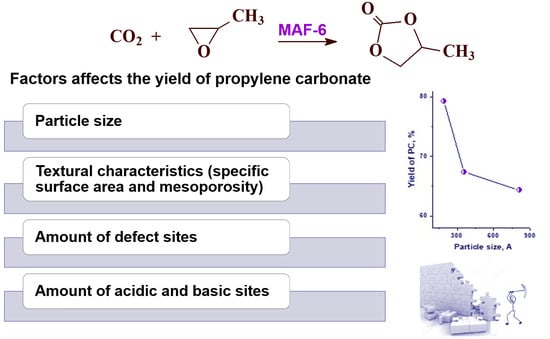
Effect of MAF-6 Crystal Size on Its Physicochemical and Catalytic Properties in the Cycloaddition of CO2 to Propylene Oxide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of MAF-6 and MAF-5
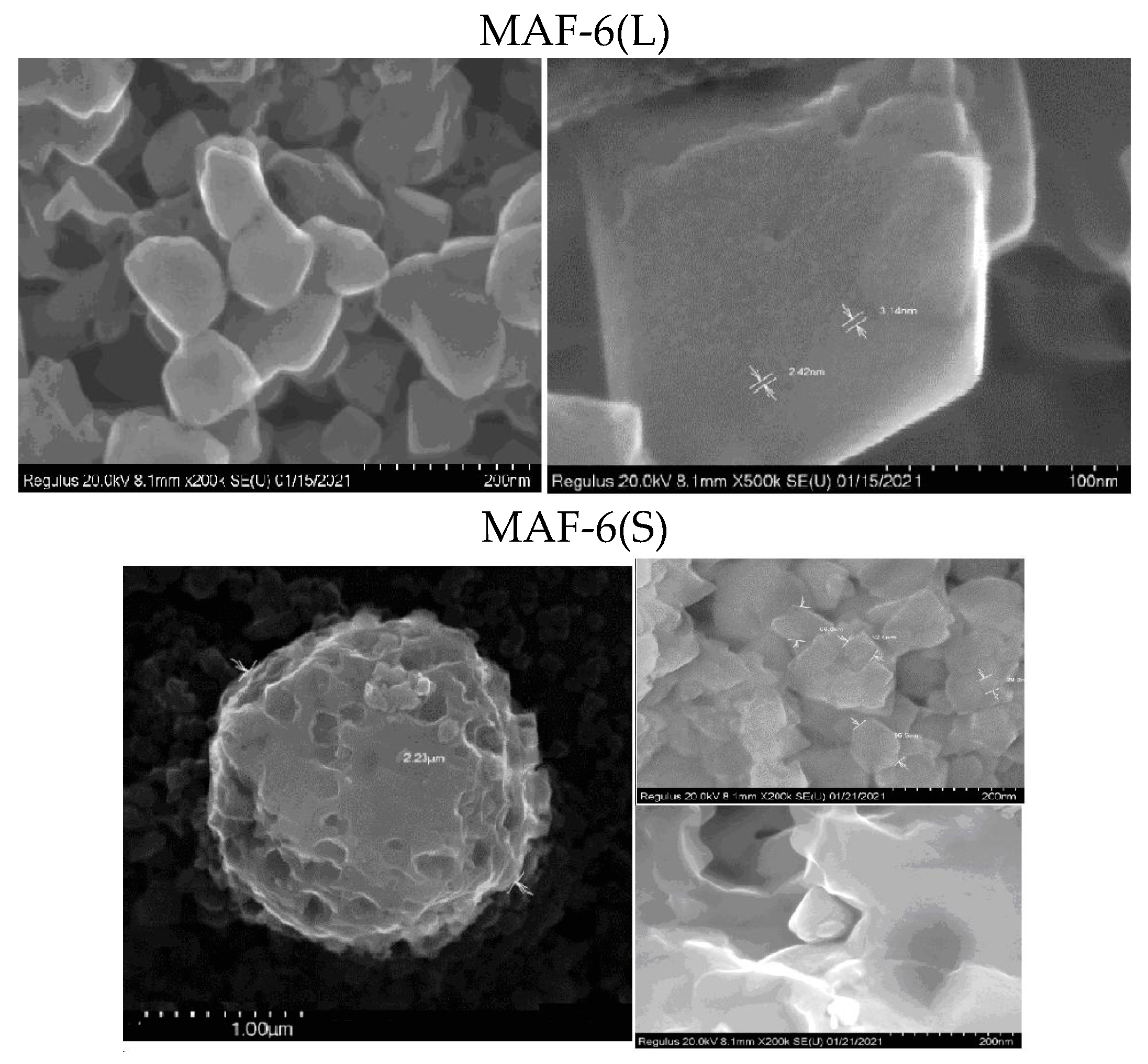
2.1.1. Structural and Textural Properties of MAF-6
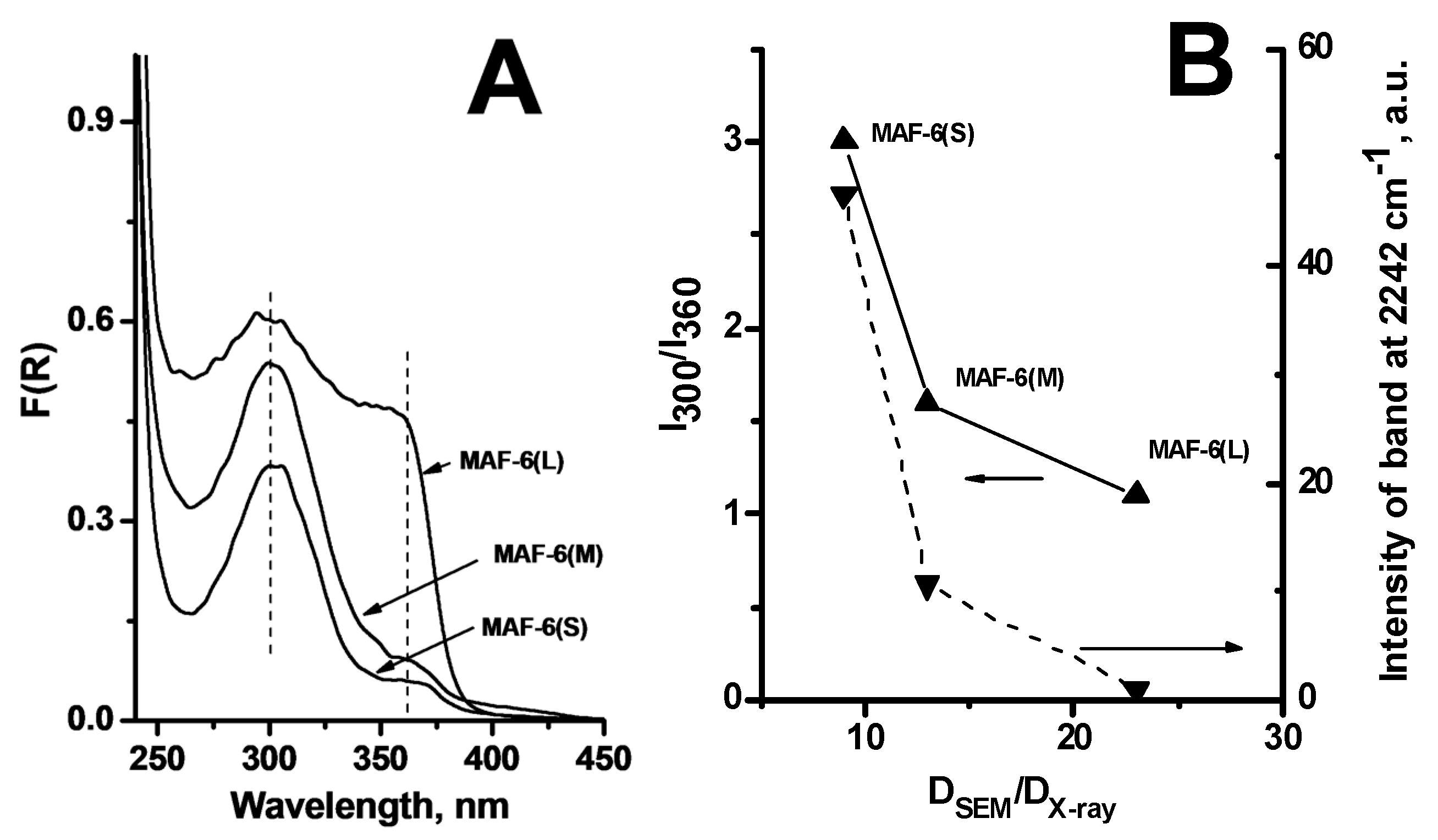
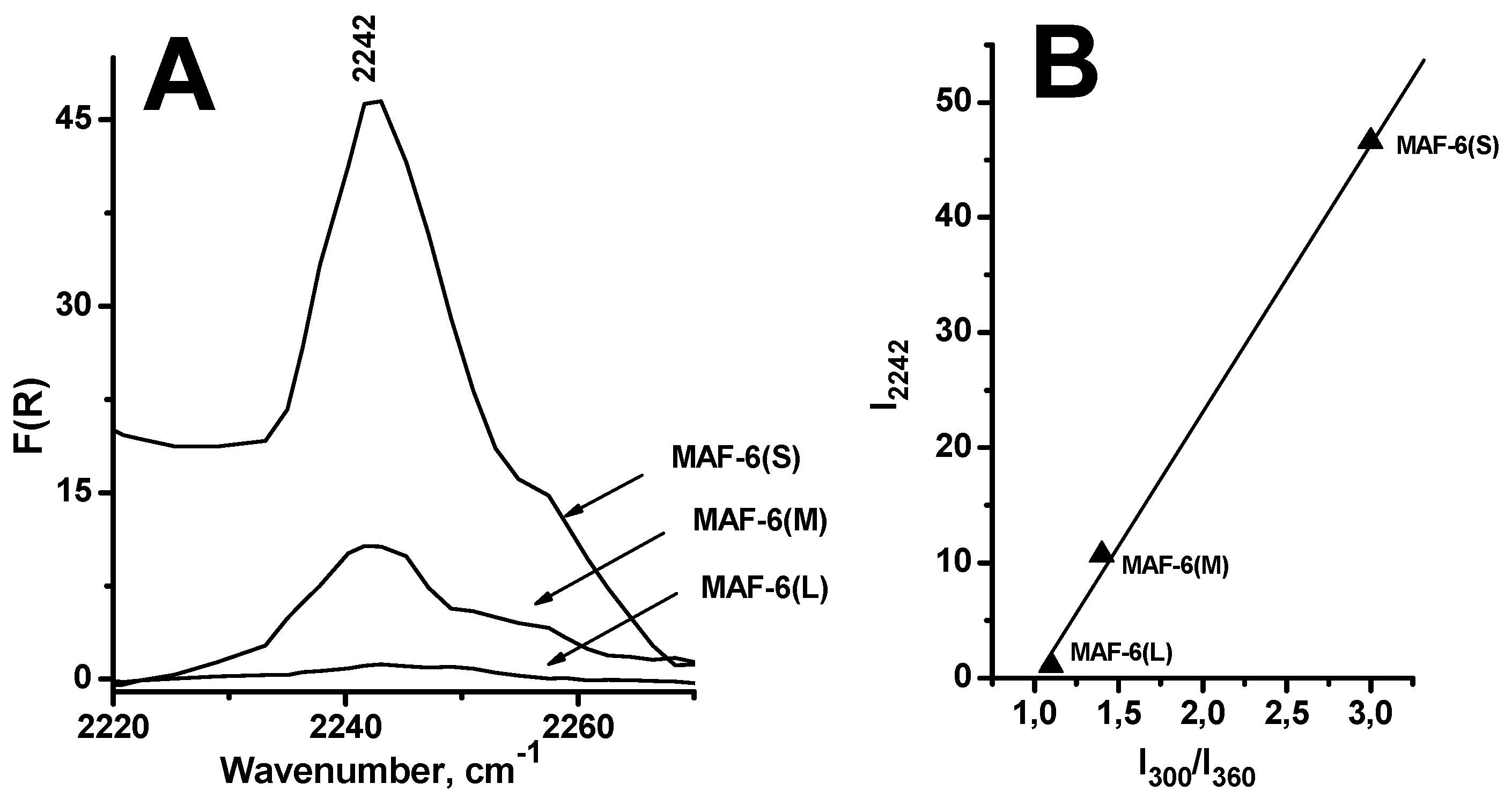
2.1.2. Nature of Active Sites of MAF-6
2.2. Catalytic Properties of MAF-6
2.3. Stability and Efficiency of MAF-6
3. Materials and Methods
3.1. Materials
3.2. Instrumental Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Academies of Sciences, Engineering, and Medicine. Chemical Utilization of CO2 into Chemicals and Fuels. In Gaseous Carbon Waste Streams Utilization: Status and Research Needs; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- Quadrelli, E.A.; Centi, G.; Duplan, J.-L.; Perathoner, S. Carbon dioxide recycling: Emerging large-scale technologies with industrial potential. ChemSusChem 2011, 4, 1194–1215. [Google Scholar] [CrossRef]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable conversion of carbon dioxide: An integrated review of catalysis and life cycle assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Nocito, F.; Dibenedetto, A. What catalysis can do for boosting CO2 utilization. Adv. Catal. 2018, 62, 49–109. [Google Scholar]
- VCI & DECHEMA Position Paper Utilization and Storage of CO2; DECHEMA: Frankfurt, Germany, 2009.
- Suib, S.L. (Ed.) New and Future Developments in Catalysis: Activation of Carbon Dioxide; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Bhanage, B.M.; Arai, M. (Eds.) Transformation and Utilization of Carbon Dioxide; Springer: New York, NY, USA, 2014. [Google Scholar]
- NexantECA. Market Analytics: Ammonia and Urea. Available online: https://www.nexanteca.com/news-and-media/new-analysis-market-analytics-ammonia-and-urea-2020-0 (accessed on 10 August 2021).
- ADROIT Market Research: Salicylic Acid Market. Available online: https://www.adroitmarketresearch.com/infographics/salicylic-acid-industry (accessed on 10 August 2021).
- Fukuoka, S.; Kawamura, M.; Komiya, K.; Tojo, M.; Hachiya, H.; Hasegawa, K.; Aminaka, M.; Okamoto, H.; Fukawa, I.; Konno, S. A novel non-phosgene polycarbonate production process using by-product CO2 as starting material. Green Chem. 2003, 5, 497–507. [Google Scholar] [CrossRef]
- Langanke, J.; Wolf, A.; Hofmann, J.; Böhm, K.; Subhani, M.A.; Müller, T.E.; Leitner, W.; Gürtler, C. Carbon dioxide (CO2) as sustainable feedstock for polyurethane production. Green Chem. 2014, 16, 1865–1870. [Google Scholar] [CrossRef]
- Allied Marked Research: Ethylene Carbonate Market. Available online: https://www.alliedmarketresearch.com/ethylene-carbonate-market-A07307 (accessed on 10 August 2021).
- Market.us: Propylene Carbonate Market. Available online: https://market.us/report/propylene-carbonate-market (accessed on 30 August 2021).
- Kamphuis, A.J.; Picchioni, F.; Pescarmona, P.P. CO2-fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef] [Green Version]
- Lopes, E.J.C.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. New trends in the conversion of CO2 to cyclic carbonates. Catalysts 2020, 10, 479. [Google Scholar] [CrossRef]
- Yano, T.; Matsui, H.; Koike, T.; Ishiguro, H.; Fujihara, H.; Yoshihara, M.; Maeshima, T. Magnesium oxide-catalysed reaction of carbon dioxide with an epoxide with retention of stereo chemistry. Chem Commun. 1997, 1129, 1130. [Google Scholar]
- Bhanage, B.M.; Fujita, S.; Ikushima, Y.; Torii, K.; Arai, M. Synthesis of dimethyl carbonate and glycols from carbon dioxide, epoxides and methanol using heterogeneous basic metal oxide catalysts with high activity and selectivity. Appl. Catal. A Gen. 2001, 219, 259–266. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.A.P.; Frizzo, C.P.; Moreira, D.N.; Zanatta, N.; Bonacorso, H.G. Ionic liquids in heterocyclic synthesis. Chem. Rev. 2008, 108, 2015–2050. [Google Scholar] [CrossRef]
- Sun, J.; Fujita, S.; Arai, M. Development in the green synthesis of cyclic carbonate from carbon dioxide using ionic liquids. J. Organomet. Chem. 2005, 690, 3490–3497. [Google Scholar] [CrossRef]
- Lan, D.H.; Fan, N.; Wang, Y.; Gao, X.; Zhang, P.; Chen, L.; Au, C.T.; Yin, S.F. Recent advances in metal-free catalysts for the synthesis of cyclic carbonates from CO2 and epoxides. Chin. J. Catal. 2016, 37, 826–845. [Google Scholar] [CrossRef]
- Girard, A.L.; Simon, N.; Zanatta, M.; Marmitt, S.; Goncalves, P.; Dupont, J. Insights on recyclable catalytic system composed of task-specific ionic liquids for the chemical fixation of carbon dioxide. Green Chem. 2014, 16, 2815–2825. [Google Scholar] [CrossRef]
- Beyzavi, M.H.; Stephenson, C.J.; Liu, Y.; Karagiaridi, O.; Hupp, J.T.; Farha, O.K. Metal-organic framework-based catalysts: Chemical fixation of CO2 with epoxides leading to cyclic organic carbonates. Front. Energy Res. 2015, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Perman, J.A.; Zhu, G.; Ma, S. Metal-organic frameworks for CO2 chemical transformations. Small 2016, 12, 6309–6324. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Z.; Hu, S.; Wu, T.; Jiang, T.; Han, B. MOF-5/n-Bu4NBr: An efficient catalyst system for the synthesis of cyclic carbonates from epoxides and CO2under mild conditions. Green. Chem. 2009, 11, 1301–1336. [Google Scholar] [CrossRef]
- Xiang, W.; Shen, C.; Lu, Z.; Chen, S.; Li, X.; Zou, R.; Zhang, Y.; Liu, C. CO2 cycloaddition over ionic liquid immobilized hybrid zeolitic imidazolate frameworks: Effect of Lewis acid/base sites. Chem. Eng. Sci. 2021, 233, 116429. [Google Scholar] [CrossRef]
- Ryu, H.; Roshan, R.; Kim, M.-I.; Kim, D.-W.; Selvaraj, M.; Park, D.-W. Cycloaddition of carbon dioxide with propylene oxide using zeolitic imidazolate framework ZIF-23 as a catalyst. Korean J. Chem. Eng. 2017, 34, 928–934. [Google Scholar] [CrossRef]
- Tharun, J.; Mathai, G.; Kathalikkatti, A.C.; Roshan, R.; Won, Y.-S.; Cho, S.J.; Chang, J.-S.; Park, D.-W. Exploring the catalytic potential of ZIF-90: Solventless and Co-Catalyst-Free Synthesis of propylene carbonate from propylene oxide and CO2. Chem Plus Chem. 2015, 80, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Tharun, J.; Bhin, K.-M.; Roshan, R.; Kim, D.W.; Kathalikkattil, A.C.; Babu, R.; Ahn, H.Y.; Won, Y.S.; Park, D.-W. Ionic liquid tethered post functionalized ZIF-90 framework for the cycloaddition of propylene oxide and CO2. Green Chem. 2016, 18, 2479–2487. [Google Scholar] [CrossRef]
- Jose, T.; Hwang, Y.; Kim, D.-W.; Kim, M.-I.; Park, D.-W. Functionalized zeolitic imidazolate framework F-ZIF-90 as efficient catalyst for the cycloaddition of carbon dioxide to allyl glycidyl ether. Catal. Today 2015, 245, 61–67. [Google Scholar] [CrossRef]
- Bhin, K.M.; Tharun, J.; Roshan, K.R.; Kim, D.-W.; Chung, Y.; Park, D.-W. Catalytic performance of zeolitic imidazolate framework ZIF-95 for the solventless synthesis of cyclic carbonates from CO2 and epoxides. J. CO2 Util. 2017, 17, 112–118. [Google Scholar] [CrossRef]
- Kuruppathparambil, R.R.; Babu, R.; Jeong, H.; Hwang, G.-Y.; Jeong, G.S.; Kim, M.-I.; Dong-Kim, W.; Park, D.-W. A solid solution zeolitic imidazolate framework as a room temperature efficient catalyst for the chemical fixation of CO2. Green Chem. 2016, 18, 6349–6356. [Google Scholar] [CrossRef]
- Babu, R.; Kathalikkattil, A.C.; Roshan, R.; Tharun, J.; Kim, D.W.; Park, D.W. Dual-porous metal organic framework for room temperature CO2 fixation via cyclic carbonate synthesis. Green Chem. 2016, 18, 232–242. [Google Scholar] [CrossRef]
- Hu, L.; Yan, Z.; Mo, X.; Peng, X.; Chen, L. Morphology control synthesis of ZIF-8 as highly efficient catalyst for the cycloaddition of CO2 to cyclic carbonate. ChemCatChem. 2019, 11, 3212–3219. [Google Scholar] [CrossRef]
- Schejn, A.; Balan, L.; Falk, V.; Aranda, V.; Medjahdi, L.; Schneider, G.; Schneider, R. Controlling ZIF-8 nano- and microcrystal formation and reactivity through zinc salt variations. Cryst. Eng. Comm. 2014, 16, 4493–4500. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, Y.; Wu, X.; Jiang, H.; Wang, W.; Li, H. Hollow zeolitic imidazolate framework nanospheres as highly efficient cooperative catalysts for [3+3] cycloaddition reactions. J. Am. Chem. Soc. 2014, 136, 13963–13966. [Google Scholar] [CrossRef]
- Timofeeva, M.; Paukshtis, E.; Panchenko, V.; Shefer, K.; Isaeva, V.; Kustov, L.; Gerasimov, E. Tuning the catalytic performance of the novel composites based on ZIF-8 and Nafen through dimensional and concentration effects in the synthesis of propylene glycol methyl ether. Eur. J. Org. Chem. 2019, 26, 4215–4225. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Lukoyanov, I.A.; Jhung, S.H. Proceedings of the 2nd International Conference on Reaction Kinetics, Mechanisms and Catalysis, Budapest, Hungary, 20–22 May 2021.
- Timofeeva, M.N.; Lykoyanov, I.A.; Panchenko, V.N.; Shefer, K.I.; Bhadra, B.N.; Jhung, S.H. Zeolitic imidazolate frameworks ZIF-8 and MAF-5 as highly efficient heterogeneous catalysts for synthesis of 1-methoxy-2-propanol from methanol and propylene oxide. Ind. Eng. Chem. Res. 2019, 58, 10750–10758. [Google Scholar] [CrossRef]
- Bustamante, E.; Fernandez, J.L.; Zamaro, J.M. Influence of the solvent in the synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals at room temperature. J. Colloid. Interface Sci. 2014, 424, 37–43. [Google Scholar] [CrossRef]
- Marcus, Y. The Effectiveness of solvents as hydrogen bond donors. J. Solut. Chem. 1991, 20, 929–944. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Nalt. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Self, K.; Telfer, M.; Greer, H.F.; Zhou, W. Reversed crystal growth of RHO zeolitic imidazolate framework (ZIF). Chem. Eur. J. 2015, 21, 19090–19095. [Google Scholar] [CrossRef] [Green Version]
- Mortada, B.; Chaplais, G.; Nouali, H.; Marichal, C.; Patarin, J. Phase transformations of Metal-Organic Frameworks MAF-6 and ZIF-71 during intrusion-extrusion experiments. J. Phys. Chem. C 2019, 123, 4319–4328. [Google Scholar] [CrossRef]
- Gao, M.; Wang, J.; Rong, Z.; Shi, Q.; Dong, J. A combined experimental-computational investigation on water adsorption in various ZIFs with the SOD and RHO topologies. RSC Adv. 2018, 8, 39627–39634. [Google Scholar] [CrossRef] [Green Version]
- Abdel-wahab, M.S.; Jilani, A.; Yahia, I.S.; Al-Ghamdi, A.A. Enhanced the photocatalytic activity of Ni-doped ZnO thin films: Morphological, optical and XPS analysis. Superlatt. Microstruct. 2016, 94, 108–118. [Google Scholar] [CrossRef]
- Jilani, A.; Abdel-wahab, M.S.; Al-ghamdi Attieh, A.; Dahlan, A.; Sadik Yahia, I.S. Nonlinear optical parameters of nanocrystalline AZO thin film measured at different substrate temperatures. Phys. B Condens. Matter 2016, 481, 97–103. [Google Scholar] [CrossRef]
- Bettens, B.; Dekeyzer, S.; van der Bruggen, B.; Degreve, J.; Vandecasteele, C. Transport of pure components in pervaporation through a microporous silica membrane. J. Phys. Chem. B 2005, 109, 5216–5222. [Google Scholar] [CrossRef]
- Huang, X.-C.; Lin, Y.-Y.; Zhang, J.-P.; Chen, X.-M. Ligand-directed strategy for zeolite-type metal-organic frameworks: Zinc(II) imidazolates with unusual zeolitic topologies. Angew. Chem. Int. Ed. 2006, 45, 1557–1559. [Google Scholar] [CrossRef]
- Tanaka, S.; Fujita, K.; Miyake, Y.; Miyamoto, M.; Hasegawa, Y.; Makino, T.; van der Perre, S.; Remi, J.C.S.; van Assche, T.; Baron, G.V.; et al. Adsorption and diffusion phenomena in crystal size engineered ZIF-8 MOF. J. Phys. Chem. C 2015, 119, 28430–28439. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Gee, J.A.; Sholl, D.S.; Lively, R.P. Crystal-size-dependent structural transitions in nanoporous crystals: Adsorption-induced transitions in ZIF-8. J. Phys. Chem. C. 2014, 118, 20727–20733. [Google Scholar] [CrossRef]
- Karaagas, D.; Kurkcuoglu, G.S. Synthesis and characterizations of the cyanide-bridged heteronuclear polymeric complexes with 2-ethylimidazole. Bull. Chem. Soc. Ethiop. 2016, 30, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Arivazhagan, M.; Manivel, S.; Jeyavijayan, S.; Meenakshi, R. Vibrational spectroscopic (FTIR and FT-Raman), first-order hyperpolarizablity, HOMO, LUMO, NBO, Mulliken charge analyses of 2-ethylimidazole based on Hartree-Fock and DFT calculations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 134, 493–501. [Google Scholar] [CrossRef]
- Zhang, C.; Han, C.; Sholl, D.S.; Schmidt, J.R. Computational characterization of defects in metal-organic frameworks: Spontaneous and water induced point defects in ZIF-8. J. Phys. Chem. Lett. 2016, 7, 459–464. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Saikia, G.; Jana, S. Physical and structural characterization of biofield treated imidazole derivatives. Nat. Prod. Chem. Res. 2015, 3, 187–195. [Google Scholar] [CrossRef]
- Godlewska, S.; Jezierska, J.; Baranowska, K.; Augustin, E.; Dołęga, A. Copper(II) complexes with substituted imidazole and chlorido ligands: X-ray, UV-Vis, magnetic and EPR studies and chemotherapeutic potential. Polyhedron 2013, 65, 288–297. [Google Scholar] [CrossRef]
- Isaia, F.; Aragoni, M.C.; Arca, M.; Bettoschi, A.; Caltagirone, C.; Castellano, C.; Demartin, F.; Lippolis, V.; Pivetta, T.; Valletta, E. Zinc(II)-methimazole complexes: Synthesis and reactivity. Dalton. Trans. 2015, 44, 9805–9814. [Google Scholar] [CrossRef] [Green Version]
- Chizallet, C.; Lazare, S.; Bazer-Bachi, D.; Bonnier, F.; Lecocq, V.; Soyer, E.; Quoineaud, A.-A.; Bats, N. Catalysis of trans-esterification by a nonfunctionalized metal-organic framework: Acid-basicity at the external surface of ZIF-8 probed by FTIR and ab Initio calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Jun, J.W.; Hasan, Z.; Matrosova, M.M.; Jhung, S.H. Effects of linker substitution on catalytic properties of porous zirconium terephthalate UiO-66 in acetalization of benzyldehyde with methanol. Appl. Catal. A Gen. 2014, 471, 91–97. [Google Scholar] [CrossRef]
- Panchenko, V.N.; Matrosova, M.M.; Jeon, J.; Jun, J.W.; Timofeeva, M.N.; Jhung, S.H. Catalytic behavior of metal-organic frameworks in the Knoevenagel condensation reaction. J. Catal. 2014, 316, 251–259. [Google Scholar] [CrossRef]
- The National Institute of Standards and Technology (NIST) Is an Agency of the U.S. Commerce Department. Available online: https://webbook.nist.gov (accessed on 20 October 2018).
- Ueda, T.; Nakai, M.; Yamatani, T. A solid-state 1H-NMR study of the dynamic structure of ZIF-8 and its role in the adsorption of bulky molecules. Adsorption 2017, 23, 887–901. [Google Scholar] [CrossRef]
- Parlie, D.; Thiubaut, D.; Caude, M.; Rosset, R. Supercritical fluid chromatography of imidazole derivatives. Chromatographia 1991, 31, 293–296. [Google Scholar] [CrossRef]
- Matuszak, C.A.; Matuszak, A.J. Imidazole-versatile today, prominent tomorrow. J. Chem. Educ. 1976, 53, 280–284. [Google Scholar] [CrossRef]
- Mori, K.; Mitani, Y.; Hara, T.; Mizugaki, T.; Ebitani, K.; Kaneda, K. A single-site hydroxyapatite-bound zinc catalyst for highly efficient chemical fixation of carbon dioxide with epoxides. Chem. Commun. 2005, 3331, 3333. [Google Scholar]
- Vitillo, J.G.; Crocella, V.; Bonino, F. ZIF-8 as a catalyst in ethylene oxide and propylene oxide reaction with CO2 to cyclic organic carbonates. Chem. Eng. 2019, 3, 60. [Google Scholar] [CrossRef] [Green Version]
- Biswal, B.P.; Panda, T.; Banerjee, R. Solution mediated phase transformation (RHO to SOD) in porous Co-imidazolate based zeolitic frameworks with high water stability. Chem. Commun. 2012, 48, 11868–11870. [Google Scholar] [CrossRef] [PubMed]
- Linder-Patton, O.M.; de Prinse, T.J.; Furukawa, S.; Bell, S.G.; Sumida, K.; Doonan, C.J.; Sumby, C.J. Influence of nanoscale structuralisation on the catalytic performance of ZIF-8: A cautionary surface catalysis study. Cryst. Eng. Comm. 2018, 20, 4926–4934. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Seo, P.W.; Khan, N.A.; Jhung, S.H. Hydrophobic cobalt-ethylimidazolate frameworks: Phase-pure syntheses and possible application in cleaning of contaminated water. Inorg. Chem. 2016, 55, 11362–11371. [Google Scholar] [CrossRef] [PubMed]
- He, C.-T.; Jiang, L.; Ye, Z.-M.; Krishna, R.; Zhong, Z.-S.; Liao, P.-Q.; Xu, J.; Ouyang, G.; Zhang, J.-P.; Chen, X.-M. Exceptional hydrophobicity of a large-pore metal-organic zeolite. J. Am. Chem. Soc. 2015, 137, 7217–7223. [Google Scholar] [CrossRef]
- Paukshtis, E.A.; Kotsarenko, N.S.; Karakchiev, L.G. Investigation of proton-acceptor properties of oxide surfaces by IR spectroscopy of hydrogen-bonded complexes. React. Kinet. Catal. Lett. 1979, 12, 315–319. [Google Scholar] [CrossRef]




| Catalyst | Experimental Conditions | Catalytic Test | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| (mmol) a | PO (mmol) | PCO2 MPa | T (°C) | Time (h) | PO Conversion (%) | PC Yield (%) | ||
| MAF-6(L) | 0.85 | 24.0 | 0.8 | 80 | 5 | 8.0 | 7.9 | This work |
| MAF-6(M) | 0.85 | 24.0 | 0.8 | 80, 100 | 5 | 12.2, 34.1 | 11.0, 33.8 | This work |
| MAF-6(S) | 0.85 | 24.0 | 0.8 | 80 | 5 | 22.3 | 22.0 | This work |
| MAF-5 | 0.85 | 24.0 | 0.8 | 80 | 5 | 8.3 | 8.2 | This work |
| ZIF-8 | 0.52 | 25 | 1.0 | 110 | 3 | <5 | <5 | [26] |
| ZIF-23 | 0.8 | 42.9 | 3.0 | 140 | 24 | 71.2 | 70.3 | [27] |
| ZIF-90 | 0.52 | 42.9 | 1.2 | 90, 120 | 8 | 13, 88 | 11, 81 | [28] |
| ZIF-90 | 0.49 | 18.1 | 1.0 | 120 | 3 | 51.0 | 49.0 | [29] |
| F-ZIF-90 | 0.177 | 18.1 | 1.2 | 120 | 6 | 89 | 87.8 | [30] |
| ZIF-95 | 0.4 | 18.6 | 1.2 | 80 | 24 | 91.0 | 90 | [31] |
| CoZn-ZIF | 0.7 | 25 | 0.7 | 100 | 4 | 81 | 99 | [32] |
| Catalytic System | Experimental Conditions | Catalytic Test | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (mmol) a | [n-Bu4N]Br (mmol) a | PO (mmol) | PCO2 MPa | T (°C) | Time (h) | PO Conversion (%) | PC Yield (%) | ||
| MAF-6(L) | 0.85 | 0.85 | 24 | 0.8 | 80 | 5 | 65.0 | 64.3 | This work |
| MAF-6(M) | 0.85 | 0.85 | 24 | 0.8 | 80 | 5 | 67.6 | 67.2 | This work |
| MAF-6(S) | 0.85 | 0.85 | 24 | 0.8 | 80 | 5 | 80.5 | 79.6 | This work |
| MAF-5 | 0.85 | 0.85 | 24 | 0.8 | 80, 100 | 5 | 67.3, 80.7 | 66.6, 79.9 | This work |
| ZIF-23 | 0.8 | 0.8 | 42.9 | 1.2 | 80, 100, 120 | 6 | 55.6, 58.8, 61.9 | 55, 58.2, 61.3 | [27] |
| ZIF-95 | 0.4 | 0.4 | 18.6 | 1.2 | 80, 100 | 2 | 83.2, 97.2 | 84.0, 96.2 | [31] |
| MOF-5 | 2.5 | 2.5 | 20.0 | 6 | 50 | 4 | - | 97.6 | [25] |
| UMCM-1-NH2 | 0.64 | 0.64 | 42.8 | 1.2 | RT | 24 | 90 | 90 | [33] |
| Experimental Conditions | Crystal Size b (nm) | Textural Properties | ||||||
|---|---|---|---|---|---|---|---|---|
| Solvent | α a | SBET (m2/g) | VΣ (cm3/g) | Vµ (cm3/g) | Vµ/VΣ | |||
| (vol./vol.) | ||||||||
| MAF-6(S) | PrOH/cyclohexane | 28.5:1.5 | 0.84 | 190 ± 20 | 1258 | 0.59 | 0.43 | 0.73 |
| MAF-6(M) | EtOH/cyclohexane | 28:2 | 0.86 | 360 ± 30 | 1395 | 0.71 | 0.58 | 0.82 |
| MAF-6(L) | MeOH/cyclohexane | 27:3 | 0.98 | 810 ± 30 | 1579 | 0.68 | 0.63 | 0.93 |
| MAF-5 | MeOH | - | - | 190–260 | 464 | 0.32 | 0.17 | 0.53 |
| νC-D (cm−1) | PA (kJ/mol) | |
|---|---|---|
| CDCl3 | 2268 | - |
| 2-Methylimidazole [61] | - | 963.4 |
| 2-Ethylimidazole [61] | - | 978 |
| MAF-6 | 2250 | 839 |
| 2242 | 870 | |
| MAF-5 [39] | 2250 | 839 |
| 2243 | 864 | |
| 2235 | 884 | |
| ZIF-8 [37] | 2255 | 812 |
| 2245 | 858 | |
| NH2-UiO-66(Zr) [60] | 2253 | 839 |
| 2245 | 867 | |
| UiO-66(Zr) [60] | 2253 | 839 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timofeeva, M.N.; Lukoyanov, I.A.; Panchenko, V.N.; Bhadra, B.N.; Gerasimov, E.Y.; Jhung, S.H. Effect of MAF-6 Crystal Size on Its Physicochemical and Catalytic Properties in the Cycloaddition of CO2 to Propylene Oxide. Catalysts 2021, 11, 1061. https://doi.org/10.3390/catal11091061
Timofeeva MN, Lukoyanov IA, Panchenko VN, Bhadra BN, Gerasimov EY, Jhung SH. Effect of MAF-6 Crystal Size on Its Physicochemical and Catalytic Properties in the Cycloaddition of CO2 to Propylene Oxide. Catalysts. 2021; 11(9):1061. https://doi.org/10.3390/catal11091061
Chicago/Turabian StyleTimofeeva, Maria N., Ivan A. Lukoyanov, Valentina N. Panchenko, Biswa Nath Bhadra, Evgenii Yu Gerasimov, and Sung Hwa Jhung. 2021. "Effect of MAF-6 Crystal Size on Its Physicochemical and Catalytic Properties in the Cycloaddition of CO2 to Propylene Oxide" Catalysts 11, no. 9: 1061. https://doi.org/10.3390/catal11091061
APA StyleTimofeeva, M. N., Lukoyanov, I. A., Panchenko, V. N., Bhadra, B. N., Gerasimov, E. Y., & Jhung, S. H. (2021). Effect of MAF-6 Crystal Size on Its Physicochemical and Catalytic Properties in the Cycloaddition of CO2 to Propylene Oxide. Catalysts, 11(9), 1061. https://doi.org/10.3390/catal11091061