Abstract
Ru-based eggshell-type catalysts, in which Ru is located at the outer region of the pellet, were prepared by the impregnation method, using spherically shaped γ-Al2O3 pellets for steam-methane reforming (SMR). Ru was only supported on the external region of the pellet because of the strong interaction between its precursor and the alumina pellet. The Ru precursor penetrated the inside of the pellet by adding nitric acid to the impregnation solution. The distribution and thickness of the Ru layer in the catalyst can be controlled using the HNO3/Ru molar ratio and contact time at the impregnation step. Among the catalysts, the graded eggshell-type catalyst showed the highest activity and long-term stability in the SMR reaction. In addition, in the daily startup and shutdown (DSS) operation, similar to the hydrogen production environment for domestic polymer electrolyte membrane fuel cells (PEMFC), the graded eggshell-type catalyst showed high activity and stability after multiple cycles. Based on the experimental studies, it was confirmed that Ru-based catalysts are suitable for steam-methane reforming for PEMFC.
1. Introduction
Hydrogen is a prospective energy carrier and it has attracted attention because it can be stored and transported, efficiently, and burns to produce only water as a byproduct. Among the various ways of using hydrogen, polymer electrolyte membrane fuel cells (PEMFCs) are employed in residential fuel cells due to their large energy density, high calorific value, and abundant resources [1]. Residential fuel cells are designed to operate with city gas supplied through well-developed pipeline infrastructures. Thus, the H2 gas will be generated onsite by reforming available fuels, such as natural gas (mostly methane), before the fuel gas enters the fuel cell stack. Several processes for producing hydrogen using methane reforming are available: steam reforming, dry reforming, partial oxidation, etc. [2,3,4]. Steam-methane reforming (SMR) is the most commonly used process, described by Equation (1). The reformer operates at high temperatures (1073 K–1373 K) because SMR is an endothermic reaction, and the steam is fed more than CH4 to avoid coke formation, based on methane decomposition (2) and the Boudouard reaction (3).
Various types of catalysts are used to increase the efficiency of this process [4,5,6,7,8,9,10,11,12]. Noble metals, such as Ru, Rh, and Pt catalysts, show high catalytic activity, even at a low concentration, and have resistance to coking or active metal oxidation, which is a disadvantage of nickel catalysts. Experimental and kinetic studies on various noble metal catalysts have been reported. In particular, Ru has displayed a high reforming activity compared to other noble metals, such as Ir and Pt [9,10,11,12,13]. This indicated that Ru is a promising catalyst for methane reforming.
To operate in a fixed-bed configuration, on an industrial scale, catalytically active components should be supported on an inert porous pellet to avoid the pressure drop inside the fixed-bed reactors. In addition, in the case of a catalyst using pellet-type support, the distribution of catalytically active components is various such as eggshell, egg-yolk, and homogeneous type [14,15,16,17]. This difference in the distribution of catalytically active components depends on the strength of the metal precursor–support interactions and may appear as a difference in activity in the reaction. In the platinum catalyst on alumina, where the interaction between the platinum precursor and aluminum is strong, the distribution of the platinum precursor has been adjusted using various additives [14,15]. The generally accepted description of the strong metal precursor–support interaction is the revised physical adsorption model provided by Spieker and Regalbuto [18]. Porta et al. reported that varied distributions of a metal precursor, which interact strongly with the support, can be obtained through the addition of an inorganic acid to the impregnating solution. This is because the acid addition is expected to decrease the pH of the impregnating solution, thereby reducing the surface potential of the alumina. Consequently, it reduces the interaction between the negatively charged Ru complex and the support [16].
In this study, Ru catalysts supported on spherical-shaped γ-Al2O3 pellets were prepared using the impregnation method. They showed various distributions of Ru by controlling the HNO3/Ru molar ratio and impregnating contact time in the catalyst-synthesizing process. The effect of Ru distribution on catalytic activity in the SMR reaction was investigated. The catalytic activity and stability, in daily startup and shutdown (DSS) operation, of the selected eggshell-type catalyst was evaluated. Moreover, the Ru/γ-Al2O3 was analyzed using Brunauer–Emmett–Teller (BET) analysis, X-ray diffraction (XRD), scanning electron microscopy (SEM) with energy-dispersive X-ray spectroscopy (EDS), and wavelength-dispersive X-ray fluorescence spectrometry (XRF).
2. Results and Discussion
2.1. Characteristics of the Ru/γ-Al2O3 Catalysts
Prepared catalysts are designated as RA (a, b h), where “RA” indicates “Ru/Al2O3” catalysts, “a” designates the HNO3/Ru molar ratio, and “b” indicates the contact time (e.g., RA (10, 1 h) is a catalyst with an HNO3/Ru molar ratio of 10 and a contact time of 1 h.)
Figure 1 shows (a) external surface and (b) cross-sectional images of prepared catalysts using a digital microscope, based on the HNO3/Ru molar ratio and contact time. All pellets had a black color due to Ru oxide, and the surface increased in brightness with the HNO3/Ru molar ratio when the contact time was 1 h. This is because alumina can be redeposited on the catalyst surface, in highly acidic environments, during the precipitation and Ru can be covered [19]. Figure 1b shows that, in the absence of HNO3, Ru was loaded only on the external surface of the pellet because of the strong metal precursor–support interaction, regardless of the contact time (delimited eggshell-type catalyst). In the presence of HNO3, Ru for catalysts penetrated the pellet.
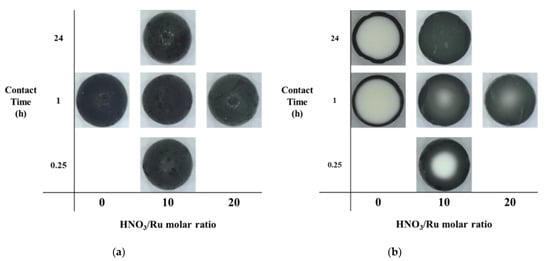
Figure 1.
(a) External surface and (b) cross-sectional images of prepared catalysts.
In addition, the Ru distribution in the pellet interior was different, depending on the HNO3/Ru molar ratio and the contact time. Ru penetrated the pellets better as the HNO3/Ru molar ratio increased. The difference in contact time exhibited a notable difference inside the pellet when the HNO3/Ru molar ratio was fixed at 10. The RA (10, 0.25 h) showed the eggshell profile, indicating that the concentration of Ru was low in the center of the pellet. When the contact time reached 1 h, i.e., (RA (10, 1 h)), the boundary became more blurred. RA (10, 24 h) with sufficient contact time showed a uniform color distribution (homogeneous type). The shell thickness of the eggshell layer could be effectively adjusted by controlling the HNO3/Ru molar ratio and contact time until a homogeneously impregnated pellet was obtained.
To investigate the distribution of the pellets inside the Ru, the concentration of Ru was measured using SEM-EDS in the cross-section of the catalysts from the edge to the center (Figure 2). Figure 2a shows the difference in Ru distribution based on the HNO3/Ru molar ratio. For the RA (0, 1 h) catalyst, a high concentration of Ru was detected only on the external surface of the catalyst (r/R of ~0.05). This is because the strong metal precursor–support interaction prevented the Ru precursor from penetrating the pellet. However, Ru was found inside the pellet when HNO3 was added. The concentration of the Ru inside the pellet gradually increased as the amount of the HNO3 added in the impregnation solution increased, whereas the concentration of Ru on the external surface of the pellet decreased. In addition, we confirmed the relationship between the distribution of Ru and contact time in Figure 2b. The total Ru concentration of RA (10, 0.25 h) inside a pellet was less than those of the others. For the homogeneous catalyst (RA (10, 24 h)), the concentration of Ru was similar (~2 wt%) in all regions, including the external surface of the pellet. The change in Ru concentration, on the external surface, decreased with the contact time.
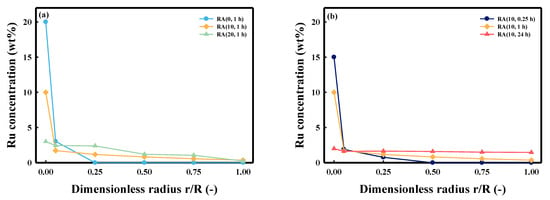
Figure 2.
Ru concentration profile measured in prepared catalysts by SEM-EDS: (a) different HNO3/Ru molar ratio; (b) different contact times.
Figure 3a shows the XRD patterns of calcined Ru/γ-Al2O3 catalysts with different HNO3/Ru molar ratios. In addition to the γ-alumina peaks (JCPDS No. 04-0875), the catalysts showed RuO2 peaks (JCPDS No. 73-1469); their intensity decreased with HNO3/Ru molar ratio. After the reduction, the peaks corresponding to RuO2 disappeared, and peaks of Ru metal (JCPDS No. 07-0274) were observed, which means that all Ru oxide was reduced through a reduction process. The calculated RuO2 and Ru crystallite sizes decreased with the increase in the amount of nitric acid (Table S1). In the absence of HNO3, the crystallite sizes of RuO2 and Ru metal were larger than the others. In addition, the XRD patterns were almost the same, regardless of the contact time, when HNO3/Ru molar ratio was 10 (Figure S1). In contrast to RuO2 and Ru, no change in the γ-alumina peaks was observed based on the differences in reduction, contact time, and amount of nitric acid. The BET surface area, pore size, pore volume, and Ru content of Ru/Al2O3 catalysts in the calcined and reduced states were almost the same, regardless of the molar ratio or contact time (Table S1).
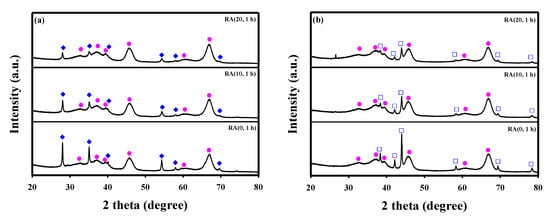
Figure 3.
XRD patterns of the Ru/Al2O3 catalysts with different HNO3/Ru molar ratios in its fresh (a) and reduced (b) states. (●) γ-Al2O3, (♦) RuO2, and (□) metallic Ru.
2.2. Catalytic Performance of the Ru/γ-Al2O3 Catalysts
2.2.1. Catalytic Test in Long-Term Stability Test
Figure 4 shows the CH4 conversion as a function of time on stream (T.O.S) for five kinds of Ru/Al2O3 catalysts. Figure 4a shows that RA (0, 1 h) exhibited the lowest initial XCH4 (~91%). On the other hand, RA (10, 1 h) and RA (20, 1 h) showed higher catalytic activities than RA (0, 1 h) did. The RA (10, 1 h) catalyst showed the highest XCH4 (98%), close to the equilibrium conversion (98%). The RA (20, 1 h) catalyst showed slightly lower XCH4 because RA (10, 1 h) showed a higher Ru concentration on the external surface of pellets. In the absence of HNO3, the catalyst (RA (0, 1 h)) was deactivated (from 91% to 72%) due to the poisoning of NH3 by the byproduct of the reaction (N2 + 3H2 = 2NH3) [20]. The RA (0, 1 h) catalyst did not show deactivation when nitrogen was replaced with argon (Figure S2). Deactivation was not observed with RA (10, 1 h), even in a nitrogen atmosphere, and this difference seemed to be due to the smaller Ru crystallite size. From these results, we confirmed that the addition of HNO3 increased the Ru dispersion, but not the methane conversion [21]. The CO selectivity of RA (0, 1 h) was lower than those of other catalysts, attributed to the water–gas shift reaction (WGS, CO + H2O = CO2 + H2) (Figure 5a) [22]. During the SMR reaction using the RA (0, 1 h) catalyst, more water was available because of the lower CH4 conversion, thus increasing the H2O/CO ratio, favoring WGS equilibrium. In the case of RA (10, 1 h), the CO, CO2 carbon-based selectivity did not change over time; however, the CO selectivity of RA (0, 1 h) decreased because the methane conversion rate decreased with T.O.S. (Figure S3). For this reason, the H2/CO ratio of the RA (0, 1 h) catalyst was higher than those of other catalysts (Table S2). When the HNO3/Ru molar ratio was 10, the activity of the SMR reaction was similar for all catalysts because of the similar Ru crystallite size (Figure 4b). In addition, Figure 5b shows that the selectivity of catalysts with a fixed HNO3/Ru molar ratio at 10 was not significantly different.
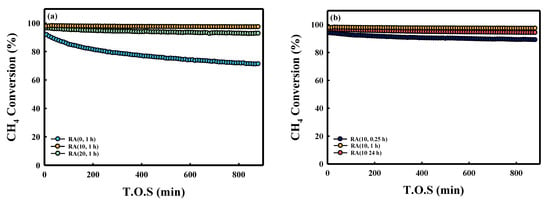
Figure 4.
Long-term stability tests of the Ru/Al2O3 catalysts in the SMR reaction at 700 °C, S/C ratio of 3, and WHSV of 12,000 mL/g/h: (a) different HNO3/Ru molar ratios and contact time fixed at 1 h; (b) HNO3/Ru molar ratio fixed at 10 and different contact times.
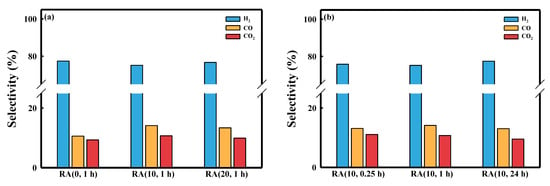
Figure 5.
Selectivity in long-term stability tests of the Ru/Al2O3 catalysts: (a) different HNO3/Ru molar ratios and contact time fixed at 1 h; (b) HNO3/Ru ratio fixed at 10 and different contact times.
In the long-term stability test, the catalytic activity was slightly different, depending on the Ru distribution on pellets. The RA (0, 1 h) catalyst showed a lower SMR activity than the other catalysts did due to the poisoning of NH3. This is a disadvantage because the H2 gas entering the fuel cell will be generated onsite by reforming natural gas, including N2 gas. The homogeneous catalysts (RA (10, 24 h)) showed a similar catalytic activity to graded eggshell-type catalysts (RA (10, 1 h)); however, it required longer preparation times.
Figure 6 shows the XRD patterns of spent Ru/γ-Al2O3 catalysts. No difference was observed between the reduced and spent catalysts in any catalyst. In addition, no coke diffraction peaks in the XRD patterns of the catalysts were observed. The BET surface areas and average pore sizes of spent catalysts changed after 15 h of reaction (Table 1). However, compared to the reduced catalysts, the Ru crystallite sizes of all catalysts did not change after the reaction. Figure S4 shows the SEM images of the prepared and the spent Ru/γ-Al2O3 catalysts with a fixed contact time at 1 h. For all catalysts, there was no significant difference between the prepared and the spent catalysts.
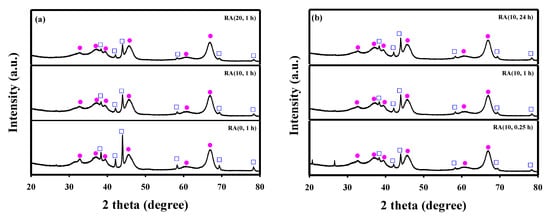
Figure 6.
XRD patterns of the Ru/Al2O3 catalysts after long-term stability tests: (a) different HNO3/Ru molar ratios and contact time fixed at 1 h; (b) HNO3/Ru ratio fixed at 10 and different contact times.

Table 1.
BET surface area, pore volume, average pore size, and Ru crystallite size of Ru/Al2O3 catalysts in their spent states.
2.2.2. Catalytic Test in DSS Operation
To confirm the efficiency of the actual fuel cell, the RA (10, 1 h) catalyst showing the best activity and stability was tested in DSS operation [23]. Before the catalytic test, the catalyst was reduced in an H2/N2 gas mixture (10/90 mL/min) for 3 h at 800 °C. After the reduction, the catalysts were cooled to 700 °C under N2 gas, and then the reaction was started in a CH4/H2O/N2 (10/30/60 mL/min). After a 90 min reaction, the catalysts were cooled to 200 °C at a rate of 10 °C/min under purge conditions. The purge condition consisted of H2O/N2 (30/60 mL/min) in which only the CH4 supply was stopped under reaction conditions. After the catalysts bed temperature was maintained at 200 °C for 30 min, the temperature was increased to 700 °C under purge conditions. CH4 gas was added to start the reaction; the reaction was held for 90 min. To perform the DSS operation, this reaction was repeated 5 times. The results of the DSS operation are shown in Figure 7. RA (10, 1 h) showed no significant deactivation during the 5 cycles of DSS operation. The catalyst prepared using an appropriate HNO3/Ru molar ratio and contact time was suitable for SMR reaction.
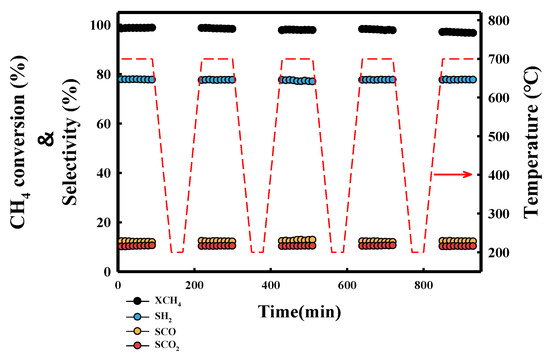
Figure 7.
Methane conversion and selectivity of RA (10, 1 h) catalyst in DSS operation.
3. Materials and Methods
3.1. Materials
The Ru(III) nitrosyl nitrate (Ru(NO3)3(NO), Ru 31.3% min) was purchased from Alfa-Aesar (Ward Hill, MA, USA). Spherical-shaped γ-Al2O3 pellets were purchased from SASOL (Sandton, South Africa). HNO3 (64–66 wt%) was purchased from DUKSAN (An-San, Korea).
3.2. Catalysts Preparation
All catalysts were synthesized using impregnation methods. Before preparation, the pellet was dried at 150 °C for 2 h. The Ru concentration was 2 wt% based on Ru/γ-Al2O3, and the nitric acid was added based on the molar ratio of Ru. The desired amount of pellets, Ru(III) nitrosyl nitrate, and HNO3 were dispersed in deionized water, and the mixture was stirred at different contact times, for each catalyst. The mixed solution was evaporated in an oven at 150 °C for 5 h and calcined in a muffle furnace, under air, at 700 °C for 4 h; the temperature ramp rate was 10 °C/min.
3.3. Characterization
Cross-sectional images of the catalysts after preparation were observed using a Dino-Lite Premier Digital Microscope (AM3113T, AnMo Electronics Corp., New Taipei City, Taiwan). The change in the Ru concentrations inside the alumina pellet was measured using scanning electron microscopy (FE-SEM, SU-8230, Hitachi, Tokyo, Japan) with EDS (Oxford Instruments, Abingdon, UK). The crystal structure of the catalysts was analyzed using a Cu Kα radiation source in a Phillips X’PERT X-ray diffractometer (PANalytical, Amsterdam, Netherlands) located at the Korea Basic Science Institute, Daegu. The surface area, pore volume, and pore size were measured by the BET method, using ASAP2020 (Norcross, GA, USA). The chemical composition of catalysts was measured using a wavelength-dispersive X-ray fluorescence spectrometer (XRF, Bruker Corp., Billerica, MA, USA).
3.4. Steam-Methane Reforming Tests
The SMR tests were determined using gas chromatography (GC). The Ru/Al2O3 pellets (0.5 g) were packed into a fixed-bed reactor with a diameter of ½ inch (1.27 cm), and the reactor was placed in an electric furnace at atmospheric pressure. The thermocouple was placed at the center of the catalyst bed. Before the SMR, 10 vol% H2 and N2 balance at 100 mL/min (WHSV: 12,000 mL/g/h) were passed through the packed bed at 800 °C for 3 h to reduce the catalysts. After purging the reactor with N2, the reaction temperature was fixed at 700 °C, and the gas composition was changed to 30 vol% H2O, 10 vol% CH4 (steam/carbon ratio: 3), and N2 balance at 100 mL/min. To prevent the condensation of water vapor, the inlet and outlet lines of the reactor were maintained at 120 °C using heating tape. In addition, the reactor outlet stream was passed through a condenser to capture water before entering the reactor and GC column. The dried gases were analyzed using a gas chromatograph (Agilent 6890, Agilent Technologies, Inc., Santa Clara, CA, USA), equipped with two thermal conductivity detectors (TCDs). The first Carboxen 1000-packed column was connected to one TCD for analysis of the N2, CO, CH4, and CO2 gases, and the second Carboxen 1000-packed column was connected to the other TCD for analysis of the H2 gas.
The conversion of CH4 (XCH4) and selectivity of the products (CO, CO2, and H2) were calculated using Equations (4)–(7)
4. Conclusions
In this study, Ru/Al2O3 catalysts were prepared using the impregnation method at various HNO3/Ru molar ratios and contact times. HNO3 was added to reduce the strength of the metal precursor–support interactions. In the presence of HNO3, at the impregnation step, Ru penetrated the pellet. Therefore, it is possible to make various types, from delimited eggshell type to homogenous type, by controlling the HNO3/Ru molar ratio and contact time. Ru/Al2O3 catalysts showed a similar CH4 conversion in the SMR reaction except for RA (0, 1 h), which showed a low CH4 conversion due to the poisoning of NH3. Among them, the graded eggshell-type RA (10, 1 h) catalyst showed the best activity (98%) and stability in the long-term test. In addition, the RA (10, 1 h) catalyst needed shorter preparation times than the homogeneous-type catalysts that showed similar catalytic activity. Therefore, the RA (10, 1 h) catalyst was selected as the optimal catalyst in our study. In the DSS operation, which was tested under conditions similar to those of an actual fuel cell, the catalytic activity of the graded eggshell-type RA (10, 1 h) catalyst was maintained for five cycles. Based on the experimental studies, it was confirmed that Ru-based catalysts are suitable for SMR for PEMFC.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal11080951/s1, Table S1: BET surface area, pore volume, average pore size, RuO2, Ru crystallite size, and Ru content of Ru/Al2O3 catalysts in its fresh and reduced states. Table S2: H2/CO ratio of the Ru/Al2O3 catalysts in SMR reaction long-term stability test at 700 °C, S/C ratio of 3, and WHSV of 12,000 mL/g/h. Figure S1: XRD patterns of the Ru/Al2O3 catalysts with different contact times in its fresh (a) and reduced (b) states. Figure S2: Methane conversion of the RA (0, 1 h) catalysts in SMR reaction under different balance gases. Figure S3: Carbon-based selectivity of the RA (0, 1 h) and RA (10, 1 h) catalysts in the SMR reaction long-term stability test at 700 °C, S/C ratio of 3, and WHSV of 12,000 mL/g/h. Figure S4: SEM images of Ru/γ-Al2O3 catalysts: (a) RA (0, 1 h), (b) RA (10, 1 h), and (c) RA (20, 1 h)
Author Contributions
Conceptualization, J.-H.L., S.J. and M.-S.K.; formal analysis, J.-H.L., T.-Y.K., J.-H.W., Y.L. and H.-O.P.; investigation, J.-H.L., T.-Y.K. and J.-H.W.; supervision, S.-C.L. and J.-C.K.; writing—original draft, J.-H.L. and S.J.; writing—review and editing, S.J. and S.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Evaluation Institute of Industrial Technology (KEIT) and the Ministry of Trade, Industry and Energy (MOTIE) of the Republic of Korea (No. 20015460). This work was also supported by the Sudokwon Landfill Site Management Corporation (SLC) through the joint research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. Data are contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dodds, P.E.; Staffell, I.; Hawkes, A.D.; Li, F.; Grünewald, P.; McDowall, W.; Ekins, P.J. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrogen Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Z.; Jiang, B.; Hongmanorom, P.; Zhong, W.; Kawi, S. A review on perovskite catalysts for reforming of methane to hydrogen production. Renew. Sustain. Energy Rev. 2020, 134, 110291. [Google Scholar] [CrossRef]
- Summa, P.; Samojeden, B.; Motak, M. Dry and steam reforming of methane. Comparison and analysis of recently investigated catalytic materials. A short review. Pol. J. Chem. Technol. 2019, 21, 31–37. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic hydrogen production from methane: A review on recent progress and prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Cho, E.H.; Koo, K.Y.; Lee, H.W.; Park, Y.-K.; Yoon, W.L.; Ko, C.H. Preparation of egg-shell-type Ni/Ru bimetal alumina pellet catalysts: Steam methane reforming for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 18350–18357. [Google Scholar] [CrossRef]
- Ligthart, D.; Van Santen, R.; Hensen, E.J.M. Influence of particle size on the activity and stability in steam methane reforming of supported Rh nanoparticles. J. Catal. 2011, 280, 206–220. [Google Scholar] [CrossRef]
- Miyata, T.; Li, D.; Shiraga, M.; Shishido, T.; Oumi, Y.; Sano, T.; Takehira, K. Promoting effect of Rh, Pd and Pt noble metals to the Ni/Mg(Al)O catalysts for the DSS-like operation in CH4 steam reforming. Appl. Catal. A Gen. 2006, 310, 97–104. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Reaction pathways and site requirements for the activation and chemical conversion of methane on Ru− based catalysts. J. Phys. Chem. B 2004, 108, 7253–7262. [Google Scholar] [CrossRef]
- Jones, G.; Jakobsen, J.G.; Shim, S.S.; Kleis, J.; Andersson, M.P.; Rossmeisl, J.; Abild-Pedersen, F.; Bligaard, T.; Helveg, S.; Hinnemann, B. First principles calculations and experimental insight into methane steam reforming over transition metal catalysts. J. Catal. 2008, 259, 147–160. [Google Scholar] [CrossRef]
- Kikuchi, E.; Tanaka, S.; Yamazaki, Y.; Morita, Y. Steam Reforming of Hydrocarbons on Noble Metal Catalysts (Part 1) The Catalytic Activity in Methane-Steam Reaction. Bull. Jpn. Pet. Inst. 1974, 16, 95–98. [Google Scholar] [CrossRef][Green Version]
- Qin, D.; Lapszewicz, J. Study of mixed steam and CO2 reforming of CH4 to syngas on MgO-supported metals. Catal. Today 1994, 21, 551–560. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam reforming of methane: Current states of catalyst design and process upgrading. Renew. Sustain. Energy Rev. 2021, 149, 111330. [Google Scholar] [CrossRef]
- Morales-Cano, F.; Lundegaard, L.F.; Tiruvalam, R.R.; Falsig, H.; Skjøth-Rasmussen, M.S. Improving the sintering resistance of Ni/Al2O3 steam-reforming catalysts by promotion with noble metals. Appl. Catal. A Gen. 2015, 498, 117–125. [Google Scholar] [CrossRef]
- Mang, T.; Breitscheidel, B.; Polanek, P.; Knözinger, H. Adsorption of platinum complexes on silica and alumina: Preparation of non-uniform metal distributions within support pellets. Appl. Catal. A Gen. 1993, 106, 239–258. [Google Scholar] [CrossRef]
- Pinna, F. Supported metal catalysts preparation. Catal. Today 1998, 41, 129–137. [Google Scholar] [CrossRef]
- Porta, A.; Falbo, L.; Visconti, C.G.; Lietti, L.; Bassano, C.; Deiana, P. Synthesis of Ru-based catalysts for CO2 methanation and experimental assessment of intraporous transport limitations. Catal. Today 2020, 343, 38–47. [Google Scholar] [CrossRef]
- Zhuang, Y.; Claeys, M.; Van Steen, E. Novel synthesis route for egg-shell, egg-white and egg-yolk type of cobalt on silica catalysts. Appl. Catal. A Gen. 2006, 301, 138–142. [Google Scholar] [CrossRef]
- Spieker, W.; Regalbuto, J. A fundamental model of platinum impregnation onto alumina. Chem. Eng. Sci. 2001, 56, 3491–3504. [Google Scholar] [CrossRef]
- Fenoglio, R.; Alvarez, W.; Nunez, G.; Resasco, D. Interactions of the Impregnating Solution with the Support during the Preparation of Rh/Tio2 Catalysts. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1991; Volume 63, pp. 77–86. [Google Scholar]
- Watanabe, F.; Kaburaki, I.; Shimoda, N.; Satokawa, S. Influence of nitrogen impurity for steam methane reforming over noble metal catalysts. Fuel Process. Technol. 2016, 152, 15–21. [Google Scholar] [CrossRef]
- Carvalho, L.S.; Martins, A.R.; Reyes, P.; Oportus, M.; Albonoz, A.; Vicentini, V.; do Carmo Rangel, M. Preparation and characterization of Ru/MgO-Al2O3 catalysts for methane steam reforming. Catal. Today 2009, 142, 52–60. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Sanz, O.; Italiano, C.; Vita, A.; Montes, M.; Specchia, S. Analysis of Ru/La-Al2O3 catalyst loading on alumina monoliths and controlling regimes in methane steam reforming. Chem. Eng. J. 2018, 334, 1792–1807. [Google Scholar] [CrossRef]
- Ohi, T.; Miyata, T.; Li, D.; Shishido, T.; Kawabata, T.; Sano, T.; Takehira, K. Sustainability of Ni loaded Mg–Al mixed oxide catalyst in daily startup and shutdown operations of CH4 steam reforming. Appl. Catal. A Gen. 2006, 308, 194–203. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).