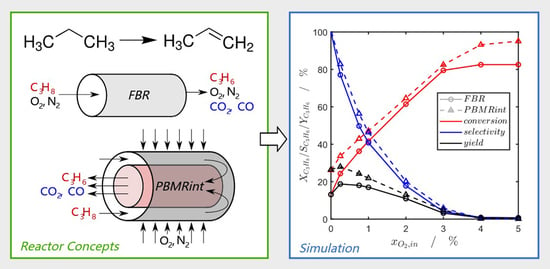
Process Intensification of the Propane Dehydrogenation Considering Coke Formation, Catalyst Deactivation and Regeneration—Transient Modelling and Analysis of a Heat-Integrated Membrane Reactor
Abstract
:1. Introduction
2. Results and Discussion
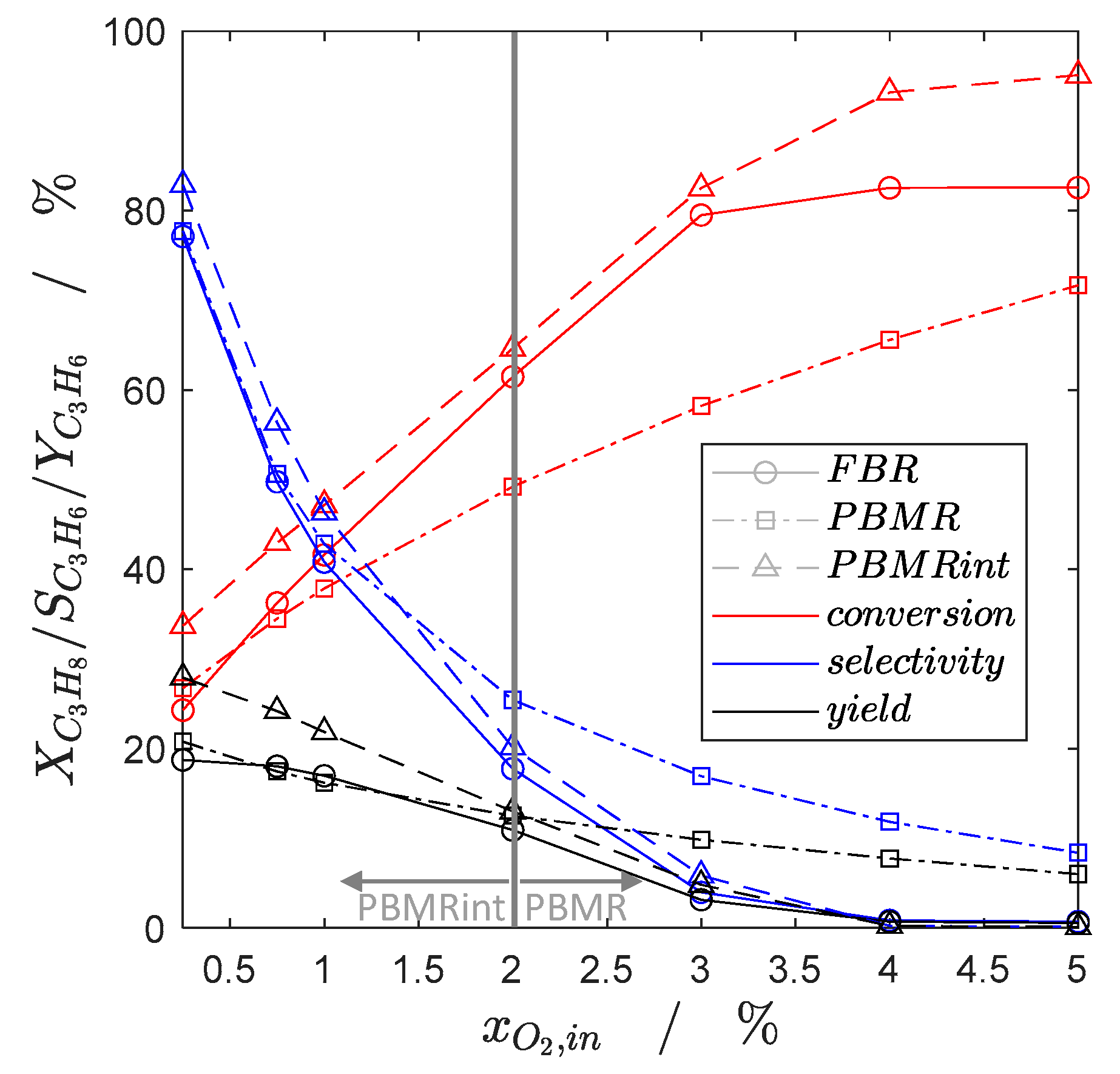
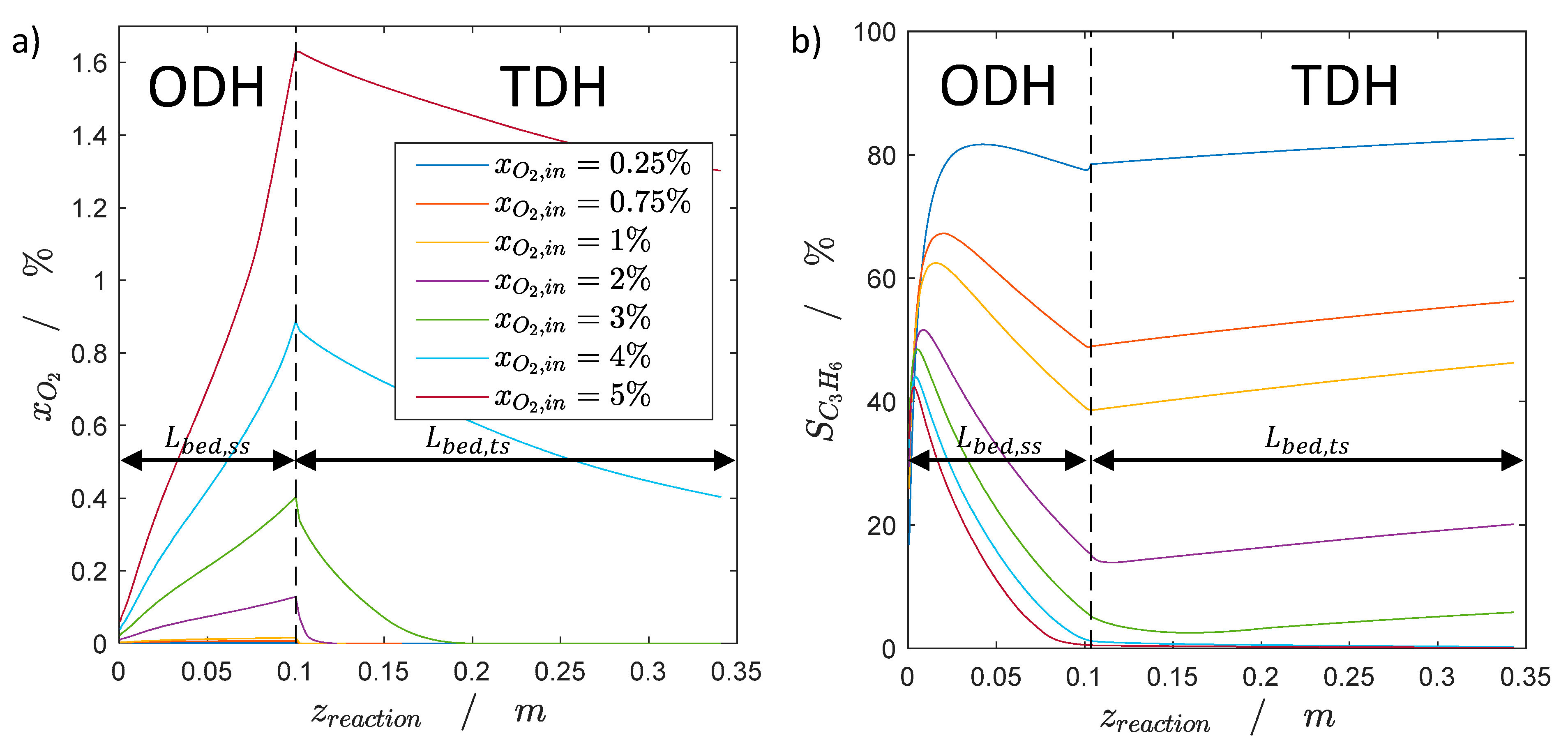
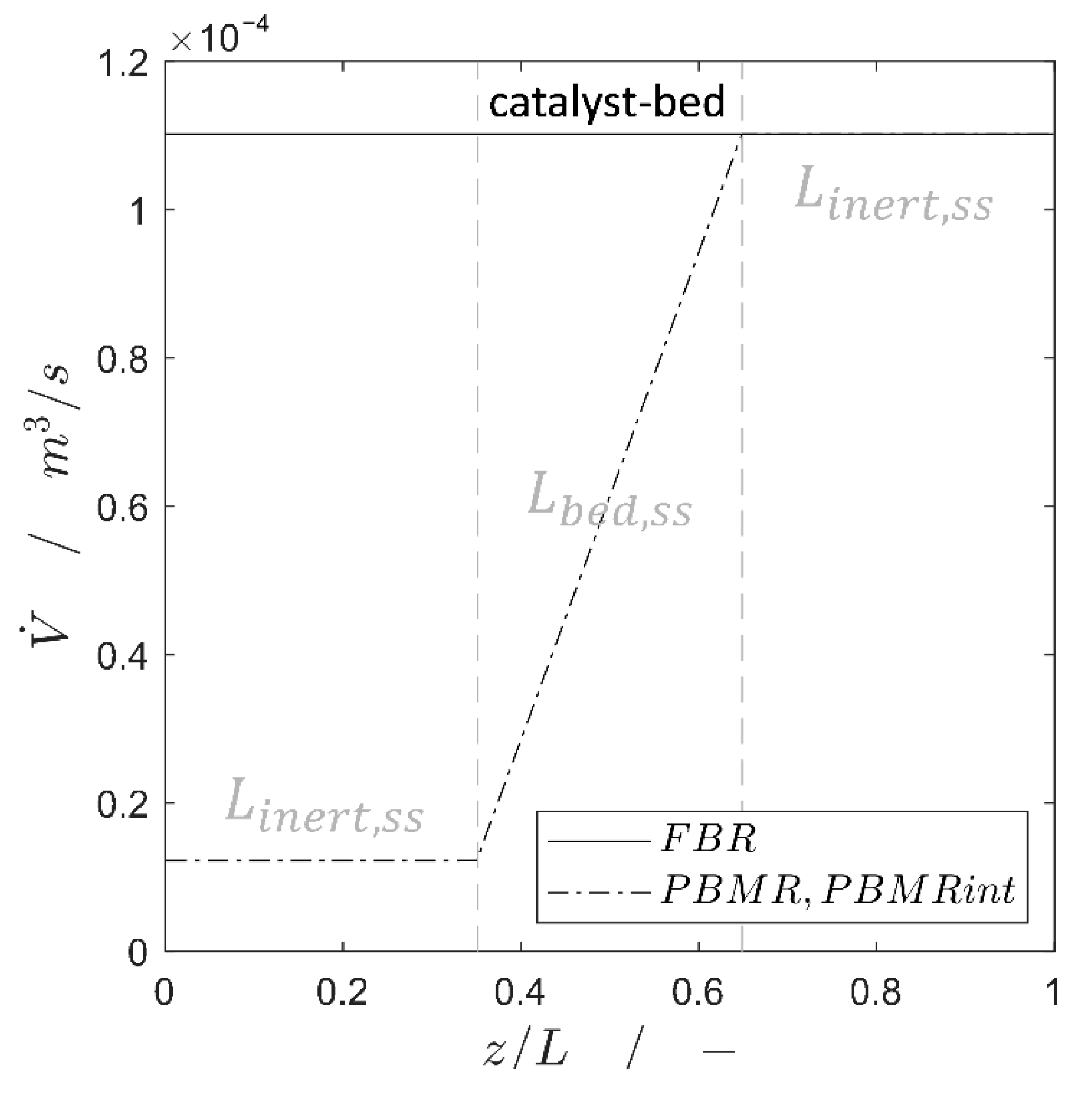
2.1. Steady-State Simulation Studies
2.2. Transient Simulation Studies
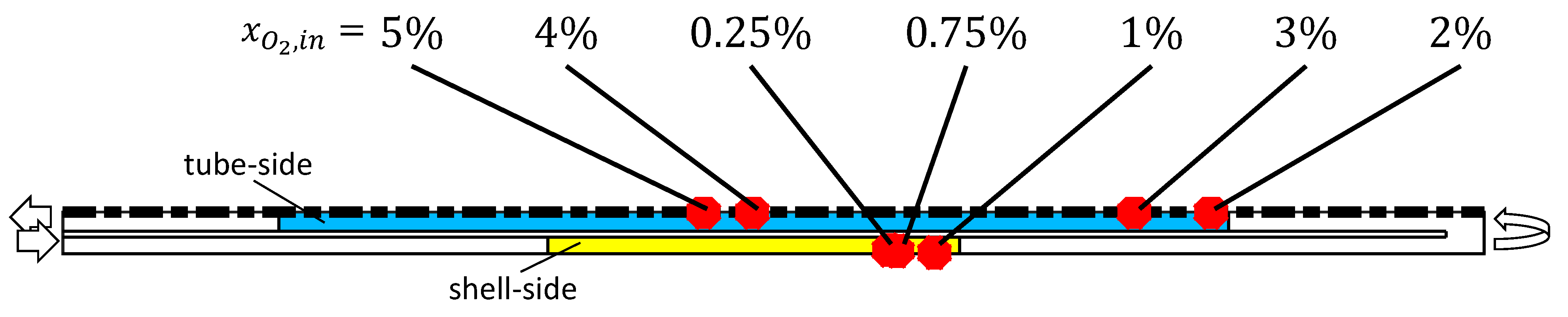
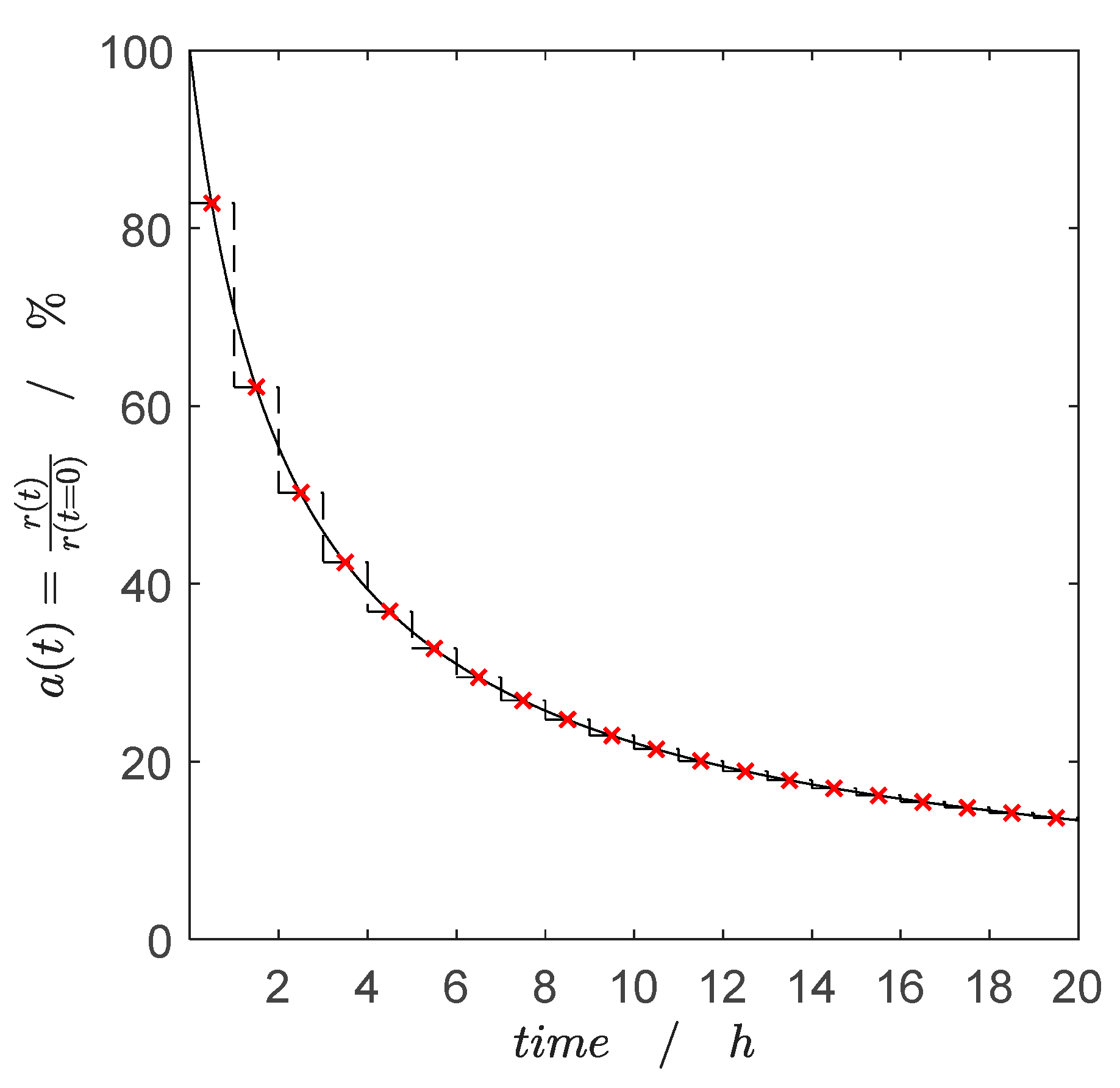
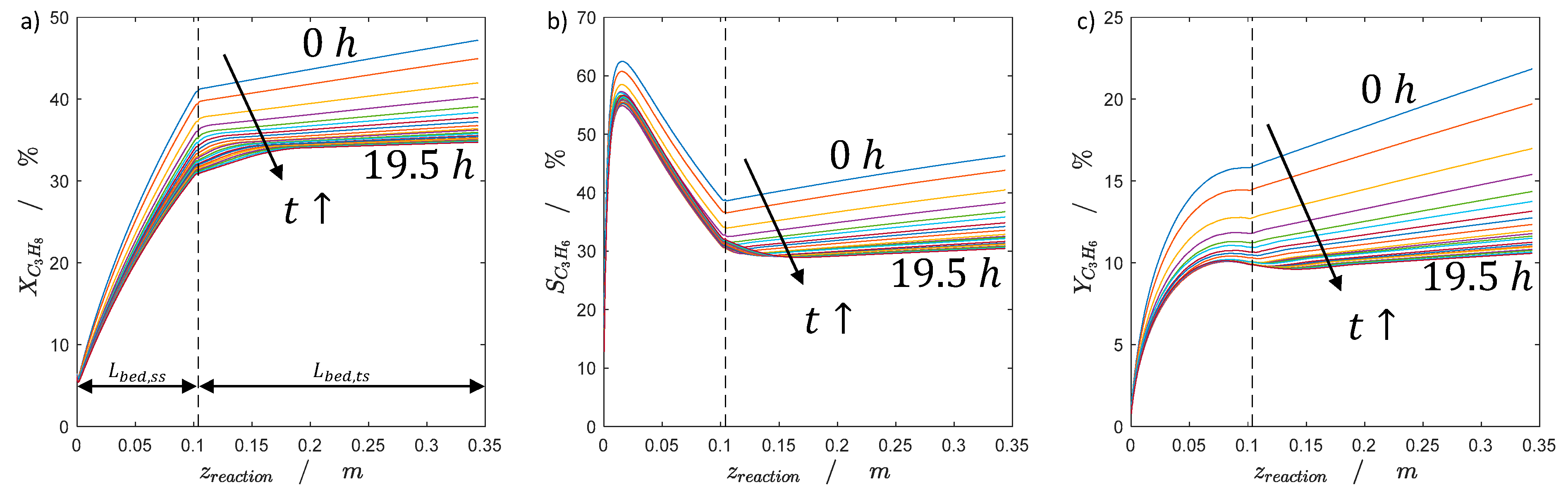
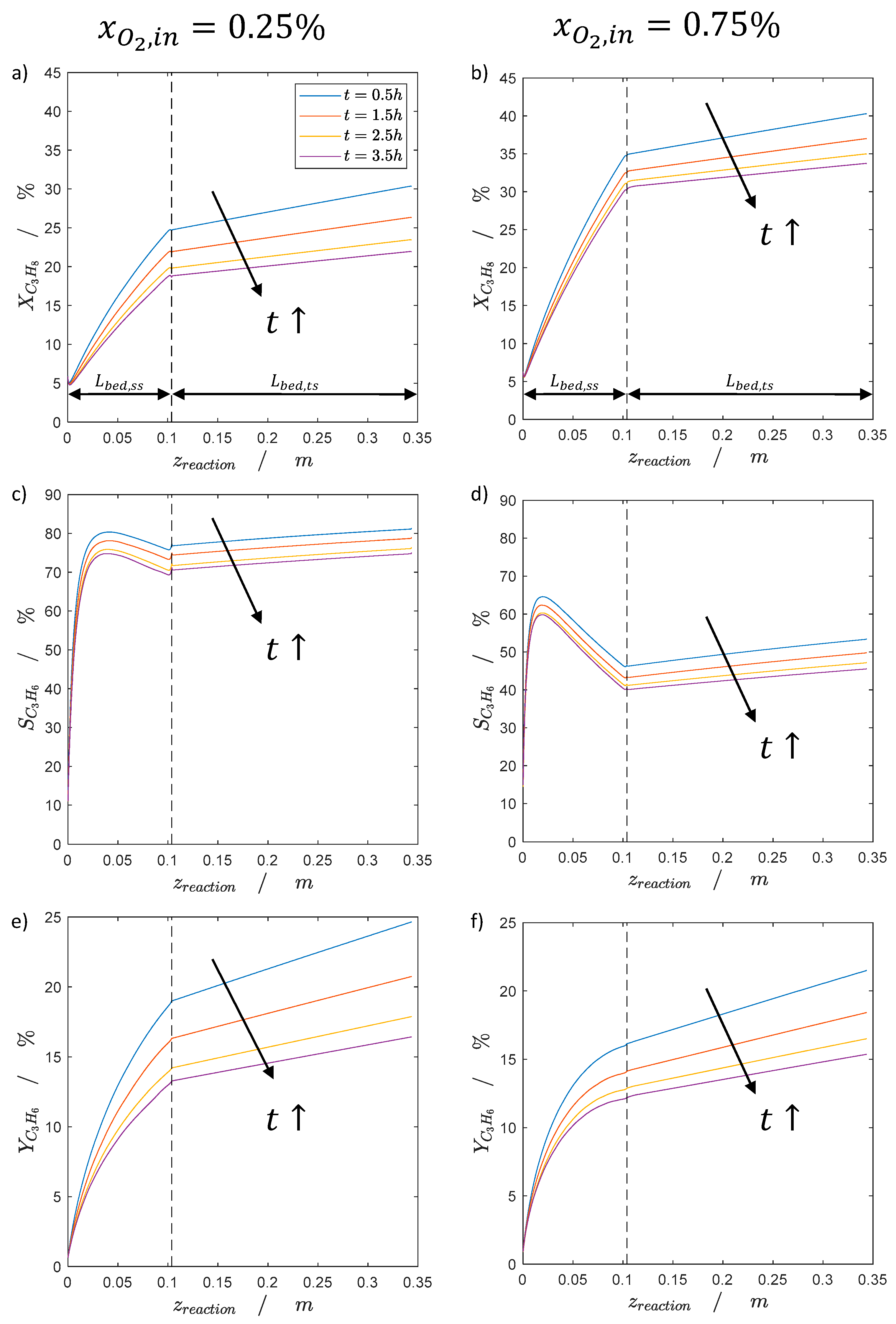
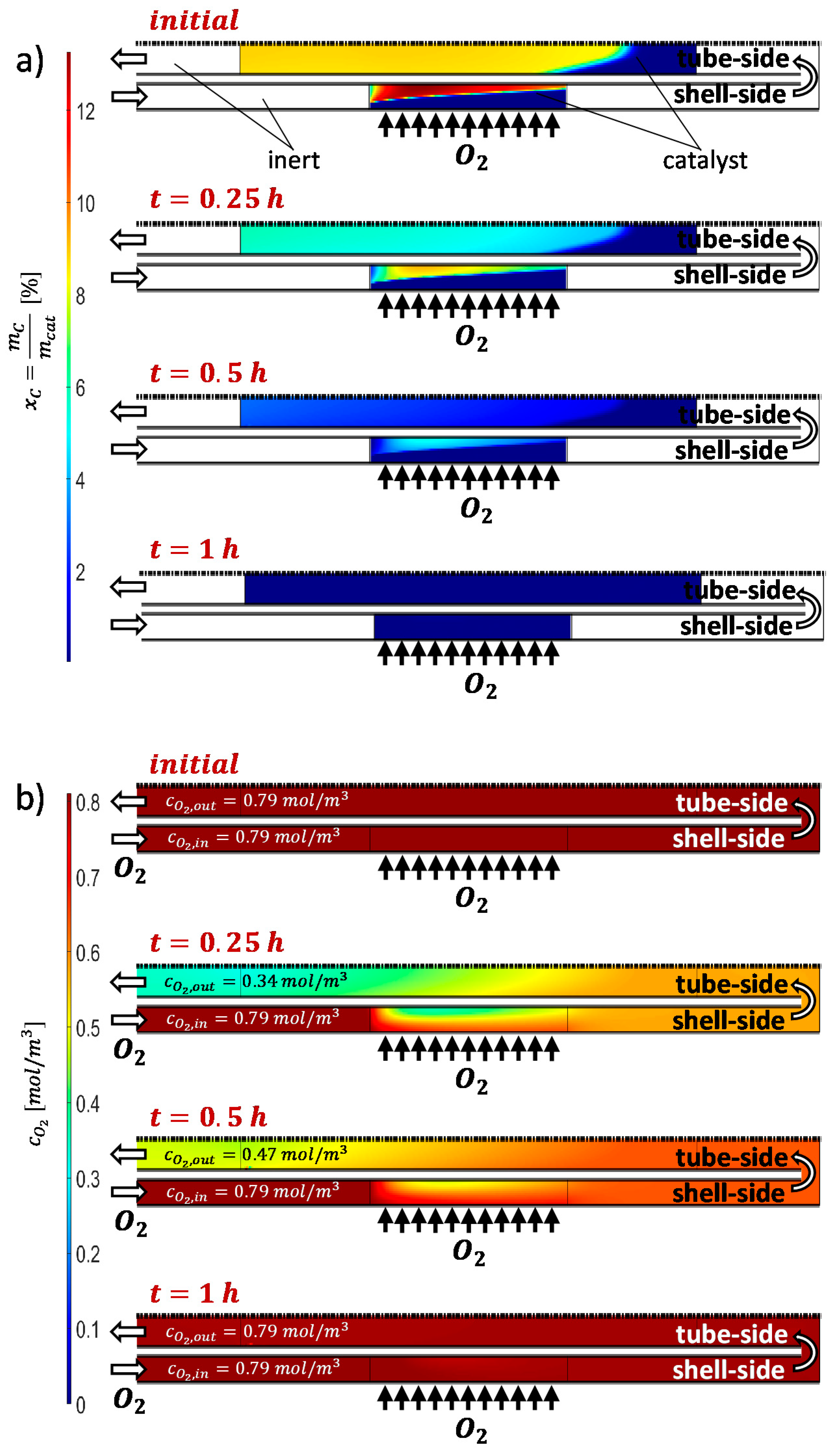
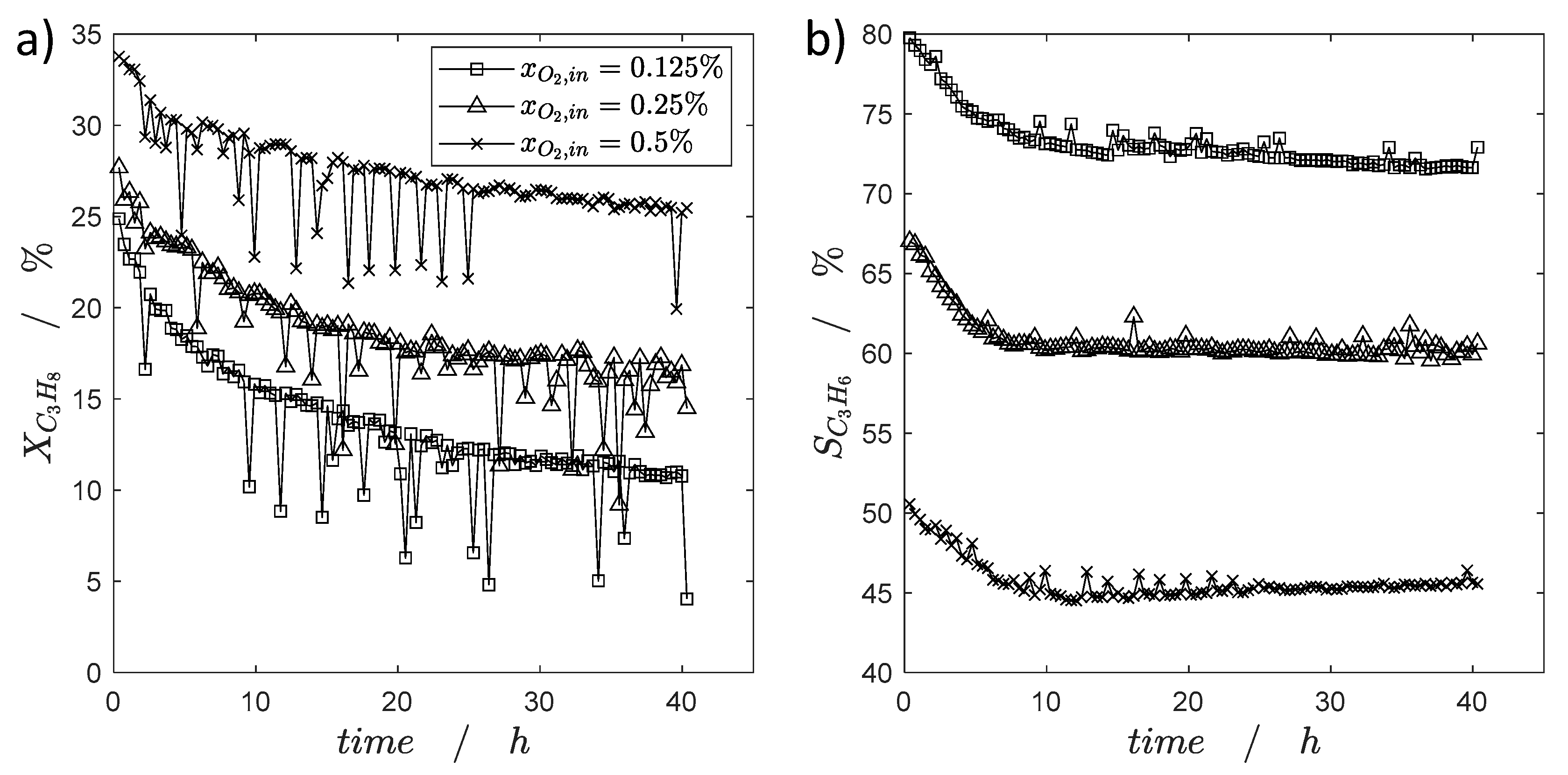
2.2.1. Catalyst Coking and Deactivation
2.2.2. Catalyst Regeneration by Coke Burning
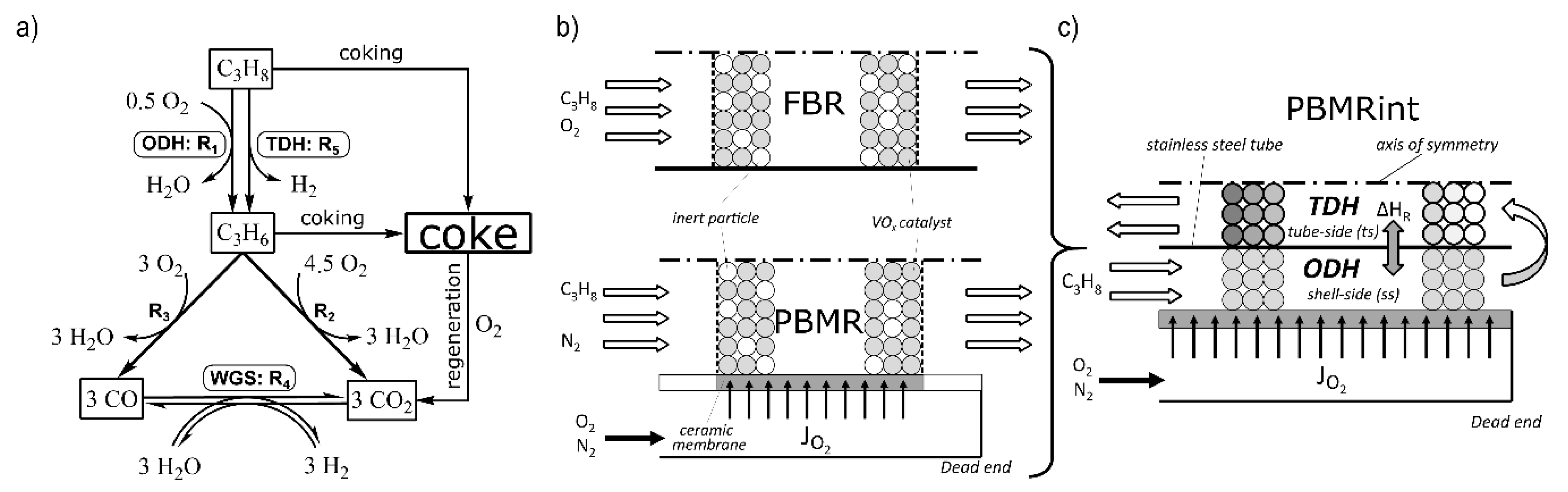
3. Modelling
3.1. Reaction Kinetics
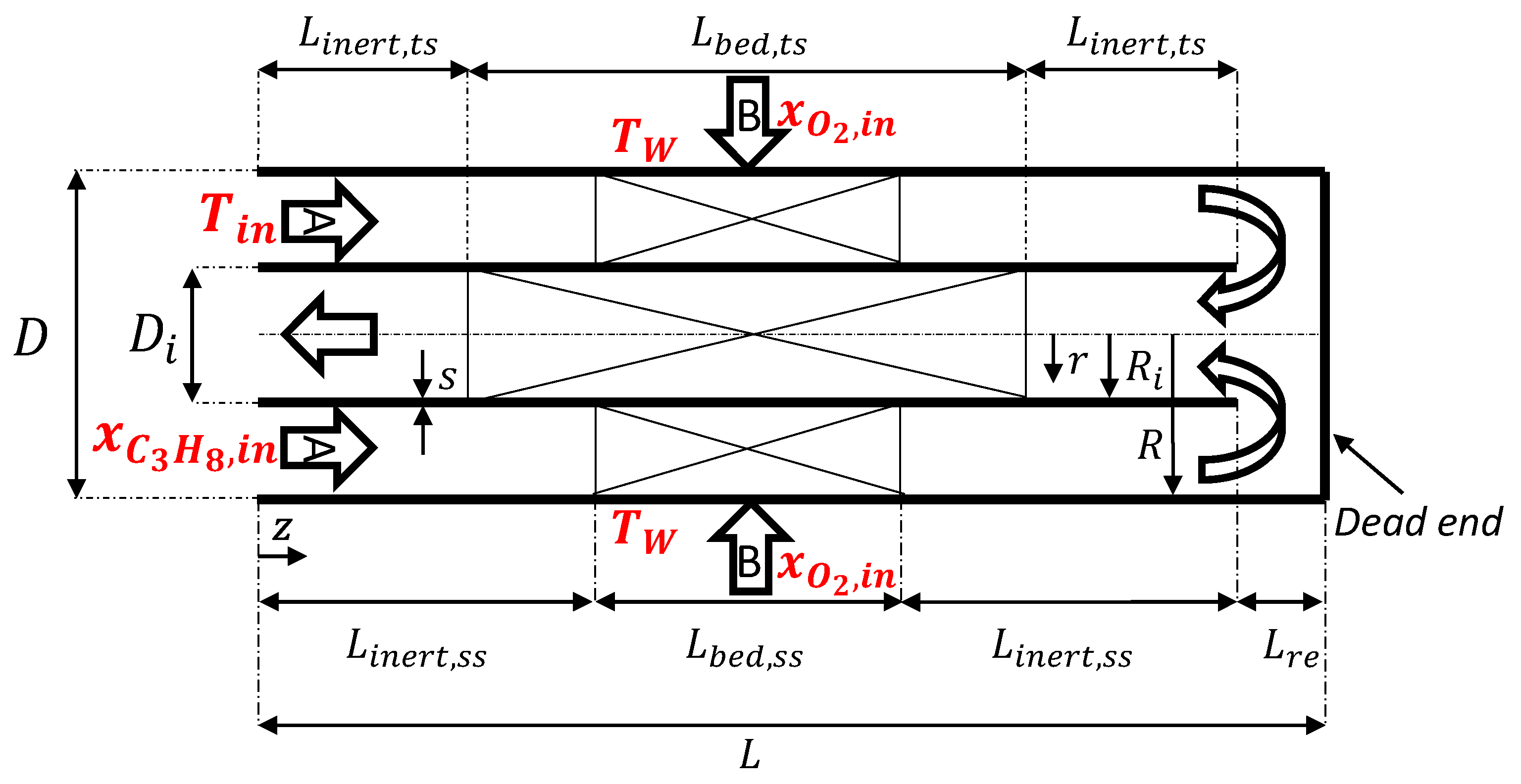
3.2. Steady-State Balance Equations
3.2.1. Component Mass Balance
3.2.2. Heat Balance
3.2.3. Momentum Balance
3.3. Transient Behavior
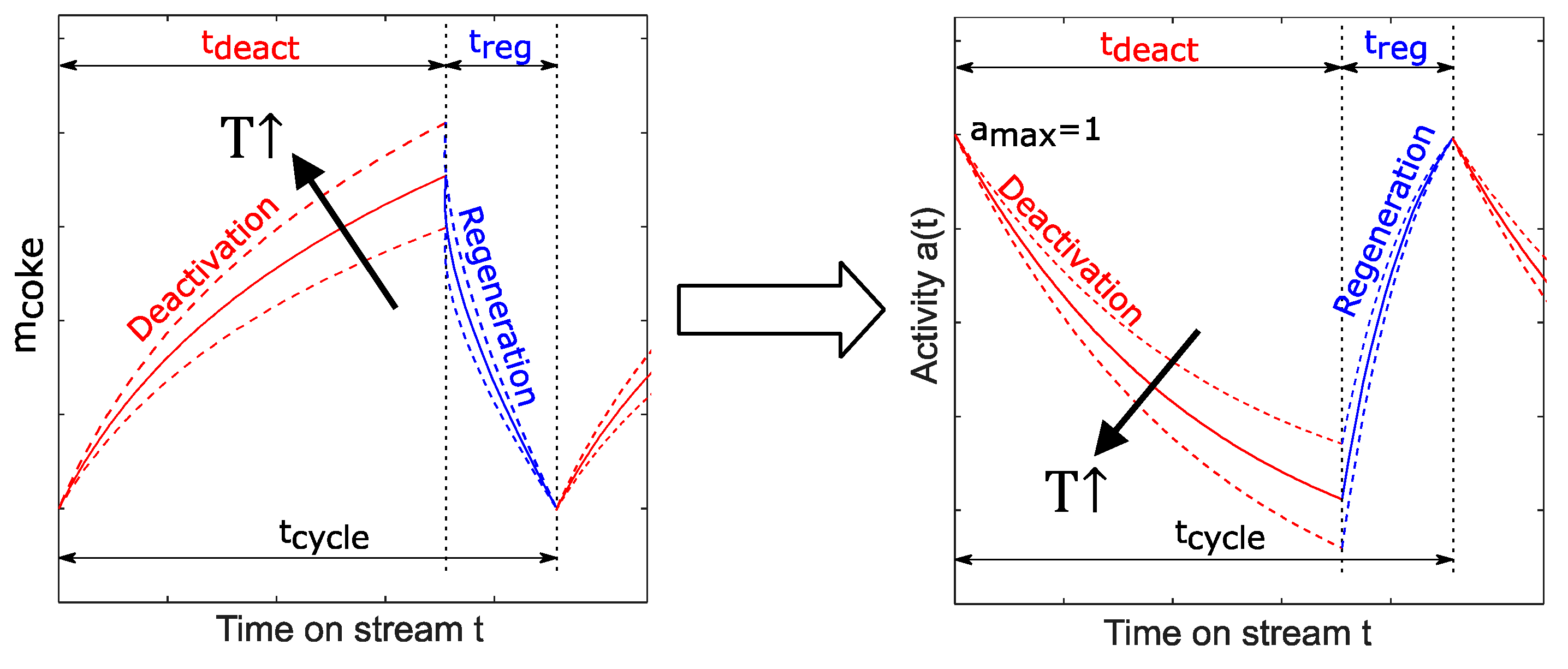
3.3.1. Coke Formation and Regeneration
3.3.2. Activity–Time Relationship
3.3.3. Transient Reactor Modelling of Coke Growth Considering Catalyst Activity
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Glossary
| Symbols | ||
| Symbol | Unit | Meaning |
| - | catalyst activity | |
| - | exponent | |
| A | area | |
| exponent | ||
| concentration | ||
| specific heat capacity | ||
| inner diameter of the membrane tube | ||
| inner diameter of the inner tube | ||
| effective mass dispersion coefficient | ||
| activation energy | ||
| molar flux | ||
| reaction rate constant | ||
| - | equilibrium constant | |
| reactor length | ||
| m | length of the catalyst-bed | |
| length of the inert zone | ||
| length of the recycle zone | ||
| mass | ||
| - | exponent | |
| molar flow | ||
| - | number of experiments | |
| - | number of measurements per experiment | |
| pressure | ||
| reaction rate | ||
| radial coordinate | ||
| tube radius | ||
| s | m | thickness of the inner tube |
| - | selectivity | |
| t | s | time |
| s | mean time of a time interval | |
| temperature | ||
| velocity | ||
| volumetric flow | ||
| reactor volume | ||
| - | molar fraction | |
| % | Coke concentration () | |
| - | conversion | |
| - | yield | |
| axial coordinate | ||
| Greek Letters | ||
| - | performance indicator (conversion, selectivity, yield) | |
| reaction enthalpy | ||
| - | porosity | |
| dynamic viscosity | ||
| effective heat dispersion coefficient | ||
| - | stoichiometric coefficient | |
| density | ||
| catalyst density | ||
| Subscripts | ||
| a | reactivation | |
| c | coking | |
| c | cross section | |
| cat | catalyst | |
| d | deactivation | |
| exp | experimental | |
| f | fluid | |
| i | component index | |
| in | inlet | |
| j | reaction index | |
| mem | membrane | |
| r | radial direction | |
| r | regeneration | |
| sim | simulated | |
| ss | shell-side | |
| ts | tube-side | |
| W | wall | |
| z | axial direction | |
| 0 | ambient | |
| Abbreviations | ||
| BC | boundary condition | |
| eq. | equation | |
| FBR | fixed-bed reactor | |
| ODH | oxidative dehydrogenation | |
| OF | objective function | |
| PBMR | packed-bed membrane reactor | |
| PBMRint | Heat-integrated packed-bed membrane reactor | |
| RSS | residual sum of squares | |
| TDH | thermal dehydrogenation | |
| A/B | inlet ratio | |
| WHSV | weight hourly space velocity | |
References
- Isac-García, J.; Dobado, J.A.; Calvo-Flores, F.G.; Martínez-García, H. Experimental Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Dittmeyer, R.; Caro, J. Handbook of Heterogeneous Catalysis; Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Perea, L.A.; Wolff, T.; Hamel, C.; Seidel-Morgenstern, A. Experimental Study of the Deactivation of Ni/AlMCM-41 Catalyst in the Direct Conversion of Ethene to Propene. Appl. Catal. A Gen. 2017, 533, 121–131. [Google Scholar] [CrossRef]
- Sundmacher, K.; Kienle, A.; Seidel-Morgenstern, A. Integrated Chemical Processes: Synthesis, Operation, Analysis, and Control; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Dingerdissen, U.; Martin, A.; Herein, D.; Wernicke, H.J. The Development of Industrial Heterogeneous Catalysis. In Handbook of Heterogeneous Catalysis; Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; ISBN 3527312412. [Google Scholar]
- Keil, F.J. Process Intensification. Rev. Chem. Eng. 2018, 34, 135–200. [Google Scholar] [CrossRef] [Green Version]
- Agar, D.W. Multifunctional Reactors: Old Preconceptions and New Dimensions. Chem. Eng. Sci. 1999, 54, 1299–1305. [Google Scholar] [CrossRef]
- Jurtz, N.; Srivastava, U.; Moghaddam, A.A.; Kraume, M. Particle-Resolved Computational Fluid Dynamics as the Basis for Thermal Process Intensification of Fixed-Bed Reactors on Multiple Scales. Energies 2021, 14, 2913. [Google Scholar] [CrossRef]
- Gallucci, F. Membrane Reactors: A Way to Increase Efficiency. J. Membr. Sci. Res. 2019, 5, 259–260. [Google Scholar] [CrossRef]
- Marín, P.; Hamel, C.; Ordóñez, S.; Díez, F.V.; Tsotsas, E.; Seidel-Morgenstern, A. Analysis of a fluidized bed membrane reactor for butane partial oxidation to maleic anhydride: 2D modelling. Chem. Eng. Sci. 2010, 65, 3538–3548. [Google Scholar] [CrossRef]
- Zimmermann, H. Propene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; ISBN 3527306730. [Google Scholar]
- Zimmermann, H.; Walzl, R. Ethylene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; ISBN 3527306730. [Google Scholar]
- Bai, P.; Etim, U.J.; Yan, Z.; Mintova, S.; Zhang, Z.; Zhong, Z.; Gao, X. Fluid Catalytic Cracking Technology: Current Status and Recent Discoveries on Catalyst Contamination. Catal. Rev. 2019, 61, 333–405. [Google Scholar] [CrossRef]
- Najari, S.; Saeidi, S.; Gallucci, F.; Drioli, E. Mixed Matrix Membranes for Hydrocarbons Separation and Recovery: A Critical Review. Rev. Chem. Eng. 2021, 37, 363–406. [Google Scholar] [CrossRef]
- Won, W.; Lee, K.S.; Lee, S.; Jung, C. Repetitive Control and Online Optimization of Catofin Propane Process. Comput. Chem. Eng. 2010, 34, 508–517. [Google Scholar] [CrossRef]
- Staszak, K.; Wieszczycka, K.; Tylkowski, B.; Staszak, K. Chemical Technologies and Processes; Staszak, K., Wieszczycka, K., Tylkowski, B., Eds.; De Gruyter: Berlin, Germany, 2020; ISBN 9783110656275. [Google Scholar]
- Brune, A.; Seidel-Morgenstern, A.; Hamel, C. Analysis and Model-Based Description of the Total Process of Periodic Deactivation and Regeneration of a VOx Catalyst for Selective Dehydrogenation of Propane. Catalysts 2020, 10, 1374. [Google Scholar] [CrossRef]
- Lian, Z.; Si, C.; Jan, F.; Zhi, S.; Li, B. Coke Deposition on Pt-Based Catalysts in Propane Direct Dehydrogenation: Kinetics, Suppression, and Elimination. ACS Catal. 2021, 11, 9279–9292. [Google Scholar] [CrossRef]
- Cavani, F.; Ballarini, N.; Cericola, A. Oxidative Dehydrogenation of Ethane and Propane: How Far From Commercial Implementation? Catal. Today 2007, 127, 113–131. [Google Scholar] [CrossRef]
- Bhasin, M.M.; McCain, J.H.; Vora, B.V.; Imai, T.; Pujadó, P.R. Dehydrogenation and Oxydehydrogenation of Paraffins to Olefins. Appl. Catal. A Gen. 2001, 221, 397–419. [Google Scholar] [CrossRef]
- Albonetti, S.; Cavani, F.; Trifirò, F. Key Aspects of Catalyst Design for the Selective Oxidation of Paraffins. Catal. Rev. 1996, 38, 413–438. [Google Scholar] [CrossRef]
- Walter, J.P.; Brune, A.; Seidel-Morgenstern, A.; Hamel, C. Model-based Analysis of Fixed-bed and Membrane Reactors of Various Scale. Chem. Ing. Tech. 2021, 93, 819–824. [Google Scholar] [CrossRef]
- Brune, A.; Wolff, T.; Seidel-Morgenstern, A.; Hamel, C. Analysis of Membrane Reactors for Integrated Coupling of Oxidative and Thermal Dehydrogenation of Propane. Chem. Ing. Tech. 2019, 91, 645–650. [Google Scholar] [CrossRef] [Green Version]
- Hamel, C.; Tóta, Á.; Klose, F.; Tsotsas, E.; Seidel-Morgenstern, A. Analysis of Single and Multi-Stage Membrane Reactors for the Oxidation of Short-Chain Alkanes—Simulation Study and Pilot Scale Experiments. Chem. Eng. Res. Des. 2008, 86, 753–764. [Google Scholar] [CrossRef]
- Klose, F.; Wolff, T.; Thomas, S.; Seidel-Morgenstern, A. Concentration and Residence Time Effects in Packed Bed Membrane Reactors. Catal. Today 2003, 82, 25–40. [Google Scholar] [CrossRef]
- Steen, H. Handbuch des Explosionsschutzes; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Winterberg, M.; Tsotsas, E.; Krischke, A.; Vortmeyer, D. A Simple and Coherent Set of Coefficients for Modelling of Heat and Mass Transport With and Without Chemical Reaction in Tubes Filled with Spheres. Chem. Eng. Sci. 2000, 55, 967–979. [Google Scholar] [CrossRef]
- Huš, M.; Kopač, D.; Likozar, B. Kinetics of Non-Oxidative Propane Dehydrogenation on Cr2O3 and the Nature of Catalyst Deactivation from First-Principles Simulations. J. Catal. 2020, 386, 126–138. [Google Scholar] [CrossRef]
- Phadke, N.M.; Mansoor, E.; Bondil, M.; Head-Gordon, M.; Bell, A.T. Mechanism and Kinetics of Propane Dehydrogenation and Cracking over Ga/H-MFI Prepared via Vapor-Phase Exchange of H-MFI with GaCl3. J. Am. Chem. Soc. 2019, 141, 1614–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, K.; Hughes, R. The Kinetics of Methane Steam Reforming over a Ni/α-Al2O Catalyst. Chem. Eng. J. 2001, 82, 311–328. [Google Scholar] [CrossRef]
- Chandrasekhara, B.C.; Vortmeyer, D. Flow Model for Velocity Distribution in Fixed Porous Beds under Isothermal Conditions. Wärme- und Stoffübertragung 1979, 12, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Ergun, S. Fluid Flow through Packed Columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Brinkman, H.C. A Calculation of the Viscous Force Exerted by a Flowing Fluid on a Dense Swarm of Particles. Appl. Sci. Res. 1949, 1. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Farrauto, R.J. Fundamentals of Industrial Catalytic Processes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 9780471730071. [Google Scholar]
- Castleman, A.W.; Schäfer, F.P.; Toennies, J.P.; Zinth, W. Basic Principles in Applied Catalysis; Baerns, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; ISBN 978-3-642-07310-6. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; Wiley: Hoboken, NJ, USA, 1999; ISBN 047125424X. [Google Scholar]
- Janssens, T.V.W.; Svelle, S.; Olsbye, U. Kinetic Modeling of Deactivation Profiles in the Methanol-to-hydrocarbons (MTH) Reaction: A Combined Autocatalytic–Hydrocarbon Pool Approach. J. Catal. 2013, 308, 122–130. [Google Scholar] [CrossRef]
- Nawaz, Z. Light Alkane Dehydrogenation to Light Olefin Technologies: A Comprehensive Review. Rev. Chem. Eng. 2015, 31. [Google Scholar] [CrossRef]





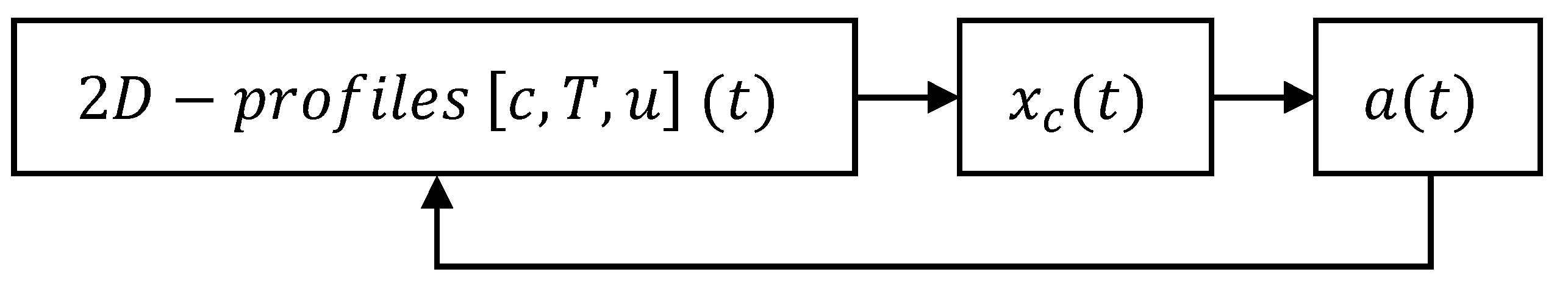

| Parameter | Value | Description |
|---|---|---|
| TW | 600 °C | Wall temperature |
| Tin | 600 °C | Inlet temperature |
| WHSV | Weight hourly space velocity | |
| mcat,ss | 0.017 kg | Mass of catalyst in the shell-side |
| 1% | Inlet concentration of propane |
| Parameter | Value | Description |
|---|---|---|
| 0.24 m | Length of catalyst-bed of tube-side | |
| 0.055 m | Length of inert zone of tube-side | |
| 0.0098 m | Inner diameter of inner tube | |
| 0.0015 m | Thickness of inner tube | |
| 0.104 m | Length of catalyst-bed of shell-side | |
| 0.123 m | Length of inert zone of shell-side | |
| 0.021 m | Inner diameter of membrane tube | |
| 0.01 m | Length of the reversal zone |
| Parameter | Opt. Value | Confidence Intervals | Unit | |
|---|---|---|---|---|
| - | ||||
| - | ||||
| Parameter | Opt. Value | Confidence Intervals | Unit |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walter, J.P.; Brune, A.; Seidel-Morgenstern, A.; Hamel, C. Process Intensification of the Propane Dehydrogenation Considering Coke Formation, Catalyst Deactivation and Regeneration—Transient Modelling and Analysis of a Heat-Integrated Membrane Reactor. Catalysts 2021, 11, 1056. https://doi.org/10.3390/catal11091056
Walter JP, Brune A, Seidel-Morgenstern A, Hamel C. Process Intensification of the Propane Dehydrogenation Considering Coke Formation, Catalyst Deactivation and Regeneration—Transient Modelling and Analysis of a Heat-Integrated Membrane Reactor. Catalysts. 2021; 11(9):1056. https://doi.org/10.3390/catal11091056
Chicago/Turabian StyleWalter, Jan P., Andreas Brune, Andreas Seidel-Morgenstern, and Christof Hamel. 2021. "Process Intensification of the Propane Dehydrogenation Considering Coke Formation, Catalyst Deactivation and Regeneration—Transient Modelling and Analysis of a Heat-Integrated Membrane Reactor" Catalysts 11, no. 9: 1056. https://doi.org/10.3390/catal11091056
APA StyleWalter, J. P., Brune, A., Seidel-Morgenstern, A., & Hamel, C. (2021). Process Intensification of the Propane Dehydrogenation Considering Coke Formation, Catalyst Deactivation and Regeneration—Transient Modelling and Analysis of a Heat-Integrated Membrane Reactor. Catalysts, 11(9), 1056. https://doi.org/10.3390/catal11091056