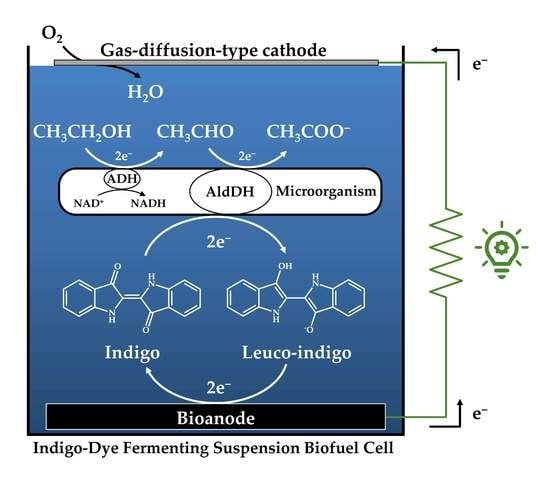
Indigo-Mediated Semi-Microbial Biofuel Cell Using an Indigo-Dye Fermenting Suspension
Abstract
:1. Introduction
2. Results and Discussion
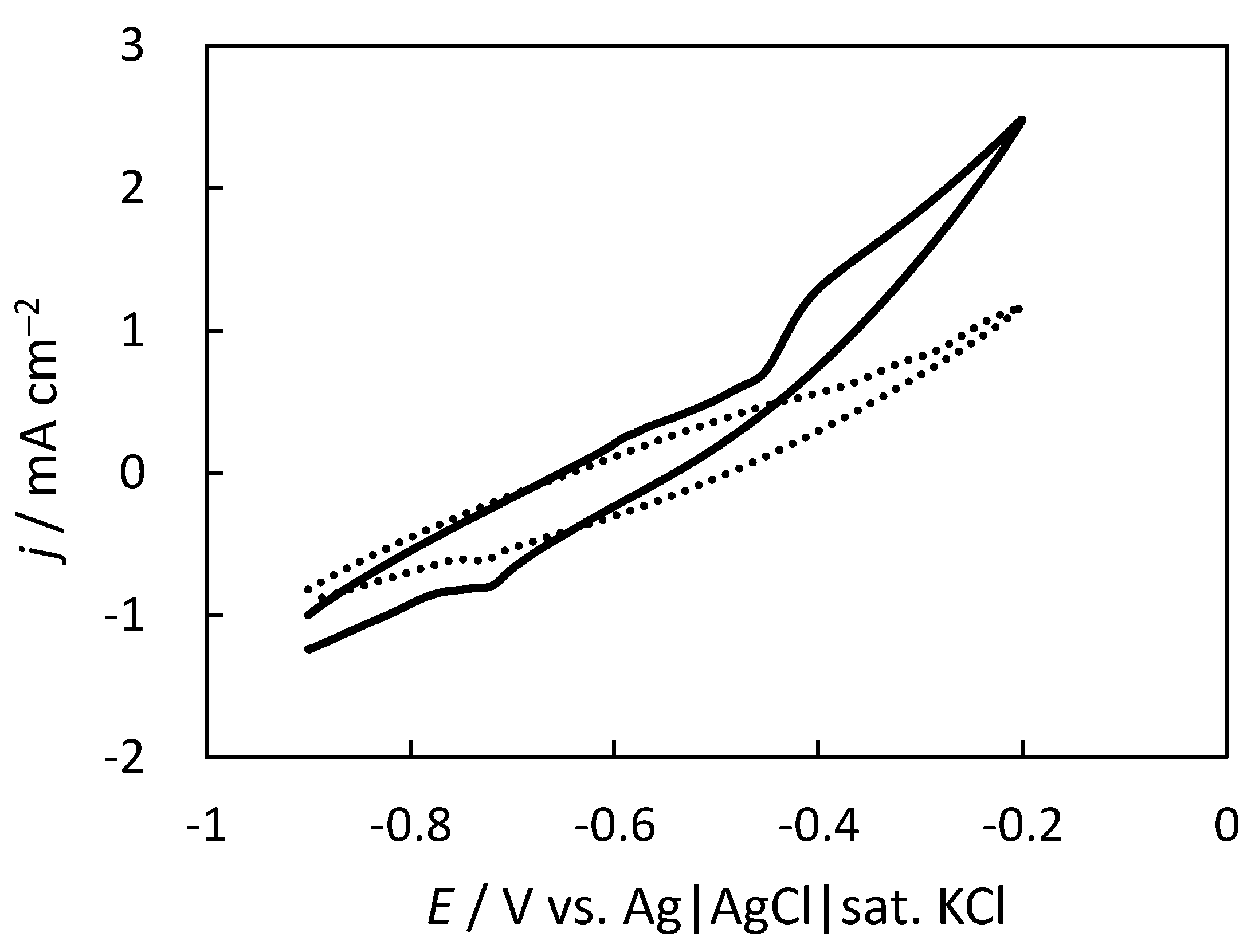
2.1. Electrochemical Performance of the Carbon Felt Electrode (CFE) Anode in the Indigo-Dye Fermenting Suspension
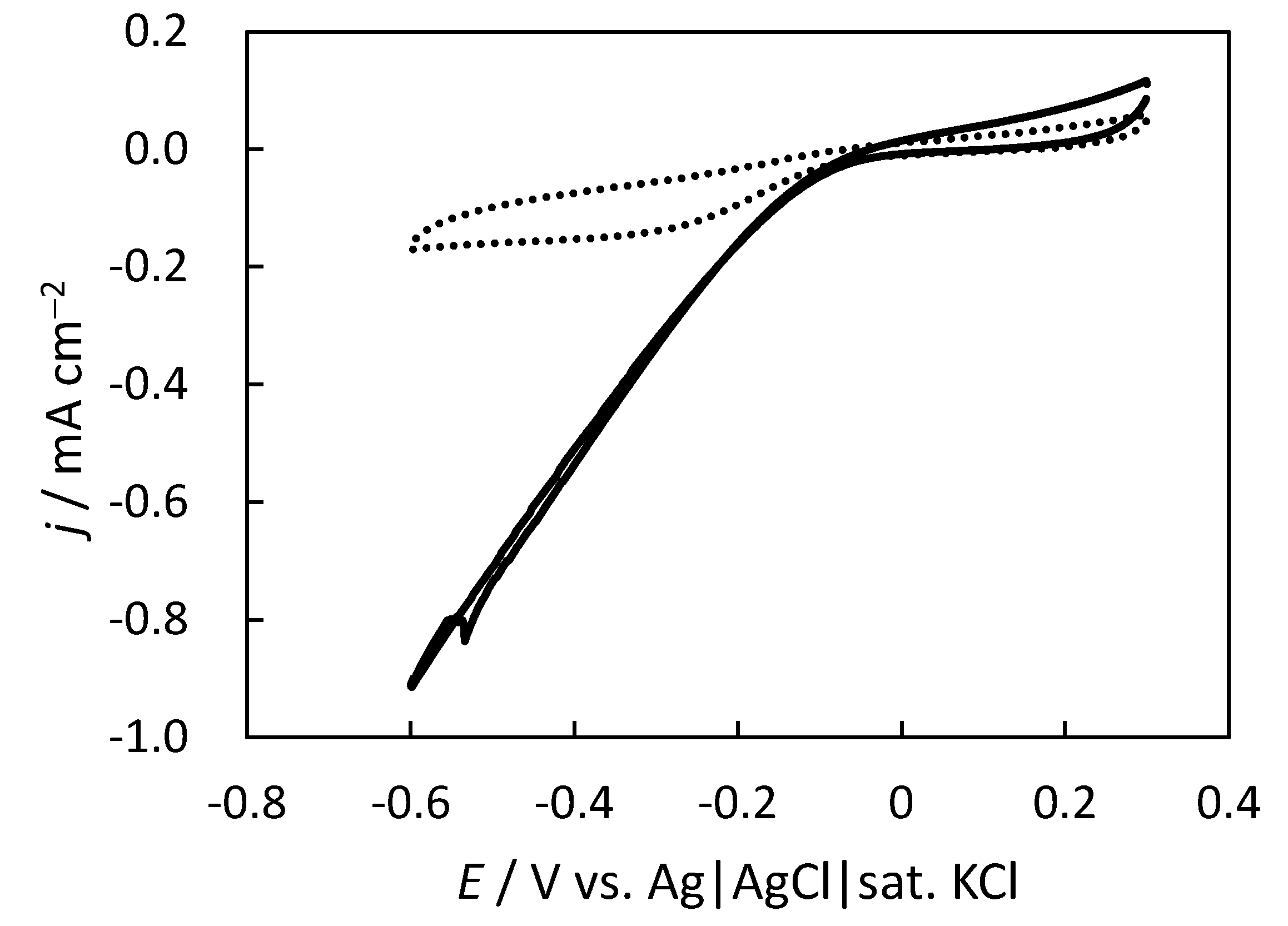
2.2. Gas-Diffusion-Type O2 Reduction with the Pt-Mesh Cathode
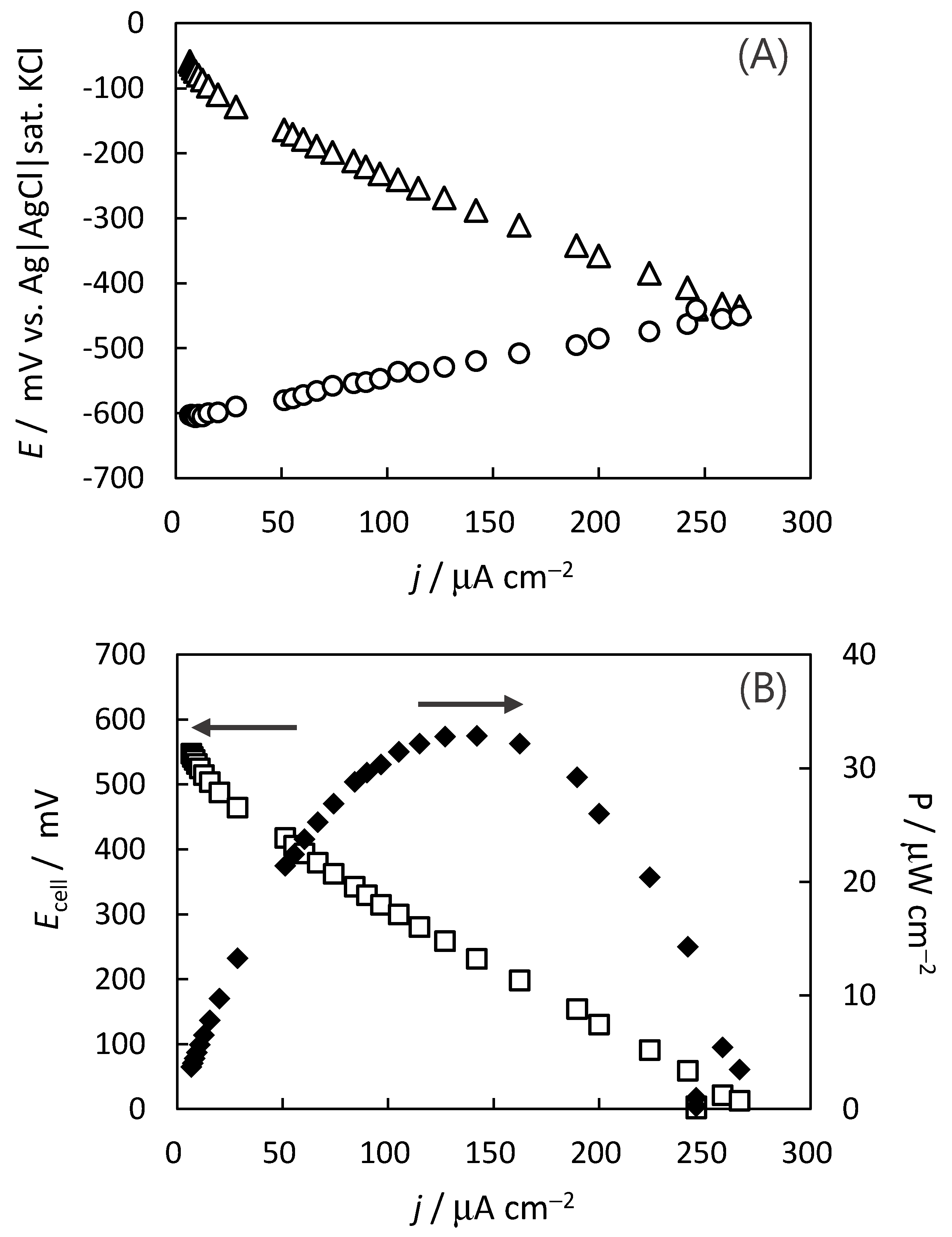
2.3. Construction of Indigo-Dye Fermenting Suspension Biofuel Cell
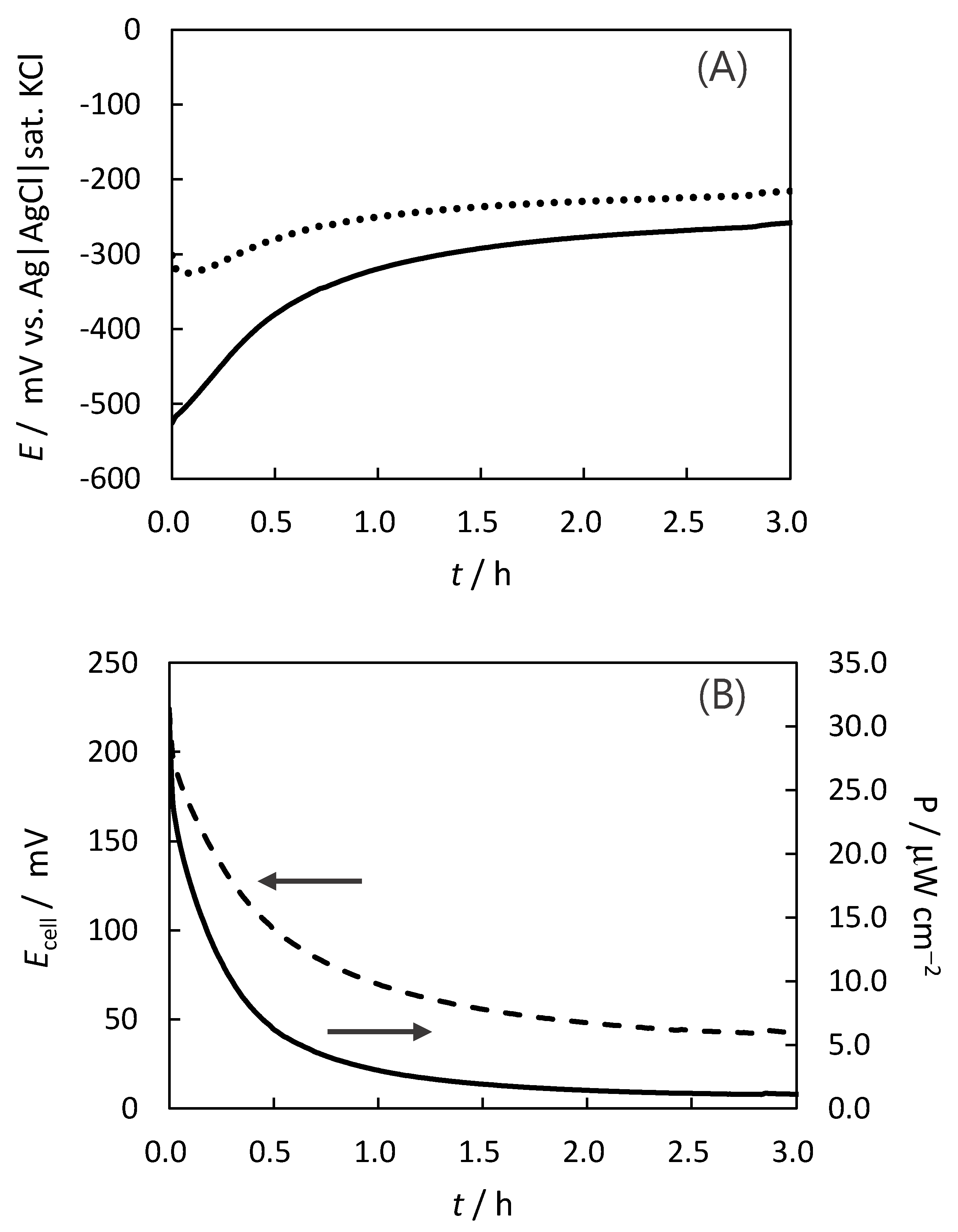
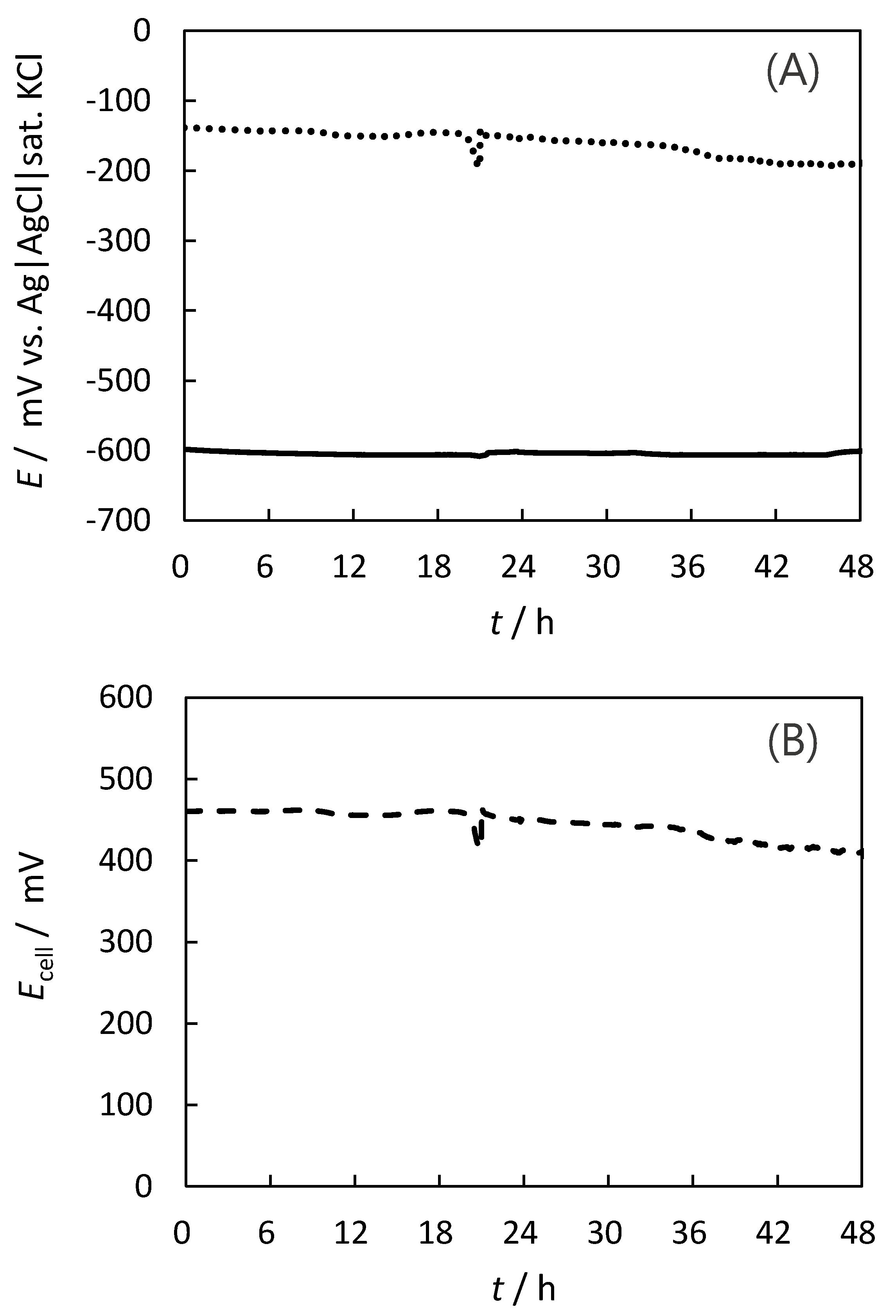
2.4. Stability for the Indigo-Dye Fermenting Suspension Biofuel Cell
2.5. Challenges in Achieving High-Performance Biofuel Cells
3. Materials and Methods
3.1. Preparation of the Indigo-Dye Fermenting Suspension
3.2. Preparation of Lye
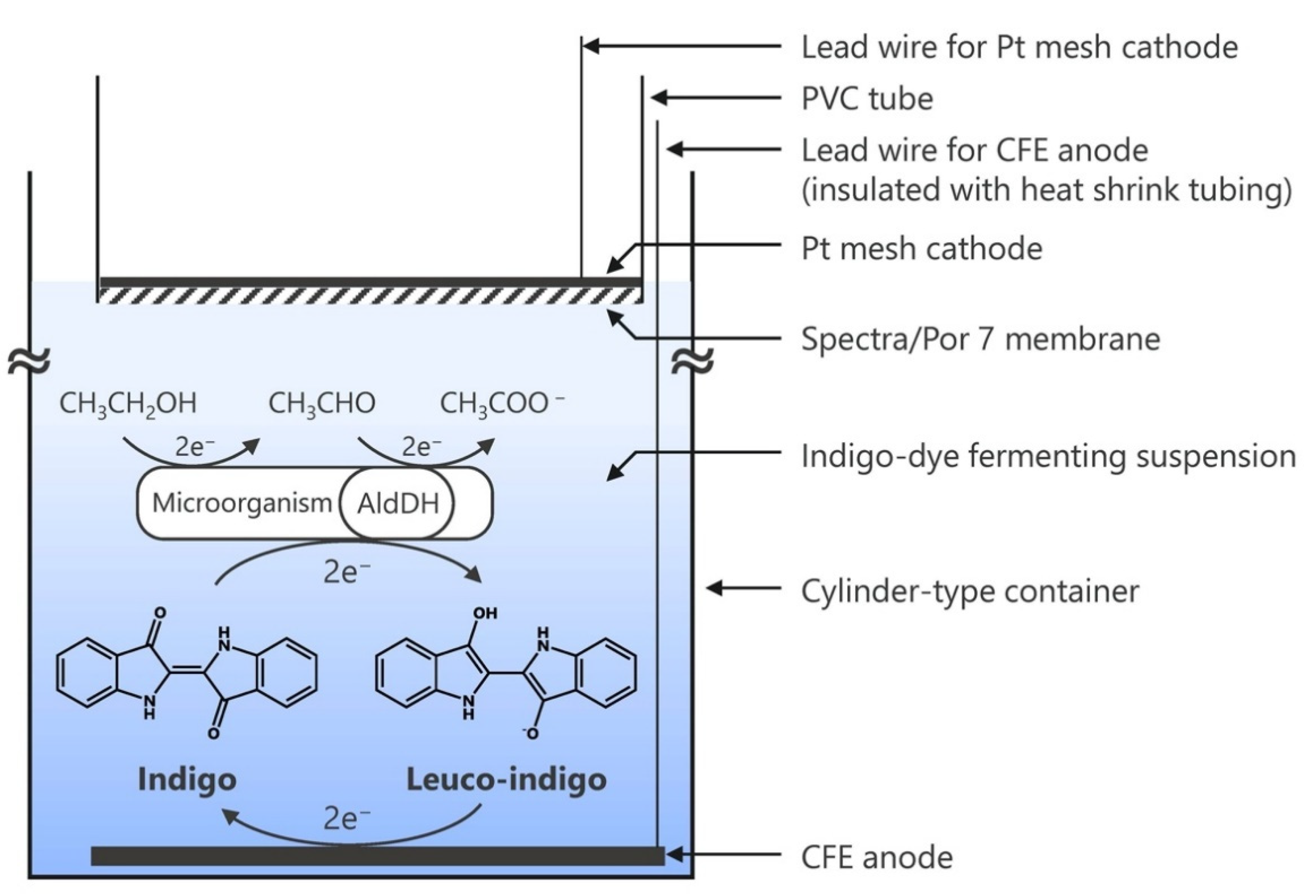
3.3. Preparation of the Semi-Microbial Fuel Cell
3.4. Evaluation of the Pt Electrode under an Alkaline Condition
3.5. Evaluation of the CFE Anode and Pt-Mesh Cathode
3.6. Indigo-Dye Fermenting Suspension Biofuel Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabaey, K.; Lissens, G.; Siciliano, S.D.; Verstraete, W. A microbial fuel cell capable of converting glucose to electricity at high rate and efficiency. Biotechnol. Lett. 2003, 25, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mo, G.; Li, X.; Zhang, W.; Zhang, J.; Ye, J.; Huang, X.; Yu, C. A graphene modified anode to improve the performance of microbial fuel cells. J. Power Sources 2011, 196, 5402–5407. [Google Scholar] [CrossRef]
- Jung, S.; Regan, J.M. Comparison of anode bacterial communities and performance in microbial fuel cells with different electron donors. Appl. Microbiol. Biotechnol. 2007, 77, 393–402. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Lovley, D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 2003, 21, 1229–1232. [Google Scholar] [CrossRef]
- Ringeisen, B.R.; Henderson, E.; Wu, P.K.; Pietron, J.; Ray, R.; Little, B.; Biffinger, J.C.; Jones-Meehan, J.M. High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10. Environ. Sci. Technol. 2006, 40, 2629–2634. [Google Scholar] [CrossRef] [Green Version]
- Bretschger, O.; Obraztsova, A.; Sturm, C.A.; Chang, I.S.; Gorby, Y.A.; Reed, S.B.; Culley, D.E.; Reardon, C.L.; Barua, S.; Romine, M.F.; et al. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl. Environ. Microbiol. 2007, 73, 7003–7012. [Google Scholar] [CrossRef] [Green Version]
- Watson, V.J.; Logan, B.E. Power production in MFCs inoculated with Shewanella oneidensis MR-1 or mixed cultures. Biotechnol. Bioeng. 2010, 105, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Cotta, M.A.; Angenent, L.T. Aerated Shewanella oneidensis in continuously fed bioelectrochemical systems for power and hydrogen production. Biotechnol. Bioeng. 2010, 105, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Jung, S.H.; Regan, J.M.; Logan, B.E. Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour. Technol. 2007, 98, 2568–2577. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Sund, C.J.; McMasters, S.; Crittenden, S.R.; Harrell, L.E.; Sumner, J.J. Effect of electron mediators on current generation and fermentation in a microbial fuel cell. Appl. Microbiol. Biotechnol. 2007, 76, 561–568. [Google Scholar] [CrossRef]
- Rabaey, K.; Boon, N.; Höfte, M.; Verstraete, W. Microbial phenazine production enhances electron transfer in biofuel cells. Environ. Sci. Technol. 2005, 39, 3401–3408. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, R.; Sugimoto, Y.; Kitazumi, Y.; Shirai, O.; Ogawa, J.; Kano, K. Electrochemical study on the extracellular electron transfer pathway from Shewanella strain Hac319 to electrodes. Anal. Sci. 2018, 34, 1177–1182. [Google Scholar] [CrossRef] [Green Version]
- von Canstein, H.; Ogawa, J.; Shimizu, S.; Lloyd, J.R. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl. Environ. Microbiol. 2008, 74, 615–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin-A-Woeng, T.F.C.; Bloemberg, G.V.; Lugtenberg, B.J.J. Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol. 2003, 157, 503–523. [Google Scholar] [CrossRef] [Green Version]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Call, D.F.; Logan, B.E. Lactate oxidation coupled to iron or electrode reduction by Geobacter sulfurreducens PCA. Appl. Environ. Microbiol. 2011, 77, 8791–8794. [Google Scholar] [CrossRef] [Green Version]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996, 96, 2563–2605. [Google Scholar] [CrossRef] [PubMed]
- Tarasevich, M.R.; Yaropolov, A.I.; Bogdanovskaya, V.A.; Varfolomeev, S.D. Electrocatalysis of a cathodic oxygen reduction by laccase. J. Electroanal. Chem. 1979, 104, 393–403. [Google Scholar] [CrossRef]
- Shleev, S.; Jarosz-Wilkolazka, A.; Khalunina, A.; Morozova, O.; Yaropolov, A.; Ruzgas, T.; Gorton, L. Direct electron transfer reactions of laccases from different origins on carbon electrodes. Bioelectrochemistry 2005, 67, 115–124. [Google Scholar] [CrossRef]
- Wanibuchi, M.; Kitazumi, Y.; Shirai, O.; Kano, K. Enhancement of the direct electron transfer-type bioelectrocatalysis of bilirubin oxidase at the interface between carbon particles. Electrochemistry 2021, 89, 43–48. [Google Scholar] [CrossRef]
- Murao, S.; Tanaka, N. Purification and some properties of bilirubin oxidase of Myrothecium verrucaria MT-1. Agric. Biol. Chem. 1982, 46, 2499–2503. [Google Scholar] [CrossRef]
- Tsujimura, S.; Nakagawa, T.; Kano, K.; Ikeda, T. Kinetic study of direct bioelectrocatalysis of dioxygen reduction with bilirubin oxidase at carbon electrodes. Electrochemistry 2004, 72, 437–439. [Google Scholar] [CrossRef] [Green Version]
- Mano, N.; Edembe, L. Bilirubin oxidases in bioelectrochemistry: Features and recent findings. Biosens. Bioelectron. 2013, 50, 478–485. [Google Scholar] [CrossRef]
- Kataoka, K.; Komori, H.; Ueki, Y.; Konno, Y.; Kamitaka, Y.; Kurose, S.; Tsujimura, S.; Higuchi, Y.; Kano, K.; Seo, D.; et al. Structure and function of the engineered multicopper oxidase CueO from Escherichia coli—Deletion of the methionine-rich helical region covering the substrate-binding site. J. Mol. Biol. 2007, 373, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Miura, Y.; Tsujimura, S.; Kurose, S.; Kamitaka, Y.; Kataoka, K.; Sakurai, T.; Kano, K. Direct electrochemistry of CueO and its mutants at residues to and near type I Cu for oxygen-reducing biocathode. Fuel Cells 2009, 9, 70–78. [Google Scholar] [CrossRef]
- Nakagawa, K.; Takeuchi, M.; Kikuchi, M.; Kiyofuji, S.; Kugo, M.; Sakamoto, T.; Kano, K.; Ogawa, J.; Sakuradani, E. Mechanistic insights into indigo reduction in indigo fermentation: A voltammetric study. Electrochemistry 2021, 89, 25–30. [Google Scholar] [CrossRef]
- Nakagawa, K.; Takeuchi, M.; Kikuchi, M.; Tada, M.; Sakamoto, T.; Kano, K.; Ogawa, J.; Sakuradani, E. Voltammetric in-situ monitoring of leuco-indigo in indigo-fermenting suspensions. J. Biosci. Bioeng. 2021, 131, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Lopes, H.F.S.; Igarashi, K.; Yumoto, I. Characterization of the microbiota in long- and short-term natural indigo fermentation. J. Ind. Microbiol. Biotechnol. 2019, 46, 1657–1667. [Google Scholar] [CrossRef]
- Aino, K.; Hirota, K.; Okamoto, T.; Tu, Z.; Matsuyama, H.; Yumoto, I. Microbial communities associated with indigo fermentation that thrive in anaerobic alkaline environments. Front. Microbiol. 2018, 9, 2196. [Google Scholar] [CrossRef]
- Gross, A.; Holzinger, M.; Cosnier, S. 1. Buckypapers for Bioelectrochemical Applications: Bioelectrochemistry: Design and Applications of Biomaterials; Cosnier, S., Ed.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2019; pp. 1–22. [Google Scholar] [CrossRef]
- Gross, A.; Chen, X.; Giroud, F.; Abreu, C.; Goff, A.; Holzinger, M.; Cosnier, S. A High Power Buckypaper Biofuel Cell: Exploiting 1,10-Phenanthroline-5,6-dione with FAD-Dependent Dehydrogenase for Catalytically-Powerful Glucose Oxidation. ACS Catal. 2017, 7, 4408. [Google Scholar] [CrossRef]
- So, K.; Ozawa, H.; Onizuka, M.; Komukai, T.; Kitazumi, Y.; Shirai, O.; Kano, K. Highly permeable gas diffusion electrodes with hollow carbon nanotubes for bilirubin oxidase-catalyzed. Electrochim. Acta. 2017, 246, 794. [Google Scholar] [CrossRef]
- Shleev, S.; Shumakovich, G.; Morozova, O.; Yaropolov, A. Stable “floating”air diffusion biocathode based on direct electron transfer reactions between carbon particles and high redox potential laccase. Fuel Cells 2010, 10, 726. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikuchi, M.; Sowa, K.; Nakagawa, K.; Matsunaga, M.; Ando, A.; Kano, K.; Takeuchi, M.; Sakuradani, E. Indigo-Mediated Semi-Microbial Biofuel Cell Using an Indigo-Dye Fermenting Suspension. Catalysts 2021, 11, 1080. https://doi.org/10.3390/catal11091080
Kikuchi M, Sowa K, Nakagawa K, Matsunaga M, Ando A, Kano K, Takeuchi M, Sakuradani E. Indigo-Mediated Semi-Microbial Biofuel Cell Using an Indigo-Dye Fermenting Suspension. Catalysts. 2021; 11(9):1080. https://doi.org/10.3390/catal11091080
Chicago/Turabian StyleKikuchi, Mayu, Keisei Sowa, Kasumi Nakagawa, Momoka Matsunaga, Akinori Ando, Kenji Kano, Michiki Takeuchi, and Eiji Sakuradani. 2021. "Indigo-Mediated Semi-Microbial Biofuel Cell Using an Indigo-Dye Fermenting Suspension" Catalysts 11, no. 9: 1080. https://doi.org/10.3390/catal11091080
APA StyleKikuchi, M., Sowa, K., Nakagawa, K., Matsunaga, M., Ando, A., Kano, K., Takeuchi, M., & Sakuradani, E. (2021). Indigo-Mediated Semi-Microbial Biofuel Cell Using an Indigo-Dye Fermenting Suspension. Catalysts, 11(9), 1080. https://doi.org/10.3390/catal11091080