The Effects of AlPO-n Additives as Catalytic Support on Pd-Catalytic Hydrogenation of 2-Amylanthraquinone Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Supports and Catalysts
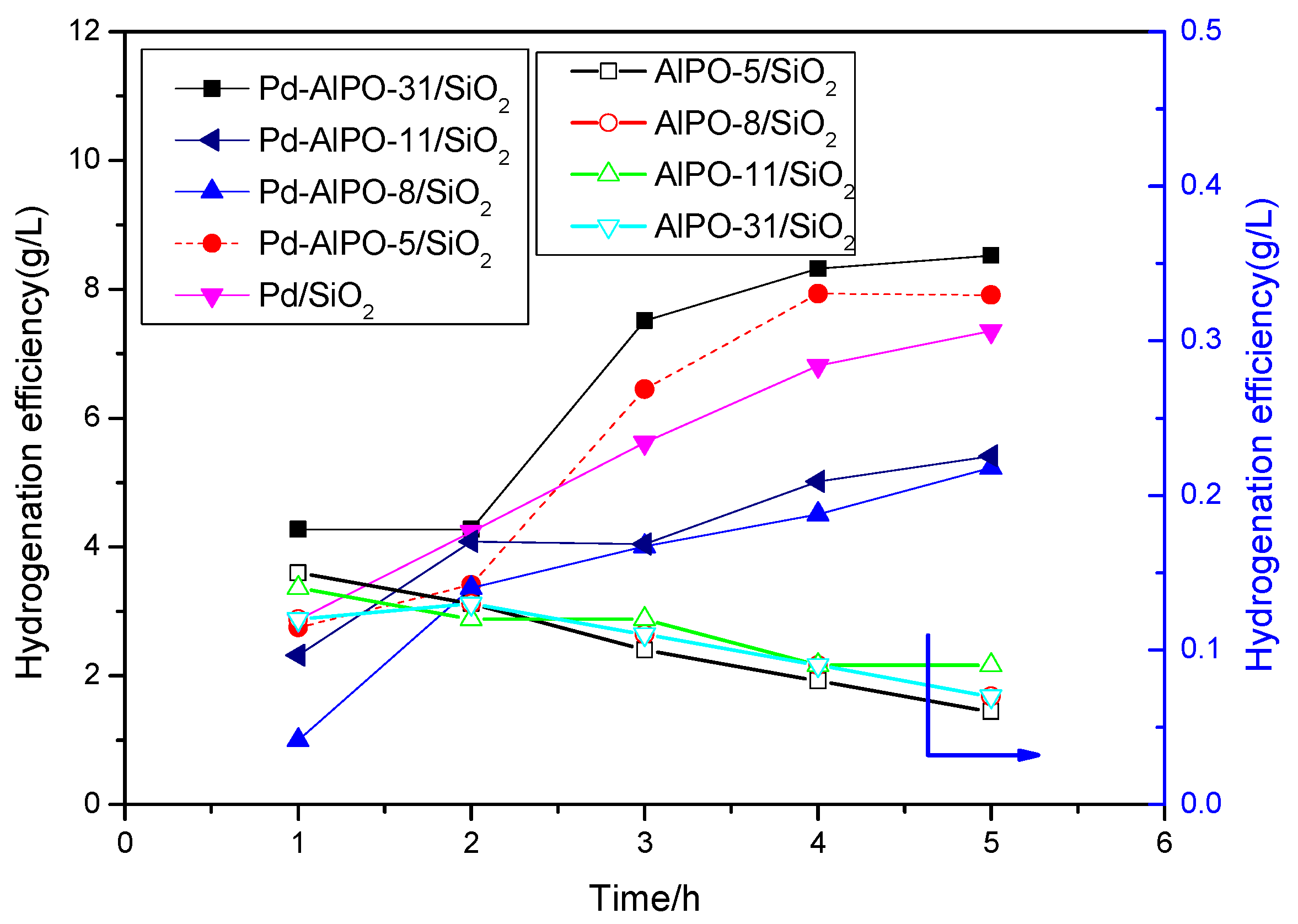
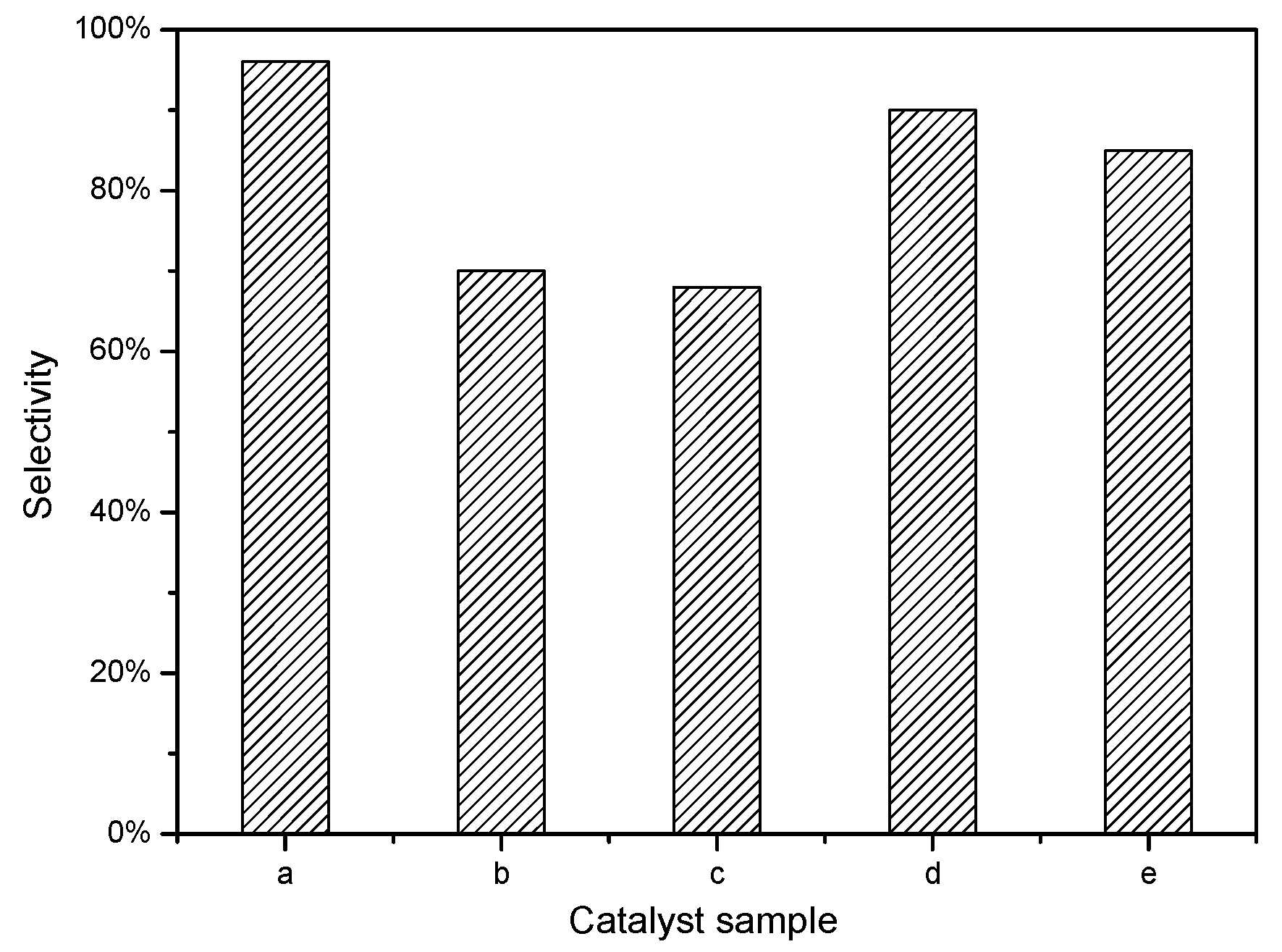
2.2. Catalytic Performance
3. Experimental Section
3.1. Catalyst Preparation
- Preparation of AlPO-5. The molar ratio of the starting gels was 1.2 TEA: Al2O3: P2O5: 50 H2O. The gels were prepared by adding pseudoboehmite (Sasol, Sandton, South Africa) to a solution of phosphoric acid (Sinopharm Chemical Reagent Co., Shanghai, China) and stirred for 3 h. Tetraethylamine (TEA, Sinopharm Chemical Reagent Co., Shanghai, China) was added to the mixture and stirred for 24 h. The resulting gels were introduced into Teflon-lined stainless-steel autoclaves and heated statically at 200 °C (with a heating rate of 2 °C·min−1) for 36 h. After hydrothermal treatment, the zeolite was separated from the mother liquor, rinsed repeatedly with distilled water and dried. The dried zeolite was finally calcined at 600 °C (with a heating rate of 2 °C·min−1) for 6 h.
- Preparation of AlPO-8. The molar ratio of the starting gels was DPA: Al2O3: P2O5:34 H2O. The gels were prepared by adding pseudoboehmite to a solution of phosphoric acid and stirred for 3 h. Then, Dipropylamine (DPA, Sinopharm Chemical Reagent Co., Shanghai, China) was added to the mixture and stirred for 24 h. The resulting gels were introduced into Teflon-lined stainless-steel autoclaves and heated statically at 144 °C (with a heating rate of 2 °C·min−1) for 12 h. After hydrothermal treatment, the zeolite was separated from the mother liquor, rinsed repeatedly with distilled water and dried. The dried zeolite was finally calcined at 600 °C (with a heating rate of 2 °C·min−1) for 6 h.
- Preparation of AlPO-11. The molar ratio of the starting gels was 4 DPA: Al2O3: P2O5:40 H2O. The gels were prepared by adding pseudoboehmite to a solution of phosphoric acid and stirred for 3 h. Then, Dipropylamine (DPA, Sinopharm Chemical Reagent Co., Shanghai, China) was added to the mixture and stirred for 24 h. The resulting gels were introduced into Teflon-lined stainless-steel autoclaves and heated statically at 200 °C (with a heating rate of 2 °C·min−1) for 24 h. After hydrothermal treatment, the zeolite was separated from the mother liquor, rinsed repeatedly with distilled water and dried. The dried zeolite was finally calcined at 600 °C (with a heating rate of 2 °C·min−1) for 6 h.
- Preparation of AlPO-31. The molar ratio of the starting gels was 4 DPA: Al2O3: P2O5:40 H2O. The gels were prepared by adding pseudoboehmite to a solution of phosphoric acid and stirred for 3 h. Then, Dipropylamine (DPA, Sinopharm Chemical Reagent Co., Shanghai, China) was added to the mixture and stirred for 24 h. The resulting gels were introduced into Teflon-lined stainless-steel autoclaves and heated statically at 200 °C (with a heating rate of 2 °C·min−1) for 48 h. After hydrothermal treatment, the zeolite was separated from the mother liquor, rinsed repeatedly with distilled water and dried. The dried zeolite was finally calcined at 600 °C (with a heating rate of 2 °C·min−1) for 6 h.
- Preparation of complex supports. First, 12 g of silica sol (ludox-30) and 1 g of AlPO-n were mixed and injected into an oil column at 90 °C, in which the spherical complex supports were generated and then dried in a vacuum oven at 60 °C for 10h. The supports were calcinated at 700 °C (with a heating rate of 2 °C·min−1) for 12 h, while they were used at 120–200 mesh to prepare catalysts.
- Preparation of catalysts. A total of 2 g of the prepared support was impregnated in 4 mL of a Pd(NH3)4(NO3)2 aqueous solution (1.00 mg·ml−1, calculated by the quality of Pd) for 2 h, followed by drying and calcination at 400 °C (with a heating rate of 2 °C·min−1) for 2 h. All catalysts were reduced by H2 at 120 °C for 2 h before the reaction.
3.2. Characterization of the Catalysts
3.3. Evaluation of Catalytic Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santacesaria, E.; Di Serio, M.; Russo, A.; Leone, U.; Velotti, R. Kinetic and catalytic aspects in the hydrogen peroxide production via anthraquinone. Chem. Eng. Sci. 1999, 54, 2799–2806. [Google Scholar] [CrossRef]
- Samanta, C. Direct synthesis of hydrogen peroxide from hydrogen and oxygen: An overview of recent developments in the process. Appl. Catal. A Gen. 2008, 350, 133–149. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro, J.L.G. Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process. Angew. Chem. Int. Ed. 2006, 45, 6962–6984. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Petersson, L.-G. Spillover of hydrogen, oxygen and carbon monoxide in oxidation reactions on SiO2 supported Pd. Surf. Sci. 1994, 311, 139–152. [Google Scholar] [CrossRef]
- Kamachi, T.; Ogata, T.; Mori, E.; Iura, K.; Okuda, N.; Nagata, M.; Yoshizawa, K. Computational Exploration of the Mechanism of the Hydrogenation Step of the Anthraquinone Process for Hydrogen Peroxide Production. J. Phys. Chem. C 2015, 119, 8748–8754. [Google Scholar] [CrossRef]
- Drelinkiewicz, A.; Waksmundzka-Góra, A.; Makowski, W.; Stejskal, J. Pd/polyaniline(SiO2) a novel catalyst for the hydrogenation of 2-ethylanthraquinone. Catal. Commun. 2005, 6, 347–356. [Google Scholar] [CrossRef]
- Meyer, K.H. Zur Kenntnis des Anthracens. I. Über Anthranol und Anthrahydrochinon. Eur. J. Org. Chem. 1911, 379, 37–78. [Google Scholar] [CrossRef]
- Li, X.; Su, H.; Ren, G.; Wang, S. A highly stable Pd/SiO2/cordierite monolith catalyst for 2-ethyl-anthraquinone hydrogenation. RSC Adv. 2015, 5, 100968–100977. [Google Scholar] [CrossRef]
- Tang, P.; Chai, Y.; Feng, J.; Feng, Y.; Li, Y.; Li, D. Highly dispersed Pd catalyst for anthraquinone hydrogenation supported on alumina derived from a pseudoboehmite precursor. Appl. Catal. A Gen. 2014, 469, 312–319. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Y.; Xu, J.; Lu, Y.; Luo, G. Preparation and the hydrogenation performance of a novel catalyst-Pd nanoparticles loaded on glass beads with an egg–shell structure. Chem. Eng. J. 2011, 173, 226–232. [Google Scholar] [CrossRef]
- Kosydar, R.; Drelinkiewicz, A.; Lalik, E.; Gurgul, J. The role of alkali modifiers (Li, Na, K, Cs) in activity of 2%Pd/Al2O3 catalysts for 2-ethyl-9, 10-anthraquione hydrogenation. Appl. Catal. A Gen. 2011, 402, 121–131. [Google Scholar] [CrossRef]
- Drelinkiewicz, A.; Pukkinen, A.; Kangas, R.; Laitinen, R. Hydrogenation of 2-Ethylanthraquinone over Pd/SiO2 and Pd/Al2O3 in the Fixed-Bed Reactor. The Effect of the Type of Support. Catal. Lett. 2004, 94, 157–170. [Google Scholar] [CrossRef]
- Wilson, S.T.; Lok, B.M.; Messina, C.A.; Cannan, T.R.; Flanigen, E.M. Aluminophosphate molecular sieves: A new class of microporous crystalline inorganic solids. J. Am. Chem. Soc. 1982, 104, 1146–1147. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef]
- Li, X.; Su, H.; Li, D.; Chen, H.; Yang, X.; Wang, S. Highly dispersed Pd/AlPO-5 catalyst for catalytic hydrogenation of 2-ethylanthraquinone. Appl. Catal. A Gen. 2016, 528, 168–174. [Google Scholar] [CrossRef]
- Yu, J.-S.; Kurshev, V.; Kevan, L. Identification of the Site Locations of Two Pd(I) Species in PdH-SAPO-5 and PdH-SAPO-11 Molecular Sieves from Phosphorus-31 Nuclear Modulation. J. Phys. Chem. 1994, 98, 10225–10228. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.-H.; Ren, J.; Zhang, J.-L.; Li, W.; Guo, C.-L. Enhanced catalytic activity and stability over P-modified alumina supported Pd for anthraquinone hydrogenation. Appl. Catal. A Gen. 2020, 593, 117422. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Wang, Q.; Ni, L.; Song, Z.; Li, R. Development and application of hydrogenation catalyst EK-Ⅲ for producing hydrogen peroxide through anthraquinone route. Pet. Process. Petrochem. 2021, 52, 39. Available online: http://www.sylzyhg.com/EN/Y2021/V52/I6/39 (accessed on 1 June 2021).
- Liang, W.; Fu, J.; Chen, H.; Zhang, X.; Deng, G. Pd-Ce/AC catalyst with high productivity for direct synthesis of H2O2 at ambient temperature. Mater. Lett. 2021, 283, 128857. [Google Scholar] [CrossRef]
- Belykh, L.B.; Skripov, N.I.; Sterenchuk, T.P.; Akimov, V.V.; Tauson, V.L.; Milenkaya, E.A.; Schmidt, F.K. Structurally Disordered Pd−P Nanoparticles as Effective Catalysts for the Production of Hydrogen Peroxide by the Anthraquinone Method. Eur. J. Inorg. Chem. 2021, 2021, 4586–4593. [Google Scholar] [CrossRef]
- Tapp, N.; Milestone, N.; Bibby, D. Synthesis of AIPO4-11. Zeolites 1988, 8, 183–188. [Google Scholar] [CrossRef]
- Guo, Z.; Guo, C.; Jin, Q.; Li, B.; Ding, D. Synthesis and Structure of Large AlPO4-5 Crystals. J. Porous Mater. 2005, 12, 29–33. [Google Scholar] [CrossRef]
- Chaudhari, K.; Das, T.; Soni, H.; Rajmohanan, P.; Jacob, N.; Chandwadkar, A. n-DBA-VPI-5: Synthesis and characterization. Stud. Surf. Sci. Catal. 1998, 113, 651–658. [Google Scholar] [CrossRef]
- Bennett, J.; Kirchner, R.M. The structure of calcined AIP4-31: A new framework topology containing one-dimensional 12-ring pores. Zeolites 1992, 12, 338–342. [Google Scholar] [CrossRef]
- Grenev, I.; Gavrilov, V.Y. Calculating henry adsorption constants of molecular hydrogen at 77 K on alumophosphate zeolites with different microchannel sizes. Russ. J. Phys. Chem. A 2013, 88, 127–133. [Google Scholar] [CrossRef]
- Grenev, I.; Gavrilov, V. Calculation of microchannel parameters in aluminophosphate zeolites. Microporous Mesoporous Mater. 2015, 208, 36–43. [Google Scholar] [CrossRef]
- Pan, C.-J.; Tsai, M.-C.; Su, W.-N.; Rick, J.; Akalework, N.G.; Agegnehu, A.K.; Cheng, S.-Y.; Hwang, B.-J. Tuning/exploiting Strong Metal-Support Interaction (SMSI) in Heterogeneous Catalysis. J. Taiwan Inst. Chem. Eng. 2017, 74, 154–186. [Google Scholar] [CrossRef]
- Neimark, A. Percolation Theory of Capillary Hysteresis Phenomena and Its Application for Characterization of Porous Solids. Stud. Surf. Sci. Catal. 1991, 62, 67–74. [Google Scholar] [CrossRef]
- Guo, Z.; Feng, J.; Feng, Y.; Evans, D.G.; Li, D. In situ synthesis of solid base catalysts for the regeneration of degradation products formed during the anthraquinone process for the manufacture of hydrogen peroxide. Appl. Catal. A Gen. 2011, 401, 163–169. [Google Scholar] [CrossRef]
- Bai, H.; Fang, X.; Peng, C. Synthesis of Tailored Egg-Shell Pd@Al2O3 Catalyst for Catalytic Hydrogenation of 2-Alkylanthraquinone. ACS Sustain. Chem. Eng. 2019, 7, 7700–7707. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Zhang, X.; Sun, M.; Hu, J.; Zhai, Y.; Lv, H.; Lv, G. Highly dispersed Pd nanoparticles supported on γ-Al2O3 modified by minimal 3-aminopropyltriethoxysilane as effective catalysts for 2-ethyl-anthraquinone hydrogenation. Appl. Catal. A Gen. 2021, 619, 118124. [Google Scholar] [CrossRef]
- Yu, J.S.; Comets, J.M.; Kevan, L. Study of palladium(I) adsorbate interactions in Pd monohydride-SAPO 5 molecular sieves by electron spin resonance and electron spin echo modulation. J. Phys. Chem. 1993, 97, 10433–10439. [Google Scholar] [CrossRef]

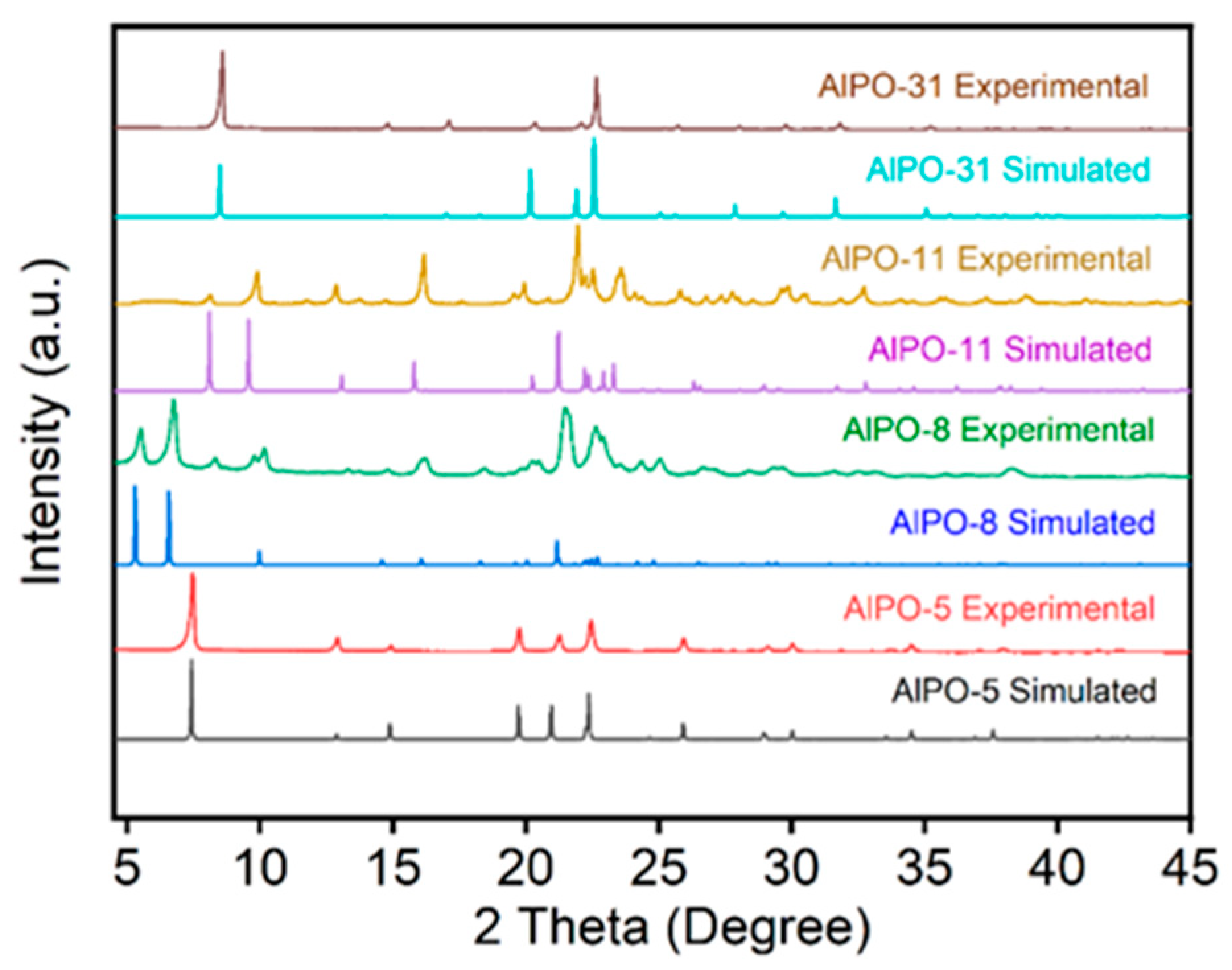
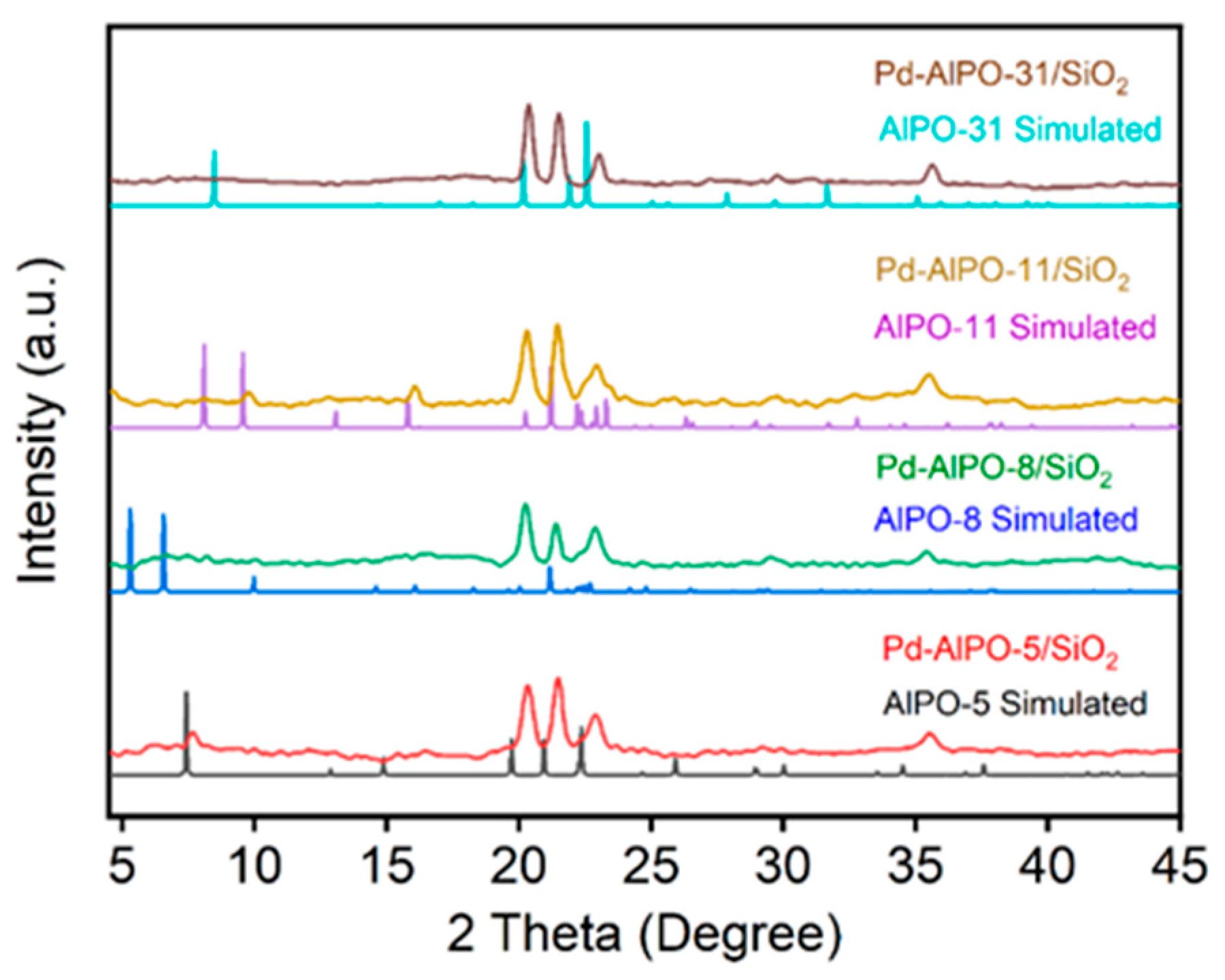
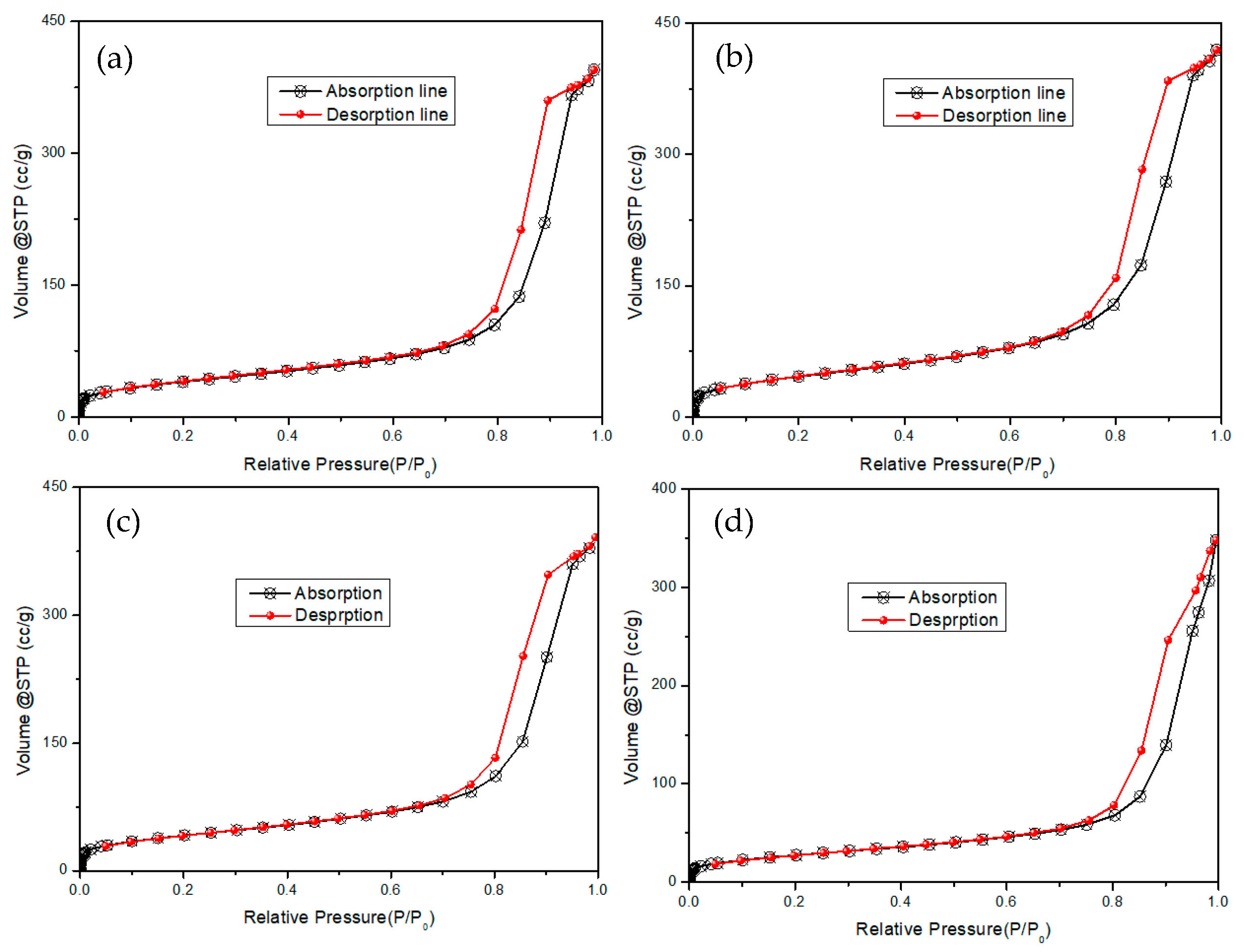

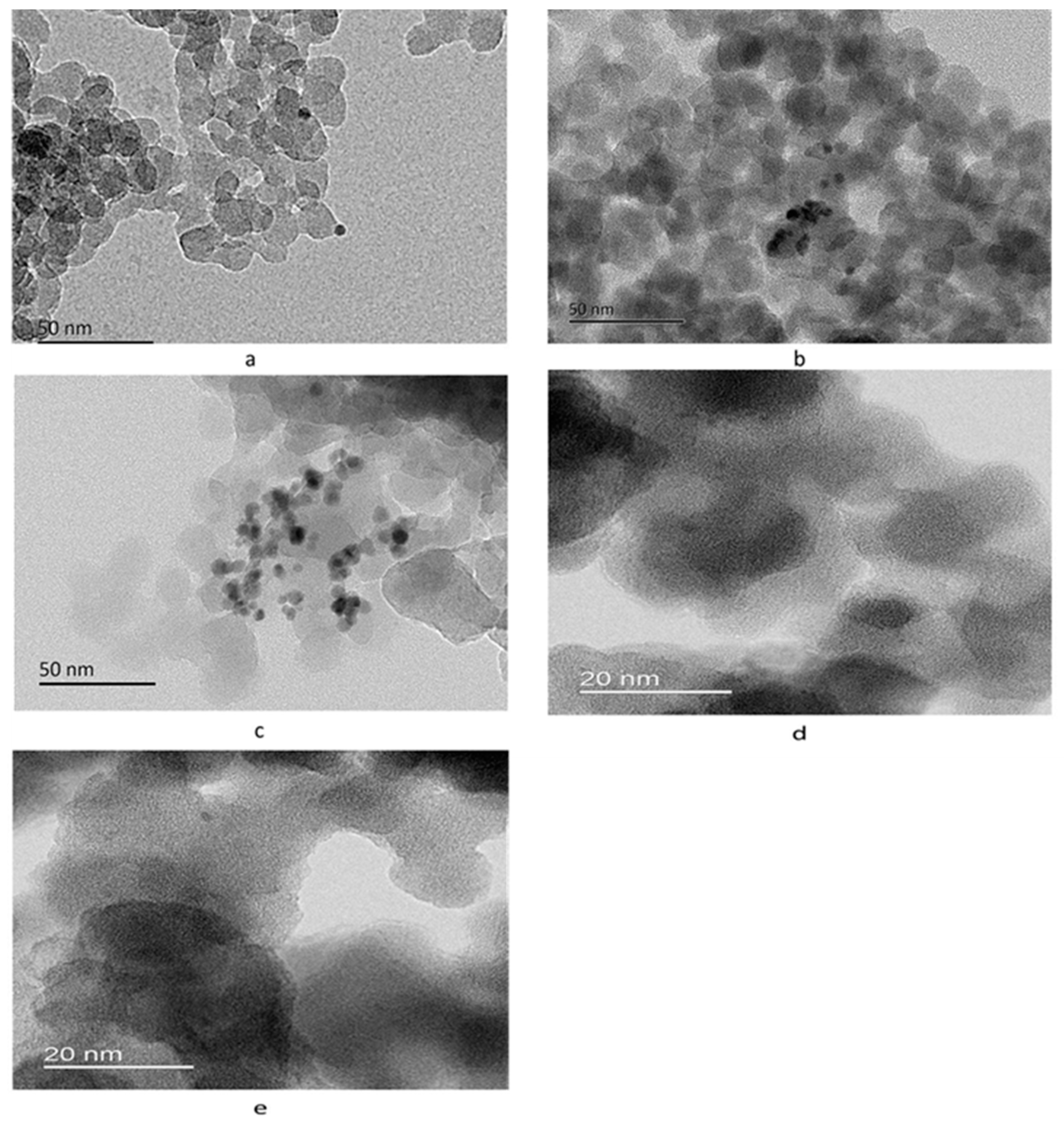
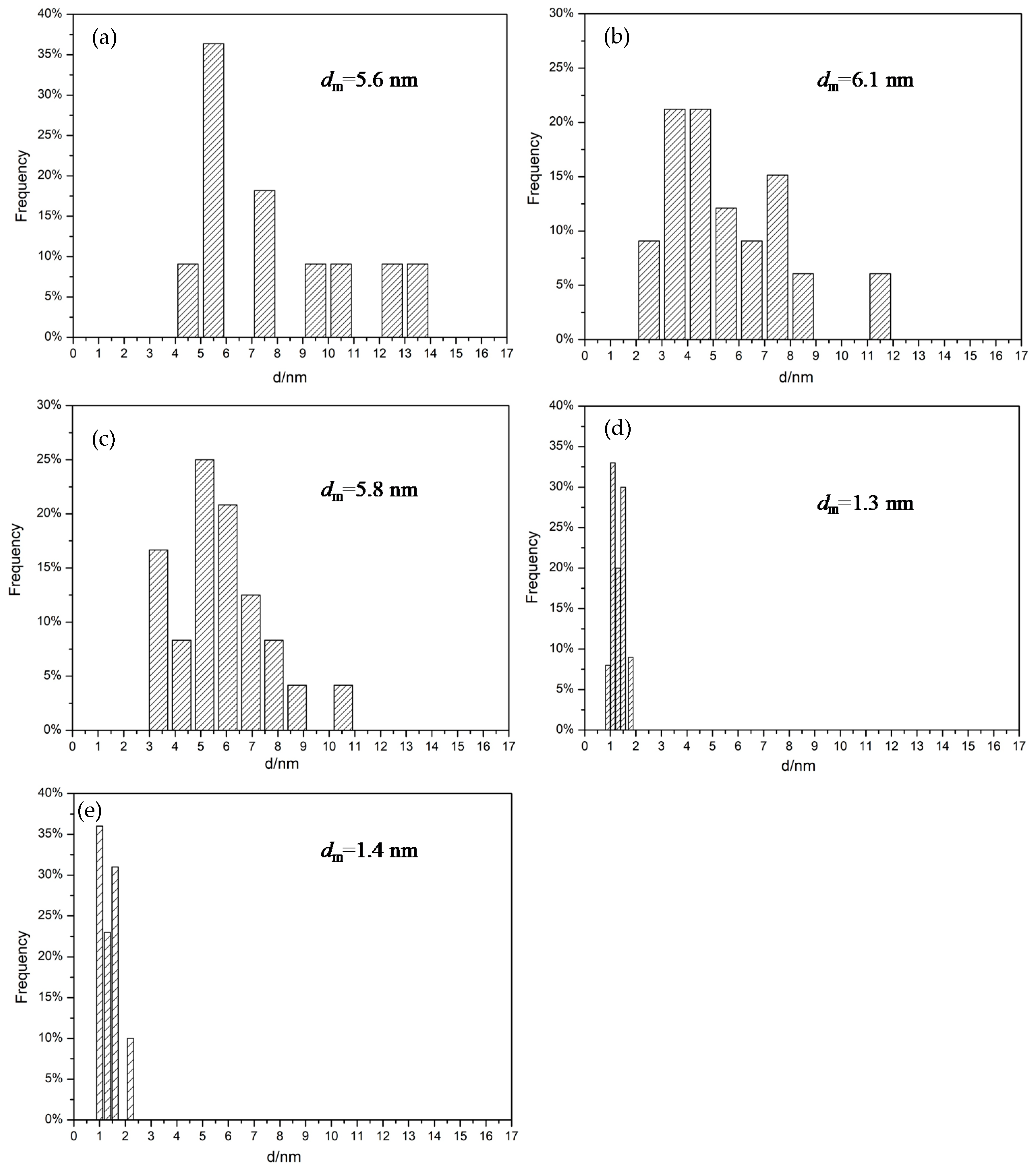
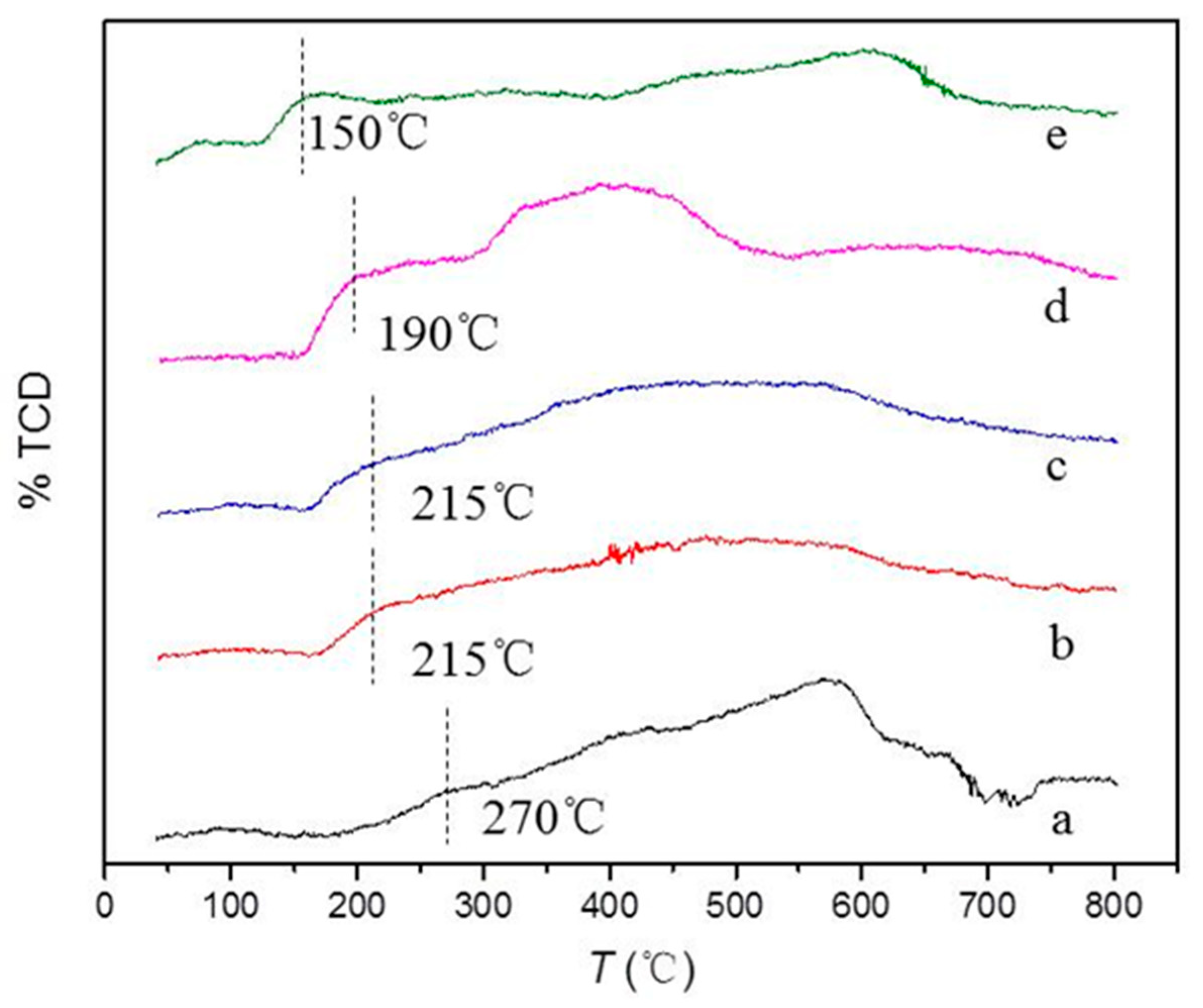
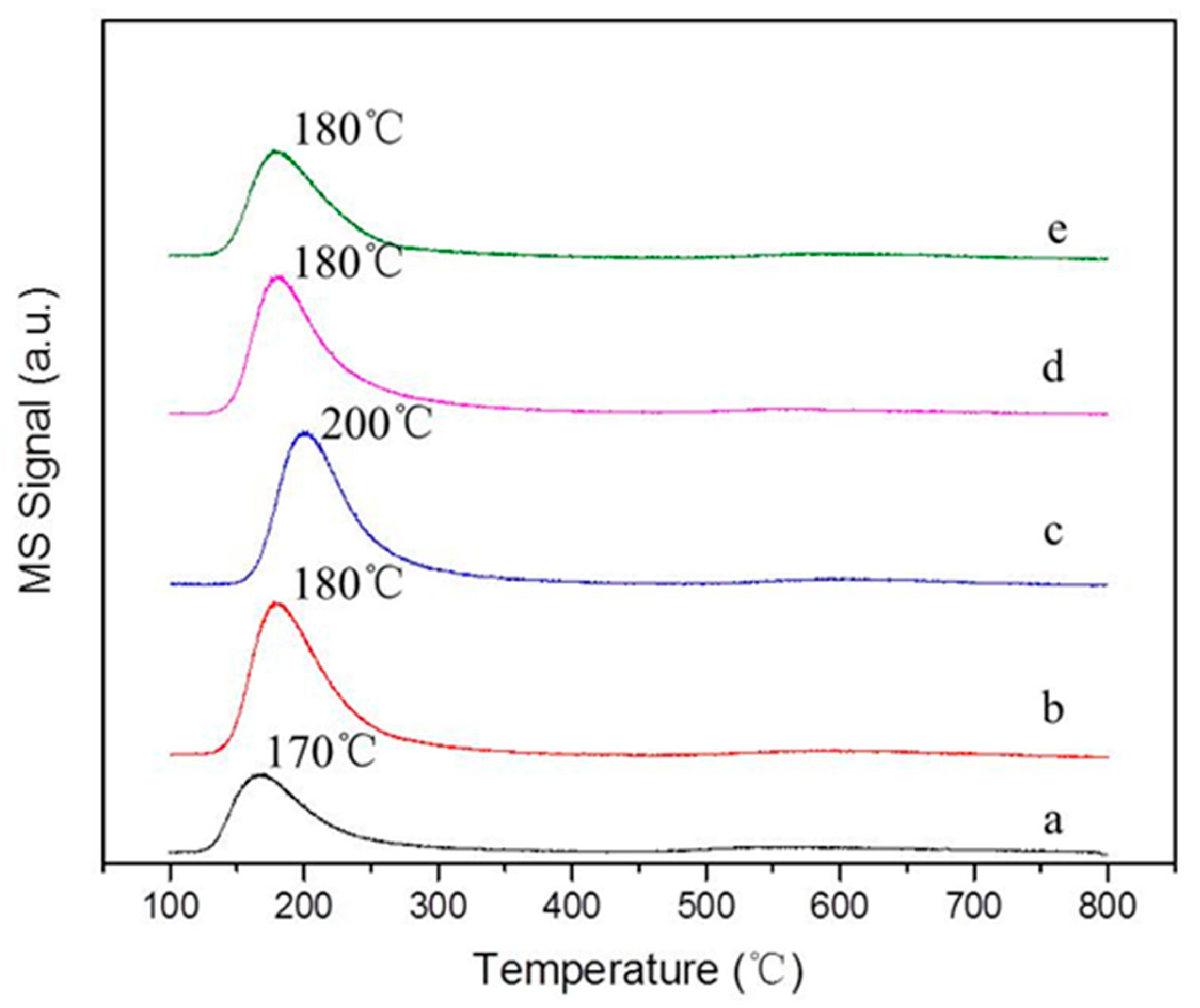
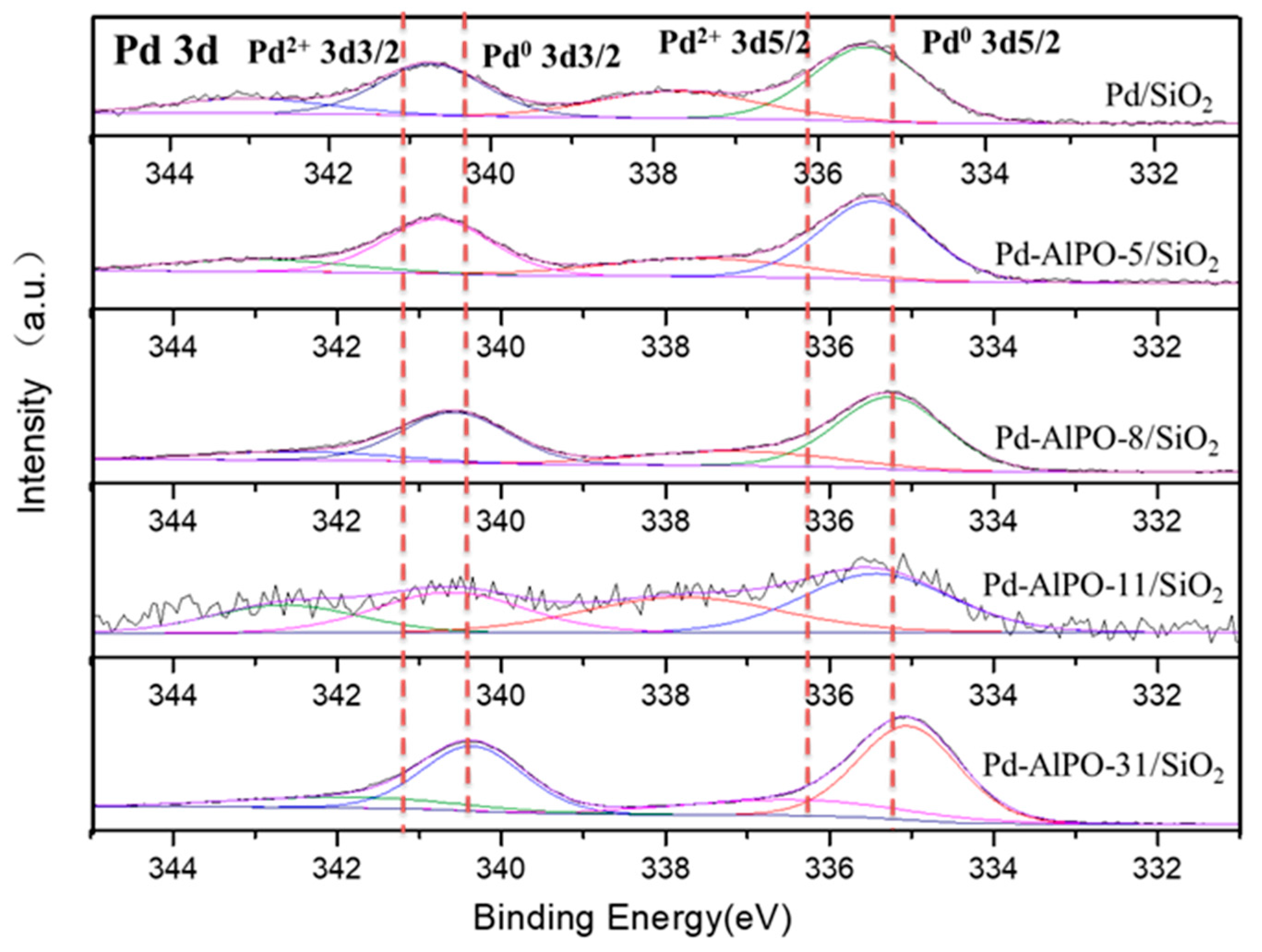
| Sample | a, Å | b, Å | c, Å | Ve.c, Å |
|---|---|---|---|---|
| AlPO-5 | 13.706 | 13.705 | 8.470 | 1377.535 |
| AlPO-8 | 33.162 | 14.814 | 8.376 | 4114.809 |
| AlPO-11 | 13.369 | 18.710 | 8.451 | 2113.882 |
| AlPO-31 | 20.830 | 20.830 | 4.999 | 1878.340 |
| No. | AlPO-n Zeolite | S* (m2·g−1) | VS (mL·g−1) | Vμ (mL·g−1) | Dmicro (nm) |
|---|---|---|---|---|---|
| 1 | AlPO-5 | 48 | 0.276 | 0.111 | 0.73 |
| 2 | AlPO-8 | 145 | 0.25 | 0.078 | 0.83 |
| 3 | AlPO-11 | 35 | 0.11 | 0.051 | 0.49 |
| 4 | AlPO-31 | 57 | 0.25 | 0.038 | 0.53 |
| Sample | Pd Loading (wt.%) | Pd Dispersion (%) | Specific Surface Area (m2/g) |
|---|---|---|---|
| Pd/SiO2 | 0.1964 | 5.21 | 185.0 |
| Pd-AlPO-5/SiO2 | 0.1971 | 7.33 | 187.7 |
| Pd-AlPO-8/SiO2 | 0.1952 | 7.52 | 187.5 |
| Pd-AlPO-11/SiO2 | 0.1960 | 18.45 | 186.4 |
| Pd-AlPO-31/SiO2 | 0.1970 | 20.20 | 186.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Su, H.; Yan, H.; Yang, X.; Zhou, J.; Wang, S. The Effects of AlPO-n Additives as Catalytic Support on Pd-Catalytic Hydrogenation of 2-Amylanthraquinone Process. Catalysts 2022, 12, 1156. https://doi.org/10.3390/catal12101156
Li D, Su H, Yan H, Yang X, Zhou J, Wang S. The Effects of AlPO-n Additives as Catalytic Support on Pd-Catalytic Hydrogenation of 2-Amylanthraquinone Process. Catalysts. 2022; 12(10):1156. https://doi.org/10.3390/catal12101156
Chicago/Turabian StyleLi, Dawei, Hongjiu Su, Hua Yan, Xiaoye Yang, Junhong Zhou, and Shudong Wang. 2022. "The Effects of AlPO-n Additives as Catalytic Support on Pd-Catalytic Hydrogenation of 2-Amylanthraquinone Process" Catalysts 12, no. 10: 1156. https://doi.org/10.3390/catal12101156
APA StyleLi, D., Su, H., Yan, H., Yang, X., Zhou, J., & Wang, S. (2022). The Effects of AlPO-n Additives as Catalytic Support on Pd-Catalytic Hydrogenation of 2-Amylanthraquinone Process. Catalysts, 12(10), 1156. https://doi.org/10.3390/catal12101156







