Effect of Potential and Chlorides on Photoelectrochemical Removal of Diethyl Phthalate from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of TiO2 Nanotubes
2.2. Photoelectrochemical Tests
2.3. Analytical Methods
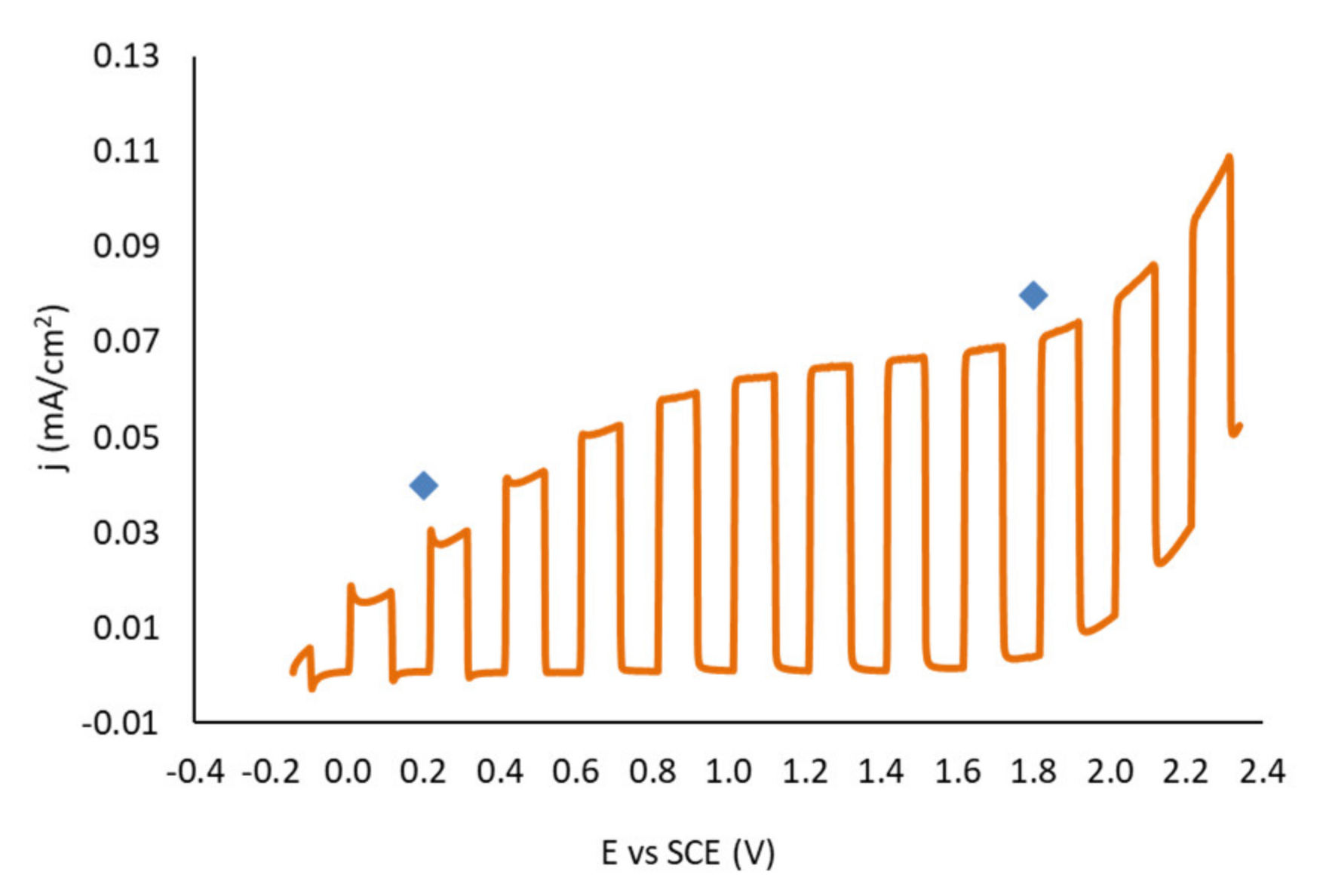
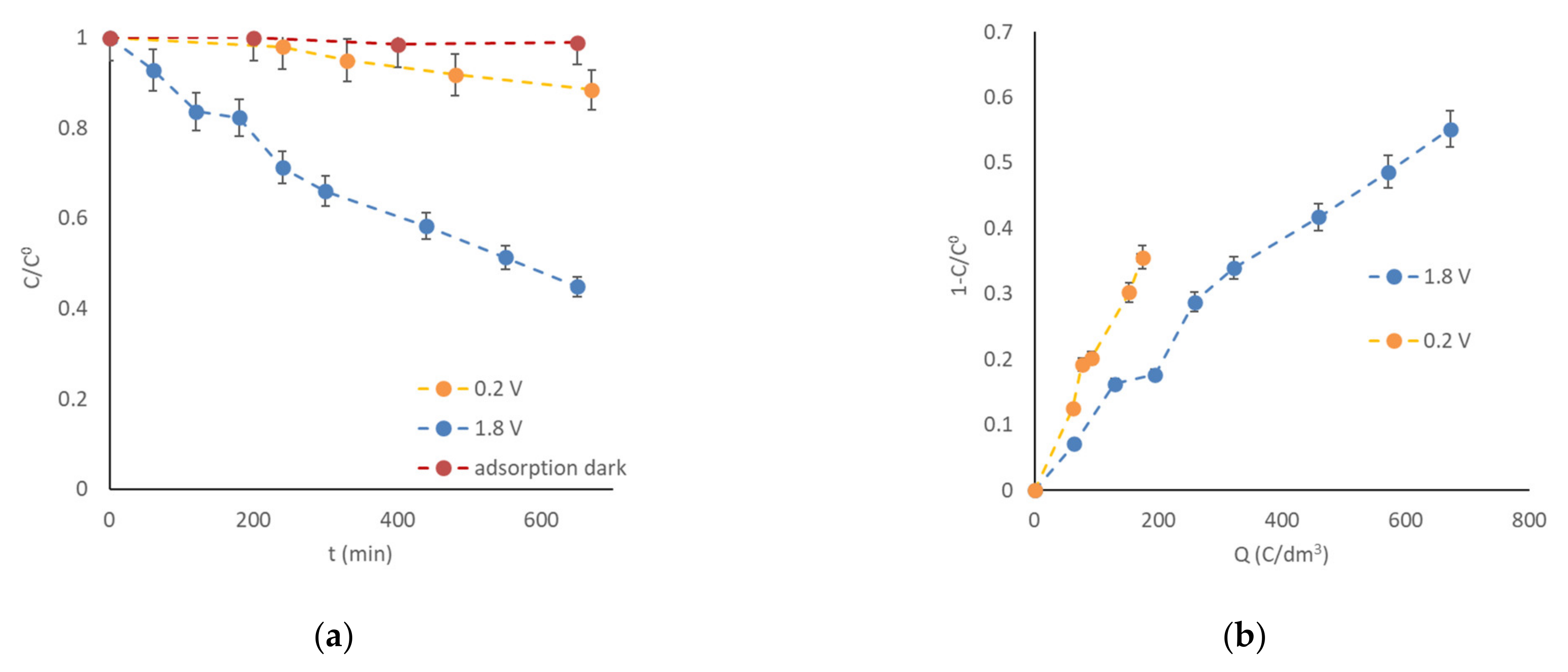
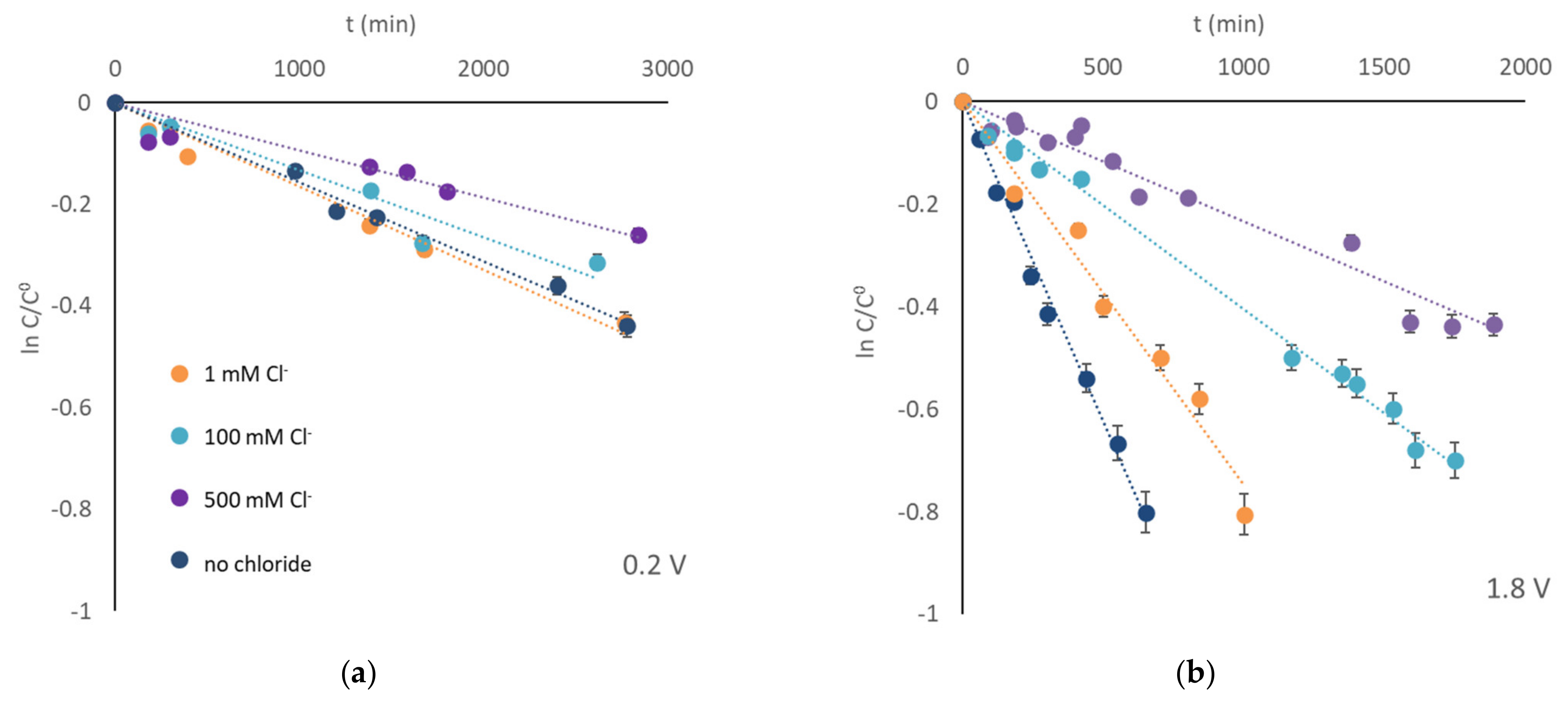
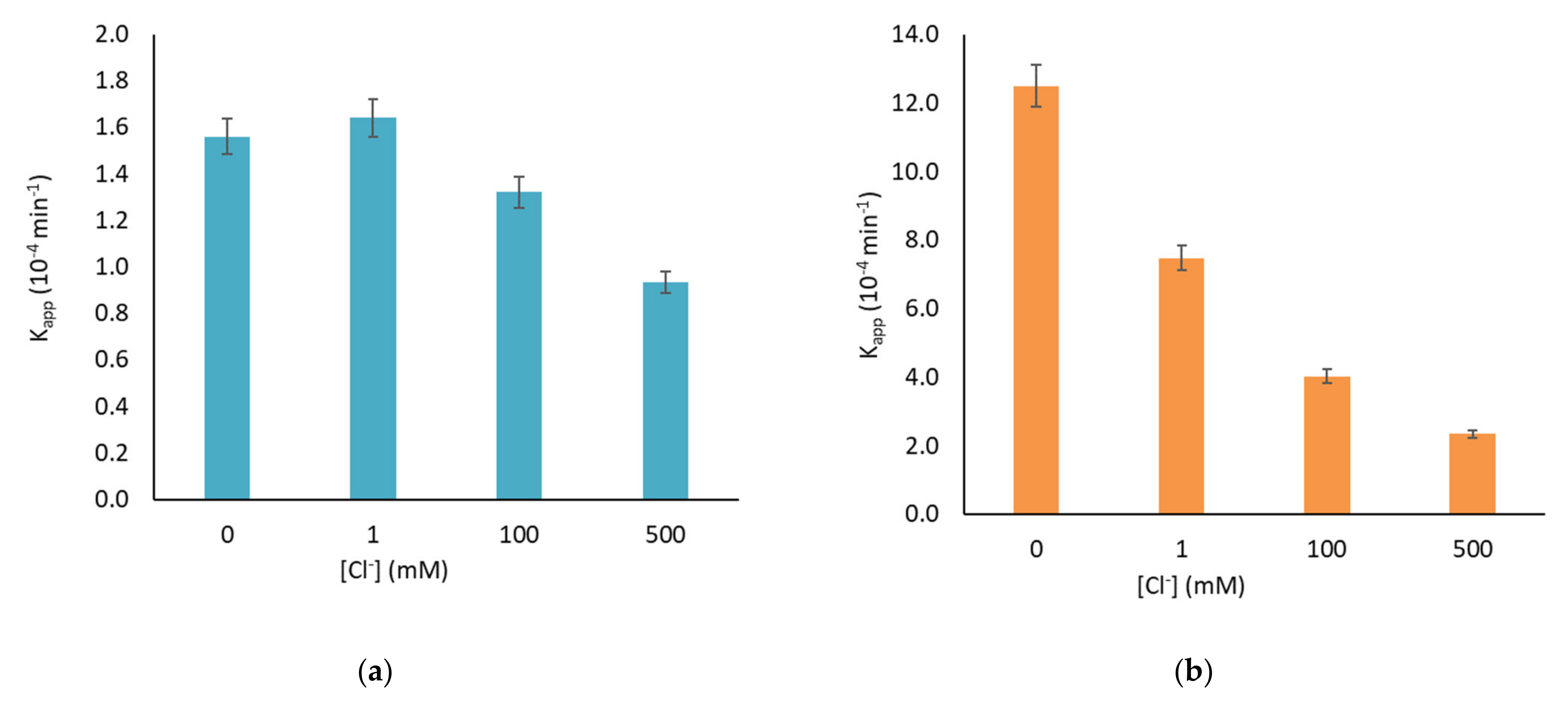
3. Results and Discussion
- (1)
- (2)
- (3)
- chloride acting as surface-charge-recombination center for photogenerated charge carriers [38].
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Palmas, S.; Mais, L.; Mascia, M.; Vacca, A. Trend in using TiO2 nanotubes as photoelectrodes in PEC processes for wastewater treatment. Curr. Opin. Electrochem. 2021, 28, 100699. [Google Scholar]
- van de Krol, R. Principles of Photoelectrochemical Cells. In Photoelectrochemical Hydrogen Production; van de Krol, R., Grätzel, M., Eds.; Electronic Materials: Science & Technology; Springer: Boston, MA, USA, 2012; Volume 102, pp. 13–67. [Google Scholar]
- Krivec, M.; Dillert, R.; Bahnemann, D.W.; Mehle, A.; Strancar, J.; Drazic, G. The nature of chlorine-inhibition of photocatalytic degradation of dichloroacetic acid in a TiO2-based microreactor. Phys. Chem. Chem. Phys. 2014, 16, 14867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of produced water with photocatalysis: Recent advances, affecting factors and future research prospects. Catalysts 2020, 10, 924. [Google Scholar] [CrossRef]
- Piscopo, A.; Robert, D.; Weber, J.V. Influence of pH and chloride anion on the photocatalytic degradation of organic compounds Part I. Effect on the benzamide and para-hydroxybenzoic acid in TiO2 aqueous solution. Appl. Catal. B Environ. 2001, 35, 117–124. [Google Scholar] [CrossRef]
- Selcuk, H.; Bekbolet, M. Photocatalytic and photoelectrocatalytic humic acid removal and selectivity of TiO2 coated photoanode. Chemosphere 2008, 73, 854–858. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, Y.; Zhou, J.; Zhou, S. Degradation kinetics of photoelectrocatalysis on landfill leachate using codoped TiO2/Ti photoelectrodes. J. Nanomater. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Moser, J.; Gratzel, M. Photoelectrochemistry with colloidal semiconductors; laser studies of halide oxidation in colloidal dispersions of TiO2 and α-Fe2O3. Helv. Chim. Acta 1982, 65, 1436–1444. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Sirtori, C.; Agüera, A.; Gernjak, W.; Malato, S. Effect of water-matrix composition on Trimethoprim solar photodegradation kinetics and pathways. Water Res. 2010, 44, 2735–2744. [Google Scholar] [CrossRef]
- Brüninghoff, R.; van Duijne, A.K.; Braakhuis, L.; Saha, P.; Jeremiasse, A.W.; Mei, B.; Mul, G. Comparative Analysis of Photocatalytic and Electrochemical Degradation of 4-Ethylphenol in Saline Conditions. Environ. Sci. Technol. 2019, 53, 8725–8735. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.P.; Yassitepe, E.; Yeh, Y.T.; Ruzybayev, I.; Shah, S.I.; Huang, C.P. Photoelectrochemical degradation of azo dye over pulsed laser deposited nitrogen-doped TiO2 thin film. Appl. Catal. B Environ. 2012, 125, 465–472. [Google Scholar] [CrossRef]
- Vacca, A.; Mais, L.; Mascia, M.; Usai, E.M.; Palmas, S. Design of experiment for the optimization of pesticide removal from wastewater by photo-electrochemical oxidation with TiO2 nanotubes. Catalysts 2020, 10, 512. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.Q.; Fu, X.; Zhang, N.; Xu, Y.J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.V.B.; Sene, J.J.; Anderson, M.A. Photoelectrocatalytic degradation of Remazol Brilliant Orange 3R on titanium dioxide thin-film electrode. J. Photochem. Photobiol. A Chem. 2003, 157, 55–63. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Z.; Liu, Y.; Fang, M. Investigation on the Photoelectrocatalytic Activity of Well-Aligned TiO2 Nanotube Arrays. Int. J. Photoenergy 2012, 2012, 832516. [Google Scholar] [CrossRef] [Green Version]
- An, T.; Zhang, W.; Xiao, X.; Sheng, G.; Fu, J.; Zhu, X. Photoelectrocatalytic degradation of quinoline with a novel three-dimensional electrode-packed bed photocatalytic reactor. J. Photochem. Photobiol. A Chem. 2004, 161, 233–242. [Google Scholar] [CrossRef]
- Zhang, W.; An, T.; Cui, M.; Sheng, G.; Fu, J. Effects of anions on the photocatalytic and photoelectrocatalytic degradation of reactive dye in a packed-bed reactor. J. Chem. Technol. Biotechnol. 2005, 80, 223–229. [Google Scholar] [CrossRef]
- Zanoni, M.V.B.; Sene, J.J.; Selcuk, H.; Anderson, M.A. Photoelectrocatalytic production of active chlorine on nanocrystalline titanium dioxide thin-film electrodes. Environ. Sci. Technol. 2004, 38, 3203–3208. [Google Scholar] [CrossRef]
- Xu, B.; Gao, N.Y.; Sun, X.F.; Xia, S.J.; Rui, M.; Simonnot, M.O.; Causserand, C.; Zhao, J.F. Photochemical degradation of diethyl phthalate with UV/H2O2. J. Hazard. Mater. 2007, 139, 132–139. [Google Scholar] [CrossRef]
- Chang, B.V.; Liao, C.S.; Yuan, S.Y. Anaerobic degradation of diethyl phthalate, di-n-butyl phthalate, and di-(2-ethylhexyl) phthalate from river sediment in Taiwan. Chemosphere 2005, 58, 1067–1601. [Google Scholar] [CrossRef]
- Wang, H.; Sun, D.Z.; Bian, Z.Y. Degradation mechanism of diethyl phthalate with electrogenerated hydroxyl radical on a Pd/C gas-diffusion electrode. J. Hazard. Mater. 2010, 180, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Peterson, D.R.; Parkarton, T.F.; Adams, W.J. The environmental fate of phthalate esters: A literature review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Staples, C.A.; Parkerton, T.F.; Peterson, D.R. A risk assessment of selected phthalate esters in North American and western European surface waters. Chemosphere 2000, 40, 885–891. [Google Scholar] [CrossRef]
- Wang, R.; Ji, M.; Zhai, H.; Liub, Y. Occurrence of phthalate esters and microplastics in urban secondary effluents, receiving water bodies and reclaimed water. Sci. Total Environ. 2020, 737, 140219. [Google Scholar] [CrossRef]
- Ma, F.; Shi, T.; Gao, J.; Chen, L.; Guo, W.; Guo, Y.; Wang, S. Comparison and understanding of the different simulated sunlight photocatalytic activity between the saturated and monovacant Keggin unit functionalized titania materials. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 116–125. [Google Scholar] [CrossRef]
- Cai, J.; Niu, B.; Zhao, H.; Zhao, G. Selective photoelectrocatalytic removal for group-targets of phthalic esters. Environ. Sci. Technol. 2021, 55, 2618–2627. [Google Scholar] [CrossRef]
- Vacca, A.; Mais, L.; Mascia, M.; Usai, M.E.; Rodriguez, J.; Palmas, S. Mechanistic insights into 2,4-D photoelectrocatalytic removal from water with TiO2 nanotubes under dark and solar light irradiation. J. Hazard. Mater. 2021, 412, 125202. [Google Scholar] [CrossRef] [PubMed]
- Varghese, O.K.; Gong, D.W.; Paulose, M.; Grimes, C.A.; Dickey, E.C. Crystallization and high-temperature structural stability of titanium oxide nanotube arrays. J. Mater. Res. 2003, 18, 156–165. [Google Scholar] [CrossRef]
- Allam, N.K.; El-Sayed, M.A. Photoelectrochemical water oxidation characteristics of Anodically Fabricated TiO2 nanotube arrays: Structural and optical properties. J. Phys. Chem. C 2010, 114, 12024–12029. [Google Scholar] [CrossRef]
- Palmas, S.; Da Pozzo, A.; Mascia, M.; Vacca, A.; Ardu, A.; Matarrese, R.; Nova, I. Effect of the preparation conditions on the performance of TiO2 nanotube arrays obtained by electrochemical oxidation. Int. J. Hydrogen Energy 2011, 36, 8894–8901. [Google Scholar] [CrossRef]
- Calza, P.; Pelizzetti, E. Photocatalytic transformation of organic compounds in the presence of inorganic ions. Pure Appl. Chem. 2001, 73, 1839–1848. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Chen, Y.; Lou, L.; Wu, X. involvement of chloride anion in photocatalytic process. J. Environ. Sci. 2005, 17, 761–765. [Google Scholar]
- Burns, R.A.; Crittenden, J.C.; Hand, D.W.; Selzer, V.H.; Sutter, L.L.; Salman, S.R. Effect of inorganic ions in heterogeneous photocatalysis of TCE. J. Environ. Eng. 1999, 125, 77–85. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zahraa, O.; Bouchy, M. Inhibition of the adsorption and photocatalytic degradation of an organic contaminant in an aqueous suspension of TiO2 by inorganic ions. J. Photochem. Photobiol. A 1997, 108, 37–44. [Google Scholar] [CrossRef]
- Sunada, F.; Heller, A. Effects of water, salt water, and silicone overcoating of the TiO2 photocatalyst on the rates and products of photocatalytic oxidation of liquid 3-octanol and 3-octanone. Environ. Sci. Technol. 1998, 32, 282–286. [Google Scholar] [CrossRef]
- Abdullah, M.; Low, G.K.C.; Matthews, R.W. Effects of common inorganic anions on rates of photocatalytic oxidation of organic carbon over illuminated titanium dioxide. J. Phys. Chem. 1990, 94, 6820–6825. [Google Scholar] [CrossRef]
- Azevedo, E.B.; de Aquino Neto, F.R.; Dezotti, M. TiO2-photocatalyzed degradation of phenol in saline media: Lumped kinetics, intermediates, and acute toxicity. Appl. Catal. B 2004, 54, 165–173. [Google Scholar] [CrossRef]
- Adishkumar, S.; Kanmani, S.; Rajesh Banu, J. Solar photocatalytic treatment of phenolic wastewaters: Influence of chlorides, sulphates, aeration, liquid volume and solar light intensity. Desalin. Water Treat. 2014, 52, 7957–7963. [Google Scholar] [CrossRef]
- Denisov, N.; Yoo, J.E.; Schmuki, P. Effect of different hole scavengers on the photoelectrochemical properties and photocatalytic hydrogen evolution performance of pristine and Pt-decorated TiO2 nanotubes. Electrochim. Acta 2019, 319, 61–71. [Google Scholar] [CrossRef]
- Peter, L.M.; Upul Wijayantha, K.G.; Tahir, A.A. Kinetics of light-driven oxygen evolution at α-Fe2O3 electrodes. Faraday Discuss. 2012, 155, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Qu, J.; Liu, H.; Zhao, X.; Wan, D. Fabrication of TiO2/Ti nanotube electrode and the photoelectrochemical behaviors in NaCl solutions. J. Solid State Electrochem. 2009, 13, 1959–1964. [Google Scholar] [CrossRef]
- Spadavecchia, F.; Ardizzone, S.; Cappelletti, G.; Falciola, L.; Ceotto, M.; Lotti, D. Investigation and optimization of photocurrent transient measurements on nano-TiO2. J. Appl. Electrochem. 2013, 43, 217–225. [Google Scholar] [CrossRef]
- Souza, F.L.; Aquino, J.M.; Irikura, K.; Miwa, D.W.; Rodrigo, M.A.; Motheo, A.J. Electrochemical degradation of the dimethyl phthalate ester on a fluoride-doped Ti/b-PbO2 anode. Chemosphere 2014, 109, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Miwa, D.W.; Malpass, G.R.P.; Machado, S.A.S.; Motheo, A.J. Electrochemical degradation of carbaryl on oxide electrodes. Water Res. 2006, 40, 3281–3289. [Google Scholar] [CrossRef] [PubMed]




| Applied Potential (V) | [Cl−] mM | TOC Removal | φ |
|---|---|---|---|
| 0.2 V | 0 | 31% | 0.87 |
| 1 | 28% | 0.87 | |
| 100 | 26% | 0.96 | |
| 500 | 11% | 0.98 | |
| 1.8 V | 0 | 53% | 0.96 |
| 1 | 46% | 0.99 | |
| 100 | 49% | 1.00 | |
| 500 | 39% | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mais, L.; Palmas, S.; Mascia, M.; Vacca, A. Effect of Potential and Chlorides on Photoelectrochemical Removal of Diethyl Phthalate from Water. Catalysts 2021, 11, 882. https://doi.org/10.3390/catal11080882
Mais L, Palmas S, Mascia M, Vacca A. Effect of Potential and Chlorides on Photoelectrochemical Removal of Diethyl Phthalate from Water. Catalysts. 2021; 11(8):882. https://doi.org/10.3390/catal11080882
Chicago/Turabian StyleMais, Laura, Simonetta Palmas, Michele Mascia, and Annalisa Vacca. 2021. "Effect of Potential and Chlorides on Photoelectrochemical Removal of Diethyl Phthalate from Water" Catalysts 11, no. 8: 882. https://doi.org/10.3390/catal11080882
APA StyleMais, L., Palmas, S., Mascia, M., & Vacca, A. (2021). Effect of Potential and Chlorides on Photoelectrochemical Removal of Diethyl Phthalate from Water. Catalysts, 11(8), 882. https://doi.org/10.3390/catal11080882









