A Review: Photocatalysts Based on BiOCl and g-C3N4 for Water Purification
Abstract
:1. Introduction
2. Synthesis of g-C3N4/BiOCl Heterojunction
2.1. Hydrothermal Method
2.2. Deposition-Precipitation Method
| Catalyst (Mass Ratio %) | Template | Morphology | Size | BET Surface Area | Year | Ref. |
|---|---|---|---|---|---|---|
| BiOCl/g-C3N4 (97/3) | - | Hierarchical flowerlike | 0.15 μm/10 nm | 19.04 | 2014 | [6] |
| g-C3N4/BiOCl (20/80) | - | Nanoplate | 1 μm | - | 2014 | [21] |
| C3N4/BiOCl (20/80) | Arabic gum | Flower-like | 200 nm/5–8 nm | 49.37 | 2014 | [34] |
| ng-CN/BOC-010 (70/30) | - | Nanoparticle- nanosheet | - | 18.10 | 2015 | [61] |
| (OV)BiOCl/g-C3N4-10 | - | Flower-like | 2 μm/33.7 nm | 11.66 | 2020 | [62] |
| BiOCl/g-C3N4 (10/90) | - | Sheet-like+ microplate | 51.8 nm | - | 2019 | [63] |
| g-C3N4 /BiOCl (55/45) | - | Hierarchical flower-like | 1 μm/10 nm | 44.2 | 2017 | [65] |
| BiOCl-g-C3N4 | - | Two-dimensional structure | 10 nm | - | 2014 | [66] |
| BiOCl-g-C3N4 (50/50) | CTAC | Wrinkle two-dimensional structure | 10 nm | - | 2014 | [67] |
| g-C3N4/BiOCl (20/80) | - | Nanoplate + sheets | 1 μm | - | 2015 | [68] |
| BiOCl/(0.1g)g-C3N4 | - | nanosheet | 20 nm | 6.60 | 2017 | [69] |
2.3. Solvent-Thermal Method
2.4. Calcination Method
3. Applications of g-C3N4/BiOCl Heterojunction
3.1. Dye Degradation
3.2. Other Applications
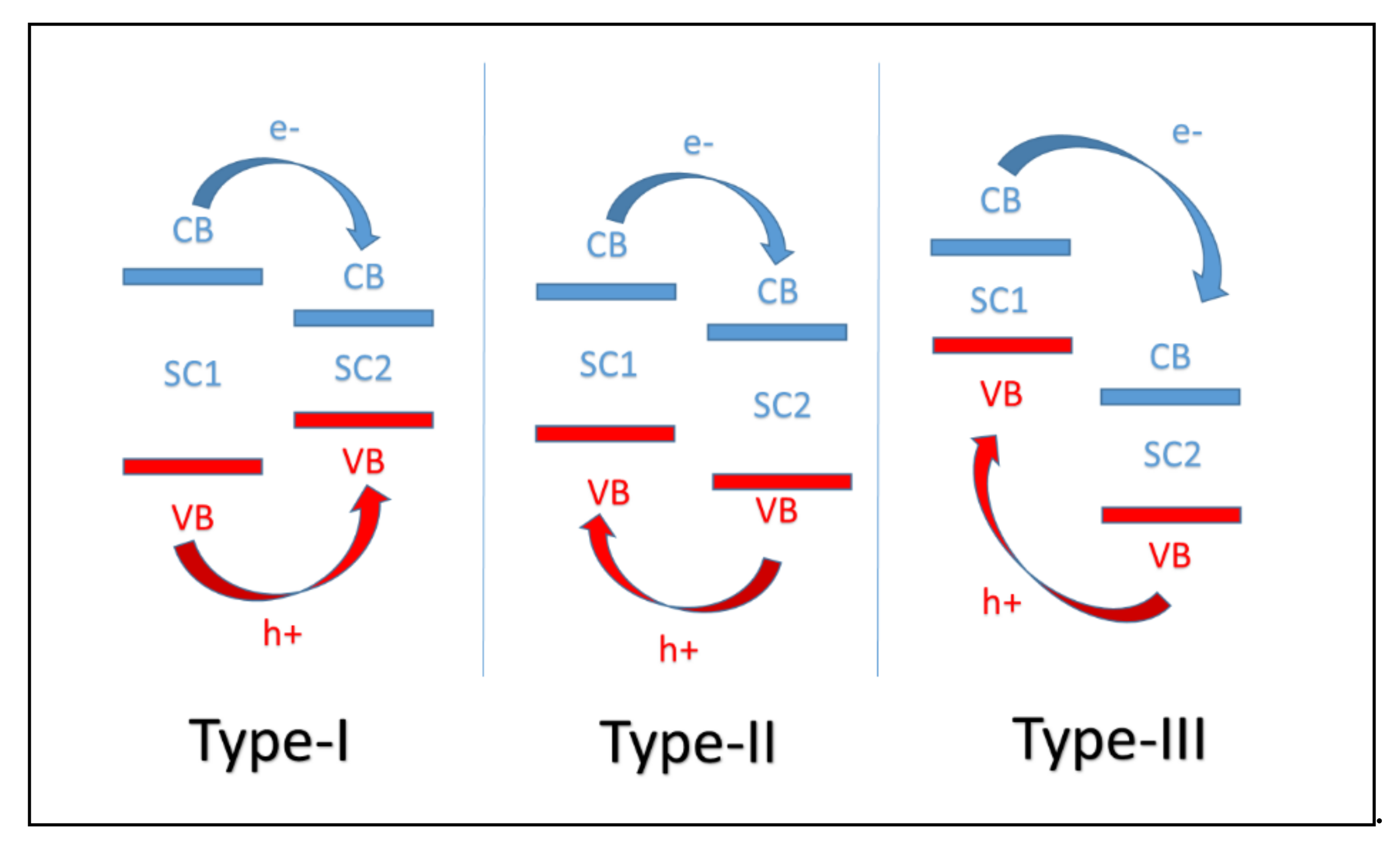
4. Mechanisms of the BiOCl/g-C3N4 Heterojunctions
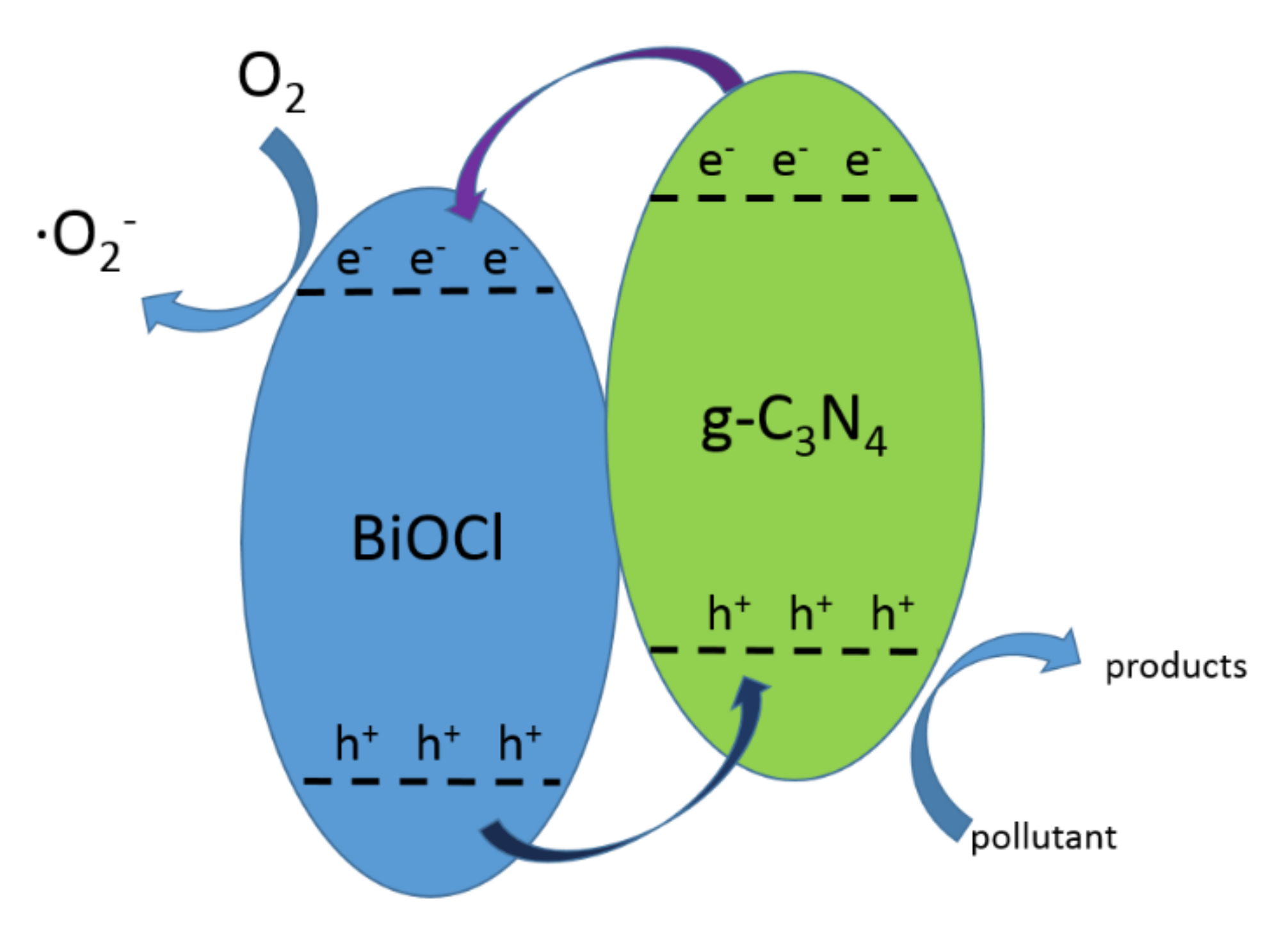
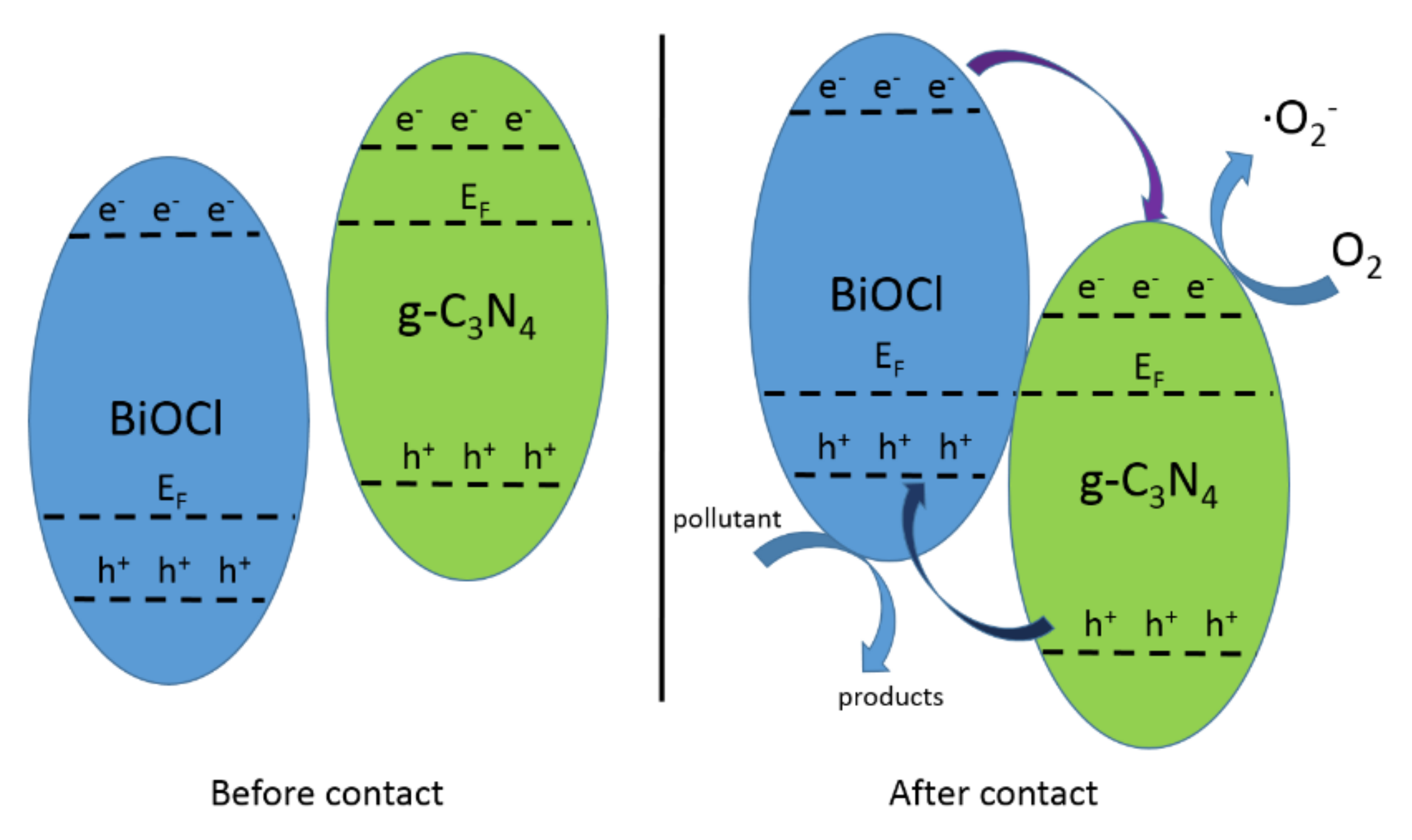
4.1. CNB Heterojunction
4.2. PCNB Heterojunction
4.3. Z-Scheme Heterojunction
5. Other Methods to Improve Photoactivity of Catalysts Based on CN and BOC
6. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Pan, M.; Wong, C.K.C.; Chu, L.M. Distribution of Antibiotics in Wastewater-Irrigated Soils and Their Accumulation in Vegetable Crops in the Pearl River Delta, Southern China. J. Agric. Food Chem. 2014, 62, 11062–11069. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Anquandah, G.A.K.; Yngard, R.A.; Kim, H.; Fekete, J.; Bouzek, K.; Ray, A.K.; Golovko, D. Nonylphenol, octylphenol, and bisphenol-A in the aquatic environment: A review on occurrence, fate, and treatment. J. Environ. Sci. Health Part A 2009, 44, 423–442. [Google Scholar] [CrossRef]
- Yesilada, O.; Asma, D.; Cing, S. Decolorization of textile dyes by fungal pellets. Process. Biochem. 2003, 38, 933–938. [Google Scholar] [CrossRef]
- Ahern, J.; Fairchild, R.; Thomas, J.S.; Carr, J.; Patterson, H.H. Characterization of BiOX compounds as photocatalysts for the degradation of pharmaceuticals in water. Appl. Catal. B Environ. 2015, 179, 229–238. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Zhang, L.; Zhou, L.; Yin, W.; Shang, M. Visible Light-Induced Efficient Contaminant Removal by Bi5O7I. Environ. Sci. Technol. 2009, 43, 2005–2010. [Google Scholar] [CrossRef]
- Chang, F.; Xie, Y.; Zhang, J.; Chen, J.; Li, C.; Wang, J.; Luo, J.; Deng, B.; Hu, X. Construction of exfoliated g-C3N4 nanosheets–BiOCl hybrids with enhanced photocatalytic performance. RSC Adv. 2014, 4, 28519–28528. [Google Scholar] [CrossRef]
- Zhou, C.; Lai, C.; Xu, P.; Zeng, G.; Huang, D.; Li, Z.; Zhang, C.; Cheng, M.; Hu, L.; Wan, J.; et al. Rational Design of Carbon-Doped Carbon Nitride/Bi12O17Cl2 Composites: A Promising Candidate Photocatalyst for Boosting Visible-Light-Driven Photocatalytic Degradation of Tetracycline. ACS Sustain. Chem. Eng. 2018, 6, 6941–6949. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Cheng, M.; Wang, J.; Chen, F.; Zhou, C.; Xiong, W. BiOX (X = Cl, Br, I) photocatalytic nanomaterials: Applications for fuels and environmental management. Adv. Colloid Interface Sci. 2018, 254, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Ke, J.; Zhang, J.; Tian, W.; Xu, X.; Duan, X.; Sun, P.H.; Tade, M.; Wang, S. 0D (MoS2)/2D (g-C3N4) heterojunctions in Z-scheme for enhanced photocatalytic and electrochemical hydrogen evolution. Appl. Catal. B Environ. 2018, 228, 64–74. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Li, M.; Shi, Y.; Wen, H. 2D/2D Z-scheme heterojunction of CuInS2/g-C3N4 for enhanced visible-light-driven photocatalytic activity towards the degradation of tetracycline. Sep. Purif. Technol. 2019, 210, 608–615. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, D.; Pu, X.; Yao, X.; Han, R.; Yin, J.; Ren, X. A novel Ag2O/g-C3N4 p-n heterojunction photocatalysts with enhanced visible and near-infrared light activity. Sep. Purif. Technol. 2019, 210, 786–797. [Google Scholar] [CrossRef]
- Liu, G.; Wang, G.; Hu, Z.; Su, Y.; Zhao, L. Ag2O nanoparticles decorated TiO2 nanofibers as a p-n heterojunction for enhanced photocatalytic decomposition of RhB under visible light irradiation. Appl. Surf. Sci. 2019, 465, 902–910. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Wang, H.; Han, M.; Guan, W.; Huang, H.; Liu, Y.; Kang, Z. Study on highly enhanced photocatalytic tetracycline degradation of type II AgI/CuBi2O4 and Z-scheme AgBr/CuBi2O4 heterojunction photocatalysts. J. Hazard. Mater. 2018, 349, 111–118. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L.; Liu, X.; Yi, H.; Cheng, M.; Li, L.; et al. Fabrication of CuS/BiVO4 (0 4 0) binary heterojunction photocatalysts with enhanced photocatalytic activity for Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2019, 358, 891–902. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, L.; Jiang, B.; Zheng, J.; Hu, P.; Li, S.; Wu, M.; Wu, W. Fabrication of Z-scheme Ag3PO4/MoS2 composites with enhanced photocatalytic activity and stability for organic pollutant degradation. Appl. Surf. Sci. 2016, 377, 99–108. [Google Scholar] [CrossRef]
- Hao, Q.; Niu, X.; Nie, C.; Hao, S.; Zou, W.; Ge, J.; Chen, D.; Yao, W. A highly efficient g-C3N4/SiO2 heterojunction: The role of SiO2 in the enhancement of visible light photocatalytic activity. Phys. Chem. Chem. Phys. 2016, 18, 31410–31418. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Huang, C.; Doong, R.-A. Photocatalytic degradation of bisphenol A over a ZnFe2O4/TiO2 nanocomposite under visible light. Sci. Total Environ. 2019, 646, 745–756. [Google Scholar] [CrossRef]
- Luo, J.; Li, R.; Chen, Y.; Zhou, X.; Ning, X.; Zhan, L.; Ma, L.; Xu, X.; Xu, L.; Zhang, L. Rational design of Z-scheme LaFeO3/SnS2 hybrid with boosted visible light photocatalytic activity towards tetracycline degradation. Sep. Purif. Technol. 2019, 210, 417–430. [Google Scholar] [CrossRef]
- He, R.; Zhou, J.; Fu, H.; Zhang, S.; Jiang, C. Room-temperature in situ fabrication of Bi2O3/g-C3N4 direct Z-scheme photocatalyst with enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 430, 273–282. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, G.; Hojamberdiev, M.; Gao, J.; Zhu, R.; Wang, C.; Wei, X.; Liu, P. Three-dimensional Ag2O/Bi5O7I p–n heterojunction photocatalyst harnessing UV–vis–NIR broad spectrum for photodegradation of organic pollutants. J. Hazard. Mater. 2018, 344, 42–54. [Google Scholar] [CrossRef]
- Shi, S.; Gondal, M.; Al-Saadi, A.; Fajgar, R.; Kupcik, J.; Chang, X.; Shen, K.; Xu, Q.; Seddigi, Z. Facile preparation of g-C3N4 modified BiOCl hybrid photocatalyst and vital role of frontier orbital energy levels of model compounds in photoactivity enhancement. J. Colloid Interface Sci. 2014, 416, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Shuai, D.; Naraginti, S.; Wang, D.; Zhang, W. Visible-light-driven photocatalytic inactivation of MS2 by metal-free g-C3N4: Virucidal performance and mechanism. Water Res. 2016, 106, 249–258. [Google Scholar] [CrossRef]
- Goettmann, F.; Fischer, A.; Antonietti, M.; Thomas, A. Metal-free catalysis of sustainable Friedel–Crafts reactions: Direct activation of benzene by carbon nitrides to avoid the use of metal chlorides and halogenated compounds. Chem. Commun. 2006, 4530–4532. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S.Z. Graphitic carbon nitride materials: Controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731. [Google Scholar] [CrossRef]
- Pawar, R.; Kang, S.; Ahn, S.H.; Lee, C.S. Gold nanoparticle modified graphitic carbon nitride/multi-walled carbon nanotube (g-C3N4/CNTs/Au) hybrid photocatalysts for effective water splitting and degradation. RSC Adv. 2015, 5, 24281–24292. [Google Scholar] [CrossRef]
- Lan, M.; Fan, G.; Yang, L.; Li, F. Enhanced visible-light-induced photocatalytic performance of a novel ternary semiconductor coupling system based on hybrid Zn–In mixed metal oxide/g-C3N4 composites. RSC Adv. 2014, 5, 5725–5734. [Google Scholar] [CrossRef]
- Huang, L.; Xu, H.; Li, Y.; Li, H.; Cheng, X.; Xia, J.; Xu, Y.; Cai, G. Visible-light-induced WO3/g-C3N4 composites with enhanced photocatalytic activity. Dalton Trans. 2013, 42, 8606–8616. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Muhammad, S.; He, J. Graphite-like C3N4 hybridized ZnWO4 nanorods: Synthesis and its enhanced photocatalysis in visible light. CrystEngComm 2012, 14, 5065–5070. [Google Scholar] [CrossRef]
- Xing, C.; Wu, Z.; Jiang, D.; Chen, M. Hydrothermal synthesis of In2S3/g-C3N4 heterojunctions with enhanced photocatalytic activity. J. Colloid Interface Sci. 2014, 433, 9–15. [Google Scholar] [CrossRef]
- Han, G.; Li, D.Y.; Zheng, Y.F.; Song, X.C. Enhanced Visible-Light-Responsive Photocatalytic Properties of Bi2MoO6-BiOCl Nanoplate Composites. J. Nanosci. Nanotechnol. 2018, 18, 5575–5581. [Google Scholar] [CrossRef]
- Lei, L.; Gao, D.; Jin, H.; Zhang, Q.; Xu, J.; Fu, Z. A novel enhanced visible-light-driven photocatalyst via hybridization of nanosized BiOCl and graphitic C3N4. Dalton Trans. 2015, 44, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Meneses, E.; Valencia-Barrón, J.P.; Hernández-Pérez, M.A.; Domínguez-Crespo, M.A.; Torres, A.; Palacios, E. Synthesis and Characterization of BiOCl Powders with Soft Templates. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2350–2364. [Google Scholar] [CrossRef]
- Lin, W.; Yu, X.; Shen, Y.; Chen, H.; Zhu, Y.; Zhang, Y.; Meng, H. Carbon dots/BiOCl films with enhanced visible light photocatalytic performance. J. Nanoparticle Res. 2017, 19, 56. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, R.; Khanuja, M. A review and recent developments on strategies to improve the photocatalytic elimination of organic dye pollutants by BiOX (X=Cl, Br, I, F) nanostructures. Korean J. Chem. Eng. 2018, 35, 1955–1968. [Google Scholar] [CrossRef]
- Yu, C.L.; Chen, J.C.; Zhou, W.Q.; Wei, L.F.; Fan, Q.Z. Grinding calcination preparation of WO3/BiOCl heterostructures with enhanced visible light photocatalytic activity. Mater. Res. Innov. 2014, 19, 54–59. [Google Scholar] [CrossRef]
- Cui, Z.; Song, H.; Ge, S.; He, W.; Liu, Y. Fabrication of BiOCl/BiOBr hybrid nanosheets with enhanced superoxide radical dominating visible light driven photocatalytic activity. Appl. Surf. Sci. 2019, 467, 505–513. [Google Scholar] [CrossRef]
- Junxiu, W.; Zhenzong, Z.; Xi, W.; Yi, S.; Yongfu, G.; Keung, W.P.; Renbi, B. Synthesis of novel p-n heterojunction m-Bi2O4/BiOCl nanocomposite with excellent photocatalytic activity through ion-etching method. Chin. J. Catal. 2018, 39, 1792–1803. [Google Scholar]
- Song, L.; Pang, Y.; Zheng, Y.; Chen, C.; Ge, L. Design, preparation and enhanced photocatalytic activity of porous BiOCl/BiVO4 microspheres via a coprecipitation-hydrothermal method. J. Alloys Compd. 2017, 710, 375–382. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X.; Li, G.; Cao, Y.; Shao, Y.; Li, D. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye-sensitization under visible light. Appl. Catal. B Environ. 2016, 187, 301–309. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, Y.; Wu, S.; Zhu, Y.; Chen, H.; Yu, X.; Zhang, Y. Facile Fabrication of BiOI/BiOCl Immobilized Films with Improved Visible Light Photocatalytic Performance. Front. Chem. 2018, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J.; Wang, Q.; Li, F.-T.; Yang, W.-Y.; Zhao, Y.; Hao, Y.-J.; Liu, S.-J. Novel BiOCl–C3N4 heterojunction photocatalysts: In situ preparation via an ionic-liquid-assisted solvent-thermal route and their visible-light photocatalytic activities. Chem. Eng. J. 2013, 234, 361–371. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2019, 214, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Dai, G.; Bao, X.; Huang, N.; Peng, R.; Zhou, Y. Synthesis and characterization of novel Bi2S3/BiOCl/g-C3N4 composite with efficient visible-light photocatalytic activity. Mater. Lett. 2019, 241, 190–193. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Z.; Zhang, X.; Li, C.; Zheng, S. Construction of BiOCl/g-C3N4/kaolinite composite and its enhanced photocatalysis performance under visible-light irradiation. J. Taiwan Inst. Chem. Eng. 2018, 84, 203–211. [Google Scholar] [CrossRef]
- Asadzadeh-Khaneghah, S.; Habibi-Yangjeh, A.; Yubuta, K. Novel g-C3N4 nanosheets/CDs/BiOCl photocatalysts with exceptional activity under visible light. J. Am. Ceram. Soc. 2018, 102, 1435–1453. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Zhou, Y.; Fang, J.; Wang, Y.; Zhang, C.; Chen, W. Fabrication of sandwich-structured g-C3N4/Au/BiOCl Z-scheme photocatalyst with enhanced photocatalytic performance under visible light irradiation. J. Mater. Sci. 2018, 53, 6008–6020. [Google Scholar] [CrossRef]
- Xue, J.; Li, X.; Ma, S.; Xu, P.; Wang, M.; Ye, Z. Facile fabrication of BiOCl/RGO/protonated g-C3N4 ternary nanocomposite as Z-scheme photocatalyst for tetracycline degradation and benzyl alcohol oxidation. J. Mater. Sci. 2018, 54, 1275–1290. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, J.; Wang, Y.; Ren, F. Hybrid density functional study on the mechanism for the enhanced photocatalytic properties of the ultrathin hybrid layered nanocomposite g-C3N4/BiOCl. Appl. Surf. Sci. 2018, 435, 1351–1360. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Xu, H.; Yan, J.; Xu, Y.; Song, Y.; Li, H.; Xia, J.; Huang, C.; Wan, H. Novel visible-light-driven AgX/graphite-like C3N4 (X=Br, I) hybrid materials with synergistic photocatalytic activity. Appl. Catal. B Environ. 2013, 129, 182–193. [Google Scholar] [CrossRef]
- Iqbal, W.; Yang, B.; Zhao, X.; Rauf, M.; Waqas, M.; Gong, Y.; Zhang, J.; Mao, Y. Controllable synthesis of graphitic carbon nitride nanomaterials for solar energy conversion and environmental remediation: The road travelled and the way forward. Catal. Sci. Technol. 2018, 8, 4576–4599. [Google Scholar] [CrossRef]
- Rono, N.; Kibet, J.K.; Martincigh, B.S.; Nyamori, V.O. A comparative study between thermal etching and liquid exfoliation of bulk graphitic carbon nitride to nanosheets for the photocatalytic degradation of a model environmental pollutant, Rhodamine B. J. Mater. Sci. Mater. Electron. 2021, 32, 687–706. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, K.; Xiao, X.; Zhang, L. Synthesis and Facet-Dependent Photoreactivity of BiOCl Single-Crystalline Nanosheets. J. Am. Chem. Soc. 2012, 134, 4473–4476. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y. BiOCl Nanoplates Decorated on g-C3N4 for Enhanced Photocatalytic Activities. Int. J. Electrochem. Sci. 2017, 4351–4359. [Google Scholar] [CrossRef]
- Song, L.; Pang, Y.; Zheng, Y.; Ge, L. Hydrothermal synthesis of novel g-C3N4/BiOCl heterostructure nanodiscs for efficient visible light photodegradation of Rhodamine B. Appl. Phys. A 2017, 123, 500. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, F.; Zhan, S.; Liu, Y.; Yin, Y. Enhanced Photocatalytic Activity of BiOCl Hybridized with g-C3N4. J. Inorg. Organomet. Polym. Mater. 2016, 26, 91–99. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, X.; Yang, J.; Jia, C.-J.; Jin, Z.; Fan, W. Exploring the effects of nanocrystal facet orientations in g-C3N4/BiOCl heterostructures on photocatalytic performance. Nanoscale 2015, 7, 18971–18983. [Google Scholar] [CrossRef]
- Hou, W.; Deng, C.; Xu, H.; Li, D.; Zou, Z.; Xia, H.; Xia, D. n–p BiOCl@g-C3N4 Heterostructure with Rich-oxygen Vacancies for Photodegradation of Carbamazepine. ChemistrySelect 2020, 5, 2767–2777. [Google Scholar] [CrossRef]
- AlMarzouqi, F.; Al Farsi, B.; Kuvarega, A.T.; Al Lawati, H.A.J.; Al Kindy, S.M.Z.; Kim, Y.; Selvaraj, R. Controlled Microwave-Assisted Synthesis of the 2D-BiOCl/2D-g-C3N4 Heterostructure for the Degradation of Amine-Based Pharmaceuticals under Solar Light Illumination. ACS Omega 2019, 4, 4671–4678. [Google Scholar] [CrossRef] [Green Version]
- Tompsett, G.A.; Conner, W.C.; Yngvesson, K.S. Microwave Synthesis of Nanoporous Materials. Chemphyschem Eur. J. Chem. Phys. Phys. Chem. 2006, 7, 296–319. [Google Scholar] [CrossRef]
- Jia, T.; Li, J.; Long, F.; Fu, F.; Zhao, J.; Deng, Z.; Wang, X.; Zhang, Y. Ultrathin g-C3N4 Nanosheet-Modified BiOCl Hierarchical Flower-Like Plate Heterostructure with Enhanced Photostability and Photocatalytic Performance. Crystals 2017, 7, 266. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, P.-Q.; Liu, J.-Y.; Liu, X.-J. Enhanced photocatalytic performance of direct Z-scheme BiOCl–g-C3N4 photocatalysts. RSC Adv. 2014, 4, 19456–19461. [Google Scholar] [CrossRef]
- Zheng, C.-Z.; Zhang, C.-Y.; Zhang, G.-H.; Zhao, D.-J.; Wang, Y.-Z. Enhanced photocatalytic performance of g-C3N4 with BiOCl quantum dots modification. Mater. Res. Bull. 2014, 55, 212–215. [Google Scholar] [CrossRef]
- Sun, J.; Song, J.; Gondal, M.A.; Shi, S.; Lu, Z.; Xu, Q.; Chang, X.; Xiang, D.; Shen, K. Preparation of g-C3N4/BiOX (X = Cl, Br, I) composites, and their photocatalytic activity under visible light irradiation. Res. Chem. Intermed. 2014, 41, 6941–6955. [Google Scholar] [CrossRef]
- Song, L.; Zheng, Y.; Chen, C. Sonication-assisted deposition–precipitation synthesis of graphitic C3N4/BiOCl heterostructured photocatalysts with enhanced rhodamine B photodegradation activity. J. Mater. Sci. Mater. Electron. 2017, 28, 15861–15869. [Google Scholar] [CrossRef]
- Cai, W.; Tang, J.; Shi, Y.; Wang, H.; Jiang, X. Improved in Situ Synthesis of Heterostructured 2D/2D BiOCl/g-C3N4 with Enhanced Dye Photodegradation under Visible-Light Illumination. ACS Omega 2019, 4, 22187–22196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Wang, W.; Zhong, L.; Liu, D.; Cao, X.; Cui, F. Oxygen vacancy-rich 2D/2D BiOCl-g-C3N4 ultrathin heterostructure nanosheets for enhanced visible-light-driven photocatalytic activity in environmental remediation. Appl. Catal. B Environ. 2018, 220, 290–302. [Google Scholar] [CrossRef]
- Zhang, X.; An, D.; Feng, D.; Liang, F.; Chen, Z.; Liu, W.; Yang, Z.; Xian, M. In situ surfactant-free synthesis of ultrathin BiOCl/g-C3N4 nanosheets for enhanced visible-light photodegradation of rhodamine B. Appl. Surf. Sci. 2019, 476, 706–715. [Google Scholar] [CrossRef]
- Liu, W.; Qiao, L.; Zhu, A.; Liu, Y.; Pan, J. Constructing 2D BiOCl/C3N4 layered composite with large contact surface for visible-light-driven photocatalytic degradation. Appl. Surf. Sci. 2017, 426, 897–905. [Google Scholar] [CrossRef]
- Yin, S.; Di, J.; Li, M.; Sun, Y.; Xia, J.; Xu, H.; Fan, W.; Li, H. Ionic liquid-assisted synthesis and improved photocatalytic activity of p-n junction g-C3N4/BiOCl. J. Mater. Sci. 2016, 51, 4769–4777. [Google Scholar] [CrossRef]
- Shan, W.; Hu, Y.; Bai, Z.; Zheng, M.; Wei, C. In situ preparation of g-C3N4/bismuth-based oxide nanocomposites with enhanced photocatalytic activity. Appl. Catal. B Environ. 2016, 188, 1–12. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Luo, J.; Duan, X.; Crittenden, J.; Liu, C.; Zhang, S.; Pei, Y.; Zeng, Y.; Duan, X. Self-Optimization of the Active Site of Molybdenum Disulfide by an Irreversible Phase Transition during Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. Engl. 2017, 56, 7610–7614. [Google Scholar] [CrossRef]
- Che, H.; Che, G.; Dong, H.; Hu, W.; Hu, H.; Liu, C.; Li, C. Fabrication of Z-scheme Bi3O4Cl/g-C3N4 2 D/2 D heterojunctions with enhanced interfacial charge separation and photocatalytic degradation various organic pollutants activity. Appl. Surf. Sci. 2018, 455, 705–716. [Google Scholar] [CrossRef]
- Adepu, A.K.; Anumula, R.; Narayanan, V. Photocatalytic degradation of Rhodamine B over a novel mesoporous titanosilicate/g-C3N4 nanocomposite under direct sunlight irradiation. Microporous Mesoporous Mater. 2017, 247, 86–94. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009–2026. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Miino, M.C.; Damiani, S. Treatments for color removal from wastewater: State of the art. J. Environ. Manag. 2019, 236, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Descorme, C. Catalytic wastewater treatment: Oxidation and reduction processes. Recent studies on chlorophenols. Catal. Today 2017, 297, 324–334. [Google Scholar] [CrossRef]
- Radke, E.; Braun, J.M.; Nachman, R.M.; Cooper, G.S. Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environ. Int. 2020, 137, 105408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jia, Y.; Wang, J.; Hu, X.; Zhao, Z.; Cheng, Y. Cysteine-assisted photoelectrochemical immunoassay for the carcinoembryonic antigen by using an ITO electrode modified with C3N4-BiOCl semiconductor and CuO nanoparticles as antibody labels. Microchim. Acta 2019, 186, 633. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, P.; Ma, R.; Luo, C.; Wen, T.; Zhao, G.; Cheng, W.; Wang, X. Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: A critical review. Catal. Today 2019, 335, 65–77. [Google Scholar] [CrossRef]
- Bellamkonda, S.; Rao, G.R. Nanojunction-mediated visible light photocatalytic enhancement in heterostructured ternary BiOCl/ CdS/g-C3N4 nanocomposites. Catal. Today 2019, 321–322, 18–25. [Google Scholar] [CrossRef]
- Aghdam, S.M.; Haghighi, M.; Allahyari, S.; Yosefi, L. Precipitation dispersion of various ratios of BiOI/BiOCl nanocomposite over g-C3N4 for promoted visible light nanophotocatalyst used in removal of acid orange 7 from water. J. Photochem. Photobiol. A Chem. 2017, 338, 201–212. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Wang, Y.; Yao, W.; Zhu, Y. Enhanced oxidation ability of g-C3N4 photocatalyst via C60 modification. Appl. Catal. B Environ. 2014, 152–153, 262–270. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ai, Z.; Jia, F.; Zhang, L. Oxygen Vacancy-Mediated Photocatalysis of BiOCl: Reactivity, Selectivity, and Perspectives. Angew. Chem. Int. Ed. 2018, 57, 122–138. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L. Oxygen vacancy induced selective silver deposition on the {001} facets of BiOCl single-crystalline nanosheets for enhanced Cr(vi) and sodium pentachlorophenate removal under visible light. Nanoscale 2014, 6, 7805–7810. [Google Scholar] [CrossRef]
- Li, H.; Shang, J.; Zhu, H.; Yang, Z.; Ai, Z.; Zhang, L. Oxygen Vacancy Structure Associated Photocatalytic Water Oxidation of BiOCl. ACS Catal. 2016, 6, 8276–8285. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Q.; Luo, Y. Determining the Charge-Transfer Direction in a p-n Heterojunction BiOCl/g-C3N4 Photocatalyst by Ultrafast Spectroscopy. ChemPhotoChem 2017, 1, 350–354. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thampi, K.R.; Tayade, R.J. Visible light driven redox-mediator-free dual semiconductor photocatalytic systems for pollutant degradation and the ambiguity in applying Z-scheme concept. Appl. Catal. B Environ. 2018, 227, 296–311. [Google Scholar] [CrossRef]
- Guo, Q.; Li, H.; Zhang, Q.; Zhang, Y. Fabrication, characterization and mechanism of a novel Z-scheme Ag3PO4/NG/polyimide composite photocatalyst for microcystin-LR degradation. Appl. Catal. B Environ. 2018, 229, 192–203. [Google Scholar] [CrossRef]
- Maeda, K. Z-Scheme Water Splitting Using Two Different Semiconductor Photocatalysts. ACS Catal. 2013, 3, 1486–1503. [Google Scholar] [CrossRef]
- Abe, R.; Sayama, K.; Sugihara, H. Development of New Photocatalytic Water Splitting into H2 and O2 Using Two Different Semiconductor Photocatalysts and a Shuttle Redox Mediator IO3−/I−. J. Phys. Chem. B 2005, 36, 16052–16061. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.; Jaroniec, M. All-Solid-State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef]
- Sayama, K.; Mukasa, K.; Abe, R.; Abe, Y.; Arakawa, H. A new photocatalytic water splitting system under visible light irradiation mimicking a Z-scheme mechanism in photosynthesis. J. Photochem. Photobiol. A Chem. 2002, 148, 71–77. [Google Scholar] [CrossRef]
- Sayama, K.; Mukasa, K.; Abe, R.; Abe, Y.; Arakawa, H. Stoichiometric water splitting into H2 and O2 using a mixture of two different photocatalysts and an IO3−/I− shuttle redox mediator under visible light irradiation. Chem. Commun. 2001, 2416–2417. [Google Scholar] [CrossRef]
- Tada, H.; Mitsui, T.; Kiyonaga, T.; Akita, T.; Tanaka, K. All-solid-state Z-scheme in CdS–Au–TiO2 three-component nanojunction system. Nat. Mater. 2006, 5, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, A.; Wang, Y.; Lv, C.; Zhu, W.; Dou, S.; Wang, Q.; Zhong, Q. Accessible fabrication and mechanism insight of heterostructured BiOCl/Bi2MoO6/g-C3N4 nanocomposites with efficient photosensitized activity. J. Alloys Compd. 2017, 726, 164–172. [Google Scholar] [CrossRef]
- Shakeel, M.; Zhang, X.; Yasin, G.; Arif, M.; Abbas, Z.; Zaman, U.; Li, B. Fabrication of Amorphous BiOCl/TiO2-C3N4 Heterostructure for Efficient Water Oxidation. ChemistrySelect 2019, 4, 8277–8282. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, Y. Facile Synthesis of Ternary g-C3N4@BiOCl/Bi12O17Cl2 Composites with Excellent Visible Light Photocatalytic Activity for NO Removal. Front. Chem. 2019, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.; Jo, W.-K. g-C3N4/oxygen-deficient BiOCl nanocomposite assisted by distinguished properties of graphene quantum dots for the efficient photocatalytic removal of organic vapors. Appl. Surf. Sci. 2019, 493, 873–881. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Wang, B.; Li, H.; Dong, W. Novel g-C3N4/BiOClxI1-x nanosheets with rich oxygen vacancies for enhanced photocatalytic degradation of organic contaminants under visible and simulated solar light. Appl. Catal. B Environ. 2019, 256, 117789. [Google Scholar] [CrossRef]
- Feng, Y.; Du, Y.; Du, M.; Tian, X.; Jiang, N.; Liu, Y. Synthesis and enhanced visible light photocatalytic activity of g- C3N4/BiOClxBr1-x heterojunctions with adjustable energy band structure. J. Phys. Chem. Solids 2019, 132, 222–229. [Google Scholar] [CrossRef]
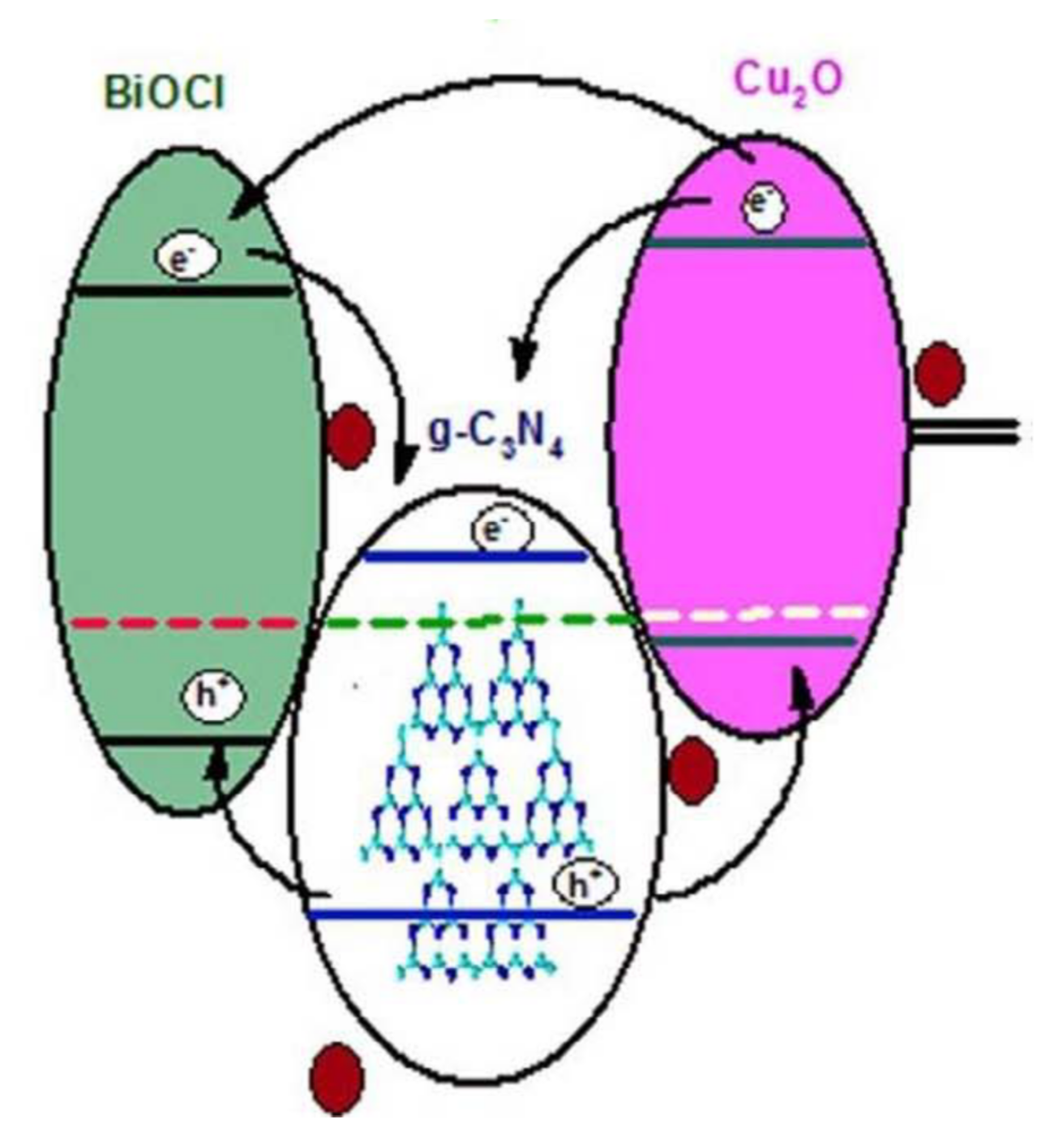
- Kumar, A.; Kumar, A.; Sharma, G.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Stadler, F.J. Quaternary magnetic BiOCl/g-C3N4/Cu2O/Fe3O4 nano-junction for visible light and solar powered degradation of sulfamethoxazole from aqueous environment. Chem. Eng. J. 2018, 334, 462–478. [Google Scholar] [CrossRef]
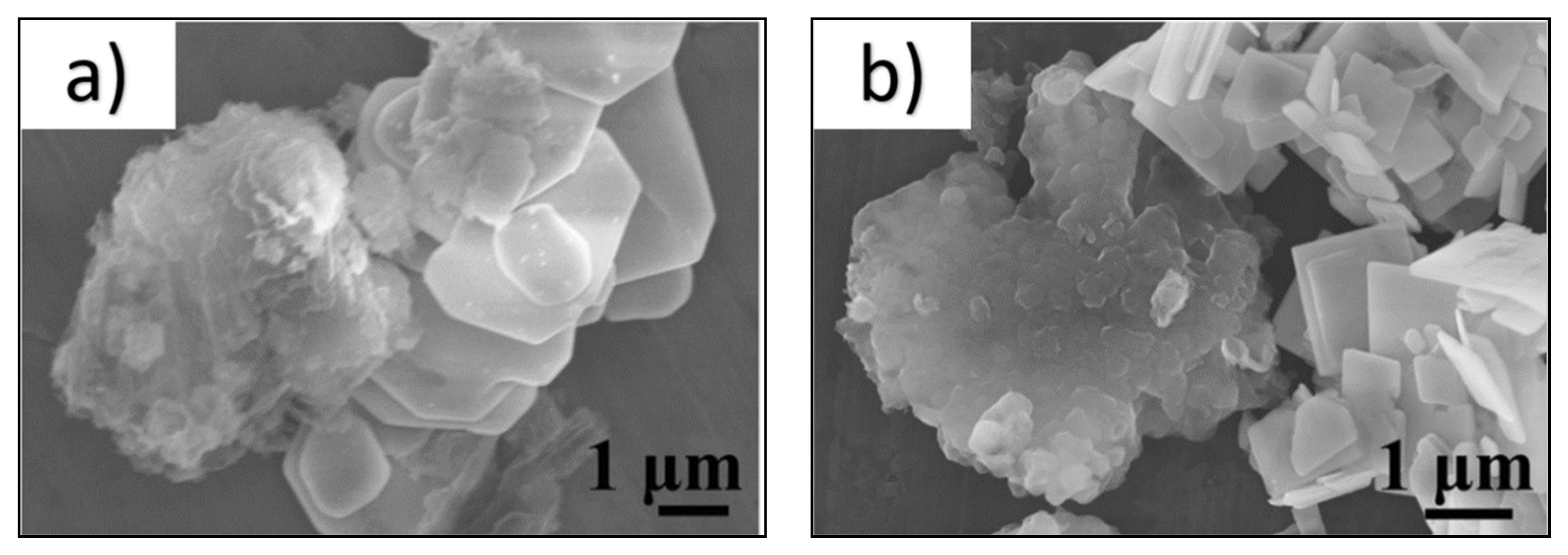
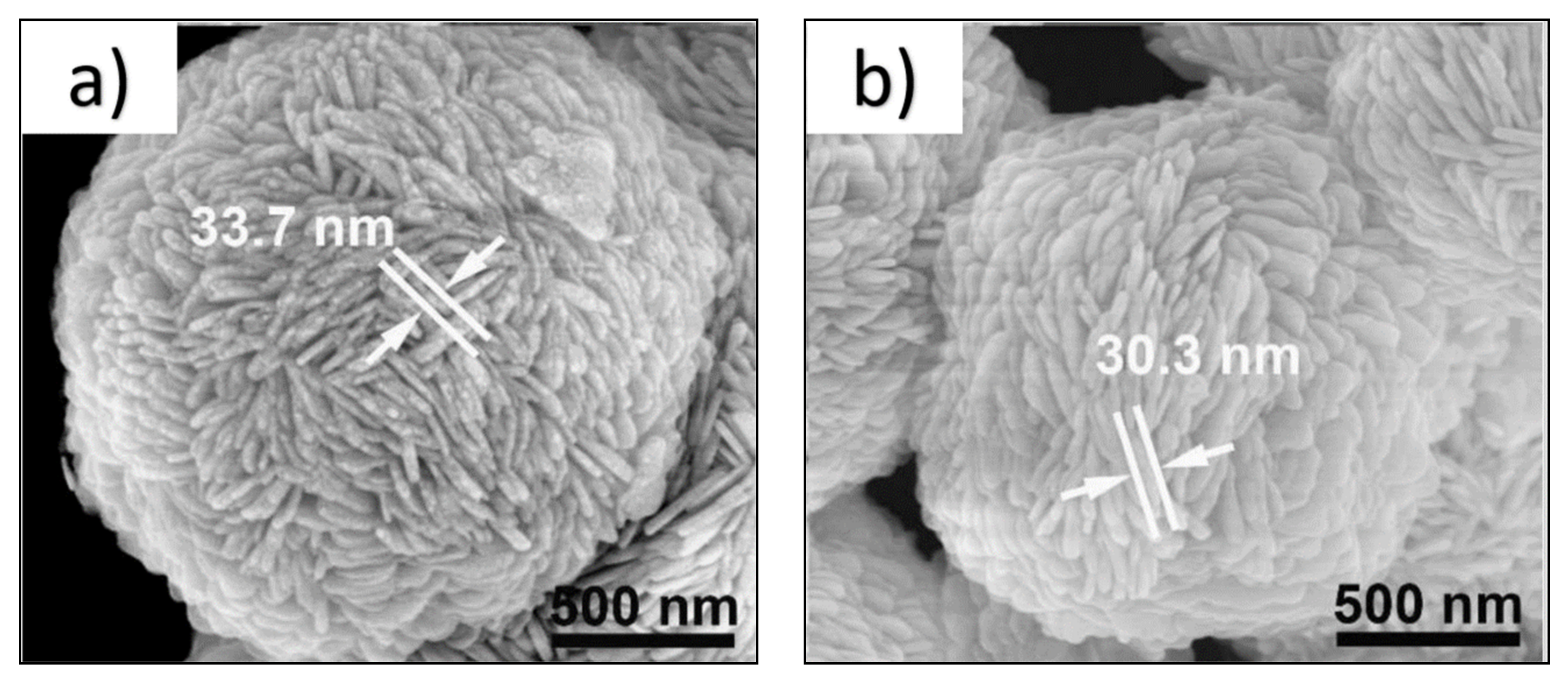
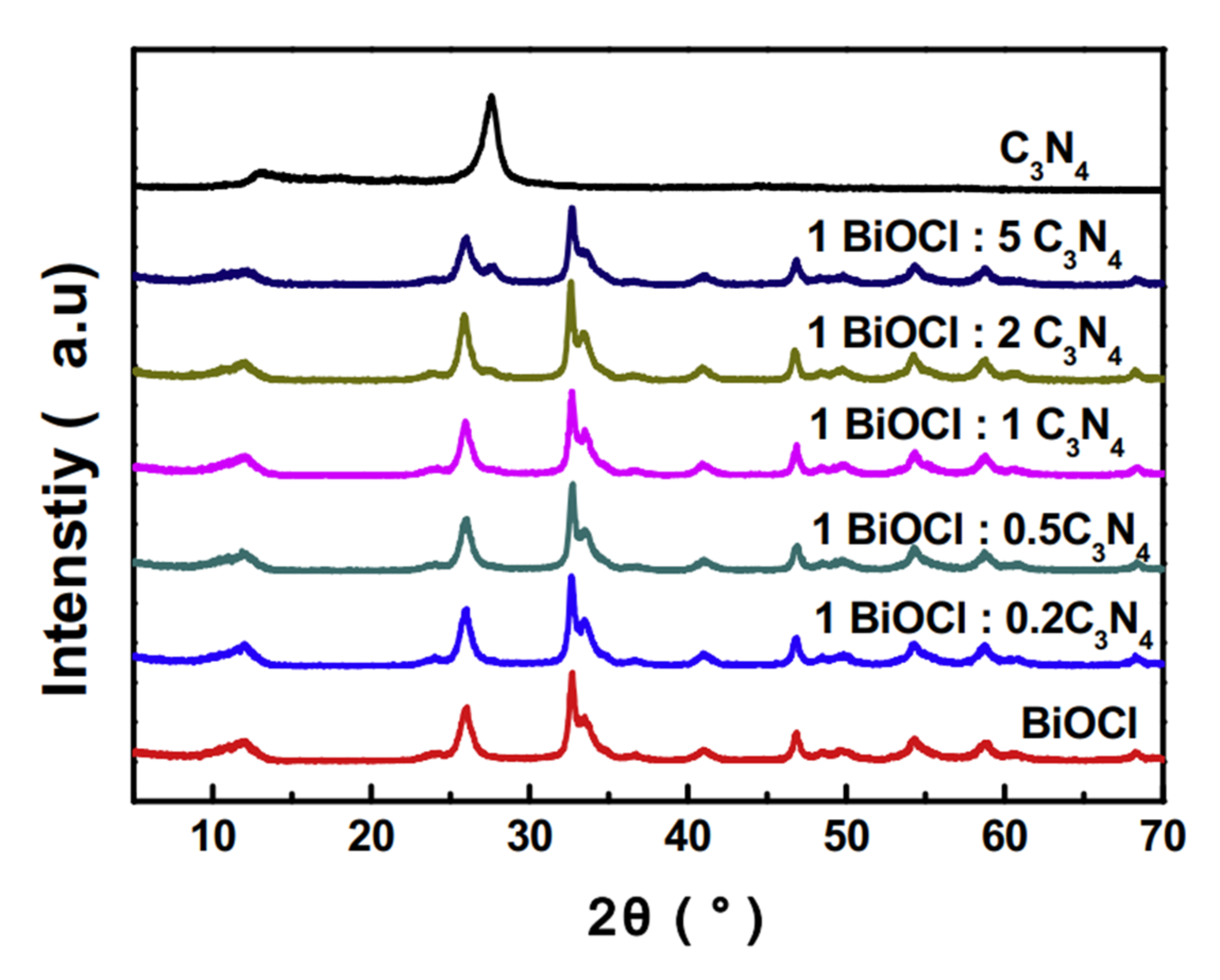
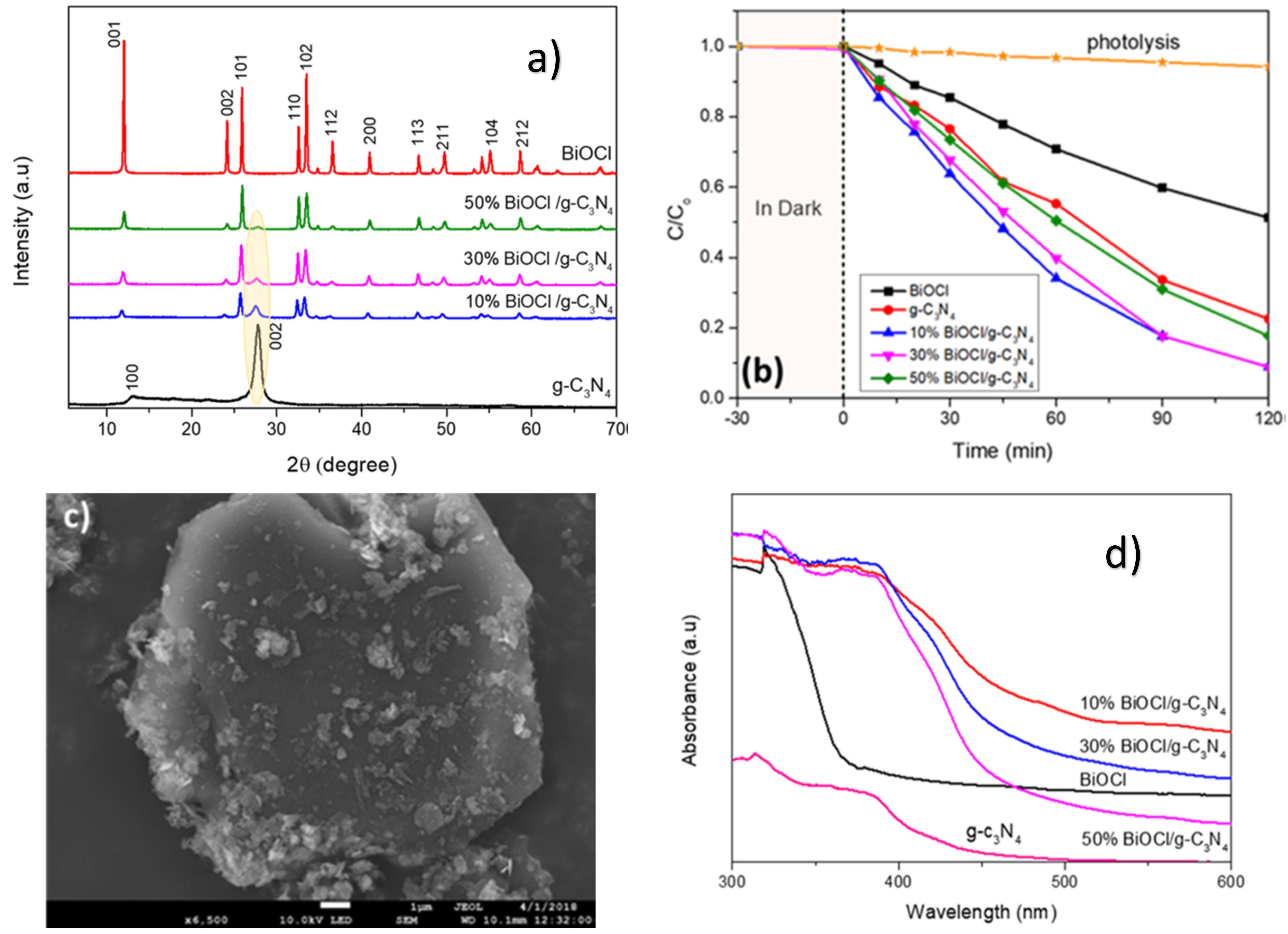
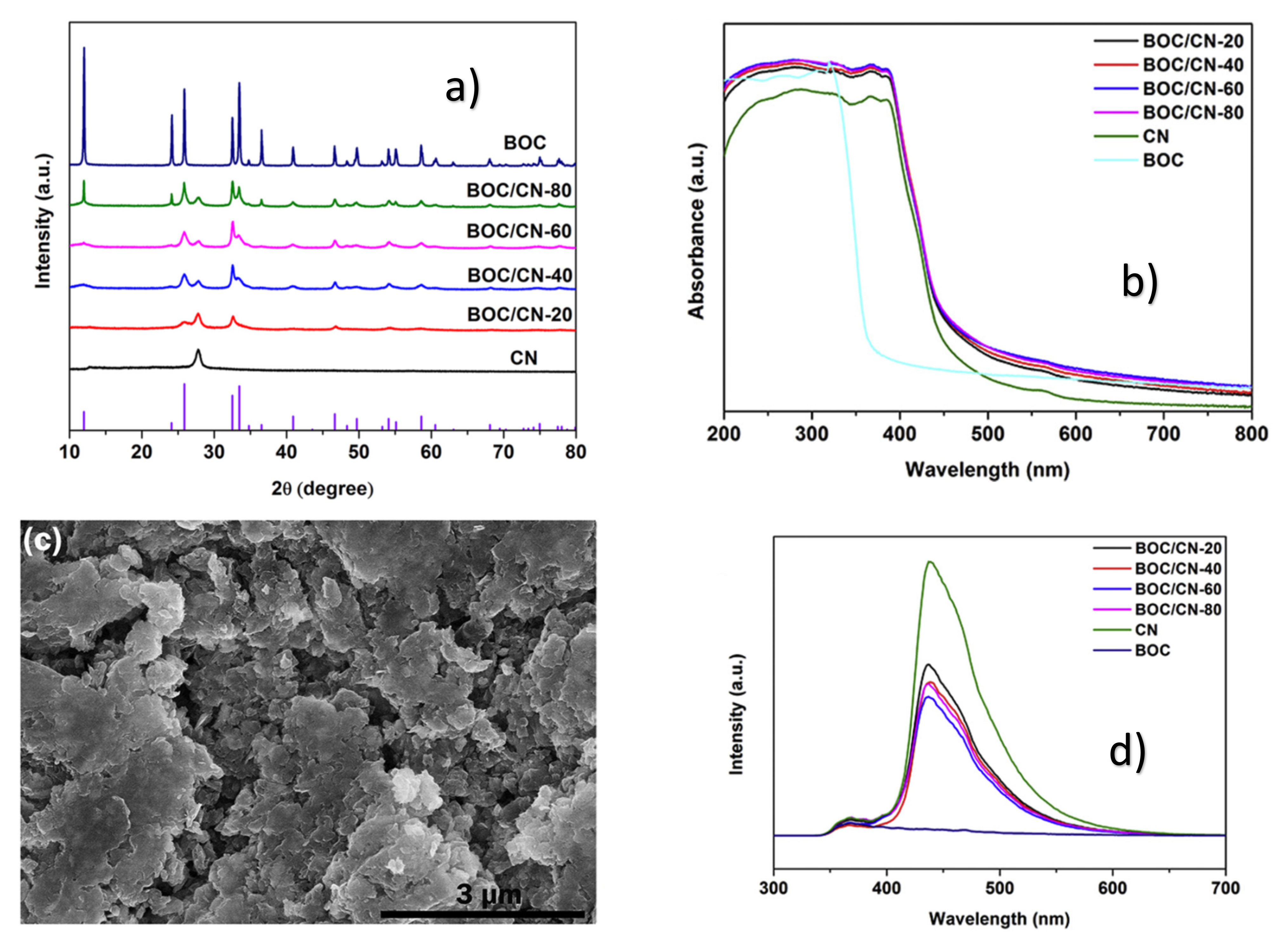
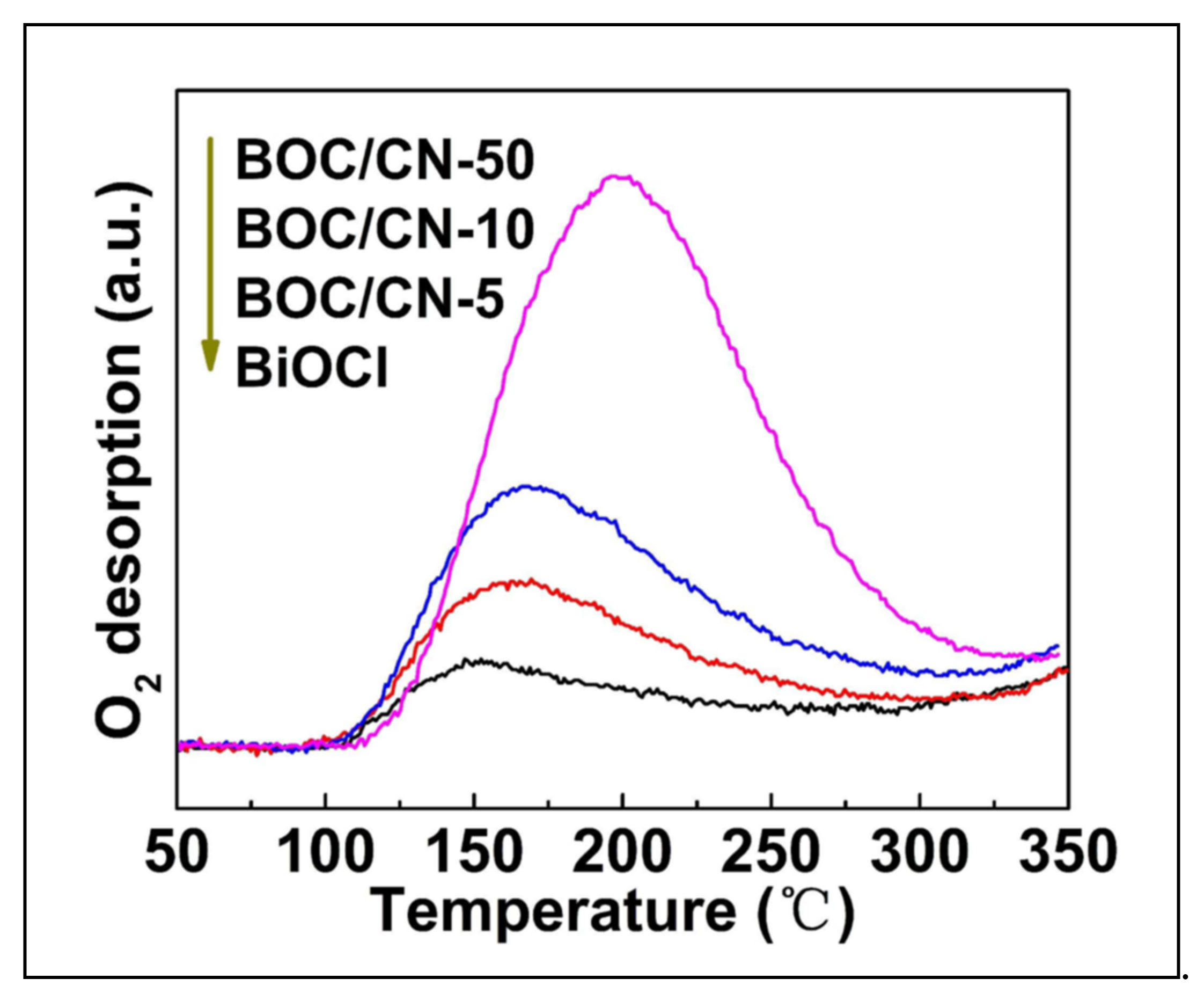
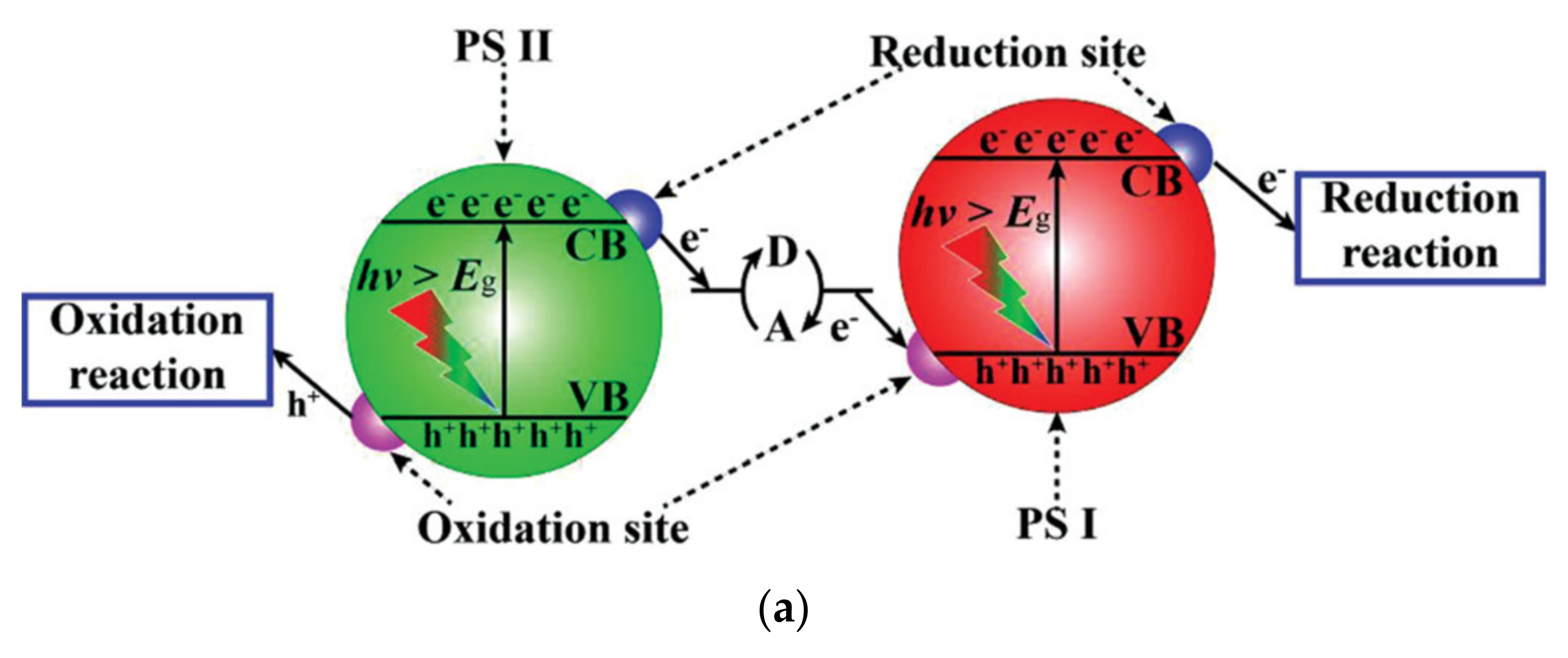
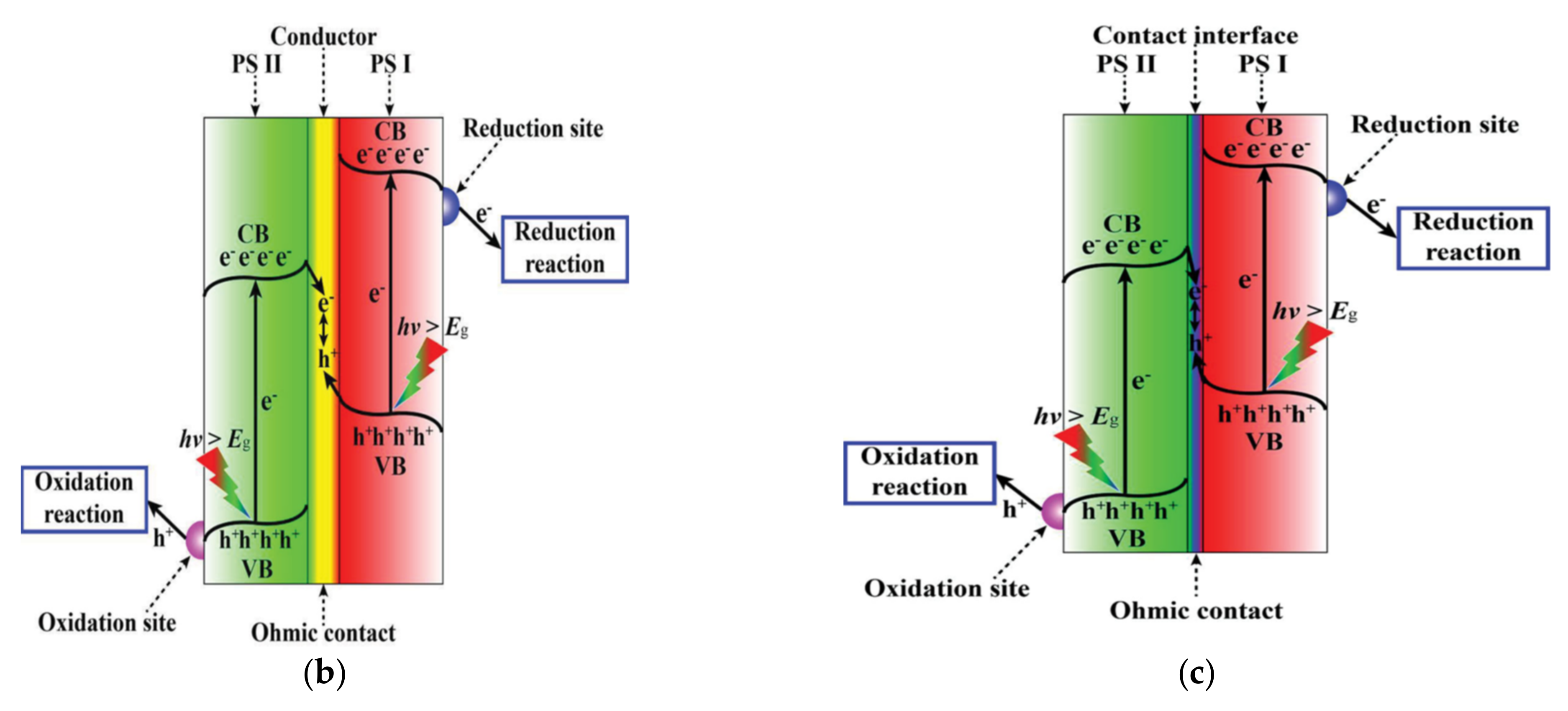
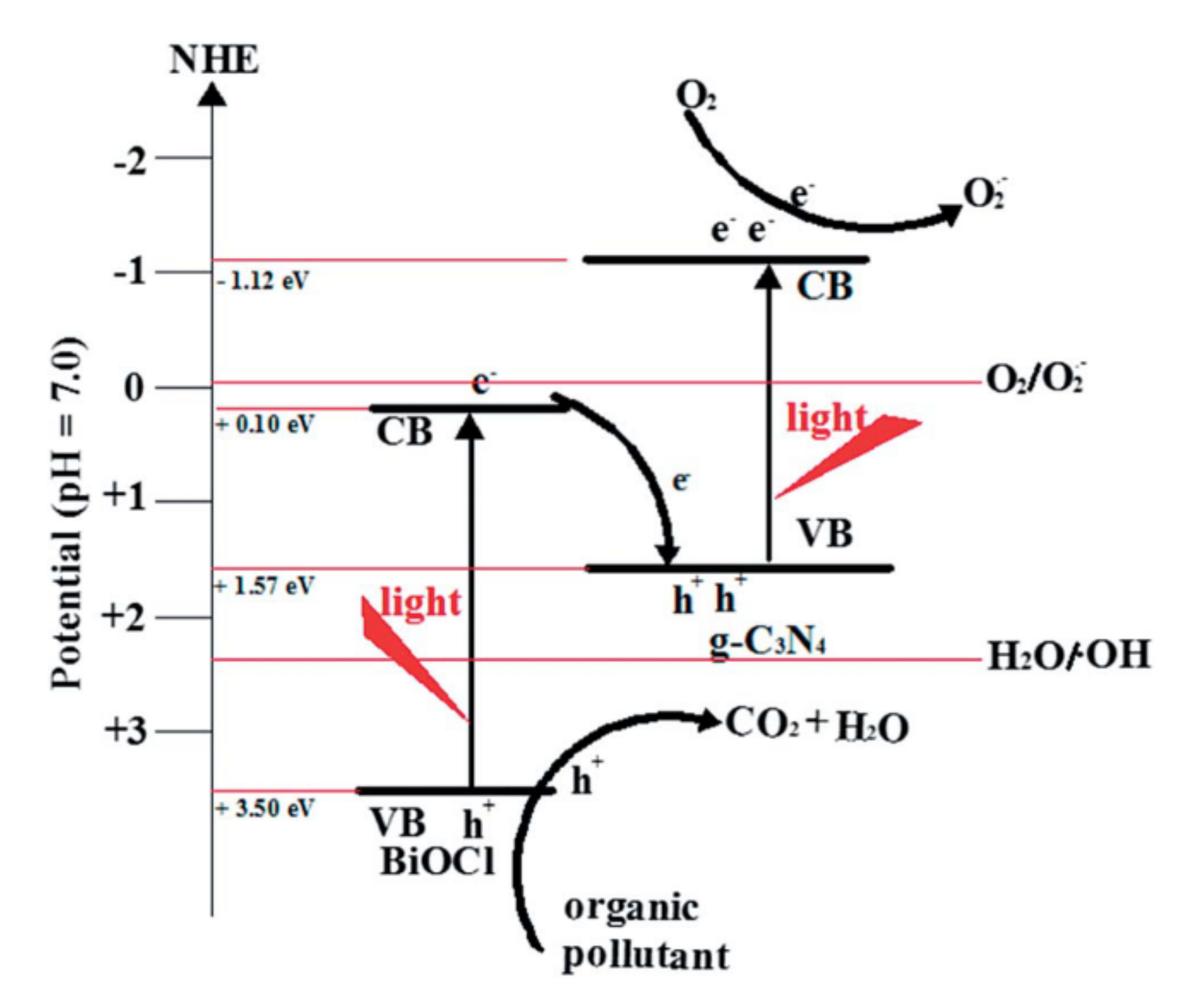
| Catalyst (Mass Ratio %) | Template | Morphology | Size | Year | Ref. |
|---|---|---|---|---|---|
| BiOCl/g-C3N4 (50/50) | SDBS | nanoplate | <5 nm | 2017 | [58] |
| g-C3N4/BiOCl (23.03/76.97) | - | nanodisc | 35–50 nm | 2017 | [59] |
| BiOCl/g-C3N4 (40/60) | - | - | 2 μm | 2015 | [60] |
| Catalyst (Mass Ratio %) | Template | g-C3N4 Precursor | Morphology | Diameter or Thickness | BET Surface Area (m2/g) | Year | Ref. |
|---|---|---|---|---|---|---|---|
| BiOCl-C3N4 (50/50) | IL: [HMIm]Cl | melamine | nanoflowers | - | 24.26 | 2013 | [44] |
| BiOCl/g-C3N4 (85/15) | CTAB | melamine | rolled flake+ lamellar | 70 nm | 47.1 | 2019 | [70] |
| (OV)BiOCl-g-C3N4 (50/50) | PVP | urea | Ultrathin nanosheet | ∼4.3 nm | 62.0 | 2017 | [71] |
| BOC/CN (60/40) | - | melamine | ultrathin layered structure | around 3.5 nm | 68.5 | 2019 | [72] |
| BiOCl/C3N4 | - | urea | Nanoplate+ rough slice | - | 47.1 | 2017 | [73] |
| g-C3N4/BiOCl (1/99) | [C16mim]Cl | Dicyandiamide‘ | three-dimensional spherical structure | 1 μm | 22.58 | 2016 | [74] |
| Catalyst (Mass Ratio%) | Application | Efficiency/Time | Light Source | Concentration of the Pollutant | Main Reactive Species | Stability and Reusability | Ref. |
|---|---|---|---|---|---|---|---|
| BiOCl/g-C3N4 (97/3) | Degradation of RhB | 100%/40 min | 400 W halogen lamp sodium nitrite solution (2 M) to eliminate UV light (λ < 400 nm) and thermal effect | 50 mg/L | •O2− | stable after 7 irradiation cycles | [6] |
| g-C3N4/BiOCl (20/80) (001) | Degradation of RhB | 100%/35 min | 500 W Xenon lamp with a light filter 400–800 nm | 7 mg/L | - | 65% after 10 irradiation cycles | [21] |
| C3N4/BiOCl (20/80) | Degradation of RhB | ~100%/20 min | 500 W Xe arc lamp UV-cut off filter (λ > 420 nm) | 20 mg/L | •O2−, hole | 90% after 5 irradiation cycles | [34] |
| BiOCl-C3N4 (50/50) (001) | Degradation methyl orange (MO) | 95%/80 min | 300 W xenon arc lamp 400 nm cutoff filter | 10 mg/L | hole | stable after 6 irradiation cycles | [44] |
| BiOCl/g-C3N4 (50/50) (001) | Degradation of RhB | >90%/50 min | 300 W Xelamp 400 nm cutoff filter | 10 mg/L | - | - | [58] |
| g-C3N4/BiOCl (23.03/76.97) | Degradation of RhB | 100%/30 min | 300 W Xe arc lamp 400 nm cutoff filter 35 mW/cm2 | 10 mg/L | •O2−, hole | stable after 4 irradiation cycles | [59] |
| BiOCl/g-C3N4 (40/60) (001) | Degradation of methylene-blue (MB) | 80%120 min | 500 W Xenon lamp 420 nm cutoff filter | 5–10 mol/L | - | stable after 5 irradiation cycles | [60] |
| ng-CN/BOC-010 (70/30) | Degradation methyl orange (MO) | >90%/150 min | 300 W metal-halide lamp 420 nm cutoff filter | 20 mg/L | •O2−, hole | - | [61] |
| g-C3N4 /BiOCl (55/45) | Degradation of methylene blue (MB) | 100%/30 min | daylight lamp 60 W, λ ≥ 400 nm | 5–10 mol/L | hole | ~99% after 5 irradiation cycles | [65] |
| BiOCl-g-C3N4 | Degradation of RhB | 99%/60 min | 300 W xenon lamp incident lightpower: 6 W | 10 mg/L | •OH, •O2− | - | [66] |
| BiOCl-g-C3N4 (50/50) | Degradation of RhB | 99%/35 min | 300 W xenon lamp | 10 mg/L | - | - | [67] |
| g-C3N4/BiOCl (20/80) | Degradation of RhB | 100%/20 min | 500-W Xe lamp with a light filter 400–800 nm | 7 mg/L | - | 56% after 5 irradiation cycles | [68] |
| BiOCl/(0.1 g)g-C3N4 (001) | Degradation of RhB | 100%/50 min | 300 W Xe arc lamp 400 nm cutoff filter 35 mW/cm2 | 10 mg/L | •O2−, hole | stable after 5 irradiation cycles | [69] |
| BiOCl/g-C3N4 (85/15) | Degradation of RhB | 90%/30 min | 300 W Xe lamp 400 nm cutoff filter | 25 mg/L | •O2− | - | [70] |
| BOC/CN (60/40) | Degradation of RhB | 95.93%/80 min | 300 W Xe lamp 420 nm cutoff filter | 10 mg/L | •O2−, hole | 89% after 5 irradiation cycles | [72] |
| BiOCl/C3N4 | Degradation of methyl orange (MO) | 84.28%/180 min | 300 W Xe lamp 0.5 mol·L−1 Na2SO4 Solution (λ ≥ 420 nm) | 10 mg/L | •O2−, hole | stable after 4 irradiation cycles | [73] |
| g-C3N4/BiOCl (1/99) | Degradation of RhB | 94 %/30 min | 300 W Xe lamp 400 nm cutoff filter | 10 mg/L | •O2−, hole | - | [74] |
| g-C3N4/BiOCl (001) | degradation of methylorange (MO) | 70%/300 min | 500 W halogen tungsten lamp 420 nm cut-off filter | 20 mg/L | - | stable after 4 irradiation cycles | [75] |
| Catalyst (Mass Ratio %) | Application | Light Source | Efficiency/Time | Main Reactive Species | Stability and Reusability | Ref. |
|---|---|---|---|---|---|---|
| (OV)BiOCl/g-C3N4-10 | Degradation of carbamazepine | Visible light | 49%/240 min | •O2−, hole | ~50% after 5 irradiation cycles | [62] |
| BiOCl/g-C3N4 (10/90) | Degradation of nizatidine | LED (365 nm) | 96%/30 min | •O2−, hole | ~92% after 5 irradiation cycles | [63] |
| (OV)BiOCl-g-C3N4 (50/50) | Degradation of 4-chlorophenol | Short-arc xenon lamp 420 nm cutoff filter | 95 %/2 h | •O2−, hole | 81% after 4 irradiation cycles | [71] |
| g-C3N4/BiOCl | degradation of dibutyl phthalate and methyl orange | 500 W halogen tungsten lamp 420 nm cutoff filter | 60%/300 min (DBP) 70%/300 min (MO) | - | stable after 4 irradiation cycles | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Q.; Liu, J.; Yang, Q.; Shen, W. A Review: Photocatalysts Based on BiOCl and g-C3N4 for Water Purification. Catalysts 2021, 11, 1084. https://doi.org/10.3390/catal11091084
Ren Q, Liu J, Yang Q, Shen W. A Review: Photocatalysts Based on BiOCl and g-C3N4 for Water Purification. Catalysts. 2021; 11(9):1084. https://doi.org/10.3390/catal11091084
Chicago/Turabian StyleRen, Qiang, Juming Liu, Qi Yang, and Wei Shen. 2021. "A Review: Photocatalysts Based on BiOCl and g-C3N4 for Water Purification" Catalysts 11, no. 9: 1084. https://doi.org/10.3390/catal11091084
APA StyleRen, Q., Liu, J., Yang, Q., & Shen, W. (2021). A Review: Photocatalysts Based on BiOCl and g-C3N4 for Water Purification. Catalysts, 11(9), 1084. https://doi.org/10.3390/catal11091084





