A Review: Scanning Electrochemical Microscopy (SECM) for Visualizing the Real-Time Local Catalytic Activity
Abstract
1. Introduction
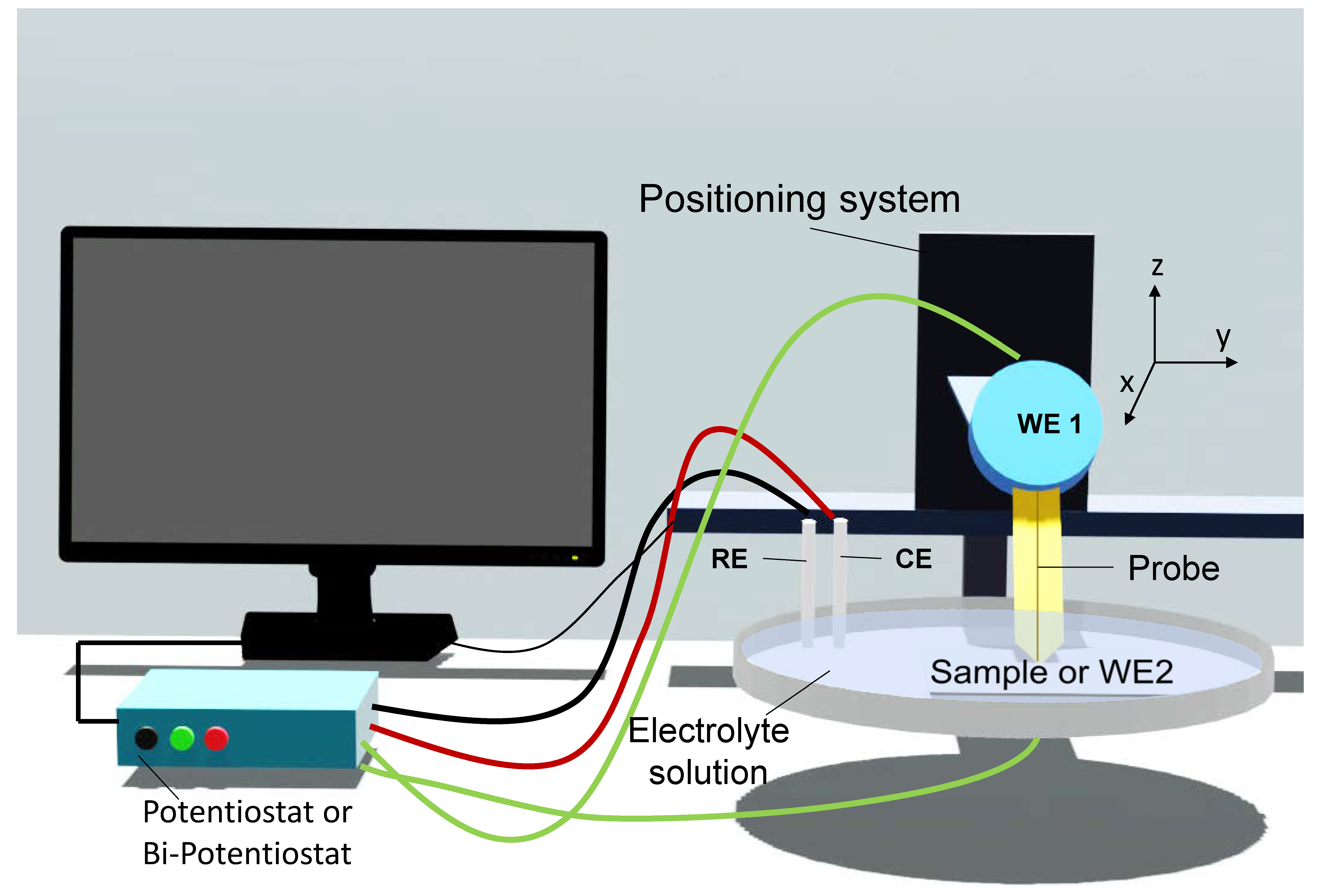
Introduction to SECM
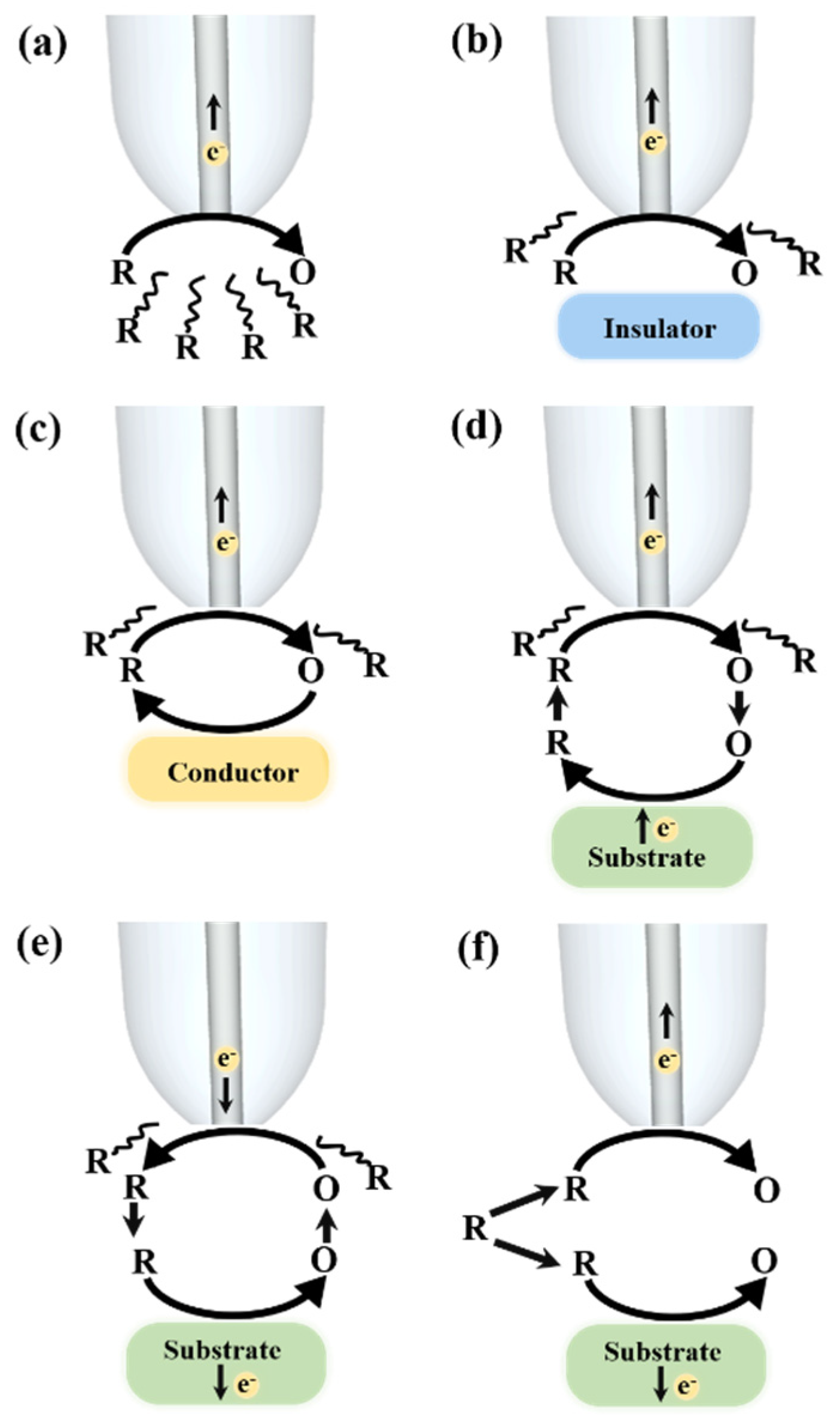
2. SECM Operation Modes
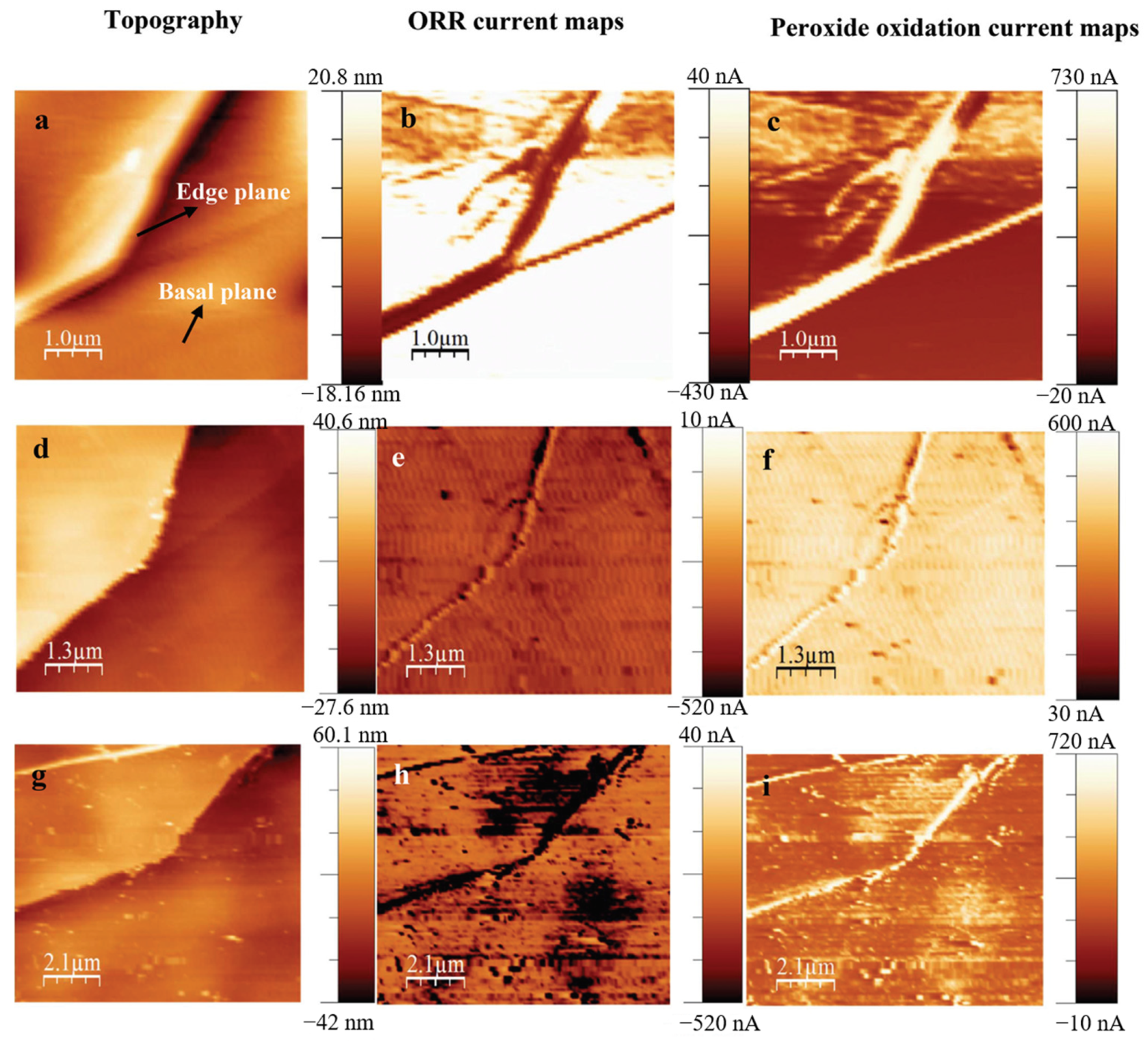
3. The Study of Oxygen Reduction Reaction (ORR) by SECM
4. The Study of Oxygen Evolution Reaction (OER) by SECM
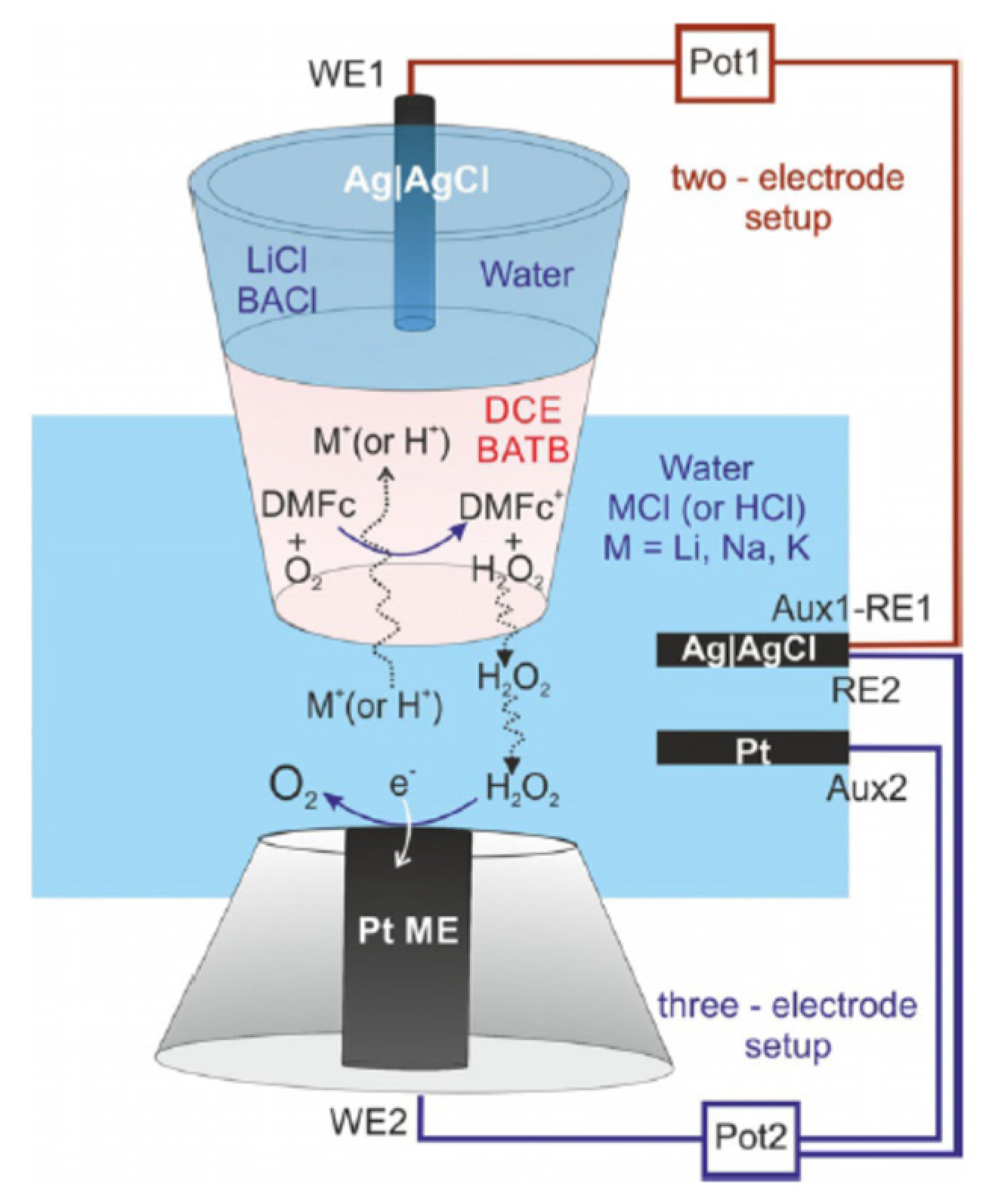
5. The Study of Hydrogen Evolution Reaction (HER) by SECM
6. The Emerging Applications of SECM
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Daviddi, E.; Shkirskiy, V.; Kirkman, P.M.; Robin, M.P.; Bentley, C.L.; Unwin, P.R. Nanoscale electrochemistry in a copper/aqueous/oil three-phase system: Surface structure–activity-corrosion potential relationships. Chem. Sci. 2021, 12, 3055–3069. [Google Scholar] [CrossRef]
- Wang, S.; Yang, X.; Wu, F.; Min, L.; Chen, X.; Hou, X. Inner Surface Design of Functional Microchannels for Microscale Flow Control. Small 2019, 16, e1905318. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhou, W.; Zhang, H.; Zhou, W.; Zhao, X.; Li, Y.; Liu, M.; Cheng, W.; Liu, Q. Dynamic Evolution of Solid–Liquid Electrochemical Interfaces over Single-Atom Active Sites. J. Am. Chem. Soc. 2020, 142, 12306–12313. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Huang, H.; Yan, S.; Luo, W.; Yu, T.; Li, Z.; Zou, Z. Non-oxide semiconductors for artificial photosynthesis: Progress on photoelectrochemical water splitting and carbon dioxide reduction. Nano Today 2020, 30, 100830. [Google Scholar] [CrossRef]
- Suk, M.; Chung, M.W.; Han, M.H.; Oh, H.-S.; Choi, C.H. Selective H2O2 production on surface-oxidized metal-nitrogen-carbon electrocatalysts. Catal. Today 2021, 359, 99–105. [Google Scholar] [CrossRef]
- Von Kurnatowski, M.; Bortz, M. Modeling and Multi-Criteria Optimization of a Process for H2O2 Electrosynthesis. Processes 2021, 9, 399. [Google Scholar] [CrossRef]
- Ye, W.; Guo, X.; Ma, T. A review on electrochemical synthesized copper-based catalysts for electrochemical reduction of CO2 to C2+ products. Chem. Eng. J. 2021, 414, 128825. [Google Scholar] [CrossRef]
- Boutin, E.; Robert, M. Molecular Electrochemical Reduction of CO2 beyond Two Electrons. Trends Chem. 2021, 3, 359–372. [Google Scholar] [CrossRef]
- Sidhureddy, B.; Prins, S.; Wen, J.; Thiruppathi, A.R.; Govindhan, M.; Chen, A. Synthesis and Electrochemical Study of Mesoporous Nickel-Cobalt Oxides for Efficient Oxygen Reduction. ACS Appl. Mater. Interfaces 2019, 11, 18295–18304. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Charge Transfer and Biocompatibility Aspects in Conducting Polymer-Based Enzymatic Biosensors and Biofuel Cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef]
- Limani, N.; Boudet, A.; Blanchard, N.; Jousselme, B.; Cornut, R. Local probe investigation of electrocatalytic activity. Chem. Sci. 2021, 12, 71–98. [Google Scholar] [CrossRef]
- Bertoncello, P. Advances on scanning electrochemical microscopy (SECM) for energy. Energy Environ. Sci. 2010, 3, 1620–1633. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.-K.; Cho, S.K.; Bard, A.J. Tunneling Ultramicroelectrode: Nanoelectrodes and Nanoparticle Collisions. J. Am. Chem. Soc. 2014, 136, 8173–8176. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Pebay, C.; Thouin, L.; Wang, A.; Warkocz, J.-S. Difference between Ultramicroelectrodes and Microelectrodes: Influence of Natural Convection. Anal. Chem. 2010, 82, 6933–6939. [Google Scholar] [CrossRef]
- Liberman, I.; He, W.; Shimoni, R.; Ifraemov, R.; Hod, I. Spatially confined electrochemical conversion of metal-organic frameworks into metal-sulfides and their in situ electrocatalytic investigation via scanning electrochemical microscopy. Chem. Sci. 2019, 11, 180–185. [Google Scholar] [CrossRef]
- Kolagatla, S.; Subramanian, P.; Schechter, A. Nanoscale mapping of catalytic hotspots on Fe, N-modified HOPG by scanning electrochemical microscopy-atomic force microscopy. Nanoscale 2018, 10, 6962–6970. [Google Scholar] [CrossRef]
- Rastgar, S.; Santos, K.T.; Angelucci, C.A.; Wittstock, G. Catalytic Activity of Alkali Metal Cations for the Chemical Oxygen Reduction Reaction in a Biphasic Liquid System Probed by Scanning Electrochemical Microscopy. Chem. A Eur. J. 2020, 26, 10882–10890. [Google Scholar] [CrossRef]
- Yu, Z. In-Situ and Real-Time Monitoring of Oxygen Evolution during Kolbe Reaction by Scanning Electrochemical Microscopy. Int. J. Electrochem. Sci. 2021, 16, 210240. [Google Scholar] [CrossRef]
- Jin, Z.; Bard, A.J. Surface Interrogation of Electrodeposited MnOx and CaMnO3 Perovskites by Scanning Electrochemical Microscopy: Probing Active Sites and Kinetics for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2021, 60, 794–799. [Google Scholar] [CrossRef]
- Kumar, S.; Sahoo, P.K.; Satpati, A.K. Electrochemical and SECM Investigation of MoS2/GO and MoS2/rGO Nanocomposite Materials for HER Electrocatalysis. ACS Omega 2017, 2, 7532–7545. [Google Scholar] [CrossRef]
- Djire, A.; Wang, X.; Xiao, C.; Nwamba, O.C.; Mirkin, M.V.; Neale, N.R. Basal Plane Hydrogen Evolution Activity from Mixed Metal Nitride MXenes Measured by Scanning Electrochemical Microscopy. Adv. Funct. Mater. 2020, 30, 2001136. [Google Scholar] [CrossRef]
- Iffelsberger, C.; Ng, S.; Pumera, M. Catalyst coating of 3D printed structures via electrochemical deposition: Case of the transition metal chalcogenide MoSx for hydrogen evolution reaction. Appl. Mater. Today 2020, 20, 100654. [Google Scholar] [CrossRef]
- Ahn, H.S.; Bard, A.J. Surface Interrogation of CoPi Water Oxidation Catalyst by Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2015, 137, 612–615. [Google Scholar] [CrossRef]
- Soldà, A.; Valenti, G.; Marcaccio, M.; Giorgio, M.; Pelicci, P.G.; Paolucci, F.; Rapino, S. Glucose and Lactate Miniaturized Biosensors for SECM-Based High-Spatial Resolution Analysis: A Comparative Study. ACS Sensors 2017, 2, 1310–1318. [Google Scholar] [CrossRef]
- Jayathilake, N.M.; Koley, D. Glucose Microsensor with Covalently Immobilized Glucose Oxidase for Probing Bacterial Glucose Uptake by Scanning Electrochemical Microscopy. Anal. Chem. 2020, 92, 3589–3597. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.D.; Hosseini-Benhangi, P.; Sánchez-Sánchez, C.M.; Asselin, E.; Gyenge, E.L. Scanning electrochemical microscopy screening of CO2 electroreduction activities and product selectivities of catalyst arrays. Commun. Chem. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- Strange, L.E.; Yadav, J.; Li, X.; Pan, S. Editors’ Choice—Review—Creating Electrocatalytic Heterojunctions for Efficient Photoelectrochemical CO2 Reduction to Chemical Fuels. J. Electrochem. Soc. 2020, 167, 146518. [Google Scholar] [CrossRef]
- Wittstock, G.; Burchardt, M.; Pust, S.E.; Shen, Y.; Zhao, C. Scanning Electrochemical Microscopy for Direct Imaging of Reaction Rates. Angew. Chem. Int. Ed. 2007, 46, 1584–1617. [Google Scholar] [CrossRef]
- Sun, X.; Li, W.; Mi, H.; Li, Y.; Zhang, P.; Ren, X. Nitrogen and sulfur co-doped graphene supported PdW alloys as highly active electrocatalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2018, 43, 5530–5540. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, S.-C.; Lee, C.; Kim, M.H.; Lee, Y. Evolution of silver to a better electrocatalyst: Water-assisted oxygen reduction reaction at silver chloride nanowires in alkaline solution. Nano Energy 2018, 48, 134–143. [Google Scholar] [CrossRef]
- Singh, V.; Tiwari, A.; Nagaiah, T.C. Facet-controlled morphology of cobalt disulfide towards enhanced oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 22545–22554. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, V.; Nagaiah, T.C. Tuning the MnWO4 morphology and its electrocatalytic activity towards oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 2681–2692. [Google Scholar] [CrossRef]
- Michalak, M.; Roguska, A.; Nogala, W.; Opallo, M. Patterning Cu nanostructures tailored for CO2 reduction to electrooxidizable fuels and oxygen reduction in alkaline media. Nanoscale Adv. 2019, 1, 2645–2653. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, P.; Zhu, Z.; Zhang, J.; Cao, F. The study of the H2O2 during oxygen reduction process on typically corroding metal surface using tip generation-substrate collection mode of SECM. Corros. Sci. 2020, 164, 108312. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, V.; Mandal, D.; Nagaiah, T.C. Nitrogen containing carbon spheres as an efficient electrocatalyst for oxygen reduction: Microelectrochemical investigation and visualization. J. Mater. Chem. A 2017, 5, 20014–20023. [Google Scholar] [CrossRef]
- Xin, S.; Liu, Z.; Ma, L.; Sun, Y.; Xiao, C.; Li, F.; Du, Y. Visualization of the electrocatalytic activity of three-dimensional MoSe2@reduced graphene oxide hybrid nanostructures for oxygen reduction reaction. Nano Res. 2016, 9, 3795–3811. [Google Scholar] [CrossRef]
- Dobrzeniecka, A.; Zeradjanin, A.R.; Masa, J.; Blicharska, M.; Wintrich, D.; Kulesza, P.J.; Schuhmann, W. Evaluation of kinetic constants on porous, non-noble catalyst layers for oxygen reduction—A comparative study between SECM and hydrodynamic methods. Catal. Today 2016, 262, 74–81. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, H.; Xin, S.; Xiao, C.; Li, F.; Ding, S. Characterization of local electrocatalytical activity of nanosheet-structured ZnCo2O4/carbon nanotubes composite for oxygen reduction reaction with scanning electrochemical microscopy. Electrochim. Acta 2015, 178, 767–777. [Google Scholar] [CrossRef]
- Kolagatla, S.; Subramanian, P.; Schechter, A. Simultaneous Mapping of Oxygen Reduction Activity and Hydrogen Peroxide Generation on Electrocatalytic Surfaces. ChemSusChem 2019, 12, 2708–2714. [Google Scholar] [CrossRef]
- O’Connell, M.A.; Lewis, J.R.; Wain, A.J. Electrochemical imaging of hydrogen peroxide generation at individual gold nanoparticles. Chem. Commun. 2015, 51, 10314–10317. [Google Scholar] [CrossRef]
- Konkena, B.; Masa, J.; Botz, A.J.R.; Sinev, I.; Xia, W.; Koßmann, J.; Drautz, R.; Muhler, M.; Schuhmann, W. Metallic NiPS3@NiOOH Core–Shell Heterostructures as Highly Efficient and Stable Electrocatalyst for the Oxygen Evolution Reaction. ACS Catal. 2017, 7, 229–237. [Google Scholar] [CrossRef]
- Steimecke, M.; Seiffarth, G.; Bron, M. In Situ Characterization of Ni and Ni/Fe Thin Film Electrodes for Oxygen Evolution in Alkaline Media by a Raman-Coupled Scanning Electrochemical Microscope Setup. Anal. Chem. 2017, 89, 10679–10686. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, S.; Mukherjee, A.; Su, W.-N.; Basu, S. Improved bi-functional ORR and OER catalytic activity of reduced graphene oxide supported ZnCo2O4 microsphere. Int. J. Hydrogen Energy 2019, 44, 1565–1578. [Google Scholar] [CrossRef]
- Barwe, S.; Andronescu, C.; Engels, R.; Conzuelo, F.; Seisel, S.; Wilde, P.; Chen, Y.-T.; Masa, J.; Schuhmann, W. Cobalt metalloid and polybenzoxazine derived composites for bifunctional oxygen electrocatalysis. Electrochim. Acta 2019, 297, 1042–1051. [Google Scholar] [CrossRef]
- Tavakkoli, M.; Flahaut, E.; Peljo, P.; Sainio, J.; Davodi, F.; Lobiak, E.V.; Mustonen, K.; Kauppinen, E.I. Mesoporous Single-Atom-Doped Graphene–Carbon Nanotube Hybrid: Synthesis and Tunable Electrocatalytic Activity for Oxygen Evolution and Reduction Reactions. ACS Catal. 2020, 10, 4647–4658. [Google Scholar] [CrossRef]
- Jasion, D.; Barforoush, J.M.; Qiao, Q.; Zhu, Y.; Ren, S.; Leonard, K.C. Low-Dimensional Hyperthin FeS2 Nanostructures for Efficient and Stable Hydrogen Evolution Electrocatalysis. ACS Catal. 2015, 5, 6653–6657. [Google Scholar] [CrossRef]
- Xiaolin, Z.; Du, M.; Mleczko, M.J.; Koh, A.L.; Nishi, Y.; Pop, E.; Bard, A.J.; Zheng, X. Kinetic Study of Hydrogen Evolution Reaction over Strained MoS2 with Sulfur Vacancies Using Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2016, 138, 5123–5129. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, H.; Wang, X.; Liu, J.; Xiao, C.; Nanayakkara, S.U.; Blackburn, J.L.; Mirkin, M.V.; Miller, E.M. Nanoscale mapping of hydrogen evolution on metallic and semiconducting MoS2 nanosheets. Nanoscale Horiz. 2018, 4, 619–624. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Sun, T.; Huang, J.; Zhang, W.; Wang, Q.; Cao, R. Karst landform-featured monolithic electrode for water electrolysis in neutral media. Energy Environ. Sci. 2020, 13, 174–182. [Google Scholar] [CrossRef]
- Jedraszko, J.; Adamiak, W.; Nogala, W.; Girault, H.H.; Opallo, M. SECM study of hydrogen photogeneration in a 1,2-dichloroethane | water biphasic system with decamethylruthenocene electron donor regeneration. J. Electroanal. Chem. 2018, 819, 101–106. [Google Scholar] [CrossRef]
- Arrocha-Arcos, A.; Cervantes-Alcalá, R.; Huerta-Miranda, G.; Miranda-Hernández, M. Electrochemical reduction of Bicarbonate to Formate with Silver Nanoparticles and Silver Nanoclusters supported on Multiwalled Carbon Nanotubes. Electrochim. Acta 2017, 246, 1082–1087. [Google Scholar] [CrossRef]
- Kai, T.; Zhou, M.; Duan, Z.; Henkelman, G.A.; Bard, A.J. Detection of CO2•– in the Electrochemical Reduction of Carbon Dioxide in N,N-Dimethylformamide by Scanning Electrochemical Microscopy. J. Am. Chem. Soc. 2017, 139, 18552–18557. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Darvishi, S.; Preet, A.; Huang, T.-Y.; Lin, S.-H.; Girault, H.H.; Wang, L.; Lin, T.-E. A Review: Electrochemical Biosensors for Oral Cancer. Chemosensors 2020, 8, 54. [Google Scholar] [CrossRef]
- Lin, T.-E.; Rapino, S.; Girault, H.H.; Lesch, A. Electrochemical imaging of cells and tissues. Chem. Sci. 2018, 9, 4546–4554. [Google Scholar] [CrossRef] [PubMed]
- Polcari, D.; Dauphin-Ducharme, P.; Mauzeroll, J. Scanning Electrochemical Microscopy: A Comprehensive Review of Experimental Parameters from 1989 to 2015. Chem. Rev. 2016, 116, 13234–13278. [Google Scholar] [CrossRef]
- Izquierdo, J.; Knittel, P.; Kranz, C. Scanning electrochemical microscopy: An analytical perspective. Anal. Bioanal. Chem. 2017, 410, 307–324. [Google Scholar] [CrossRef]
- Lin, T.-E.; Lesch, A.; Li, C.-L.; Girault, H.H. Mapping the antioxidant activity of apple peels with soft probe scanning electrochemical microscopy. J. Electroanal. Chem. 2017, 786, 120–128. [Google Scholar] [CrossRef]
- Bondarenko, A.; Lin, T.-E.; Stupar, P.; Lesch, A.; Cortés-Salazar, F.; Girault, H.H.; Pick, H.M. Fixation and Permeabilization Approaches for Scanning Electrochemical Microscopy of Living Cells. Anal. Chem. 2016, 88, 11436–11443. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-E.; Cortés-Salazar, F.; Lesch, A.; Qiao, L.; Bondarenko, A.; Girault, H.H. Multiple scanning electrochemical microscopy mapping of tyrosinase in micro-contact printed fruit samples on polyvinylidene fluoride membrane. Electrochim. Acta 2015, 179, 57–64. [Google Scholar] [CrossRef]
- Darvishi, S.; Pick, H.M.; Lin, T.-E.; Zhu, Y.; Li, X.; Ho, P.-C.; Girault, H.H.; Lesch, A. Tape-Stripping Electrochemical Detection of Melanoma. Anal. Chem. 2019, 91, 12900–12908. [Google Scholar] [CrossRef]
- Salomo, M.; Pust, S.E.; Wittstock, G.; Oesterschulze, E. Integrated cantilever probes for SECM/AFM characterization of surfaces. Microelectron. Eng. 2010, 87, 1537–1539. [Google Scholar] [CrossRef]
- Chen, C.C.; Zhou, Y.; Baker, L.A. Scanning ion conductance microscopy. Ann. Rev. Anal. Chem. 2012, 5, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kumatani, A.; Shiku, H.; Matsue, T. Scanning Probe Microscopy for Nanoscale Electrochemical Imaging. Anal. Chem. 2016, 89, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-E.; Bondarenko, A.; Lesch, A.; Pick, H.; Cortés-Salazar, F.; Girault, H.H. Monitoring Tyrosinase Expression in Non-metastatic and Metastatic Melanoma Tissues by Scanning Electrochemical Microscopy. Angew. Chem. Int. Ed. 2016, 55, 3813–3816. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-E.; Lu, Y.-J.; Sun, C.-L.; Pick, H.; Chen, J.-P.; Lesch, A.; Girault, H.H. Soft Electrochemical Probes for Mapping the Distribution of Biomarkers and Injected Nanomaterials in Animal and Human Tissues. Angew. Chem. Int. Ed. 2017, 56, 16498–16502. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, Z.; Lou, Y.; Cao, F.; Zhang, D.; Li, X. Recent Advances in Scanning Electrochemical Microscopy for Biological Applications. Materials 2018, 11, 1389. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Küngas, R.; Lin, T.-E.; Diethelm, S.; Maréchal, F.; Van Herle, J. Power-to-fuels via solid-oxide electrolyzer: Operating window and techno-economics. Renew. Sustain. Energy Rev. 2019, 110, 174–187. [Google Scholar] [CrossRef]
- Ma, S.; Lin, M.; Lin, T.-E.; Lan, T.; Liao, X.; Maréchal, F.; Van Herle, J.; Yang, Y.; Dong, C.; Wang, L. Fuel cell-battery hybrid systems for mobility and off-grid applications: A review. Renew. Sustain. Energy Rev. 2021, 135, 110119. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Pérez-Fortes, M.; Aubin, P.; Lin, T.-E.; Yang, Y.; Maréchal, F.; Van Herle, J. Reversible solid-oxide cell stack based power-to-x-to-power systems: Comparison of thermodynamic performance. Appl. Energy 2020, 275, 115330. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Qu, Y.; Yuan, T.; Wang, W.; Wu, Y.; Li, Y. Review of Metal Catalysts for Oxygen Reduction Reaction: From Nanoscale Engineering to Atomic Design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. NPJ Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Fernández, J.L.; Walsh, D.A.; Bard, A.J. Thermodynamic Guidelines for the Design of Bimetallic Catalysts for Oxygen Electroreduction and Rapid Screening by Scanning Electrochemical Microscopy. M−Co (M: Pd, Ag, Au). J. Am. Chem. Soc. 2005, 127, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Eckhard, K.; Schuhmann, W. Localised visualisation of O2 consumption and H2O2 formation by means of SECM for the characterisation of fuel cell catalyst activity. Electrochim. Acta 2007, 53, 1164–1169. [Google Scholar] [CrossRef]
- Nagaiah, T.C.; Maljusch, A.; Chen, X.; Bron, M.; Schuhmann, W. Visualization of the Local Catalytic Activity of Electrodeposited Pt-Ag Catalysts for Oxygen Reduction by means of SECM. ChemPhysChem 2009, 10, 2711–2718. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, Z.; Duan, X.; Huang, Y. Nanoscale Structure Design for High-Performance Pt-Based ORR Catalysts. Adv. Mater. 2019, 31, e1802234. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.L.; Mano, N.; Heller, A.; Bard, A.J. Optimization Of “Wired” Enzyme O2-Electroreduction Catalyst Compositions by Scanning Electrochemical Microscopy. Angew. Chem. Int. Ed. 2004, 43, 6355–6357. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, A.; Zaharieva, I.; Dau, H.; Strasser, P. Electrochemical water splitting by layered and 3D cross-linked manganese oxides: Correlating structural motifs and catalytic activity. Energy Environ. Sci. 2013, 6, 2745–2755. [Google Scholar] [CrossRef]
- Li, P.; Jin, Z.; Qian, Y.; Fang, Z.; Xiao, D.; Yu, G. Supramolecular confinement of single Cu atoms in hydrogel frameworks for oxygen reduction electrocatalysis with high atom utilization. Mater. Today 2020, 35, 78–86. [Google Scholar] [CrossRef]
- Sun, T.; Yu, Y.; Zacher, B.J.; Mirkin, M.V. Scanning Electrochemical Microscopy of Individual Catalytic Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 14120–14123. [Google Scholar] [CrossRef] [PubMed]
- Kromer, M.L.; Simpson, B.H.; Rodríguez-López, J. Evaluating the impact of catalyst selection and semiconductor band edge on the photoelectrochemical production of H2O2 via a real-time in situ probe. J. Electroanal. Chem. 2020, 875, 114677. [Google Scholar] [CrossRef]
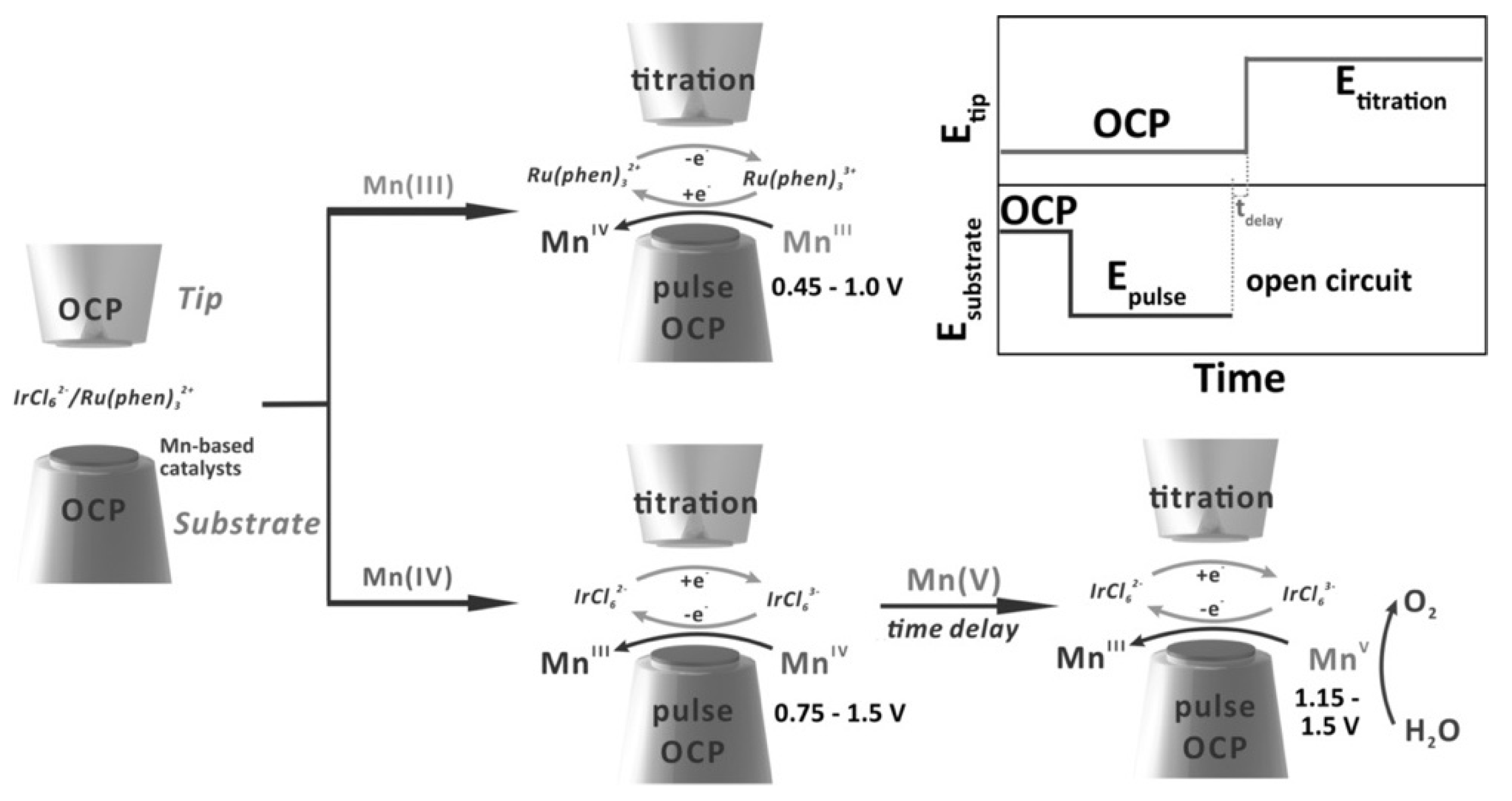
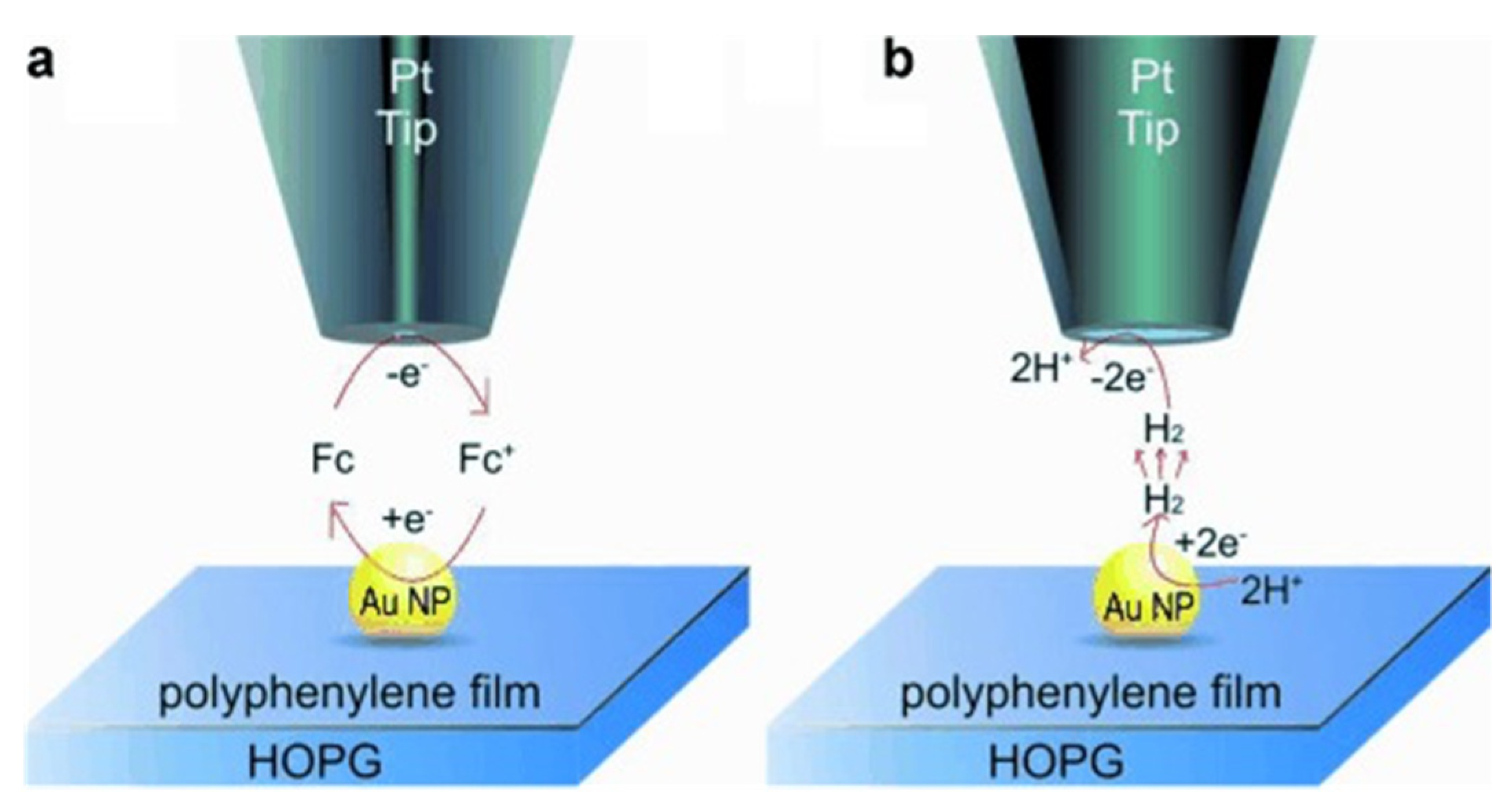
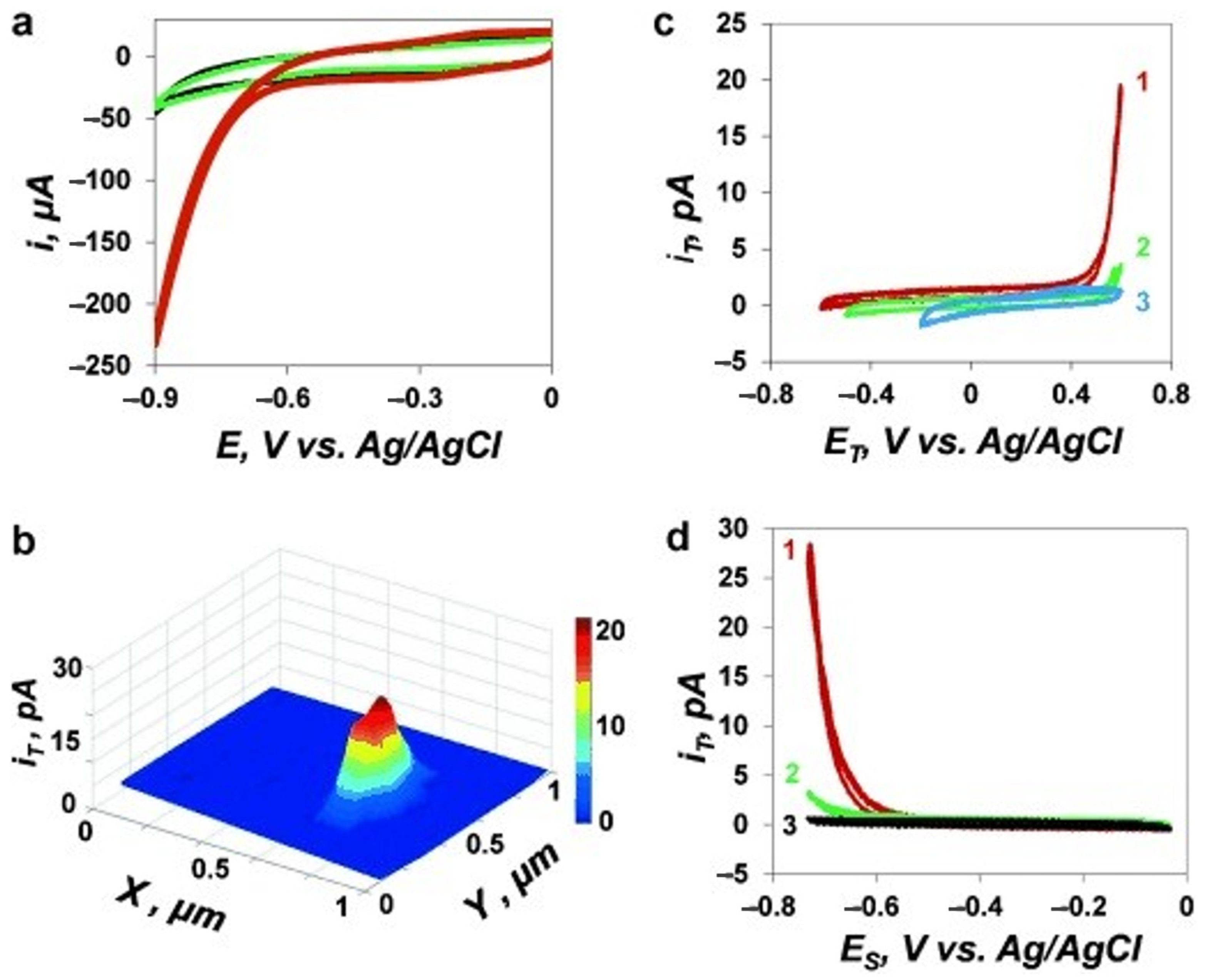
| Instrumentation | Material of the Probe | Mode | Catalysts | Catalytic Reaction | References |
|---|---|---|---|---|---|
| SECM | Pt | RC | PdW nanoparticles supported on nitrogen and sulfur co-doped graphene (NSG) | ORR | [29] |
| SECM | Au | SG/TC | Nanowires of silver chloride and bromide (AgClNWs and AgBrNWs) | ORR | [30] |
| SECM | Pt | RC | Octahedral cobalt sulfide (CoS2) | ORR | [31] |
| SECM | Pt | RC | Manganese tungstate (MnWO4) | ORR | [32] |
| AFM-SECM | Pt | SG/TC; Direct mode | Fe–N doped planar graphite | ORR; H2O2 production | [16] |
| SECM | Pt | SG/TC | nickel and cobalt-based oxides (NiO, Co3O4 and NixCo3-xO4) | HO2− production in ORR | [9] |
| SECM | Au | FB | Copper nanostructures (CuNSs) | ORR and CO2 reduction | [33] |
| SECM | Cu and Fe | TG/SC | Copper | ORR; HER; CO2RR | [34] |
| SECM | Pt | SG/TC; RC | Nitrogen-bearing carbon spheres (NCSs) | ORR; H2O2 production | [35] |
| SECM | Pt | RC | Nanostructured hybrids based on MoSe2 on reduced graphene oxide (rGO) nanosheets | ORR | [36] |
| SECM | Pt | SG/TC; TG/SC; RC | Multiwalled Carbon nanotubes (MWCNTs) with cobalt(IX) protoporphyrin (MWCNTs/CoP) | ORR; H2O2 production | [37] |
| SECM | Pt | RC | ZnCo2O4 on carbon nanotubes (ZnCo2O4/CNTs) and Pt/C | ORR | [38] |
| AFM-SECM | Au-c-Pt tip | SG/TC | Platinum nanoparticles (Pt NPs) | ORR; H2O2 production | [39] |
| SECM-SICM | Pt coated pyrolytic carbon | SG/TC; RC | Gold nanoparticles (Au NPs) | ORR; H2O2 generation | [40] |
| Raman-SECM | Glassy carbon | SG/TC | Lithium intercalated nickel phosphorus trisulfide (NiPS3) | OER | [41] |
| SI-SECM | Au | FB | CoPi nanosheets | Water oxidation, OER | [23] |
| Raman-SECM | Pt | SG/TC | Ni/Fe and Ni thin films | OER | [42] |
| SECM | Pt | SG/TC | reduced graphene oxide supported ZnCo2O4 microsphere (rGO-ZnCo2O4) | ORR; OER | [43] |
| SECM | Pt | SG/TC | Cobalt-based metalloids (CoxB and CoxP) composites | ORR, OER; | [44] |
| SECM | Pt | SG/TC | Mesoporous single-atom-doped graphene–carbon nanotube hybrid | ORR; OER | [45] |
| SECM | Pt | SG/TC; FB | MXenes (2D early transition metal carbide) | HER | [21] |
| SECM | Pt | SG/TC | Iron sulfide (FeS2) nanostructures (1D, wires and 2D, discs) | HER | [46] |
| SECM | Pt | SG/TC | Metal-organic frameworks (CoSx MOFs) | HER | [15] |
| SECM | Pt | SG/TC | Sulfur vacancies on Molybdenum disulfide (SV-MoS2) | HER | [47] |
| SECM | Pt | FB; SG/TC | Mixed-phase MoS2 nanosheets | HER | [48] |
| SECM | Pt | SG/TC | Ni and Ni/α-Ni(OH)2 heterostructures | HER, OER | [49] |
| SECM | Pt | SG/TC | Decamethylruthenocene (DMRc) in 1,2-dichloroethane/water (DCE|W) biphasic system | HER | [50] |
| SECM | Carbon; Pt | FB; SG/TC | Silver nanoparticles (Ag NPs) and silver nanoclusters (Ag NCs) on multi-wall carbon nanotubes (MWCNT) | Bicarbonate reduction | [51] |
| SECM | Hg/Pt | TG/SC | Au metal surface | Intermediate of CO2 reduction | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preet, A.; Lin, T.-E. A Review: Scanning Electrochemical Microscopy (SECM) for Visualizing the Real-Time Local Catalytic Activity. Catalysts 2021, 11, 594. https://doi.org/10.3390/catal11050594
Preet A, Lin T-E. A Review: Scanning Electrochemical Microscopy (SECM) for Visualizing the Real-Time Local Catalytic Activity. Catalysts. 2021; 11(5):594. https://doi.org/10.3390/catal11050594
Chicago/Turabian StylePreet, Anant, and Tzu-En Lin. 2021. "A Review: Scanning Electrochemical Microscopy (SECM) for Visualizing the Real-Time Local Catalytic Activity" Catalysts 11, no. 5: 594. https://doi.org/10.3390/catal11050594
APA StylePreet, A., & Lin, T.-E. (2021). A Review: Scanning Electrochemical Microscopy (SECM) for Visualizing the Real-Time Local Catalytic Activity. Catalysts, 11(5), 594. https://doi.org/10.3390/catal11050594







