Thermal Stability of Potassium-Promoted Cobalt Molybdenum Nitride Catalysts for Ammonia Synthesis
Abstract
:1. Introduction
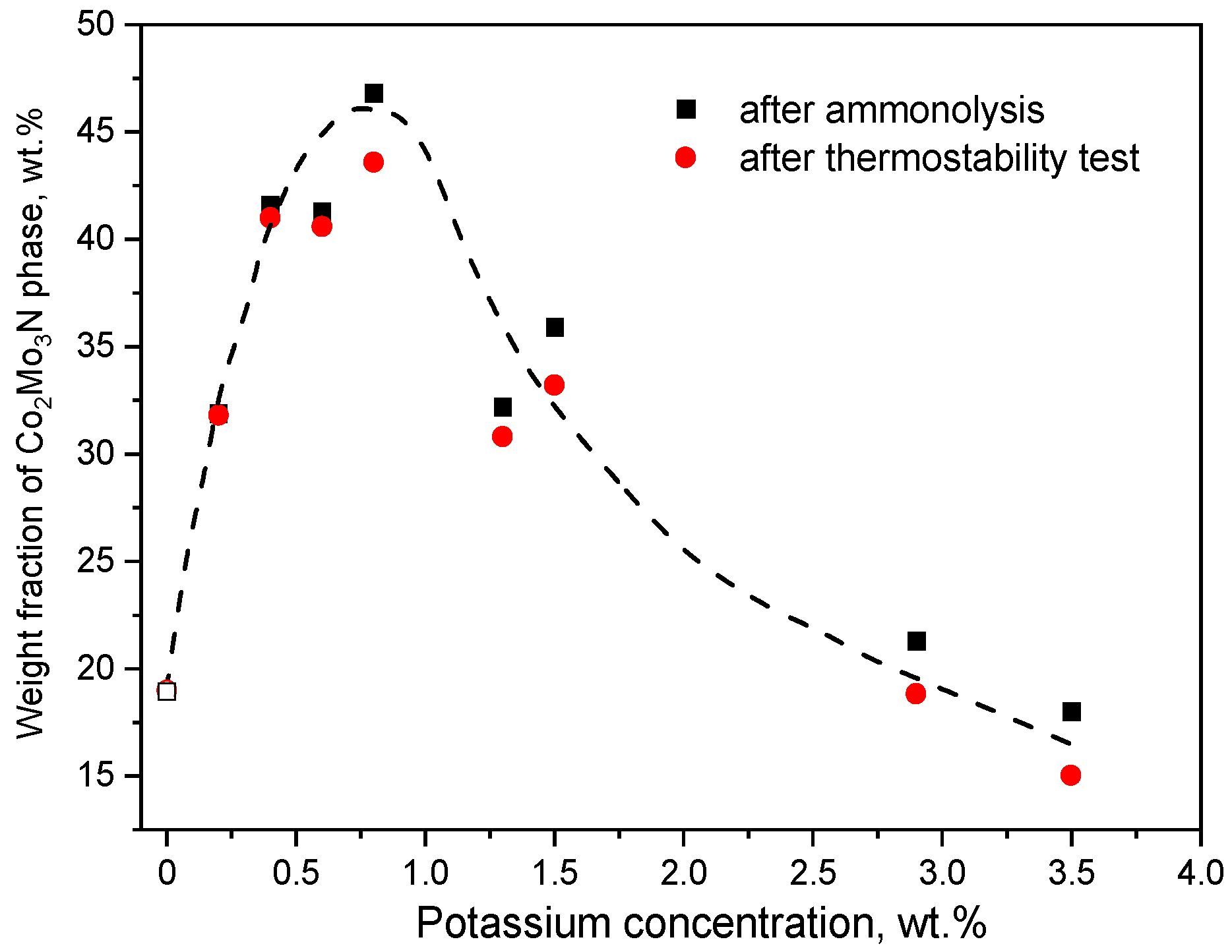
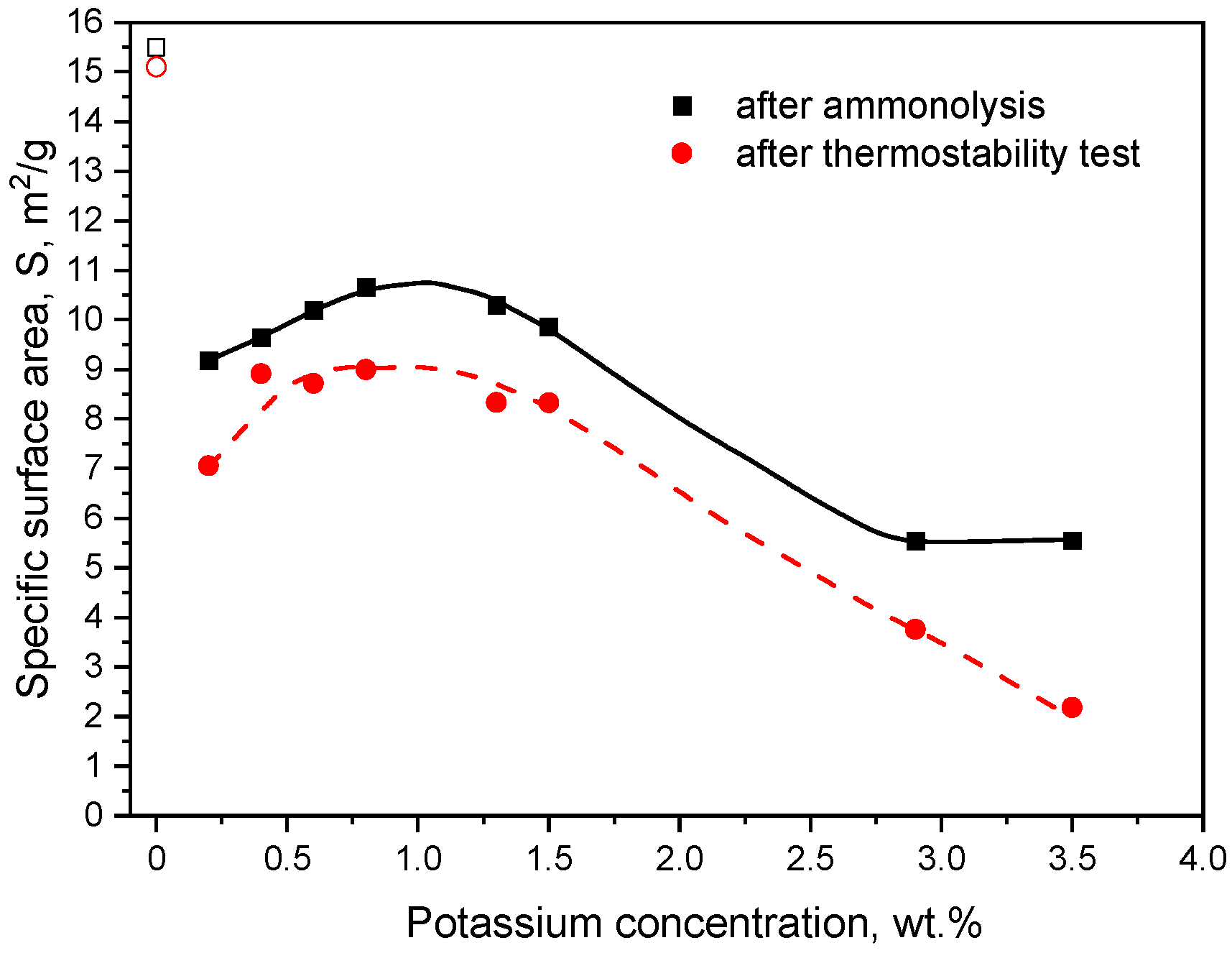
2. Results
3. Discussion
4. Materials and Methods
4.1. Precursor Synthesis
4.2. Nitriding of Precursor
4.3. Material Characterization
4.4. Catalytic Activity Tests
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hargreaves, J.S.J. Nitrides as ammonia synthesis catalysts and as potential nitrogen transfer reagents. Appl. Petrochem. Res. 2014, 4, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Kotarba, A.; Dmytrzyk, J.; Raróg-Pilecka, W.; Kowalczyk, Z. Surface heterogeneity and ionization of Cs promoter in carbon-based ruthenium catalyst for ammonia synthesis. Appl. Surf. Sci. 2003, 207, 327–333. [Google Scholar] [CrossRef]
- Karolewska, M.; Truszkiewicz, E.; Mierzwa, B.; Kępiński, L.; Raróg-Pilecka, W. Ammonia synthesis over cobalt catalysts doped with cerium and barium. Effect of the ceria loading. Appl. Catal. A Gen. 2012, 445–446, 280–286. [Google Scholar] [CrossRef]
- Dongil, A.B. Recent Progress on Transition Metal Nitrides Nanoparticles as Heterogeneous Catalysts. Nanomaterials 2019, 9, 1111. [Google Scholar] [CrossRef] [Green Version]
- Boisen, A.; Dahl, S.; Jacobsen, C.J.H. Promotion of Binary Nitride Catalysts: Isothermal N2 Adsorption, Microkinetic Model, and Catalytic Ammonia Synthesis Activity. J. Catal. 2002, 208, 180–186. [Google Scholar] [CrossRef]
- She, Y.; Tang, B.; Li, D.; Tang, X.; Qiu, J.; Shang, Z.; Hu, W. Mixed Nickel-Cobalt-Molybdenum Metal Oxide Nanosheet Arrays for Hybrid Supercapacitor Applications. Coatings 2018, 8, 340. [Google Scholar] [CrossRef] [Green Version]
- Hada, K.; Tanabe, J.; Omi, S.; Nagai, M. Characterization of cobalt molybdenum nitrides for thiophene HDS by XRD, TEM, and XPS. J. Catal. 2002, 207, 10–22. [Google Scholar] [CrossRef]
- Hada, K.; Nagai, M.; Omi, S. Characterization and HDS activity of cobalt molybdenum nitrides. J. Phys. Chem. B 2001, 105, 4084–4093. [Google Scholar] [CrossRef]
- Logan, J.W.; Heiser, J.L.; McCrea, K.R.; Gates, B.D.; Bussell, M.E. Thiophene hydrodesulfurization over bimetallic and promoted nitride catalysts. Catal. Lett. 1998, 56, 165–171. [Google Scholar] [CrossRef]
- Furimsky, E. Metal carbides and nitrides as potential catalysts for hydroprocessing. Appl. Catal. A Gen. 2003, 240, 1–28. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Zheng, M.; Wu, Z.; Wu, W.; Li, C. The reaction route and active site of catalytic decomposition of hydrazine over molybdenum nitride catalyst. J. Catal. 2004, 224, 473–478. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, A.M.; Yang, X.F.; Au, C.T. NO Reduction with Hydrogen over Cobalt Molybdenum Nitride and Molybdenum Nitride: A Comparison Study. Catal. Lett. 2004, 97, 9–16. [Google Scholar] [CrossRef]
- Kojima, R.; Aika, K.I. Cobalt molybdenum bimetallic nitride catalysts for ammonia synthesis. Part 2. Kinetic study. Appl. Catal. A 2001, 218, 121–128. [Google Scholar] [CrossRef]
- Jacobsen, C.J.H.; Dahl, S.; Clausen, B.S.; Bahn, S.; Logadottir, A.; Norskov, J.K. Catalyst Design by Interpolation in the Periodic Table: Bimetallic Ammonia Synthesis Catalysts. J. Am. Chem. Soc. 2001, 123, 8404–8405. [Google Scholar] [CrossRef] [PubMed]
- Kojima, R.; Aika, K.-I. Cobalt molybdenum bimetallic nitride catalysts for ammonia synthesis Part 1. Preparation and characterization. Appl. Catal. A Gen. 2001, 215, 149–160. [Google Scholar] [CrossRef]
- Jacobsen, C.J.H. Novel class of ammonia synthesis catalysts. Chem. Commun. 2000, 1057–1058. [Google Scholar] [CrossRef]
- Kojima, R.; Aika, K. Cobalt molybdenum bimetallic nitride catalysts for ammonia synthesis. Chem. Lett. 2000, 29, 514–515. [Google Scholar] [CrossRef]
- Moszyński, D.; Jędrzejewski, R.; Ziebro, J.; Arabczyk, W. Surface and catalytic properties of potassium-modified cobalt molybdenum catalysts for ammonia synthesis. Appl. Surf. Sci. 2010, 256, 5581–5584. [Google Scholar] [CrossRef]
- Adamski, P.; Nadziejko, M.; Komorowska, A.; Sarnecki, A.; Albrecht, A.; Moszyński, D. Chromium-modified cobalt molybdenum nitrides as catalysts for ammonia synthesis. Open Chem. 2019, 17, 127–131. [Google Scholar] [CrossRef]
- Moszyński, D. Controlled phase composition of mixed cobalt molybdenum nitrides. Int. J. Refract. Met. Hard Mater. 2013, 41, 449–452. [Google Scholar] [CrossRef]
- Moszyński, D.; Adamski, P.; Nadziejko, M.; Komorowska, A.; Sarnecki, A. Cobalt molybdenum nitrides co-promoted by chromium and potassium as catalysts for ammonia synthesis. Chem. Pap. 2018, 72, 425–430. [Google Scholar] [CrossRef]
- Adamski, P.; Moszyński, D.; Komorowska, A.; Nadziejko, M.; Sarnecki, A.; Albrecht, A. Ammonolysis of Cobalt Molybdenum Oxides—In Situ XRD Study. Inorg. Chem. 2018, 57, 9844–9850. [Google Scholar] [CrossRef] [PubMed]
- Liu, H. Ammonia Synthesis Catalysts: Innovation and Practice; World Scientific: Singapore, 2013. [Google Scholar]
- Kojima, R.; Aika, K.I. Cobalt molybdenum bimetallic nitride catalysts for ammonia synthesis. Part 3. Reactant gas treatment. Appl. Catal. A 2001, 219, 157–170. [Google Scholar] [CrossRef]
- Adamski, P.; Moszyński, D.; Nadziejko, M.; Komorowska, A.; Sarnecki, A.; Albrecht, A. Thermal stability of catalyst for ammonia synthesis based on cobalt molybdenum nitrides. Chem. Pap. 2019, 73, 851–859. [Google Scholar] [CrossRef] [Green Version]
- Daisley, A.; Costley-Wood, L.; Hargreaves, J.S.J. The Role of Composition and Phase upon the Lattice Nitrogen Reactivity of Ternary Molybdenum Nitrides. Top. Catal. 2021, 73, 851–859. [Google Scholar] [CrossRef]
- Nadziejko, M.; Adamski, P.; Moszyński, D. Doped cobalt-molybdenum catalysts for the ammonia synthesis. Przem. Chem. 2020, 99, 1454–1458. [Google Scholar] [CrossRef]
- Moszyński, D.; Adamski, P.; Pelech, I.; Arabczyk, W. Cobalt-molybdenum catalysts doped with cesium for ammonia synthesis. Przem. Chem. 2015, 94, 1399–1403. [Google Scholar] [CrossRef]
- Dry, M.E.; du Plessis, J.A.K.; Leuteritz, G.M. The Influence of Structural Promoters on the Surface Properties of Reduced Magnetite Catalysts. J. Catal. 1966, 6, 194–199. [Google Scholar] [CrossRef]
- Raje, A.P.; O’Brien, R.J.; Davies, B.H. Effect of potassium promotion on iron-based catalysts for Fischer-Tropsch synthesis. J. Catal. 1998, 180, 36–43. [Google Scholar] [CrossRef]
- Choi, J.-G.; Curl, R.L.; Thompson, L.T. Molybdenum Nitride Catalysts I. Influence of the Synthesis Factors on Structural Properties. J. Catal. 1994, 146, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Prior, T.J.; Battle, P.D. Facile synthesis of interstitial metal nitrides with the filled b-manganese structure. J. Solid State Chem. 2003, 172, 138–147. [Google Scholar] [CrossRef]
- Alconchel, S.; Sapiña, F.; Beltran, D.; Beltran, A. Chemistry of interstitial molybdenum ternary nitrides MnMo3N (M = Fe, Co, n = 3; M = Ni, n = 2). J. Mater. Chem. 1998, 8, 1901–1909. [Google Scholar] [CrossRef]
- Arabczyk, W.; Kałucki, K.; Kaleńczuk, R.J.; Śpiewak, Z.; Morawski, A.W.; Pajewski, R.; Ludwiczak, S.; Stołecki, K.; Janecki, Z. Badanie aktywności kontaktów żelazowych do syntezy amoniaku. Przem. Chem. 1986, 65, 532. [Google Scholar]
- Kowalczyk, Z.; Jodzis, S.; Środa, J.; Diduszko, R.; Kowalczyk, E. Influence of aluminium and potassium on activity and texture of fused iron Catalysts for ammonia synthesis. Appl. Catal. A Gen. 1992, 87, 1–14. [Google Scholar] [CrossRef]
- Yan, P.; Guo, W.; Liang, Z.; Meng, W.; Yin, Z.; Li, S.; Li, M.; Zhang, M.; Yan, J.; Xiao, D.; et al. Highly efficient K-Fe/C catalysts derived from metal-organic frameworks towards ammonia synthesis. Nano Res. 2019, 12, 2341–2347. [Google Scholar] [CrossRef]
- Spencer, M. On the rate-determining step and the role of potassium in the catalytic synthesis of ammonia. Catal. Lett. 1992, 13, 45–53. [Google Scholar] [CrossRef]
- Arabczyk, W.; Narkiewicz, U.; Moszyński, D. Double-layer model of the fused iron catalyst for ammonia synthesis. Langmuir 1999, 15, 5785–5789. [Google Scholar] [CrossRef]
- Bem, D.S.; Olsen, H.P.; zur Loye, H.-C. Synthesis, Electronic and Magnetic Characterization of the Ternary Nitride (Fe0.8Mo0.2)MoN2. Chem. Mater. 1995, 7, 1824. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Hill, R.; Howard, C. Quantitative phase analysis from neutron powder diffraction data using the Rietveld method. J. Appl. Crystallogr. 1987, 20, 467–474. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- Arabczyk, W. The state of studies on iron catalyst for ammonia synthesis. Pol. J. Chem. Technol. 2005, 7, 8–17. [Google Scholar]
- Arabczyk, W.; Moszyński, D.; Narkiewicz, U.; Pelka, R.; Podsiadły, M. Poisoning of iron catalyst by sulfur. Catal. Today 2007, 124, 43–48. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamski, P.; Czerwonko, W.; Moszyński, D. Thermal Stability of Potassium-Promoted Cobalt Molybdenum Nitride Catalysts for Ammonia Synthesis. Catalysts 2022, 12, 100. https://doi.org/10.3390/catal12010100
Adamski P, Czerwonko W, Moszyński D. Thermal Stability of Potassium-Promoted Cobalt Molybdenum Nitride Catalysts for Ammonia Synthesis. Catalysts. 2022; 12(1):100. https://doi.org/10.3390/catal12010100
Chicago/Turabian StyleAdamski, Paweł, Wojciech Czerwonko, and Dariusz Moszyński. 2022. "Thermal Stability of Potassium-Promoted Cobalt Molybdenum Nitride Catalysts for Ammonia Synthesis" Catalysts 12, no. 1: 100. https://doi.org/10.3390/catal12010100
APA StyleAdamski, P., Czerwonko, W., & Moszyński, D. (2022). Thermal Stability of Potassium-Promoted Cobalt Molybdenum Nitride Catalysts for Ammonia Synthesis. Catalysts, 12(1), 100. https://doi.org/10.3390/catal12010100





