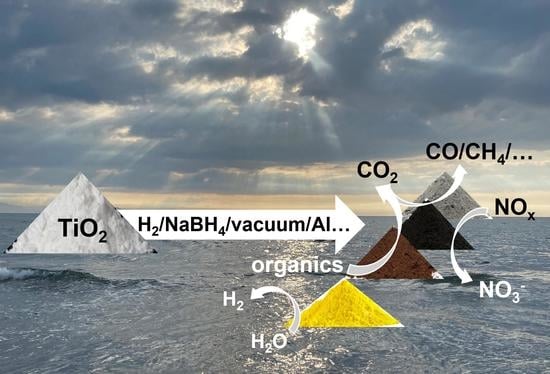
Defective Dopant-Free TiO2 as an Efficient Visible Light-Active Photocatalyst
Abstract
:1. Introduction
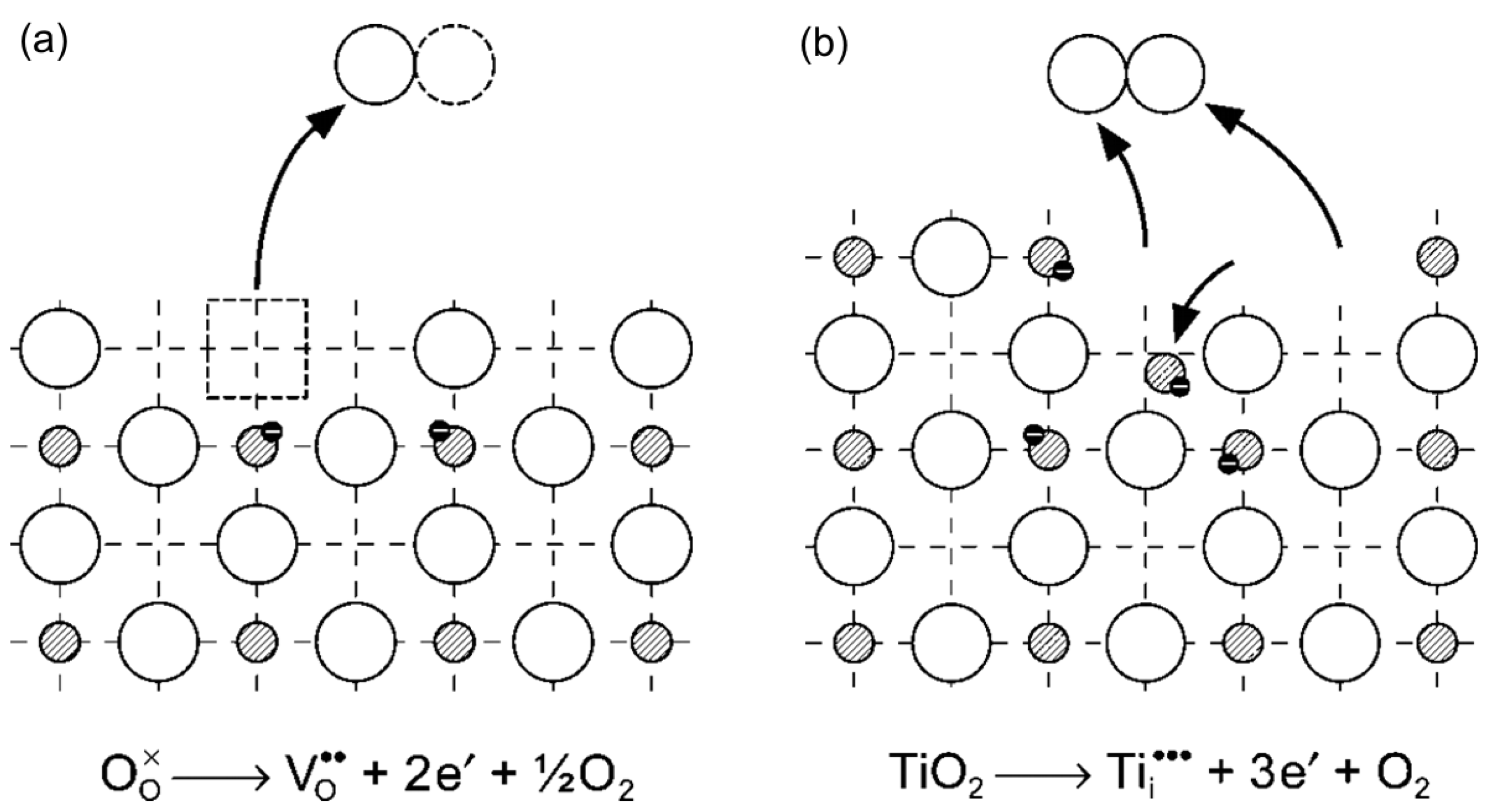
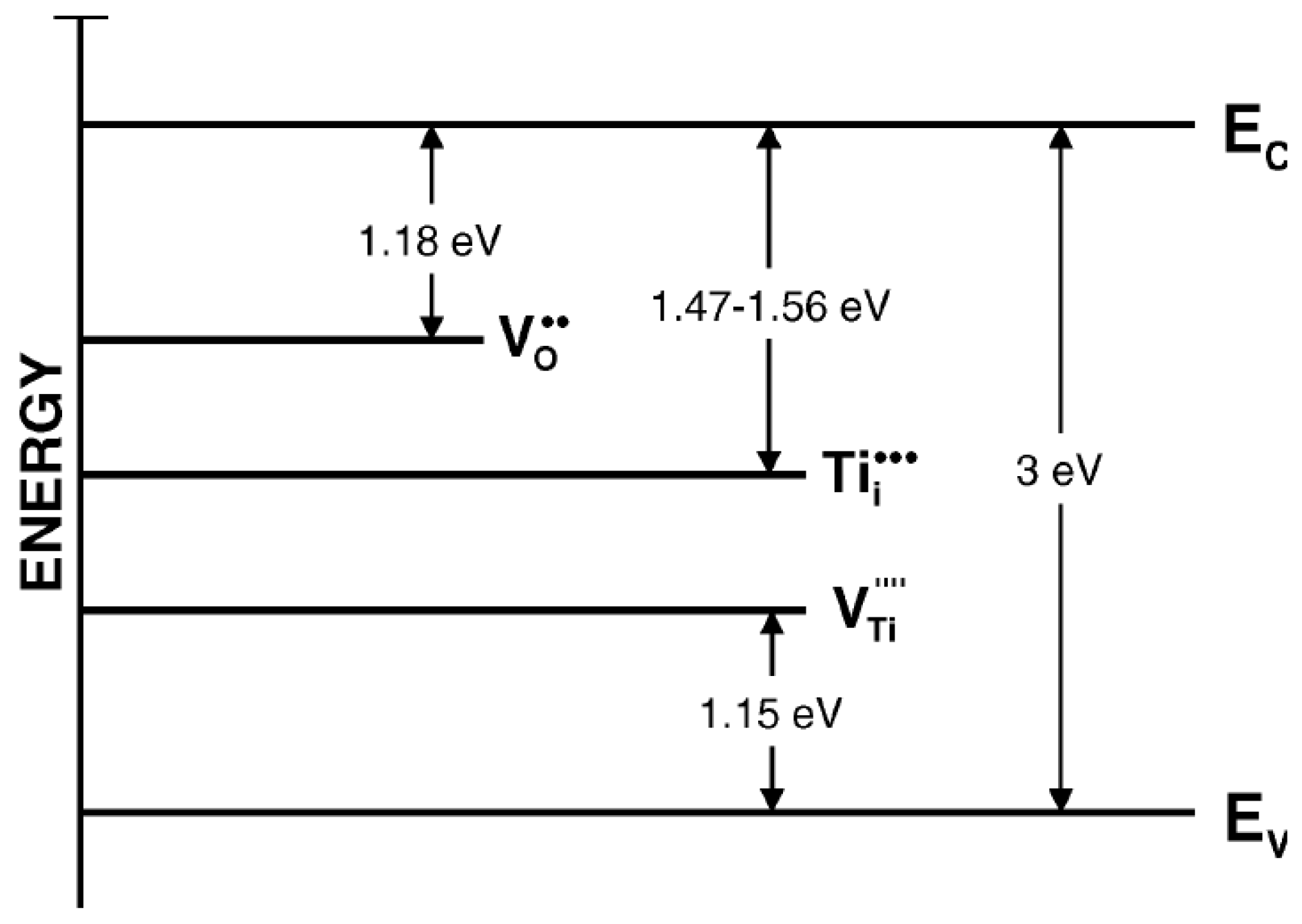
2. The Types of Defect Disorders in Titania
- —O2− ion in the oxygen lattice site,
- —oxygen vacancy,
- —Ti3+ ion in the titanium lattice site (quasi-free electron),
- —Ti4+ ion in the titanium lattice site,
- —Ti3+ in the interstitial site.
- titanium vacancy,
- —O− ion in the oxygen lattice site (quasi-free electron hole).
3. Dopant-Free Defective Titania Nanomaterials—Preparation Strategies and Properties
3.1. Thermal Treatment in the Presence of Hydrogen Gas
3.2. Application of Other Reductants
3.3. Oxidative Treatment
3.4. Other Methods
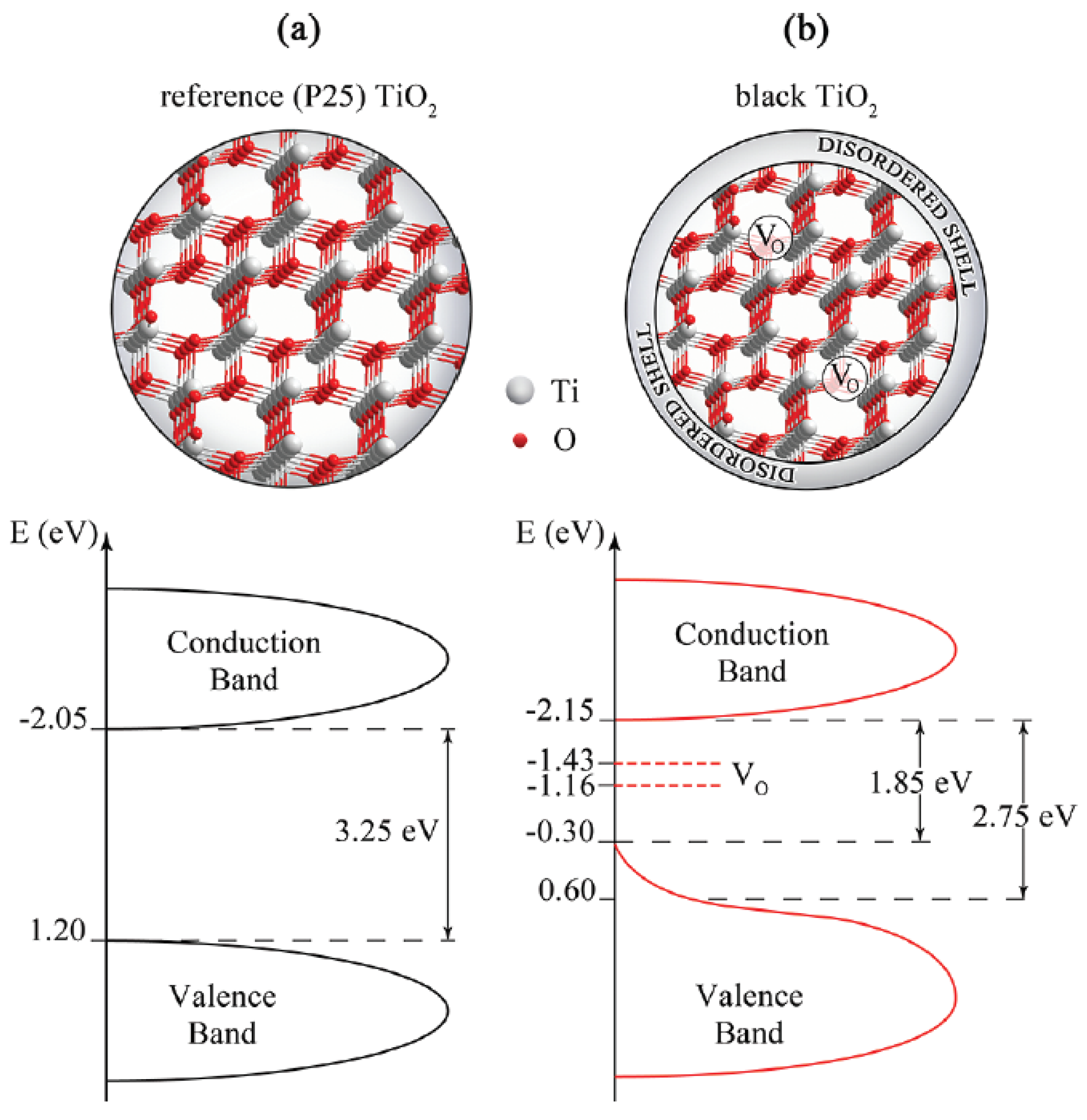
4. Defect-Depending Photocatalytic Activity of Self-Doped TiO2
5. Summary and Conclusions
- -
- The existence of optimal defects’ concentration—stronger photoabsorption properties in the visible-light range does not directly mean higher photocatalytic activity, as charge carriers’ recombination effect on defect sites exists;
- -
- The preferential role of surface defects in comparison to bulk defects was shown;
- -
- The surface-to-bulk ratio is higher for rutile than anatase (the role of crystal phase);
- -
- The beneficial impact of the disordered surface layer of titania should be clarified.
Author Contributions
Funding
Conflicts of Interest
References
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Bahnemann, D.W. Photocatalytic water treatment: Solar energy applications. Sol. Energy 2004, 77, 445–459. [Google Scholar] [CrossRef]
- Colon, G. Towards the hydrogen production by photocatalysis. Appl. Catal. A Gen. 2016, 518, 48–59. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef] [Green Version]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernandez-Ibanez, P.A.; Polo-Lopez, I.; Sharma, P.K.; Vennard, A.S.M. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [Green Version]
- Clarizia, L.; Spasiano, D.; Di Somma, I.; Marotta, R.; Andreozzi, R.; Dionysiou, D.D. Copper modified-TiO2 catalysts for hydrogen generation through photoreforming of organics. A short review. Int. J. Hydrogen. Energ. 2014, 39, 16812–16831. [Google Scholar] [CrossRef]
- Palmisano, G.; Augugliaro, V.; Pagliaro, M.; Palmisano, L. Photocatalysis: A promising route for 21st century organic chemistry. Chem. Comm. 2007, 3425–3437. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, S.; Kisch, H. Daylight photocatalysis by carbon-modified titanium dioxide. Angew. Chem. Int. Ed. 2003, 42, 4908–4911. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Robert, D.; Weber, J.-V. Photocatalytic activity of Cu2O/TiO2, Bi2O3/TiO2 and ZnMn2O4/TiO2 heterojunctions. Catal. Today 2005, 101, 315–321. [Google Scholar] [CrossRef]
- Kowalska, E.; Prieto Mahaney, O.O.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Endo, M.; Zhang, D.; Wang, K.; Kowalska, E. Enhanced photocatalytic and antimicrobial performance of cuprous oxide/titania: The effect of titania matrix. Materials 2018, 11, 2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielinska-Jurek, A.; Wysocka, I.; Janczarek, M.; Stampor, W.; Hupka, J. Preparation and characterization of Pt–N/TiO2 photocatalysts and their efficiency in degradation of recalcitrant chemicals. Sep. Purif. Technol. 2015, 156, 369–378. [Google Scholar] [CrossRef]
- Meng, S.; Sun, W.; Zhang, S.; Zheng, X.; Fu, X.; Chen, S. Insight into the transfer mechanism of photogenerated carriers for WO3/TiO2 heterojunction photocatalysts: Is it the transfer of band–band or Z-scheme? Why? J. Phys. Chem. C 2018, 122, 26326–26336. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Artiglia, L.; Granozzi, G.; Ohtani, B.; Selli, E. Photocatalytic activity vs structural features of titanium dioxide materials singly doped or codoped with fluorine and boron. J. Phys. Chem. C 2014, 118, 25579–25589. [Google Scholar] [CrossRef]
- Kuznetsov, V.N.; Serpone, N. On the origin of the spectral bands in the visible absorption spectra of visible-light-active TiO2 specimens analysis and assignment. J. Phys. Chem. C 2009, 113, 15110–15123. [Google Scholar] [CrossRef]
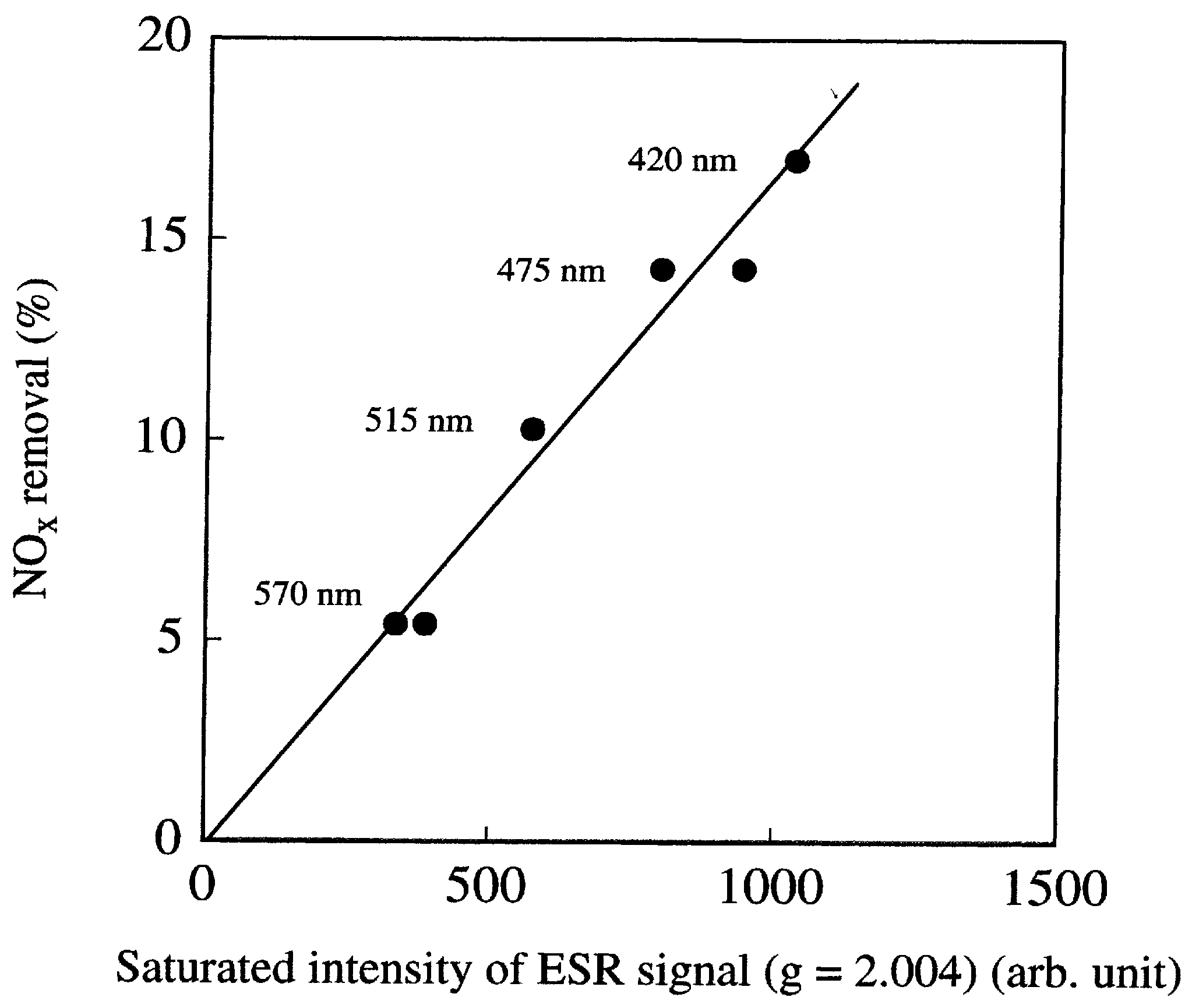
- Nakamura, I.; Negishi, N.; Kotsuna, S.; Ihara, T.; Sugihara, S.; Takeuchi, K. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal. J. Mol. Catal. A Chem. 2000, 161, 205–212. [Google Scholar] [CrossRef]
- Justicia, I.; Ordejon, P.; Canto, G.; Mozos, J.L.; Fraxedas, J.; Battiston, G.A.; Gerbasi, R.; Figueras, A. Designed self-doped titanium oxide thin films for efficient visible-light photocatalysis. Adv. Mater. 2002, 14, 1399–1402. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T. TiO2-based photocatalysis: Surface defects, oxygen and charge transfer. Top. Catal. 2005, 35, 197–210. [Google Scholar] [CrossRef]
- Nowotny, J. Titanium dioxide-based semiconductors for solar-driven environmentally friendly applications: Impact of point defects on performance. Energy Environ. Sci. 2008, 1, 565–572. [Google Scholar] [CrossRef]
- Nowotny, M.K.; Sheppard, L.R.; Bak, T.; Nowotny, J. Defect chemistry of titanium dioxide. Application of defect engineering in processing of TiO2-based photocatalysts. J. Phys. Chem. C 2008, 112, 5275–5300. [Google Scholar] [CrossRef]
- Zuo, F.; Wu, T.; Zhang, Z.; Borchardt, D.; Feng, P. Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. J. Am. Chem. Soc. 2010, 132, 11856–11857. [Google Scholar] [CrossRef] [PubMed]
- Bak, T.; Nowotny, J.; Sucher, N.J.; Wachsman, E. Effect of crystal imperfections on reactivity and photoreactivity of TiO2 (rutile) with oxygen, water, and bacteria. J. Phys. Chem. C 2011, 115, 15711–15738. [Google Scholar] [CrossRef]
- Dhumal, S.Y.; Daulton, T.L.; Jiang, J.; Khomami, B.; Biswas, P. Synthesis of visible light-active nanostructured TiOx (x < 2) photocatalysts in a flame aerosol reactor. Appl. Catal. B Environ. 2009, 86, 145–151. [Google Scholar]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappeli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, S.; Xu, H.; Lou, Z.; Wang, W.; Huang, B.; Dai, Y. Green synthetic approach for Ti3+ self-doped TiO2-x nanoparticles with efficient visible light photocatalytic activity. Nanoscale 2013, 5, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Fang, W.; Nasir, M.; Ma, Y.; Zhang, J.; Anpo, M. Self-doped Ti3+-enhanced TiO2 nanoparticles with a high-performance photocatalysis. J. Catal. 2013, 297, 236–243. [Google Scholar] [CrossRef]
- Katal, R.; Eshkalak, S.K.; Masudy-panah, S.; Kosari, M.; Saeedikhani, M.; Zarinejad, M.; Ramakrishna, S. Evaluation of solar-driven photocatalytic activity of thermal treated TiO2 under various atmospheres. Nanomaterials 2019, 9, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Reduced and n-Type Doped TiO2: Nature of Ti3+ Species. J. Phys. Chem. C 2009, 113, 20543–20552. [Google Scholar] [CrossRef]
- Kako, T.; Umezawa, N.; Xie, K.; Ye, J. Undoped visible-light-sensitive titania photocatalyst. J. Mater. Sci. 2013, 48, 108–114. [Google Scholar] [CrossRef]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 nanotube arrays for photoelectrochemical water splitting. J. Mater. Chem. A 2013, 1, 5766–5774. [Google Scholar] [CrossRef]
- Zhu, Q.; Peng, Y.; Lin, L.; Fan, C.M.; Gao, G.Q.; Wang, R.X.; Xu, A.W. Stable blue TiO2-x nanoparticles for efficient visible light photocatalysts. J. Mater. Chem. A 2014, 2, 4429–4437. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, D.; Liu, K.; Wang, C.; Liu, L.; Li, B.; Zhang, Z.; Shen, D. Laser-modified black titanium oxide nanospheres and their photocatalytic activities under visible light. ACS Appl. Mater. Interfaces 2015, 7, 16070–16077. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Chen, C.; Wang, J.; Fu, X.; Ren, Z.; Qian, G.; Wang, Z. Black hydroxylated titanium dioxide prepared via ultrasonication with enhanced photocatalytic activity. Sci. Rep. 2015, 5, 11712. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Yazawa, S.; Araki, S.; Kogoshi, S.; Katayama, N.; Kudo, Y.; Nakanishi, T. Experimental study of the visible-light photocatalytic activity of oxygen-deficient TiO2 prepared with Ar/H2 plasma surface treatment. Jpn. J. Appl. Phys. 2015, 54, 01AE04. [Google Scholar] [CrossRef]
- Shah, M.W.; Zhu, Y.; Fan, X.; Zhao, J.; Li, Y.; Asim, S.; Wang, C. Facile synthesis of defective TiO2−x nanocrystals with high surface area and tailoring bandgap for visible-light photocatalysis. Sci. Rep. 2015, 5, 15804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinhamahapatra, A.; Jeon, J.P.; Yu, J.S. A new approach to prepare highly active and stable black titania for visible light-assisted hydrogen production. Energy Environ. Sci. 2015, 8, 3529–3544. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Wei, Y.; Wang, C.; Zhang, X. Vacuum heat treated titanate nanotubes for visible-light photocatalysis. New J. Chem. 2015, 39, 1281–12896. [Google Scholar] [CrossRef]
- Zhu, G.; Yin, C.; Cui, H.; Wang, Z.; Xu, J.; Lin, T.; Huang, F. Black titania for superior photocatalytic hydrogen production and photoelectrochemical water splitting. ChemCatChem 2015, 7, 2614–2619. [Google Scholar] [CrossRef]
- Amano, F.; Nakata, M.; Yamamoto, A.; Tanaka, T. Effect of Ti3+ ions and conduction band electrons on photocatalytic and photoelectrochemical activity of rutile titania for water oxidation. J. Phys. Chem. C 2016, 120, 6467–6474. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Wang, J.; Gan, Y.; Liu, L.; Ju, M. Microwave-assisted ionic liquid synthesis of Ti3+ self-doped TiO2 hollow nanocrystals with enhanced visible-light photoactivity. Appl. Catal. B Environ. 2016, 191, 94–105. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Zhao, H.; Chen, J.; Cheng, J.; Yang, K.; Li, Y. Engineering coexposed {001} and {101} facets in oxygen-deficient TiO2 nanocrystals for enhanced CO2 photoreduction under visible light. ACS Catal. 2016, 6, 1097–1108. [Google Scholar] [CrossRef]
- Liu, N.; Schneider, C.; Freitag, D.; Zolnhofer, E.M.; Meyer, K.; Schmuki, P. Noble-metal-free photocatalytic H2 generation: Active and inactive ’black’ TiO2 nanotubes and synergistic effects. Chem. Eur. J. 2016, 22, 13810–13814. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, X.; Nguyen, N.T.; Peters, K.; Zoller, F.; Hwang, I.; Schneider, C.; Miehlich, M.E.; Freitag, D.; Meyer, K.; et al. Black magic in gray titania: Noble-metal-free photocatalytic H2 evolution from hydrogenated anatase. ChemSusChem 2017, 10, 62–67. [Google Scholar] [CrossRef]
- Nakano, T.; Ito, R.; Kogoshi, S.; Katayama, N. Optimal levels of oxygen deficiency in the visible light photocatalyst TiO2-x and long-term stability of catalytic performance. J. Phys. Chem. Solid. 2016, 98, 136–142. [Google Scholar] [CrossRef]
- Qiu, M.; Tian, Y.; Chen, Z.; Yang, Z.; Li, W.; Wang, K.; Wang, L.; Wang, K.; Zhang, W. Synthesis of Ti3+ self-doped TiO2 nanocrystals based on Le Chatelier’s principle and their application in solar light photocatalysis. RSC Adv. 2016, 6, 74376–74383. [Google Scholar] [CrossRef]
- Sarkar, D.; Ishchuk, S.; Taffa, D.H.; Kaynan, N.; Berke, B.A.; Bendikov, T.; Yerushalmi, R. Oxygen-deficient titania with adjustable band positions and defects; molecular layer deposition of hybrid organic−inorganic thin films as precursors for enhanced photocatalysis. J. Phys. Chem. C 2016, 120, 3853–3862. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Periyat, P. A ‘one pot’ gel combustion strategy towards Ti3+ self-doped ‘black’ anatase TiO2-x solar photocatalyst. J. Mater. Chem. A 2016, 4, 5854–5858. [Google Scholar] [CrossRef]
- Zhu, G.L.; Shan, Y.F.; Lin, T.Q.; Zhao, W.L.; Xu, J.J.; Tian, Z.L.; Zhang, H.; Zheng, C.; Huang, F.Q. Hydrogenated blue titania with high solar absorption and greatly improved photocatalysis. Nanoscale 2016, 8, 4705–4712. [Google Scholar] [CrossRef]
- Wang, W.K.; Gao, M.; Zhang, X.; Fujitsuka, M.; Majima, T.; Yu, H.Q. One-step synthesis of nonstoichiometric TiO2 with designed (101) facets for enhanced photocatalytic H2 evolution. Appl. Catal. B. 2017, 205, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Park, J.H. Surface localization of defects in black TiO2: Enhancing photoactivity or reactivity. J. Phys. Chem. Lett. 2017, 8, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Zimbone, M.; Cacciato, G.; Boutinguiza, M.; Privitera, V.; Grimaldi, M.G. Laser irradiation in water for the novel, scalable synthesis of black TiOx photocatalyst for environmental remediation. Beilstein J. Nanotechnol. 2017, 8, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Katal, R.; Salehi, M.; Farahani, M.H.D.A.; Panah-Masudy, S.; Ong, S.L.; Hu, J. Preparation of a new type of black TiO2 under a vacuum atmosphere for sunlight photocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 35316–35326. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, P.; Fang, Y.; Li, C.; Yuan, X.; Zheng, X.; Gao, J. Effect of heat treatment under vacuum on structure and visible-light photocatalytic activity of nano-TiO2. RSC Adv. 2019, 9, 32691–32698. [Google Scholar] [CrossRef] [Green Version]
- Lan, K.; Wang, R.; Wei, Q.; Wang, Y.; Hong, A.; Feng, P.; Zhao, D. Stable Ti3+ defects in oriented mesoporous titania frameworks for efficient photocatalysis. Angew. Chem. Int. Ed. 2020, 59, 17676–17683. [Google Scholar] [CrossRef]
- Wang, Y.; Saitow, K. Mechanochemical synthesis of red-light-active green TiO2 photocatalysts with disorder: Defect-rich, with polymorphs, and no metal loading. Chem. Mater. 2020, 32, 9190–9200. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, F.; He, J.; Dan, M.; Cao, Y.; Yu, S.; Zhou, Y. Oxygen defect boosted photocatalytic hydrogen evolution from hydrogen sulfide over active {0 0 1} facet in anatase TiO2. Appl. Surf. Sci. 2020, 517, 146198. [Google Scholar] [CrossRef]
- Hu, Q.; Li, G.; Lan, H.; Li, J.; Hu, B.; Guo, W.; Huang, J.; Huang, X. Facile coengineering of oxygen defects and highly active {110} facets in TiO2 nanorods for efficient water splitting. Cryst. Growth Des. 2019, 19, 1680–1688. [Google Scholar] [CrossRef]
- Jiang, Y.; Ning, H.; Tian, C.; Jiang, B.; Li, Q.; Yan, H.; Zhang, X.; Wang, J.; Jing, L.; Fu, H. Single-crystal TiO2 nanorods assembly for efficient and stable cocatalyst-free photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 229, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Yan, J.; Wang, T.; Zhao, Z.J.; Zhang, J.; Gong, J.; Guan, N. Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production. Nat. Commun. 2015, 6, 5881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naldoni, A.; Altomare, M.; Zopellaro, G.; Liu, N.; Kment, S.; Zboril, R.; Schmuki, P. Photocatalysis with reduced TiO2: From black TiO2 to cocatalyst-free hydrogen production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Bian, J.; Guo, Y.; Huang, M.; Zhang, R.Q. Thermal vacuum de-oxygenation and post oxidation of TiO2 nanorod arrays for enhanced photoelectrochemical properties. J. Mater. Chem. A 2019, 7, 5434–5441. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, T.; Le, L.; Ruan, X.; Fang, P.; Pan, C.; Xiong, R.; Shi, J.; Wei, J. Quick and facile preparation of visible light-driven TiO2 photocatalyst with high absorption and photocatalytic activity. Sci. Rep. 2014, 4, 7045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Gang, L.; Hong, L.; Shaodan, W.; Qiang, W. Preparation and photocatalytic activities of lack TiO2 in vacuum and ambient temperature environment. J. Nanosci. Nanotech. 2019, 19, 81–90. [Google Scholar]
- Ozer, L.Y.; Apostoleris, H.; Ravaux, F.; Shylin, S.I.; Mamedov, F.; Lindblad, A.; Johansson, F.O.L.; Chiesa, M.; Sa, J.; Palmisano, G. Long-lasting non-hydrogenated dark titanium dioxide: Medium vacuum anneal for enhanced visible activity of modified multiphase photocatalysts. ChemCatChem 2018, 10, 2949–2954. [Google Scholar] [CrossRef]
- Araujo, M.A.; Gromboni, M.F.; Marken, F.; Parker, S.C.; Peter, L.M.; Turner, J.; Aspinall, H.C.; Black, K.; Mascaro, L.H. Contrasting transient photocurrent characteristics for thin films of vacuum doped “grey” TiO2 and “grey” Nb2O5. Appl. Catal. B Environ. 2018, 237, 339–352. [Google Scholar] [CrossRef]
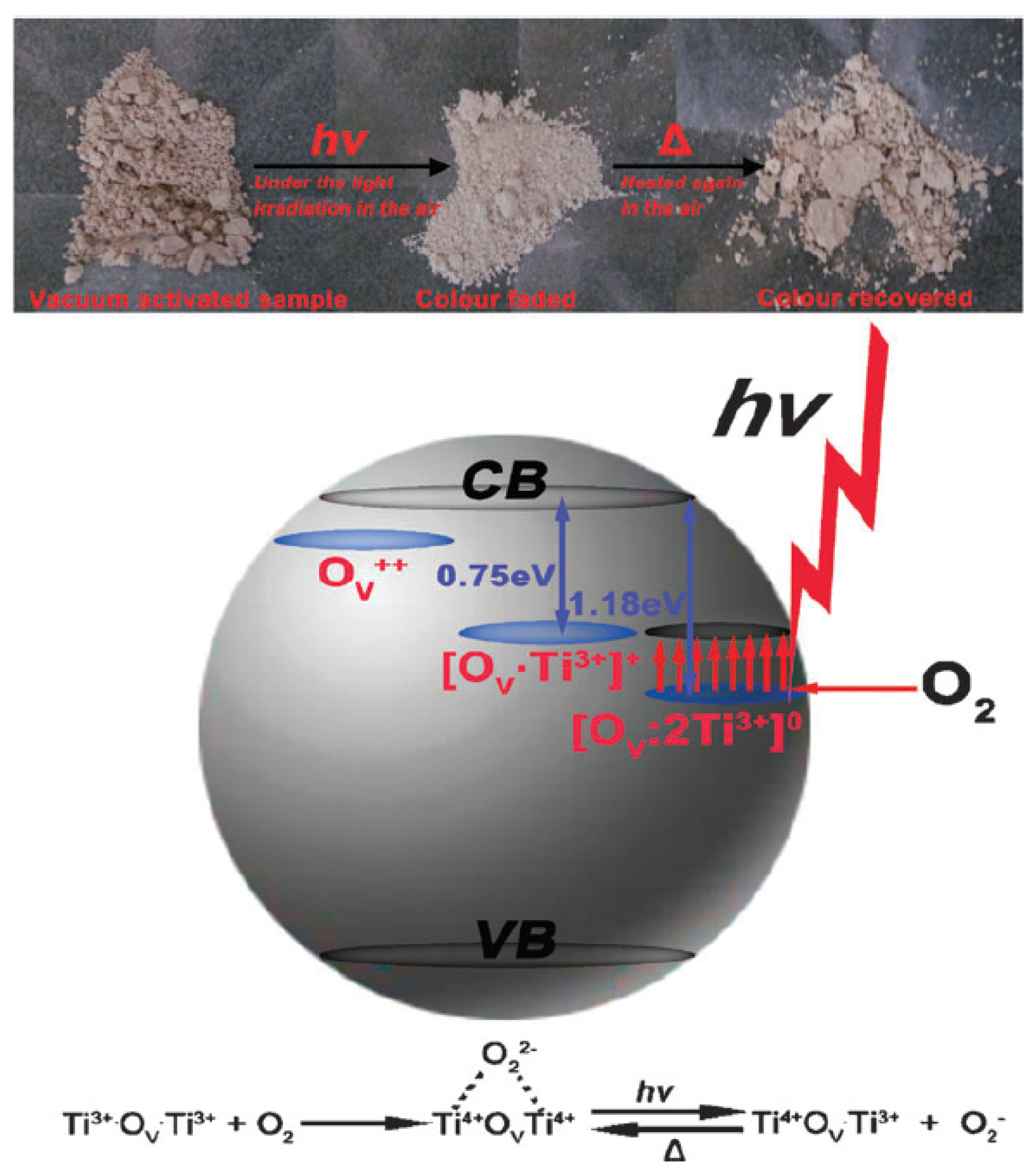
- Dong, G.; Wang, X.; Chen, Z.; Lu, Z. Enhanced photocatalytic activity of vacuum-activated TiO2 induced by oxygen vacancies. Photochem. Photobiol. 2018, 94, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Xing, M.; Zhang, J. A new approach to prepare Ti3+ self-doped TiO2 via NaBH4 reduction and hydrochloric acid treatment. Appl. Catal. B 2014, 160–161, 240–246. [Google Scholar] [CrossRef]
- Xin, L.; Liu, X. Black TiO2 inverse opals for visible-light photocatalysis. RSC Adv. 2015, 5, 71547–71550. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, J.; Chen, F.; Tian, B. An economic method to prepare vacuum activated photocatalysts with high photo-activities and photosensitivities. Chem. Commun. 2011, 47, 4947–4949. [Google Scholar] [CrossRef]
- Pei, Z.; Weng, S.; Liu, P. Enhanced photocatalytic activity by bulk trapping and spatial separation of charge carriers: A case study of defect and facet mediated TiO2. Appl. Catal. B Environ. 2016, 180, 463–470. [Google Scholar] [CrossRef]
- Wang, W.; Lu, C.H.; Ni, Y.R.; Song, J.B.; Su, M.X.; Xu, Z.Z. Enhanced visible-light photoactivity of {001} facets dominated TiO2 nanosheets with even distributed bulk oxygen vacancy and Ti3+. Catal. Commun. 2012, 22, 19–23. [Google Scholar] [CrossRef]
- Qi, D.; Lu, L.; Xi, Z.; Wang, L.; Zhang, J. Enhanced photocatalytic performance of TiO2 based on synergistic effect of Ti3+ self-doping and slow light effect. Appl. Catal. B Environ. 2014, 160–161, 621–628. [Google Scholar] [CrossRef]
- Ren, R.; Wen, Z.; Cui, S.; Hou, Y.; Guo, X.; Chen, J. Controllable synthesis andtunable photocatalytic properties of Ti3+-doped TiO2. Sci. Rep. 2015, 5, 10714. [Google Scholar] [CrossRef] [Green Version]
- Calatayud, D.G.; Flores, R.M.; Castellanos-Aliaga, A.; Peiteado, M.; Palomares, F.J.; Caballero, A.C.; Jardiel, T. Tailoring the visible light photoactivity of un-doped defective TiO2 anatase nanoparticles through a simple two-step solvothermal process. Nanotechnology 2020, 31, 045603. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, H.; Ali, A.M.; Zhang, H. Correlation between tunable oxygen defects in TiO2 nanoflower and its photocatalytic performance for the degradation of organic waste. Nano 2020, 15, 2050018. [Google Scholar] [CrossRef] [Green Version]
- Huttenhofer, L.; Eckmann, F.; Lauri, A.; Cambiasso, J.; Pensa, E.; Li, Y.; Cortes, E.; Sharp, I.D.; Maier, S.A. Anapole excitations in oxygen-vacancy-rich TiO2–x nanoresonators: Tuning the absorption for photocatalysis in the visible spectrum. ACS Nano 2020, 14, 2456–2464. [Google Scholar] [CrossRef]
- Jing, L.; Xin, B.; Yuan, F.; Wang, B.; Fu, H. Effects of surface oxygen vacancies on photophysical and photochemical processes of Zn-doped TiO2 nanoparticles and their relationships. J. Phys. Chem. B 2006, 110, 17860–17865. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Blake, G.R.; Palstra, T.M.T. Vacancies in functional materials for clean energy storage and harvesting: The perfect imperfection. Chem. Soc. Rev. 2017, 46, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, J.; Alim, M.A.; Bak, T.; Idris, M.A.; Ionescu, M.; Prince, K.; Sahdan, M.Z.; Sopian, K.; Teridi, M.A.M.; Sigmund, W. Defect chemistry and defect engineering of TiO2-based semiconductors for solar energy conversion. Chem. Soc. Rev. 2015, 44, 8424–8442. [Google Scholar] [CrossRef]
- Su, J.; Zou, X.; Chen, J.S. Self-modification of titanium dioxide materials by Ti3+ and/or oxygen vacancies: New insights into defect chemistry of metal oxides. RSC Adv. 2014, 4, 13979–13988. [Google Scholar] [CrossRef]
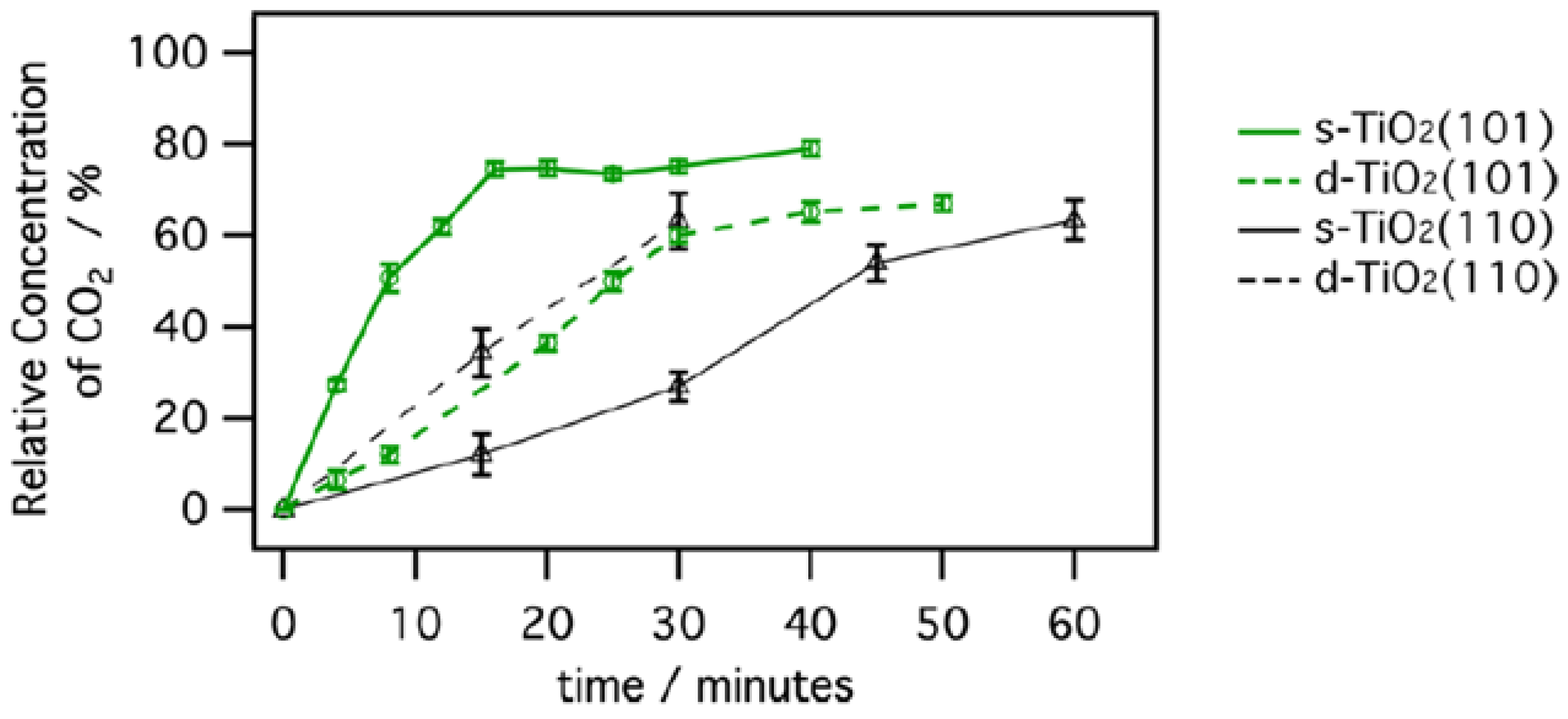
- He, Y.; Dulub, O.; Cheng, H.; Selloni, A.; Diebold, U. Evidence for the predominance of subsurface defects on reduced Anatase TiO2(101). Phys. Rev. Lett. 2009, 102, 106105. [Google Scholar] [CrossRef]
- Cronemeyer, D.C. Electrical and optical properties of rutile single crystals. Phys. Rev. 1952, 87, 876–886. [Google Scholar] [CrossRef]
- Breckenridge, R.G.; Hosler, W.R. Electrical properties of titanium dioxide semiconductors. Phys. Rev. 1953, 91, 793–801. [Google Scholar] [CrossRef]
- Cronemeyer, D.C. Infrared absorption of reduced rutile TiO, single crystals. Phys. Rev. 1959, 113, 1222–1226. [Google Scholar] [CrossRef]
- Chester, P.F. Electron spin resonance in semiconducting rutile. J. Appl. Phys. 1961, 32, 2233–2236. [Google Scholar] [CrossRef]
- Hasiguti, R.R.; Yagi, E. Electrical conductivity below 3K of slightly reduced oxygen-deficient rutile TiO2-x. Phys. Rev. B 1994, 49, 7251–7257. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Yagisawa, T.; Kamiya, N.; Mulmi, D.D.; Kurita, S.; Murakami, Y.; Kodaira, T. Defects in anatase TiO2 single crystal controlled by heat treatments. J. Phys. Soc. Jpn. 2004, 73, 703–710. [Google Scholar] [CrossRef]
- Liu, H.; Ma, H.T.; Li, X.Z.; Li, W.Z.; Wu, M.; Bao, X.H. The enhancement of TiO2 photocatalytic activity by hydrogen thermal treatment. Chemosphere 2003, 50, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Kim, B.; Kim, Y.K. Highly enhanced photoactivity of anatase TiO2 nanocrystals by controlled hydrogenation-induced surface defects. ACS Catal. 2013, 3, 2479–2486. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, P.Y.; Mao, S.S.; Shen, D.Z. Hydrogenation and disorder in engineered black TiO2. Phys. Rev. Lett. 2013, 111, 065505. [Google Scholar] [CrossRef]
- Wei, W.; Yaru, N.; Chunhua, L.; Zhongzi, X. Hydrogenation of TiO2 nanosheets with exposed {001} facets for enhanced photocatalytc activity. RSC Adv. 2012, 2, 8286–8288. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, B.; Pan, R.; Yao, J.; Qiu, J.; Luo, L.; Liu, Y. Safe and facile hydrogenation of commercial Degussa P25 at room temperature with enhanced photocatalytic activity. RSC Adv. 2014, 4, 1128–1132. [Google Scholar] [CrossRef]
- Liu, N.; Schneider, C.; Freitag, D.; Hartmann, M.; Venkatesan, U.; Muller, J.; Specker, E.; Schmuki, P. Black TiO2 nanotubes: Cocatalyst-free open-circuit hydrogen generation. Nano Lett. 2014, 14, 3309. [Google Scholar] [CrossRef]
- Leshuk, T.; Parviz, R.; Everett, P.; Krishnakumar, H.; Varin, R.A. Photocatalytic activity of hydrogenated TiO2. ACS Appl. Mater. Interfaces 2013, 5, 1892–1895. [Google Scholar] [CrossRef]
- Fang, W.; Xing, M.; Zhang, J. Modifications on reduced titanium dioxide photocatalysts: A review. J. Photochem. Photobiol. C Photochem. Rev. 2017, 32, 21–39. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 2013, 6, 3007–3014. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, W.; Yang, C.; Yin, H.; Lin, T.; Shan, Y.; Xie, Y.; Gu, H.; Huang, F. Black TiO2 nanotube arrays for high-efficiency photoelectrochemical water-splitting. J. Mater. Chem. A 2014, 2, 8612–8616. [Google Scholar] [CrossRef]
- Zhao, Z.; Tan, H.; Zhao, H.; Lv, Y.; Zhou, L.J.; Song, Y.; Sun, Z. Reduced TiO2 rutile nanorods with well-defined facets and their visible-light photocatalytic activity. Chem. Commun. 2014, 50, 2755–2757. [Google Scholar] [CrossRef]
- Grabstanowicz, L.R.; Gao, S.; Li, T.; Rickard, R.M.; Rajh, T.; Liu, D.J.; Xu, T. Facile oxidative conversion of TiH2 to high-concentration Ti3+-self-doped rutile TiO2 with visible-light photoactivity. Inorg. Chem. 2013, 52, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Ding, L.; Lin, H.; Weng, S.; Zheng, Z.; Hou, Y.; Liu, P. Facile synthesis of defect-mediated TiO2-x with enhanced visible light photocatalytic activity. J. Mater. Chem. A 2013, 1, 10099. [Google Scholar] [CrossRef]
- Teng, F.; Li, M.; Gao, C.; Zhang, G.; Zhang, P.; Wang, Y.; Chen, L.; Xie, E. Preparation of black TiO2 by hydrogen plasma assisted chemical vapor deposition and its photocatalytic activity. Appl. Catal. B Environ. 2014, 148–149, 339–343. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. H-doped black titania with very high solar absorption and excellent photocatalysis enhanced by localized surface plasmon resonance. Adv. Funct. Mater. 2013, 23, 5444. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, Y.; Murowchick, J.; Chen, X. Vacuum-treated titanium dioxide nanocrystals: Optical properties, surface disorder, oxygen vacancy, and photocatalytic activities. Catal. Today 2014, 225, 2–9. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Li, D.; Fang, F.; Huang, W. Engineering self-doped surface defects of anatase TiO2 nanosheets for enhanced photocatalytic efficiency. Appl. Surf. Sci. 2021, 540, 148330. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, Y.; Luo, L.; Shao, S.; Zhou, Y.; Shao, Y.; Zhan, F.; Yang, J.; Zhou, Y. γ-ray induced formation of oxygen vacancies and Ti3+ defects in anatase TiO2 for efficient photocatalytic organic pollutant degradation. Sci. Total Environ. 2020, 747, 141533. [Google Scholar] [CrossRef] [PubMed]
- Rex, R.E.; Yang, Y.; Knorr, F.J.; Zhang, J.Z.; Li, Y.; McHale, J.L. Spectroelectrochemical photoluminescence of trap states in H-treated rutile TiO2 nanowires: Implications for photooxidation of water. J. Phys. Chem. C 2016, 120, 3530–3541. [Google Scholar] [CrossRef] [Green Version]
- Pesci, F.M.; Wang, G.; Klug, D.R.; Li, Y.; Cowan, A.J. Efficient suppression of electron−hole recombination in oxygen-deficient hydrogen-treated TiO2 nanowires for photoelectrochemical water splitting. J. Phys. Chem. C 2013, 117, 25837–25844. [Google Scholar] [CrossRef] [PubMed]
- Diebold, U. Closing the gap. Nat. Chem. 2011, 3, 271–272. [Google Scholar] [CrossRef]
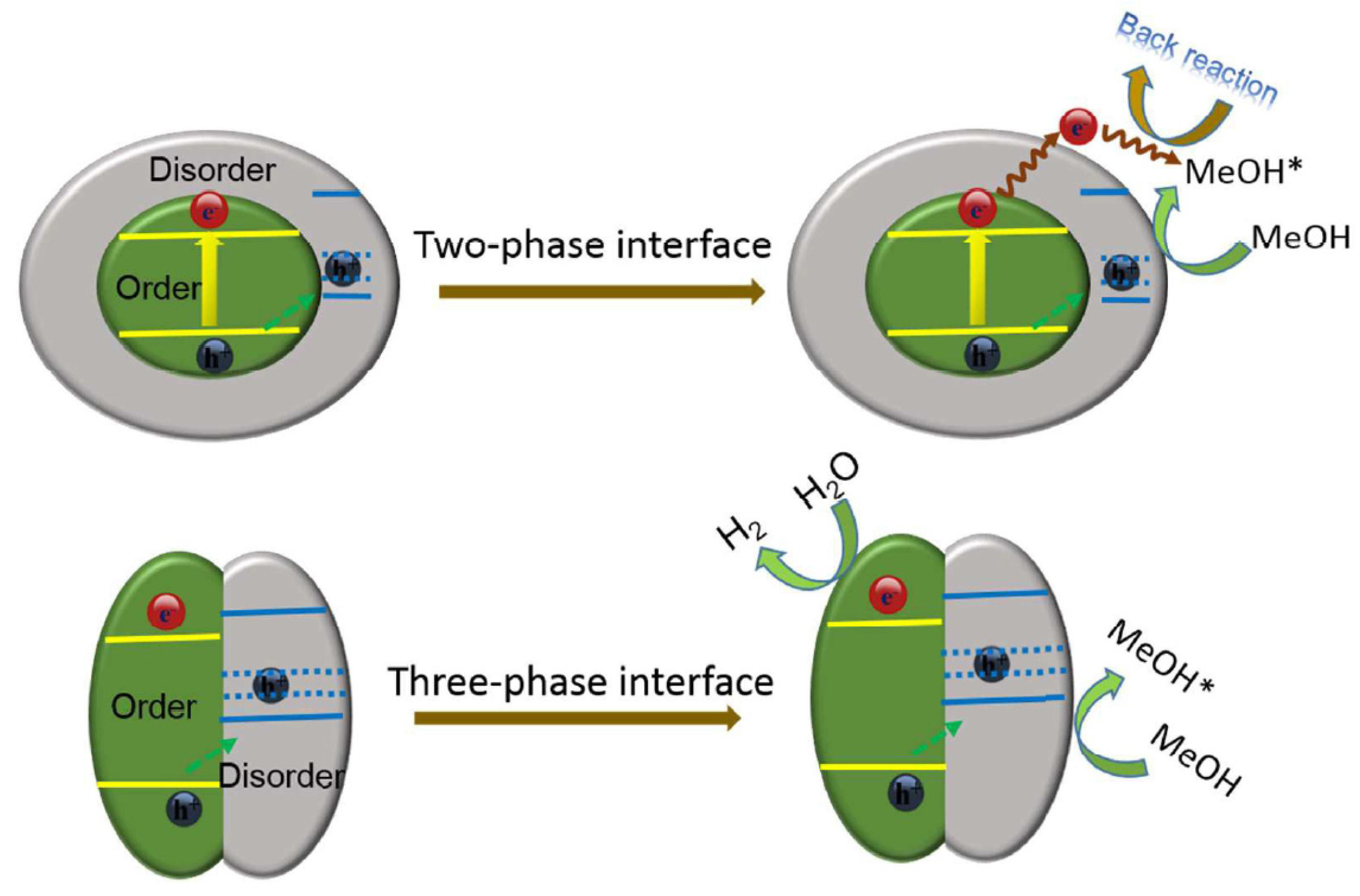
- Zhang, K.; Wang, L.; Kim, J.K.; Ma, M.; Veerappan, G.; Lee, C.L.; Kong, K.; Lee, H.; Park, J.H. An order/disorder/water junction system for highly efficient co-catalyst-free photocatalytic hydrogen generation. Energy Environ. Sci. 2016, 9, 499–503. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Niu, M.; Mao, C.; Cao, D.; Cheng, D.; Feng, P.; Sun, Z. A facile and versatile method for preparation of colored TiO2 with enhanced solar-driven photocatalytic activity. Nanoscale 2014, 6, 10216–10223. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Li, Y.Z.; Chen, X.; Tian, T.T.; Fang, P.F.; Zheng, F.; Zhao, X.J. Tuning the relative concentration ratio of bulk defects to surface defects in TiO2 nanocrystals leads to high photocatalytic efficiency. J. Am. Chem. Soc. 2011, 133, 16414–16417. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Jiang, J.; Rong, Y.; Wang, Y.; Wu, Y.; Pan, C. Characterization of oxygen vacancy associates within hydrogenated TiO2: A positron annihilation study. J. Phys. Chem. C 2012, 116, 22619–22624. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Robertson, J. Calculation of TiO2 surface and subsurface oxygen vacancy by the screened exchange functional. J. Phys. Chem. C 2015, 119, 18160–18166. [Google Scholar] [CrossRef]
- Cheng, H.; Selloni, A. Surface and subsurface oxygen vacancies in anatase TiO2 and differences with rutile. Phys. Rev. B 2009, 79, 092101. [Google Scholar] [CrossRef]
- Wagstaffe, M.; Noei, H.; Stierle, A. Elucidating the defect-induced changes in the photocatalytic activity of TiO2. J. Phys. Chem. C 2020, 124, 12539–12547. [Google Scholar] [CrossRef]
- Selloni, A. Anatase shows its reactive side. Nature Mater. 2008, 7, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yu, J.C.; Lu, G.Q.; Cheng, H.-M. Crystal facet engineering of semiconductor photocatalysts: Motivations, advances and unique properties. Chem. Commun. 2011, 47, 6763–6783. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Kowalska, E.; Verret, J.; Colbeau-Justin, C.; Remita, H.; Ohtani, B. Morphology-dependent photocatalytic activity of octahedral anatase particles prepared by ultrasonication-hydrothermal reaction of titanates. Nanoscale 2015, 7, 12392–12404. [Google Scholar] [CrossRef] [Green Version]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Janczarek, M.; Endo, M.; Wang, K.; Balcytis, A.; Nitta, A.; Mendez-Medrano, M.G.; Colbeau-Justin, C.; Juodkazis, S.; Ohtani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl. Catal. B 2018, 237, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Janczarek, M.; Wei, Z.; Endo, M.; Ohtani, B.; Kowalska, E. Silver- and copper-modified decahedral anatase titania particles as visible light-responsive plasmonic photocatalyst. J. Photon. Energy 2017, 7, 012008. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janczarek, M.; Kowalska, E. Defective Dopant-Free TiO2 as an Efficient Visible Light-Active Photocatalyst. Catalysts 2021, 11, 978. https://doi.org/10.3390/catal11080978
Janczarek M, Kowalska E. Defective Dopant-Free TiO2 as an Efficient Visible Light-Active Photocatalyst. Catalysts. 2021; 11(8):978. https://doi.org/10.3390/catal11080978
Chicago/Turabian StyleJanczarek, Marcin, and Ewa Kowalska. 2021. "Defective Dopant-Free TiO2 as an Efficient Visible Light-Active Photocatalyst" Catalysts 11, no. 8: 978. https://doi.org/10.3390/catal11080978
APA StyleJanczarek, M., & Kowalska, E. (2021). Defective Dopant-Free TiO2 as an Efficient Visible Light-Active Photocatalyst. Catalysts, 11(8), 978. https://doi.org/10.3390/catal11080978