Understanding the Catalytic Activity of Microporous and Mesoporous Zeolites in Cracking by Experiments and Simulations
Abstract
:1. Introduction
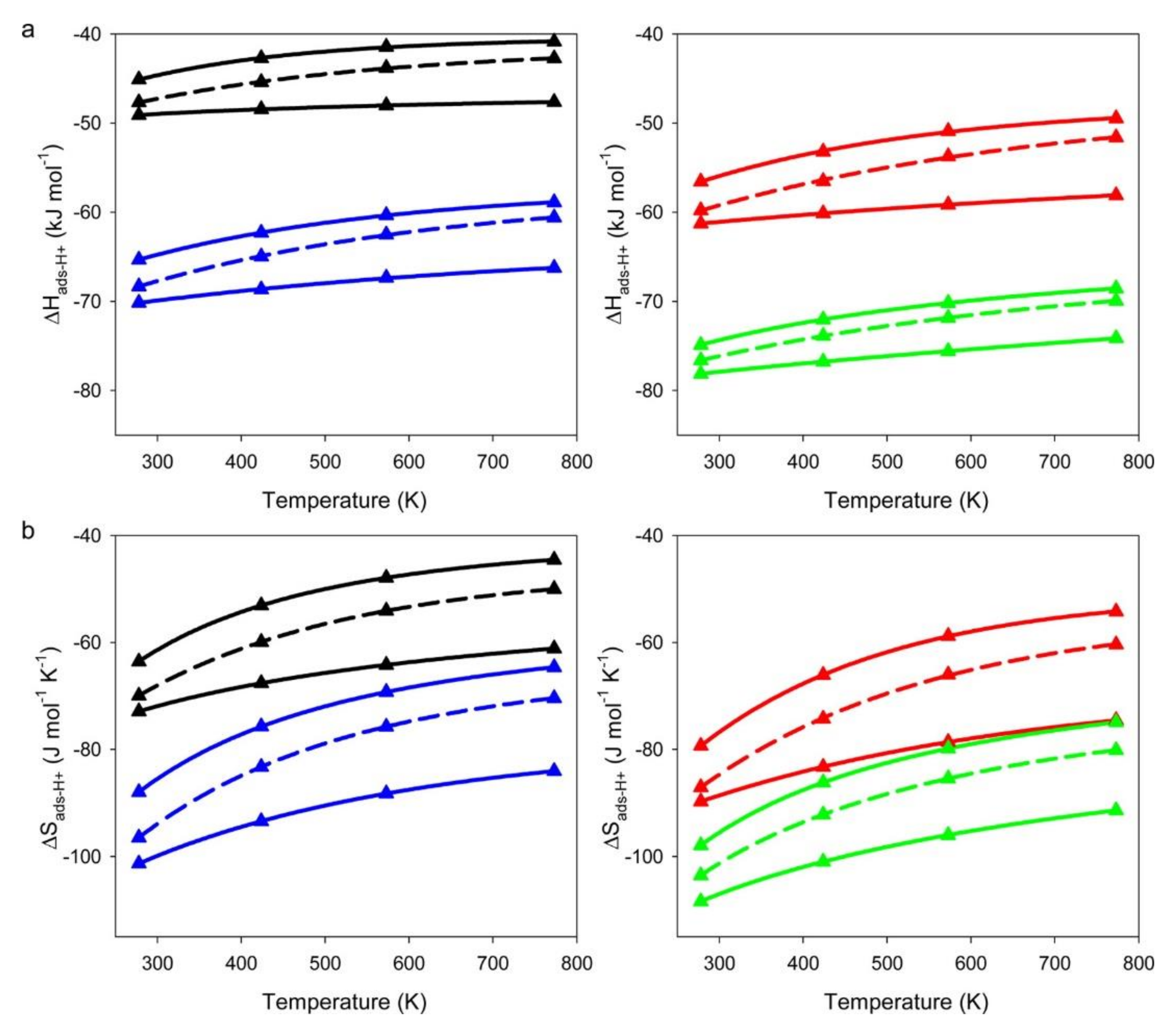
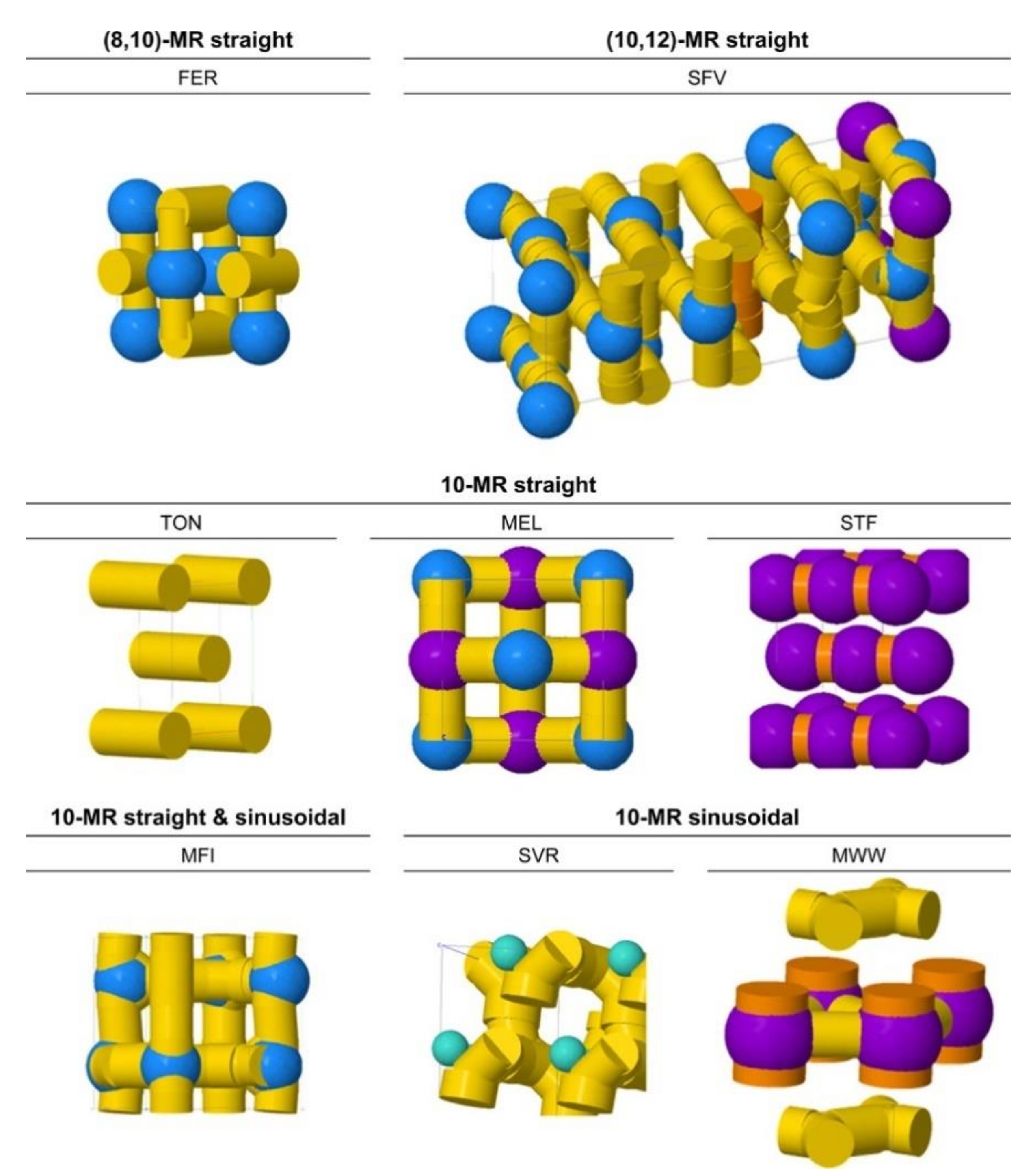
2. The Effects of Zeolite Structures on Alkane Cracking
3. The Effects of Mesoporosity on Alkane Cracking
4. Different Active Sites in Zeolites
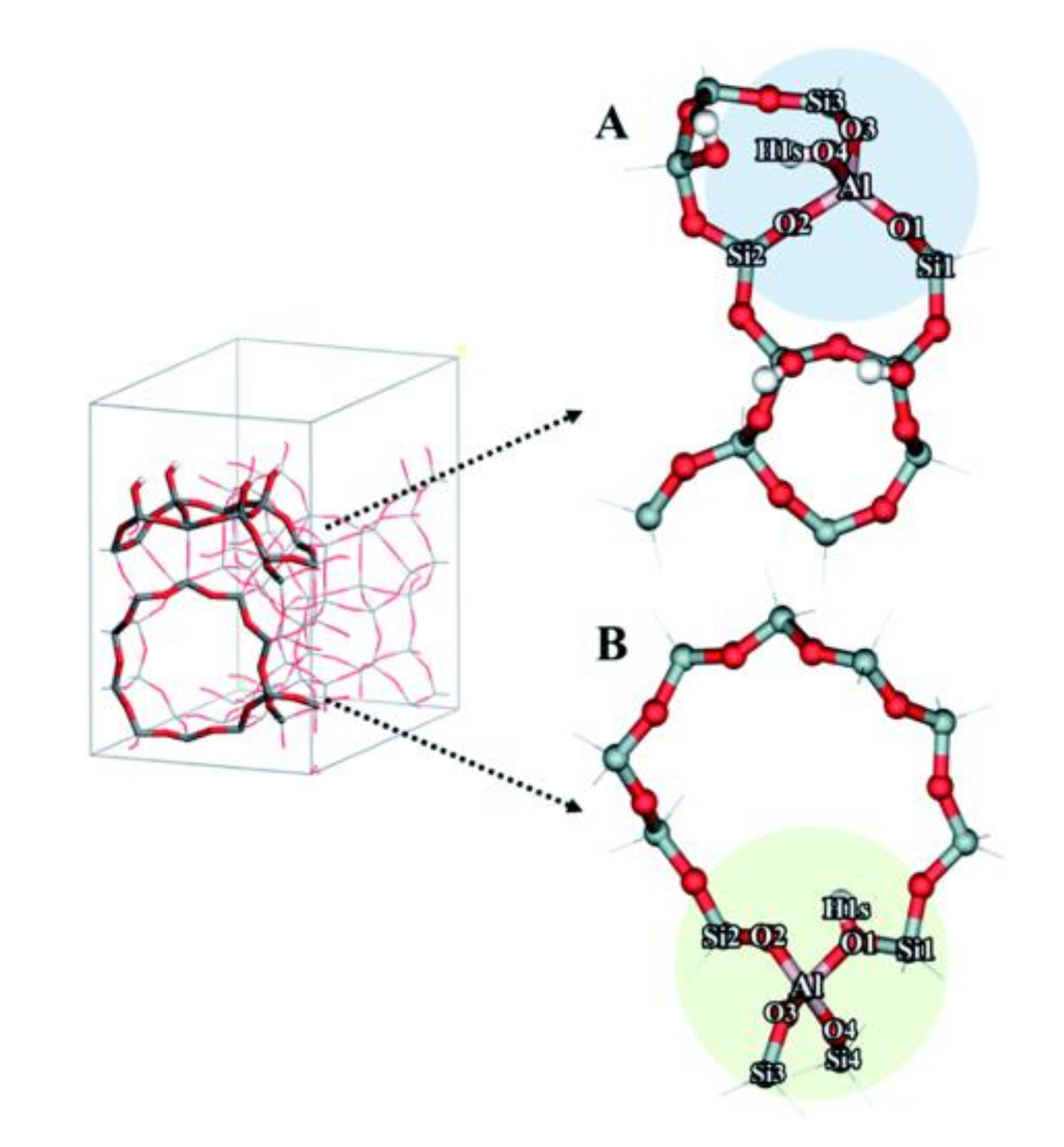
4.1. Brønsted Acid Sites in Conventional Zeolites
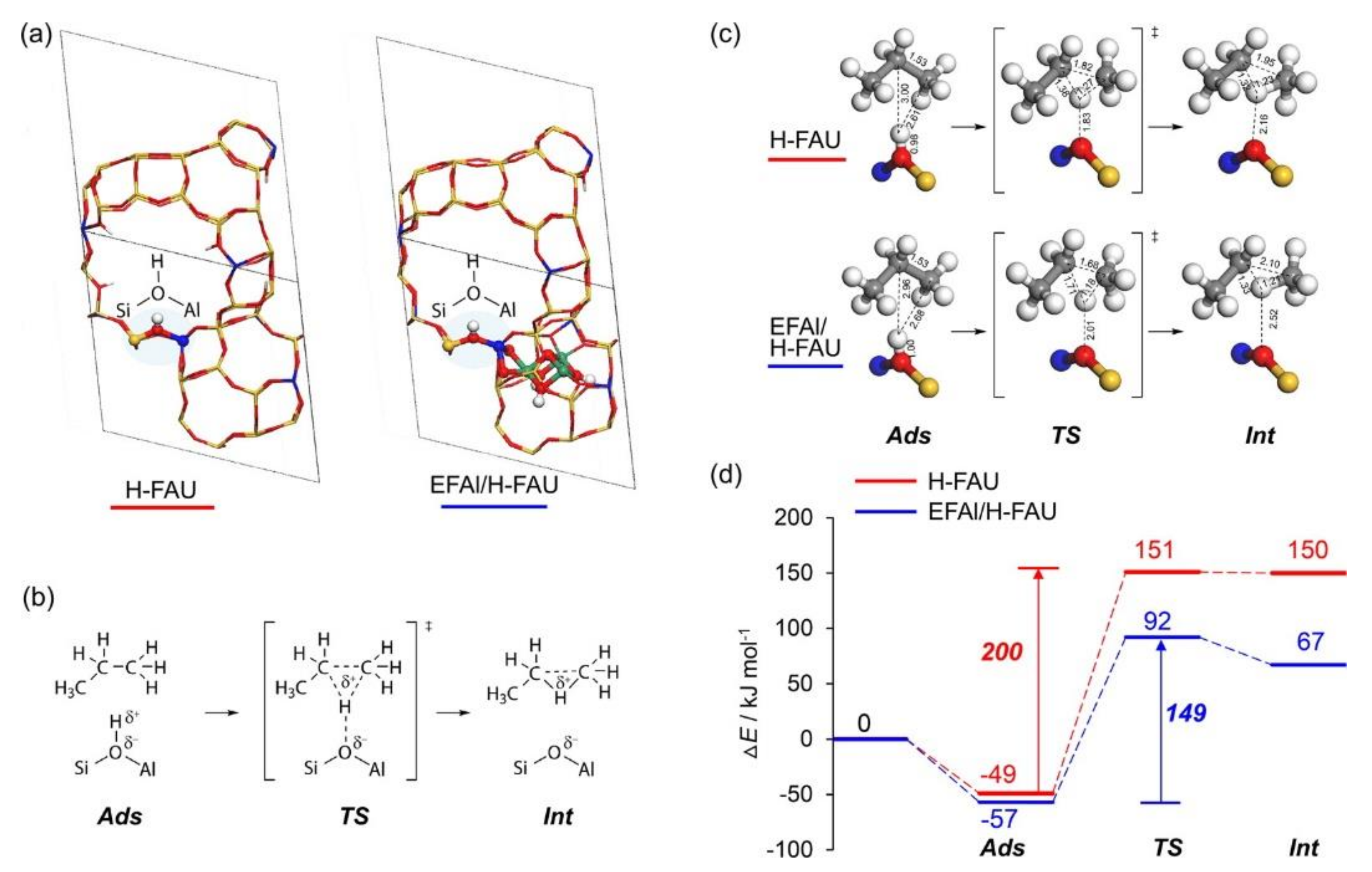
4.2. Extra-Framework Species
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CBMC | configurational-bias Monte Carlo |
| DFT | density functional theory |
| DPE | deprotonation energy |
| DQ-MAS | double quantum magic-angle spinning |
| EFAL | extra-framework aluminum |
| FCC | fluidized catalytic cracking |
| LCO | light cycle oil |
| MBH | mobile block Hessian |
| MD | molecule dynamics |
| QM/MM | quantum mechanics/molecular mechanics |
| QM:QM | quantum mechanics:quantum mechanics |
| quasi-RRHO | quasi-rigid rotor harmonic oscillator |
| VGO | vacuum gas oil |
References
- Komvokis, V.; Tan, L.X.L.; Clough, M.; Pan, S.S.; Yilmaz, B. Zeolites in fluid catalytic cracking (FCC). In Zeolites in Sustainable Chemistry; Springer: Berlin/Heidelberg, Germany, 2016; pp. 271–297. [Google Scholar]
- Bender, M. An overview of industrial processes for the production of olefins—C4Hydrocarbons. ChemBioEng Rev. 2014, 1, 136–147. [Google Scholar] [CrossRef]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Tomas, M.; Vakili, M. A review on production of light olefins via fluid catalytic cracking. Energies 2021, 14, 1089. [Google Scholar] [CrossRef]
- Zacharopoulou, V.; Lemonidou, A.A. Olefins from biomass intermediates: A review. Catalysts 2017, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Almutairi, S.M.; Mezari, B.; Filonenko, G.A.; Magusin, P.C.; Rigutto, M.S.; Pidko, E.A.; Hensen, E.J. Influence of extraframe-work aluminum on the Brønsted acidity and catalytic reactivity of faujasite zeolite. ChemCatChem 2013, 5, 452–466. [Google Scholar] [CrossRef]
- Xu, B.; Sievers, C.; Hong, S.B.; Prins, R.; van Bokhoven, J.A. Catalytic activity of Brønsted acid sites in zeolites: Intrinsic activity, rate-limiting step, and influence of the local structure of the acid sites. J. Catal. 2006, 244, 163–168. [Google Scholar] [CrossRef]
- Jones, A.J.; Zones, S.I.; Iglesia, E. Implications of transition state confinement within small voids for acid catalysis. J. Phys. Chem. C 2014, 118, 17787–17800. [Google Scholar] [CrossRef]
- Janda, A.; Bell, A. Effects of Si/Al ratio on the distribution of framework Al and on the rates of alkane monomolecular cracking and dehydrogenation in H-MFI. J. Am. Chem. Soc. 2013, 135, 19193–19207. [Google Scholar] [CrossRef] [Green Version]
- Janda, A.; Vlaisavljevich, B.; Lin, L.-C.; Smit, B.; Bell, A.T. Effects of zeolite structural confinement on adsorption thermodynamics and reaction kinetics for monomolecular cracking and dehydrogenation of n-butane. J. Am. Chem. Soc. 2016, 138, 4739–4756. [Google Scholar] [CrossRef]
- Van der Mynsbrugge, J.; Janda, A.; Sharada, S.M.; Lin, L.-C.; Van Speybroeck, V.; Head-Gordon, M.; Bell, A.T. Theoretical analysis of the influence of pore geometry on monomolecular cracking and dehydrogenation of n-butane in Brønsted acidic zeolites. ACS Catal. 2017, 7, 2685–2697. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A Gen. 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Blay, V.; Louis, B.; Miravalles, R.; Yokoi, T.; Peccatiello, K.A.; Clough, M.; Yilmaz, B. Engineering zeolites for catalytic cracking to light olefins. ACS Catal. 2017, 7, 6542–6566. [Google Scholar] [CrossRef]
- Janda, A.; Vlaisavljevich, B.; Lin, L.-C.; Mallikarjun Sharada, S.; Smit, B.; Head-Gordon, M.; Bell, A.T. Adsorption thermo-dynamics and intrinsic activation parameters for monomolecular cracking of n-alkanes on Brønsted acid sites in zeolites. J. Phys. Chem. C 2015, 119, 10427–10438. [Google Scholar] [CrossRef]
- Yang, C.-T.; Janda, A.; Bell, A.T.; Lin, L.-C. Atomistic investigations of the effects of Si/Al ratio and Al distribution on the adsorption selectivity of n-alkanes in Brønsted-acid zeolites. J. Phys. Chem. C 2018, 122, 9397–9410. [Google Scholar] [CrossRef] [Green Version]
- Van Bokhoven, J.A.; Xu, B. Towards predicting catalytic performances of zeolites. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 170, pp. 1167–1173. [Google Scholar]
- Gounder, R.; Iglesia, E. Catalytic consequences of spatial constraints and acid site location for monomolecular alkane activation on zeolites. J. Am. Chem. Soc. 2009, 131, 1958–1971. [Google Scholar] [CrossRef]
- Kadam, S.A.; Li, H.; Wormsbecher, R.F.; Travert, A. Impact of zeolite structure on entropic-enthalpic contributions to alkane monomolecular cracking: An IR operando study. Chem. Eur. J. 2018, 24, 5489–5492. [Google Scholar] [CrossRef]
- Berger, F.; Rybicki, M.; Sauer, J. Adsorption and cracking of propane by zeolites of different pore size. J. Catal. 2021, 395, 117–128. [Google Scholar] [CrossRef]
- Mallikarjun Sharada, S.; Zimmerman, P.M.; Bell, A.T.; Head-Gordon, M. Insights into the kinetics of cracking and dehydro-genation reactions of light alkanes in H-MFI. J. Phys. Chem. C 2013, 117, 12600–12611. [Google Scholar] [CrossRef]
- Niwa, M.; Suzuki, K.; Morishita, N.; Sastre, G.; Okumura, K.; Katada, N. Dependence of cracking activity on the Brønsted acidity of Y zeolite: DFT study and experimental confirmation. Catal. Sci. Technol. 2013, 3, 1919–1927. [Google Scholar] [CrossRef]
- Borges, P.; Pinto, R.R.; Lemos, M.A.; Lemos, F.; Védrine, J.; Derouane, E.; Ribeiro, F.R. Activity–acidity relationship for alkane cracking over zeolites: N-hexane cracking over HZSM-5. J. Mol. Catal. A Chem. 2005, 229, 127–135. [Google Scholar] [CrossRef]
- Katada, N.; Sota, S.; Morishita, N.; Okumura, K.; Niwa, M. Relationship between activation energy and pre-exponential factor normalized by the number of Brønsted acid sites in cracking of short chain alkanes on zeolites. Catal. Sci. Technol. 2014, 5, 1864–1869. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, C.; Zhuo, Z.; Zhang, D.; Zhao, S.; Wu, H.; Liu, Y.; He, M. Acid strength controlled reaction pathways for the catalytic cracking of 1-butene to propene over ZSM-5. J. Catal. 2013, 309, 136–145. [Google Scholar] [CrossRef]
- Garcia-Martinez, J.; Johnson, M.M.; Valla, J.; Li, K.; Ying, J. Mesostructured zeolite Y—High hydrothermal stability and superior FCC catalytic performance. Catal. Sci. Technol. 2012, 2, 987–994. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Li, K.; Valla, J.; Garcia-Martinez, J. Realizing the commercial potential of hierarchical zeolites: New opportunities in catalytic cracking. ChemCatChem 2013, 6, 46–66. [Google Scholar] [CrossRef]
- Srivastava, R. Synthesis and applications of ordered and disordered mesoporous zeolites: Present and future prospective. Catal. Today 2018, 309, 172–188. [Google Scholar] [CrossRef]
- Peng, P.; Gao, X.-H.; Yan, Z.-F.; Mintova, S. Diffusion and catalyst efficiency in hierarchical zeolite catalysts. Natl. Sci. Rev. 2020, 7, 1726–1742. [Google Scholar] [CrossRef]
- Chapellière, Y.; Daniel, C.; Tuel, A.; Farrusseng, D.; Schuurman, Y. Kinetics of n-hexane cracking over mesoporous HY zeolites based on catalyst descriptors. Catalysts 2021, 11, 652. [Google Scholar] [CrossRef]
- Zhou, F.; Gao, Y.; Wu, G.; Ma, F.; Liu, C. Improved catalytic performance and decreased coke formation in post-treated ZSM-5 zeolites for methanol aromatization. Microporous Mesoporous Mater. 2017, 240, 96–107. [Google Scholar] [CrossRef]
- Zhao, T.; Li, F.; Yu, H.; Ding, S.; Li, Z.; Huang, X.; Li, X.; Wei, X.; Wang, Z.; Lin, H. Synthesis of mesoporous ZSM-5 zeolites and catalytic cracking of ethanol and oleic acid into light olefins. Appl. Catal. A Gen. 2019, 575, 101–110. [Google Scholar] [CrossRef]
- Khalil, U.; Liu, Z.; Peng, C.; Hikichi, N.; Wakihara, T.; García-Martínez, J.; Okubo, T.; Bhattacharya, S. Ultrafast surfactant-templating of BEA zeolite: An efficient catalyst for the cracking of polyethylene pyrolysis vapours. Chem. Eng. J. 2021, 412, 128566. [Google Scholar] [CrossRef]
- Pala-Rosas, I.; Contreras, J.; Salmones, J.; Zeifert, B.; López-Medina, R.; Navarrete-Bolaños, J.; Hernández-Ramírez, S.; Pérez-Cabrera, J.; de Oca, A.F.-M. Catalytic deactivation of HY zeolites in the dehydration of glycerol to acrolein. Catalysts 2021, 11, 360. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, R.; Sanchez, M.; Haller, G.L.; Hu, J.; Bermejo-Deval, R.; Liu, Y.; Lercher, J.A. Promotion of protolytic pentane conversion on H-MFI zeolite by proximity of extra-framework aluminum oxide and Brønsted acid sites. J. Catal. 2019, 370, 424–433. [Google Scholar] [CrossRef]
- Mezari, B.; Magusin, P.C.; Almutairi, S.M.; Pidko, E.A.; Hensen, E.J. Nature of enhanced Brønsted acidity induced by ex-traframework aluminum in an ultrastabilized faujasite zeolite: An in situ NMR study. J. Phys. Chem. C 2021, 125, 9050–9059. [Google Scholar] [CrossRef]
- Li, G.; Pidko, E.A. The nature and catalytic function of cation sites in zeolites: A computational perspective. ChemCatChem 2018, 11, 134–156. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Li, G.; Hensen, E.J.M.; Pidko, E.A. Nature and catalytic role of extraframework aluminum in faujasite zeolite: A theoretical perspective. ACS Catal. 2015, 5, 7024–7033. [Google Scholar] [CrossRef]
- Li, S.; Zheng, A.; Su, Y.; Zhang, H.; Chen, L.; Yang, J.; Ye, A.C.; Deng, F. Brønsted/Lewis acid synergy in dealuminated HY zeolite: A combined solid-state NMR and theoretical calculation study. J. Am. Chem. Soc. 2007, 129, 11161–11171. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, G.; Hensen, E.J.; Pidko, E.A. Relationship between acidity and catalytic reactivity of faujasite zeolite: A periodic DFT study. J. Catal. 2016, 344, 570–577. [Google Scholar] [CrossRef]
- Gounder, R.; Jones, A.J.; Carr, R.T.; Iglesia, E. Solvation and acid strength effects on catalysis by faujasite zeolites. J. Catal. 2012, 286, 214–223. [Google Scholar] [CrossRef]
- Phadke, N.M.; Mansoor, E.; Bondil, M.; Head-Gordon, M.; Bell, A.T. Mechanism and kinetics of propane dehydrogenation and cracking over Ga/H-MFI prepared via vapor-phase exchange of H-MFI with GaCl3. J. Am. Chem. Soc. 2018, 141, 1614–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phadke, N.M.; Mansoor, E.; Head-Gordon, M.; Bell, A.T. Mechanism and kinetics of light alkane dehydrogenation and cracking over isolated Ga species in Ga/H-MFI. ACS Catal. 2021, 11, 2062–2075. [Google Scholar] [CrossRef]
- Yeh, J.Y.; Li, S.C.; Chen, C.H.; Wu, K.C.W.; Li, Y.P. Quantum mechanical calculations for biomass valorization over Metal-Organic Frameworks (MOFs). Chem. Asian J. 2021, 16, 1049–1056. [Google Scholar] [CrossRef]
- Li, Y.-P.; Gomes, J.; Mallikarjun Sharada, S.; Bell, A.T.; Head-Gordon, M. Improved force-field parameters for QM/MM simulations of the energies of adsorption for molecules in zeolites and a free rotor correction to the rigid rotor harmonic oscillator model for adsorption enthalpies. J. Phys. Chem. C 2015, 119, 1840–1850. [Google Scholar] [CrossRef]
- Li, Y.-P.; Bell, A.T.; Head-Gordon, M. Thermodynamics of anharmonic systems: Uncoupled mode approximations for molecules. J. Chem. Theory Comput. 2016, 12, 2861–2870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghysels, A.; Van Neck, D.; Van Speybroeck, V.; Verstraelen, T.; Waroquier, M. Vibrational modes in partially optimized molecular systems. J. Chem. Phys. 2007, 126, 224102. [Google Scholar] [CrossRef]
- Grimme, S. Supramolecular binding thermodynamics by dispersion-corrected density functional theory. Chem. Eur. J. 2012, 18, 9955–9964. [Google Scholar] [CrossRef] [PubMed]
- Sklenak, S.; Dedecek, J.; Li, C.; Wichterlova, B.; Gábová, V.; Sierka, M.; Sauer, J. Aluminium siting in the ZSM-5 framework by combination of high resolution 27Al NMR and DFT/MM calculations. Phys. Chem. Chem. Phys. 2009, 11, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- First, E.L.; Gounaris, C.; Wei, J.; Floudas, C.A. Computational characterization of zeolite porous networks: An automated approach. Phys. Chem. Chem. Phys. 2011, 13, 17339–17358. [Google Scholar] [CrossRef]
- Bai, P.; Haldoupis, E.; Dauenhauer, P.J.; Tsapatsis, M.; Siepmann, J.I. Understanding diffusion in hierarchical zeolites with house-of-cards nanosheets. ACS Nano 2016, 10, 7612–7618. [Google Scholar] [CrossRef]
- Bu, L.; Nimlos, M.R.; Robichaud, D.J.; Kim, S. Diffusion of aromatic hydrocarbons in hierarchical mesoporous H-ZSM-5 zeolite. Catal. Today 2018, 312, 73–81. [Google Scholar] [CrossRef]
- Josephson, T.R.; Dauenhauer, P.J.; Tsapatsis, M.; Siepmann, J.I. Adsorption of furan, hexanoic acid, and alkanes in a hierarchical zeolite at reaction conditions: Insights from molecular simulations. J. Comput. Sci. 2021, 48, 101267. [Google Scholar] [CrossRef]
- Shetsiri, S.; Thivasasith, A.; Saenluang, K.; Wannapakdee, W.; Salakhum, S.; Wetchasat, P.; Nokbin, S.; Limtrakul, J.; Wattanakit, C. Sustainable production of ethylene from bioethanol over hierarchical ZSM-5 nanosheets. Sustain. Energy Fuels 2018, 3, 115–126. [Google Scholar] [CrossRef]
- Li, Y.-P.; Head-Gordon, M.; Bell, A. Analysis of the reaction mechanism and catalytic activity of metal-substituted beta zeolite for the isomerization of glucose to fructose. ACS Catal. 2014, 4, 1537–1545. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-P.; Head-Gordon, M.; Bell, A.T. Computational study of p-Xylene synthesis from ethylene and 2,5-dimethylfuran catalyzed by H-BEA. J. Phys. Chem. C 2014, 118, 22090–22095. [Google Scholar] [CrossRef]
- Li, Y.-P.; Head-Gordon, M.; Bell, A.T. Theoretical study of 4-(hydroxymethyl) benzoic acid synthesis from ethylene and 5-(hydroxymethyl) furoic acid catalyzed by Sn-BEA. ACS Catal. 2016, 6, 5052–5061. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Shi, B.; Yang, H.; Yan, W. Synthesis of isomorphous MFI nanosheet zeolites for supercritical catalytic cracking of n-dodecane. Appl. Catal. A Gen. 2017, 533, 90–98. [Google Scholar] [CrossRef]
- Qin, F.; Wang, Y.; Lu, Y.; Osuga, R.; Gies, H.; Kondo, J.N.; Yokoi, T. Synthesis of novel aluminoborosilicate isomorphous to zeolite TUN and its acidic and catalytic properties. Microporous Mesoporous Mater. 2021, 323, 111237. [Google Scholar] [CrossRef]
- Yabushita, M.; Osuga, R.; Muramatsu, A. Control of location and distribution of heteroatoms substituted isomorphously in framework of zeolites and zeotype materials. CrystEngComm 2021. [Google Scholar] [CrossRef]
- Yabushita, M.; Kobayashi, H.; Neya, A.; Nakaya, M.; Maki, S.; Matsubara, M.; Kanie, K.; Muramatsu, A. Precise control of density and strength of acid sites of MFI-type zeolite nanoparticles via simultaneous isomorphous substitution by Al and Fe. CrystEngComm 2020, 22, 7556–7564. [Google Scholar] [CrossRef]
- Jones, A.J.; Iglesia, E. The strength of Brønsted acid sites in microporous aluminosilicates. ACS Catal. 2015, 5, 5741–5755. [Google Scholar] [CrossRef]
- Jungsuttiwong, S.; Lomratsiri, J.; Limtrakul, J. Characterization of acidity in [B], [Al], and [Ga] isomorphously substituted ZSM-5: Embedded DFT/UFF approach. Int. J. Quantum Chem. 2011, 111, 2275–2282. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Kijima, N.; Hayakawa, T.; Murata, K.; Suzuki, K.; Mizukami, F.; Matano, K.; Konishi, T.; Oikawa, T.; Saito, M.; et al. Catalytic cracking of naphtha to light olefins. Catal. Surv. Jpn. 2001, 4, 157–167. [Google Scholar] [CrossRef]
- Wakui, K.; Satoh, K.; Sawada, G.; Shiozawa, K.; Matano, K.; Suzuki, K.; Hayakawa, T.; Yoshimura, Y.; Murata, K.; Mizukami, F. Dehydrogenative Cracking of n-butane over modified HZSM-5 catalysts. Catal. Lett. 2002, 81, 83–88. [Google Scholar] [CrossRef]
- Xiaoning, W.; Zhen, Z.; Chunming, X.; Aijun, D.; Li, Z.; Guiyuan, J. Effects of light rare earth on acidity and catalytic per-formance of HZSM-5 zeolite for catalytic cracking of butane to light olefins. J. Rare Earths 2007, 25, 321–328. [Google Scholar] [CrossRef]
- Sun, Y.; Brown, T.C. Catalytic cracking, dehydrogenation, and aromatization of isobutane over Ga/HZSM-5 and Zn/HZSM-5 at low pressures. Int. J. Chem. Kinet. 2002, 34, 467–480. [Google Scholar] [CrossRef]
- Xue, N.; Chen, X.; Nie, L.; Guo, X.; Ding, W.; Chen, Y.; Gu, M.; Xie, Z. Understanding the enhancement of catalytic performance for olefin cracking: Hydrothermally stable acids in P/HZSM-5. J. Catal. 2007, 248, 20–28. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, M.; Sun, L.; Su, H.; Zou, X.; Qi, C. The performance of catalytic conversion of ZSM-5 comodified with gold and lanthanum for increasing propylene production. Ind. Eng. Chem. Res. 2019, 58, 14695–14704. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Long, H.; Sun, L.; Murayama, T.; Qi, C. Insights into Au nanoparticle size and chemical state of Au/ZSM-5 catalyst for catalytic cracking of n-octane to increase propylene production. J. Phys. Chem. C 2021, 125, 29. [Google Scholar] [CrossRef]
- Pidko, E.A.; Hensen, E.J.M.; Van Santen, R.A. Self-organization of extraframework cations in zeolites. Proc. R. Soc. A Math. Phys. Eng. Sci. 2012, 468, 2070–2086. [Google Scholar] [CrossRef]
- Yu, Z.; Zheng, A.; Wang, Q.; Chen, L.; Xu, J.; Amoureux, J.-P.; Deng, F. Insights into the dealumination of zeolite HY revealed by sensitivity-enhanced 27Al DQ-MAS NMR spectroscopy at high field. Angew. Chem. Int. Ed. 2010, 122, 8839–8843. [Google Scholar] [CrossRef]
- Yu, Z.; Li, S.; Wang, Q.; Zheng, A.; Jun, X.; Chen, L.; Deng, F. Brønsted/Lewis acid synergy in H–ZSM-5 and H–MOR zeolites studied by 1H and 27Al DQ-MAS solid-state NMR spectroscopy. J. Phys. Chem. C 2011, 115, 22320–22327. [Google Scholar] [CrossRef]
- Yi, X.; Liu, K.; Chen, W.; Li, J.; Xu, S.; Li, C.; Xiao, Y.; Liu, H.; Guo, X.; Liu, S.-B.; et al. Origin and structural characteristics of tri-coordinated extra-framework aluminum species in dealuminated zeolites. J. Am. Chem. Soc. 2018, 140, 10764–10774. [Google Scholar] [CrossRef] [PubMed]
- Schüßler, F.; Pidko, E.A.; Kolvenbach, R.; Sievers, C.; Hensen, E.J.M.; van Santen, R.A.; Lercher, J.A. Nature and location of cationic lanthanum species in high alumina containing faujasite type zeolites. J. Phys. Chem. C 2011, 115, 21763–21776. [Google Scholar] [CrossRef]
- Zheng, A.; Yi, X.; Xiao, Y.; Liu, Z.; Li, G.; Chen, W.; Liu, S. Observation of Multinuclear Extra-Framework Aluminum Species in Dealuminated Zeolite Catalysts. Research Square. Available online: https://assets.researchsquare.com/files/rs-50498/v1/0ef49198-624e-43a3-a79a-b1d936b0bbca.pdf (accessed on 29 August 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.-C.; Lin, Y.-C.; Li, Y.-P. Understanding the Catalytic Activity of Microporous and Mesoporous Zeolites in Cracking by Experiments and Simulations. Catalysts 2021, 11, 1114. https://doi.org/10.3390/catal11091114
Li S-C, Lin Y-C, Li Y-P. Understanding the Catalytic Activity of Microporous and Mesoporous Zeolites in Cracking by Experiments and Simulations. Catalysts. 2021; 11(9):1114. https://doi.org/10.3390/catal11091114
Chicago/Turabian StyleLi, Shih-Cheng, Yen-Chun Lin, and Yi-Pei Li. 2021. "Understanding the Catalytic Activity of Microporous and Mesoporous Zeolites in Cracking by Experiments and Simulations" Catalysts 11, no. 9: 1114. https://doi.org/10.3390/catal11091114
APA StyleLi, S.-C., Lin, Y.-C., & Li, Y.-P. (2021). Understanding the Catalytic Activity of Microporous and Mesoporous Zeolites in Cracking by Experiments and Simulations. Catalysts, 11(9), 1114. https://doi.org/10.3390/catal11091114






