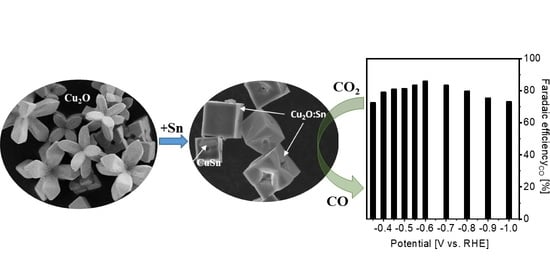
Efficient CO2 Electroreduction on Tin Modified Cuprous Oxide Synthesized via a One-Pot Microwave-Assisted Route
Abstract
:1. Introduction
2. Results and Discussion
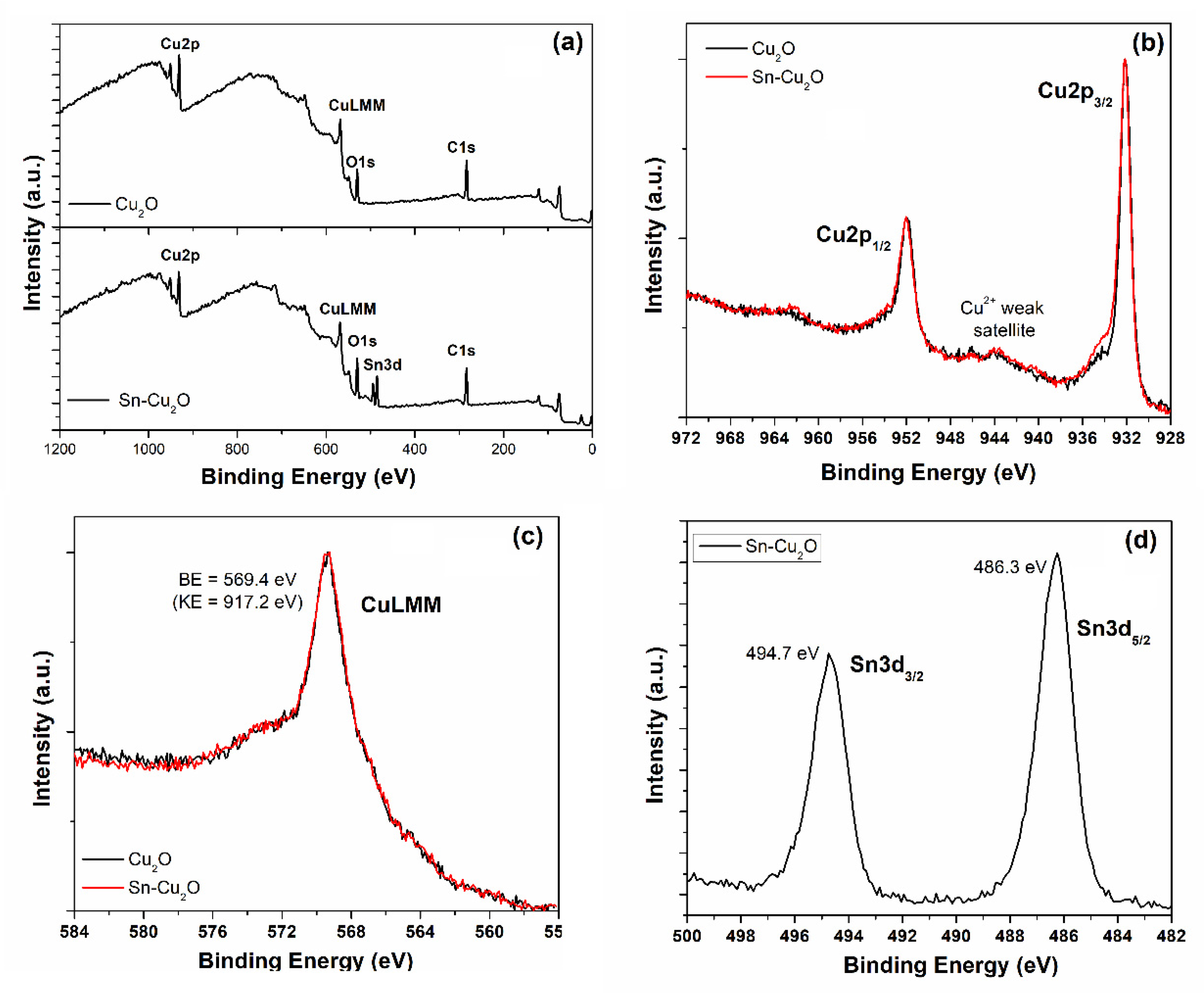
2.1. Physical and Chemical Characterizations of the As-Prepared Catalysts
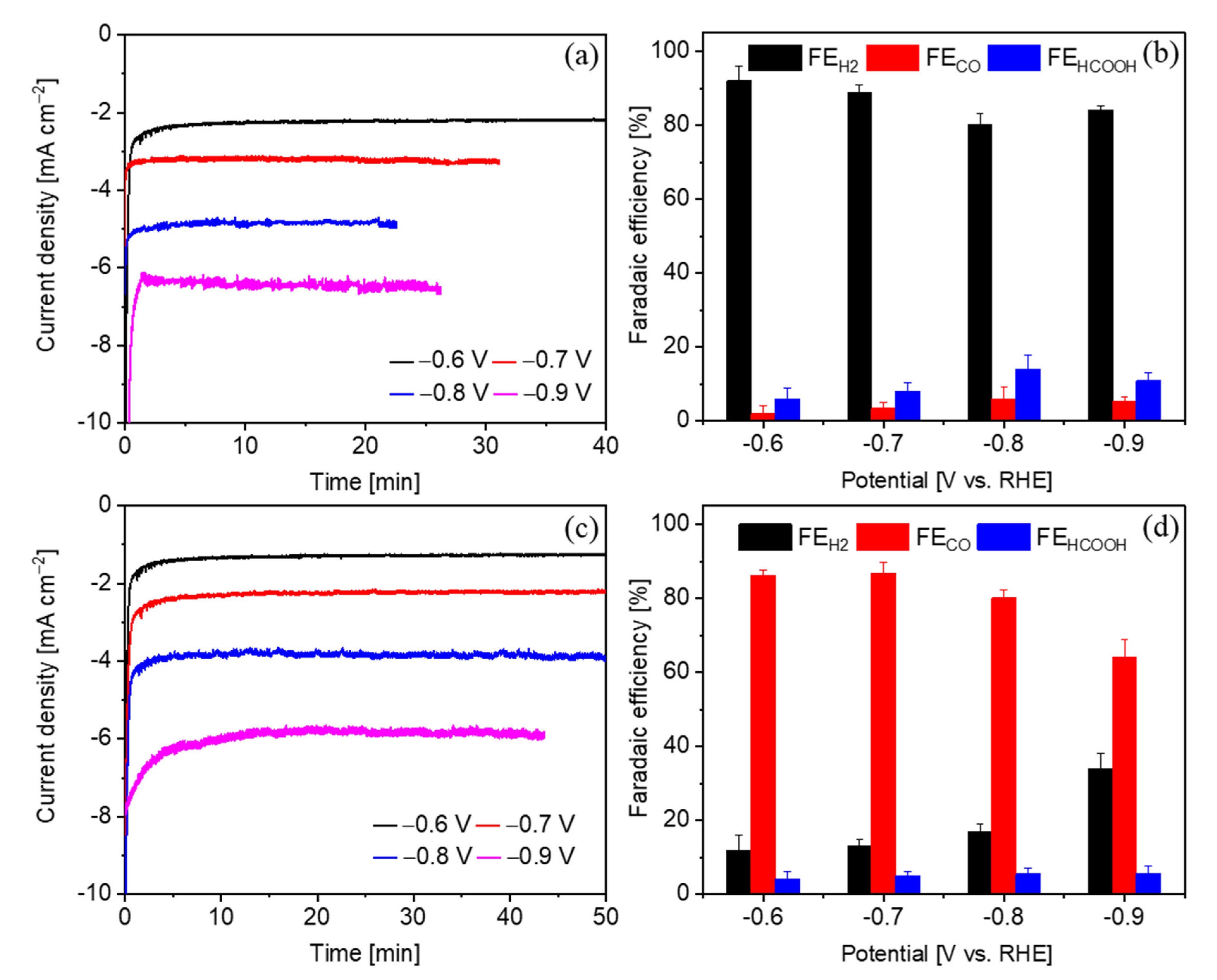
2.2. Comparison of the CO2RR Performance on Various Samples
2.3. Study of the CO2RR on the Sn-Cu2O Electrode in a Semi-Flow Cell
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Physical and Chemical Characterizations
3.4. Electrode Preparation
3.5. CO2 Electrolysis and Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; De Luna, P.; Dinh, C.T.; Tao, L.; Saur, G.; Van de Lagemaat, J.; Kelley, S.O.; Sargent, E.H. What Should We Make with CO2 and How Can We Make It? Joule 2018, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, D.U.; Hu, X.-M.; Daasbjerg, K.; Skrydstrup, T. Chemically and electrochemically catalysed conversion of CO2 to CO with follow-up utilization to value-added chemicals. Nat. Catal. 2018, 1, 244–254. [Google Scholar] [CrossRef]
- Zeng, J.; Rino, T.; Bejtka, K.; Castellino, M.; Sacco, A.; Farkhondehfal, M.A.; Chiodoni, A.; Drago, F.; Pirri, C.F. Coupled Copper-Zinc Catalysts for Electrochemical Reduction of Carbon Dioxide. ChemSusChem 2020, 13, 4128–4139. [Google Scholar] [CrossRef] [PubMed]
- Hoang, V.C.; Gomes, V.G.; Kornienko, N. Metal-based nanomaterials for efficient CO2 electroreduction: Recent advances in mechanism, material design and selectivity. Nano Energy 2020, 78, 105311. [Google Scholar] [CrossRef]
- Burdyny, T.; Smith, W.A. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy Environ. Sci. 2019, 12, 1442–1453. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.N.; Liu, Z.; Wen, J.; Chen, A. Enhanced catalytic activity of nanoporous Au for the efficient electrochemical reduction of carbon dioxide. Appl. Catal. B Environ. 2018, 236, 483–489. [Google Scholar] [CrossRef]
- Han, X.; Liu, L.; Yuan, J.; Zhang, X.; Niu, D. Polyacrylamide-Mediated Silver Nanoparticles for Selectively Enhancing Electroreduction of CO2 towards CO in Water. ChemSusChem 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Koshy, D.M.; Chen, S.; Lee, D.U.; Stevens, M.B.; Abdellah, A.M.; Dull, S.M.; Chen, G.; Nordlund, D.; Gallo, A.; Hahn, C.; et al. Understanding the Origin of Highly Selective CO2 Electroreduction to CO on Ni,N-doped Carbon Catalysts. Angew. Chem. Int. Ed. 2020, 59, 4043–4050. [Google Scholar] [CrossRef]
- Jia, M.; Hong, S.; Wu, T.-S.; Li, X.; Soo, Y.-L.; Sun, Z. Single Sb sites for efficient electrochemical CO2 reduction. Chem. Commun. 2019, 55, 12024–12027. [Google Scholar] [CrossRef]
- Sarfraz, S.; Garcia-Esparza, A.T.; Jedidi, A.; Cavallo, L.; Takanabe, K. Cu-Sn Bimetallic Catalyst for Selective Aqueous Electroreduction of CO2 to CO. ACS Catal. 2016, 6, 2842–2851. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Bejtka, K.; Ju, W.; Castellino, M.; Chiodoni, A.; Sacco, A.; Farkhondehfal, M.A.; Hernández, S.; Rentsch, D.; Battaglia, C.; et al. Advanced Cu-Sn foam for selectively converting CO2 to CO in aqueous solution. Appl. Catal. B Environ. 2018, 236, 475–482. [Google Scholar] [CrossRef]
- Morimoto, M.; Takatsuji, Y.; Yamasaki, R.; Hashimoto, H.; Nakata, I.; Sakakura, T.; Haruyama, T. Electrodeposited Cu-Sn Alloy for Electrochemical CO2 Reduction to CO/HCOO−. Electrocatalysis 2018, 9, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Ju, W.; Zeng, J.; Bejtka, K.; Ma, H.; Rentsch, D.; Castellino, M.; Sacco, A.; Pirri, C.F.; Battaglia, C. Sn-Decorated Cu for Selective Electrochemical CO2 to CO Conversion: Precision Architecture beyond Composition Design. ACS Appl. Energy Mater. 2019, 2, 867–872. [Google Scholar] [CrossRef]
- Yoo, C.J.; Dong, W.J.; Park, J.Y.; Lim, J.W.; Kim, S.; Choi, K.S.; Ngome, F.O.O.; Choi, S.-Y.; Lee, J.-L. Compositional and Geometrical Effects of Bimetallic Cu–Sn Catalysts on Selective Electrochemical CO2 Reduction to CO. ACS Appl. Energy Mater. 2020, 3, 4466–4473. [Google Scholar] [CrossRef]
- Dong, W.J.; Lim, J.W.; Hong, D.M.; Park, J.Y.; Cho, W.S.; Baek, S.; Yoo, C.J.; Kin, W.; Lee, J.-L. Evidence of Local Corrosion of Bimetallic Cu-Sn Catalysts and Its Effects on the Selectivity of Electrochemical CO2 Reduction. ACS Appl. Energy Mater. 2020, 3, 10568–10577. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Garg, S.; Rufford, T.E.; Zhao, P.; Wu, Y.; Yago, A.J.; Rudolph, V.; Wang, G. Modulated Sn Oxidation States over a Cu2O-Derived Substrate for Selective Electrochemical CO2 Reduction. ACS Appl. Mater. Interfaces 2020, 12, 22760–22770. [Google Scholar] [CrossRef]
- Singh, M.R.; Clark, E.L.; Bell, A.T. Effects of electrolyte, catalyst, and membrane composition and operating conditions on the performance of solar-driven electrochemical reduction of carbon dioxide. PCCP 2015, 17, 18924–18936. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.N.; Dinh, C.-T. Gas diffusion electrode design for electrochemical carbon dioxide reduction. Chem. Soc. Rev. 2020, 49, 7488–7504. [Google Scholar] [CrossRef]
- Rabiee, H.; Ge, L.; Zhang, X.; He, S.; Li, M.; Yuan, Z. Gas diffusion electrodes (GDEs) for electrochemical reduction of carbon dioxide, carbon monoxide, and dinitrogen to value-added products: A review. Energy Environ. Sci. 2021, 14, 1959–2008. [Google Scholar] [CrossRef]
- Zeng, J.; Bejtka, K.; Di Martino, G.; Sacco, A.; Castellino, M.; Re Fiorentin, M.; Risplendi, F.; Farkhondehfal, M.A.; Hernández, S.; Cicero, G.; et al. Microwave-Assisted Synthesis of Copper-Based Electrocatalysts for Converting Carbon Dioxide to Tunable Syngas. ChemElectroChem 2020, 7, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Knödler, H. On the Crystal Structure and Structure Relationships of the γ and ε Phases in the Cu-Sn System. Metall 1966, 20, 823–829. [Google Scholar]
- Arnberg, L.; Jönsson, A.; Westman, S. The Structure of the delta-Phase in the Cu--Sn System. A Phase of gamma-Brass Type with an 18 Å Superstructure. Acta Chem. Scand. Ser. A 1976, 30, 187–192. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, Y.; Chen, H.; Xu, C. CTAB-assisted synthesis of eight-horn-shaped Cu2O crystals via a simple solution approach. J. Mater. Sci. Mater. Electron. 2018, 29, 4256–4260. [Google Scholar] [CrossRef]
- Chang, Y.; Zeng, H.C. Manipulative Synthesis of Multipod Frameworks for Self-Organization and Self-Amplification of Cu2O Microcrystals. Cryst. Growth Des. 2004, 4, 273–278. [Google Scholar] [CrossRef]
- Biesinger, M. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Garino, N.; Zeng, J.; Castellino, M.; Sacco, A.; Risplendi, F.; Re Fiorentin, M.; Bejtka, K.; Chiodoni, A.; Salomon, D.; Segura-Ruiz, J.; et al. Facilely synthesized nitrogen-doped reduced graphene oxide functionalized with copper ions as electrocatalyst for oxygen reduction. NPJ 2D Mater. Appl. 2021, 5, 2. [Google Scholar] [CrossRef]
- Zhang, B.A.; Ozel, T.; Elias, J.S.; Costentin, C.; Nocera, D.G. Interplay of homogeneous reactions, mass transport, and kinetics in determining selectivity of the reduction of CO2 on Gold electrodes. ACS Cent. Sci. 2019, 5, 1097–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasileff, A.; Zhi, X.; Xu, C.; Ge, L.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Selectivity control for electrochemical CO2 reduction by charge redistribution on the surface of copper alloys. ACS Catal. 2019, 9, 9411−9417. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, M.; Shao, Q.; Pi, Y.; Zhu, X.; Li, Y.; Huang, X. Phase and structure engineering of copper tin heterostructures for efficient electrochemical carbon dioxide reduction. Nat. Commun. 2018, 9, 4933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, M.; Vaes, J.; Klemm, E.; Pant, D. Solvents and Supporting Electrolytes in the Electrocatalytic Reduction of CO2. iScience 2019, 19, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Dunwell, M.; Lu, Q.; Heyes, J.M.; Rosen, J.; Chen, J.G.; Yan, Y.; Jiao, F.; Xu, B. The central role of bicarbonate in the electrochemical reduction of carbon dioxide on gold. J. Am. Chem. Soc. 2017, 139, 3774–3783. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fu, J.; Zhu, W.; Chen, Z.; Shen, B.; Wu, L.; Xi, Z.; Wang, T.; Lu, G.; Zhu, J.J.; et al. Tuning Sn-Catalysis for Electrochemical Reduction of CO2 to CO via the Core/Shell Cu/SnO2 Structure. J. Am. Chem. Soc. 2017, 139, 4290–4293. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Jiang, F.; Ma, H.; Pan, Z.; Zhao, Y.-B.; Pagani, F.; Rentsch, D.; Wang, J.; Battaglia, C. Electrocatalytic Reduction of Gaseous CO2 to CO on Sn/Cu-Nanofiber-Based Gas Diffusion Electrodes. Adv. Energy Mater. 2019, 9, 1901514. [Google Scholar] [CrossRef]
- Wang, J.; Ji, Y.; Shao, Q.; Yin, R.; Guo, J.; Li, Y.; Huang, X. Phase and structure modulating of bimetallic CuSn nanowires boosts electrocatalytic conversion of CO2. Nano Energy 2019, 59, 138–145. [Google Scholar] [CrossRef]
- Li, C.W.; Kanan, M.W. CO2 Reduction at Low Overpotential on Cu Electrodes Resulting from the Reduction of Thick Cu2O Films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Cryst. 2018, 51, 210–218. [Google Scholar] [CrossRef] [Green Version]
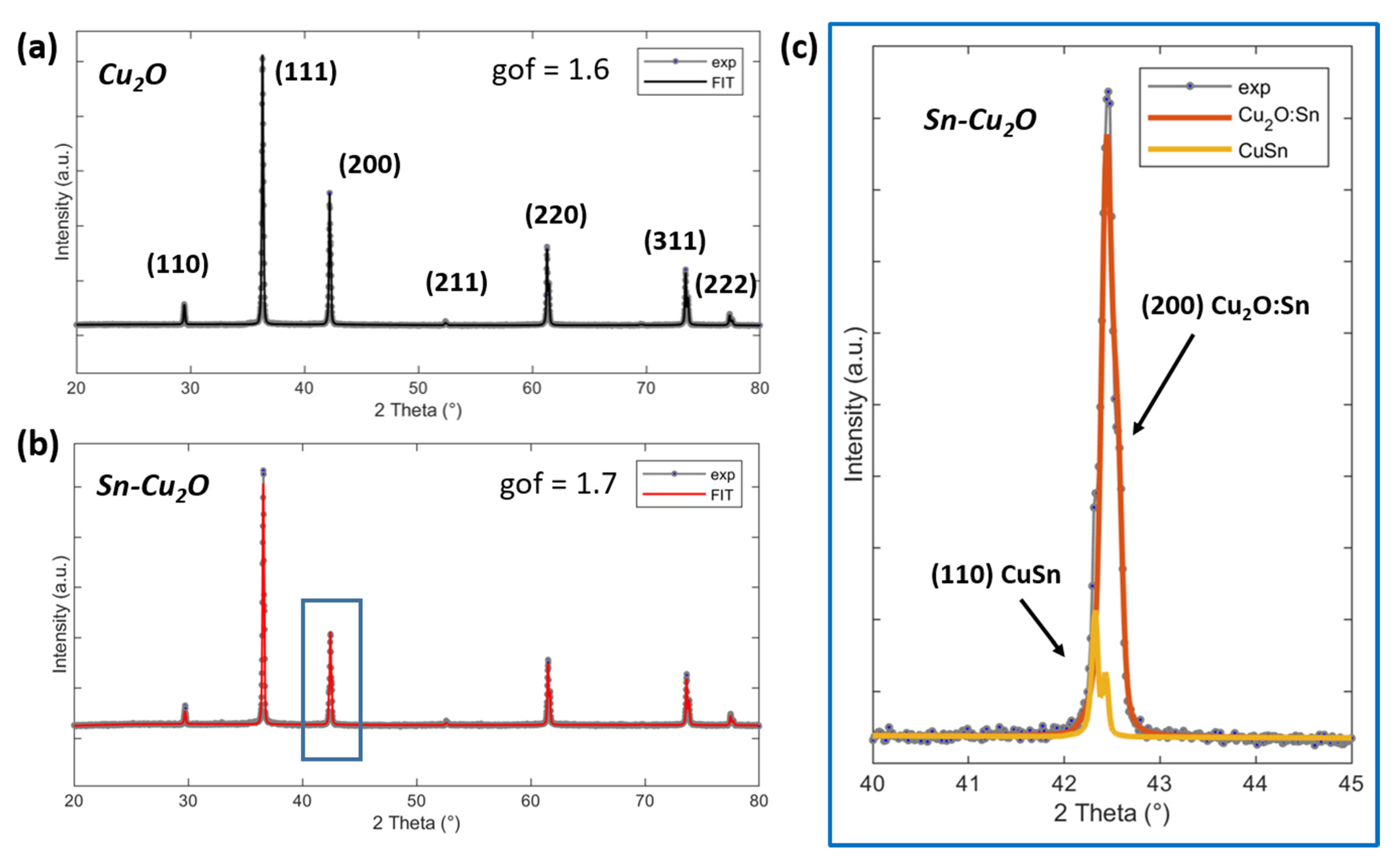



| Cu2O | Sn-Cu2O | ||||||
|---|---|---|---|---|---|---|---|
| Phase | Cu2O | a (Å) | Size (nm) | Phase | Cu2O:Sn | a (Å) | Size (nm) |
| - | cubic | 4.268 | 108 | - | cubic | 4.269 | 88 |
| - | Pn-3m | - | - | - | Pn-3m | - | - |
| - | - | - | - | - | - | - | - |
| - | - | - | - | phase | CuSn | a (Å) | size (nm) |
| - | - | - | - | - | cubic | 3.027 | 285 |
| - | - | - | - | - | Im-3m | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Fontana, M.; Castellino, M.; Sacco, A.; Farkhondehfal, M.A.; Drago, F.; Pirri, C.F. Efficient CO2 Electroreduction on Tin Modified Cuprous Oxide Synthesized via a One-Pot Microwave-Assisted Route. Catalysts 2021, 11, 907. https://doi.org/10.3390/catal11080907
Zeng J, Fontana M, Castellino M, Sacco A, Farkhondehfal MA, Drago F, Pirri CF. Efficient CO2 Electroreduction on Tin Modified Cuprous Oxide Synthesized via a One-Pot Microwave-Assisted Route. Catalysts. 2021; 11(8):907. https://doi.org/10.3390/catal11080907
Chicago/Turabian StyleZeng, Juqin, Marco Fontana, Micaela Castellino, Adriano Sacco, M. Amin Farkhondehfal, Filippo Drago, and Candido Fabrizio Pirri. 2021. "Efficient CO2 Electroreduction on Tin Modified Cuprous Oxide Synthesized via a One-Pot Microwave-Assisted Route" Catalysts 11, no. 8: 907. https://doi.org/10.3390/catal11080907
APA StyleZeng, J., Fontana, M., Castellino, M., Sacco, A., Farkhondehfal, M. A., Drago, F., & Pirri, C. F. (2021). Efficient CO2 Electroreduction on Tin Modified Cuprous Oxide Synthesized via a One-Pot Microwave-Assisted Route. Catalysts, 11(8), 907. https://doi.org/10.3390/catal11080907