Effect of Calcium Doping Using Aqueous Phase Reforming of Glycerol over Sonochemically Synthesized Nickel-Based Supported ZrO2 Catalyst
Abstract
1. Introduction
2. Results and Discussion
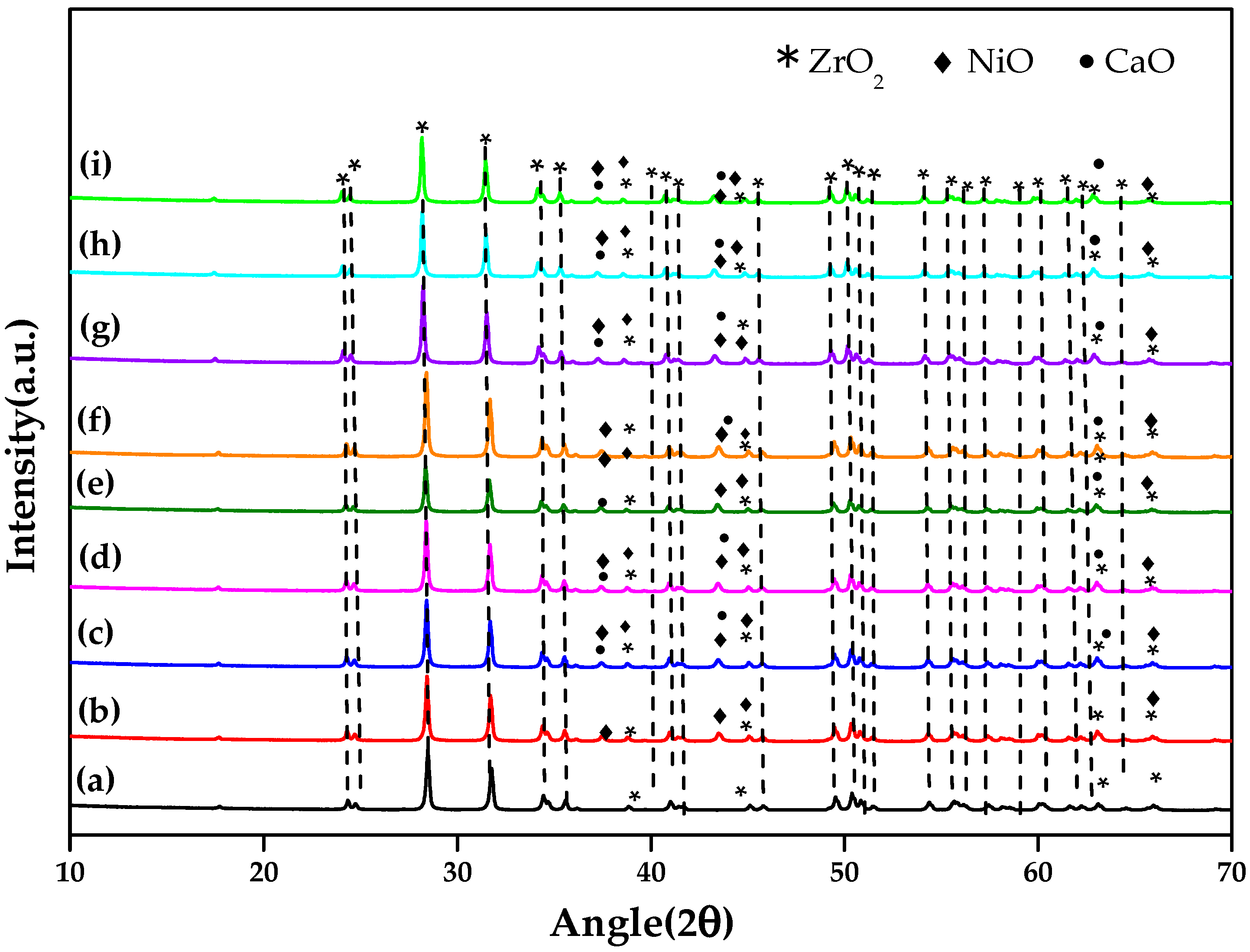
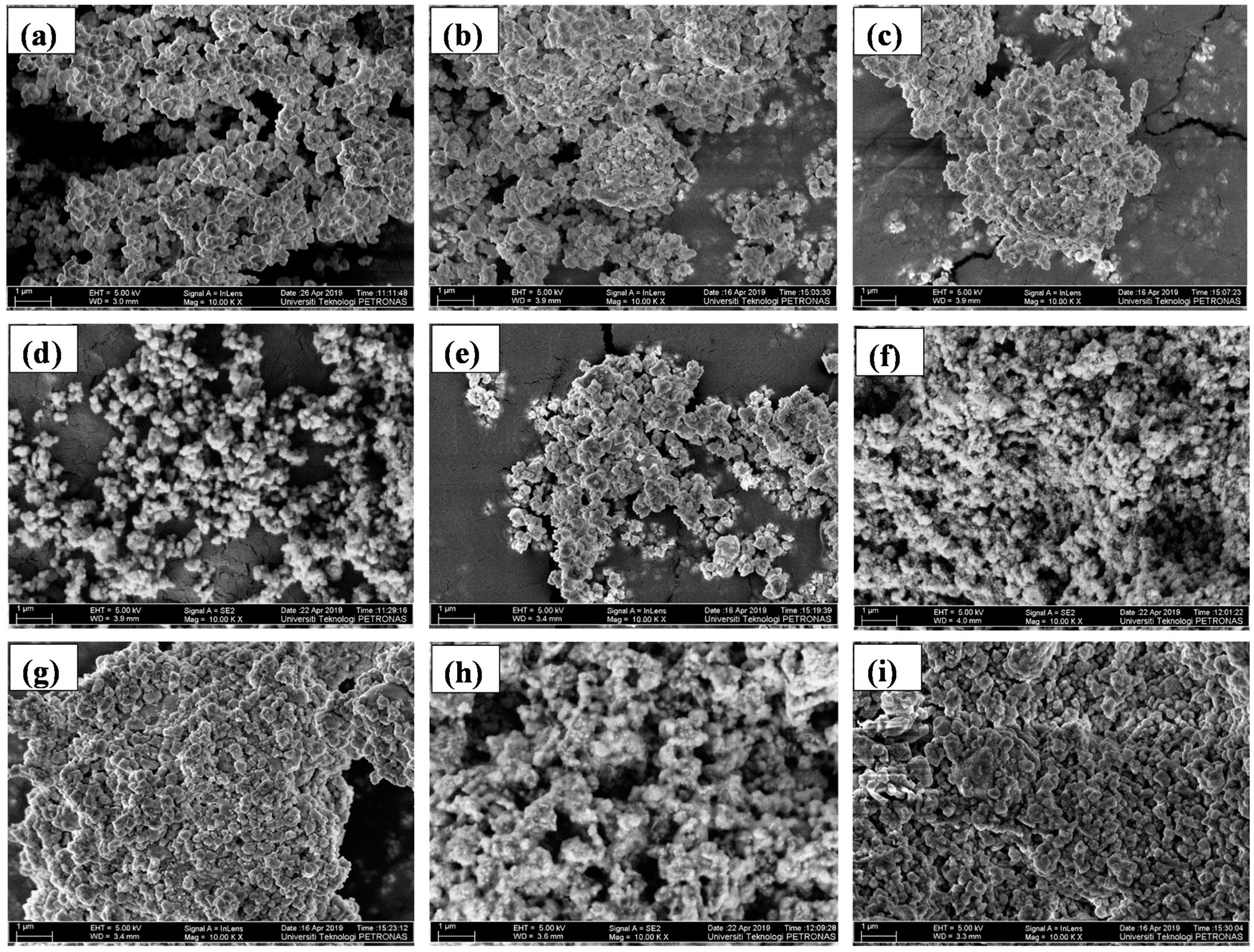
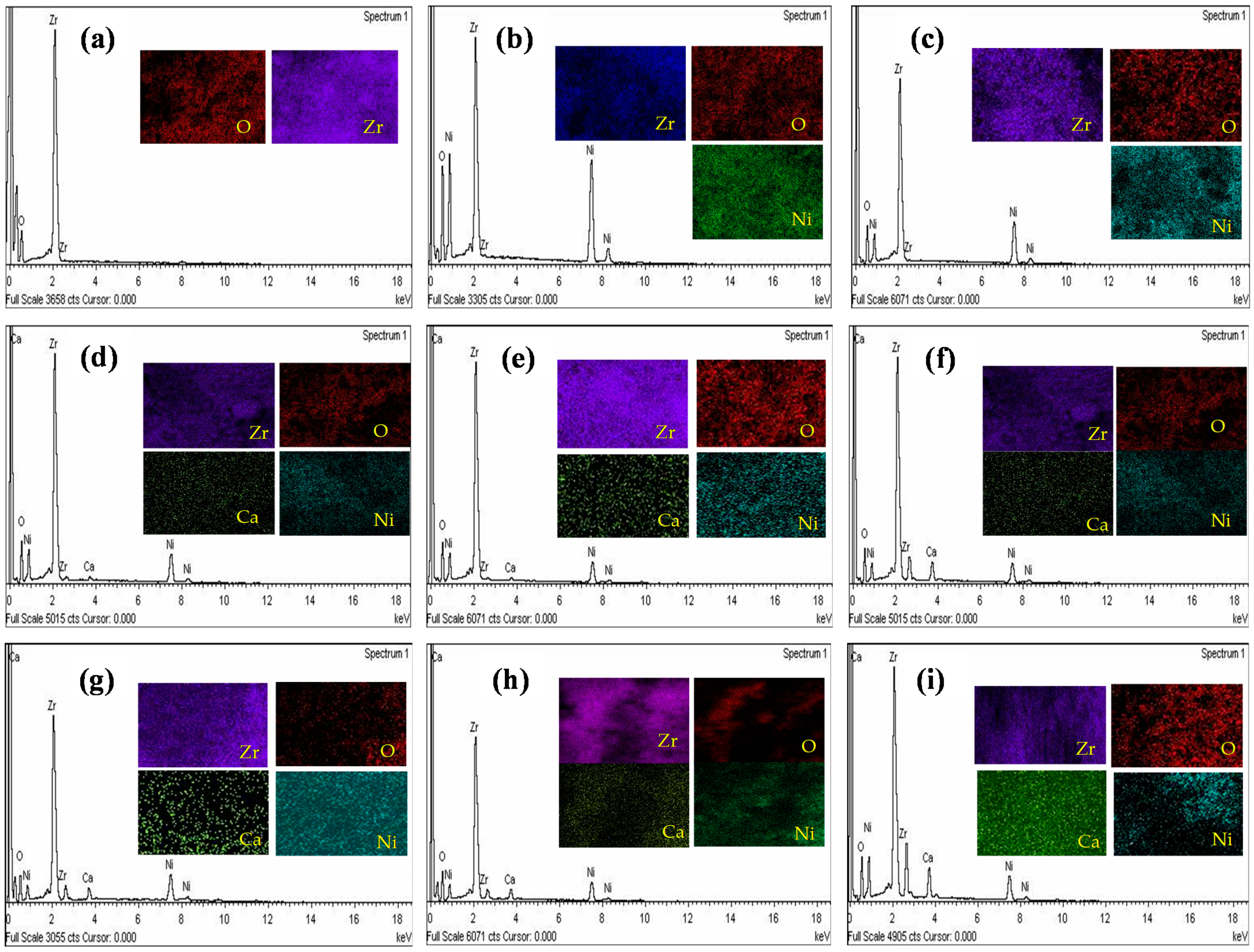
2.1. Structural and Morphological Analysis
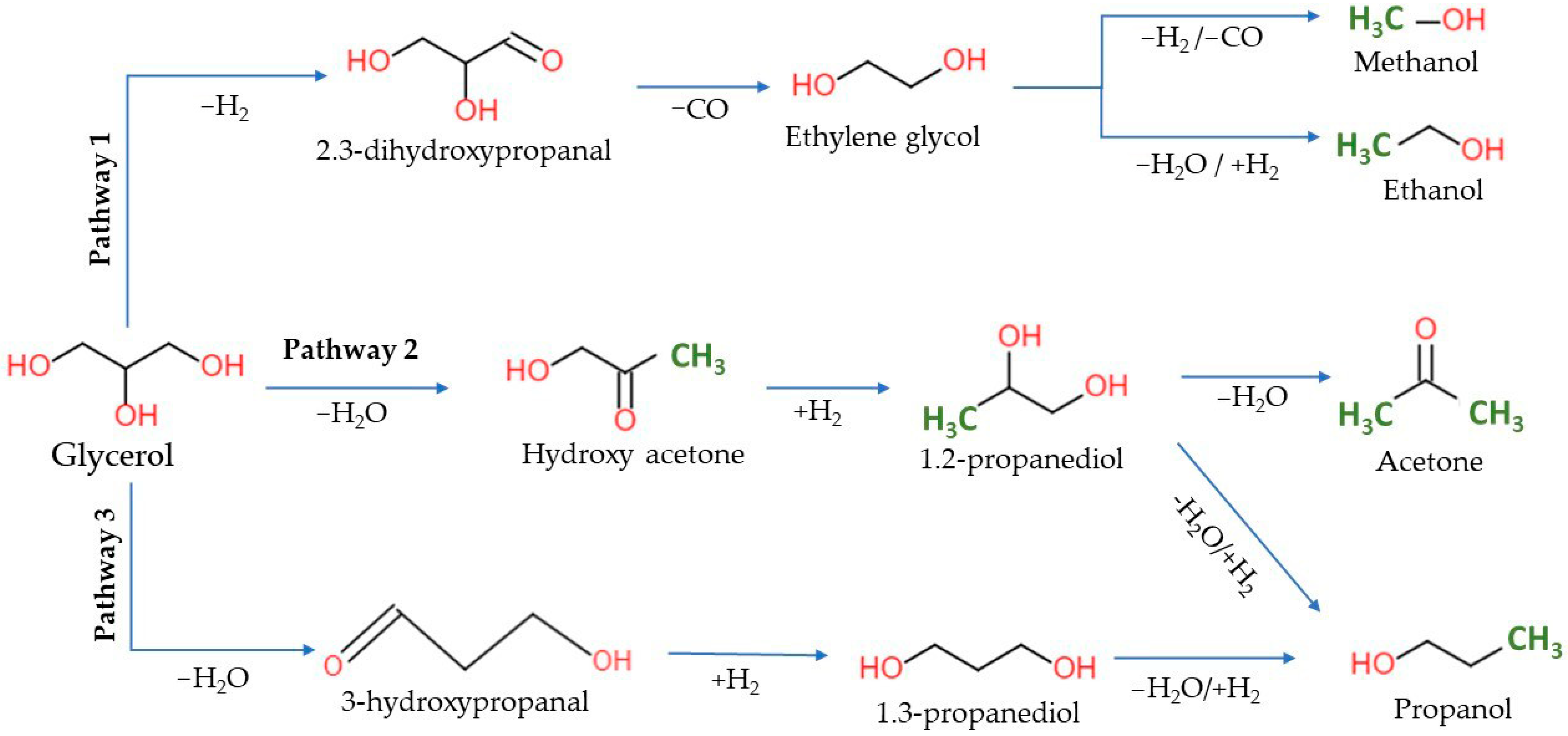
2.2. Performance of Catalysts for Glycerol APR
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Catalyst
3.3. Characterization of Catalyst
3.4. Catalyst Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yumashev, A.; Ślusarczyk, B.; Kondrashev, S.; Mikhaylov, A. Global indicators of sustainable development: Evaluation of the influence of the human development index on consumption and quality of energy. Energies 2020, 13, 2768. [Google Scholar] [CrossRef]
- Sadaf, S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Nisar, J.; Iqbal, M. Biodiesel production from waste cooking oil: An efficient technique to convert waste into biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar]
- Teo, S.H.; Rashid, U.; Thomas Choong, S.Y.; Taufiq-Yap, Y.H. Heterogeneous calcium-based bimetallic oxide catalyzed transesterification of Elaeis guineensis derived triglycerides for biodiesel production. Energy Convers. Manag. 2017, 141, 20–27. [Google Scholar] [CrossRef]
- Keera, S.; El Sabagh, S.; Taman, A. Castor oil biodiesel production and optimization. Egypt. J. Pet. 2018, 27, 979–984. [Google Scholar] [CrossRef]
- Ciriminna, R.; Pina, C.D.; Rossi, M.; Pagliaro, M. Understanding the glycerol market. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439. [Google Scholar] [CrossRef]
- Rodriguez, A.; Wojtusik, M.; Ripoll, V.; Santos, V.E.; Garcia-Ochoa, F. 1,3-Propanediol production from glycerol with a novel biocatalyst Shimwellia blattae ATCC 33430: Operational conditions and kinetics in batch cultivations. Bioresour. Technol. 2016, 200, 830–837. [Google Scholar] [CrossRef][Green Version]
- Wei, R.; Qu, X.; Xiao, Y.; Fan, J.; Geng, G.; Gao, L.; Xiao, G. Hydrogenolysis of glycerol to propanediols over silicotungstic acid catalysts intercalated with CuZnFe hydrotalcite-like compounds. Catal. Today 2021. [Google Scholar] [CrossRef]
- Callison, J.; Subramanian, N.D.; Rogers, S.M.; Chutia, A.; Gianolio, D.; Catlow, C.R.A.; Wells, P.P.; Dimitratos, N. Directed aqueous-phase reforming of glycerol through tailored platinum nanoparticles. Appl. Catal. B Environ. 2018, 238, 618–628. [Google Scholar] [CrossRef]
- Azizan, M.T.; Aqsha, A.; Ameen, M.; Syuhada, A.; Klaus, H.; Abidin, S.Z.; Sher, F. Catalytic reforming of oxygenated hydrocarbons for the hydrogen production: An outlook. Biomass Convers. Biorefin. 2020. [Google Scholar] [CrossRef]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef]
- Morales-Marín, A.; Ayastuy, J.; Iriarte-Velasco, U.; Gutiérrez-Ortiz, M. Nickel aluminate spinel-derived catalysts for the aqueous phase reforming of glycerol: Effect of reduction temperature. Appl. Catal. B Environ. 2019, 244, 931–945. [Google Scholar] [CrossRef]
- Nadzru, N.A.; Syuhada, A.; Azizan, M.T.; Ameen, M. Thermodynamic analysis of aqueous phase reforming of sorbitol. J. Comput. Theor. Nanosci. 2020, 17, 1004–1008. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.; Jeong, H.; Kim, Y.; Choi, S.H.; Ham, H.C.; Lee, S.W.; Kim, J.Y.; Song, K.H.; Yoon, C.W. High purity hydrogen production via aqueous phase reforming of xylose over small Pt nanoparticles on a γ-Al2O3 support. Int. J. Hydrogen Energy 2020, 45, 13848–13861. [Google Scholar] [CrossRef]
- Reynoso, A.J.; Iriarte-Velasco, U.; Gutiérrez-Ortiz, M.A.; Ayastuy, J.L. Highly stable Pt/CoAl2O4 catalysts in Aqueous-Phase Reforming of glycerol. Catal. Today 2021, 367, 278–289. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, N. Hydrogen Production from Ethylene Glycol Aqueous Phase Reforming over Ni-Al Layered Hydrotalcite-Derived Catalysts. Catalysts 2020, 10, 54. [Google Scholar] [CrossRef]
- Larimi, A.S.; Kazemeini, M.; Khorasheh, F. Aqueous phase reforming of glycerol using highly active and stable Pt0.05CexZr0.95−xO2 ternary solid solution catalysts. Appl. Catal. A Gen. 2016, 523, 230–240. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha, A. Comparative study of hydrogen production from steam reforming of acetic acid over synthesized catalysts via MOF and wet impregnation methods. Int. J. Hydrogen Energy 2020, 45, 11512–11526. [Google Scholar] [CrossRef]
- Luo, W.; Lyu, Y.; Gong, L.; Du, H.; Jiang, M.; Ding, Y. Alcohol-treated SiO2 as the support of Ir-Re/SiO2 catalysts for glycerol hydrogenolysis. Chin. J. Catal. 2016, 37, 2009–2017. [Google Scholar] [CrossRef]
- Pamphile-Adrián, A.J.; Florez-Rodriguez, P.P.; Pires, M.H.M.; Perez, G.; Passos, F.B. Selective hydrogenolysis of glycerol over Ir-Ni bimetallic catalysts. Catal. Today 2017, 289, 302–308. [Google Scholar] [CrossRef]
- Ahmadi, F.; Haghighi, M.; Ajamein, H. Sonochemically coprecipitation synthesis of CuO/ZnO/ZrO2/Al2O3 nanocatalyst for fuel cell grade hydrogen production via steam methanol reforming. J. Mol. Catal. A Chem. 2016, 421, 196–208. [Google Scholar] [CrossRef]
- Sing Yu, F.W.; Ameen, M.; Aqsha, A.; Izham, M.; Azizan, M.T.; Sher, F. Effects of ultrasound irradiations time over Ni–Mo/γ-Al2O3 catalyst synthesis for 1,3-Propanediol selectively via aqueous phase reforming of glycerol. Case Stud. Chem. Environ. Eng. 2021, 3, 100096. [Google Scholar] [CrossRef]
- Shahbudin, M.I.; Jacob, D.M.; Ameen, M.; Aqsha, A.; Azizan, M.T.; Yusoff, M.H.M.; Sher, F. Liquid value-added chemicals production from aqueous phase reforming of sorbitol and glycerol over sonosynthesized Ni-based catalyst. J. Environ. Chem. Eng. 2021, 9, 105766. [Google Scholar] [CrossRef]
- Pei, L.; Xie, Y.; Pei, Y.; Yuan, C. Synthesis and formation process of zirconium dioxide nanorods. Mater. Sci. Pol. 2013, 31, 186–192. [Google Scholar] [CrossRef]
- Radzi, M.R.M.; Azizan, M.T.; Daud, N.; Topek, M.A.; Abidin, S.Z. Aqueous phase reforming of sorbitol over Ca doped Ni/Al2O3 for value-added chemicals production. Mater. Today Proc. 2018, 5, 21728–21736. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Ramli, A.; Yusup, S.; Yasir, M. Physicochemical Properties of Ni-Mo/γ-Al2O3 Catalysts Synthesized via Sonochemical Method. Procedia Eng. 2016, 148, 64–71. [Google Scholar] [CrossRef]
- Mahboob, S.; Haghighi, M.; Rahmani, F. Sonochemically preparation and characterization of bimetallic Ni-Co/Al2O3-ZrO2 nanocatalyst: Effects of ultrasound irradiation time and power on catalytic properties and activity in dry reforming of CH4. Ultrason. Sonochem. 2017, 38, 38–49. [Google Scholar] [CrossRef]
- Taghizadeh, M.T.; Vatanparast, M. Ultrasonic-assisted synthesis of ZrO2 nanoparticles and their application to improve the chemical stability of Nafion membrane in proton exchange membrane (PEM) fuel cells. J. Colloid Interface Sci. 2016, 483, 1–10. [Google Scholar] [CrossRef]
- Bastan, F.; Kazemeini, M.; Larimi, A.S. Aqueous-phase reforming of glycerol for production of alkanes over Ni/CexZr1−xO2 nano-catalyst: Effects of the support’s composition. Renew. Energy 2017, 108, 417–424. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Ramli, A.; Yusup, S.; Alnarabiji, M.S. Catalytic hydrodeoxygenation of rubber seed oil over sonochemically synthesized Ni-Mo/γ-Al2O3 catalyst for green diesel production. Ultrason. Sonochem. 2019, 51, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Numpilai, T.; Cheng, C.K.; Seubsai, A.; Faungnawakij, K.; Limtrakul, J.; Witoon, T. Sustainable utilization of waste glycerol for 1,3-propanediol production over Pt/WOx/Al2O3 catalysts: Effects of catalyst pore sizes and optimization of synthesis conditions. Environ. Pollut. 2021, 272, 116029. [Google Scholar] [CrossRef]
- Chen, A.; Guo, H.; Song, Y.; Chen, P.; Lou, H. Recyclable CeO2–ZrO2 and CeO2–TiO2 mixed oxides based Pt catalyst for aqueous-phase reforming of the low-boiling fraction of bio-oil. Int. J. Hydrogen Energy 2017, 42, 9577–9588. [Google Scholar] [CrossRef]
- Rahbar Shamskar, F.; Meshkani, F.; Rezaei, M. Ultrasound assisted co-precipitation synthesis and catalytic performance of mesoporous nanocrystalline NiO-Al2O3 powders. Ultrasonics Sonochem. 2017, 34, 436–447. [Google Scholar] [CrossRef]
- Daroughegi, R.; Meshkani, F.; Rezaei, M. Enhanced activity of CO2 methanation over mesoporous nanocrystalline Ni-Al2O3 catalysts prepared by ultrasound-assisted co-precipitation method. Int. J. Hydrogen Energy 2017, 42, 15115–15125. [Google Scholar] [CrossRef]
- Shang, Z.; Li, S.; Li, L.; Liu, G.; Liang, X. Highly active and stable alumina supported nickel nanoparticle catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 201, 302–309. [Google Scholar] [CrossRef]
- Remón, J.; Giménez, J.; Valiente, A.; García, L.; Arauzo, J. Production of gaseous and liquid chemicals by aqueous phase reforming of crude glycerol: Influence of operating conditions on the process. Energy Convers. Manag. 2016, 110, 90–112. [Google Scholar] [CrossRef]
- Han, L.; Liu, Q.; Lin, K.; Wang, Q.; Rong, N.; Liang, X.; Shaikh, A.R.; Feng, Y.; Zhong, Y. Enhanced hydrogen production via catalytic toluene reforming with in situ carbon dioxide capture: Effects of a hybrid iron-calcium composite prepared by impregnation. Energy Convers. Manag. 2020, 214, 112834. [Google Scholar] [CrossRef]
- Stummann, M.Z.; Elevera, E.; Hansen, A.B.; Hansen, L.P.; Beato, P.; Davidsen, B.; Wiwel, P.; Gabrielsen, J.; Jensen, P.A.; Jensen, A.D. Catalytic hydropyrolysis of biomass using supported CoMo catalysts–Effect of metal loading and support acidity. Fuel 2020, 264, 116807. [Google Scholar] [CrossRef]

| Catalyst | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| ZrO2 | 24.00 | 0.42 | 5.08 |
| 10%Ni/ZrO2_Us | 25.50 | 0.48 | 6.85 |
| 10%Ni/ZrO2_WI | 15.50 | 0.39 | 4.58 |
| 10%Ni-0.5%Ca/ZrO2_Us | 24.30 | 0.34 | 5.77 |
| 10%Ni-0.5%Ca/ZrO2_WI | 14.40 | 0.31 | 3.64 |
| 10%Ni-3%Ca/ZrO2_Us | 23.90 | 0.26 | 4.04 |
| 10%Ni-3%Ca/ZrO2_WI | 12.50 | 0.24 | 2.21 |
| 10%Ni-5%Ca/ZrO2_Us | 22.40 | 0.21 | 3.46 |
| 10%Ni-5%Ca/ZrO2_WI | 11.70 | 0.20 | 1.01 |
| Catalyst | Cglycerol (%) | Selectivity (%) | C Balance (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1,3-PDO | 1,2-PDO | EG | Propanol | Acetone | Ethanol | Methanol | Others Unidentified Compounds | |||
| 10%Ni/ZrO2_Us | 50.8 ± 0.2 | 23.8 ± 2.3 | 17.2 ± 1.3 | 10.5 ± 2.1 | 5.41 ± 2.2 | 7.32 ± 1.0 | 5.21 ± 1.3 | 6.43 ± 0.9 | 18.1 ± 0.8 | 94 ± 1.3 |
| 10%Ni/ZrO2_WI | 49.5 ± 1.3 | 12.3 ± 1.3 | 10.2 ± 2.2 | 6.78 ± 1.2 | 3.54 ± 3.4 | 5.67 ± 0.7 | 4.93 ± 1.8 | 5.78 ± 1.2 | 38.8 ± 1.2 | 88 ± 4.0 |
| 10%Ni-0.5%Ca/ZrO2_Us | 49.5 ± 0.9 | 19.8 ± 1.0 | 21.9 ± 1.0 | 12.2 ± 0.8 | 9.81 ± 1.2 | 8.82 ± 0.7 | 7.45 ± 2.3 | 8.67 ± 2.5 | 3.35 ± 3.5 | 92 ± 3.3 |
| 10%Ni-0.5%Ca/ZrO2_WI | 48.2 ± 3.4 | 10.9 ± 2.5 | 12.3 ± 1.1 | 8.76 ± 1.7 | 5.21 ± 1.0 | 7.89 ± 3.0 | 6.98 ± 2.4 | 7.84 ± 3.2 | 30.1 ± 5.4 | 90 ± 1.2 |
| 10%Ni-3%Ca/ZrO2_Us | 49.3 ± 1.3 | 15.8 ± 4.7 | 24.8 ± 3.4 | 14.2 ± 3.2 | 10.1 ± 1.2 | 9.43 ± 1.7 | 9.72 ± 3.4 | 9.67 ± 1.4 | 1.28 ± 2.9 | 95 ± 1.0 |
| 10%Ni-3%Ca/ZrO2_WI | 42.7 ± 4.5 | 9.98 ± 3.4 | 14.2 ± 2.9 | 9.23 ± 2.4 | 7.89 ± 3.4 | 9.21 ± 2.3 | 8.98 ± 1.2 | 8.43 ± 3.2 | 16.1 ± 3.4 | 84 ± 1.2 |
| 10%Ni-5%Ca/ZrO2_Us | 48.2 ± 0.6 | 14.2 ± 2.3 | 24.9 ± 2.8 | 14. 7 ± 1.7 | 12.3 ± 2.3 | 10.0 ± 3.2 | 9.91 ± 0.8 | 9.76 ± 4.1 | 1.23 ± 3.7 | 97 ± 3.4 |
| 10%Ni-5%Ca/ZrO2_WI | 40.2 ± 3.4 | 7.89 ± 3.2 | 15.7 ± 4.0 | 11.4 ± 1.5 | 10.5 ± 1.3 | 9.98 ± 4.2 | 9.34 ± 0.7 | 8.98 ± 3.2 | 11.2 ± 1.0 | 85 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syuhada, A.; Ameen, M.; Sher, F.; Azizan, M.T.; Aqsha, A.; Yusoff, M.H.M.; Ruslan, M.S.H. Effect of Calcium Doping Using Aqueous Phase Reforming of Glycerol over Sonochemically Synthesized Nickel-Based Supported ZrO2 Catalyst. Catalysts 2021, 11, 977. https://doi.org/10.3390/catal11080977
Syuhada A, Ameen M, Sher F, Azizan MT, Aqsha A, Yusoff MHM, Ruslan MSH. Effect of Calcium Doping Using Aqueous Phase Reforming of Glycerol over Sonochemically Synthesized Nickel-Based Supported ZrO2 Catalyst. Catalysts. 2021; 11(8):977. https://doi.org/10.3390/catal11080977
Chicago/Turabian StyleSyuhada, Ain, Mariam Ameen, Farooq Sher, Mohammad Tazli Azizan, Aqsha Aqsha, Mohd Hizami Mohd Yusoff, and Muhamad Syafiq Hazwan Ruslan. 2021. "Effect of Calcium Doping Using Aqueous Phase Reforming of Glycerol over Sonochemically Synthesized Nickel-Based Supported ZrO2 Catalyst" Catalysts 11, no. 8: 977. https://doi.org/10.3390/catal11080977
APA StyleSyuhada, A., Ameen, M., Sher, F., Azizan, M. T., Aqsha, A., Yusoff, M. H. M., & Ruslan, M. S. H. (2021). Effect of Calcium Doping Using Aqueous Phase Reforming of Glycerol over Sonochemically Synthesized Nickel-Based Supported ZrO2 Catalyst. Catalysts, 11(8), 977. https://doi.org/10.3390/catal11080977








