Simulated Ageing of Crude Oil and Advanced Oxidation Processes for Water Remediation since Crude Oil Pollution
Abstract
1. Introduction
2. Results and Discussion
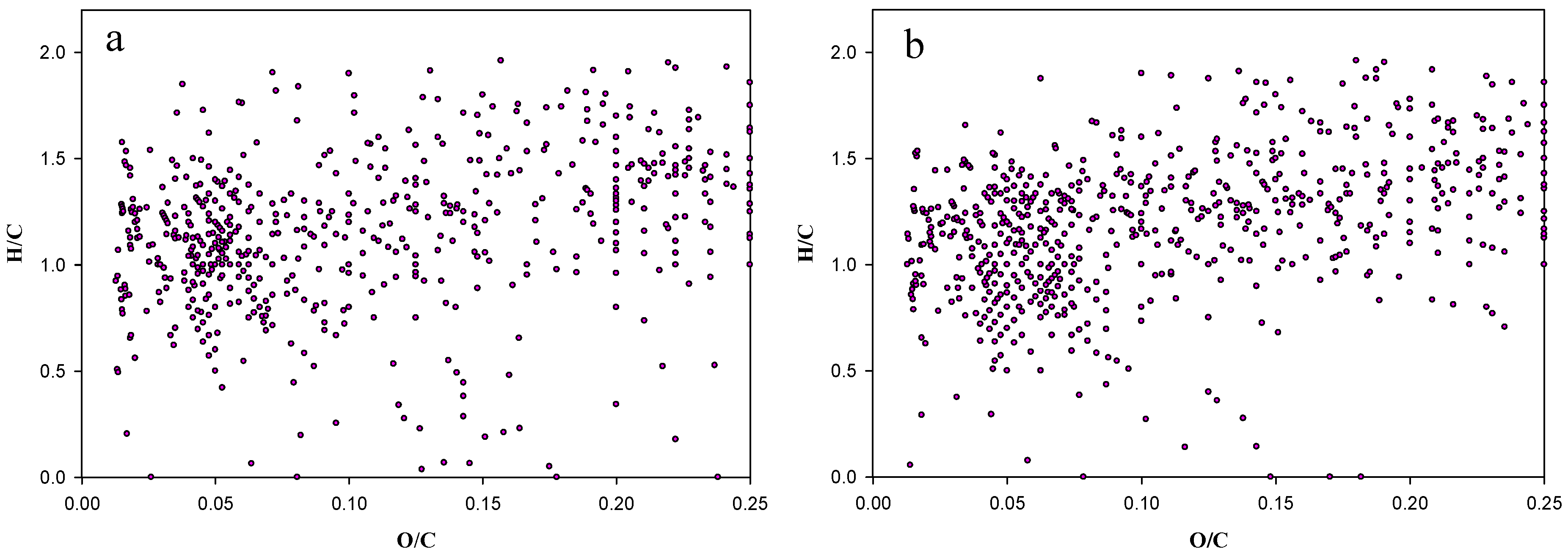
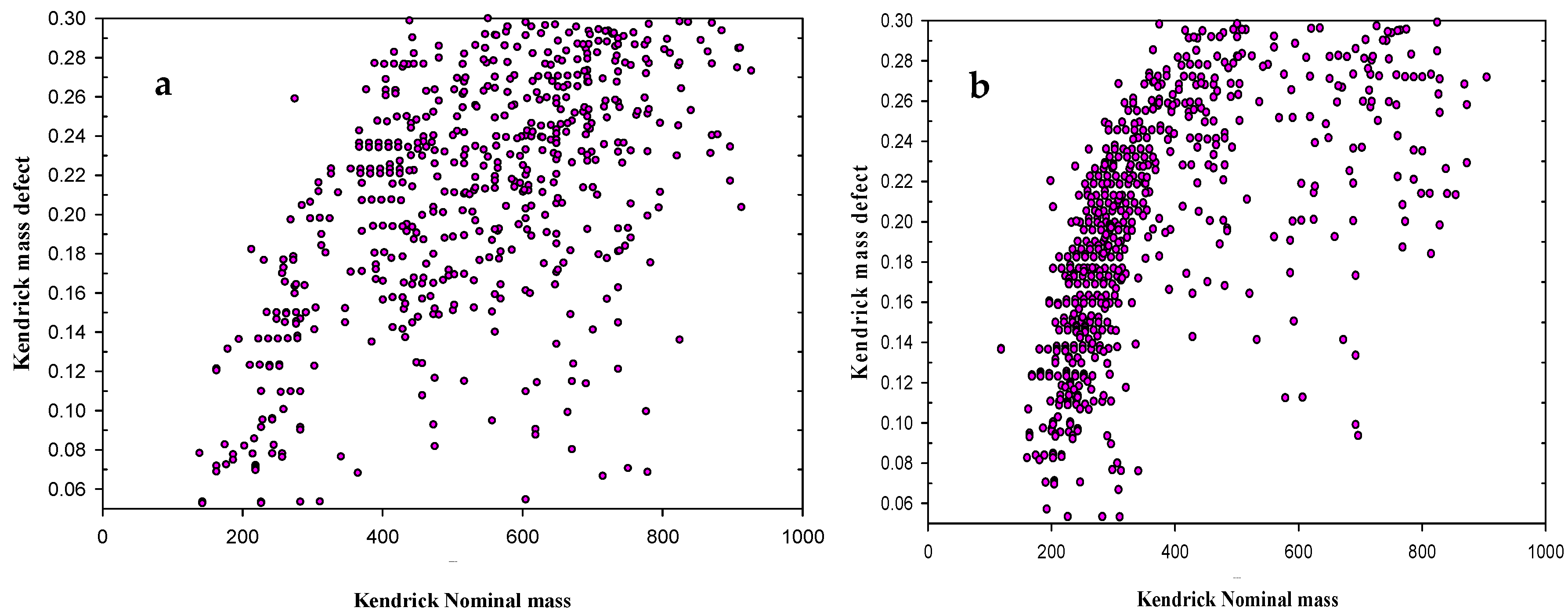
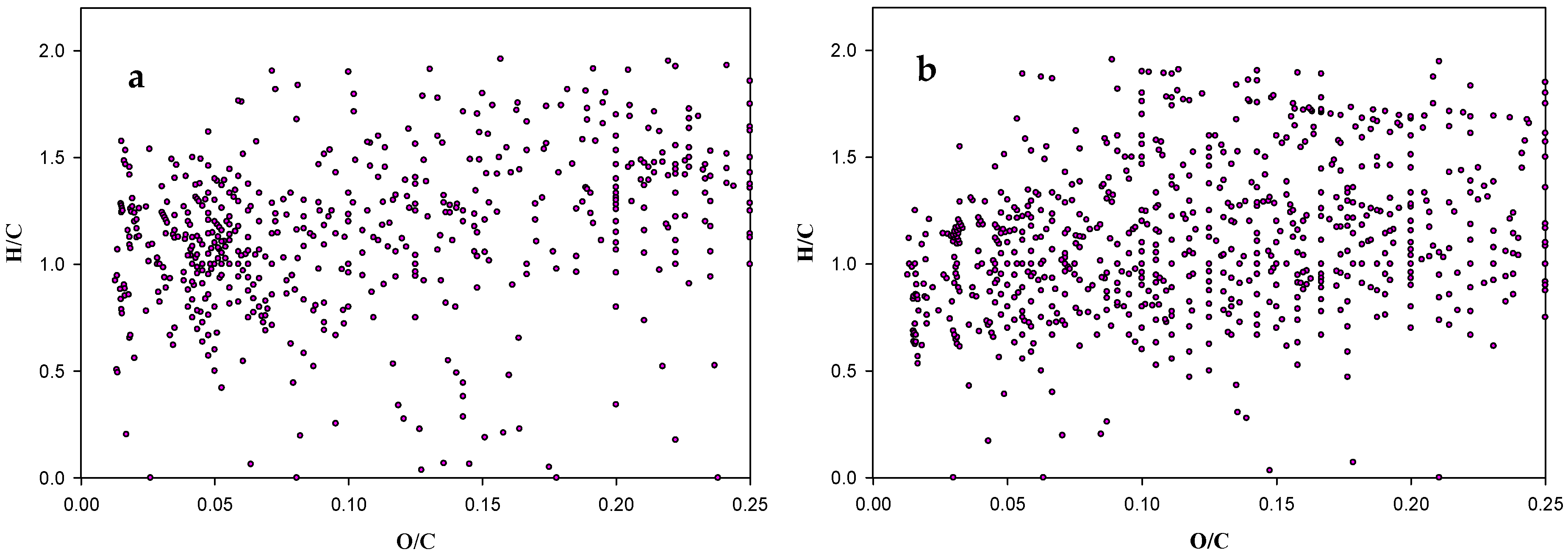
2.1. Ageing Study of Crude Oil by FT-ICR-MS
2.2. Remediation of Oil-Polluted Water
2.2.1. Photocatalytic Degradation
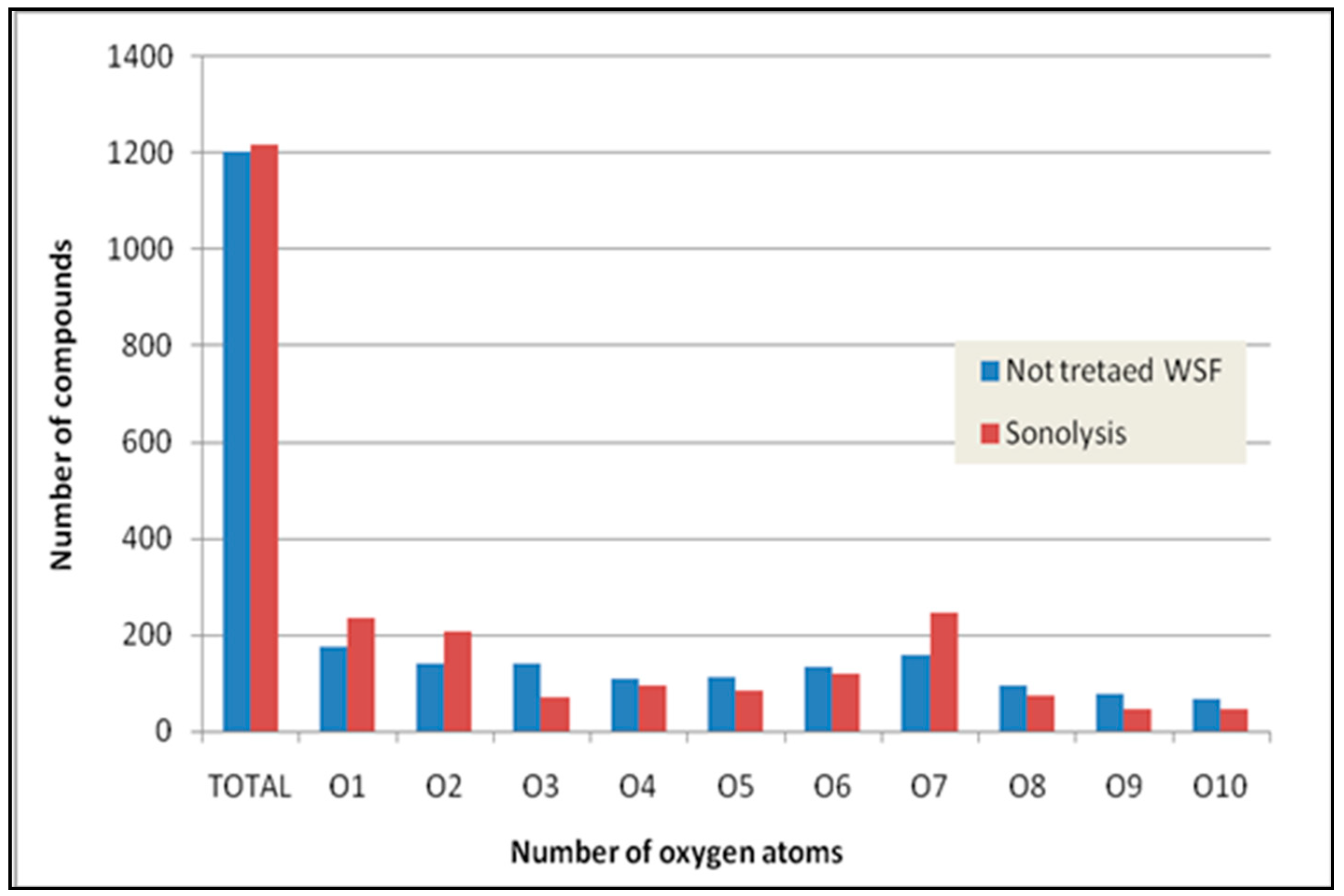
2.2.2. Ultrasonic Irradiation
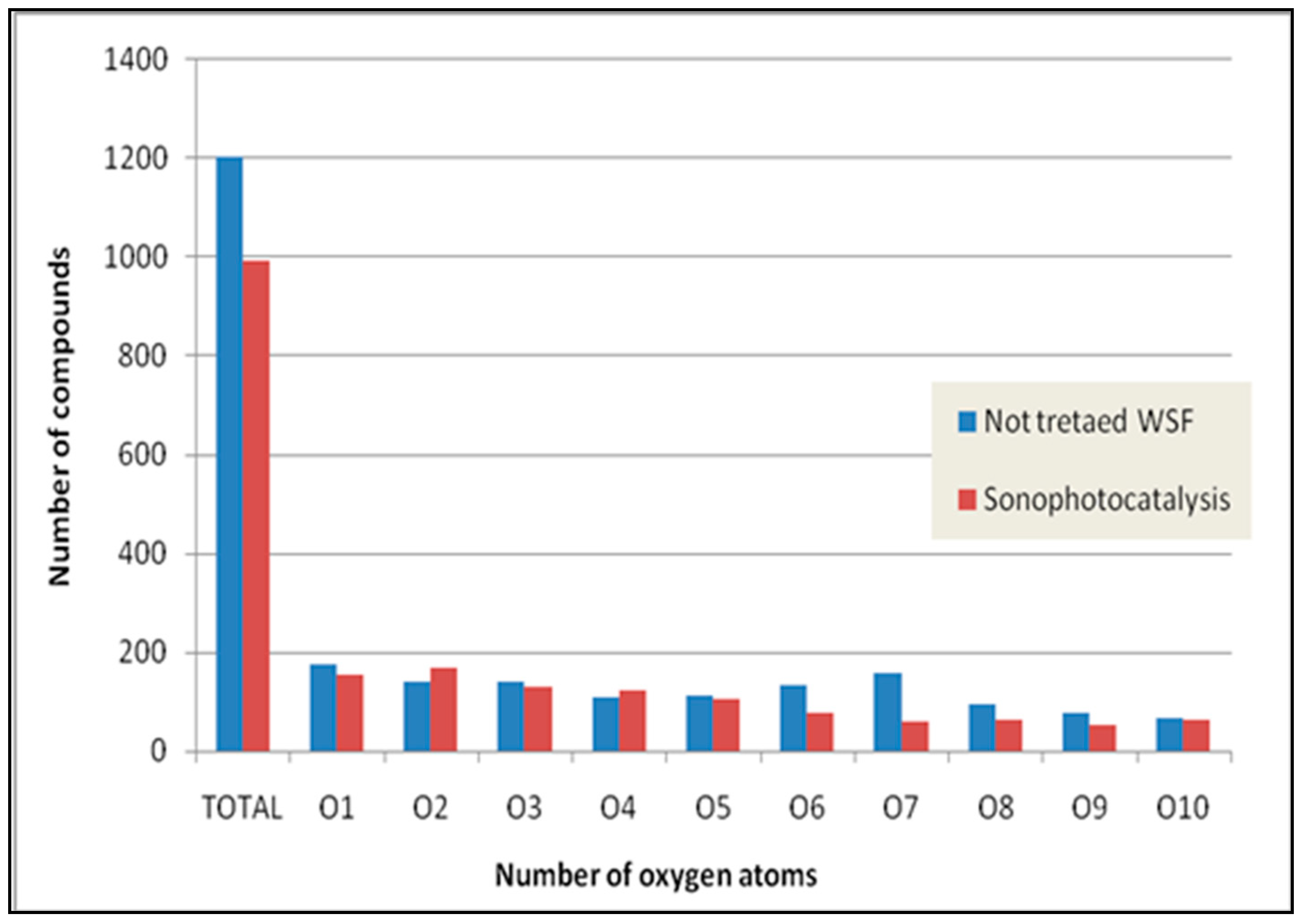
2.2.3. Sonophotocatalytic Degradation
3. Materials and Methods
3.1. Crude Oil and Chemicals
3.2. Photodegradation Apparatus
3.3. Photodegradation Process and Sample Preparation for ESI FT-ICR MS
3.4. Ultrasonic Irradiation of WSF Samples
3.5. Photocatalytic and Sonophotocatalytic Degradation of WSF Samples
3.6. Liquid–Liquid Extraction (LLE)
3.7. Analysis of Fluorescence
3.8. 1H-NMR Analysis
3.9. Mass Spectrometry of Polar Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richard Parent. Available online: https://www.experts.com/articles/toxicity-of-crude-oil-and-its-vapors-by-richard-parent (accessed on 17 April 2020).
- McKee, R.H.; White, R. The Mammalian Toxicological Hazards of Petroleum-Derived Substances: An Overview of the Petroleum Industry Response to the High Production Volume Challenge Program. Int. J. Toxicol. 2014, 33 (Suppl. 1), 4S–16S. [Google Scholar] [CrossRef]
- Laffon, B.; Pásaro, E.; Valdiglesias, V. Effects of exposure to oil spills on human health: Updated review. J. Toxicol. Environ. Health Part B 2016, 19, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Guarino, C.; Spada, V.; Sciarrillo, R. Assessment of three approaches of bioremediation (Natural Attenuation, Landfarming and Bioagumentation–Assistited Landfarming) for a petroleum hydrocarbons contaminated soil. Chemosphere 2017, 170, 10–16. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, L.; Faustorilla, V.; Megharaj, M.; Naidu, R.; Chen, Z. Integrated electrochemical treatment systems for facilitating the bioremediation of oil spill contaminated soil. Chemosphere 2017, 175, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Safdari, M.-S.; Kariminia, H.-R.; Rahmati, M.; Fazlollahi, F.; Polasko, A.; Mahendra, S.; Wilding, W.V.; Fletcher, T.H. Development of bioreactors for comparative study of natural attenuation, biostimulation, and bioaugmentation of petroleum-hydrocarbon contaminated soil. J. Hazard. Mater. 2018, 342, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.T.; Wang, L.N.; Sun, P.Y.; Cao, L.X.; Zou, J.; Li, Y.M. Biodegradation of crude oil using an efficient microbial consortium in a simulated marine environment. Mar. Pollut. Bull. 2012, 64, 1177–1185. [Google Scholar] [CrossRef]
- Adzigbli, L.; Bacosa, H.P.; Deng, Y. Response of microbial communities to oil spill in the Gulf of Mexico: A review. Afr. J. Microbiol. Res. 2018, 12, 536–545. [Google Scholar] [CrossRef][Green Version]
- Al-Gharabally, D.; Al-Barood, A. Kuwait environmental remediation program (KERP): Remediation demonstration strategy. Biol. Chem. Res. 2015, 2015, 289–296. Available online: http://www.ss-pub.org/wp-content/uploads/2015/09/BCR2015051501.pdf. (accessed on 12 September 2020).
- Shankar, R.; Won, J.-S.; An, J.-G.; Yim, U.-H. A practical review on photooxidation of crude oil: Laboratory lamp setup and factors affecting it. Water Res. 2015, 68, 304–315. [Google Scholar] [CrossRef]
- Ray, P.Z.; Chen, H.; Podgorski, D.C.; McKenna, A.M.; Tarr, M.A. Sunlight creates oxygenated species in water-soluble fractions of deep water horizon oil. J. Hazard. Mater. 2014, 280, 636–643. [Google Scholar] [CrossRef]
- Vaughan, P.P.; Wilson, T.; Kamerman, R.; Hagy, M.E.; McKenna, A.; Chen, H.; Jeffrey, W.H. Photochemical changes in water accommodated fractions of MC252 and surrogate oil created during solar exposure as determined by FT-ICR MS. Mar. Pollut. Bull. 2016, 104, 262–268. [Google Scholar] [CrossRef]
- Lee, K.; Boufadel, M.; Chen, B.; Foght, J.; Hodson, P.; Swanson, S.; Venosa, A. Expert Panel Report on the Behaviour and Environmental Impacts of Crude Oil Released into Aqueous Environments; Royal Society of Canada: Ottawa, ON, Canada, 2015; pp. 38–415. ISBN 978-1-928140-02-3. Available online: https://www.rsc-src.ca/sites/default/files/OIW%20Report_1.pdf. (accessed on 12 September 2020).
- Cho, E.; Park, M.; Hur, M.; Kang, G.; Kim, Y.H.; Kim, S. Molecular-level investigation of soils contaminated by oil spilled during the Gulf War. J. Hazard. Mater. 2019, 373, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Cho, Y.; Yim, U.H.; Shim, W.J.; Kim, Y.H.; Kim, S. The comparison of naturally weathered oil and artificially photo-degraded oil at the molecular level by a combination of SARA fractionation and FT-ICR MS. J. Hazard. Mater. 2013, 263, 404–411. [Google Scholar] [CrossRef]
- Lelario, F.; Brienza, M.; Bufo, S.A.; Scrano, L. Effectiveness of different advanced oxidation processes (AOPs) on the abatement of the model compound mepanipyrim in water. J. Photochem. Photobiol. A Chem. 2016, 321, 187–201. [Google Scholar] [CrossRef]
- Hall, G.J.; Frysinger, G.S.; Aeppli, C.; Carmichael, C.A.; Gros, J.; Lemkau, K.L.; Nelson, R.K.; Reddy, C.M. Oxygenated weathering products of Deepwater Horizon oil come from surprising precursors. Mar. Pollut. Bull. 2013, 75, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Prince, R.C.; McFarlin, K.M.; Butler, J.D.; Febbo, E.J.; Wang, F.C.; Nedwed, T.J. The primary biodegradation of dispersed crude oil in the sea. Chemosphere 2013, 90, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Gros, J.; Reddy, C.M.; Aeppli, C.; Nelson, R.K.; Carmichael, C.A.; Arey, J.S. Resolving biodegradation patterns of persistent saturated hydrocarbons in weathered oil samples from the Deepwater Horizon disaster. Environ. Sci. Technol. 2014, 48, 1628–1637. [Google Scholar] [CrossRef]
- Seidel, M.; Kleindienst, S.; Dittmar, T.; Joye, S.B.; Medeiros, P.M. Biodegradation of crude oil and dispersants in deep seawater from the Gulf of Mexico: Insights from ultra-high resolution mass spectrometry. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 129, 108–118. [Google Scholar] [CrossRef]
- Bacosa, H.P.; Erdner, D.L.; Liu, Z. Differentiating the roles of photooxidation and biodegradation in the weathering of Light Louisiana Sweet crude oil in surface water from the Deepwater Horizon site. Mar. Pollut. Bull. 2015, 95, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Barrow, M.P.; Peru, K.M.; Fahlman, B.; Hewitt, L.M.; Frank, R.A.; Headley, J.V. Beyond naphthenic acids: Environmental screening of water from natural sources and the Athabasca oil sands industry using atmospheric pressure photoionisation Fourier transform ion cyclotron resonance mass spectrometry. J. Am. Soc. Mass Spectrom. 2015, 26, 1508–1521. [Google Scholar] [CrossRef]
- D’Auria, M.; Racioppi, R.; Velluzzi, V. Photodegradation of crude oil: Liquid injection and headspace solid-phase microextraction for crude oil analysis by gas chromatography with mass spectrometer detector. J. Chromatogr. Sci. 2008, 46, 339–344. [Google Scholar] [CrossRef]
- D’Auria, M.; Emanuele, L.; Racioppi, R.; Velluzzi, V. Photochemical degradation of crude oil: Comparison between direct irradiation, photocatalysis, and photocatalysis on zeolite. J. Hazard. Mater. 2009, 164, 32–38. [Google Scholar] [CrossRef]
- Kim, D.; Ha, S.Y.; An, J.G.; Cha, S.; Yim, U.H.; Kim, S. Estimating degree of degradation of spilled oils based on relative abundance of aromatic compounds observed by paper spray ionisation mass spectrometry. J. Hazard. Mater. 2018, 359, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Christopher, M.R.; Quinn, J.G. GC-MS analysis of total petroleum hydrocarbons and polycyclic aromatic hydrocarbons in seawater samples after the North Cape oil spill. Mar. Pollut. Bull. 1999, 38, 126–135. [Google Scholar] [CrossRef]
- de Oteyza, T.G.; Grimalt, J.O. GC and GC–MS characterisation of crude oil transformation in sediments and microbial mat samples after the 1991 oil spill in the Saudi Arabian Gulf coast. Environ. Pollut. 2006, 139, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.J.; Vinas, L.; Franco, M.A.; Fumega, J.; Soriano, J.A.; Grueiro, G.; Muniategui, S.; Lopez-Mahıa, P.; Prada, D.; Bayona, J.M.; et al. Spatial and temporal distribution of dissolved/dispersed aromatic hydrocarbons in seawater in the area affected by the Prestige oil spill. Mar. Pollut. Bull. 2006, 53, 250–259. [Google Scholar] [CrossRef]
- McKenna, A.M.; Nelson, R.K.; Reddy, C.M.; Savory, J.J.; Kaiser, N.K.; Fitzsimmons, J.E.; Marshall, A.G.; Rodgers, R.P. Expansion of the analytical window for oil spill characterisation by ultrahigh resolution mass spectrometry: Beyond gas chromatography. Environ. Sci. Technol. 2013, 47, 7530–7539. [Google Scholar] [CrossRef] [PubMed]
- Haleyur, N.; Shahsavari, E.; Mansur, A.A.; Koshlaf, E.; Morrison, P.D.; Osborn, A.M.; Ball, A.S. Comparison of rapid solvent extraction systems for the GC–MS/MS characterisation of polycyclic aromatic hydrocarbons in aged, contaminated soil. MethodsX 2016, 3, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, T.B.; Jones, M.; Huang, H.; Bennett, B.; Shafiee, N.S.; Head, I.; Larter, S.R. The controls on the composition of biodegraded oils in the deep subsurface—Part 4. Destruction and production of high molecular weight non-hydrocarbon species and destruction of aromatic hydrocarbons during progressive in-reservoir biodegradation. Org. Geochem. 2017, 114, 57–80. [Google Scholar] [CrossRef]
- Griffiths, M.T.; Da Campo, R.; O’Connor, P.B.; Barrow, M.P. Throwing light on petroleum: Simulated exposure of crude oil to sunlight and characterisation using atmospheric pressure photoionisation Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 2013, 86, 527–534. [Google Scholar] [CrossRef]
- Lemkau, K.L.; McKenna, A.M.; Podgorski, D.C.; Rodgers, R.P.; Reddy, C.M. Molecular evidence of heavy-oil weathering following the M/V Cosco Busan spill: Insights from Fourier transform ion cyclotron resonance mass spectrometry. Environ. Sci. Technol. 2014, 48, 3760–3767. [Google Scholar] [CrossRef]
- Marshall, A.G.; Rodgers, R.P. Petroleomics: The Next Grand Challenge for Chemical Analysis. Acc. Chem. Res. 2004, 37, 53–59. [Google Scholar] [CrossRef]
- Kendrick, E. A Mass Scale Based on CH2=14.0000 for High Resolution Mass Spectrometry of Organic Compounds. Anal. Chem. 1963, 35, 2146–2154. [Google Scholar] [CrossRef]
- Hughey, C.A.; Hendrickson, C.L.; Rodgers, R.P.; Marshall, A.G.; Qian, K. Kendrick mass defect spectroscopy: A compact visual analysis for ultrahigh-resolution broadband mass spectra. Anal. Chem. 2001, 73, 4676–4681. [Google Scholar] [CrossRef] [PubMed]
- Hughey, C.A.; Rodgers, R.P.; Marshall, A.G. Resolution of 11,000 compositionally distinct components in a single electrospray ionisation Fourier transform ion cyclotron resonance mass spectrum of crude oil. Anal. Chem. 2002, 74, 4145–4149. [Google Scholar] [CrossRef]
- van Krevelen, D.W. Graphical-statistical method for the study of structure and reaction processes of coal. Fuel 1950, 29, 269–284. [Google Scholar]
- Kim, S.; Kramer, R.W.; Hatcher, P.G. Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the van Krevelen diagram. Anal. Chem. 2003, 75, 5336–5344. [Google Scholar] [CrossRef]
- Hughey, C.A.; Rodgers, R.P.; Marshall, A.G.; Qian, K.; Robbins, W.R. Identification of acidic NSO compounds in crude oils of different geochemical origins by negative ion electrospray Fourier transform ion cyclotron resonance mass spectrometry. Org. Geochem. 2002, 33, 743–759. [Google Scholar] [CrossRef]
- Wu, Z.; Jernström, S.; Hughey, C.A.; Rodgers, R.P.; Marshall, A.G. Resolution of 10,000 compositionally distinct components in polar coal extractsby negative-ion electrospray ionisation Fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2003, 17, 946–953. [Google Scholar] [CrossRef]
- Finch, B.E.; Stefansson, E.S.; Langdon, C.J.; Pargee, S.M.; Blunt, S.M.; Gage, S.J.; Stubblefield, W.A. Photo-enhanced toxicity of two weathered Macondo crude oils to early life stages of the eastern oyster (Crassostrea virginica). Mar. Pollut. Bull. 2016, 113, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Ahmed, A.; Islam, A.; Kim, S. Developments in FT-ICR MS instrumentation, ionisation techniques, and data interpretation methods for petroleomics. Mass Spectrom. Rev. 2015, 34, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Pradyot, P. A Comprehensive Guide to the Hazardous Properties of Chemical Substances; Wiley & Sons: New York, NY, USA, 2007; pp. 161–568. [Google Scholar]
- Khalaf, S.; Shoqeir, J.H.; Lelario, F.; Bufo, S.A.; Karaman, R.; Scrano, L. TiO2 and Active Coated Glass Photodegradation of Ibuprofen. Catalysts 2020, 10, 560. [Google Scholar] [CrossRef]
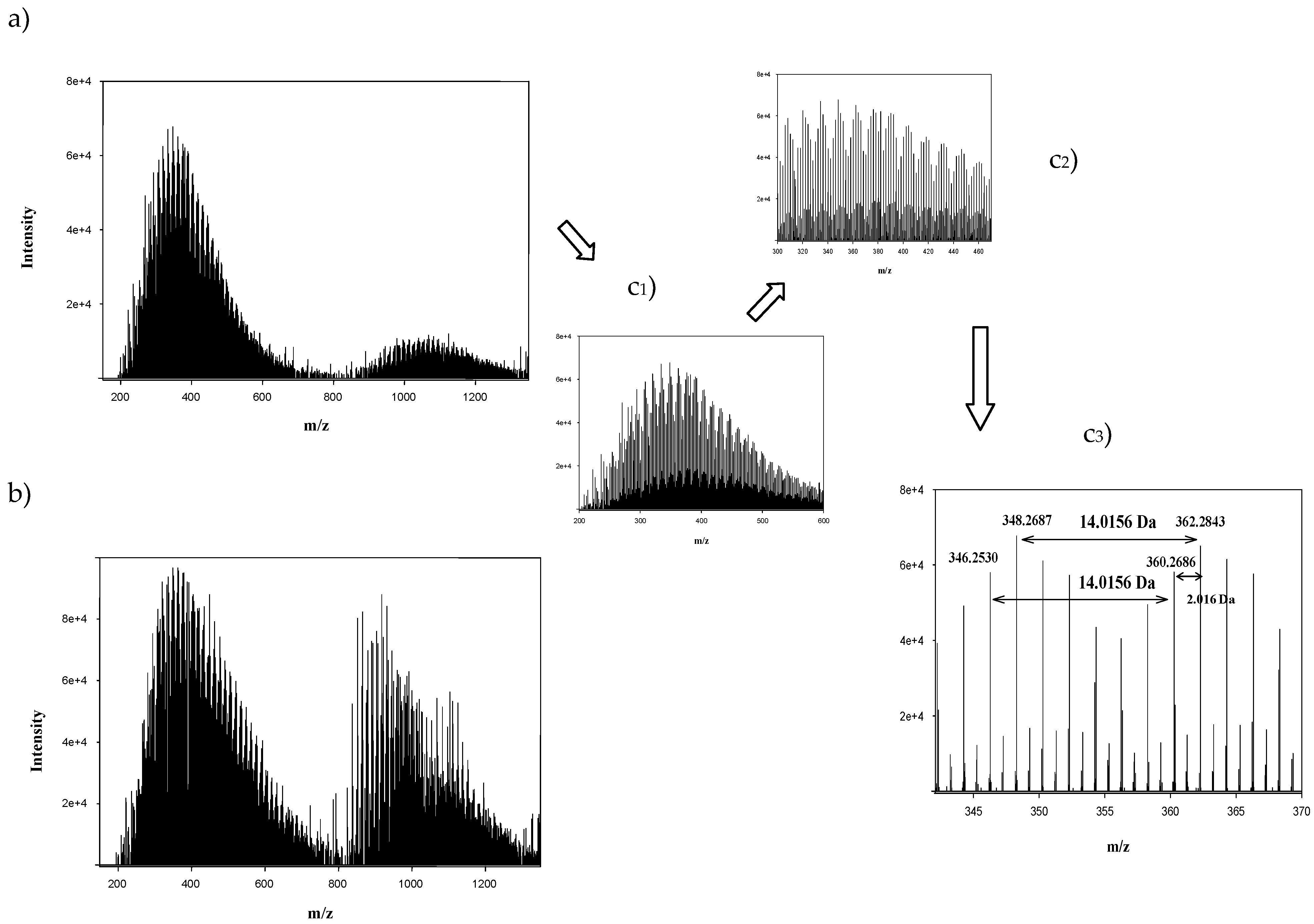
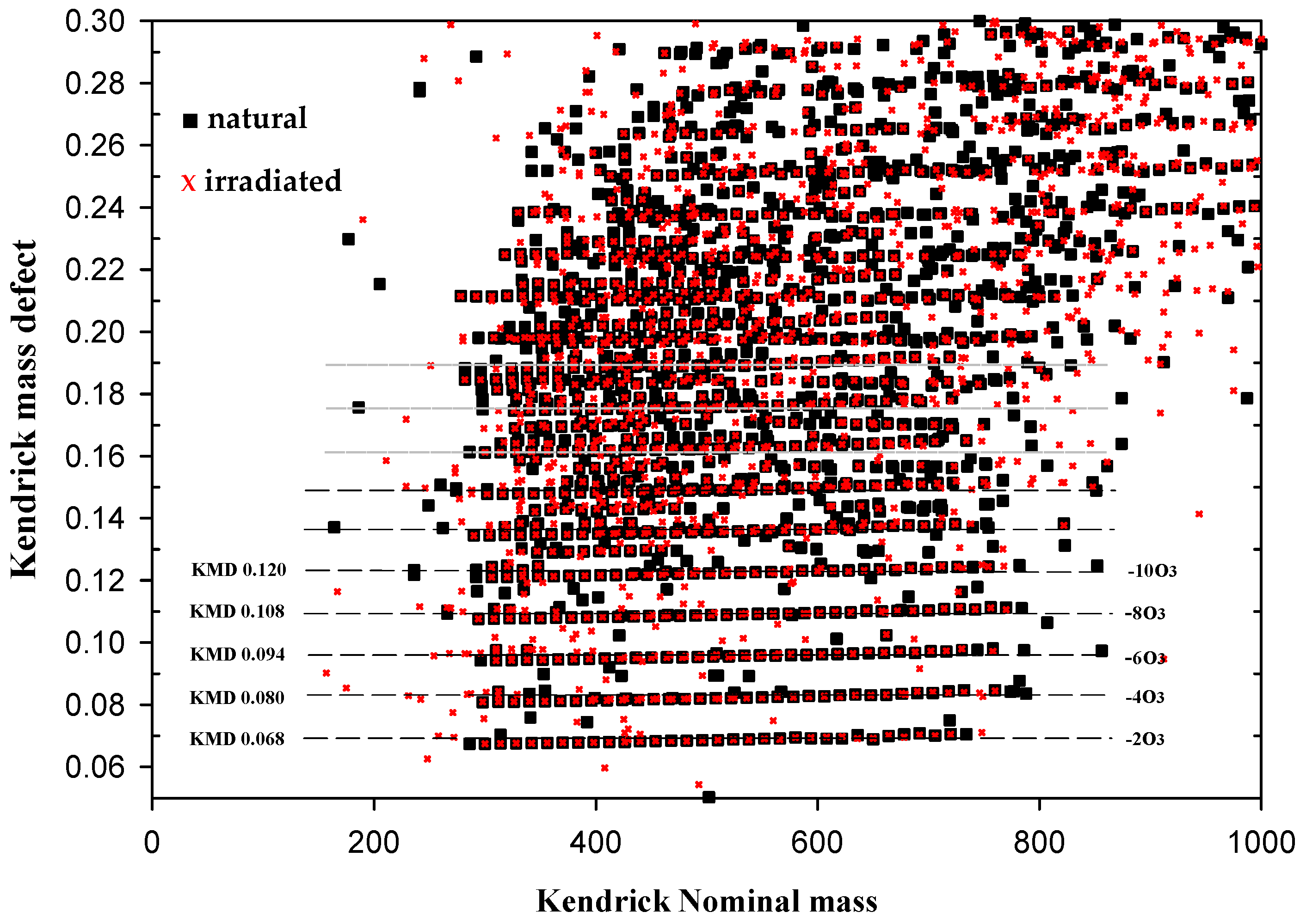
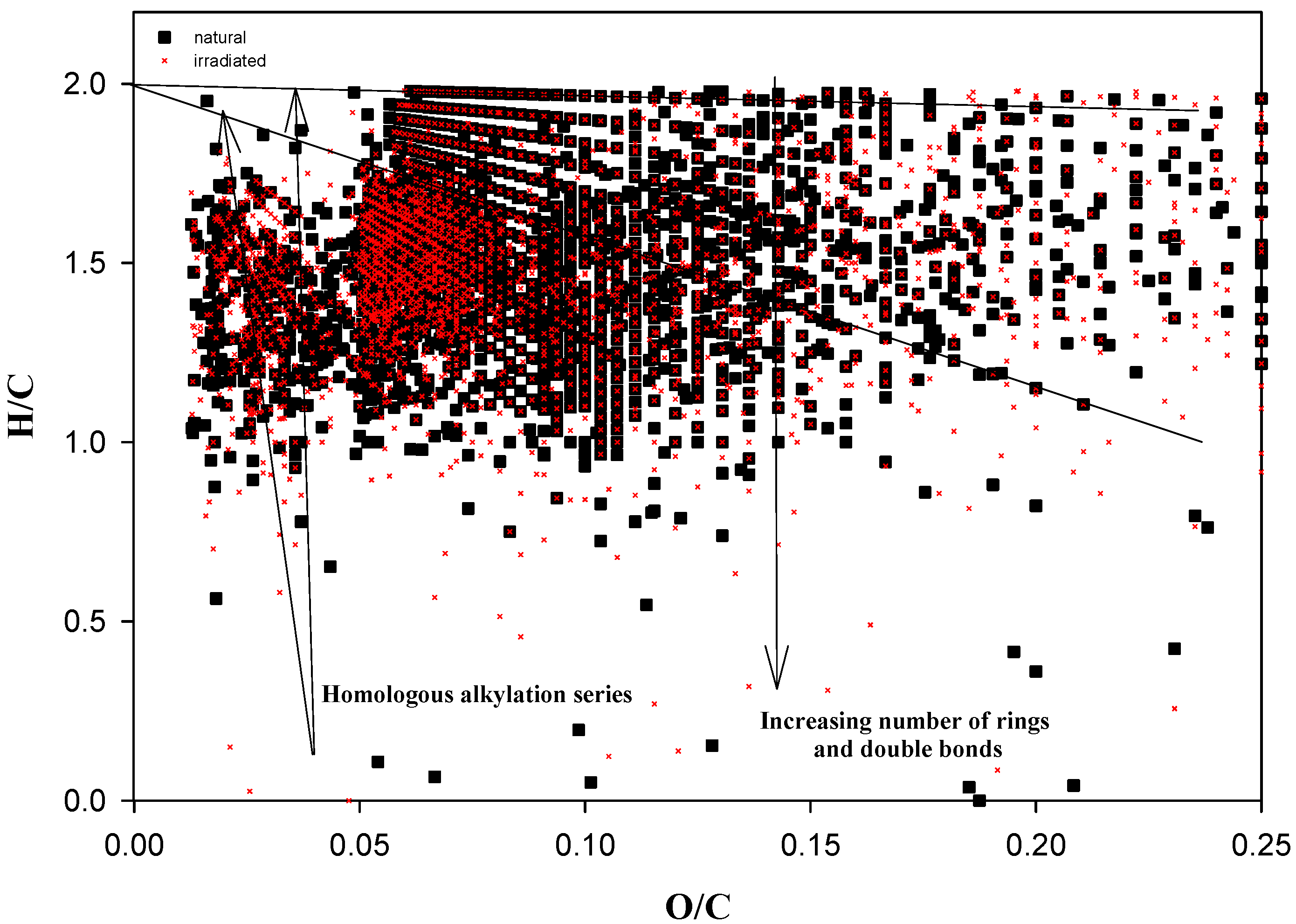




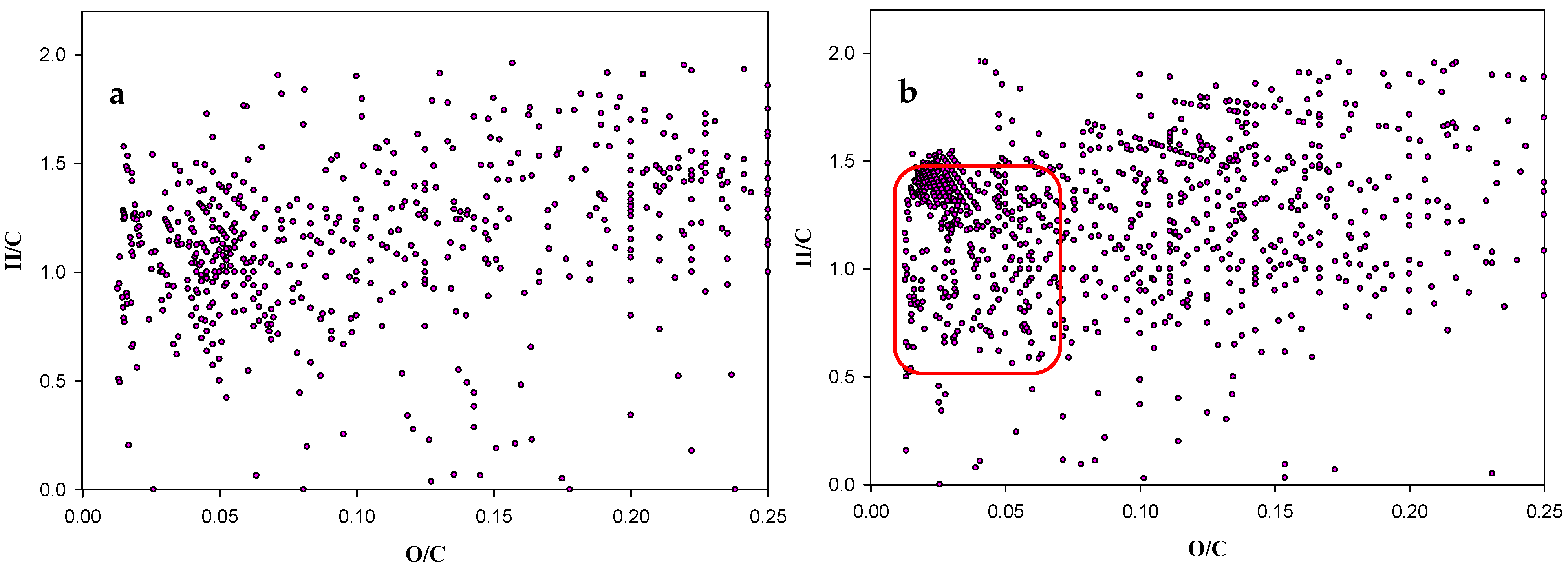

| m/z | Intensity | Composition | KNM | KMD |
|---|---|---|---|---|
| 337.1818 | 3941.7 | C22 H25 O3 | 4721 | 0.45494 |
| 351.1972 | 5150.2 | C23 H27 O3 | 4917 | 0.23920 |
| 365.2128 | 3112.4 | C24 H29 O3 | 5113 | 0.02038 |
| 379.2270 | 4609.2 | C25 H31 O3 | 5310 | 0.82256 |
| 393.2424 | 4252.4 | C26 H33 O3 | 5506 | 0.60640 |
| 407.2582 | 3311.8 | C27 H35 O3 | 5702 | 0.38464 |
| 421.2737 | 3427.8 | C28 H37 O3 | 5898 | 0.16848 |
| 435.2893 | 4966.3 | C29 H39 O3 | 6095 | 0.94924 |
| 449.3052 | 3587.2 | C30 H41 O3 | 6291 | 0.72748 |
| 463.3202 | 4252.1 | C31 H43 O3 | 6487 | 0.51692 |
| 477.3362 | 4772.3 | C32 H45 O3 | 6683 | 0.29390 |
| 505.3668 | 7455.6 | C34 H49 O3 | 7076 | 0.86536 |
| 533.3981 | 5506.9 | C36 H53 O3 | 7468 | 0.42618 |
| 547.4131 | 6265.8 | C37 H55 O3 | 7664 | 0.21674 |
| 561.4292 | 6183.2 | C38 H57 O3 | 7861 | 0.99120 |
| 575.4446 | 5847.4 | C39 H59 O3 | 8057 | 0.77504 |
| 589.4606 | 5685.7 | C40 H61 O3 | 8253 | 0.55118 |
| 603.4760 | 6234.8 | C41 H63 O3 | 8449 | 0.33586 |
| 617.4921 | 5529.1 | C42 H65 O3 | 8645 | 0.11018 |
| 631.5075 | 3522.3 | C43 H67 O3 | 8842 | 0.89486 |
| 645.5230 | 4399.5 | C44 H69 O3 | 9038 | 0.67870 |
| 659.5385 | 5131.9 | C45 H71 O3 | 9234 | 0.46086 |
| 673.5545 | 4308.2 | C46 H73 O3 | 9430 | 0.23700 |
| 687.5703 | 4191.7 | C47 H75 O3 | 9626 | 0.01566 |
| 701.5854 | 2133.1 | C48 H77 O3 | 9823 | 0.80454 |
| 715.6014 | 3178.9 | C49 H79 O3 | 10,019 | 0.58068 |
| 729.6165 | 3507.6 | C50 H81 O3 | 10,215 | 0.36872 |
| 743.6323 | 3063.6 | C51 H83 O3 | 10,411 | 0.14836 |
| Degradation Method/System | GC-MS | 1H-NMR | FT-ICR MS | Fluorescence |
|---|---|---|---|---|
| Photolysis (UV)/ Ageing of crude oil |
|
| ||
| Photocatalysis (UV + TiO2)/ Oil Water-Soluble Fraction (WSF) |
|
|
|
|
| Sonolysis (US)/ Oil Water-Soluble Fraction (WSF) |
|
|
|
|
| Sonophotocatalysis (UV + TiO2 + US)/ Oil Water-Soluble Fraction (WSF) |
|
|
|
|
| C | H | N | O b | S |
|---|---|---|---|---|
| 85.4 ± 2.8 | 11.8 ± 1.4 | 0.3 ± 0.1 | 0.6 ± 0.4 | 1.9 ± 0.9 |
| Experiments Performed | Treated System | Analytical Methods Applied | |||
|---|---|---|---|---|---|
| GC-MS | 1H-NMR | FT-ICR MS | Fluorescence | ||
| Artificial ageing process by photolysis (UV) | Crude oil | X | X | ||
| Remediation process by photocatalysis (UV + TiO2) | Oil WSF | X | X | X | X |
| Remediation process by sonolysis (US) | Oil WSF | X | X | X | X |
| Remediation process by sonophotocatalysis (UV + TiO2 + US) | Oil WSF | X | X | X | X |
| Instrument | Solvent | Acquisition Time | Spectral Width | Line Broadening | Number of Scans |
|---|---|---|---|---|---|
| Varian Oxford AS400 | CDCl3 | 2.049 s | 6410.3 Hz | 0.20 Hz | 512 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lelario, F.; Bianco, G.; Bufo, S.A.; Scrano, L. Simulated Ageing of Crude Oil and Advanced Oxidation Processes for Water Remediation since Crude Oil Pollution. Catalysts 2021, 11, 954. https://doi.org/10.3390/catal11080954
Lelario F, Bianco G, Bufo SA, Scrano L. Simulated Ageing of Crude Oil and Advanced Oxidation Processes for Water Remediation since Crude Oil Pollution. Catalysts. 2021; 11(8):954. https://doi.org/10.3390/catal11080954
Chicago/Turabian StyleLelario, Filomena, Giuliana Bianco, Sabino Aurelio Bufo, and Laura Scrano. 2021. "Simulated Ageing of Crude Oil and Advanced Oxidation Processes for Water Remediation since Crude Oil Pollution" Catalysts 11, no. 8: 954. https://doi.org/10.3390/catal11080954
APA StyleLelario, F., Bianco, G., Bufo, S. A., & Scrano, L. (2021). Simulated Ageing of Crude Oil and Advanced Oxidation Processes for Water Remediation since Crude Oil Pollution. Catalysts, 11(8), 954. https://doi.org/10.3390/catal11080954









