Synthesis, Property Characterization and Photocatalytic Activity of the Ag3PO4/Gd2BiTaO7 Heterojunction Catalyst under Visible Light Irradiation
Abstract
:1. Introduction
2. Result and Discussion
2.1. XRD Analysis
2.2. UV-Vis Diffuse Reflectance Spectra
2.3. Characterization of Ag3PO4, Ag3PO4/Gd2BiTaO7 Heterojunction
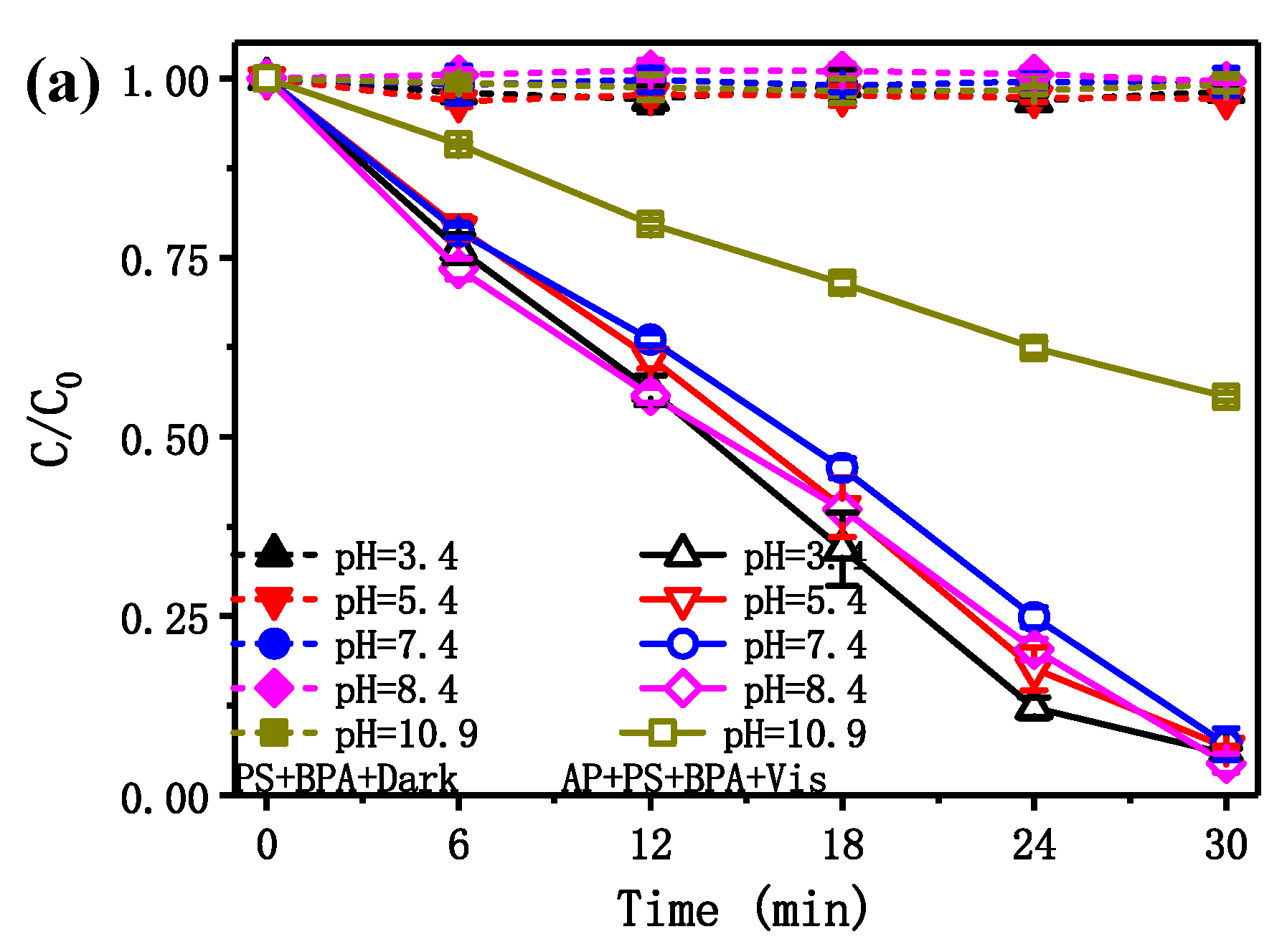
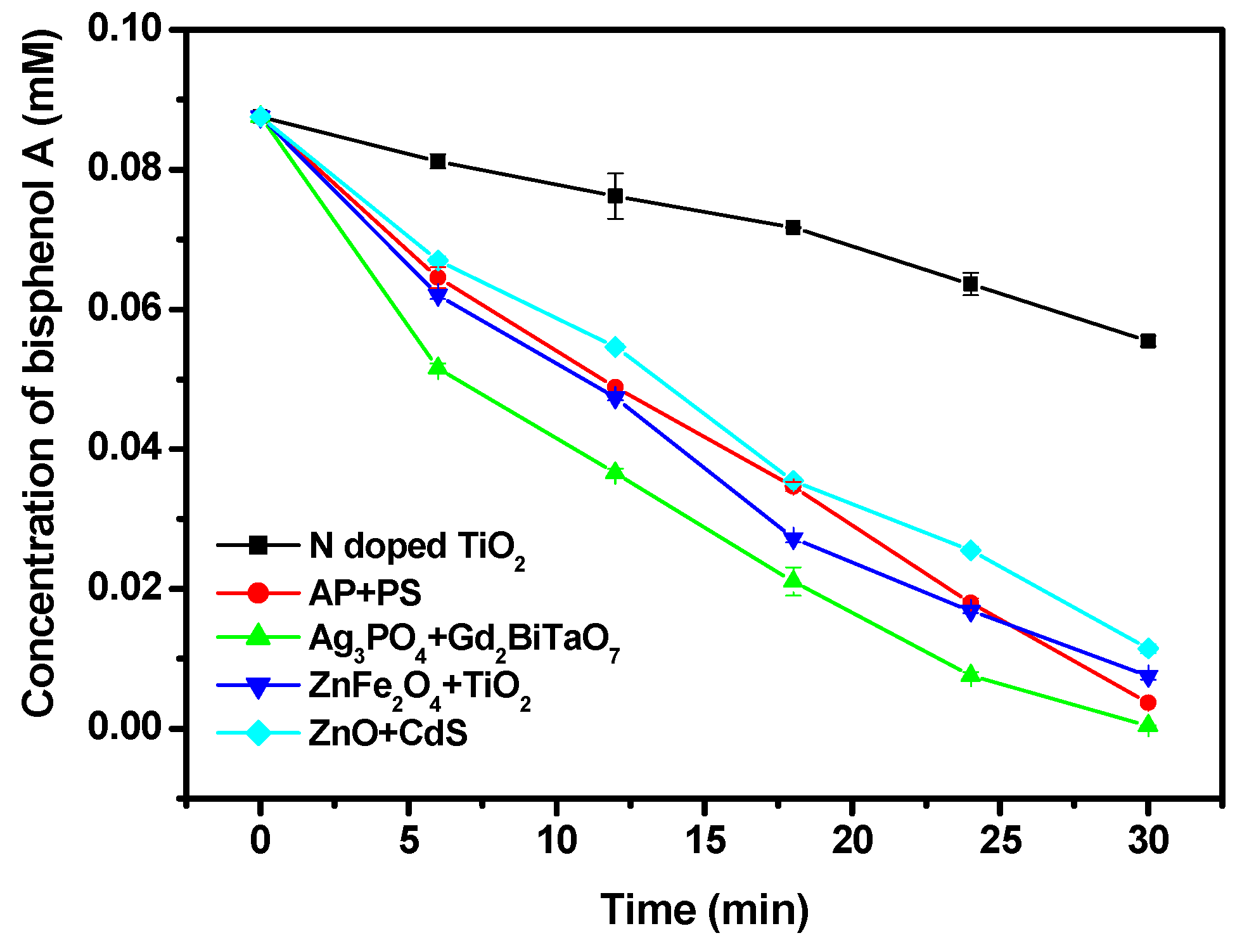
2.4. Photocatalytic Activity
2.5. Possible Degradation Mechanism Analysis
3. Experimental Section
3.1. Materials and Reagents
3.2. Synthesis of Tetrahedral Ag3PO4
3.3. Preparation Method of Gd2BiTaO7
3.4. Synthesis of N-Doped TiO2
3.5. Synthesis of Ag3PO4/Gd2BiTaO7 Heterojunction Photocatalyst, ZnFe2O4-TiO2 Nanocomposite and ZnO/CdS Hierarchical Heterojunction
3.6. Characterizations
3.7. Experimental Setup and Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- You, J.; Zhang, C.; Wu, Z.; Ao, Z.; Sun, W.; Xiong, Z.; Lai, B. N-doped graphite encapsulated metal nanoparticles catalyst for removal of Bisphenol A via activation of peroxymonosulfate: A singlet oxygen-dominated oxidation process. Chem. Eng. J. 2021, 415, 128890. [Google Scholar] [CrossRef]
- Jia, C.; Wang, Y.; Zhang, C.; Qin, Q.; Kong, S.; Yao, S. Photocatalytic degradation of bisphenol A in aqueous suspensions of titanium dioxide. Environ. Eng. Sci. 2012, 29, 630–637. [Google Scholar] [CrossRef]
- Robertson, P.K.J.; Robertson, J.M.C.; Bahnemann, D.W. Removal of microorganisms and their chemical metabolites from water using semiconductor photocatalysis. J. Hazard. Mater. 2012, 211, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahmasebi, N.; Esmaeilpour, H.; Movahedifard, F.; Hakimyfard, A.; Moayeri, H. Fabrication of a novel MoO3/Cs3PMo12O40 composite for photocatalytic decolorization of rhodamine B. Mat. Sci. Semicon. Proc. 2021, 131, 105876. [Google Scholar] [CrossRef]
- Mokhtari, F.; Tahmasebi, N. Hydrothermal synthesis of W-doped BiOCl nanoplates for photocatalytic degradation of rhodamine B under visible light. J. Phys. Chem. Solids 2021, 149, 109804. [Google Scholar] [CrossRef]
- Subhan, M.A.; Rifat, T.P.; Saha, P.C.; Alam, M.M.; Asiri, A.M.; Rahman, M.M.; Akter, S.; Raihan, T.; Azad, A.K.; Uddin, J. Enhanced visible light-mediated photocatalysis, antibacterial functions and fabrication of a 3-chlorophenol sensor based on ternary Ag2O·SrO·CaO. RSC Adv. 2020, 10, 11274–11291. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Ray, A.K. Removal of toxic metal ions from wastewater by semiconductor photocatalysis. Chem. Eng. Sci. 2001, 56, 1561–1570. [Google Scholar] [CrossRef]
- Ramos-Delgado, N.A.; Gracia-Pinilla, M.A.; Maya-Trevino, L.; Hinojosa-Reyes, L.; Guzman-Mar, J.L.; Hernandez-Ramirez, A. Solar photocatalytic activity of TiO2 modified with WO3 on the degradation of an organophosphorus pesticide. J. Hazard. Mater. 2013, 263(Pt. 1), 36–44. [Google Scholar] [CrossRef]
- Rizzo, L.; Della Sala, A.; Fiorentino, A.; Li Puma, G. Disinfection of urban wastewater by solar driven and UV lamp—TiO2 photocatalysis: Effect on a multi drug resistant Escherichia coli strain. Water Res. 2014, 53, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Da Loves, A.; Okada, T.; Mishima, S. Titania/CnTAB nanoskeleton as adsorbent and photocatalyst for removal of alkylphenols dissolved in water. J. Hazard. Mater. 2013, 248, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Volnistem, E.A.; Bini, R.D.; Silva, D.M.; Rosso, J.M.; Dias, G.S.; Cotica, L.F.; Santos, I.A. Intensifying the photocatalytic degradation of methylene blue by the formation of BiFeO3/Fe3O4 nanointerfaces. Ceram. Int. 2020, 46, 18768–18777. [Google Scholar] [CrossRef]
- Volnistem, E.A.; Bini, R.D.; Dias, G.S.; Cotica, L.F.; Santos, I.A. Photodegradation of methylene blue by mechanosynthesized BiFeO3 submicron particles. Ferroelectrics 2018, 534, 190–198. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef]
- Hu, S.Z.; Li, F.Y.; Fan, Z.P.; Gui, J.Z. The effect of H2-CCl4 mixture plasma treatment on TiO2 photocatalytic oxidation of aromatic air contaminants under both UV and visible light. Chem. Eng. J. 2014, 236, 285–292. [Google Scholar] [CrossRef]
- Zou, X.; Li, X.; Zhao, Q.; Liu, S. Synthesis of LaVO4/TiO2 heterojunction nanotubes by sol-gel coupled with hydrothermal method for photocatalytic air purification. J. Colloid. Interf. Sci. 2012, 383, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Medina-Valtierra, J.; Frausto-Reyes, C.; Ramirez-Ortiz, J.; Camarillo-Martinez, G. Self-cleaning test of doped TiO2-coated glass plates under solar exposure. Ind. Eng. Chem. Res. 2009, 48, 598–606. [Google Scholar] [CrossRef]
- Hebeish, A.A.; Abdelhady, M.M.; Youssef, A.M. TiO2 nanowire and TiO2 nanowire doped Ag-PVP nanocomposite for antimicrobial and self-cleaning cotton textile. Carbohyd. Polym. 2013, 91, 549–559. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.J.; Yin, L.; Li, B.; Hong, X.; Fan, Z.; Chen, B.; Xue, C.; Zhang, H. One-pot synthesis of CdS nanocrystals hybridized with single-layer transition-metal dichalcogenide nanosheets for efficient photocatalytic hydrogen evolution. Angew. ChemInt. Ed. Engl. 2015, 54, 1210–1214. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, A.; Cao, S.W.; Bosman, M.; Li, S.; Xue, C. Direct evidence of plasmon enhancement on photocatalytic hydrogen generation over Au/Pt-decorated TiO2 nanofibers. Nanoscale 2014, 6, 5217–5222. [Google Scholar] [CrossRef]
- Zhang, N.; Shi, J.; Mao, S.S.; Guo, L. Co3O4 quantum dots: Reverse micelle synthesis and visible-light-driven photocatalytic overall water splitting. Chem. Commun. 2014, 50, 2002–2004. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Li, M.; Shen, S.; Wang, Y.; Feng, Z.; Shi, J.; Han, H.; Li, C. Photocatalytic overall water splitting promoted by an alpha-beta phase junction on Ga2O3. Angew. Chem. Int. 2012, 51, 13089–13092. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.G.; Zhao, X.G.; Zheng, M.Y.; Li, S.; Wang, Y.; Liu, X.J. Preparation of N-doped TiO2 by oxidizing TiN and its application on phenol degradation. Water Sci. Technol. 2013, 68, 934–939. [Google Scholar] [CrossRef]
- Mohammadi, K.; Sadeghi, M.; Azimirad, R. Facile synthesis of SrFe12O19 nanoparticles and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2017, 28, 10042–10047. [Google Scholar] [CrossRef]
- Mohammadi, K.; Sadeghi, M.; Azimirad, R.; Ebrahimi, M. Barium hexaferrite nanoparticles: Morphology-controlled preparation, characterization and investigation of magnetic and photocatalytic properties. J. Mater. Sci. Mater. Electron. 2017, 28, 9983–9988. [Google Scholar] [CrossRef]
- Shieh, D.L.; Lin, Y.S.; Yeh, J.H.; Chen, S.C.; Lin, B.C.; Lin, J.L. N-doped, porous TiO2 with rutile phase and visible light sensitive photocatalytic activity. Chem. Commun. 2012, 48, 2528–2530. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, J.; Jiang, J.; Li, X.; Lu, M.; Yuan, G.; Wang, Z.; Zheng, M.; Seo, H.J. Simple fabrication of N-doped mesoporous TiO2 nanorods with the enhanced visible light photocatalytic activity. Nanoscale Res. Lett. 2014, 9, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Burda, C. The electronic origin of the visible-light absorption properties of C-, N- and S-doped TiO2 nanomaterials. J. Am. Chem. Soc. 2008, 130, 5018–5019. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Huo, Y.; Zhu, J. Supercritical preparation of a highly active S-doped TiO2 photocatalyst for methylene blue mineralization. Environ. Sci. Technol. 2007, 41, 4410–4414. [Google Scholar] [CrossRef]
- Li, Z.; Ding, D.; Ning, C. P-Type hydrogen sensing with Al-and V-doped TiO2 nanostructures. Nanoscale Res. Lett. 2013, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Wang, S.; Zou, J.J.; Huang, Z.F.; Wang, L.; Zhang, X. Ti3+-defected and V-doped TiO2 quantum dots loaded on MCM-41. Chem. Commun. 2014, 50, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, C.; Zhang, L.; Li, X.; Liu, Y. Electrospun nanofibers of V-doped TiO2 with high photocatalytic activity. J. Colloid. Interf. Sci. 2010, 351, 57–62. [Google Scholar] [CrossRef]
- Liu, G.; Han, C.; Pelaez, M.; Zhu, D.; Liao, S.; Likodimos, V.; Ioannidis, N.; Kontos, A.G.; Falaras, P.; Dunlop, P.S.; et al. Synthesis, characterization and photocatalytic evaluation of visible light activated C-doped TiO2 nanoparticles. Nanotechnology 2012, 23, 294003. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, P.; Li, Q. New insight into the enhanced visible-light photocatalytic activities of B-, C- and B/C-doped anatase TiO2 by first-principles. Phys. Chem. Chem. Phys. 2013, 15, 12040–12047. [Google Scholar] [CrossRef]
- Zhang, W.J.; Ma, Z.; Du, L.; Li, H. Role of PEG4000 in sol-gel synthesis of Sm2Ti2O7 photocatalyst for enhanced activity. J. Alloy. Compd. 2017, 704, 26–31. [Google Scholar] [CrossRef]
- Wang, S.X.; Li, W.; Wang, S.; Jiang, J.M.; Chen, Z.H. Synthesis of well-defined hierarchical porous La2Zr2O7 monoliths via non-alkoxidesolegel process accompanied by phase separation. Micropor. Mesopor. Mat. 2016, 221, 32–39. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Arakawa, H. Preparation, structural and photophysical properties of Bi2InNbO7 compound. J. Mater. Sci. 2000, 19, 1909–1911. [Google Scholar]
- Shin, H.; Byun, T.H. Effect of Pt loading onto ball-milled TiO2 on the visible-light photocatalytic activity to decompose rhodamine B. Res. J. Chem. Environ. 2013, 17, 41–46. [Google Scholar]
- Kanhere, P.; Shenai, P.; Chakraborty, S.; Ahuja, R.; Zheng, J.; Chen, Z. Mono- and co-doped NaTaO3 for visible light photocatalysis. Phys. Chem. Chem. Phys. 2014, 16, 16085–16094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daskalaki, V.M.; Antoniadou, M.; Li Puma, G.; Kondarides, D.I.; Lianos, P. Solar light-responsive Pt/CdS/TiO2 photocatalysts for hydrogen production and simultaneous degradation of inorganic or organic sacrificial agents in wastewater. Environ. Sci. Technol. 2010, 44, 7200–7205. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Photocatalytic removal of spiramycin from wastewater under visible light with N-doped TiO2 photocatalysts. Chem. Eng. J. 2015, 261, 3–8. [Google Scholar] [CrossRef]
- Iliev, V.; Tomova, D.; Rakovsky, S. Nanosized N-doped TiO2 and gold modified semiconductors—photocatalysts for combined UV–visible light destruction of oxalic acid in aqueous solution. Desalination 2010, 260, 101–106. [Google Scholar] [CrossRef]
- Zhao, X.D.; Chen, D.H.; Zhang, Y.R.; Huang, M.H.; Deng, Z.B.; Zhou, Z. Optimization of hydrothermal synthesis conditions of N-doped TiO2 using response surface methodology for photocatalytic degradation of methylene blue. Fresen. Environ. Bull. 2016, 25, 4287–4293. [Google Scholar]
- Collazzo, G.C.; Foletto, E.L.; Jahn, S.L.; Villetti, M.A. Degradation of Direct Black 38 dye under visible light and sunlight irradiation by N-doped anatase TIO2 as photocatalyst. J. Environ. Manag. 2012, 98, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Jhuma, S.S.; Saha, P.C.; Ahmed, J.; Asiri, A.M.; Rifat, T.P.; Raihan, T.; Azad, A.K.; Rahman, M.M. Photocatalysis, enhanced anti-bacterial performance and discerning thiourea sensing of Ag2O·SnO2·TiO2 hetero-structure. J. Environ. Chem. Eng. 2020, 8, 104051. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Safwan, J.A.; Islam, M.S.; Rahman, Z.; Karim, M.R.; Pirzada, T.J.; Samed, A.J.; Rahman, M.M. Electrochemical decolorization of Methylene blue at Pt electrode in KCl solution for environmental remediation. J. Ind. Eng. Chem. 2015, 21, 787–791. [Google Scholar] [CrossRef]
- Hossain, A.; Elias, M.; Sarker, D.R.; Diba, Z.R.; Mithun, J.M.; Azad, M.A.K.; Siddiquey, I.A.; Rahman, M.M.; Uddin, J.; Uddin, N. Synthesis of Fe- or Ag-doped TiO2–MWCNT nanocomposite thin films and their visible-light-induced catalysis of dye degradation and antibacterial activity. Res. Chem. Intermed. 2018, 44, 2667–2683. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.H.; Sun, Z.K.; Wei, J.; Zheng, G.F.; Asiri, A.M.; Khan, S.B.; Rahman, M.M.; Zhao, D.Y. Hierarchical Cu2S Microsponges Constructed from Nanosheets for Efficient Photocatalysis. Small 2013, 9, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.F.; Zhao, W.; Feng, J.W.; Cai, H.L.; Zheng, Z.; Pan, B.C.; Wu, X.S.; Zou, Z.G.; Li, Y.M. Structural, photophysical and photocatalytic properties of novel Bi2AlVO7. J. Hazard. Mater. 2009, 164, 781–789. [Google Scholar] [CrossRef]
- Xing, C.C.; Zhang, Y.; Liu, Y.P.; Wang, X.; Li, J.S.; Martinez-Alanis, P.R.; Spadaro, M.C.; Guardia, P.; Arbiol, J.; Llorca, J.; et al. Photodehydrogenation of ethanol over Cu2O/TiO2 heterostructures. Nanomaterials 2021, 11, 1399. [Google Scholar] [CrossRef]
- Stathi, P.; Solakidou, M.; Deligiannakis, Y. Lattice defects engineering in W-, Zr-doped BiVO4 by flame spray pyrolysis: Enhancing photocatalytic O2 evolution. Nanomaterials 2021, 11, 501. [Google Scholar] [CrossRef]
- Thomas, A.M.; Peter, J.; Nagappan, S.; Mohan, A.; Ha, C.S. Dual stimuli-responsive copper nanoparticles decorated SBA-15: A highly efficient catalyst for the oxidation of alcohols in water. Nanomaterials 2020, 10, 2051. [Google Scholar] [CrossRef]
- Wang, G.; Wu, F.; Zhang, X.; Luo, M.; Deng, N. Enhanced photodegradation of bisphenol A in the presence of β-cyclodextrin under UV light. J. Chem. Technol. Biot. 2006, 81, 805–811. [Google Scholar] [CrossRef]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.; Yu, J. A review of direct Z-scheme photocatalysts. Small Methods 2017, 1700080, 1–21. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Zuo, S.; Lu, X.; Luo, S.; Li, Z.; Yao, C.; Ni, C. Construction of LaFe1−xMnxO3 /attapulgite nanocomposite for photo-SCR of NOx at low temperature. Chem. Eng. J. 2017, 320, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Shi, H.; Zhu, W.; Zuo, S.; Lu, X.; Luo, S.; Yao, C.; Chen, Y. Nanocomposite LaFe1-xNixO3/Palygorskite catalyst for photo-assisted reduction of NOx: Effect of Ni doping. Appl. Catal. B Environ. 2018, 231, 92–100. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Lu, X.; Zuo, S.; Li, Z.; Yao, C.; Ni, C. Photo-assisted selective catalytic reduction of NO by Z-scheme natural clay based photocatalyst: Insight into the effect of graphene coupling. J. Catal. 2018, 357, 59–68. [Google Scholar] [CrossRef]
- Luo, J.; Chen, J.; Guo, R.; Qiu, Y.; Li, W.; Zhou, X.; Ning, X.; Zhan, L. Rational construction of direct Z-scheme LaMnO3/g-C3N4 hybrid for improved visible-light photocatalytic tetracycline degradation. Sep. Purif. Technol. 2018, 211, 882–894. [Google Scholar] [CrossRef]
- Tang, Y.; Tao, Y.; Zhou, T.; Yang, B.; Wang, Q.; Zhu, Z.; Xie, A.; Luo, S.; Yao, C.; Li, X. Direct Z-scheme La1-xCexMnO3 catalyst for photothermal degradation of toluene. Environ. Sci. Pollut. R. 2019, 26, 36832–36844. [Google Scholar] [CrossRef]
- Wang, J.H.; Zou, Z.G.; Ye, J.H. Synthesis, Structure and Photocatalytic Property of a New Hydrogen Evolving Photocatalyst Bi2InTaO7. Mater. Sci. Forum. 2003, 423-425, 485–490. [Google Scholar] [CrossRef]
- Kohno, M.; Ogura, S.; Sato, K.; Inoue, Y. Properties of photocatalysts with tunnel structures: Formation of a surface lattice O− radical by the UV irradiation of BaTi4O9 with a pentagonal-prism tunnel structure. Chem. Phys. Lett. 1997, 267, 72–76. [Google Scholar] [CrossRef]
- Kudo, A.; Kato, H.; Nakagawa, S. Water Splitting into H2 and O2 on New Sr2M2O7 (M = Nb and Ta) Photocatalysts with Layered Perovskite Structures: Factors Affecting the Photocatalytic Activity. J. Phys. Chem. B. 2000, 104, 571–575. [Google Scholar] [CrossRef]
- Nowak, M.; Kauch, B.; Szperlich, P. Determination of energy band gap of nanocrystalline SbSI. Rev. Sci. Instrum. 2009, 80, 046107. [Google Scholar] [CrossRef]
- Zhou, F.; Kang, K.; Maxisch, T.; Ceder, G.; Morgan, D. The electronic structure and band gap of LiFePO4 and LiMnPO4. Solid State Commun. 2004, 132, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Tauc, J.; Grigorov, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi. 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis with YFeO3 electrodes. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Cui, B.Y.; Cui, H.T.; Li, Z.R.; Dong, H.Y.; Li, X.; Zhao, L.F.; Wang, J.W. Novel Bi3O5I2 hollow microsphere and its enhanced photocatalytic activity. Catalysts 2019, 9, 709. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, W.; Cantillo, A.; Salazar, B.; Diaz-Uribe, C.; Ramos, W.; Romero, E.; Hurtado, M. Comparative study of ZnO thin films doped with transition metals (Cu and Co) for methylene blue photodegradation under visible irradiation. Catalysts 2020, 10, 528. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhou, M.; Gu, J.; Li, X. Spectrophotometric and high performance liquid chromatographic methods for sensitive determination of bisphenol A. Spectrochim. Acta. A 2013, 122, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, Q.; Wang, Z.; Lv, X.F.; Yang, Z.L. Photocatalytic degradation of trichloroethylene over BiOCl under UV irradiation. Appl. Organomet. Chem. 2018, 32, e4354. [Google Scholar] [CrossRef]
- Cheng, X.X.; Chong, R.F.; Cao, Y.Y.; Li, D.L.; Chang, Z.X.; Zhang, L. Influence of Inorganic Anions on Photocatalytic Degeneration of Methylene Blue on Ag3PO4. J. Nanosci. Nanotechnol. 2016, 16, 12489–12497. [Google Scholar] [CrossRef]
- Bhatt, D.K.; Patel, U.D. Photocatalytic degradation of Reactive Black 5 using Ag3PO4 under visible light. J. Phys. Chem. Solids 2021, 149, 109768. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Q.H.; Liu, Y.P.; Ma, D.; Xin, Y.J.; Ma, X.H.; Zhang, X.W. In situ synthesis of graphene/WO3 co-decorated TiO2 nanotube array photoelectrodes with enhanced photocatalytic activity and degradation mechanism for dimethyl phthalate. Chem. Eng. J. 2017, 337, 322–332. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Zhao, Z.Y.; Dong, F.; Dong, X.A.; Zhou, Y. N-Doped Bi2O2CO3/graphene quantum dot composite photocatalyst: Enhanced visible-light photocatalytic NO oxidation and in situ DRIFTS studies. J. Phys. Chem. C 2017, 121, 12168–12177. [Google Scholar] [CrossRef]
- Jiang, L.B.; Yuan, X.Z.; Zeng, G.M.; Liang, J.; Chen, X.H.; Yu, H.B.; Wang, H.; Wu, Z.B.; Zhang, J.; Xiong, T. In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant. Appl. Catal. B 2018, 227, 376–385. [Google Scholar] [CrossRef]
- Cao, W.; Jiang, C.Y.; Chen, C.; Zhou, H.F.; Wang, Y.P. A novel Z-scheme CdS/Bi4O5Br2 heterostructure with mechanism analysis: Enhanced photocatalytic performance. J. Alloys Compd. 2021, 861, 158554. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Z.; Yang, J. A novel composite hydrogel for adsorption and photocatalytic degradation of bisphenol A by visible light irradiation. Chem. Eng. J. 2018, 334, 1679–1690. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Liu, Y.; Ling, H.; Zheng, H.; Kim, S.H.; Dionysiou, D.D. Efficient activation of peroxymonosulfate by magnetic Mn-MGO for degradation of bisphenol A. J. Hazard. Mater. 2016, 320, 150–159. [Google Scholar] [CrossRef]
- Sharma, J.; Mishra, I.M.; Dionysiou, D.D.; Kumar, V. Oxidative removal of Bisphenol A by UV-C/peroxymonosulfate (PMS): Kinetics, influence of co-existing chemicals and degradation pathway. Chem. Eng. J. 2015, 276, 193–204. [Google Scholar] [CrossRef]
- Deonikar, V.G.; Patil, S.S.; Tamboli, M.S.; Ambekar, J.D.; Kulkarni, M.V.; Panmand, R.P.; Umarji, G.G.; Shinde, M.D.; Rane, S.B.; Munirathnam, N.R.; et al. Growth study of hierarchical Ag3PO4/LaCO3OH heterostructures. Phys. Chem. Chem. Phys. 2017, 19, 20541–20550. [Google Scholar] [CrossRef]
- Patil, S.S.; Tamboli, M.S.; Deonikar, V.G.; Umarji, G.G.; Ambekar, J.D.; Kulkarni, M.V.; Kolekar, S.S.; Kale, B.B.; Patil, D.R. Magnetically separable Ag3PO4/NiFe2O4 composites with enhanced photocatalytic activity. Dalton T 2015, 44, 20426–20434. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Doong, R.A. Heterostructured ZnFe2O4/TiO2 nanocomposites with a highly recyclable visible-light-response for bisphenol A degradation. RSC Adv. 2017, 7, 50006–50016. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.J.; Li, N.J.; Chen, D.Y.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. The ultrasonic-induced-piezoelectric enhanced photocatalytic performance of ZnO/CdS nanofibers for degradation of bisphenol A. J. Alloy Compd. 2021, 885, 160987. [Google Scholar] [CrossRef]




























| Atom | x | y | z | Occupation Factor |
|---|---|---|---|---|
| Gd | 0 | 0 | 0 | 1 |
| Bi | 0.5 | 0.5 | 0.5 | 0.5 |
| Ta | 0.5 | 0.5 | 0.5 | 0.5 |
| O(1) | −0.185 | 0.125 | 0.125 | 1 |
| O(2) | 0.125 | 0.125 | 0.125 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, J.; Wei, Z.; Niu, B.; Yang, G.; Huang, C.; Ma, B.; Liu, W. Synthesis, Property Characterization and Photocatalytic Activity of the Ag3PO4/Gd2BiTaO7 Heterojunction Catalyst under Visible Light Irradiation. Catalysts 2022, 12, 22. https://doi.org/10.3390/catal12010022
Luan J, Wei Z, Niu B, Yang G, Huang C, Ma B, Liu W. Synthesis, Property Characterization and Photocatalytic Activity of the Ag3PO4/Gd2BiTaO7 Heterojunction Catalyst under Visible Light Irradiation. Catalysts. 2022; 12(1):22. https://doi.org/10.3390/catal12010022
Chicago/Turabian StyleLuan, Jingfei, Zhijie Wei, Bowen Niu, Guangmin Yang, Cisheng Huang, Bingbing Ma, and Wenlu Liu. 2022. "Synthesis, Property Characterization and Photocatalytic Activity of the Ag3PO4/Gd2BiTaO7 Heterojunction Catalyst under Visible Light Irradiation" Catalysts 12, no. 1: 22. https://doi.org/10.3390/catal12010022
APA StyleLuan, J., Wei, Z., Niu, B., Yang, G., Huang, C., Ma, B., & Liu, W. (2022). Synthesis, Property Characterization and Photocatalytic Activity of the Ag3PO4/Gd2BiTaO7 Heterojunction Catalyst under Visible Light Irradiation. Catalysts, 12(1), 22. https://doi.org/10.3390/catal12010022







