Starch: A Comparison of NaOH with Organic Precipitants
Abstract
1. Introduction
2. Results
2.1. Physicochemical Characterization
2.1.1. XRD Analysis
2.1.2. SEM/EDS Analysis
2.1.3. FTIR Analysis
2.1.4. Thermal Analysis
2.1.5. H2 TPR-MS Analysis
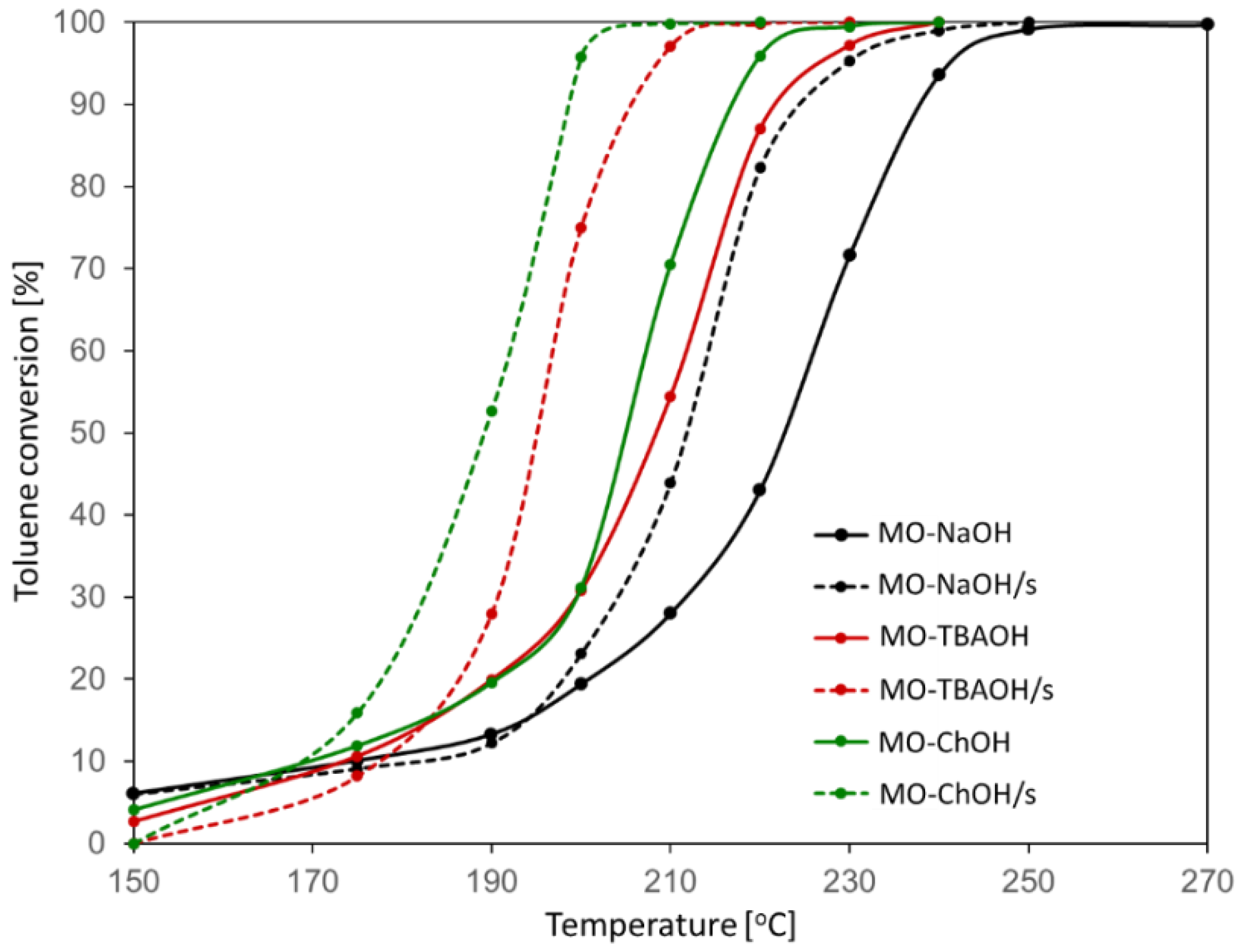
2.2. Catalytic Combustion of Toluene
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Montero-Montoya, R.; López-Vargas, R.; Arellano-Aguilar, O. Volatile Organic Compounds in Air: Sources, Distribution, Exposure and Associated Illnesses in Children. Ann. Glob. Health 2018, 84, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Kuśtrowski, P.; Rokicińska, A.; Kondratowicz, T. Abatement of volatile organic compounds emission as a target for various human activities including energy production. Adv. Inorg. Chem. 2018, 72, 385–419. [Google Scholar]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Adebayo, B.; Gelles, T.; Rownaghi, A.; Rezaei, F. Abatement of Gaseous Volatile Organic Compounds: A Process Perspective. Catal. Today 2019, 350, 100–119. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Lyu, Y.; Li, C.; Du, X.; Zhu, Y.; Zhang, Y.; Li, S. Catalytic removal of toluene over manganese oxide-based catalysts: A review. Environ. Sci. Pollut. Res. 2020, 27, 2482–2501. [Google Scholar] [CrossRef]
- Spivey, J.J. Complete catalytic oxidation of volatile organics. Ind. Eng. Chem. Res. 1987, 26, 2165–2180. [Google Scholar] [CrossRef]
- Everaert, K.; Baeyens, J. Catalytic combustion of volatile organic compounds. J. Hazard. Mater. 2004, 109, 113–139. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Shanguan, W. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Lee, J.E.; Ok, Y.S.; Tsang, D.C.W.; Song, J.; Jung, S.C.; Park, Y.K. Recent advances in volatile organic compounds abatement by catalysis and catalytic hybrid processes: A critical review. Sci. Total Environ. 2020, 719, 137405. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Su, F.; Zhao, Q.; Li, J.; Zhang, C.; Zhang, R.; Liu, P. Catalytic oxidation of volatile organic compounds over manganese-based oxide catalysts: Performance, deactivation and future opportunities. Sep. Purif. Technol. 2022, 396, 121436. [Google Scholar] [CrossRef]
- Lamb, A.B.; Bray, W.C.; Frazer, J.C.W. The Removal of Carbon Monoxide from Air. J. Ind. Eng. Chem. 1920, 12, 213–221. [Google Scholar] [CrossRef]
- Merrill, D.R.; Scalione, C.C. The Catalytic Oxidation of Carbon Monoxide at Ordinary Temperatures. J. Am. Chem. Soc. 1921, 43, 1982–2002. [Google Scholar] [CrossRef]
- Li, W.B.; Chu, W.B.; Zhuang, M.; Hua, J. Catalytic oxidation of toluene on Mn–containing mixed oxides prepared in reverse microemulsions. Catal. Today 2004, 93–95, 205–209. [Google Scholar] [CrossRef]
- Morales, M.R.; Barbero, B.P.; Cadús, L.E. Total oxidation of ethanol and propane over Mn-Cu mixed oxide catalysts. Appl. Catal. B Environ. 2006, 67, 229–236. [Google Scholar] [CrossRef]
- Zimowska, M.; Michalik-Zym, A.; Janik, R.; Machej, T.; Gurgul, J.; Socha, R.P.; Podobiński, J.; Serwicka, E.M. Catalytic combustion of toluene over mixed Cu–Mn oxides. Catal. Today 2007, 119, 321–326. [Google Scholar] [CrossRef]
- Chen, H.; Tong, X.; Li, Y. Mesoporous Cu-Mn Hopcalite catalyst and its performance in low temperature ethylene combustion in a carbon dioxide stream. Appl. Catal. A Gen. 2009, 370, 59–65. [Google Scholar] [CrossRef]
- Vu, V.H.; Belkouch, J.; Ould-Dris, A.; Taouk, B. Removal of hazardous chlorinated VOCs over Mn–Cu mixed oxide based catalyst. J. Hazard. Mater. 2009, 169, 758–765. [Google Scholar] [CrossRef]
- Palacio, L.A.; Velásquez, J.; Echavarría, A.; Faro, A.; Ribeiro, F.R.; Ribeiro, M.F. Total oxidation of toluene over calcined trimetallic hydrotalcites type catalysts. J. Hazard. Mater. 2010, 177, 407–413. [Google Scholar] [CrossRef]
- Aguilera, D.A.; Perez, A.; Molina, R.; Moreno, S. Cu-Mn and Co-Mn catalysts synthesized from hydrotalcites and their use in the oxidation of VOCs. Appl. Catal. B Environ. 2011, 104, 144–150. [Google Scholar] [CrossRef]
- Behar, S.; Gonzalez, P.; Agulhon, P.; Quignard, F.; Świerczyński, D. New synthesis of nanosized Cu-Mn spinels as efficient oxidation catalysts. Catal. Today 2012, 189, 35–41. [Google Scholar] [CrossRef]
- Machej, T.; Serwicka, E.M.; Zimowska, M.; Dula, R.; Michalik-Zym, A.; Napruszewska, B.; Rojek, W.; Socha, R. Cu/Mn-based mixed oxides derived from hydrotalcite-like precursors as catalysts for methane combustion. Appl. Catal. A Gen. 2014, 474, 87–94. [Google Scholar] [CrossRef]
- Behar, S.; Gómez-Mendoza, N.A.; Gómez-García, M.Á.; Świerczyński, D.; Quignard, F.; Tanchoux, N. Study and modelling of kinetics of the oxidation of VOC catalyzed by nanosized Cu-Mn spinels prepared via an alginate route. Appl. Catal. A Gen. 2015, 504, 203–210. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Li, S.; Shan, X.; Liu, G.; Chen, Y. Co-nanocasting synthesis of mesoporous Cu-Mn composite oxides and their promoted catalytic activities for gaseous benzene removal. Appl. Catal. B Environ. 2015, 162, 110–121. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Han, Y.; Lu, C.; Wan, H.; Xu, Z.; Zheng, S. Enhanced catalytic toluene oxidation by interaction between copper oxide and manganese oxide in Cu-O-Mn/γ-Al2O3 catalysts. Appl. Surf. Sci. 2017, 420, 260–266. [Google Scholar] [CrossRef]
- Ye, Z.; Giraudon, J.M.; Nuns, N.; Simon, P.; De Geyter, N.; Morent, R.; Lamonier, J.F. Influence of the preparation method on the activity of copper-manganese oxides for toluene total oxidation. Appl. Catal. B Environ. 2018, 223, 154–166. [Google Scholar] [CrossRef]
- Napruszewska, B.D.; Michalik, A.; Walczyk, A.; Duraczyńska, D.; Dula, R.; Rojek, W.; Lityńska-Dobrzyńska, L.; Bahranowski, K.; Serwicka, E.M. Composites of Laponite and Cu–Mn Hopcalite-Related Mixed Oxides Prepared from Inverse Microemulsions as Catalysts for Total Oxidation of Toluene. Materials 2018, 11, 1365. [Google Scholar] [CrossRef]
- Luo, M.; Cheng, Y.; Peng, X.; Pan, W. Copper modified manganese oxide with tunnel structure as efficient catalyst for low-temperature catalytic combustion of toluene. Chem. Eng. J. 2019, 369, 758–765. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Li, S.; Zhang, L.; Zheng, G.; Guo, L. Layered copper manganese oxide for the efficient catalytic CO and VOCs oxidation. Chem. Eng. J. 2019, 357, 258–268. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, J.; Ren, R.; Li, J.; Wang, N.; Chu, W. Facile synthesis of homogeneous hollow microsphere Cu–Mn based catalysts for catalytic oxidation of toluene. Chemosphere 2020, 247, 125812. [Google Scholar] [CrossRef]
- Solt, H.E.; Németh, P.; Mohai, M.; Sajó;, I.E.; Klébert, S.; Franguelli, F.P.; Fogaca, L.A.; Pawar, R.P.; Kótai, L. Temperature-limited synthesis of copper manganites along the borderline of the amorphous/crystalline state and their catalytic activity in CO oxidation. ACS Omega 2021, 6, 1523–1533. [Google Scholar] [CrossRef]
- Li, J.R.; Zhang, W.P.; Li, C.; He, C. Efficient catalytic degradation of toluene at a readily prepared Mn-Cu catalyst: Catalytic performance and reaction pathway. J. Coll. Interface Sci. 2021, 591, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, Z.; Li, Y.; Hou, Y.; Hu, J.; Huang, Z. Effect of the A-site cation over spinel AMn2O4 (A = Cu2+, Ni2+, Zn2+) for toluene combustion: Enhancement of the synergy and the oxygen activation ability. Fuel 2021, 288, 119700. [Google Scholar] [CrossRef]
- Ding, S.; Zhu, C.; Hojo, H.; Einaga, H. Enhanced catalytic performance of spinel-type Cu-Mn oxides for benzene oxidation under microwave irradiation. J. Hazard. Mater. 2022, 424, 127523. [Google Scholar] [CrossRef]
- Liu, W.; Xiang, W.; Chen, X.; Song, Z.; Gao, C.; Tsubaki, N.; Zhang, X. A novel strategy to adjust the oxygen vacancy of CuO/MnO2 catalysts toward the catalytic oxidation of toluene. Fuel 2022, 312, 122975. [Google Scholar] [CrossRef]
- Ye, Z.; Giraudon, J.M.; Nuns, N.; Abdallah, G.; Addad, A.; Morent, R.; De Geyter, N.; Lamonier, J.F. Preferential dissolution of copper from Cu-Mn oxides in strong acid medium: Effect of the starting binary oxide to get new efficient copper doped MnO2 catalysts in toluene oxidation. Appl. Surf. Sci. 2021, 537, 147993. [Google Scholar] [CrossRef]
- Herrera-Beurnio, M.C.; Hidalgo-Carrillo, J.; López-Tenllado, F.J.; Martin-Gómez, J.; Estévez, R.C.; Urbano, F.J.; Marinas, A. Bio-Templating: An Emerging Synthetic Technique for Catalysts. A Review. Catalysts 2021, 11, 1364. [Google Scholar] [CrossRef]
- Coelho, A.; Perrone, O.M.; Gomes, E.; Da-Silva, R.; Thoméo, J.C.; Boscolo, M. Mixed metal oxides from sucrose and corn starch templated hydrotalcite-like LDHs as catalysts for ethyl biodiesel synthesis. Appl. Catal. A 2017, 532, 32–39. [Google Scholar] [CrossRef]
- Tao, X.; Liu, D.; Cong, W.; Huang, L. Controllable synthesis of starch-modified ZnMgAl-LDHs for adsorption property improvement. Appl. Surf. Sci. 2018, 457, 572–579. [Google Scholar] [CrossRef]
- Manikandan, M.; Sangeetha, P. Optimizing the Surface Properties of MgO Nanoparticles towards the Transesterification of Glycerol to Glycerol Carbonate. ChemistrySelect 2019, 4, 6672–6678. [Google Scholar] [CrossRef]
- Xiong, Z.; Li, Z.; Li, C.; Wang, W.; Lu, W.; Du, Y.; Tian, S. Green synthesis of Tungsten-doped CeO2 catalyst for selective catalytic reduction of NO with NH3 using starch bio-template. Appl. Surf. Sci. 2020, 536, 147719. [Google Scholar] [CrossRef]
- Michalik, A.; Napruszewska, B.D.; Walczyk, A.; Kryściak-Czerwenka, J.; Duraczyńska, D.; Serwicka, E.M. Synthesis of Nanocrystalline Mg-Al Hydrotalcites in the Presence of Starch—The Effect on Structure and Composition. Materials 2020, 13, 602. [Google Scholar] [CrossRef]
- Karcz, R.; Napruszewska, B.D.; Michalik, A.; Kryściak-Czerwenka, J.; Duraczyńska, D.; Serwicka, E.M. Fine Crystalline Mg-Al Hydrotalcites as Catalysts for Baeyer-Villiger Oxidation of Cyclohexanone with H2O2. Catalysts 2021, 11, 1493. [Google Scholar] [CrossRef]
- Kruissink, E.C.; Pelt, H.L.; Ross, J.R.H.; Van Reüen, L.L. The effect of sodium on the methanation activity of nickel/alumina coprecipitated catalysts. Appl. Catal. 1981, 1, 23–29. [Google Scholar] [CrossRef]
- Liu, W.; Flytzanistephanopoulos, M. Total Oxidation of Carbon Monoxide and Methane over Transition Metal Fluorite Oxide Composite Catalysts. J. Catal. 1995, 153, 304–316. [Google Scholar] [CrossRef]
- Mirzaei, A.A.; Shaterian, H.R.; Joyner, R.W.; Stockenhuber, M.; Taylor, S.H.; Hutchings, G.J. Ambient temperature carbon monoxide oxidation using copper manganese oxide catalysts: Effect of residual Na+ acting as catalyst poison. Catal. Commun. 2003, 4, 17–20. [Google Scholar] [CrossRef]
- Einaga, H.; Maeda, N.; Teraoka, Y. Effect of catalyst composition and preparation conditions on catalytic properties of unsupported manganese oxides for benzene oxidation with ozone. Appl. Catal. B Environ. 2013, 142–143, 406–413. [Google Scholar] [CrossRef]
- Castañeda, R.; Pascual, L.; Martínez-Arias, A. Influence of sodium impurities on the properties of CeO2/CuO for carbon monoxide oxidation in a hydrogen-rich stream. Catal. Commun. 2018, 108, 88–92. [Google Scholar] [CrossRef]
- Einaga, H.; Kiya, A.; Yoshioka, S.; Teraoka, Y. Catalytic properties of copper–manganese mixed oxides prepared by coprecipitation using tetramethylammonium hydroxide. Catal. Sci. Technol. 2014, 4, 3713–3722. [Google Scholar] [CrossRef]
- Shuck, C.E.; Ventura-Martinez, K.; Goad, A.; Uzun, S.; Shekhirev, M.; Gogotsi, Y. Safe Synthesis of MAX and MXene: Guidelines to Reduce Risk During Synthesis. ACS Chem. Health Saf. 2021, 28, 326–338. [Google Scholar] [CrossRef]
- Karcz, R.; Napruszewska, B.D.; Walczyk, A.; Kryściak-Czerwenka, J.; Duraczyńska, D.; Płaziński, W.; Serwicka, E.M. Comparative Physicochemical and Catalytic Study of Nanocrystalline Mg-Al Hydrotalcites Precipitated with Inorganic and Organic Bases. Nanomaterials 2022, 12, 2775. [Google Scholar] [CrossRef] [PubMed]
- Bukhtiyarova, M.V. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2019, 269, 494–596. [Google Scholar] [CrossRef]
- Porta, P.; Moretti, G.; Jacono, M.L.; Musicanti, M.; Nardella, A. Characterization of copper–manganese hydroxysalts and oxysalts. J. Mater. Chem. 1991, 1, 129–135. [Google Scholar] [CrossRef]
- Clarke, T.J.; Davies, T.E.; Kondrat, S.A.; Taylor, S.H. Mechanochemical synthesis of copper manganese oxide for the ambient temperature oxidation of carbon monoxide. Appl. Catal. B Environ. 2015, 165, 222–231. [Google Scholar] [CrossRef]
- Fubini, B.; Stone, F.S. Physico-chemical properties of MnCO3–CaCO3 and MnO–CaO solid solutions. J. Chem. Soc. Faraday Trans. 1983, 79, 1215. [Google Scholar] [CrossRef]
- Gillot, B.; Buguet, S.; Kester, E. Oxidation mechanism and valence states of copper and manganese in tetragonal CuMn2O4. J. Mater. Chem. 1997, 7, 2513–2517. [Google Scholar] [CrossRef]
- Garcia, J.C.; Barai, P.; Chen, J.; Gutierrez, A.; Wen, J.; Arslan, I.; Wang, X.; Fister, T.T.; Iddir, H.; Srinivasan, V. Predicting Morphological Evolution during Coprecipitation of MnCO3 Battery Cathode Precursors Using Multiscale Simulations Aided by Targeted Synthesis. Chem. Mater. 2020, 32, 9126–9139. [Google Scholar] [CrossRef]
- Busca, G.; Lorenzelli, V. Infrared spectroscopic identification of species arising from reactive adsorption of carbon oxides on metal oxide surfaces. Mater. Chem. 1982, 7, 89–126. [Google Scholar] [CrossRef]
- Andersen, F.A.; Brečević, L. Infrared spectra of amorphous and crystalline calcium carbonate. Acta Chem. Scand. 1991, 45, 1018–1024. [Google Scholar] [CrossRef]
- Boulard, E.; Goncharov, A.F.; Blanchard, M.; Mao, W.L. Pressure-induced phase transition in MnCO3 and its implications on the deep carbon cycle. J. Geophys. Res. Solid Earth 2015, 120, 4069–4079. [Google Scholar] [CrossRef]
- Kagunya, W.; Baddour-Hadjean, R.; Kooli, F.; Jones, W. Vibrational modes in layered double hydroxides and their calcined derivatives. Chem. Phys. 1998, 236, 225–234. [Google Scholar] [CrossRef]
- Biernacki, L.; Pokrzywnicki, S. The Thermal Decomposition of Manganese Carbonate. Thermogravimetry and Exoemission of Electrons. J. Therm. Anal. Calorim. 1999, 55, 227–232. [Google Scholar] [CrossRef]
- Velu, S.; Swamy, C.S. Synthesis and physicochemical properties of a new copper-manganese-aluminium ternary hydrotalcite-like compound. J. Mater. Sci. Lett. 1996, 15, 1674–1677. [Google Scholar] [CrossRef]
- Kovanda, F.; Grygar, T.; Dorničák, V.; Rojka, T.; Bezdička, P.; Jirátová, K. Thermal behaviour of Cu–Mg–Mn and Ni–Mg–Mn layered double hydroxides and characterization of formed oxides. Appl. Clay Sci. 2005, 28, 121–136. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Rogachev, A.S.; Aruna, S.T. Combustion synthesis in nanostructured reactive systems. Adv. Powder Technol. 2015, 26, 954–976. [Google Scholar] [CrossRef]
- Tanaka, Y. Water gas shift reaction for the reformed fuels over Cu/MnO catalysts prepared via spinel-type oxide. J. Catal. 2003, 215, 271–278. [Google Scholar] [CrossRef]
- Saqer, S.M.; Kondarides, D.I.; Verykios, X.E. Catalytic oxidation of toluene over binary mixtures of copper, manganese and cerium oxides supported on γ-Al2O3. Appl. Catal. B Environ. 2011, 103, 275–286. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Niaei, A.; Salari, D.; Alvarez-Galvan, M.C.; Fierro, J.L.G. Study of correlation between activity and structural properties of Cu-(Cr, Mn and Co)2 nano mixed oxides in VOC combustion. Ceram. Int. 2014, 40, 6157–6163. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Combined steam reforming of methanol over Cu–Mn spinel oxide catalysts. J. Catal. 2007, 251, 7–20. [Google Scholar] [CrossRef]
- Kim, S.C.; Park, Y.K.; Nah, J.W. Property of a highly active bimetallic catalyst based on a supported manganese oxide for the complete oxidation of toluene. Powder Technol. 2014, 266, 292–298. [Google Scholar] [CrossRef]
- Jabłońska, M.; Chmielarz, L.; Węgrzyn, A.; Góra-Marek, K.; Piwowarska, Z.; Witkowski, S.; Bidzińska, E.; Kuśtrowski, P.; Wach, A.; Majda, D. Hydrotalcite derived (Cu, Mn)–Mg–Al metal oxide systems doped with palladium as catalysts for low-temperature methanol incineration. Appl. Clay Sci. 2015, 114, 273–282. [Google Scholar] [CrossRef]
- Valange, S.; Védrine, J. General and Prospective Views on Oxidation Reactions in Heterogeneous Catalysis. Catalysts 2018, 8, 483. [Google Scholar] [CrossRef]
- Liu, T.; Yao, Y.; Wei, L.; Shi, Z.; Han, L.; Yuan, H.; Li, B.; Dong, L.; Wang, F.; Sun, C. Preparation and Evaluation of Copper–Manganese Oxide as a High-Efficiency Catalyst for CO Oxidation and NO Reduction by CO. J. Phys. Chem. C 2017, 121, 12757–12770. [Google Scholar] [CrossRef]
- Liu, X.S.; Jin, Z.N.; Lu, J.Q.; Wang, X.X.; Luo, M.F. Highly active CuO/OMS-2 catalysts for low-temperature CO oxidation. Chem. Eng. J. 2010, 162, 151–157. [Google Scholar] [CrossRef]
- Liu, B.; Wu, H.; Li, S.; Xu, M.; Cao, Y.; Li, Y. Solid-State Construction of CuOx/Cu1.5Mn1.5O4 Nanocomposite with Abundant Surface CuOx Species and Oxygen Vacancies to Promote CO Oxidation Activity. Int. J. Mol. Sci. 2022, 23, 6856. [Google Scholar] [CrossRef]

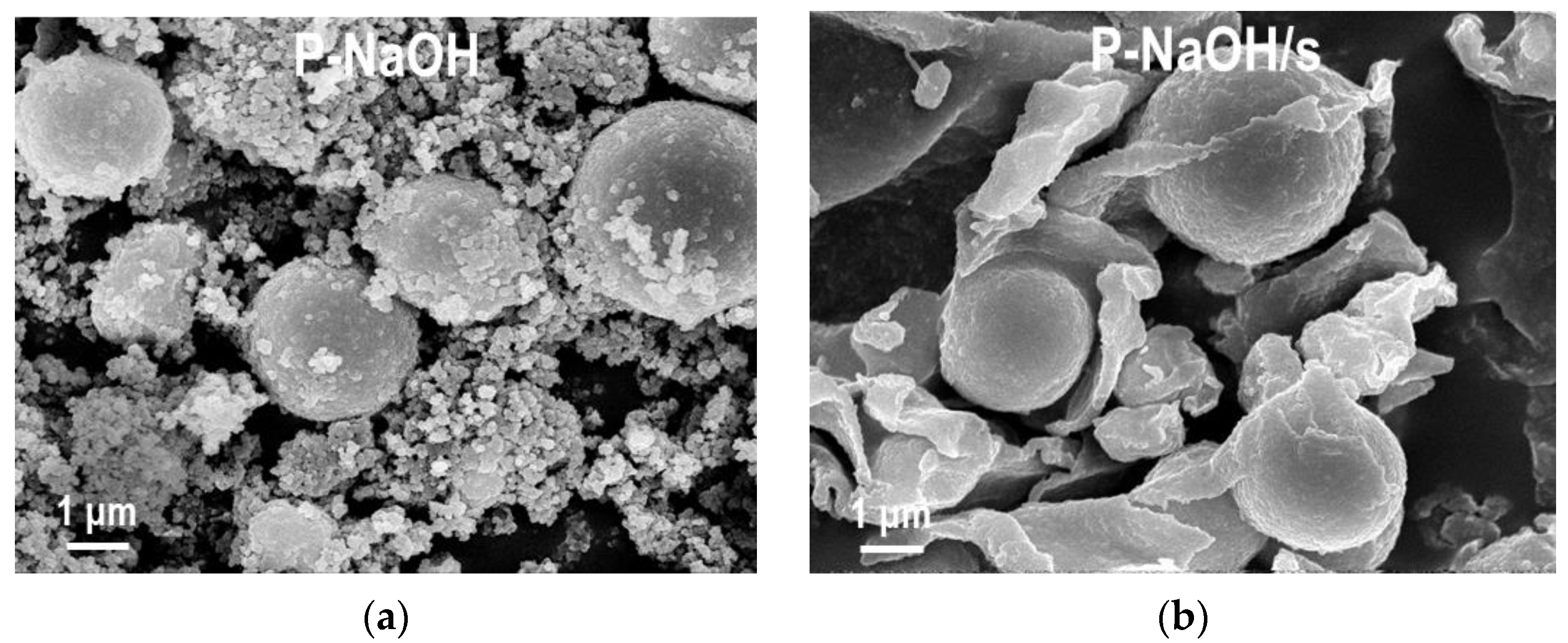
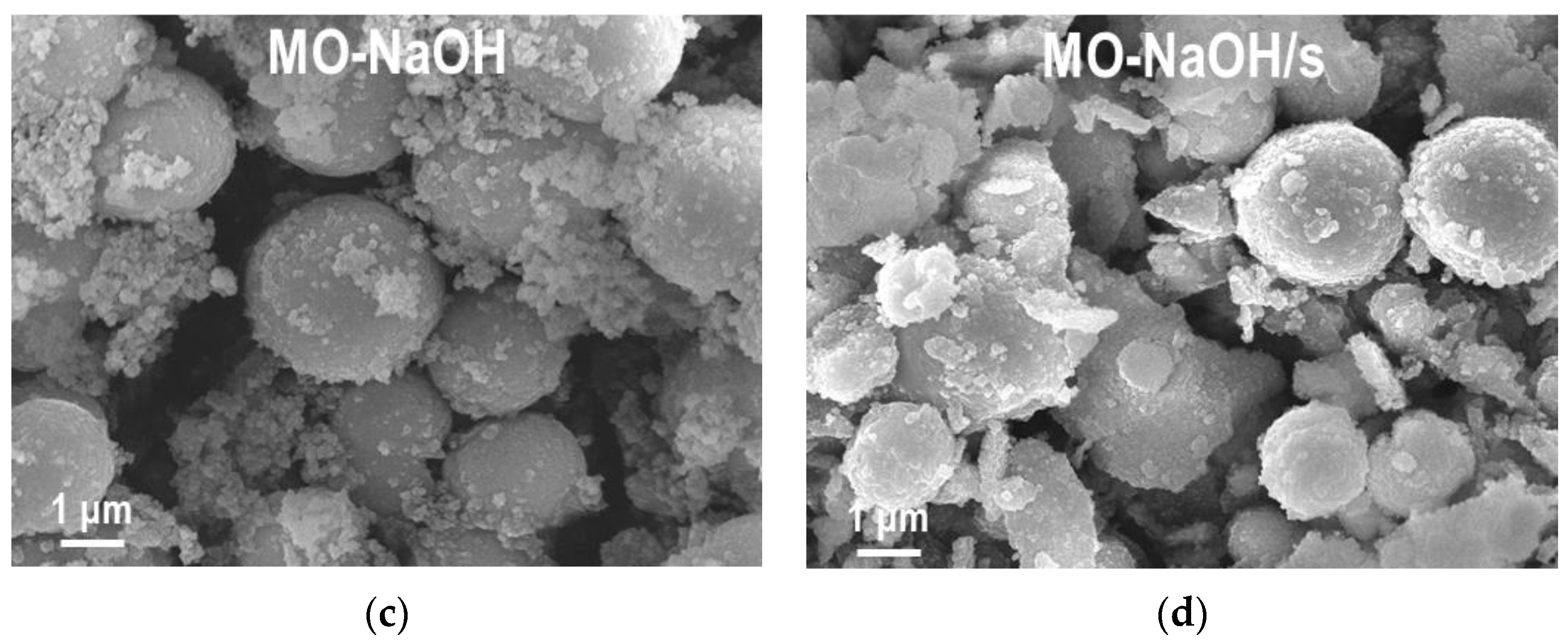
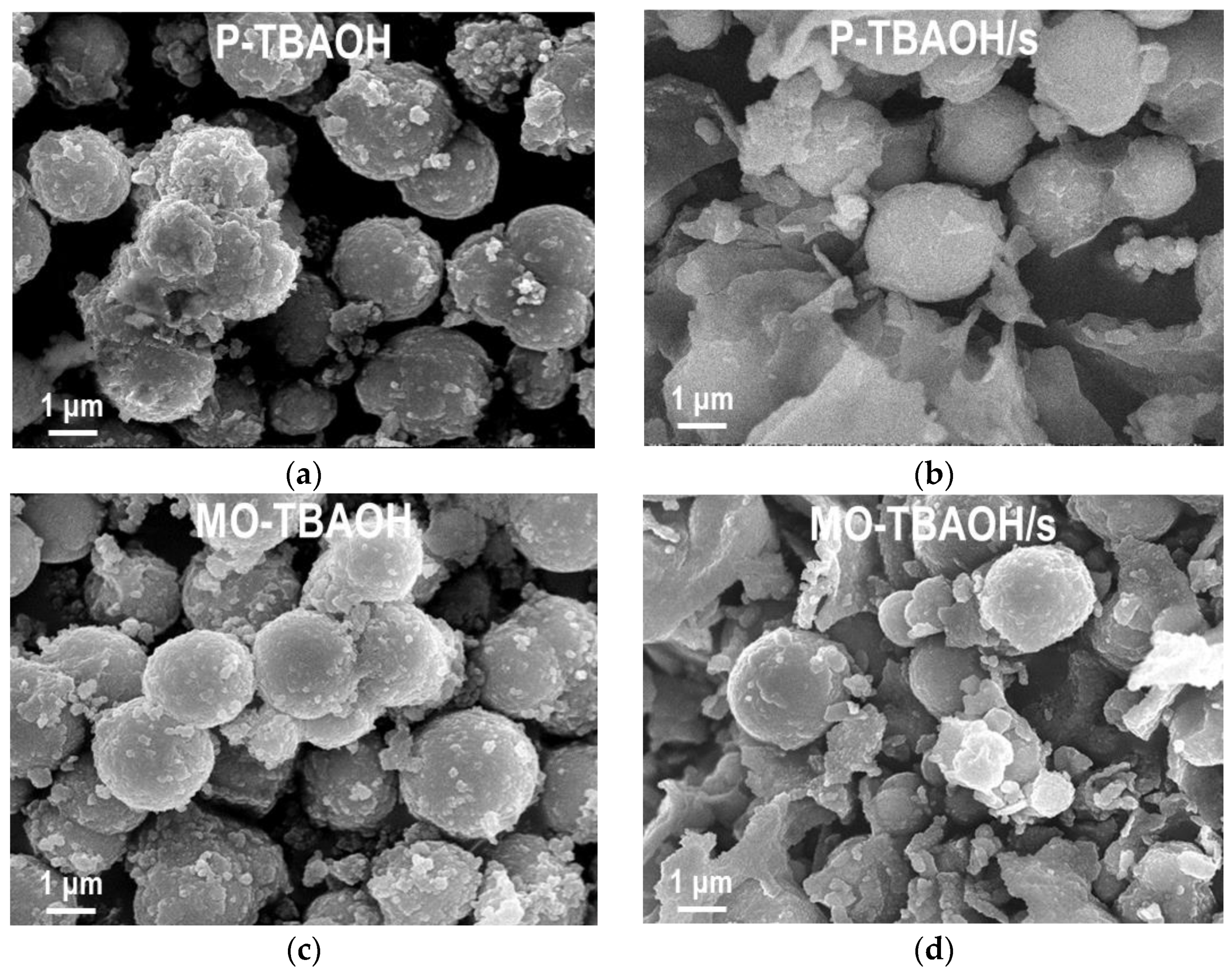


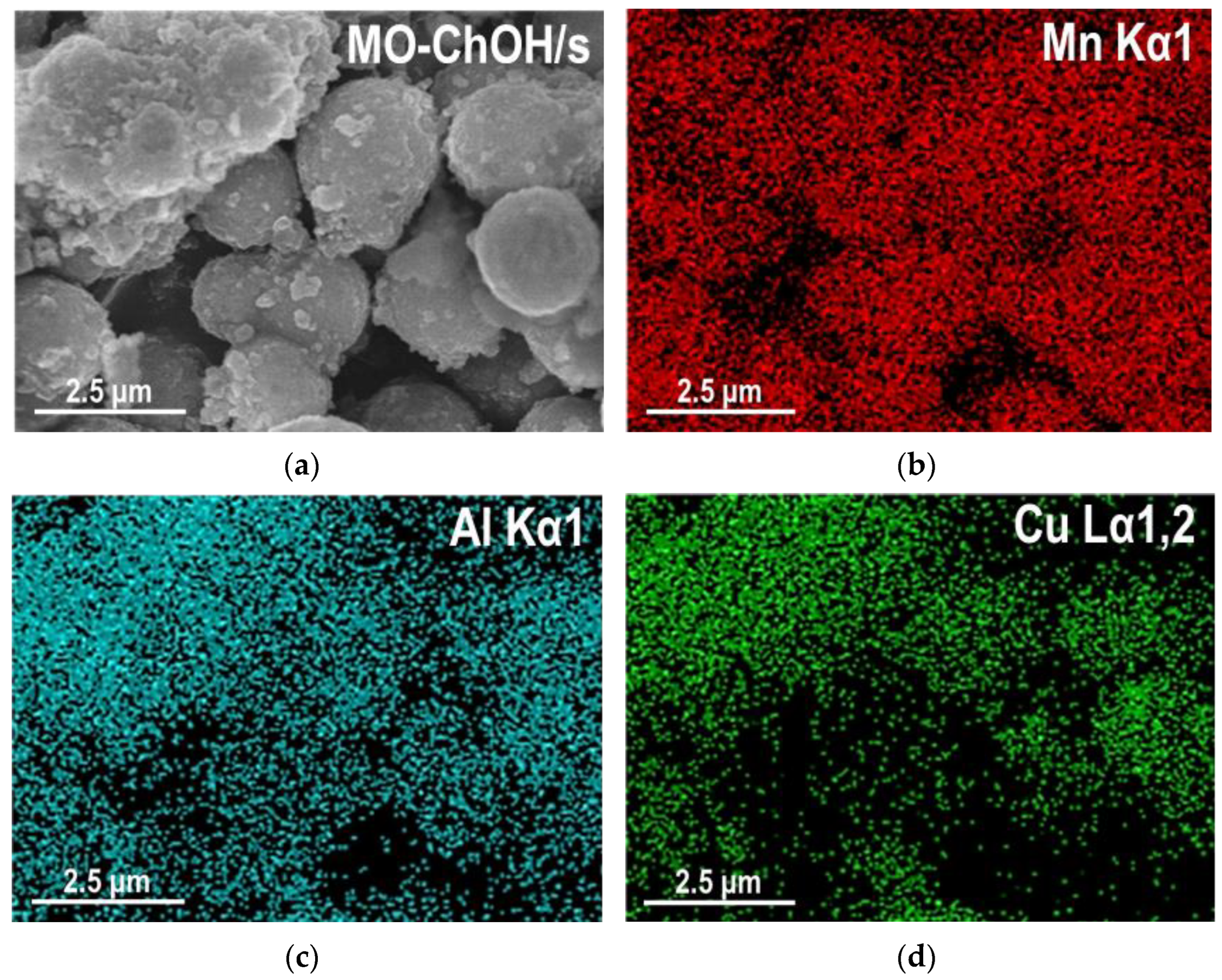


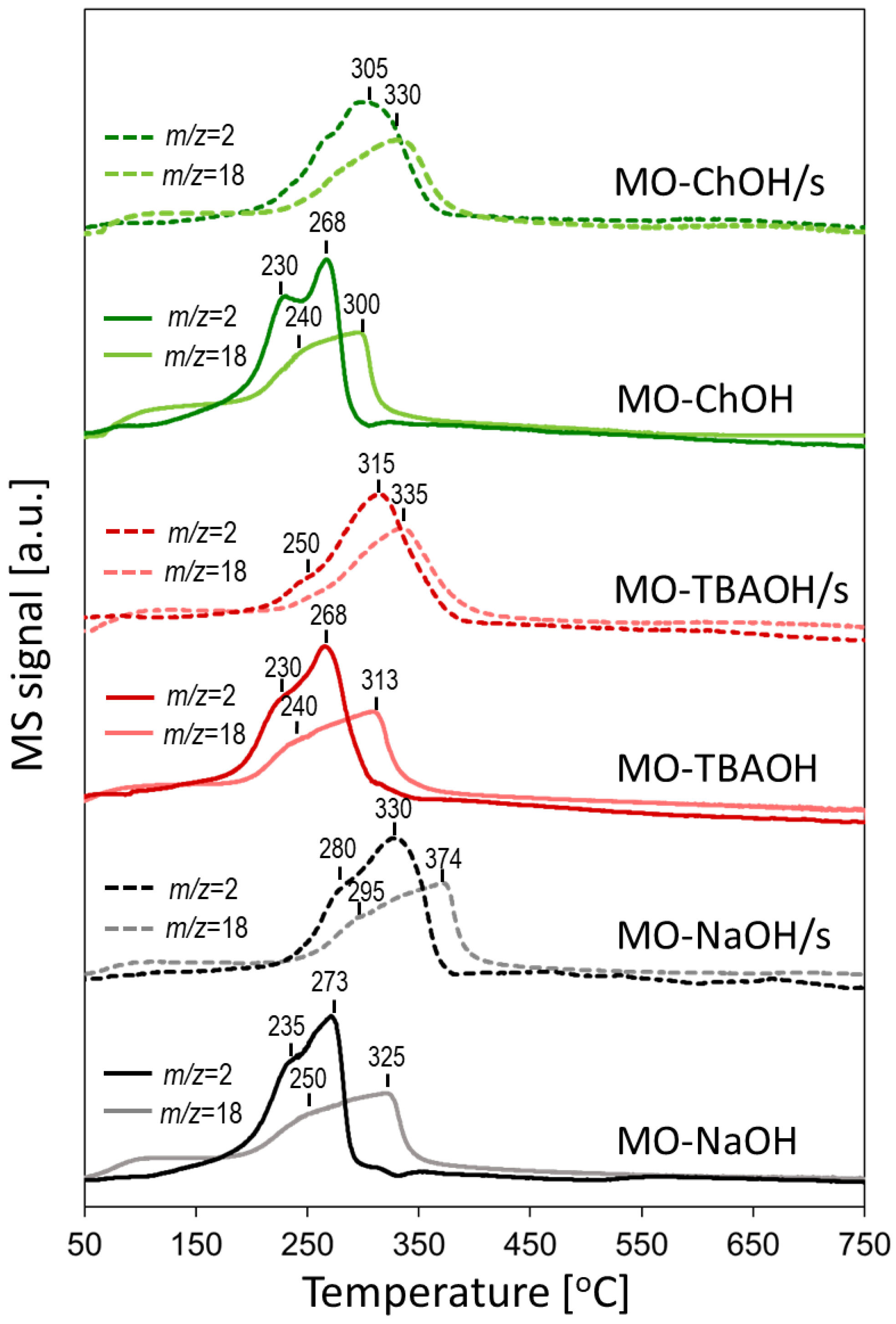
| Sample | MnCO3 D104 [nm] | Spinel D400 [nm] | Cu [at. %] | Mn [at. %] | Al [at. %] | Na [at. %] | C [wt. %] | N [wt. %] |
|---|---|---|---|---|---|---|---|---|
| P-NaOH | 8.4 | - | - | - | - | - | 5.74 | 0.09 |
| P-NaOH/s | 6.7 | - | - | - | - | - | 17.04 | 0.17 |
| MO-NaOH | - | 7.2 | 24.9 | 48.6 | 24.0 | 2.5 | - | - |
| MO-NaOH/s | - | 8.3 | 24.2 | 47.8 | 23.4 | 4.6 | - | - |
| P-TBAOH | 8.5 | - | - | - | - | - | 7.76 | 0.37 |
| P-TBAOH/s | 6.4 | - | - | - | - | - | 18.56 | 0.52 |
| MO-TBAOH | - | 7.2 | 25.6 | 49.4 | 25.0 | 0 | - | - |
| MO-TBAOH/s | - | 8.8 | 24.9 | 48.9 | 26.2 | 0 | - | - |
| P-ChOH | 8.4 | - | - | - | - | - | 6.90 | 0.26 |
| P-ChOH/s | 6.0 | - | - | - | - | - | 17.62 | 0.37 |
| MO-ChOH | - | 5.8 | 24.0 | 50.9 | 25.1 | 0 | - | - |
| MO-ChOH/s | - | 7.8 | 23.1 | 51.9 | 25.0 | 0 | - | - |
| Sample | T50 [°C] | T90 [°C] | SBET [m2g−1] |
|---|---|---|---|
| MO-NaOH | 223 | 238 | 129 |
| MO-NaOH/s | 212 | 224 | 77 |
| MO-TBAOH | 208 | 221 | 161 |
| MO-TBAOH/s | 195 | 205 | 84 |
| MO-ChOH | 205 | 217 | 188 |
| MO-ChOH/s | 189 | 199 | 97 |
| Reference | T50 [°C] | T90 [°C] | Toluene Concentration[ppm] | WHSV [mLg−1h−1] | SBET [m2g−1] |
|---|---|---|---|---|---|
| Palacio et al. [20] | 258 | n.d. | 800 | 75,000 | 108 |
| Aguilera et al. [21] | 217 | 258 | 1000 | 60,000 | 249 |
| Behar et al. [42] | 239 | n.d. | 1000 | 46,000 | 42 |
| Kim et al. [71] | 279 | 310 | 1000 | 21,000 | 128 |
| Napruszewska et al. [28] | 261 | 282 | 500 | 20,000 | 273 |
| Wang et al. [30] | 187 | 207 | 500 | 40,000 | 247 |
| Xiao et al. [31] | 228 | 237 | 1000 | 30,000 | 193 |
| Zhang et al. [34] | n.d. | 205 | 1000 | 20,000 | 144 |
| Ye at al. [37] | 202 | 222 | 800 | 30,000 | 105 |
| Li et al. [33] | 207 | 210 | 1000 | 40,000 | 45 |
| Liu et al. [36] | 224 | 234 | 500 | 60,000 | 49 |
| This work | 189 | 199 | 500 | 20,000 | 97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napruszewska, B.D.; Walczyk, A.; Duraczyńska, D.; Kryściak-Czerwenka, J.; Michalik, A.; Karcz, R.; Śliwa, M.; Serwicka, E.M. The Synthesis of Cu–Mn–Al Mixed-Oxide Combustion Catalysts by Co-Precipitation in the Presence of Starch: A Comparison of NaOH with Organic Precipitants. Catalysts 2022, 12, 1159. https://doi.org/10.3390/catal12101159
Napruszewska BD, Walczyk A, Duraczyńska D, Kryściak-Czerwenka J, Michalik A, Karcz R, Śliwa M, Serwicka EM. The Synthesis of Cu–Mn–Al Mixed-Oxide Combustion Catalysts by Co-Precipitation in the Presence of Starch: A Comparison of NaOH with Organic Precipitants. Catalysts. 2022; 12(10):1159. https://doi.org/10.3390/catal12101159
Chicago/Turabian StyleNapruszewska, Bogna D., Anna Walczyk, Dorota Duraczyńska, Joanna Kryściak-Czerwenka, Alicja Michalik, Robert Karcz, Michał Śliwa, and Ewa M. Serwicka. 2022. "The Synthesis of Cu–Mn–Al Mixed-Oxide Combustion Catalysts by Co-Precipitation in the Presence of Starch: A Comparison of NaOH with Organic Precipitants" Catalysts 12, no. 10: 1159. https://doi.org/10.3390/catal12101159
APA StyleNapruszewska, B. D., Walczyk, A., Duraczyńska, D., Kryściak-Czerwenka, J., Michalik, A., Karcz, R., Śliwa, M., & Serwicka, E. M. (2022). The Synthesis of Cu–Mn–Al Mixed-Oxide Combustion Catalysts by Co-Precipitation in the Presence of Starch: A Comparison of NaOH with Organic Precipitants. Catalysts, 12(10), 1159. https://doi.org/10.3390/catal12101159





