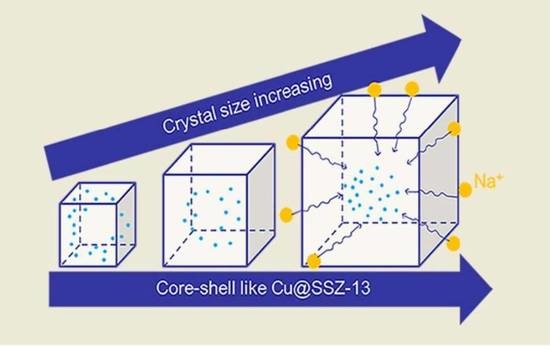
Improvement of Alkali Metal Resistance for NH3-SCR Catalyst Cu/SSZ-13: Tune the Crystal Size
Abstract
1. Introduction
2. Results and Discussions
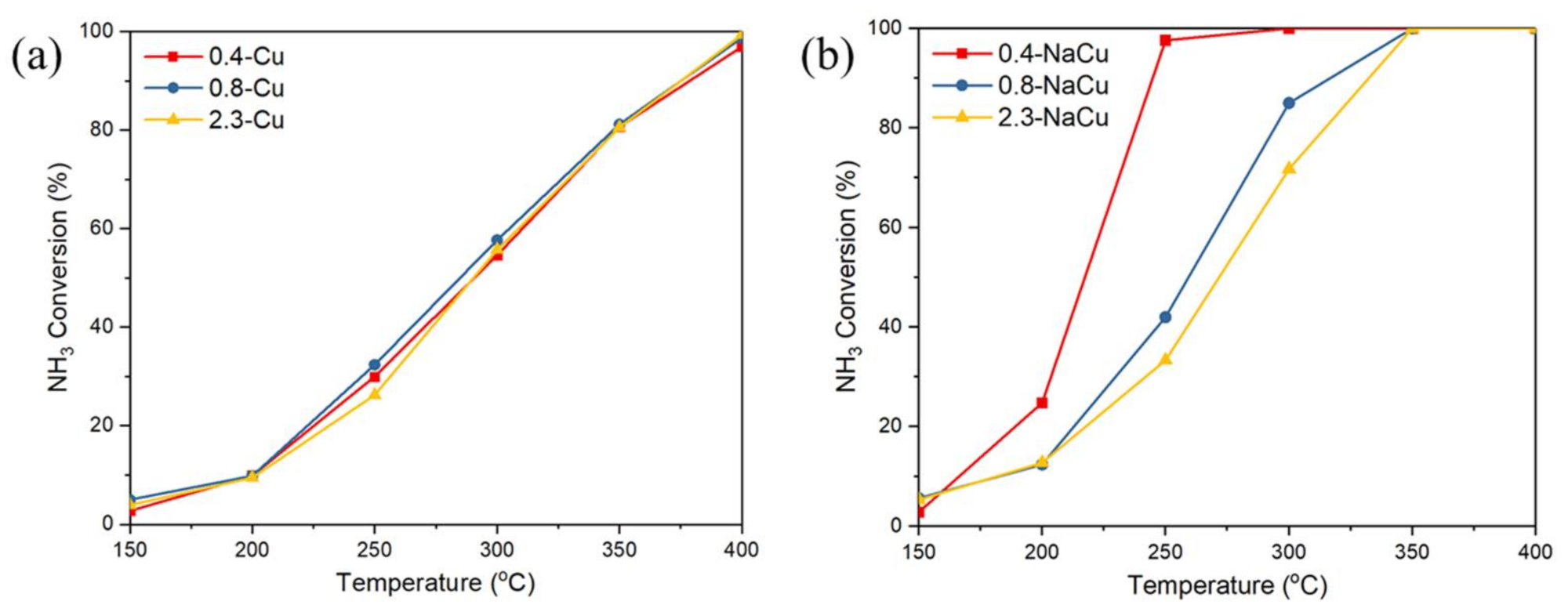
2.1. NH3-SCR and NH3 Oxidation Activity
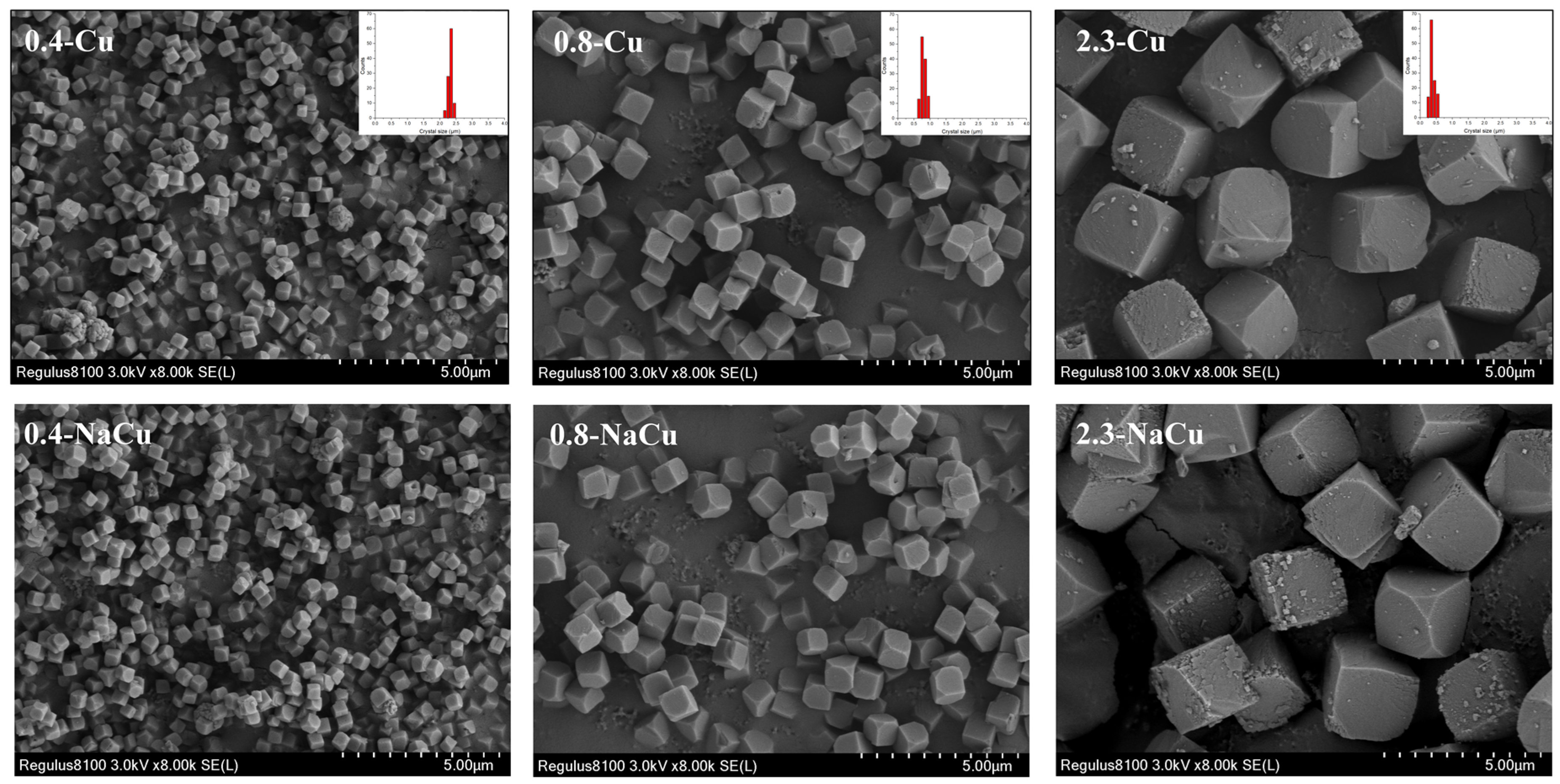
2.2. Texture Properties
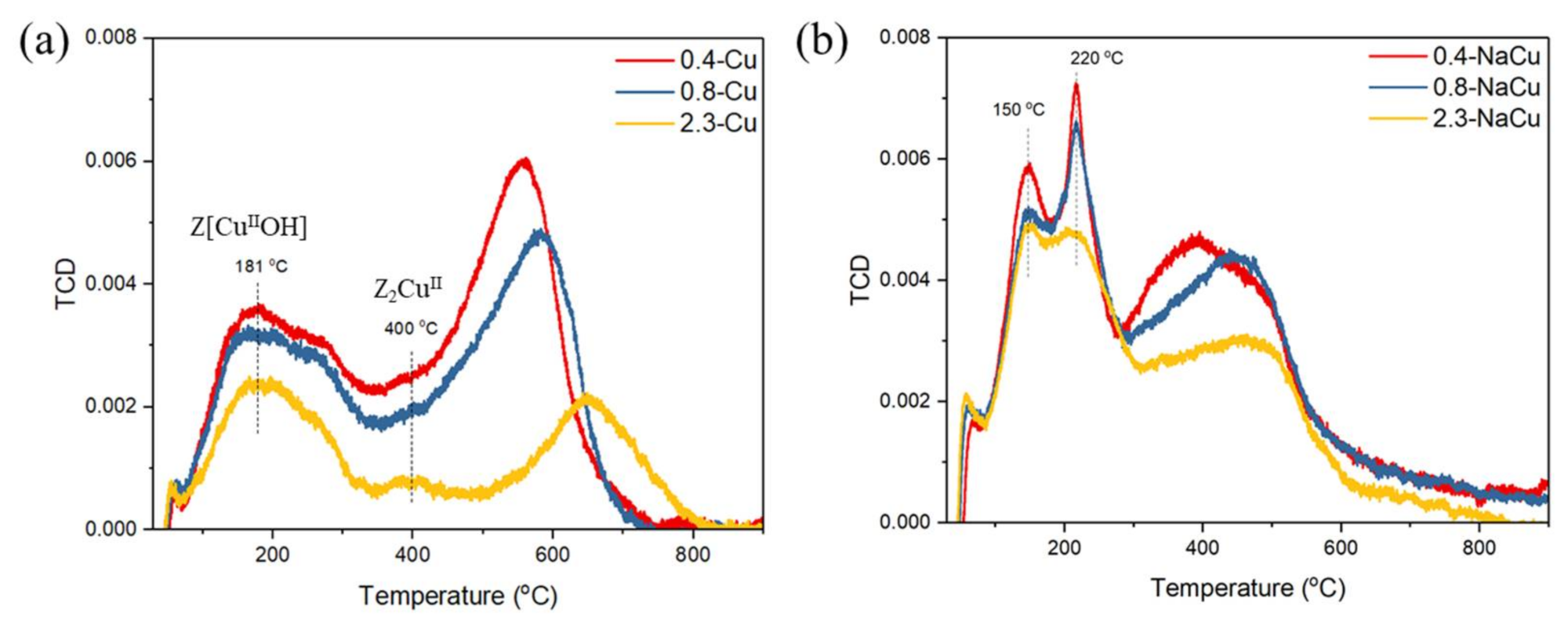
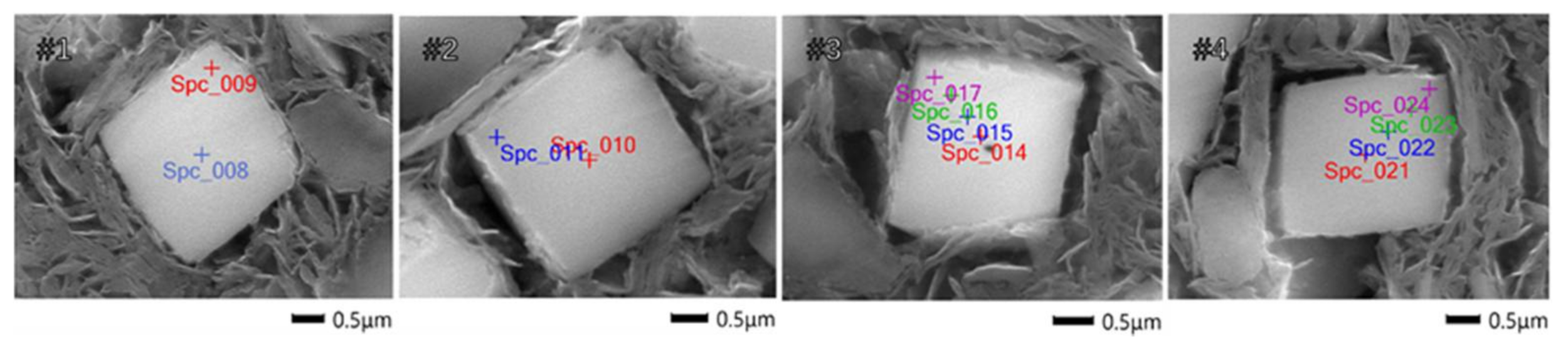
2.3. Cu Distribution
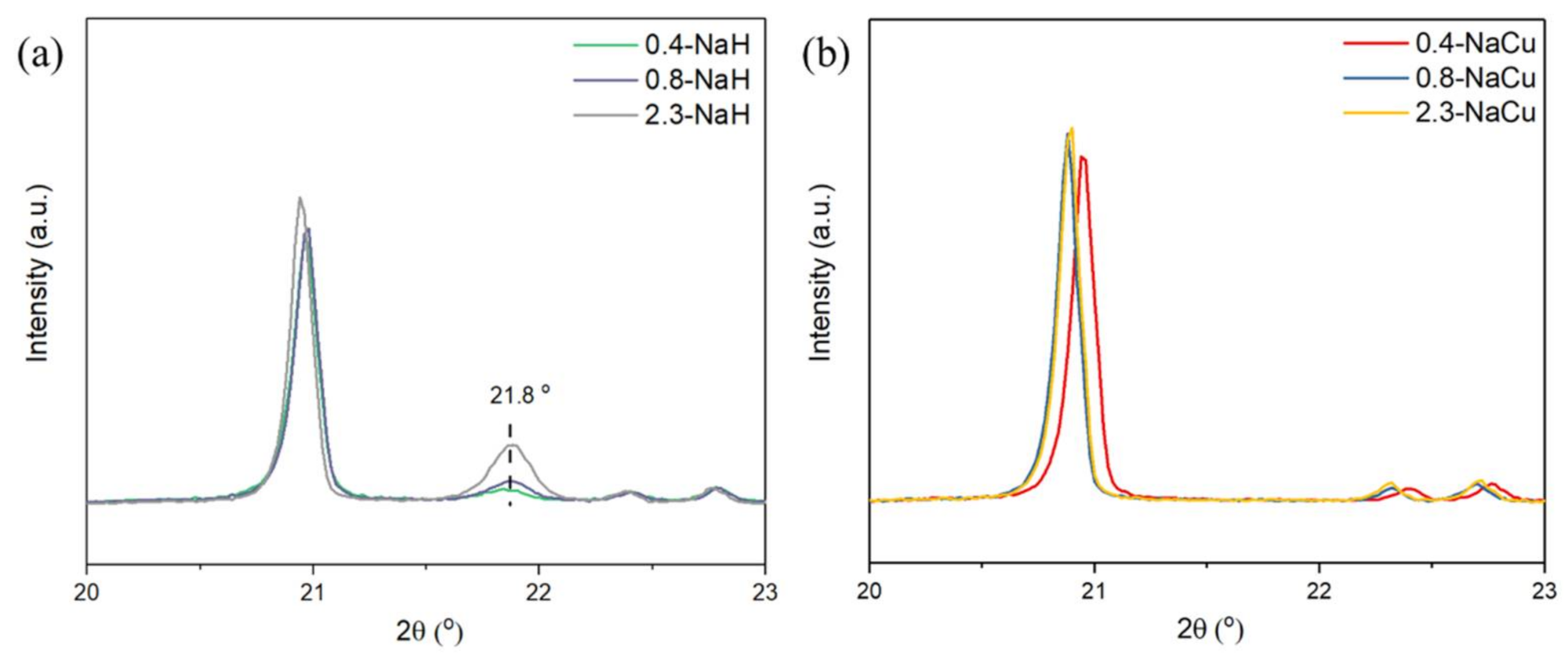
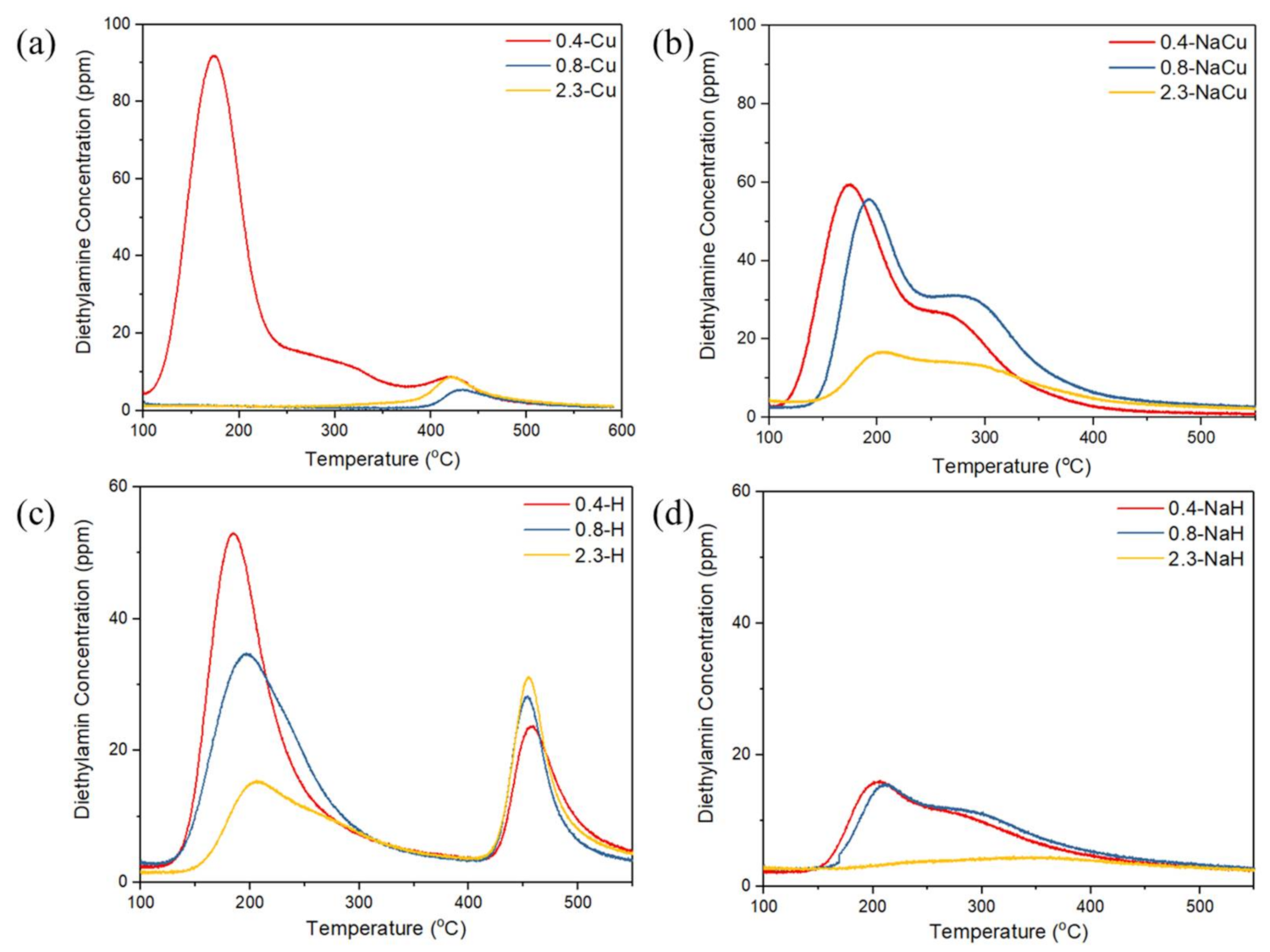
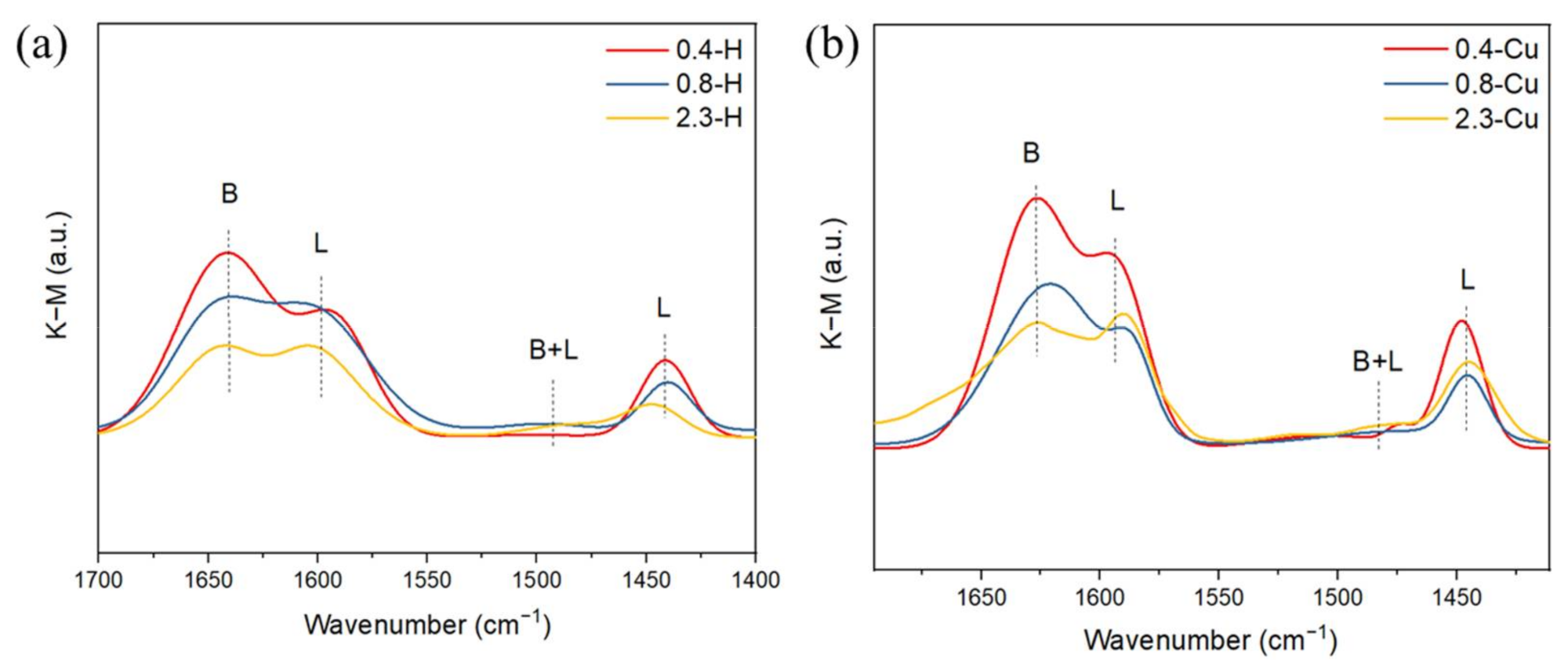
2.4. Acidic Distribution
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Characterization
3.3. Catalytic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joshi, A. Review of Vehicle Engine Efficiency and Emissions. SAE Tech. Pap. 2021. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.; Cavataio, G.; McCabe, R.W.; Fu, L.; Li, J. Characterization of commercial Cu-SSZ-13 and Cu-SAPO-34 catalysts with hydrothermal treatment for NH3-SCR of NOx in diesel exhaust. Chem. Eng. J. 2013, 225, 323–330. [Google Scholar] [CrossRef]
- Fan, C.; Chen, Z.; Pang, L.; Ming, S.; Dong, C.; Brou Albert, K.; Liu, P.; Wang, J.; Zhu, D.; Chen, H.; et al. Steam and alkali resistant Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx in diesel exhaust. Chem. Eng. J. 2018, 334, 344–354. [Google Scholar] [CrossRef]
- Jiang, H.; Guan, B.; Peng, X.; Zhan, R.; Lin, H.; Huang, Z. Influence of synthesis method on catalytic properties and hydrothermal stability of Cu/SSZ-13 for NH3-SCR reaction. Chem. Eng. J. 2020, 379, 122358. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Z.; Chen, Y.; Bao, W.; Chang, L.; Feng, G. In-situ hydrothermal synthesis of Cu-SSZ-13/cordierite for the catalytic removal of NOx from diesel vehicles by NH3. Chem. Eng. J. 2015, 263, 9–19. [Google Scholar] [CrossRef]
- Lomachenko, K.A.; Borfecchia, E.; Negri, C.; Berlier, G.; Lamberti, C.; Beato, P.; Falsig, H.; Bordiga, S. The Cu-CHA deNOx Catalyst in Action: Temperature-Dependent NH3-Assisted Selective Catalytic Reduction Monitored by Operando XAS and XES. J. Am. Chem. Soc. 2016, 138, 12025–12028. [Google Scholar] [CrossRef] [PubMed]
- Janssens, T.V.W.; Falsig, H.; Lundegaard, L.F.; Vennestrøm, P.N.R.; Rasmussen, S.B.; Moses, P.G.; Giordanino, F.; Borfecchia, E.; Lomachenko, K.A.; Lamberti, C.; et al. A Consistent Reaction Scheme for the Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. ACS Catal. 2015, 5, 2832–2845. [Google Scholar] [CrossRef]
- Bates, S.A.; Verma, A.A.; Paolucci, C.; Parekh, A.A.; Anggara, T.; Yezerets, A.; Schneider, W.F.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Identification of the active Cu site in standard selective catalytic reduction with ammonia on Cu-SSZ-13. J. Catal. 2014, 312, 87–97. [Google Scholar] [CrossRef]
- Gunter, T.; Carvalho, H.W.; Doronkin, D.E.; Sheppard, T.; Glatzel, P.; Atkins, A.J.; Rudolph, J.; Jacob, C.R.; Casapu, M.; Grunwaldt, J.D. Structural snapshots of the SCR reaction mechanism on Cu-SSZ-13. Chem. Commun. 2015, 51, 9227–9230. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, C.; Khurana, I.; Parekh, A.A.; Li, S.; Shih, A.J.; Li, H.; Di Iorio, J.R.; Albarracin-Caballero, J.D.; Yezerets, A.; Miller, J.T.; et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017, 357, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Hun Kwak, J.; Zhu, H.; Lee, J.H.; Peden, C.H.; Szanyi, J. Two different cationic positions in Cu-SSZ-13? Chem. Commun. 2012, 48, 4758–4760. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, Y.; Mei, D.; Walter, E.D.; Washton, N.M.; Holladay, J.D.; Wang, Y.; Szanyi, J.; Peden, C.H.F.; Gao, F. Revisiting effects of alkali metal and alkaline earth co-cation additives to Cu/SSZ-13 selective catalytic reduction catalysts. J. Catal. 2019, 378, 363–375. [Google Scholar] [CrossRef]
- Jiang, H.; Guan, B.; Peng, X.; Wei, Y.; Zhan, R.; Lin, H.; Huang, Z. Effect of sulfur poisoning on the performance and active sites of Cu/SSZ-13 catalyst. Chem. Eng. Sci. 2020, 226, 115855. [Google Scholar] [CrossRef]
- Olsson, L.; Wijayanti, K.; Leistner, K.; Kumar, A.; Joshi, S.Y.; Kamasamudram, K.; Currier, N.W.; Yezerets, A. A kinetic model for sulfur poisoning and regeneration of Cu/SSZ-13 used for NH3-SCR. Appl. Catal. B Environ. 2016, 183, 394–406. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Washton, N.M.; Kollár, M.; Szanyi, J.; Peden, C.H.F. Effects of Alkali and Alkaline Earth Cocations on the Activity and Hydrothermal Stability of Cu/SSZ-13 NH3-SCR Catalysts. ACS Catal. 2015, 5, 6780–6791. [Google Scholar] [CrossRef]
- Deka, U.; Lezcano-Gonzalez, I.; Weckhuysen, B.M.; Beale, A.M. Local Environment and Nature of Cu Active Sites in Zeolite-Based Catalysts for the Selective Catalytic Reduction of NOx. ACS Catal. 2013, 3, 413–427. [Google Scholar] [CrossRef]
- Jangjou, Y.; Do, Q.; Gu, Y.; Lim, L.-G.; Sun, H.; Wang, D.; Kumar, A.; Li, J.; Grabow, L.C.; Epling, W.S. Nature of Cu Active Centers in Cu-SSZ-13 and Their Responses to SO2 Exposure. ACS Catal. 2018, 8, 1325–1337. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Walter, E.D.; Washton, N.M.; Mei, D.; Kovarik, L.; Engelhard, M.H.; Prodinger, S.; Wang, Y.; Peden, C.H.F.; et al. Toward Rational Design of Cu/SSZ-13 Selective Catalytic Reduction Catalysts: Implications from Atomic-Level Understanding of Hydrothermal Stability. ACS Catal. 2017, 7, 8214–8227. [Google Scholar] [CrossRef]
- Lin, C.; Cao, Y.; Feng, X.; Lin, Q.; Xu, H.; Chen, Y. Effect of Si islands on low-temperature hydrothermal stability of Cu/SAPO-34 catalyst for NH3-SCR. J. Taiwan Inst. Chem. Eng. 2017, 81, 288–294. [Google Scholar] [CrossRef]
- Schwab, S.D.; Bennett, J.J.; Dell, S.J.; Galante-Fox, J.M.; Kulinowski, A.M.; Miller, K.T. Internal Injector Deposits in High-Pressure Common Rail Diesel Engines. SAE Int. J. Fuels Lubr. 2010, 3, 865–878. [Google Scholar] [CrossRef]
- Liu, L.; Wu, X.; Ma, Y.; Zhang, X.; Ran, R.; Si, Z.; Weng, D. Potassium deactivation of Cu-SSZ-13 catalyst for NH3-SCR: Evolution of salts, zeolite and copper species. Chem. Eng. J. 2020, 383, 123080. [Google Scholar] [CrossRef]
- Williams, A.; McCormick, R.; Lance, M.; Xie, C.; Toops, T.; Brezny, R. Effect of Accelerated Aging Rate on the Capture of Fuel-Borne Metal Impurities by Emissions Control Devices. SAE Int. J. Fuels Lubr. 2014, 7, 471–479. [Google Scholar] [CrossRef][Green Version]
- Williams, A.; Burton, J.; McCormick, R.L.; Toops, T.; Wereszczak, A.A.; Fox, E.E.; Lance, M.J.; Cavataio, G.; Dobson, D.; Warner, J.; et al. Impact of Fuel Metal Impurities on the Durability of a Light-Duty Diesel Aftertreatment System. SAE Tech. Pap. 2013. [Google Scholar] [CrossRef]
- Wang, C.; Yan, W.; Wang, Z.; Chen, Z.; Wang, J.; Wang, J.; Wang, J.; Shen, M.; Kang, X. The role of alkali metal ions on hydrothermal stability of Cu/SSZ-13 NH3-SCR catalysts. Catal. Today 2020, 355, 482–492. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Wang, J.; Wang, J.; Shen, M.; Li, W. Effects of Na(+) on Cu/SAPO-34 for ammonia selective catalytic reduction. J. Environ. Sci. 2018, 70, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Nie, Y.; Yang, R.; Cui, Y.; O’Hare, D.; Wang, Q. Highly dispersed CuyAlOx mixed oxides as superior low-temperature alkali metal and SO2 resistant NH3-SCR catalysts. Appl. Catal. A Gen. 2017, 538, 37–50. [Google Scholar] [CrossRef]
- Liu, A.; Liu, L.; Cao, Y.; Wang, J.; Si, R.; Gao, F.; Dong, L. Controlling Dynamic Structural Transformation of Atomically Dispersed CuOx Species and Influence on Their Catalytic Performances. ACS Catal. 2019, 9, 9840–9851. [Google Scholar] [CrossRef]
- Du, Y.; Huang, Z.; Zhang, J.; Jing, G. Fe2O3/HY Catalyst: A Microporous Material with Zeolite-Type Framework Achieving Highly Improved Alkali Poisoning-Resistant Performance for Selective Reduction of NOx with NH3. Environ. Sci. Technol. 2020, 54, 7078–7087. [Google Scholar] [CrossRef] [PubMed]
- Zha, K.; Kang, L.; Feng, C.; Han, L.; Li, H.; Yan, T.; Maitarad, P.; Shi, L.; Zhang, D. Improved NOx reduction in the presence of alkali metals by using hollandite Mn–Ti oxide promoted Cu-SAPO-34 catalysts. Environ. Sci. Nano. 2018, 5, 1408–1419. [Google Scholar] [CrossRef]
- Huang, S.; Wang, J.; Wang, J.; Wang, C.; Shen, M.; Li, W. The influence of crystallite size on the structural stability of Cu/SAPO-34 catalysts. Appl. Catal. B Environ. 2019, 248, 430–440. [Google Scholar] [CrossRef]
- Kumar, M.; Luo, H.; Román-Leshkov, Y.; Rimer, J.D. SSZ-13 Crystallization by Particle Attachment and Deterministic Pathways to Crystal Size Control. J. Am. Chem. Soc. 2015, 137, 13007–13017. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Kollar, M.; Wang, Y.; Szanyi, J.; Peden, C.H.F. Understanding ammonia selective catalytic reduction kinetics over Cu/SSZ-13 from motion of the Cu ions. J. Catal. 2014, 319, 1–14. [Google Scholar] [CrossRef]
- Marberger, A.; Petrov, A.W.; Steiger, P.; Elsener, M.; Kröcher, O.; Nachtegaal, M.; Ferri, D. Time-resolved copper speciation during selective catalytic reduction of NO on Cu-SSZ-13. Nat. Catal. 2018, 1, 221–227. [Google Scholar] [CrossRef]
- Gao, F.; Mei, D.; Wang, Y.; Szanyi, J.; Peden, C.H.F. Selective Catalytic Reduction over Cu/SSZ-13: Linking Homo- and Heterogeneous Catalysis. J. Am. Chem. Soc. 2017, 139, 4935–4942. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Current Understanding of Cu-Exchanged Chabazite Molecular Sieves for Use as Commercial Diesel Engine DeNOx Catalysts. Top. Catal. 2013, 56, 1441–1459. [Google Scholar] [CrossRef]
- Paolucci, C.; Parekh, A.A.; Khurana, I.; Di Iorio, J.R.; Li, H.; Albarracin Caballero, J.D.; Shih, A.J.; Anggara, T.; Delgass, W.N.; Miller, J.T.; et al. Catalysis in a Cage: Condition-Dependent Speciation and Dynamics of Exchanged Cu Cations in SSZ-13 Zeolites. J. Am. Chem. Soc. 2016, 138, 6028–6048. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Wang, J.; Wang, C.; Wang, J.; Li, W.; Shen, M. Disparate Essences of Residual, Ion-Exchanged, and Impregnated Na Ions on Topology Structure for Cu/SSZ-13 NH3 Selective Catalytic Reduction Catalysts. Ind. Eng. Chem. Res. 2019, 58, 20610–20619. [Google Scholar] [CrossRef]
- Gao, F.; Washton, N.M.; Wang, Y.; Kollár, M.; Szanyi, J.; Peden, C.H.F. Effects of Si/Al ratio on Cu/SSZ-13 NH3-SCR catalysts: Implications for the active Cu species and the roles of Brønsted acidity. J. Catal. 2015, 331, 25–38. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, R.; Zhao, R.; Shi, C.; Gies, H.; Xiao, F.-S.; De Vos, D.; Yokoi, T.; Bao, X.; Kolb, U.; et al. Cu-exchanged Al-rich SSZ-13 zeolite from organotemplate-free synthesis as NH3-SCR catalyst: Effects of Na+ ions on the activity and hydrothermal stability. Appl. Catal. B Environ. 2017, 217, 421–428. [Google Scholar] [CrossRef]
- Wang, B.; Ma, L.; Han, L.; Feng, Y.; Hu, J.; Xie, W.; Bao, W.; Chang, L.; Huang, Z.; Wang, J. Assembly-reassembly of coal fly ash into Cu-SSZ-13 zeolite for NH3-SCR of NO via interzeolite transformations. Chem. Eng. Sci. X 2021, 10, 100089. [Google Scholar]
- Fan, J.; Ning, P.; Wang, Y.; Song, Z.; Liu, X.; Wang, H.; Wang, J.; Wang, L.; Zhang, Q. Significant promoting effect of Ce or La on the hydrothermal stability of Cu-SAPO-34 catalyst for NH3-SCR reaction. Chem. Eng. J. 2019, 369, 908–919. [Google Scholar] [CrossRef]
- Zhan, H.; Huang, S.; Li, Y.; Lv, J.; Wang, S.; Ma, X. Elucidating the nature and role of Cu species in enhanced catalytic carbonylation of dimethyl ether over Cu/H-MOR. Catal. Sci. Technol. 2015, 5, 4378–4389. [Google Scholar] [CrossRef]
- Zones, S.I. Zeolite SSZ-13 and Its Method of Preparation. U.S. 4544538A, 1 October 1985. [Google Scholar]
- Fickel, D.W.; Lobo, R.F. Copper coordination in Cu-SSZ-13 and Cu-SSZ-16 investigated by variable-temperature XRD. J. Phys. Chem. C 2010, 114, 1633–1640. [Google Scholar] [CrossRef]
- Wang, J.; Shao, L.; Wang, C.; Wang, J.; Shen, M.; Li, W. Controllable preparation of various crystal size and nature of intra-crystalline diffusion in Cu/SSZ-13 NH3-SCR catalysts. J. Catal. 2018, 367, 221–228. [Google Scholar] [CrossRef]


| Samples | BET Specific Surface Area (m2/g) | Samples | BET Specific Surface Area (m2/g) |
| 0.4-H | 831 | 0.4-Cu | 820 |
| 0.8-H | 832 | 0.8-Cu | 807 |
| 2.3-H | 825 | 2.3-Cu | 843 |
| 0.4-NaH | 469 | 0.4-NaCu | 795 |
| 0.8-NaH | 500 | 0.8-NaCu | 772 |
| 2.3-NaH | 587 | 2.3-NaCu | 833 |
| Detecting Number | Cu Content from Center to Edge/wt % |
| #1 | 0.87 ± 0.06, 0.10 ± 0.03 |
| #2 | 0.76 ± 0.07, 0.08 ± 0.04 |
| #3 | 0.80 ± 0.03, 0.62 ± 0.06, 0.21 ± 0.02, 0.09 ± 0.02 |
| #4 | 0.67 ± 0.04, 0.55 ± 0.06, 0.32 ± 0.03, 0.09 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Shen, M.; Wang, C.; Wang, J.; Wang, J.; Shen, G. Improvement of Alkali Metal Resistance for NH3-SCR Catalyst Cu/SSZ-13: Tune the Crystal Size. Catalysts 2021, 11, 979. https://doi.org/10.3390/catal11080979
Chen Z, Shen M, Wang C, Wang J, Wang J, Shen G. Improvement of Alkali Metal Resistance for NH3-SCR Catalyst Cu/SSZ-13: Tune the Crystal Size. Catalysts. 2021; 11(8):979. https://doi.org/10.3390/catal11080979
Chicago/Turabian StyleChen, Zexiang, Meiqing Shen, Chen Wang, Jianqiang Wang, Jun Wang, and Gurong Shen. 2021. "Improvement of Alkali Metal Resistance for NH3-SCR Catalyst Cu/SSZ-13: Tune the Crystal Size" Catalysts 11, no. 8: 979. https://doi.org/10.3390/catal11080979
APA StyleChen, Z., Shen, M., Wang, C., Wang, J., Wang, J., & Shen, G. (2021). Improvement of Alkali Metal Resistance for NH3-SCR Catalyst Cu/SSZ-13: Tune the Crystal Size. Catalysts, 11(8), 979. https://doi.org/10.3390/catal11080979