Effects of Reaction Temperature on the Photocatalytic Activity of TiO2 with Pd and Cu Cocatalysts
Abstract
:1. Introduction
2. Results and Discussion
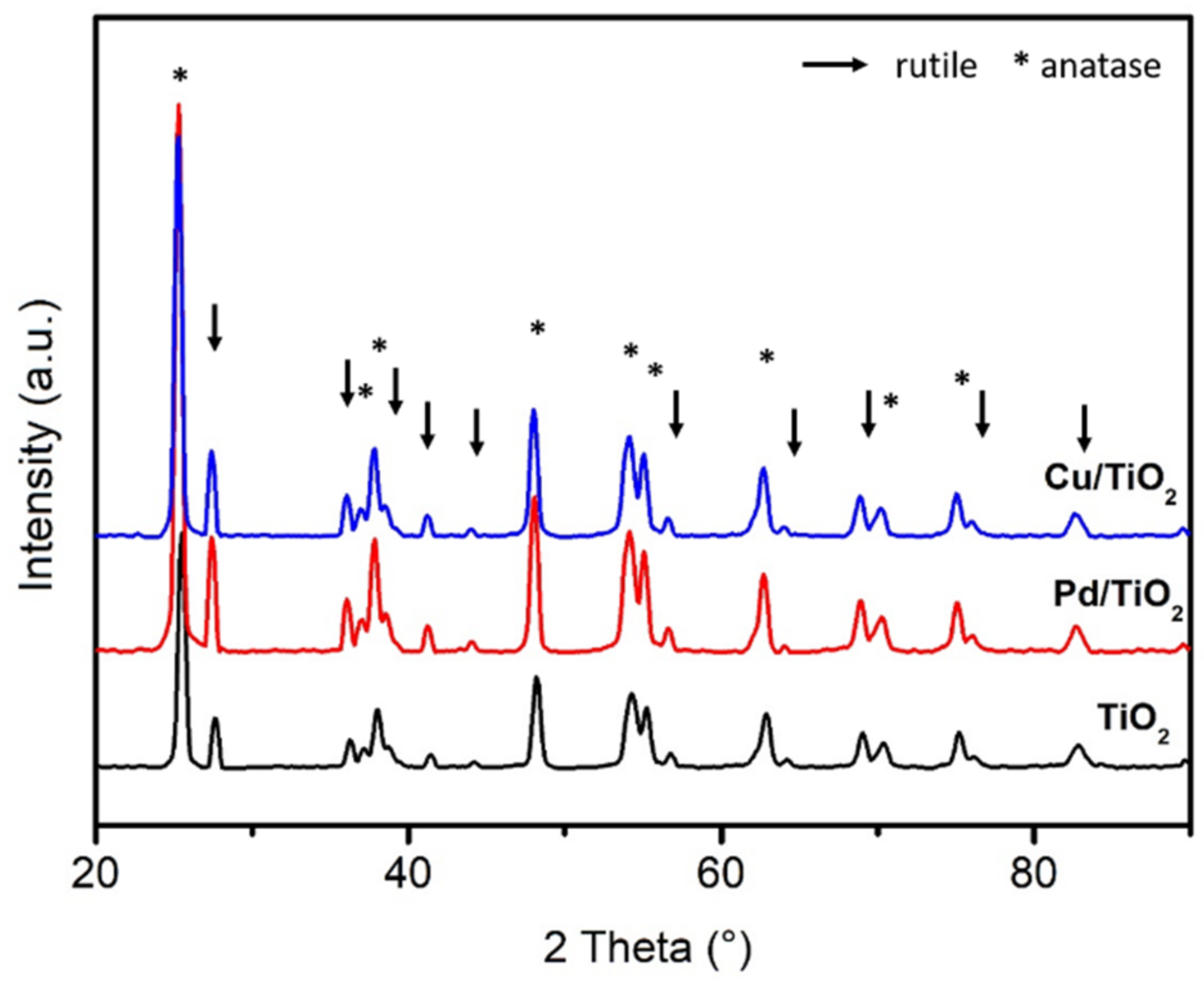
2.1. X-ray Diffraction (XRD)
2.2. Pore Structure of the Catalyst
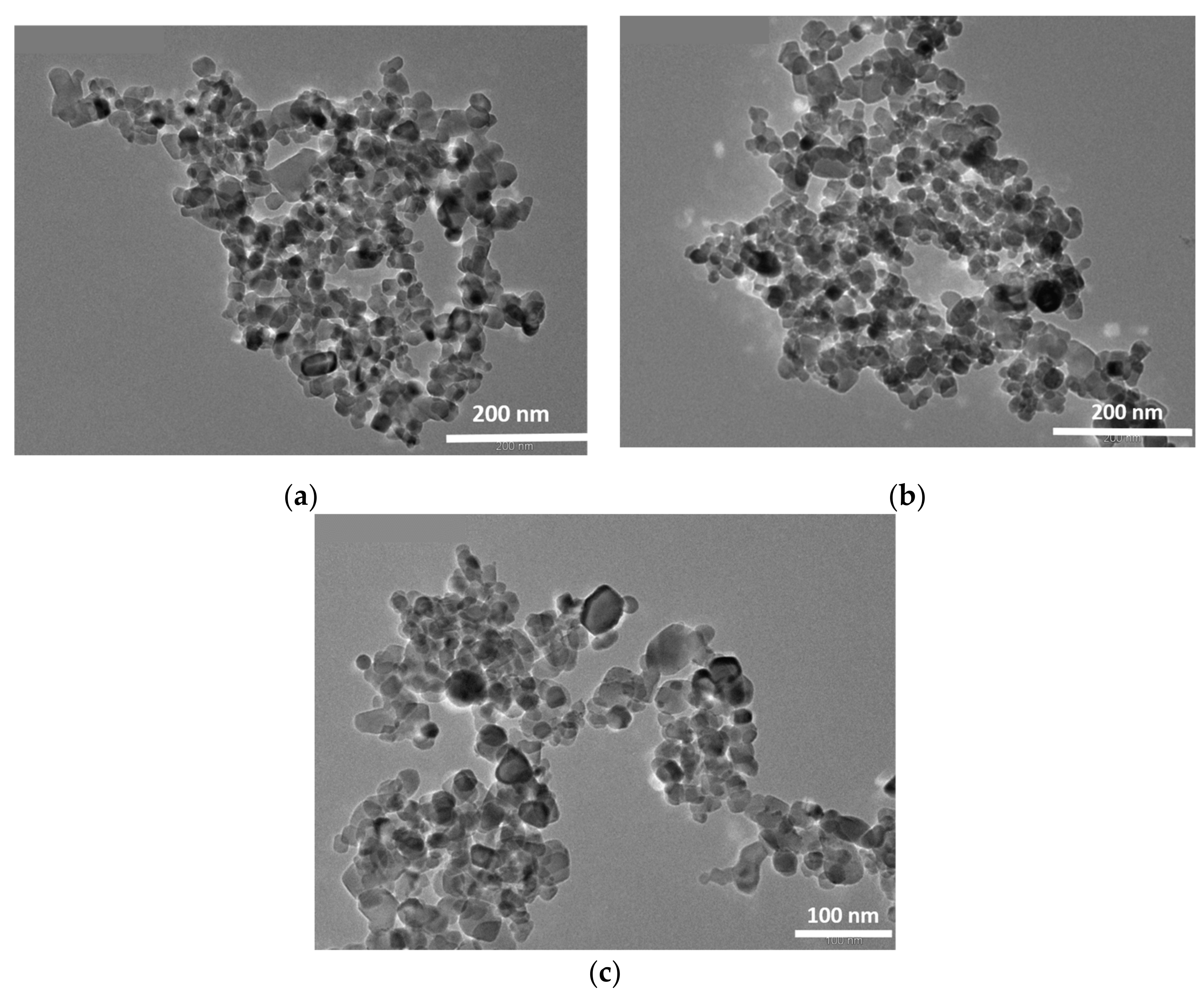
2.3. Transmission Electron Microscopy (TEM
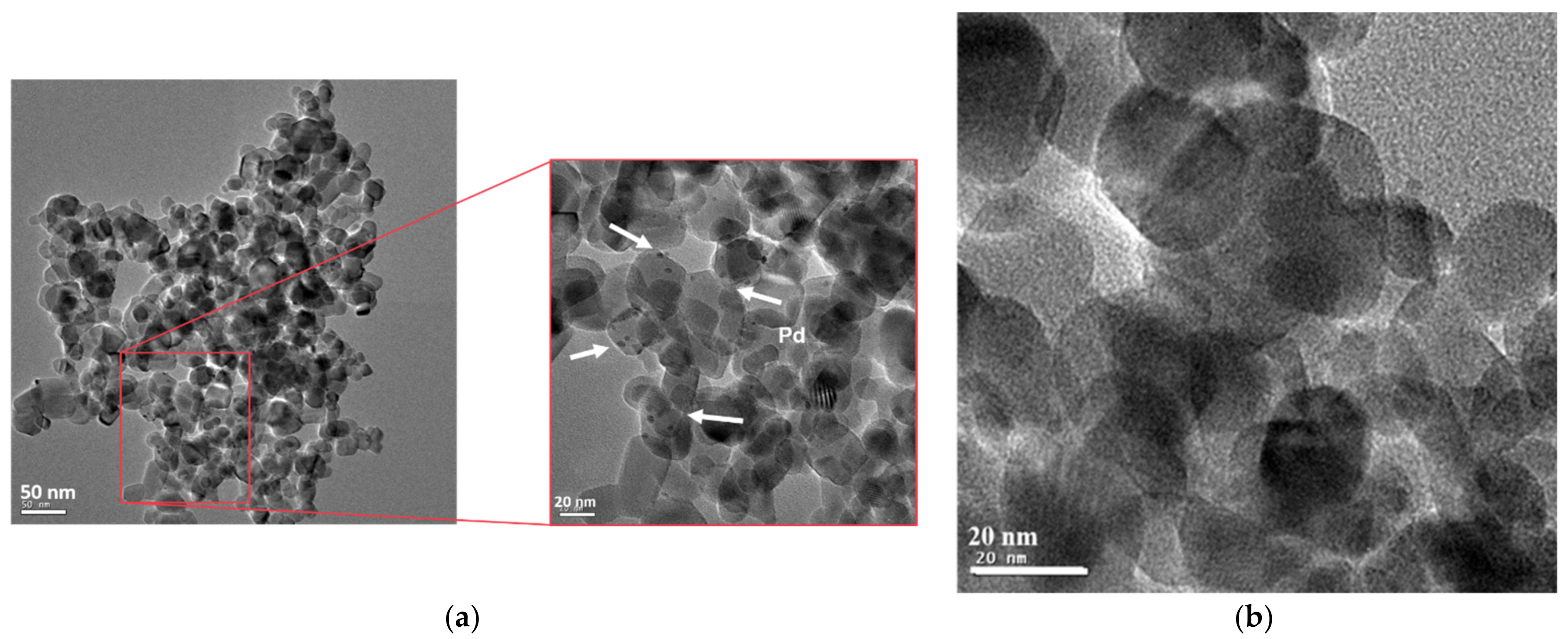
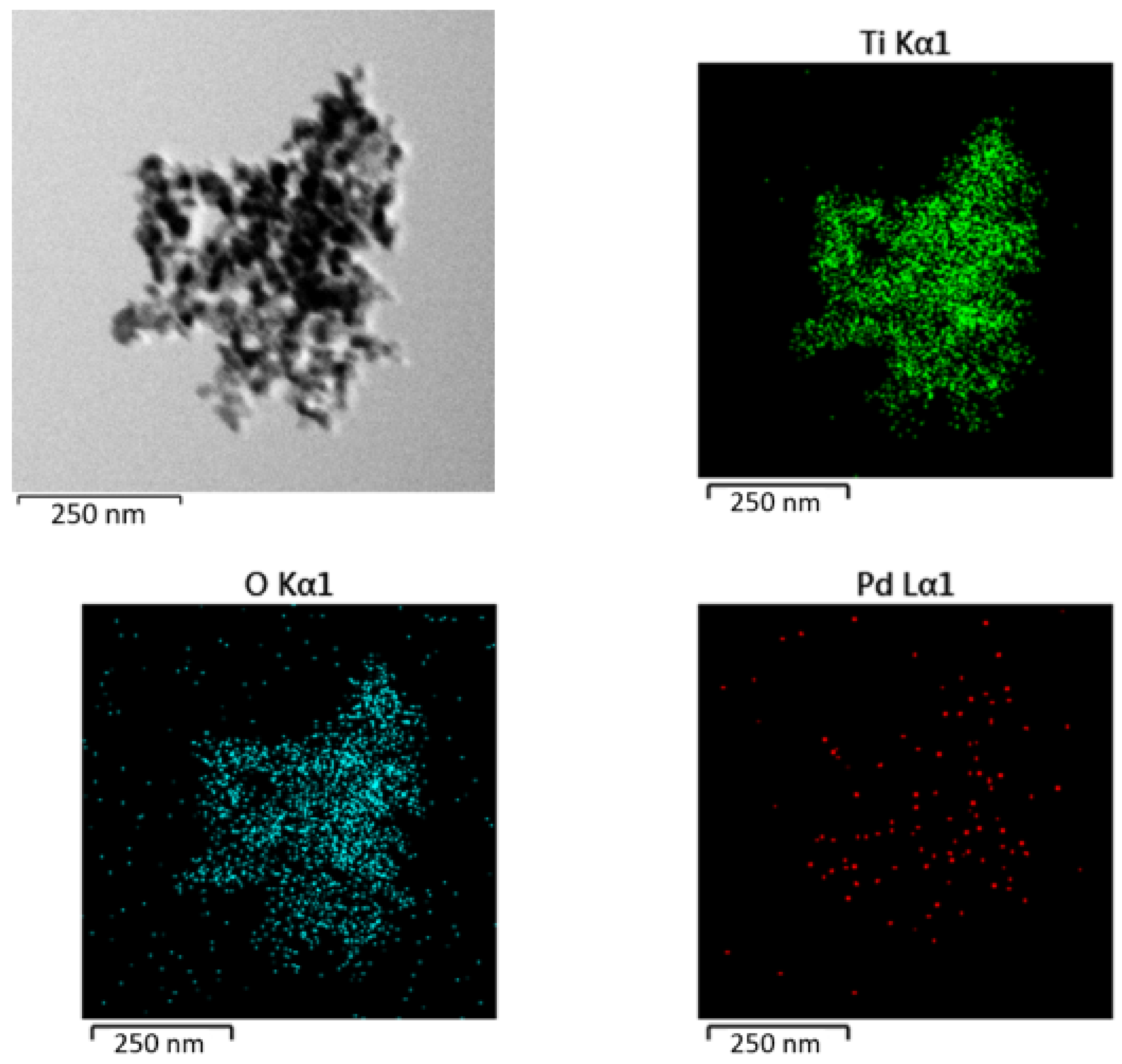
2.4. High-Resolution Transmission Electron Microscopy (HRTEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
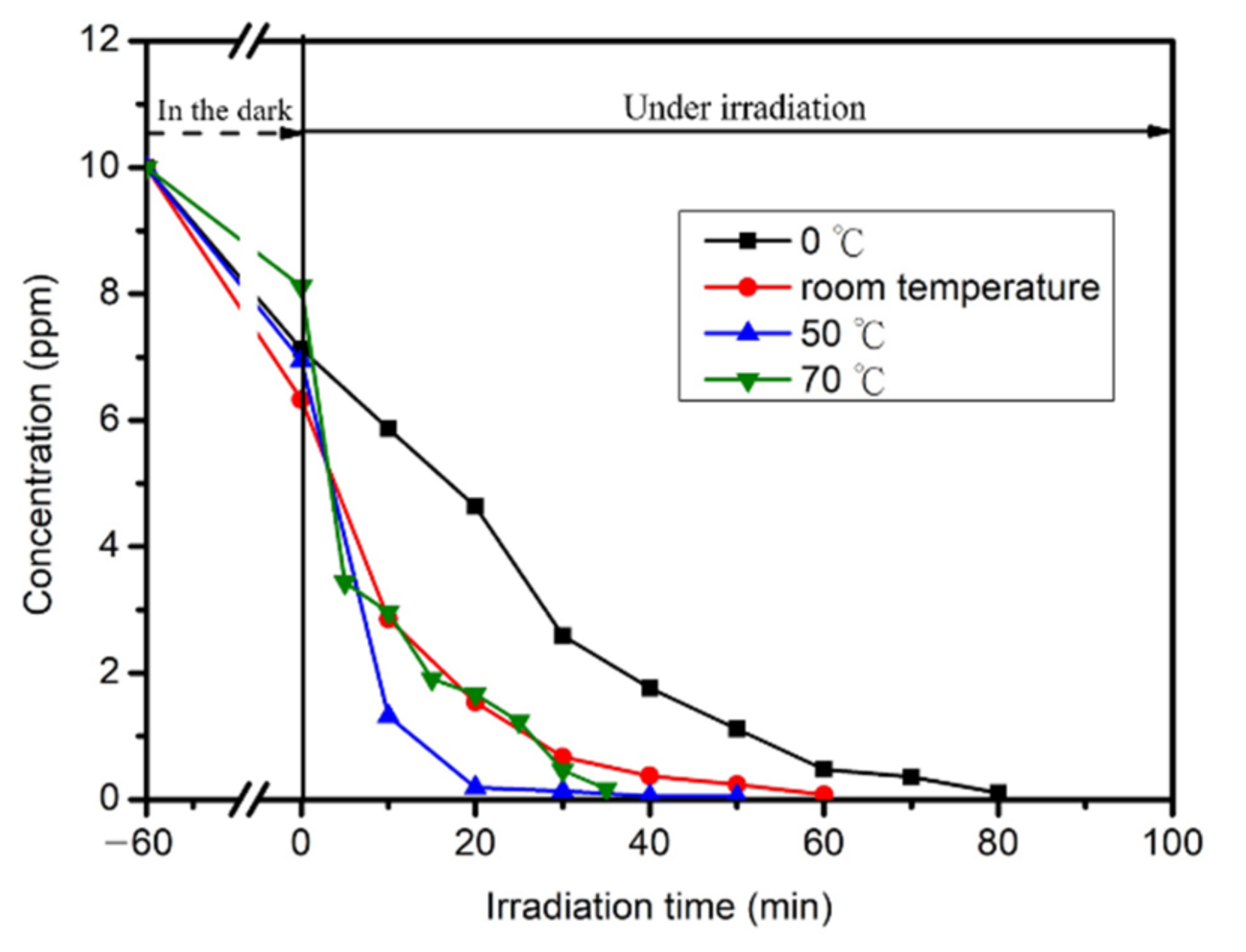
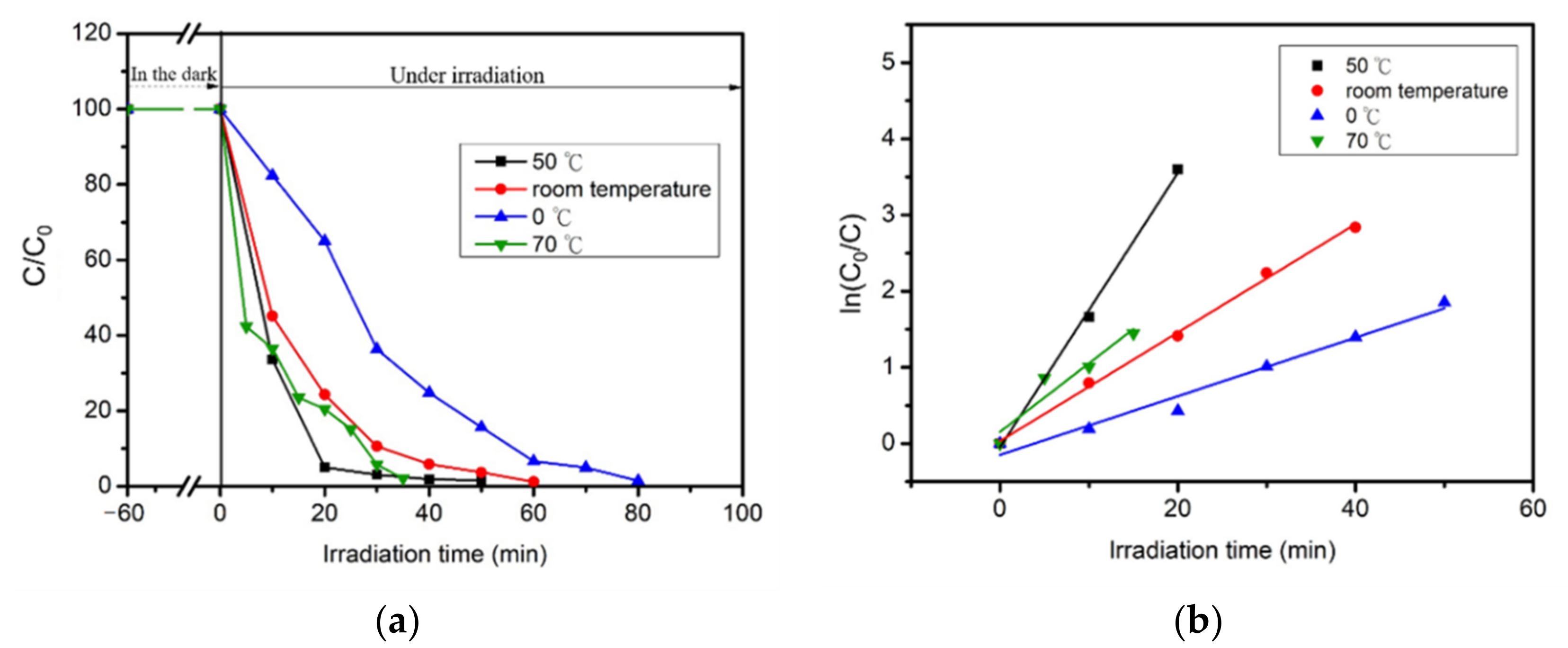
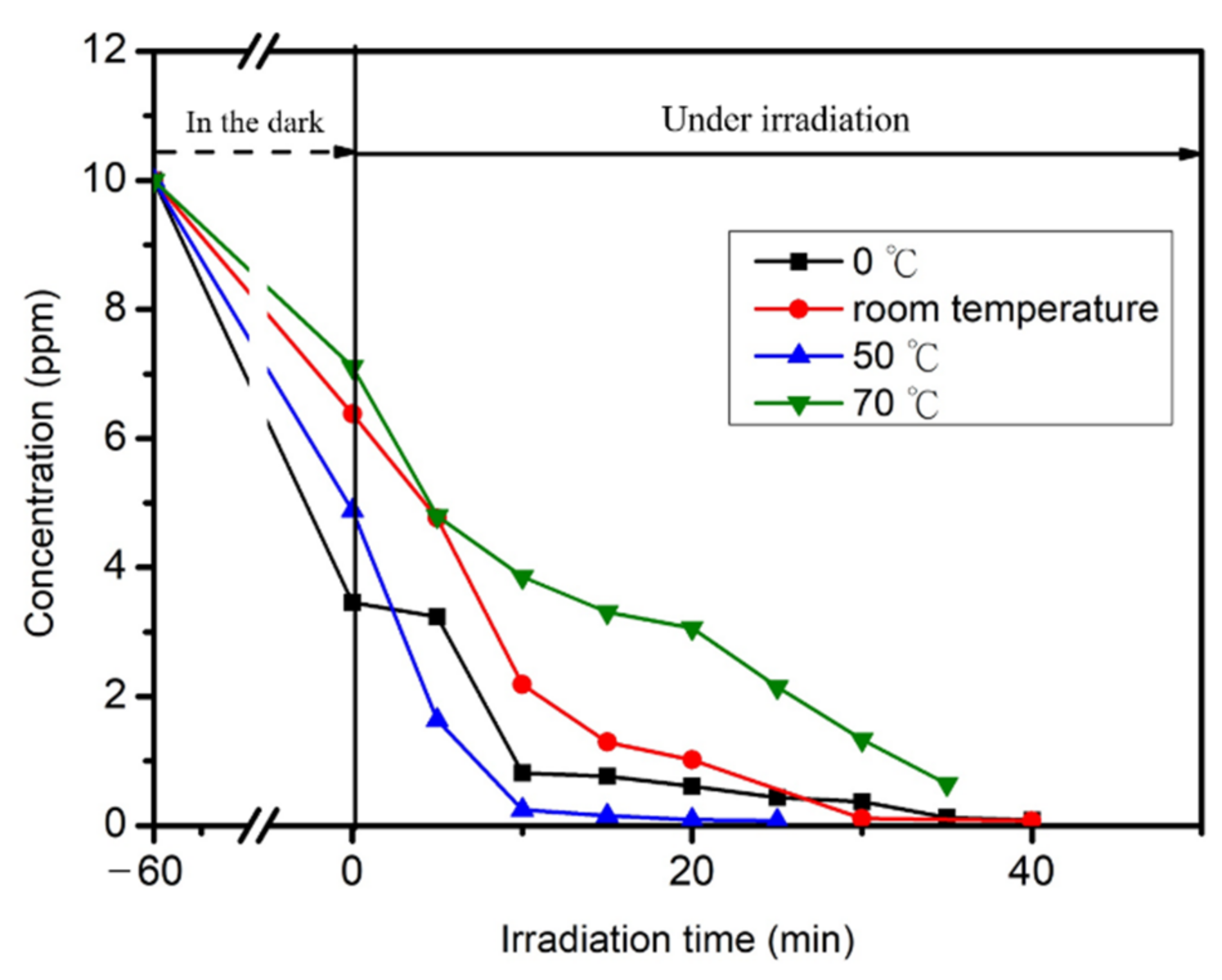
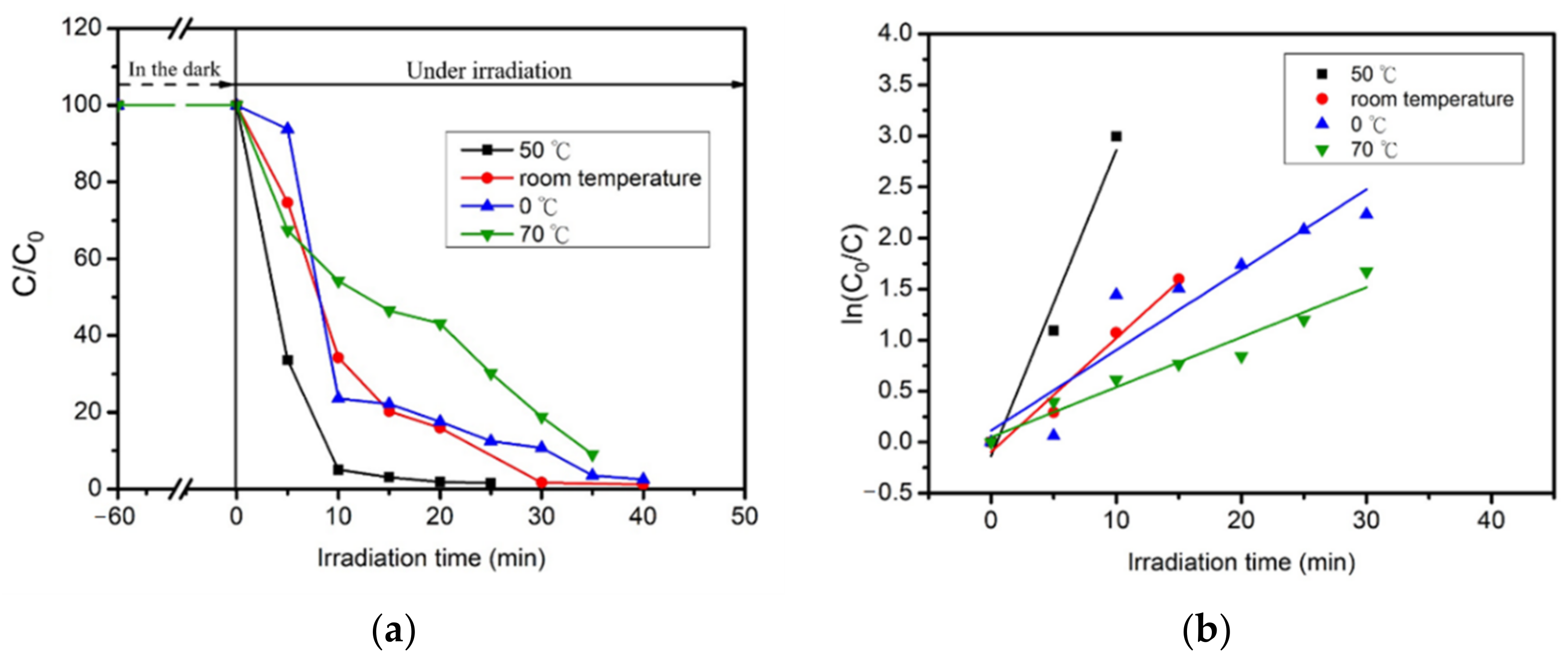
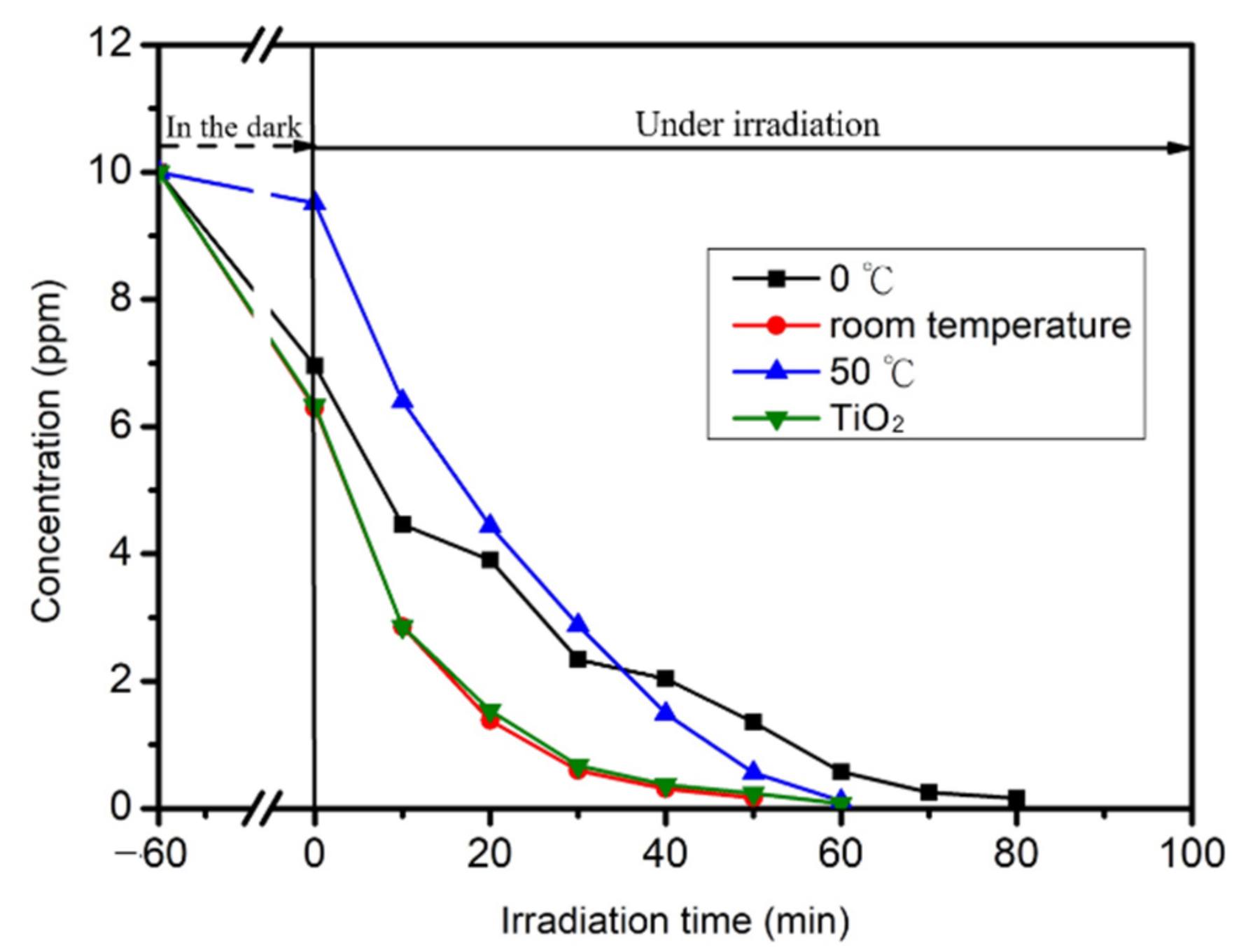
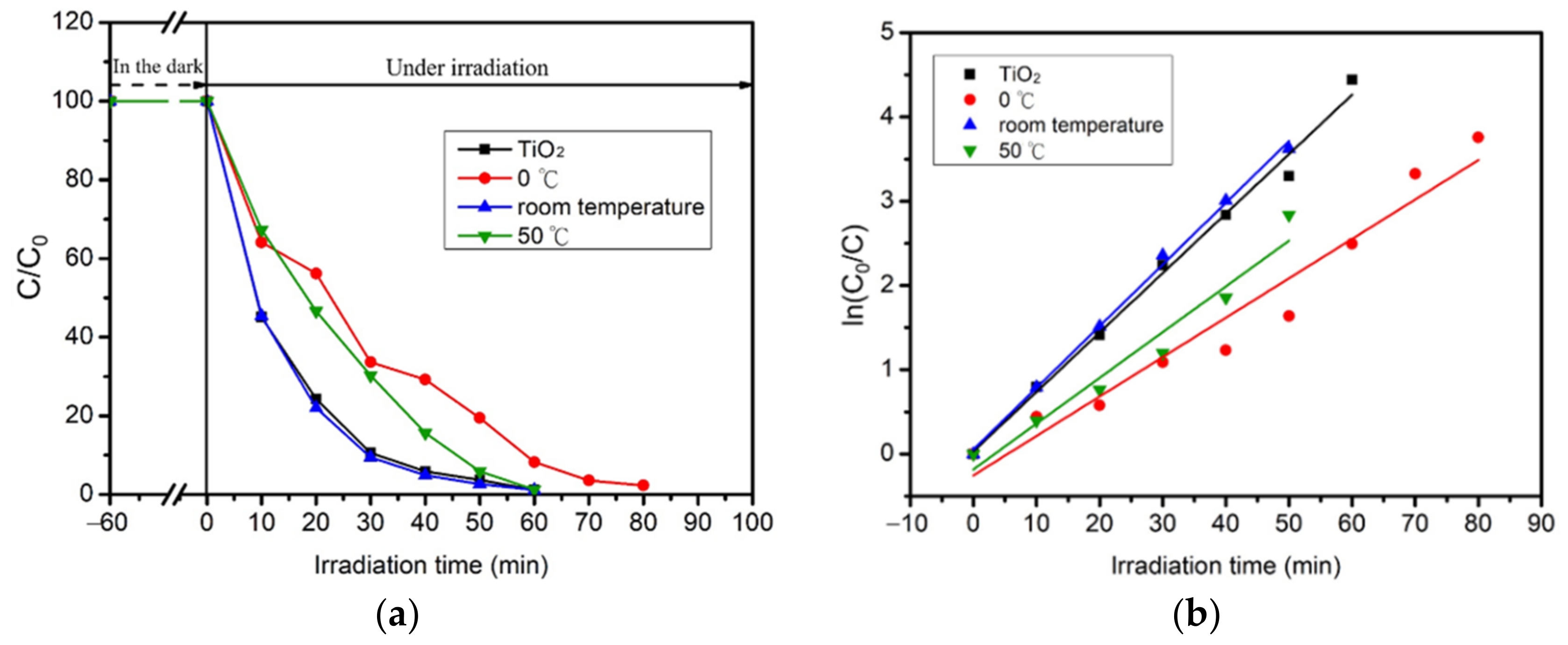
2.5. Photocatalytic Reaction
3. Materials and Methods
3.1. Materials
3.2. Catalysts Preparation
3.3. Catalysts Characterization
3.3.1. Pore Structure of the Catalyst
3.3.2. Transmission Electron Microscopy (TEM) and High-Resolution Transmission Electron Microscopy (HRTEM)
3.4. Photocatalytic Reaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, T.; Klüner, T. Photodesorption of H2O from anatase-TiO2 (101): A combined quantum chemical and quantum dynamical study. J. Phys. Chem. C 2020, 124, 11444–11455. [Google Scholar] [CrossRef]
- Liu, B.; Wu, H.; Parkin, I.P. New insights into the fundamental principle of semiconductor photocatalysis. ACS Omega 2020, 5, 14847–14856. [Google Scholar] [CrossRef]
- Gao, C.; Low, J.; Long, R.; Kong, T.; Zhu, J.; Xiong, Y. Heterogeneous single-atom photocatalysts: Fundamentals and applications. Chem. Rev. 2020, 120, 12175–12216. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Rahimi, N.; Pax, R.A.; Gray, E.M.A. Review of functional titanium oxides. I: TiO2 and its modifications. Prog. Solid State Chem. 2016, 44, 86–104. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2 photocatalyst for CO2 photocatalytic reduction: An overview. J. CO2 Util. 2017, 22, 15–32. [Google Scholar] [CrossRef]
- Ibhadon, A.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X. Titanium dioxide nanomaterials: Self-structural modifications. Chem. Rev. 2014, 114, 9890–9918. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Magalhães, P.; Andrade, L.; Nunes, O.C.; Mendes, A. Titanium dioxide photocatalysis: Fundamentals and application on photoinactivation. Rev. Adv. Mater. Sci. 2017, 51, 91–129. [Google Scholar]
- Pawar, M.; Sendoğdular, S.; Gouma, P. A brief overview of TiO2 photocatalyst for organic dye remediation: Case study of reaction mechanisms involved in Ce-TiO2 photocatalysts system. J. Nanomater. 2018, 2018, 5953609. [Google Scholar] [CrossRef] [Green Version]
- Wold, A. Photocatalytic properties of titanium dioxide (TiO2). Chem. Mater. 1993, 5, 280–283. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2019, 26, 3262–3291. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Harris, J.; Silk, R.; Smith, M.; Dong, Y.; Chen, W.T.; Waterhouse, G.I.N. Hierarchical TiO2 nanoflower photocatalysts with remarkable activity for aqueous methylene blue photo-oxidation. ACS Omega 2020, 5, 18919–18934. [Google Scholar] [CrossRef] [PubMed]
- AlSalka, Y.; Al-Madanat, O.; Curti, M.; Hakki, A.; Bahnemann, D.W. Photocatalytic H2 Evolution from Oxalic Acid: Effect of Cocatalysts and Carbon Dioxide Radical Anion on the Surface Charge Transfer Mechanisms. ACS Appl. Energy Mater. 2020, 3, 6678–6691. [Google Scholar] [CrossRef]
- Nath, R.R.; Nethravathi, C.; Rajamathi, M. Hierarchically porous, biphasic, and C-Doped TiO2 for solar photocatalytic degradation of dyes and selective oxidation of benzyl alcohol. ACS Omega 2021, 6, 12124–12132. [Google Scholar] [CrossRef]
- Pinedo-Escobar, J.F.; Fan, F.; Moctezuma, E.; Gomez-Solís, C.; Martinez, C.J.C.; Gracia-Espino, E. Nanoparticulate double-heterojunction photocatalysts comprising TiO2(anatase)/WO3/TiO2(rutile) with enhanced photocatalytic activity toward the degradation of methyl orange under near-ultraviolet and visible Light. ACS Omega 2021, 6, 11840–11848. [Google Scholar] [CrossRef]
- Günnemann, C.; Bahnemann, D.W.; Robertson, P.K.J. Isotope effects in photocatalysis: An underexplored issue. ACS Omega 2021, 6, 11113–11121. [Google Scholar] [CrossRef]
- Yaemsunthorn, K.; Kobielusz, M.; Macyk, W. TiO2 with tunable anatase-to-rutile nanoparticles ratios: How does the photoactivity depend on the phase composition and the nature of photocatalytic reaction? ACS Appl. Nano Mater. 2021, 4, 633–643. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, L.; Zhang, Z.; Gao, Z.; Weng, H.; Zhang, W.; Li, L.; Song, Y.Y. Insight of the influence of magnetic-field direction on magneto-plasmonic interfaces for tuning photocatalytical performance of semiconductors. J. Phys. Chem. Lett. 2020, 11, 9931–9937. [Google Scholar] [CrossRef]
- Sangpour, P.; Hashemi, F.; Moshfegh, A.Z. Photoenhanced degradation of methylene blue on cosputtered M: TiO2 (M: Au, Ag, Cu) nanocomposite systems: A comparative study. J. Phys. Chem. C 2010, 114, 13955–13961. [Google Scholar] [CrossRef]
- Rismanchian, A.; Chen, Y.W.; Chuang, S.S.C. In situ infrared study of photoreaction of ethanol on Au and Ag/TiO2. Catal. Today 2016, 264, 16–22. [Google Scholar] [CrossRef]
- Moongraksathum, B.; Hsu, P.T.; Chen, Y.W. Photocatalytic activity of ascorbic acid-modified TiO2 sol prepared by the peroxo sol–gel method. J. Sol Gel Sci. Technol. 2016, 78, 647–659. [Google Scholar] [CrossRef]
- Moongraksathum, B.; Lee, D.S.; Chen, Y.W. Monodispersibility and size control in the preparation of spherical titania particles by thermal hydrolysis of TiCl4 in various solvents. J. Taiwan Inst. Chem. Eng. 2016, 68, 455–460. [Google Scholar] [CrossRef]
- Moongraksathum, B.; Chen, Y.W. CeO2–TiO2 mixed oxide thin films with enhanced photocatalytic degradation of organic pollutants. J. Sol. Gel. Sci. Technol. 2017, 82, 772–782. [Google Scholar] [CrossRef]
- Moongraksathum, B.; Chen, Y.W. Anatase TiO2 co-doped with silver and ceria for antibacterial application. Catal. Today 2018, 310, 69–74. [Google Scholar] [CrossRef]
- Moongraksathum, B.; Shang, J.Y.; Chen, Y.W. Photocatalytic antibacterial effectiveness of Cu-doped TiO2 thin film prepared via peroxo sol–gel method. Catalysts 2018, 8, 352. [Google Scholar] [CrossRef] [Green Version]
- Moongraksathum, B.; Chien, M.Y.; Chen, Y.W. Antiviral and antibacterial effects of silver-doped TiO2 prepared by the peroxo sol-gel method. J. Nanosci. Nanotechnol. 2019, 19, 7356–7362. [Google Scholar] [CrossRef]
- Tseng, H.C.; Chen, Y.W. Facile synthesis of Ag/TiO2 by Photoreduction method and its degradation activity of methylene blue under UV and visible light irradiation. Mod. Res. Catal. 2020, 9, 97149. [Google Scholar] [CrossRef] [Green Version]
- Shang, J.Y.; Chen, Y.W. Photoactivity of CuO-TiO2 nanocomposite photocatalyst under twilight irradiation. Curr. Top. Catal. 2020, 14, 97–110. [Google Scholar]
- Wu, J.Y.; Chen, Y.W. Preparation of WO3-modified TiO2 thin film by peroxo sol–gel method and its photocatalytic activity in degradation of methylene blue. Res. Chem. Intermed. 2020, 46, 4627–4643. [Google Scholar] [CrossRef]
- Tseng, H.C.; Chen, Y.W. Surface modification of TiO2 by silver incorporation and its photocatalytic activity under twilight irradiation. Trends Photochem. Photobiol. 2020, 19, 23–34. [Google Scholar]
- Wu, J.Y.; Chen, Y.W. Anticorrosion of WO3-modified TiO2 thin film prepared by peroxo sol-gel method. Mod. Res. Catal. 2020, 9, 35–46. [Google Scholar] [CrossRef]
- Chen, Y.W.; Tsai, K.J. Anatase TiO2 co-doped with silver and silica for destruction of organic dye and bacteria. J. Sol. Gel. Sci. Technol. 2021, 97, 651–662. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Kanjwal, M.A.; Chronakis, I.S.; Kim, H.Y. Influence of temperature on the photodegradation process using Ag-dope TiO2 nanostructures Negative impact with the nanofibers. J. Mol. Catal. A Chem. 2013, 366, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Choi, H.J.; Kim, H.; Kim, J.; Monllor-Satoca, D.; Kim, M.; Park, H. Temperature-boosted photocatalytic H2 production and charge transfer kinetics on TiO2 under UV and visible light. Photochem. Photobiol. Sci. 2016, 15, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Zhang, G. Photocatalytic degradation of organic contaminants by TiO2/sepiolite composites prepared at low temperature. Chem. Eng. J. 2011, 173, 1–10. [Google Scholar] [CrossRef]
- Nakano, K.; Obuchi, E.; Nanri, M. Thermo-photocatalytic decomposition of acetaldehyde over Pt-TiO2/SiO2. Chem. Eng. Res. Des. 2004, 82, 297–301. [Google Scholar] [CrossRef]
- Salimi, A.; Roosta, A. Experimental solubility and thermodynamic aspects of methylene blue in different solvents. Thermochim. Acta 2019, 675, 134–139. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. A Review on the factors affecting the photocatalytic degradation of hazardous materiials. Mater. Sci. Eng. Int. J. 2017, 1, 106–114. [Google Scholar]
- Parra, S.; Stanca, E.S.; Guasaquillo, I.; Thampi, R.K. Photocatalytic degradation of atrazine using suspended and supported TiO2. Appl. Catal. B Environ. 2014, 51, 107–116. [Google Scholar] [CrossRef]
- Mateo, D.; Cerrillo, J.L.; Durini, S.; Gascon, J. Fundamentals and applications of photo-thermal catalysis. Chem. Soc. Rev. 2021, 50, 2173–2210. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xia, S.; Pan, G.; Xue, J.; Jiang, J.; Ni, Z. Preparation and photocatalytic activity of composite metal oxides derived from Salen-Cu(II) intercalated layered double hydroxides. Korean J. Chem. Eng. 2017, 34, 2331–2341. [Google Scholar] [CrossRef]
- Yamamoto, A.; Mizuno, Y.; Kentaro Teramura, K.; Shishido, T.; Tanaka, T. Effects of reaction temperature on the photocatalytic activity of photo-SCR of NO with NH3 over a TiO2 photocatalyst. Catal. Sci. Technol. 2013, 3, 1771–1775. [Google Scholar] [CrossRef]
- Ojstršek, A.; Kleinschek, K.S.; Fakin, D. Characterization of nano-sized TiO2 suspensions for functional modification of polyester fabric. Surf. Coat. Technol. 2013, 226, 68–74. [Google Scholar] [CrossRef]
- Liu, E.; Qi, L.; Bian, J.; Chen, Y.; Hu, X.; Fan, J.; Liu, H.; Zhu, C.; Wang, Q. A facile strategy to fabricate plasmonic Cu modified TiO2 nano-flower films for photocatalytic reduction of CO2 to methanol. Mater. Res. Bull. 2015, 68, 203–209. [Google Scholar] [CrossRef]
- Orudzhev, F.F.; Isaev, A.B.; Shabanov, N.S.; Gasanova, F.G.; Idrisova, A.K.; Babaeva, D.P. Photoelectrocatalytic oxidation of phenol on silver loaded TiO2 nanotube array at high oxygen pressure under luminescent light irradiation. Int. J. Electrochem. Sci. 2018, 13, 4548–4560. [Google Scholar] [CrossRef]
- Lacerda, A.M.; Larrosa, I.; Dunn, S. Plasmon enhanced visible light photocatalysis for TiO2 supported Pd nanoparticles. Nanoscale 2015, 7, 12331–12335. [Google Scholar] [CrossRef] [PubMed]
- Orudzhev, F.F.; Gasanova, F.G.; Aliev, Z.M.; Isaev, A.B. Photoelectrocatalytic oxidation of phenol on platinum-modified TiO2 nanotubes. Nanotechnol. Russ. 2012, 7, 482–485. [Google Scholar] [CrossRef]
- Esmat, M.; El-Hosainy, H.; Tahawy, R.; Jevasuwan, W.; Tsunoji, N.; Fukata, N.; Ide, Y. Nitrogen doping-mediated oxygen vacancies enhancing co-catalyst-free solar photocatalytic H2 production activity in anatase TiO2 nanosheet assembly. Appl. Catal. B Environ. 2021, 285, 119755. [Google Scholar] [CrossRef]
- Doustkhah, E.; Assadi, M.H.N.; Komaguchi, K.; Tsunoji, N.; Esmat, M.; Fukata, N.; Tomita, O.; Abe, R.; Ohtani, B.; Ide, Y. In situ Blue titania via band shape engineering for exceptional solar H2 production in rutile TiO2. Appl. Catal. B Environ. 2021, 297, 120380–120392. [Google Scholar] [CrossRef]
- Esmat, M.; Farghali, A.A.; El-Dek, S.I.; Khedr, M.H.; Yamauchi, Y.; Bando, Y.; Fukata, N.; Ide, Y. Conversion of a 2D Lepidocrocite-type layered titanate into its 1D nanowire form with enhancement of cation exchange and photocatalytic performance. Inorg. Chem. 2019, 58, 7989–7996. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Younesi, H.; Zinatizadeh, A.A. Kinetics and thermodynamics of photocatalytic degradation of organic pollutants in petroleum refinery wastewater over nano-TiO2 supported on Fe-ZSM-5. J. Taiwan Inst. Chem. Eng. 2016, 65, 357–366. [Google Scholar] [CrossRef] [Green Version]

| Sample | BET Surface Area (m2/g) | Desorption Average Pore Diameter (Å) | BJH Cumulative Volume of Pores (cm3/g) | BJH Average Pore Width (Å) |
|---|---|---|---|---|
| TiO2 | 51.3 | 283 | 0.364 0.372 0.377 | 236.0 242.4 253.7 |
| Pd/TiO2 | 50.6 | 293 | ||
| Cu/TiO2 | 48.6 | 309 |
| As-Prepared Powder | Reaction Temperature (°C) | Rate Constant (k, min−1) |
|---|---|---|
| TiO2 | 0 | 0.00309 |
| TiO2 | 25 | 0.07122 |
| TiO2 | 50 | 0.17998 |
| TiO2 | 70 | 0.08986 |
| Pd/TiO2 | 0 | 0.07874 |
| Pd/TiO2 | 25 | 0.1114 |
| Pd/TiO2 | 50 | 0.29942 |
| Pd/TiO2 | 70 | 0.04893 |
| Cu/TiO2 | 0 | 0.04677 |
| Cu/TiO2 | 25 | 0.07321 |
| Cu/TiO2 | 50 | 0.05428 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-W.; Hsu, Y.-H. Effects of Reaction Temperature on the Photocatalytic Activity of TiO2 with Pd and Cu Cocatalysts. Catalysts 2021, 11, 966. https://doi.org/10.3390/catal11080966
Chen Y-W, Hsu Y-H. Effects of Reaction Temperature on the Photocatalytic Activity of TiO2 with Pd and Cu Cocatalysts. Catalysts. 2021; 11(8):966. https://doi.org/10.3390/catal11080966
Chicago/Turabian StyleChen, Yu-Wen, and Yu-Hsuan Hsu. 2021. "Effects of Reaction Temperature on the Photocatalytic Activity of TiO2 with Pd and Cu Cocatalysts" Catalysts 11, no. 8: 966. https://doi.org/10.3390/catal11080966
APA StyleChen, Y.-W., & Hsu, Y.-H. (2021). Effects of Reaction Temperature on the Photocatalytic Activity of TiO2 with Pd and Cu Cocatalysts. Catalysts, 11(8), 966. https://doi.org/10.3390/catal11080966







